Volume 30 Number 3
Impact and implications of changing practice in pelvic floor procedures: results from a registry survey
Aruna Kartik, Rasa Ruseckaite, J Oliver Daly, Helen E O’Connell, Jennifer King, Fiona Bach, Elizabeth Gallagher, Jessica Yin, Jerome Melon, Emmanuel Karantanis, James Keck, John Short, Susannah Ahern
Licensed under CC BY 4.0
Keywords survey, clinical practice, pelvic floor procedures, clinical quality registry
For referencing Kartik A, et al. Impact and implications of changing practice in pelvic floor procedures: results from a registry survey. Australian and New Zealand Continence Journal. 2024;30(3):46-59.
DOI
10.33235/anzcj.30.3.46-59
Submitted 29 April 2024
Accepted 18 July 2024
Abstract
Introduction The Australasian Pelvic Floor Procedure Registry (APFPR) was established in 2019 to monitor safety and efficacy of pelvic floor procedures (PFP) that use prostheses. This followed increased international and Australian regulation of mesh for PFPs, resulting in an overall reduction in PFPs and changes to the procedure profile. The aim of this study was to determine contributing factors and clinician responses to clinical practice trends, and implications for the APFPR.
Methods An online clinician survey was developed and distributed between July and October 2022 to APFPR contributing clinicians and USANZ and UGSA members. Descriptive statistics were calculated and stratified analysis performed.
Results Seventy-nine valid responses were received. Approximately two-thirds of respond-ents reported a decline in procedures to implant mesh slings; forty percent reported a decline in mesh sacrocolpopexy; and 40% and 50% reported an increase in explantations of mesh used for stress urinary incontinence (SUI) and pelvic organ prolapse (POP) respectively. Contributing factors for SUI procedure changes were patient preference (83%) and litigation concerns (59%), for POP procedures it was mesh non-availability (81%). Clinician responses included changing to other procedures (SUI 54%; POP 71%); conservative management (SUI 17%); and upskilling and onward referral (14%, 10%) for POP. Responses varied by specialty group. A majority recommended adding native tissue SUI procedures to the APFPR.
Conclusion The survey provides insights into the impact and implications of the reduction in pelvic prostheses over the last 5 years. The addition of native tissue SUI procedures to the APFPR will ensure it maintains clinical relevance in a changing landscape.
Introduction
The Australasian Pelvic Floor Procedure Registry (APFPR) is a clinical quality registry (CQR) that prospectively monitors the safety and quality of pelvic floor procedures (PFPs) that involve mesh or other prostheses including implantation, revision and explantation.1 PFPs are surgical interventions to treat stress urinary incontinence (SUI) and pelvic organ prolapse (POP), commonly diagnosed pelvic floor disorders affecting women.2, 3
The success with initial outcomes and durability following the introduction of the mesh sling procedures in the late 1990s led to optimism over potentially improved efficacy for mesh-based prolapse repairs. However, this was followed by significant safety concerns with legal proceedings being brought against mesh manufacturers in Australia.4 Along with advocacy by consumer support groups, these events paved the way for a Senate Committee Inquiry to investigate transvaginal mesh complications suffered by women. The Senate Inquiry in 2018 recommended the establishment of a CQR to monitor and track PFPs that use high-risk implantable devices and to support the Therapeutic Goods Administration (TGA) with its post-marketing surveillance. Consequently, the APFPR came into existence in 2019 with funding from the Commonwealth Department of Health with aims to collect information on outcomes of PFPs involving prostheses and provide benchmarked reports to surgeons and hospitals to support continuous improvement in PFP care.5 The recently published 2023 APFPR Annual Report presents information on approximately 600 PFPs including revisions and explantations.6
Concurrently, the TGA embarked on reviews of pelvic prostheses which resulted in the withdrawal of transvaginal mesh products for POP and single-incision slings for SUI from the Australian Register of Therapeutic Goods (ARTG) in 2017 along with the up-classification of risk levels for many mesh products.7 The TGA actions combined with the medicolegal processes saw manufacturers cease development and marketing, and even withdraw mesh products which drastically reduced their availability for surgical use.8
Anecdotal claims of declines in the use of surgical prostheses for PFDs were corroborated by research examining the Medicare Benefits Schedule (MBS) procedure codes and Australian Institute of Health and Welfare (AIHW) hospital operative data.9, 10 Mid-urethral sling (MUS) insertions and total number of SUI operations halved from 2008 to 2018.10 Total POP procedures declined by 40% from 2006 to 2021.9 The authors reported constraints in determining trends for POP mesh procedures due to MBS and AIHW data prior to 2018 not distinguishing between mesh-related procedures and native tissue repair. Since 2018, transvaginal POP mesh procedures could not be claimed under the MBS item codes.11
The Australian Commission on Safety and Quality in Health Care (ACSQHC) developed resources for consumers, clinicians and health services on credentialing of practitioners to undertake mesh-related PFPs in line with position statements by the relevant colleges and medical societies.12–14 Furthermore, the ACSQHC developed a service model framework for the provision of mesh-related services in each jurisdiction.12
In addition to the above-described changes in the external environment, clinician accounts of patients preferring non-mesh interventions and increasing negative sentiment relating to pelvic mesh were emerging. A review of international registries found that most captured a mix of mesh-related and native tissue procedures to enable comparisons regarding safety and effectiveness.15 Thus the APFPR considered that a survey of surgeons from its participating specialty groups was important to understand changing practice at the practitioner level and the implications for the future scope of the registry. The aims of this study were to ascertain the impact of changes in the external environment on PFPs undertaken by practitioners, and their implications for the APFPR.
Materials and methods
This was a cross-sectional online survey that targeted surgeons from the APFPR participating specialty groups namely: urogynaecologists, urologists, and general gynaecologists from Australia and New Zealand performing PFPs. The survey tool (Appendix A) was developed with input from the clinician representatives on the APFPR clinical advisory committee. It comprised five sections, with a total of 17 questions. Demographic information included specialty group membership, clinician years of practice, jurisdiction of practice, public vs private practice and metropolitan vs regional and rural settings. The clinician survey sought information regarding previous and current surgical practice relating to common PFPs, change in referral patterns, associated factors, and how any changes were managed. Finally, the survey asked questions relating to the future scope of the registry and perceived benefits of participating in the APFPR.
The survey was finalised after pilot testing by the clinicians on the APFPR clinical advisory committee. The link to the survey included an invitation to participate, which explained the aims of the survey, its voluntary nature, and the requirements for participation. An implied consent process was utilised. The survey was administered online through Qualtrics Survey Software from July to October 2022. The survey was completed anonymously and did not seek any identifiable information.
The Qualtrics survey link was distributed by the following Australian and New Zealand surgical societies or colleges: USANZ and UGSA through their mailing lists/newsletters to all their members with a reminder after a week. In addition, the APFPR emailed the survey link to its contributing clinicians and disseminated it to surgeons at specialty group meetings/conferences.
Quantitative data were statistically analysed in two stages. Firstly, descriptive statistics were calculated for appropriate variables and responses reported as both whole numbers and proportions. Secondly, sub-analyses by participant characteristics were undertaken for questions where the participant responses were varied. Stratification by specialty group, hospital setting and years of specialist experience was also performed (this supplementary data can be found in Appendix B). Data analysis was undertaken using the STATA 17 package. Ethics approval was obtained from the Monash University Human Research Ethics Committee, Melbourne, Australia (Project I.D. 34517).
Results
The survey was distributed to approximately 750 USANZ members and 200 UGSA members. Approximately 40% of USANZ members are estimated to currently perform PFPs. This adds up to an eligible population of approximately 500 surgeons.
A total of 99 survey responses were obtained of which 20 were excluded owing to no clinical practice-related questions being completed. So, 79 valid responses were included in the analysis, representing a response rate of 15% (79/500*100).
Table 1 provides an overview of key demographic variables. All three specialty groups were represented in the sample with urogynaecologists, urologists and general gynaecologists comprising 38%, 33% and 29% of the sample respectively. Over 70% of respondents were affiliated with both public and private practice. All jurisdictions participated in the survey with NSW (35%) and Victoria (19%) having the highest representation. 80% of surgeons practiced in metropolitan areas, 18% in regional and 4% in rural settings. The sample represented specialists across a broad range of years of practice.
