Volume 30 Number 1
Recent insights into pharmaceutical treatments for underactive bladder: a scoping review of recent studies
Charlotte Phelps, Sarah Tynan, Christian Moro
Licensed under CC BY 4.0
Keywords lower urinary tract symptoms, urinary bladder, detrusor underactivity, PRISMA, bladder dysfunction
For referencing Phelps C, Tynan S and Moro C. Recent insights into pharmaceutical treatments for underactive bladder: a scoping review of recent studies. Australian and New Zealand Continence Journal 2024;30(1):4-10.
DOI
10.33235/anzcj.30.1.4-10
Submitted 23 November 2023
Accepted 11 March 2024
Abstract
Underactive bladder is a relatively prevalent condition, yet there is only limited research into its diagnosis, treatment, and management. This has increased interest in researching novel treatments for underactive bladder and enhancing understanding of the underlying mechanisms of its presentation. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews was followed as the guiding outline. An a priori protocol was developed prior to the commencement of any searching or database assessment and published online on the Open Science Framework, after which the PubMed database was searched for articles published over a five-year period from 1 April 2018 to 1 April 2023. Thirty records were identified, with eight included in the scoping review. Most studies were funded by industry, presenting the risk of considerable bias in study design or reporting. Overall, in the last five years, there have not been any new or upcoming treatments that present a consistent and evidence-based ability, across multiple articles, to alleviate underactive bladder. However, research is ongoing, and some receptor systems may hold some potential to assist in the prevention of the disorder.
Introduction
Underactive bladder (UAB) is a lower urinary tract symptom complex characterised by slow urinary stream, hesitancy, and straining to void.1 The symptoms of UAB are often associated with observations of detrusor underactivity (DU), which is the weakening or shortening of detrusor muscle contraction resulting in the incomplete or prolonged emptying of the bladder.2 Symptoms of nocturia and frequency are also common complaints associated with UAB, as a result of incomplete emptying and urinary retention.2,3 Although parasympathomimetics are often presented as the first-line pharmaceutical treatment option for UAB, the evidence supporting their success is often lacking.4 As such, there is a continued need to review and summarise advancements in the field to identify upcoming or alternative treatments and expand knowledge of underactive bladder treatments.
The prevalence of lower urinary tract symptoms (LUTS) associated with UAB is approximately 9–28% in the adult population,5 and this generally increases with age. Evaluations for non-neurogenic LUTS detected UAB in up to 28% of males aged under the age of 50 years, and 48% of those over the age of 70 years.6 Similarly, UAB was detected in up to 45% of females aged over 70 years.6 Furthermore, DU has been attributed to both myogenic and neurogenic aetiologies and may no longer be exclusively associated with the aging population.7
The absence of DU pathogenesis research corresponds to current ineffective treatment options for UAB.8 Besides parasympathomimetics, treatment options include surgery, pharmacotherapy, intermittent catheterisation, and conservative methods.8 Pharmaceutical treatments include a combination of alpha-adrenergic receptor blockers to reduce outlet obstruction pressure through decreased urethral sphincter tonicity and drugs that promote detrusor contractility, such as cholinergic agents.8 Muscarinic agonists (bethanechol) and cholinesterase inhibitors (distigmine) in combination with alpha-blockers, or prostaglandin E2 and acotiamide, as separate medications, are used for bladder outflow resistance and to increase post-void residual volume (PVR).8,9
However, the efficacy of muscarinic agonists for DU has not been supported by recent literature, due to limited improvement, as well as limited data supporting the continued use of parasympathomimetics for bladder health.4,9 In addition, cholinesterase inhibitors may have adverse side effects such as faecal incontinence, diarrhea, and urination,8 affecting compliance rates as patients are less likely to continue prescribed medication. A recent systematic review and meta-analysis assessed the effectiveness of parasympathomimetics for the treatment of UAB and reported a paucity of evidence with short follow-up periods to support their use. This highlighted the need for well-controlled future trials to further warrant their use.4 The European Association of Urology has emphasised the discontinued use of parasympathomimetics for UAB due to inadequate research and potential side effects, which must be considered for patient compliance.10 Although there is increased occurrence of UAB with ageing populations and an increase in clinical prevalence, UAB and associated DU are primarily unrecognised and, subsequently, under-researched.11
Review Question
With an increasing prevalence in the diagnosis of UAB, and a paucity of viable treatment options, this review scopes the current literature over the last five years to identify any recent research developments that may be of interest to patients and healthcare professionals. This article is guided by the research question: Does recent research in the literature identify any potential tissue targets, or pharmaceutical medications, for the future treatments of underactive bladder?
