Volume 24 Number 1
Deficits in molecular, physical and biological parameters of healing in the diabetic foot: a literature review
Isha Sikri, Timothy O’Brien, Caroline McIntosh
Keywords Diabetes, diabetic foot ulcer, wound healing, diabetic foot, impaired wound healing
For referencing Sikri I, O’Brien T, McIntosh C. Deficits in molecular, physical and biological parameters of healing in the diabetic foot: a literature review. Journal of Wound Management 2023;24(1):28-35.
DOI
https://doi.org/10.35279/jowm2023.24.01.05
Submitted 4 October 2022
Abstract
Background Diabetic foot ulcers (DFU) are complex, multifactorial and often complicated by delayed, impaired and uncoordinated wound healing. DFU are associated with devastating outcomes including infection, amputation and premature death.
Aim The aim of this narrative literature review is to obtain a broad perspective on the pathophysiological mechanisms that contribute to delayed and impaired healing in the diabetic foot.
Methods We undertook a review of the literature to critique and synthesise the evidence for pathophysiological factors that contribute to delayed and impaired healing in the diabetic foot.
Findings It is evident from the literature that molecular mechanisms that give rise to impaired inflammation will impact upon healing, whilst physical parameters such as tissue hypoxia, pressure foot-loading, wound PH, temperature and biofilm can all contribute to delayed healing in the diabetic foot.
Conclusions An understanding of the pathophysiology of impaired healing and a focus on controlling these disturbances can facilitate successful healing. To enhance the management of foot disease in diabetic patients, primary care professionals must be made aware of the significance of early referral to a specialised unit. When DFU do not heal adequately after 4 weeks of standard treatment, the underlying pathology should be re-evaluated, and the need for advanced therapy should be considered.
Implications for clinical practice It is important that clinicians involved in treating DFU have an understanding of the pathophysiological mechanisms that cause delayed and impaired healing in the diabetic foot. When a DFU fails to respond to standard care within a 4-week period, the pathophysiology of the wound should be re-evaluated and advanced therapies should be considered.
Key messages
- Diabetic foot disease is a serious complication of diabetes that is associated with devastating outcomes including diabetic foot ulceration, amputation and premature death.
- Patients with diabetes exhibit delayed, impaired and uncoordinated wound healing due to various pathophysiological factors including impaired molecular mechanisms and abnormal physical parameters.
- It is evident that uncontrolled factors such as inflammation, biofilm, tissue hypoxia, pressure, wound PH and altered temperature contribute to delayed healing. Treatments should focus on addressing these factors to improve patient outcomes.
Introduction
Diabetes mellitus (DM) is a cluster of metabolic disorders characterised by high levels of glucose in blood. A report by the International Diabetes Federation highlights that 537 million individuals aged 20–79 years are diagnosed with DM and the incidence is expected to be 46% higher in 2045 than in 20211. Diabetic foot disease is a serious complication of diabetes that is associated with devastating outcomes including diabetic foot ulcers (DFU), delayed healing, infection, amputation and premature death. Furthermore, DM patients with chronic ulcers are at increased risk of depression, anxiety and low self-esteem, which are all established risk factors for delayed wound healing2.
DFU are characterised as a full-thickness wounds that are present at a level distal to the ankle3. DFU are the most common cause of non-traumatic lower limb amputation, which has negative consequences for mortality, and high humanistic and financial costs4. Patients with DFU have a high mortality rate, which is about twice that of patients without ulceration. The cost of living with DFU is high in terms of direct and indirect costs, estimated at €11.6 billion per year in Europe in 2017 and €7.6–11 billion annually among Medicare beneficiaries in the United States from 2007 to 20145. In 2019, the international market cost of chronic wound care was US$10.12 billion, with a projected growth to US$16.36 billion by 20276.