Table 1. Respondent characteristics
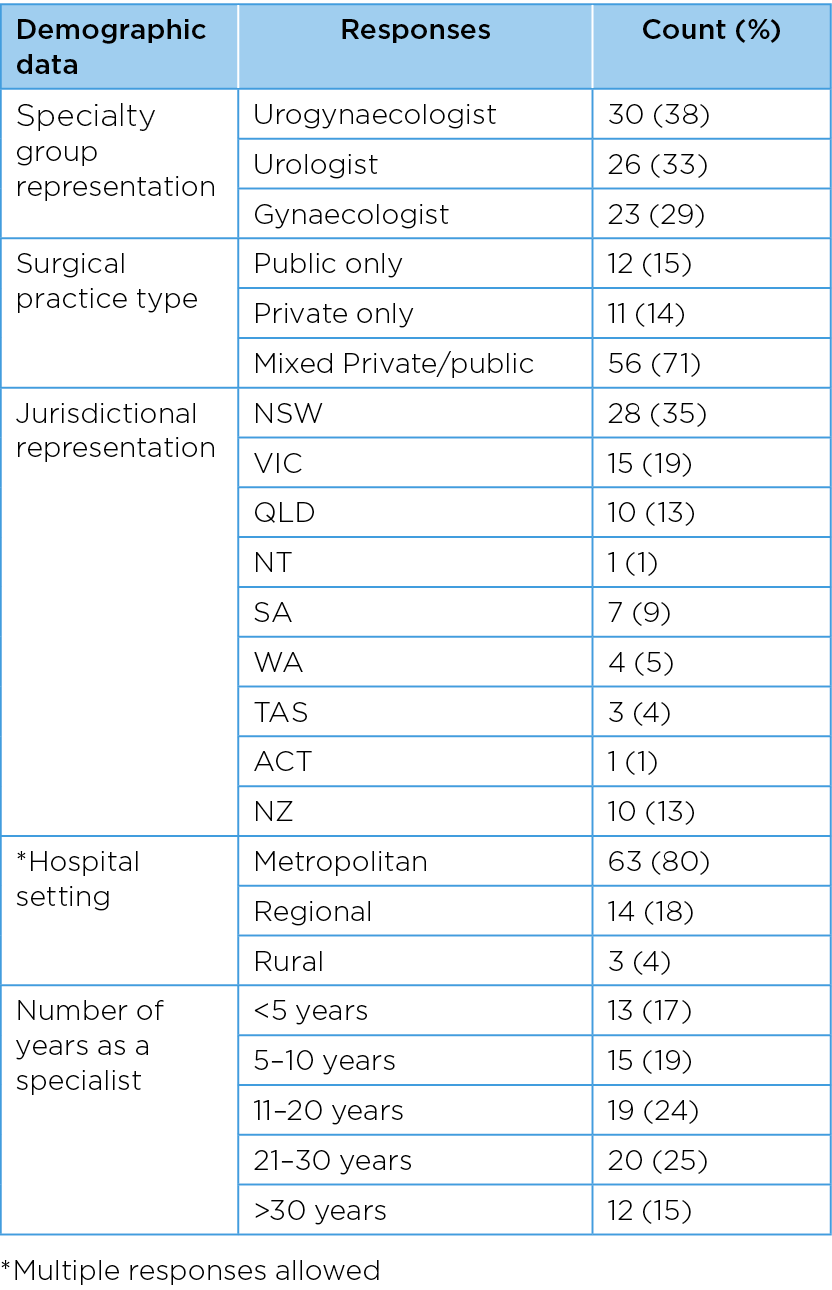
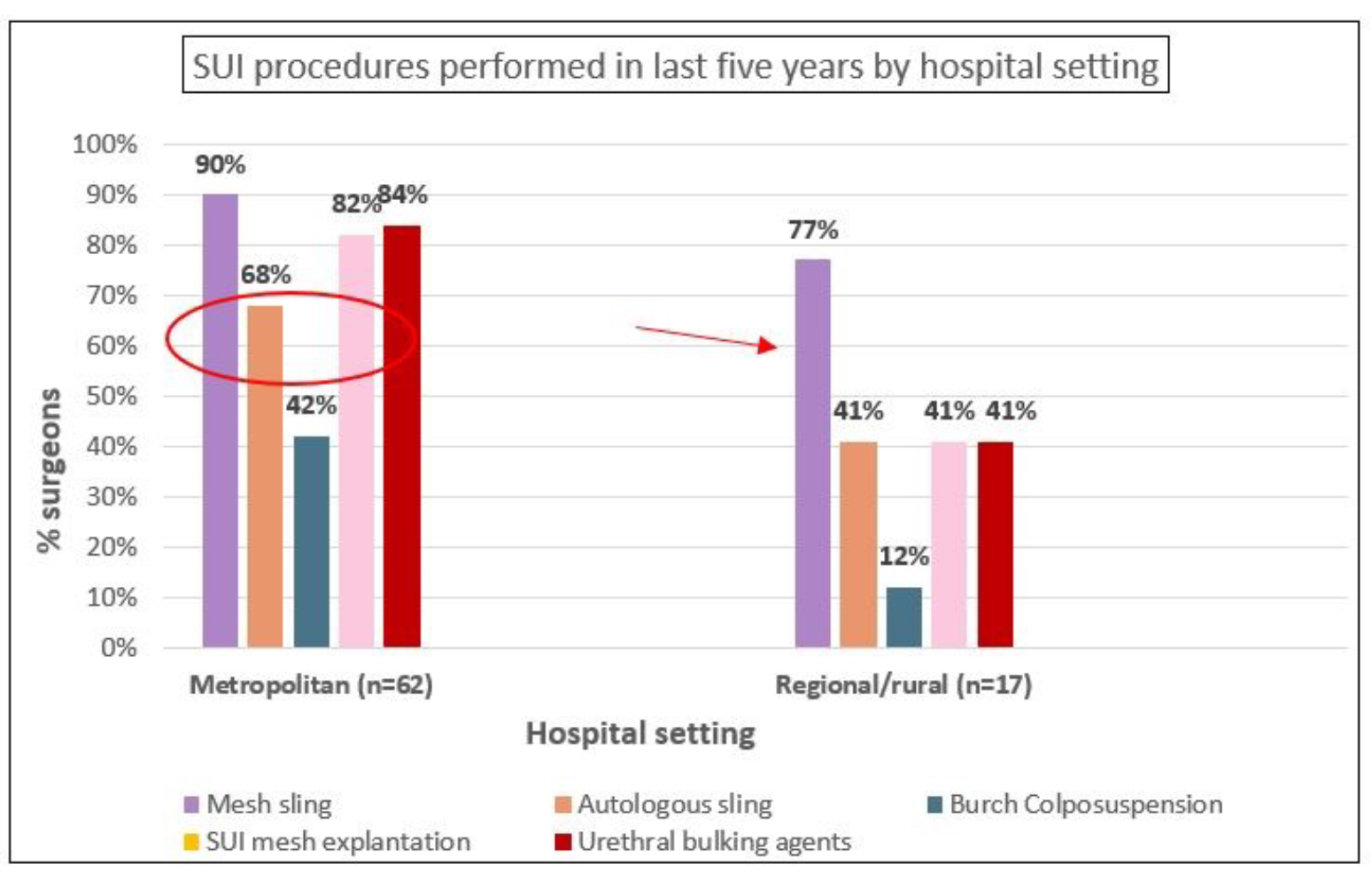
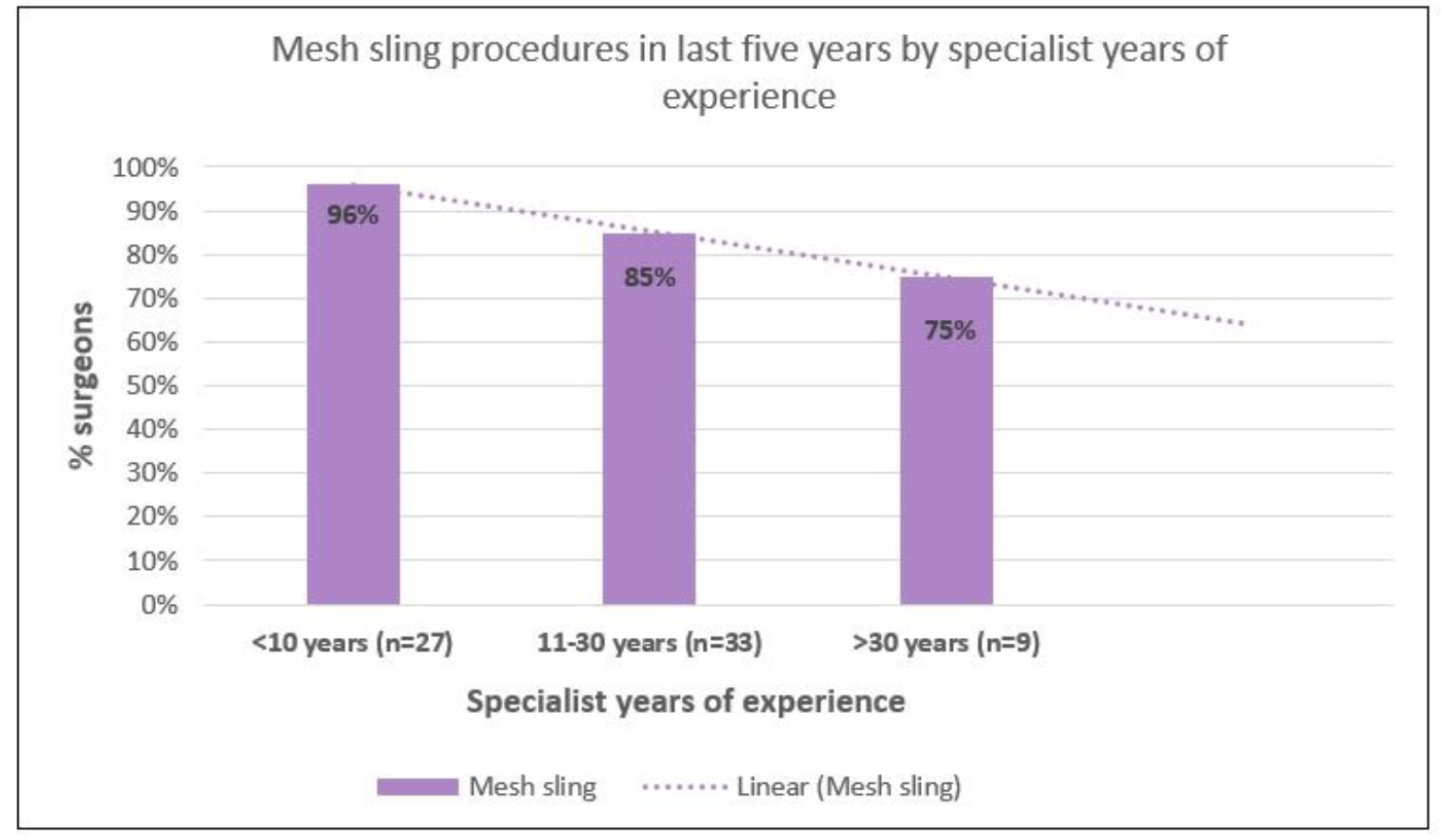
Table 2 describes pre-pandemic PFPs performed by specialty group. Regarding SUI procedures, approximately 87% of respondents reported performing mesh slings; approximately three-quarters performed urethral bulking agent procedures and SUI mesh explantations, with smaller proportions undertaking fascial slings (62%) and Burch colposuspensions (35%). Analysis by specialty group showed that majority of urogynecologists performed all SUI procedures, as did urologists (with the exception of Burch colposuspension). General gynaecologists less commonly undertook SUI procedures other than mesh sling procedures. Supplementary analysis also showed that mesh slings were by far the most commonly performed procedure in regional/rural settings (77%) compared to metropolitan settings where a wider range of SUI procedures were performed. Also, a higher proportion of early career surgeons reported performing mesh slings (96%) as compared to surgeons with more experience with reduction by 20 percentage points from early career to most experienced surgeons.