Methods
Protocol and registration
This scoping review is reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR).12 An a priori protocol was developed prior to the commencement of any searching or database assessment and published online through Open Science Framework (https://osf.io/z2hg4). It was hypothesised that underactive bladder remains an under-researched area, however, there will be suggestions in the literature for future alternative pharmaceutical therapies that may be effective. A preliminary search of MEDLINE, the Cochrane Database of Systematic Reviews and JBI Evidence Synthesis was conducted and no current or underway systematic reviews or scoping reviews on the topic were identified.
Eligibility criteria
This scoping review included all English language full-text articles published on PubMed (MEDLINE database). The PubMed database was searched for articles published over a five-year period from the dates 1 April 2018 to 1 April 2023. The search was undertaken to identify original research papers on pharmaceuticals for the treatment of any contractile disorders associated with UAB. Studies on humans and animals (both in vivo and in vitro) were included from any age, gender, or weight. Patients presenting with UAB as a consequence of surgery or injury were excluded. The review excluded reviews, abstracts, and clinical trials.
Search strategy
The following search string was employed
(formatted for the PubMed search):
(“underactive bladder”[Title/Abstract]
OR UAB[Title/Abstract]
OR “detrusor underactivity”[Title/Abstract]\
OR “bladder underactivity”[Title/Abstract]
OR “Urinary Bladder, Underactive”[Mesh])
AND (Drug[Title/Abstract]
OR medication[Title/Abstract]
OR pharmaceutical*[Title/Abstract]
OR pharmacotherapy[Title/Abstract]
OR alpha-blocker[Title/Abstract]
OR “adrenergic antagonists”[Title/Abstract]
OR “cholinesterase inhibitors”[Title/Abstract]
OR “muscarinic agonists”[Title/Abstract])
AND 2018/04/01:2023/04/01[dp]
NOT (Review[Publication Type]).
To identify the grey literature, a forward-backwards scan was undertaken by two independent authors (CP and ST) by searching the reference lists of the identified studies in the initial search to identify any additional studies not captured from the database search.
Types of Sources
This scoping review considered both experimental and quasi-experimental study designs, including: randomised controlled trials; non-randomised controlled trials; before and after studies; and interrupted time-series studies. In addition, analytical observational studies, including: prospective and retrospective cohort studies; case-control studies; and analytical cross-sectional studies were considered for inclusion. This review also considered descriptive observational study designs, including: case series; individual case reports; and descriptive cross-sectional studies for inclusion.
Study of evidence selection
Two independent authors (CP and ST) conducted the screening of the literature by an initial title and abstract scan followed by a full-text review. This was conducted using Covidence, an online screening and data extraction tool (covidence.org, Melbourne, VIC, Australia). Data extraction was conducted manually using Microsoft Excel independently by the two authors (CP and ST). Discrepancies in screening and extractions were referred to a third independent author (CM) for resolution.
Data extraction
The primary outcome of studies included in this scoping review was the effectiveness of pharmaceuticals for underactive bladder treatment and management. This included parameters such as benefits observed, alleviation of any symptoms associated with UAB (infection, retention, stones etc.), follow-up periods, and adverse side effects experienced.