DFU are prevalent worldwide. Globally, 40–60 million diabetic patients are affected with diabetic foot and lower limb complications, and the likelihood of developing a foot ulcer may be as high as 25%7. Australia has the lowest prevalence of DFU at 1.5%, the prevalence of DFU is 3.9% in Ireland, whilst the highest reported prevalence is in Belgium (16.6%); the global average is 6.4%. The rate of DFU complications is higher in male patients than female. In addition, DFU is more predominant among those with type 2 DM when compared to type 18. Both the age and the length of DM increase the incidence of foot lesions7.
Pathophysiology of DFU
The three pathological components – neuropathy, ischaemia and infection – contribute to DFU and its complications, and they often occur together as an aetiologic triad (Figure 1). The initiating causes are neuropathy and ischaemia, which are frequently combined as neuroischaemia, while infection is mostly a resultant9.
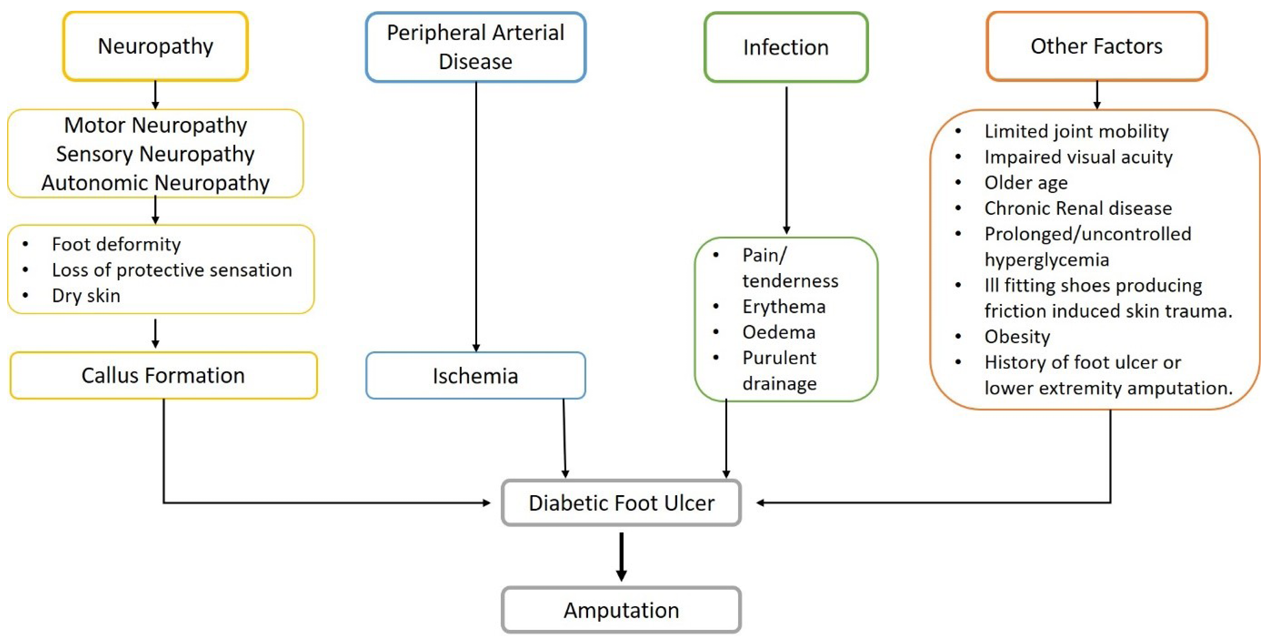
Figure 1. Risk factors associated with DFU [modified]
Diabetes-related peripheral neuropathy affects the distal nerves of the limbs, particularly those of the feet. It primarily affects symmetrical sensory function, resulting in irregular sensations and gradual numbness. Such factors make it easier for ulcers to develop as a result of external trauma and/or irregular distribution of internal bone pressure10. The incidence of diabetes-related peripheral neuropathy has been estimated to range from 16% to 87%11.