Table 2. Previous referral patterns for SUI & POP by specialty group
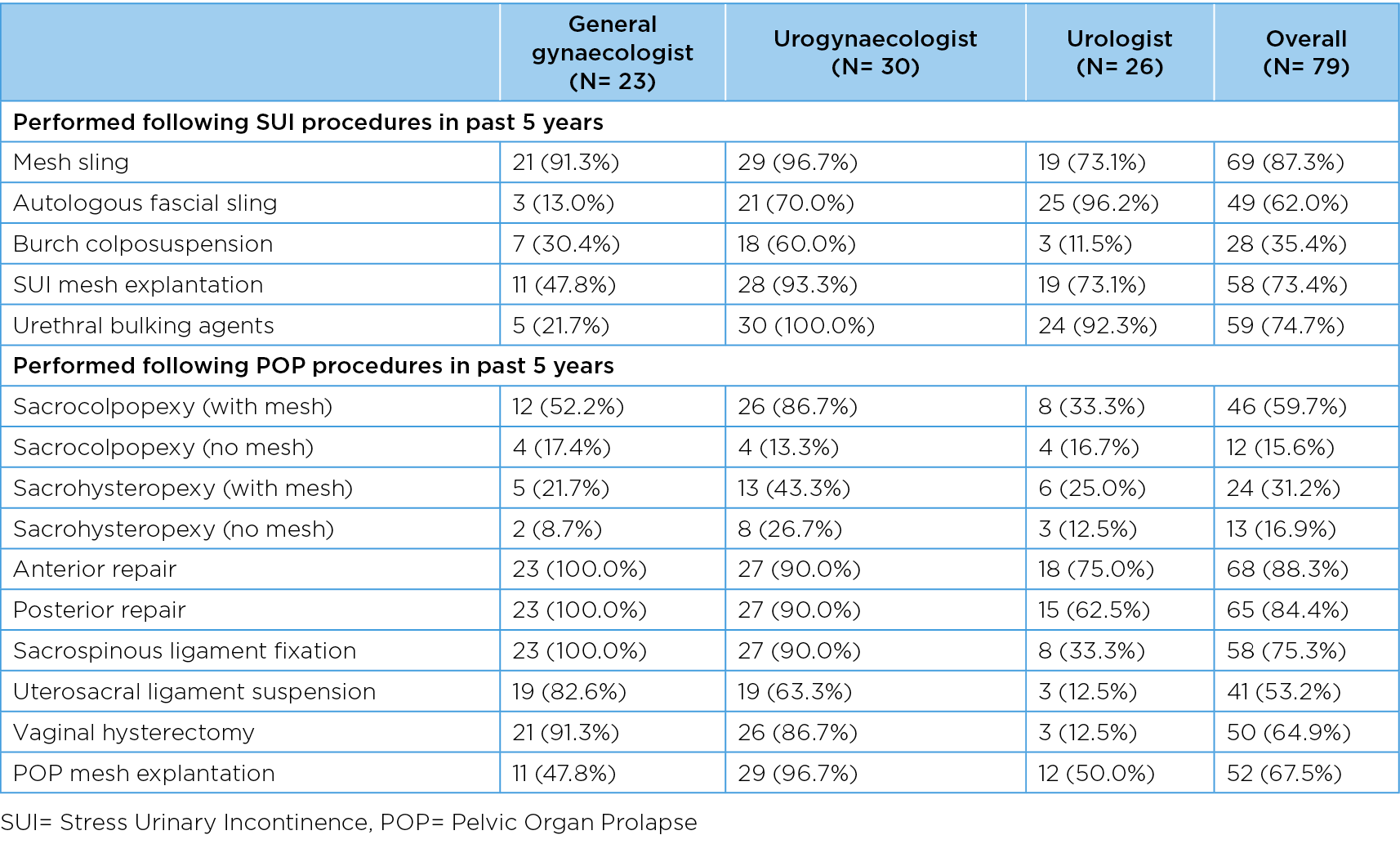
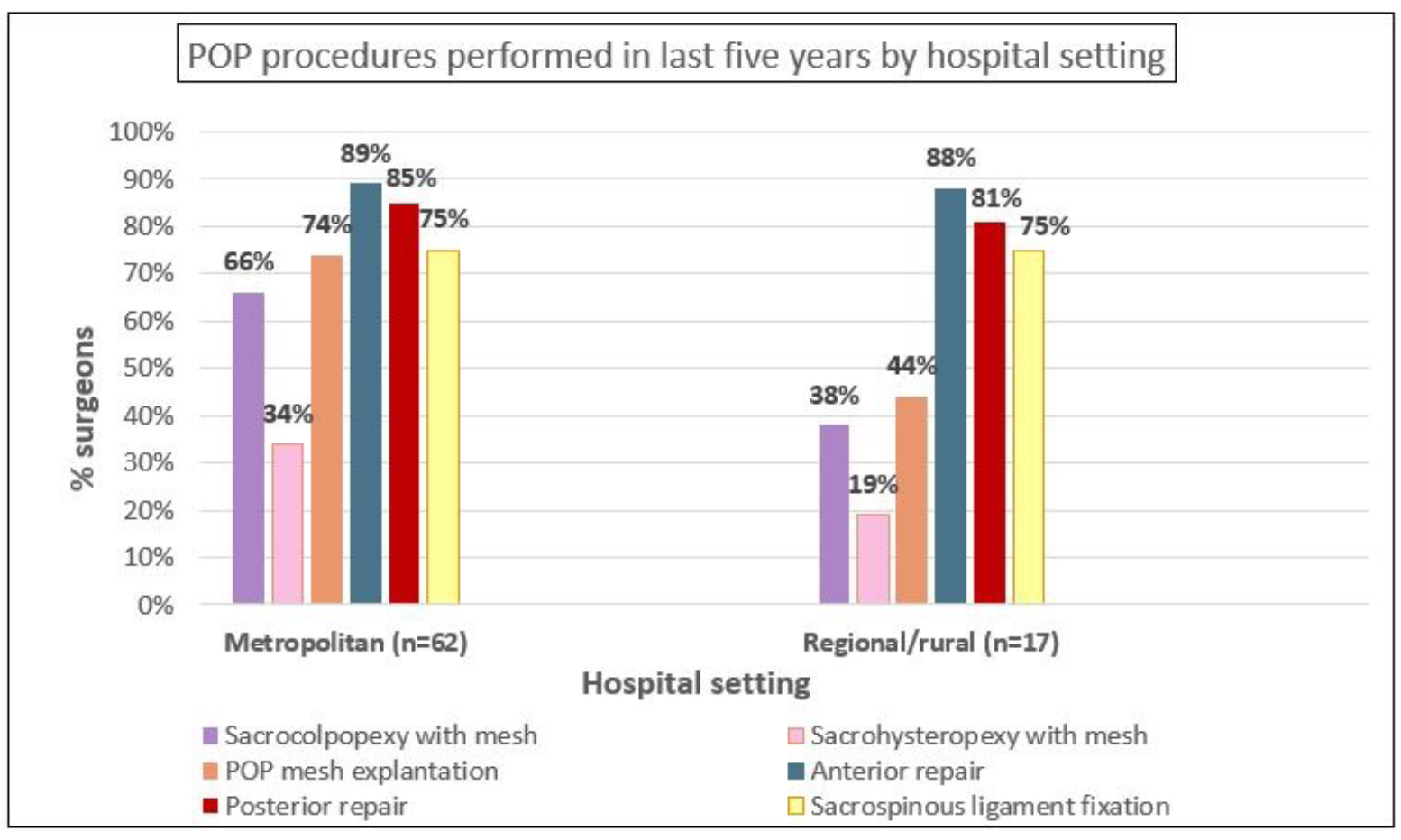
For POP procedures, the most common procedures performed were anterior and posterior repairs (over 80%) and sacrospinous ligament fixations (75%). The main POP procedures captured by the APFPR (mesh sacrocolpopexy, mesh sacrohysteropexy and explantations) were performed by approximately 60%, 30% and 68% of surgeons respectively.
Similar to the SUI procedures, the majority of urogynaecologists undertook all POP procedures, with the exception of native tissue sacrocolpopexy (13%), and mesh and native tissue sacrohysteropexy (43% and 27% respectively). A high proportion of general gynaecologists performed vaginal hysterectomy (91%), which was less commonly undertaken by urologists (13%). POP mesh explantations were undertaken by all specialty groups, including 97% of urogynaecologists, 50% of urologists and 48% of general gynaecologists. Supplementary analysis showed that POP mesh procedures, eg sacrocolpopexy with mesh (and mesh explantations) were more commonly undertaken in metropolitan areas -66% (74%) compared to 38% (44%) in regional/rural areas.
Figure 1A presents SUI procedure practice changes over the last five years. Sixty-four percent of respondents reported a decline in mesh slings; 50% noted an increase in mesh explantation; and over a third reported an increase in other procedures including the use of urethral bulking agents (37%).
Figure 1B reveals POP procedure practice changes, with over 40% of surgeons reporting a reduction in mesh sacrocolpopexy and sacrohysteropexy with a similar proportion recording an increase in mesh explantations. A third to half of respondents reported an increase in sacrohysteropexy and sacrocolpopexy using native tissue.
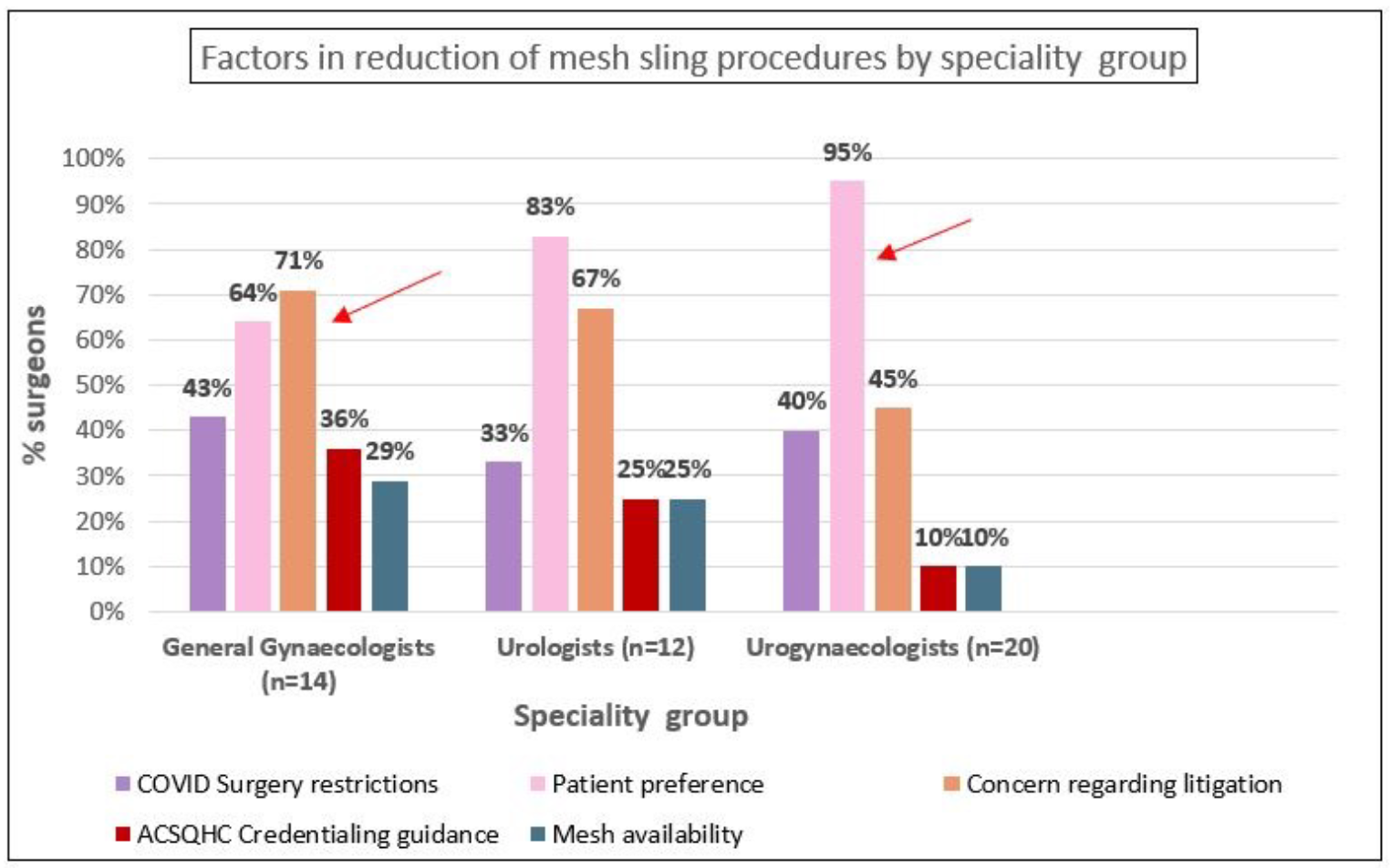
Figure 2A depicts the importance of selected factors associated with the changes in clinical practice. Patient preference (83%) and litigation concerns (59%) were reported as the two most important factors associated with the decline in mesh slings, while mesh availability (81%) followed by patient preference (62%) were most important in the reduction of mesh sacrocolpopexy. Supplementary analysis showed that gynaecologists predominantly reported concern over litigation (71%) while urogynaecologists more often cited patient preference (95%) as contributing to decline in mesh sling procedures.