Results
Search results
The electronic search on the PubMed database from 1 April 2018 to 1 April 2023 returned 28 articles, which were title and abstract screened, along with an additional two articles from forward-backwards searching. The outcomes of this screen resulted in eight articles that underwent full-text review. Of the 10 reviewed in full-text, eight met the inclusion criteria and were included in the final analysis of this scoping review (Figure 1).
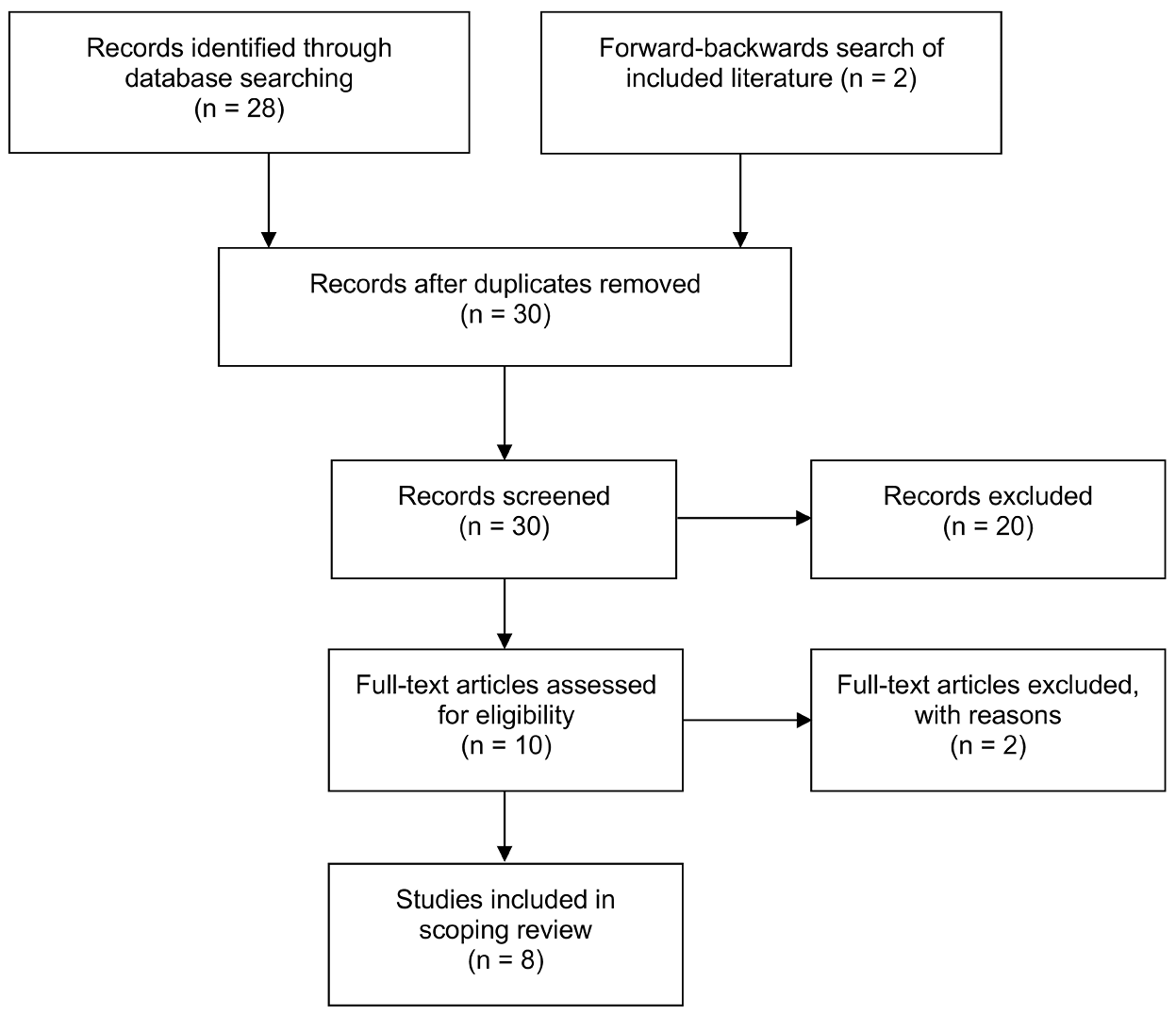
Figure 1. PRISMA flow chart for identification and inclusion of studies.
Included studies
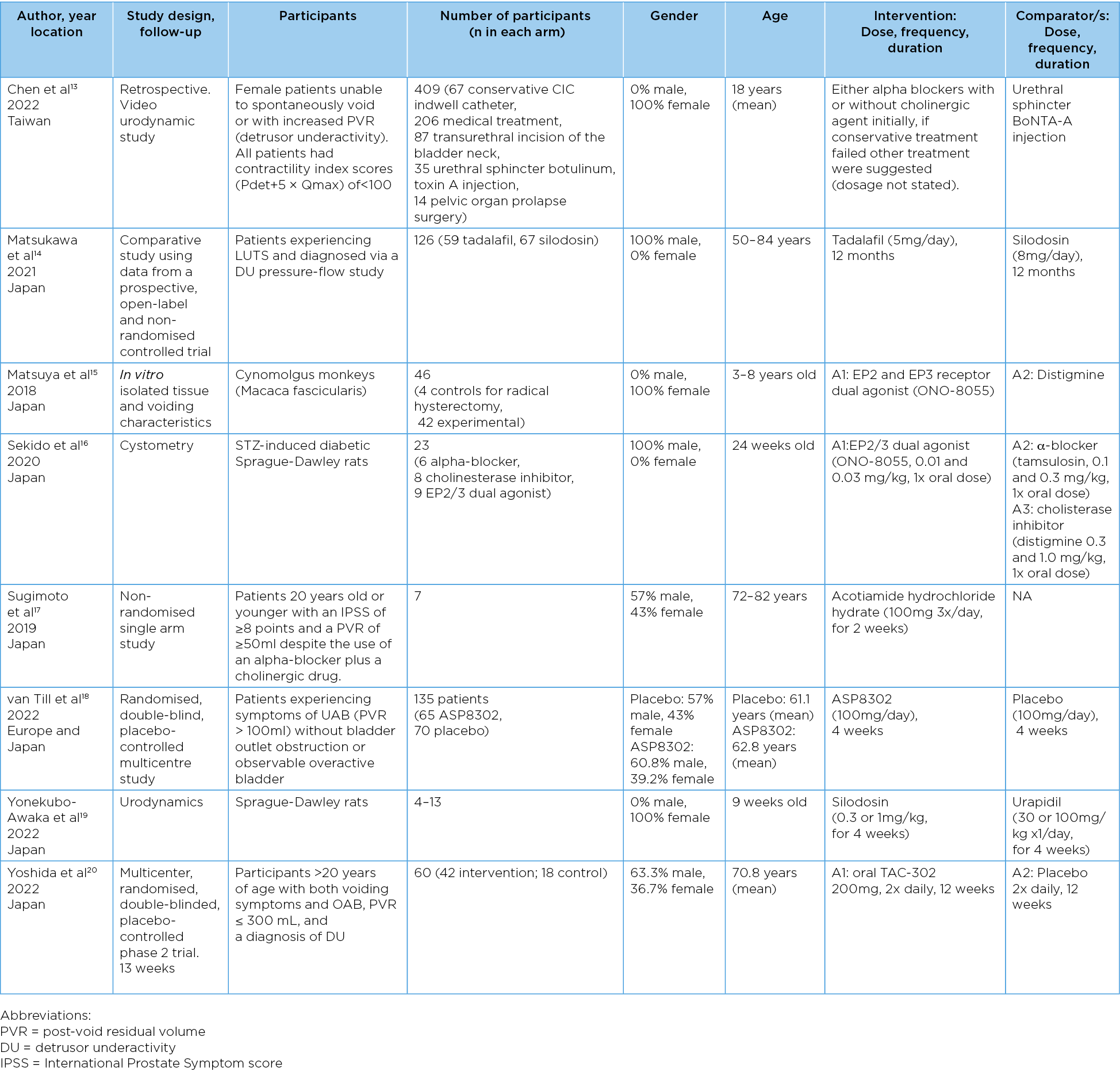
Eight studies were included in this scoping review, comprising a range of study designs and varied study populations (Table 1). Six studies were undertaken in Japan, one study in the United States and one study in Taiwan, in either research laboratories or medical institutions. Five studies included patient participants, and three studies included animals (such as monkeys and rats). Four studies included only female participants and three studies included both males (range 57–63.3%) and females (range 36.7–43%). One study did not have a formal control group. There was also variation in the age of participants, ranging from 24 weeks old to 82 years old.