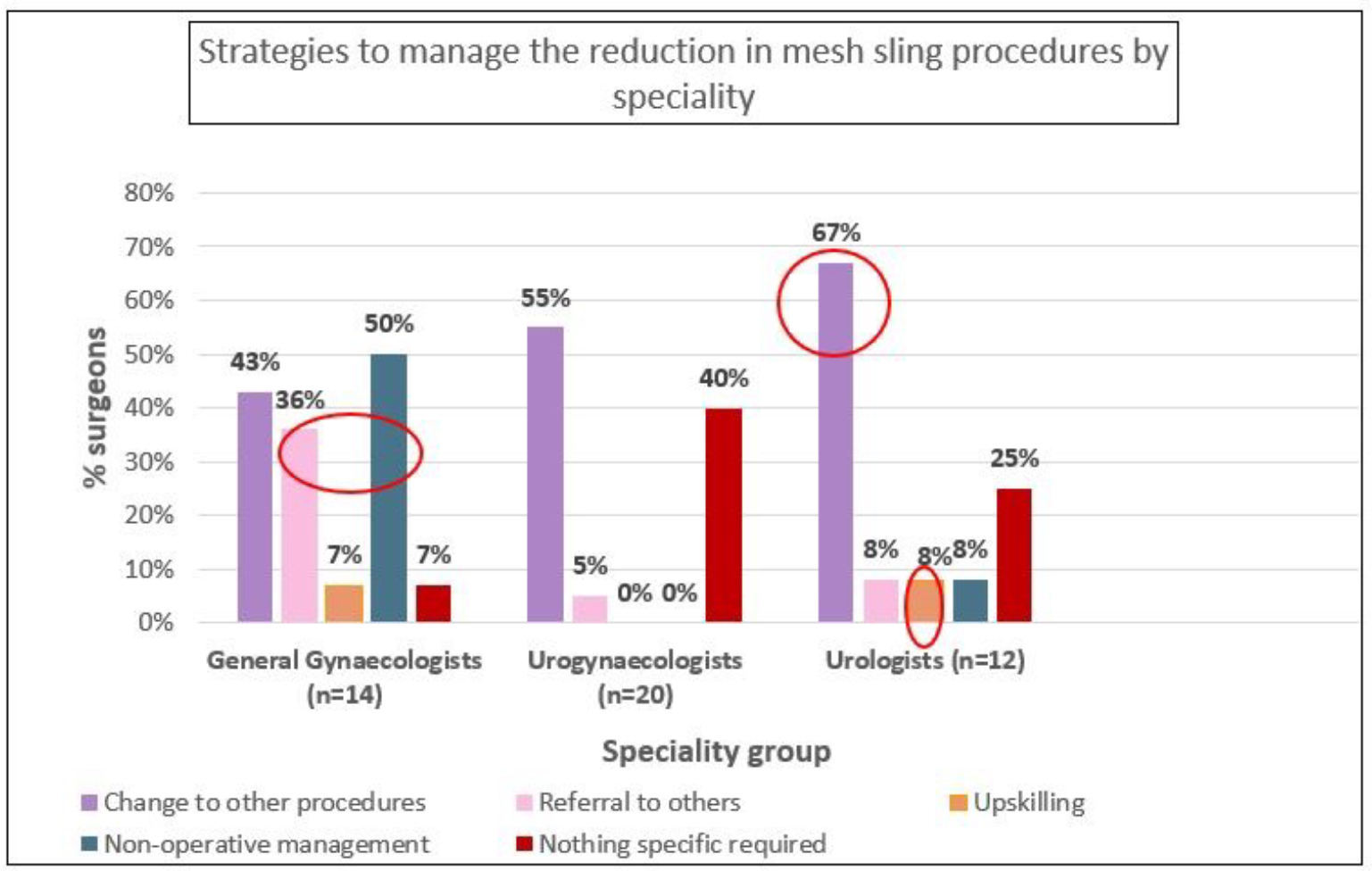
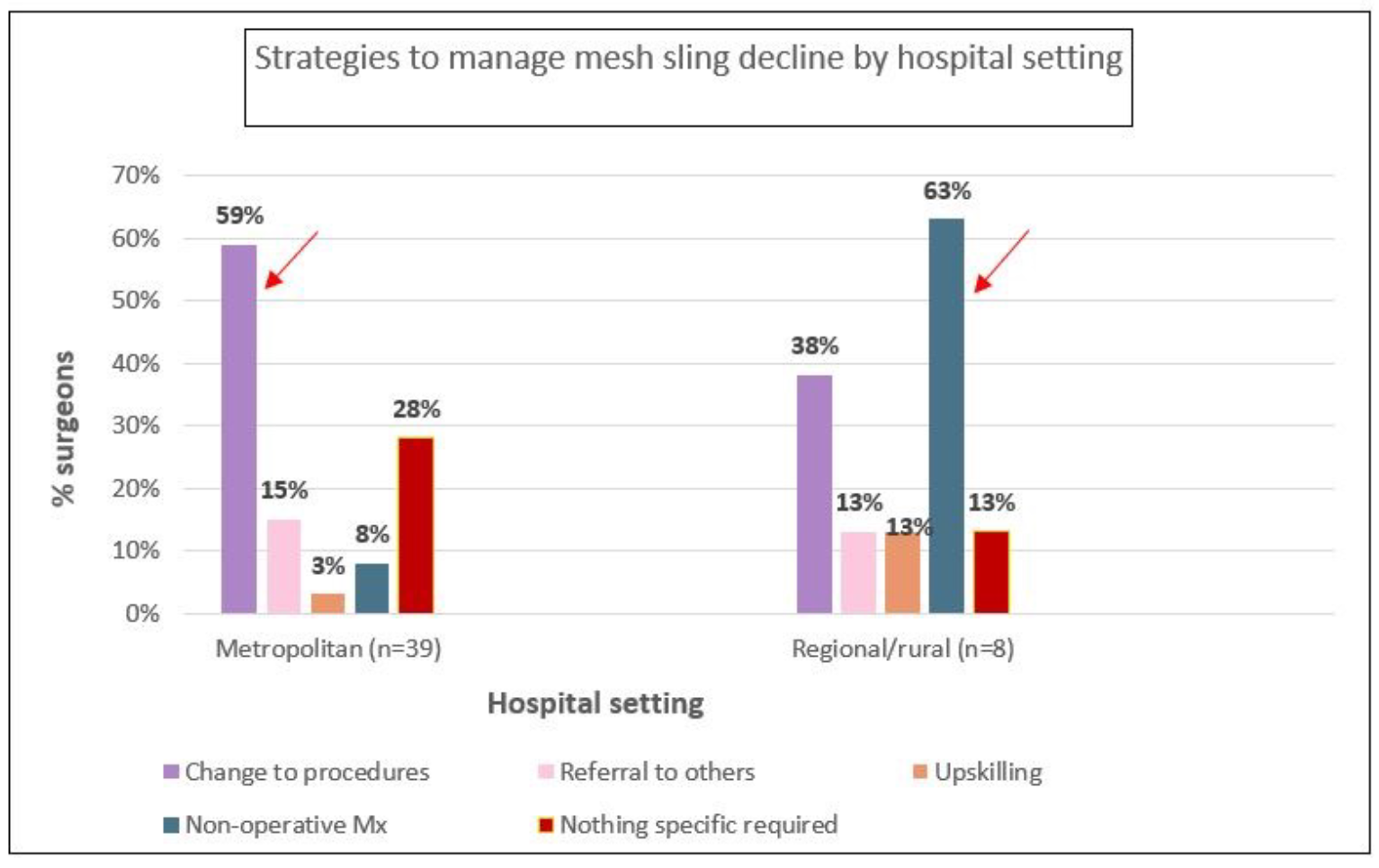
Figure 2B presents clinician responses to manage the aforementioned changes. For SUI procedures, 54% percent of surgeons managed the decline in mesh slings by changing to other procedures, with 17% choosing non-operative management and 15% referring to others. Only 2% reported upskilling in other procedures. Supplementary analysis showed that urologists were more likely than others to report changing to other procedures (67%) or upskilling (8%) to manage this change, while one third of general gynaecologists reported referring pateints to other practitioners, and half of them choose non-operative management. Practitioners in rural/regional areas were more likely to choose non-operative management (63%) while those in metropolitan areas more frequently changed to other procedures (59%).
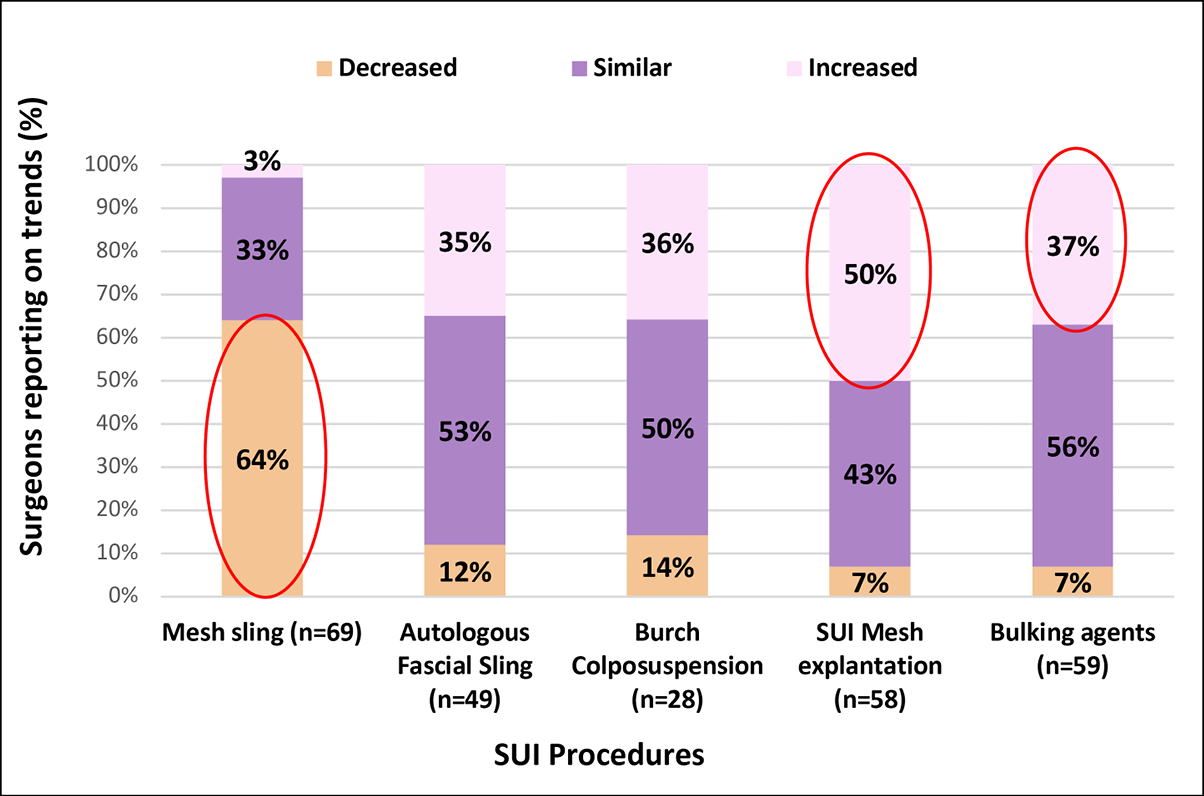
Figure 1A. SUI Procedure trends
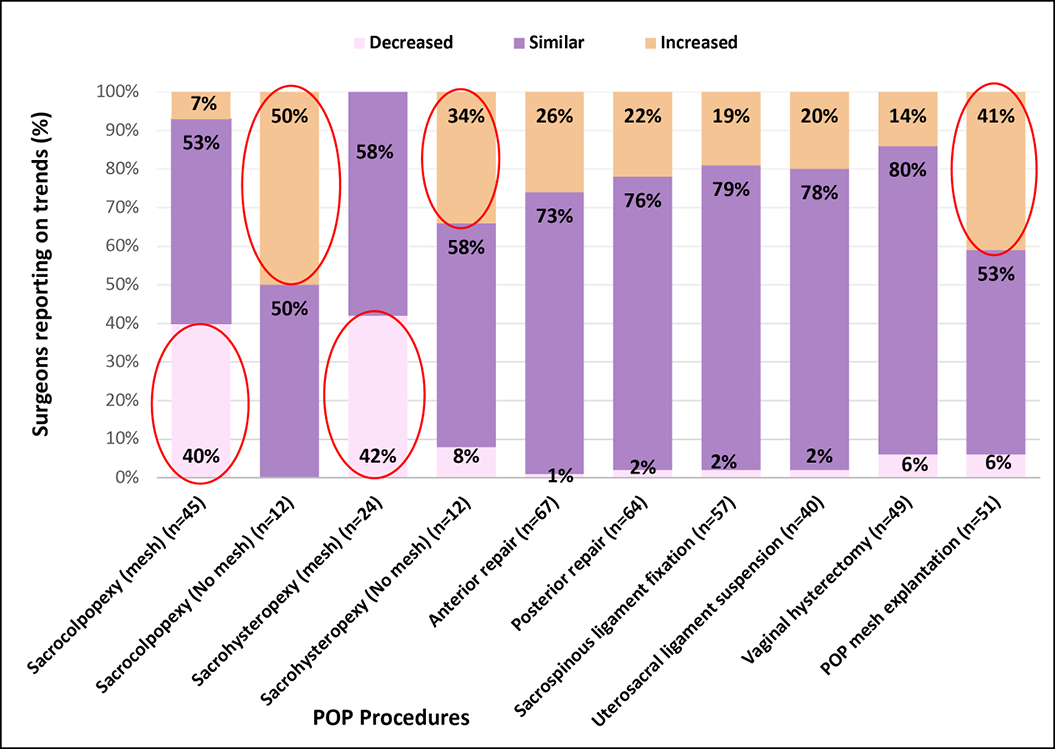
Figure 1B. POP Procedure trends
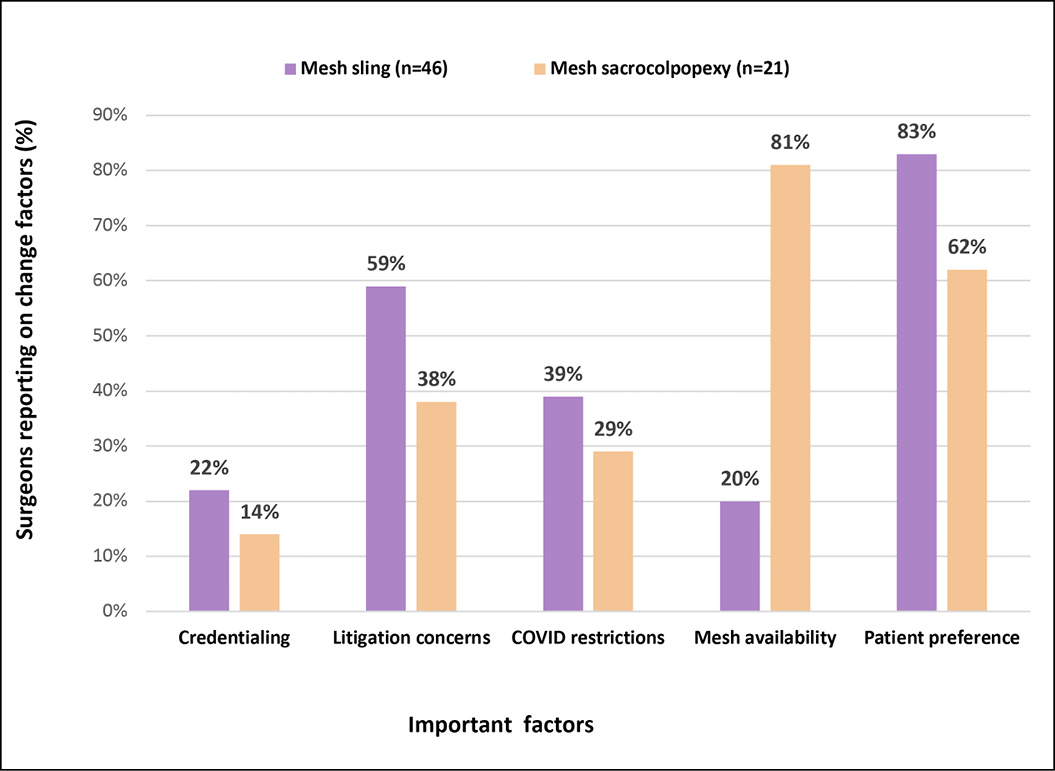
Figure 2A. Change factors for mesh sling and mesh sacrocolpopexy
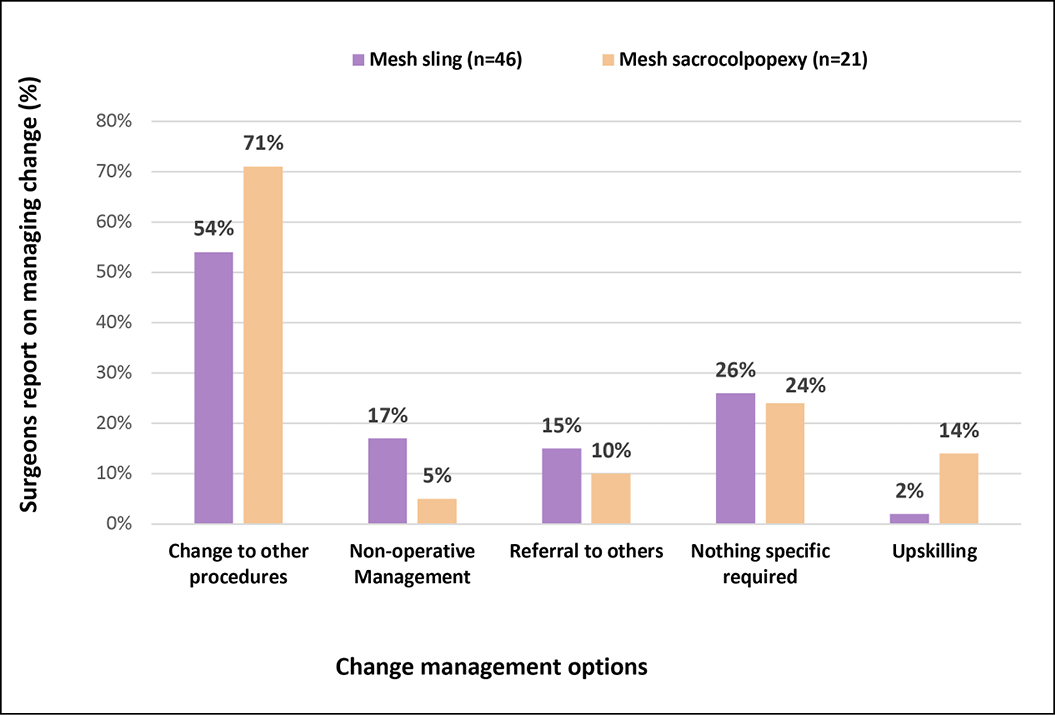
Figure 2B. Clinician responses to change-how was change managed?
Regarding POP procedures, 71% of surgeons managed the decline in mesh sacrocolpopexy by changing to other procedures, with 14% choosing to upskill, 10% referring to others and 5% choosing conservative management. For both SUI and POP, approximately one quarter responded that no specific change in practice was required.