Table 1. General characteristics of included studies: participant information and study design.
Discussion
Evaluation of the literature
The literature does provide insights into potential new treatments for underactive bladder. Although activation of prostaglandin EP2 and EP3 did not cause major improvements in the rat bladder contractions,16 there were some benefits, such as decreased postvoid residual volume. In addition, in some cases, voiding function was improved to the same degree as with parasympathomimetics15 by using an EP2/EP3 dual agonist. As such, although consistent benefits have not been clearly identified, these suggestions promote prostaglandins as a receptor system that certainly warrants further investigation and potentially important in bladder contractions.21–25
Although recent literature has suggested that parasympathomimetics are unlikely to be helpful in alleviating underactive bladder,4 Sugimoto et al17 identified promising results for acotiamide (a non-selective muscarinic antagonist) towards increasing bladder pressure during voiding. This means that although only seven patients were included in this single-arm study, the potential for parasympathomimetics may not be entirely unevidenced by the research just yet.
Within the bladder tissue itself, there are a number of systems that could be impacted in underactive bladder. The most assessed is the muscarinic receptors, responding to acetylcholine.26 This has also generated some interest in the M3 allosteric modulator ASP8302, which appears well tolerated, and may present some benefits to males with underactive bladder.18 The urothelium also has systems that induce contraction in response to agonists, such as the alpha-adrenoceptor,27 5-HT,28 and prostanoid29 receptors. It is unclear how these systems are involved in overall bladder contractions, but any inhibition of the receptors or second messenger mechanisms may, in some way, inhibit bladder function and present as underactive bladder. Alternatively, mechanisms such as activation of the nitric oxide pathway30 or beta-adrenoceptor31 system may directly induce relaxation of the tissue itself. The nitric oxide system has been of partial recent interest, with both tadalafil and silodosin demonstrating some benefits towards the reduction of lower urinary tract symptoms in men with non-neurogenic detrusor underactivity.14 As more research is conducted into the mechanisms underlying bladder physiology, and the receptors involved, systems such as these may present opportunistic areas for future research.
Of particular concern is the potential for high risk of bias within the literature. Six of the eight studies received funding from pharmaceutical companies with an interest in the treatment regimens. Although for each case the connection was clearly disclosed, this raises the prospect that independent, conflict-free research might be of interest to even the balance and reduce bias across the samples. This introduction of pharmaceutical findings would also make meta-analyses difficult, as the high risk of bias would limit the usefulness of the results presented in a broader context.