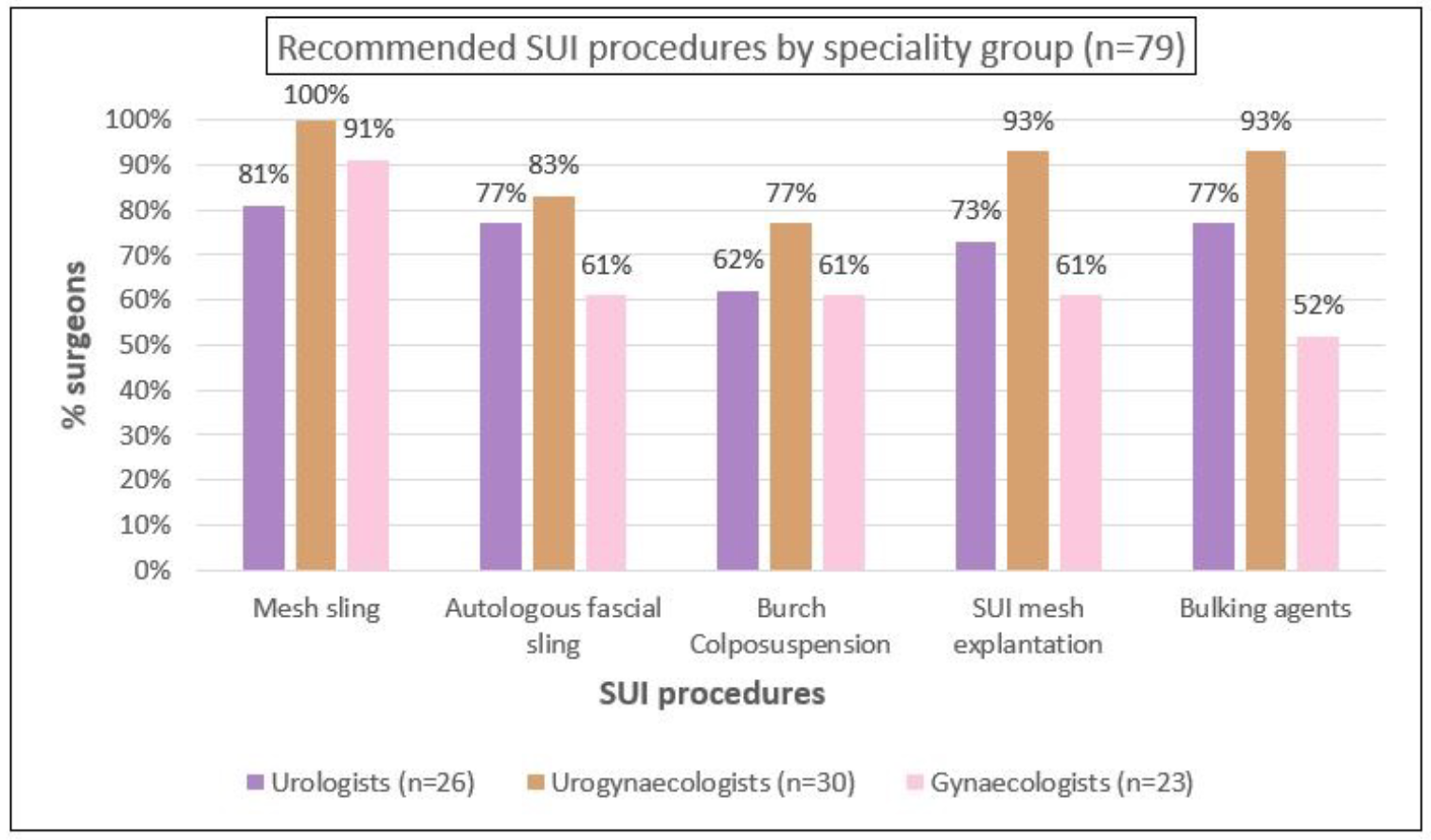
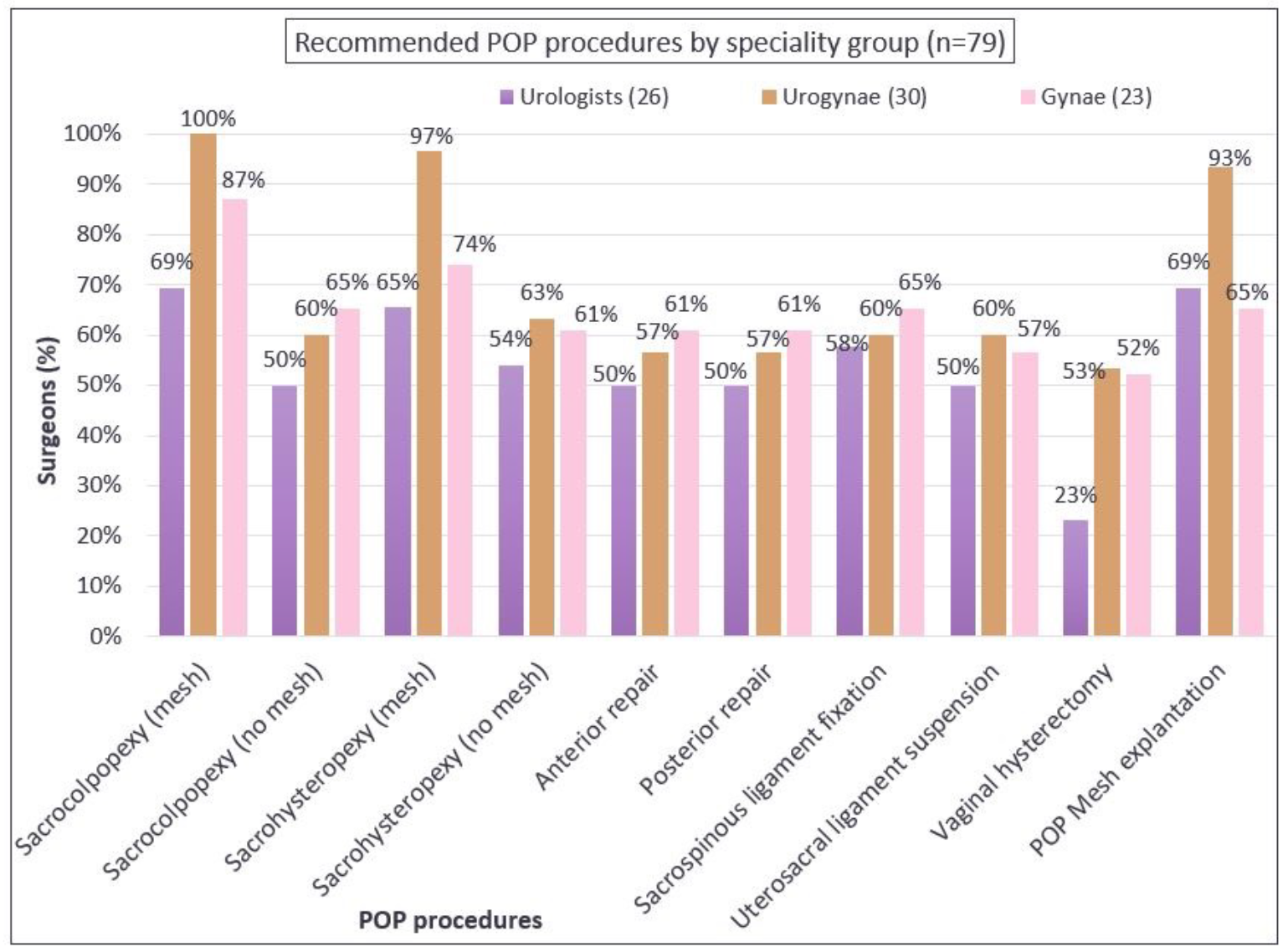
In relation to recommendations for inclusion of procedures in the scope of the registry, more than three-quarters of respondents agreed to continue capturing mesh-related SUI and POP procedures including the use of bulking agents (Figures 3A and 3B). Additionally, 75% of the respondents recommended also capturing data about autologous fascial slings and 67% suggested collecting data about Burch colposuspension. This threshold was not reached for including native tissue POP procedures, where the majority recommended that the APFPR continue to capture only mesh-related POP procedures. There was agreement among specialty groups on the recommendations (Appendix B).
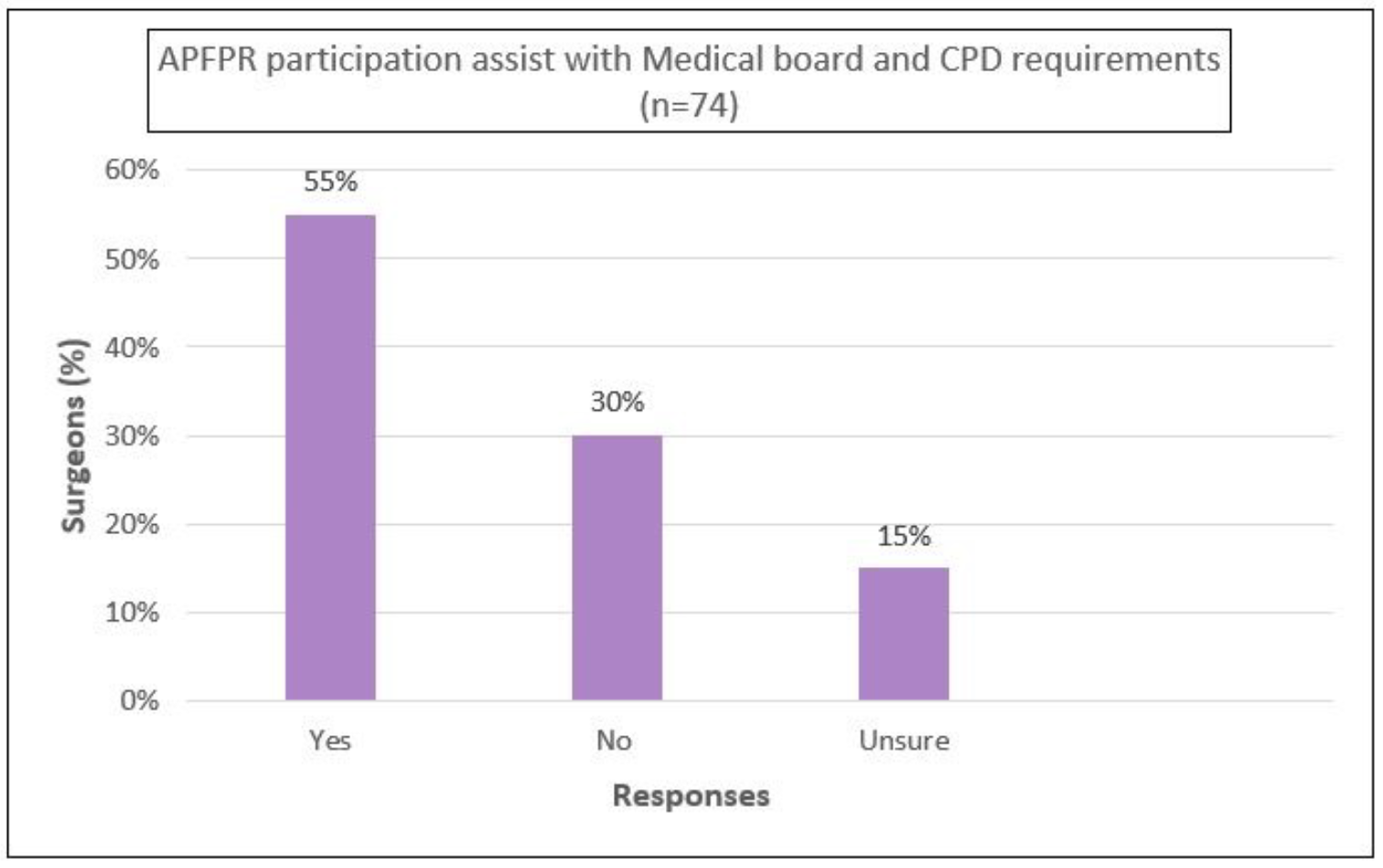
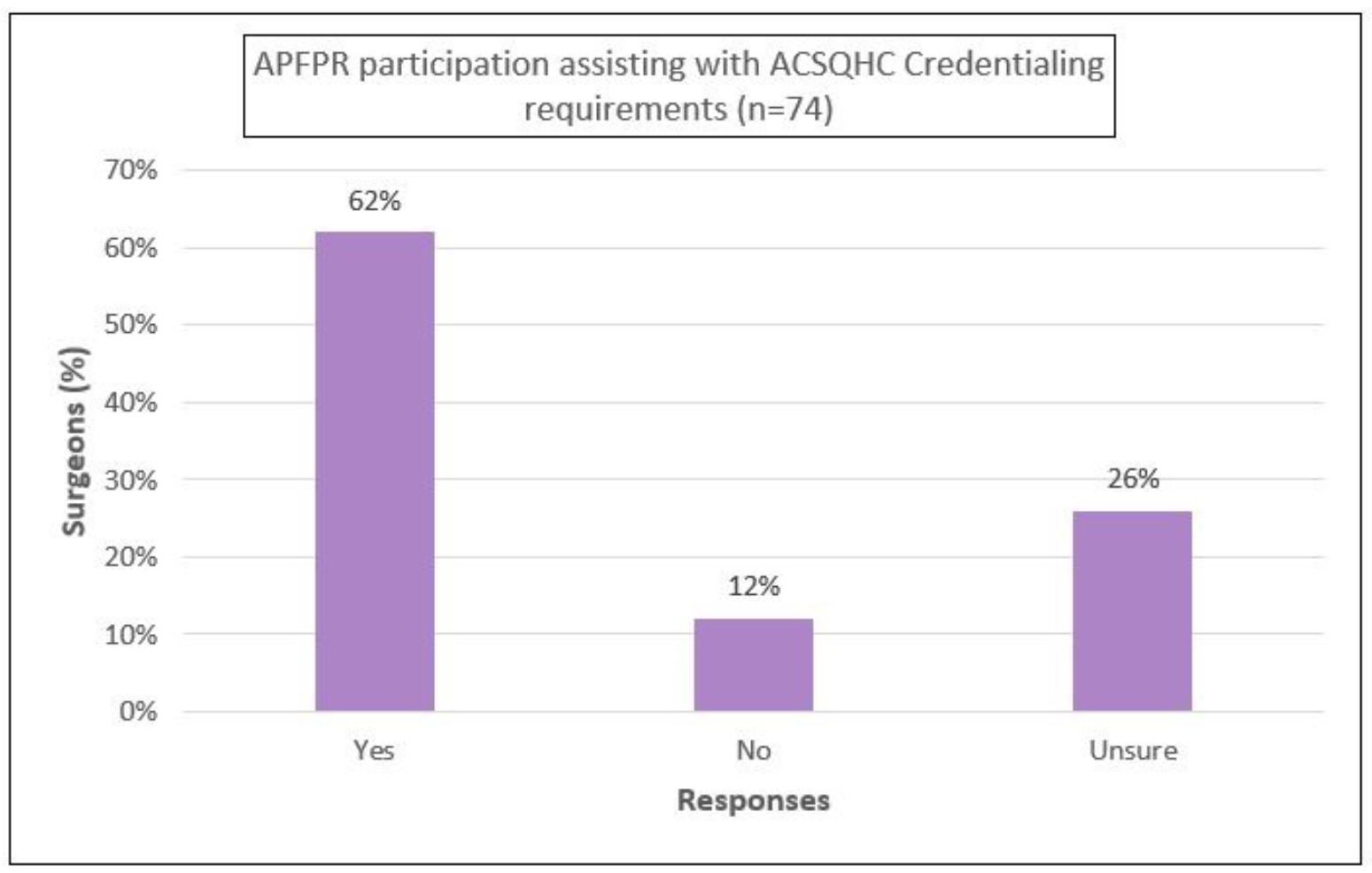
Furthermore, 55% agreed that the APFPR participation was assisting with medical board-mandated outcome monitoring, and 62% agreed that it was meeting the ACSQHC’s credentialing requirements for PFPs (Appendix B).