Conclusion
In conclusion, there remains limited recently published research into treatments for underactive bladder, and there is great variety in the methods used for their assessment, making summated and general conclusions challenging. Study findings are confounded by a potentially high risk of bias, due to a predominance of industry-funded studies. Nonetheless, pre-clinical research is ongoing and uncovering potential receptor systems that could be dysfunctional in the urinary bladder, presenting these as novel targets for future treatment development.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Charlotte Phelps
Centre for Urology Research, Faculty of Health Sciences and Medicine, Bond University, QLD, Australia
Sarah Tynan
Centre for Urology Research, Faculty of Health Sciences and Medicine, Bond University, QLD, Australia
Christian Moro*
Centre for Urology Research, Faculty of Health Sciences and Medicine, Bond University, QLD, Australia
Email cmoro@bond.edu.au
*Corresponding author
References
- Chapple CR, Osman NI, Birder L, Dmochowski R, Drake MJ, van Koeveringe G, et al. Terminology report from the International Continence Society (ICS) Working Group on Underactive Bladder (UAB). Neurourol Urodyn. 2018;37(8):2928-2931. doi:10.1002/nau.23701
- Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol. 2013;15(1):11–22. doi:10.3909/riu0558
- Uren AD, Drake MJ. Definition and symptoms of underactive bladder. Investig Clin Urol. 2017;58(s2):S61–S7. doi:10.4111/icu.2017.58.S2.S61
- Moro C, Phelps C, Veer V, Clark J, Glasziou P, Tikkinen KAO, et al. The effectiveness of parasympathomimetics for treating underactive bladder: A systematic review and meta-analysis. Neurourol Urodyn. 2021;41(1):127–139. doi:10.1002/nau.24839
- Osman NI, Esperto F, Chapple CR. Detrusor underactivity and the underactive bladder: A systematic review of preclinical and clinical studies. Eur Urol. 2018;74(5):633–643. doi:10.1016/j.eururo.2018.07.037
- Sawaqed F, Abughosh Z, Suoub M. The prevalence of detrusor underactivity and its symptoms co-relation with urodynamic study findings in patients with lower urinary tract symptoms. Res Rep Urol. 2020;12:415–422. doi:10.2147/rru.S264237
- Ahmed A, Farhan B, Vernez S, Ghoniem GM. The challenges in the diagnosis of detrusor underactivity in clinical practice: A mini-review. Arab J Urol. 2016;14(3):223–227. doi:10.1016/j.aju.2016.06.005
- Bayrak Ö, Dmochowski RR. Underactive bladder: A review of the current treatment concepts. Turk J Urol. 2019;45(6):401–409. doi:10.5152/tud.2019.37659
- Jiang Y-H, Lee C-L, Jhang J-F, Kuo H-C. Current pharmacological and surgical treatment of underactive bladder. Tzu Chi Med J. 2017;29(4):187–191. doi:10.4103/tcmj.tcmj_122_17
- Barendrecht MM, Oelke M, Laguna MP, Michel MC. Is the use of parasympathomimetics for treating an underactive urinary bladder evidence-based? BJU Int. 2007;99(4):749–752. doi:10.1111/j.1464-410X.2006.06742.x
- Yu YD, Jeong SJ. Epidemiology of underactive bladder: Common but underresearched. Investig Clin Urol. 2017;58(Suppl 2):S68–S74. doi:10.4111/icu.2017.58.S2.S68
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/m18-0850
- Chen SF, Jhang JF, Jiang YH, Kuo HC. Treatment outcomes of detrusor underactivity in women based on clinical and videourodynamic characteristics. Int Urol Nephrol. 2022;54(6):1215–1223. doi:10.1007/s11255-022-03175-8
- Matsukawa Y, Majima T, Funahashi Y, Fujita T, Ishida S, Kato M, et al. Effects of tadalafil versus silodosin on voiding function in male patients with non-neurogenic detrusor underactivity: A comparative study using propensity score matching. Int J Urol. 2021;28(4):411–416; doi:https://doi.org/10.1111/iju.14481
- Matsuya H, Sekido N, Kida J, Mashimo H, Wakamatsu D, Okada H. Effects of an EP2 and EP3 receptor dual agonist, ONO-8055, on a radical hysterectomy-induced underactive bladder model in monkeys. Low Urin Tract Symptom. 2018;10(2):204–211; doi:10.1111/luts.12166