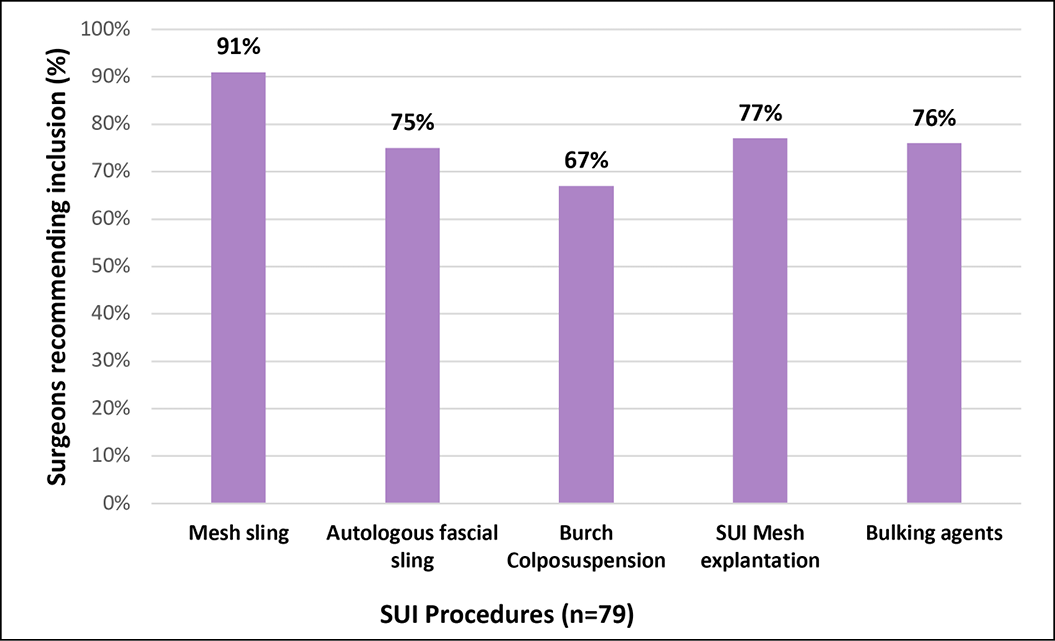
3A. Recommendation for inclusion of SUI procedures
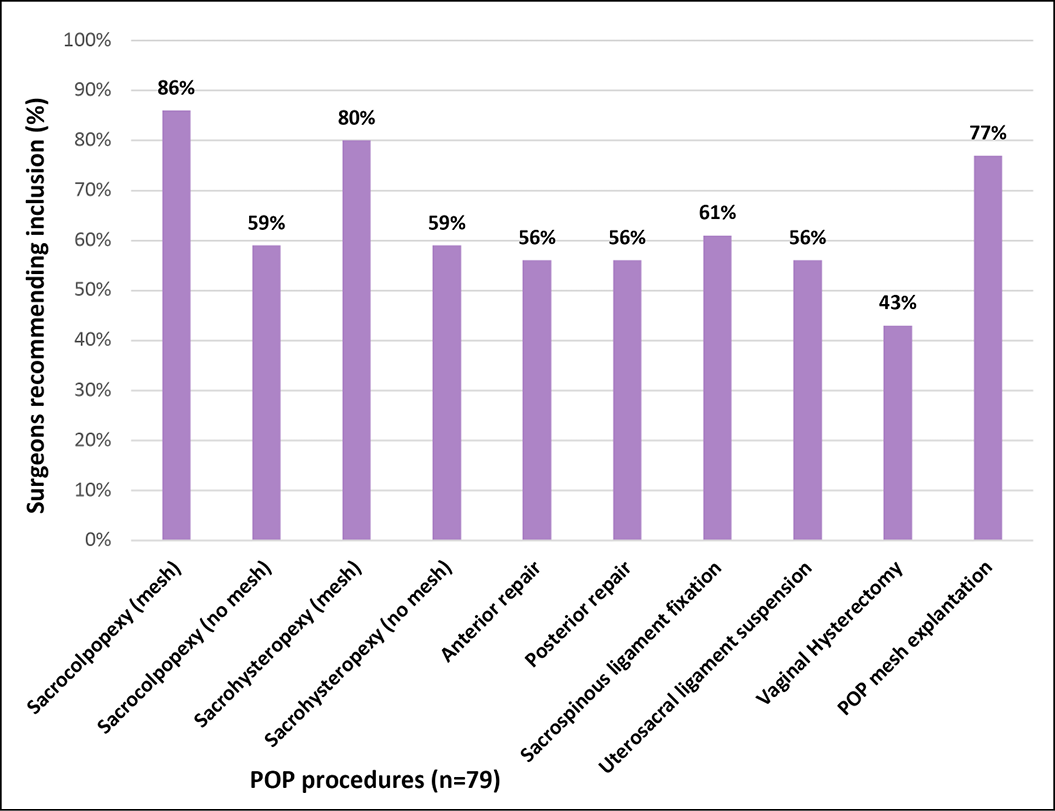
3B. Recommendation for inclusion of POP procedures
Discussion
Data provided by clinicians form the bedrock of a successful clinical quality registry. Following the Senate Inquiry into transvaginal mesh complications, the APFPR was established to systematically monitor and report on mesh-related procedures. With the continued decline in pelvic mesh use, the registry wanted to understand the implications of this on clinical practice, and the surgeon survey provided the basis for informing the future scope of the registry to keep pace with the changing external landscape. The survey received good representation across all specialty groups, hospital settings, practice types and seniority levels.
Since their introduction in the 1990s, mid-urethral slings rose to become the gold standard in minimally invasive treatment of SUI. In Australia, they came to be widely adopted by all specialist groups and are the ACSQHC’s recommended treatment for SUI.16, 17 Our survey found mesh slings to be the most commonly undertaken treatment for SUI in the last five years, and was adopted by all specialty groups, career stages and hospital settings, and continued to be a preferred treatment for SUI.
The decline in mesh sling procedures reported against the backdrop of widespread adoption of mesh slings was similar for all specialty groups, settings and years of practice; and has been verified by an analysis of MBS/ACHI procedure codes in Australia.18 The TGA withdrawal of mini-slings and other transvaginal mesh products from the ARTG in 2017 could potentially have contributed to the declining trend in pelvic mesh use in Australia.19 Not surprisingly, patient preference and litigation concerns were paramount consequent to the media scrutiny of medicolegal proceedings,20 and negative consumer sentiment affecting surgeons’ preferences as observed by Whoriskey et al.21
Most surgeons managed this decline by changing to other procedures and a smaller proportion recommended ongoing conservative management. In the UK, mesh slings for SUI and transvaginal mesh for POP procedures were paused in 2017 and the restriction remains to date.22 The British Society of Urogynaecology Audit in its annual report reveals the growing popularity of urethral bulking agent injections that now represent nearly 70% of all SUI procedures with smaller increases in other native tissue SUI procedures.23 Although the report demonstrates lower efficacy with urethral bulking agents, it nevertheless, represents an acceptable alternative especially for older patients with multiple comorbidities.24
General gynaecologists compared to the other specialists were more likely to refer patients onward or choose non-operative management consistent with the Commission’s recommendation for conservative management prior to surgery.17,25 Rural/regional practitioners were also more likely to choose non-operative management with implications for access to appropriate surgical care in rural/regional areas. If a restriction of mesh slings similar to the mesh pause in New Zealand is implemented in Australia,26 women in rural/regional Australia will be disproportionately disadvantaged, as these patients belong to a lower socio-economic background and are less able to travel for treatment further amplifying inequities in care.27
With POP procedures, the most common procedures were anterior and posterior native tissue repairs. With the imposition of hospital credentialing that requires logbook evidence of appropriate training and restriction on transvaginal mesh procedures after 2017,19, 28 most mesh-related POP procedures including explantations were performed by urogynaecologists in metropolitan settings. This underscores the complex nature of these operations that rely on credentialed surgeons. The decline in POP procedures was the result of non-availability of mesh with more surgeons switching to native tissue procedures. Regulatory authorities considered the risk benefit ratio of POP mesh and determined that this did not justify routine availability.19 New approaches are also being evaluated, such as substituting sacrocolpopexy mesh with fascia lata which may prove promising.29 However, the data in this regard is very limited at present and further comparative data is required.
Recommendations regarding the scope of the registry were in favour of including native tissue SUI procedures. This will allow the APFPR to monitor activity and outcomes for both native tissue and prosthetic slings, as well as bulking agents into the future. The lack of a similar recommendation for native tissue POP procedures suggests that these common procedures have sound outcome data available.
Strengths of our study include that it was an interdisciplinary survey that highlights the breadth of activity and change across Australia. The survey also provided an in-depth exploration of the context, contributing factors and strategies employed to manage practice change. It provides the ‘why’ to the ACHI/MBS data’s ‘what’, and highlights the impacts and implications of change in surgical practice within different specialty groups and regional settings.
Limitations of the study included the low response rate (15%), which is comparable to other surveys of similar populations,21, 30 but it yielded low numbers for the stratified analysis. However, we expect that the denominator of 500 is likely to be an overestimate, as there is no data regarding the number of clinicians who undertake PFPs. Despite this, all specialty groups were similarly represented, and overall the sample provided a broad cross-section of surgical practitioners that perform PFPs.
In conclusion, our study reports on surgeons’ perspectives on the contributing factors to declining mesh use for pelvic floor procedures. Primarily, these appear to be patient preference, litigation concerns and non-availability of mesh products. Most surgeons chose switching to other procedures to address this change. Conceived as a mesh registry following the Senate Inquiry, the APFPR embarked on evaluating its scope and future direction to better serve its stakeholders in a changing landscape. The APFPR is ideally placed to continue examining and monitoring these practice changes at a system level, thereby providing meaningful and actionable information to inform policy and practice in relation to management of pelvic floor disorders.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Acknowledgements
The authors acknowledge the professional colleges of USANZ and UGSA for mailing the survey to their members and to the clinicians who participated in the survey. We also acknowledge the APFPR coordinating team at Monash University for technical support, and assistance with data processing and analysis. The APFPR is supported by funding from the Australian Government, Department of Health and Aged Care, under the National Clinical Quality Registry Program.
Author(s)
Aruna Kartik*
APFPR Coordinator, Department of Epidemiology and Preventive Medicine, Monash University
Rasa Ruseckaite
Department of Epidemiology and Preventive Medicine, Monash University
J Oliver Daly
Department of Epidemiology and Preventive Medicine, Monash University
Mercy Werribee Hospital, Mercy Health Australia
Helen E O’Connell
Department of Epidemiology and Preventive Medicine, Monash University
Department of Surgery, University of Melbourne
Jennifer King
Urogynaecology Department, Westmead Hospital
Fiona Bach
Gynaecology Department, Christchurch Women’s Hospital
Elizabeth Gallagher
Gynaecology Department, Canberra Hospital
Jessica Yin
Urology, Ramsay Health Care-Hollywood clinic
Jerome Melon
Urogynaecology Department, Gold Coast University Hospital
Emmanuel Karantanis
Pelvic Floor Unit, University of New South Wales
James Keck
Colorectal surgery, St Vincent’s Private Hospital, Fitzroy
John Short
Gynaecology Department, Christchurch Women’s Hospital
Susannah Ahern
Department of Epidemiology and Preventive Medicine, Monash University
*Corresponding author
References
- Jayasinghe RT, Ruseckaite R, Dean J, Kartik A, Wickremasinghe AC, Daly O, et al. Establishment and initial implementation of the Australasian Pelvic Floor Procedure Registry. Int Urogynecol J. 2023.
- MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG. 2000;107(12):1460–1470.
- Smith FJ, Holman CDJ, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116(5):1096–1100.
- Parliament of Australia, The Senate Community Affairs References Committee. Number of women in Australia who have had transvaginal mesh implants and related matters: Report. Canberra: Commonwealth of Australia; 2018. https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/MeshImplants/Report
- Ministers Department of Health and Aged Care. $2.3 million to improve safety of pelvic floor surgery (Media Release). Canberra: Commonwealth of Australia Department of Health and Aged Care, 2019. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/23-million-to-improve-safety-of-pelvic-floor-surgery
- Ahern S, Lassetter C, Tapley K, Heriot N, Ruseckaite R, Hansen J, Kartik A, Merenda M, Jayasinghe R, Sharma M, King J, Yin J, Gallagher E, Short J, Keck J, Bach F, Daly OJ, Karantanis E, O'Connell HE, Brennan P, Leslie J, Honardoost MA, Melon J. The Australasian Pelvic Floor Procedure Registry Annual Report 2023. Melbourne: Monash University, School of Public Health and Preventive Medicine; 2023. Available from https://apfpr.org.au/annual-report-2023/
- Department of Health and Aged Care. Therapeutic Goods Administration. Urogynaecological(transvaginal) surgical mesh hub. Canberra: Commonwealth of Australia; 2023. https://www.tga.gov.au/products/medical-devices/urogynaecological-transvaginal-surgical-mesh-hub/australian-government-actions
- Daly JO, Ahern S, Herkes R, O’Connell HE. The Australasian Pelvic Floor Procedure Registry: Not before time. Aust N Z J Obstet Gynaecol. 2019;59(4):473–476.
- Mollah T, Brennan J. Australian trends in the treatment of pelvic organ prolapse in the non-mesh era. ANZ J Surg. 2023;93(3):469–475.
- Mathieson R, Kippen R, Manning T, Brennan J. Stress urinary incontinence in the mesh complication era: current Australian trends. BJU Int. 2021;128(1):95–102.
- MBS Online Medicare Benefits Schedule. Changes to MBS Items for the surgical repair of Pelvic Organ Prolapse (POP) via vaginal approach. Canberra: Australian Government Department of Health; 2018. http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Factsheet-POP
- Australian Commission on Safety and Quality in Health Care. Resources for consumers, clinicians and health service organisations – transvaginal mesh and sacrocolpopexy. Sydney:ACSQHC; 2018. https://www.safetyandquality.gov.au/our-work/health-conditions-and-treatments/transvaginal-mesh/resources-consumers-clinicians-and-health-service-organisations-transvaginal-mesh-and-sacrocolpopexy
- Urogynaecological Society of Australasia. UGSA Position Statements: UGSA – AGES Sacrocolpopexy Credentialing Guidance UGSA; 2022. https://www.ugsa.com.au/statements
- Urological Society of Australia and New Zealand. Credentialing to perform Abdominal Sacrocolpopexy: Position statements and guidelines. USANZ; 2022. https://www.usanz.org.au/info-resources/position-statements-guidelines.
- Ruseckaite R, Daly JO, Dean J, Ahern S. Outcomes collected in female pelvic floor surgical procedure registries and databases: a scoping review. Int Urogynecol J. 2021;32(12):3113–3130.
- Brown J, King J. Age-stratified trends in 20 years of stress incontinence surgery in Australia. Aust NZ J Obstet Gynaecol. 2016;56(2):192–198.
- Australian Commission on Safety and Quality in Health Care. Care Pathway for the Management of Stress Urinary Incontinence (SUI). Sydney: ACSQHC; 2023. https://www.safetyandquality.gov.au/our-work/health-conditions-and-treatments/transvaginal-mesh/resources-consumers-clinicians-and-health-service-organisations-transvaginal-mesh-and-sacrocolpopexy/care-pathway-management-stress-urinary-incontinence-sui
- McVey A, Qu LG, Chan G, Perera M, Brennan J, Chung E, et al. What a mesh! An Australian experience using national female continence surgery trends over 20 years. World J Urol. 2021;39(10):3931–3938.
- Therapeutic Goods Administration. TGA actions after review into urogynaecological surgical mesh implants. Canberra: Australian Government Department of Health and Aged Care; 2019. https://www.tga.gov.au/news/safety-alerts/tga-actions-after-review-urogynaecological-surgical-mesh-implants#actions
- Motamedi M, Carter SM, Degeling C. Transvaginal mesh in Australia: An analysis of news media reporting from 1996 to 2021. Health Expect. 2023;26(3):1189–1201.
- Whoriskey M, Amir B, Tennankore K, Cox A. Current practices in the surgical management of female stress urinary incontinence: a survey of Canadian urologists and gynecologists. Urology Pract. 2017;4(3):239–244.
- Department of Health and Social Care. Pause on the use of vaginally inserted surgical mesh for stress urinary incontinence: News story. London: UK Government; 2018. https://www.gov.uk/government/news/pause-on-the-use-of-vaginally-inserted-surgical-mesh-for-stress-urinary-incontinence.
- British Society of Urogynaecology. Stress Urinary Incontinence Surgery in the UK 2020-2021. Trends during the pandemic. 3rd National Report. London: BSUG Audit and Database Committee 2022; 2022. Available from: https://bsug.org.uk/budcms/includes/kcfinder/upload/files/info-leaflets/BSUG-Stress-Urinary-Incontinence-Surgery.pdf
- Sikora M, Gamper M, Zivanovic I, Münst J, Bischofberger H, Kociszewski J, et al. Current treatment of stress urinary incontinence by bulking agents and laser therapy—an update. J Clin Med [Internet]. 2024;13(5):1377. doi: https://doi.org/10.3390/jcm13051377
- Australian Commission on Safety and Quality in Health Care. Guidance for hospital credentialing of senior medical practitioners to undertake transvaginal mesh surgery for stress urinary incontinence. Sydney: ACSQHC; 2018. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/guidance-hospital-credentialing-senior-medical-practitioners-undertake-transvaginal-mesh-surgery-stress-urinary-incontinence
- New Zealand Ministry of Health. Surgical mesh: Surgical mesh statement from Director-General of Health. Wellington: New Zealand Government; 2023. https://www.health.govt.nz/our-work/hospitals-and-specialist-care/surgical-mesh#:~:text=Director%2DGeneral%20of%20Health%2C%20Dr,harm%20linked%20to%20the%20procedure
- Ng JQ, Hall SE, Holman CDAJ, Semmens JB. Inequalities in rural health care: differences in surgical intervention between metropolitan and rural Western Australia. ANZ J Surg. 2005;75(5):265–269.
- Australian Commission on Safety and Quality in Health Care. Guidance for Hospital Credentialing of Senior Medical Practitioners to Undertake Transvaginal Mesh Surgery for Pelvic Organ (Vaginal) Prolapse. Sydney: ACSQHC; 2018. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/guidance-hospital-credentialing-transvaginal-tv-mesh-surgery-pelvic-organ-prolapse-pop
- Patel S, Chaus FM, Funk JT, Twiss CO. Total autologous fascia lata sacrocolpopexy for treatment of pelvic organ prolapse: experience in thirty-four patients. Urology (Ridgewood, NJ). 2022;170:73–77.
- Miller BJ, Seman EI, O’Shea RT, Hakendorf PH, Nguyen TTT. Recent trends in the management of pelvic organ prolapse in Australia and New Zealand. Aust NZ J Obstet Gynaecol. 2019;59(1):117–122.
Appendix A. Supplementary material
Click here to download a pdf of the surgeon survey questionnaire
Appendix B. Supplementary material