- Sekido N, Otsuki T, Kida J, Mashimo H, Wakamatsu D, Okada H, et al. EP2 and EP3 receptors as therapeutic targets for underactive bladder/detrusor underactivity due to diabetic cystopathy in a type 1 diabetic rat model. Low Urin Tract Symptom. 2020;12(3):285–291; doi:10.1111/luts.12317
- Sugimoto K, Akiyama T, Matsumura N, Minami T, Uejima S, Uemura H. Efficacy of acotiamide hydrochloride hydrate added to α-blocker plus cholinergic drug combination therapy. Int J Urol. 2019;26(8):848–849; doi:10.1111/iju.14019
- van Till JWO, Arita E, Kuroishi K, Croy R, Oelke M, van Koeveringe GA, et al. Muscarinic-3-receptor positive allosteric modulator ASP8302 in patients with underactive bladder. A randomized controlled trial. Neurourol Urodyn. 2022;41(5):1139–1148.doi:10.1002/nau.24931
- Yonekubo-Awaka S, Tezuka M, Tatemichi S, Takeda H. Therapeutic effects of silodosin and urapidil on underactive bladder associated with diabetic cystopathy. Low Urin Tract Symptom. 2022;14(6):434–441. doi:10.1111/luts.12462
- Yoshida M, Gotoh M, Yokoyama O, Kakizaki H, Yamanishi T, Yamaguchi O. Efficacy of TAC-302 for patients with detrusor underactivity and overactive bladder: a randomized, double-blind, placebo-controlled phase 2 study. World J Urol. 2022;40(11):2799–2805. doi:10.1007/s00345-022-04163-4
- Stromberga Z, Chess-Williams R, Moro C. Prostaglandin E2 and F2alpha modulate urinary bladder urothelium, lamina propria and detrusor contractility via the FP receptor. Front Physiol. 2020;11(705):1–11. doi:10.3389/fphys.2020.00705
- Stromberga Z, Moro C. Which of the primary prostaglandin receptors might play a role in lower urinary tract dysfunction? A narrative review. ANZCJ. 2020;26(3):58–60. doi:10.33235/anzcj.26.3.58-60
- Phelps C, Chess-Williams R, Moro C. The dependence of urinary bladder responses on extracellular calcium varies between muscarinic, histamine, 5-HT (serotonin), neurokinin, prostaglandin, and angiotensin receptor activation. Front Physiol. 2022;13; doi:10.3389/fphys.2022.841181
- Phelps C, Chess-Williams R, Moro C. Ageing influences detrusor contractions to prostaglandin, angiotensin, histamine and 5-HT (serotonin), independent to the Rho kinase and extracellular calcium pathways. Sci Rep. 2023;13(1):18062; doi:10.1038/s41598-023-44916-8
- Phelps C, Chess-Williams R, Moro C. The role of intracellular calcium and Rho kinase pathways in G protein-coupled receptor-mediated contractions of urinary bladder urothelium and lamina propria. Am J Physiol Cell Physiol. 2023;324(3):C787–C797. doi:10.1152/ajpcell.00441.2022
- Moro C, Uchiyama J, Chess-Williams R. Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol. Urology. 2011;78(6):e9–15. doi:10.1016/j.urology.2011.08.039
- Moro C, Phelps C. Urothelium removal does not impact mucosal activity in response to muscarinic or adrenergic receptor stimulation. Tissue Barriers. 2022:2099214; doi:10.1080/21688370.2022.2099214
- Moro C, Edwards L, Chess-Williams R. 5-HT2A receptor enhancement of contractile activity of the porcine urothelium and lamina propria. Int J Urol. 2016;23(11):946–51. doi:10.1111/iju.13172
- Stromberga Z, Chess-Williams R, Moro C. The five primary prostaglandins stimulate contractions and phasic activity of the urinary bladder urothelium, lamina propria and detrusor. BMC Urol. 2020;20(1):48; doi:10.1186/s12894-020-00619-0
- Moro C, Leeds C, Chess-Williams R. Contractile activity of the bladder urothelium/lamina propria and its regulation by nitric oxide. Eur J Pharmacol. 2012;674(2-3):445–449. doi:10.1016/j.ejphar.2011.11.020
- Moro C, Tajouri L, Chess-Williams R. Adrenoceptor function and expression in bladder urothelium and lamina propria. Urology. 2013;81(1):e1–7. doi:10.1016/j.urology.2012.09.011