Patients can also have sensory, autonomic and/or motor neuropathy. Sensory neuropathy causes the loss of defensive control as well the inability to recognise the consequences of repeated trauma. In the lower limb, autonomic neuropathy can give rise to inadequate sweat gland function as a result of sudomotor dysfunction in diabetes, and is associated with dry skin, itching and anhidrosis which can contribute to the development of foot problems, including ulceration12. Callus formation is considered a symptom of DFU since the callus point is subjected to 20 times more pressure than the surrounding tissues13. Diabetes-related peripheral neuropathy can lead to devastating outcomes; approximately 50% of people with diabetes will develop a foot ulcer during their lifetime and foot ulcers often precede lower limb amputation. In addition, neuropathic pain and decreased sensation can contribute to an array of poor outcomes including falls, impaired quality of life and depressive symptoms14.
However, patients with DM and advanced peripheral arterial disease (PAD) are more susceptible to sudden ischaemia caused by progressing atherosclerosis, medio-calcinosis, thrombosis, infections and other factors15. Evidence of tissue damage becomes more apparent as the disease progresses, more often in the form of chronic non-healing foot ulcers16. Using the ankle brachial index to classify PAD, statistics indicate that 20% of patients with DM over the age of 40 years have PAD and the prevalence increases with age10.
Patients with multiple, longer duration and deeper wounds have a greater risk of infection. Ischaemia in the foot tends to be linked to a rise in infection severity as DM patients have a reduced inflammatory response. A lack of erythema or induration, which are visual indicators of infection, could be caused by reduced blood flow13. The consequence of DFU is closely linked to the use of inappropriate antibiotics to treat diabetic foot infections; DFU patients taking inappropriate antibiotics have 2.5 times higher chance of amputation as compared to appropriately treated DFU patients. Antibiotics prescribed incorrectly can also lead to the production of antibiotic-resistant pathogens17.
Other risk factors for DFU include peripheral vessel medial arterial calcification, altered foot biomechanics and limited joint mobility, skeletal disease, microangiopathy, Charcot arthropathy, trauma, autonomic neuropathy, history of foot ulceration or amputation, increased plantar pressures, prolonged and uncontrolled DM, smoking, diabetic retinopathy, nephropathy and obesity5,18.
Over the course of their lives, up to one-third of the world’s half-billion patients with diabetes will develop a DFU. More than half of DFU will become infected, out of which 17% will require amputation19. It’s significant to note that DFU occurs prior to 85% of all lower limb amputations in diabetic individuals20.
Diabetes and Impaired Wound Healing
Wound healing in the skin is a multifaceted and dynamic process that comprises chemotaxis, inflammation, neovascularisation, and cell division, synthesis of extracellular matrix (ECM) proteins and restoration of anatomic integrity. Wound healing is initiated with haemostasis that controls blood loss and regulates microbe entry to the wound area. An inflammatory phase is followed immediately that cleans up wound debris and prepares the wound site ready for healing. It generally involves three main types of cells – neutrophils, macrophages and mast cells. The proliferative process overlaps the inflammatory phase, during which new tissue, blood vessels and matrix synthesis occurs, allowing tissue regeneration that fills the wound. The ECM’s tensile strength is increased and the blood supply to the damaged area is reduced in the final remodelling process21.
Diabetes-related wounds including DFU are a major concern. Patients with DM exhibit a delayed, impaired and uncoordinated wound healing process. A persistent inflammatory process is observed in DFU healing, which is accompanied by a delay in the development of mature granulation tissue and a decrease in wound tensile strength, subsequently leading to ischaemia22.
The combined complications of neuropathy, PAD, impaired growth factor (GF) production, keratinocyte and fibroblast migration and proliferation, collagen accumulation, angiogenic response, stability between build-up of extracellular components and their remodelling by proteases, inflammation and hypoxia cause DFU healing to be delayed (Figure 2)21. A summary of scientific breakthroughs that shed light on the mechanisms underlying the delayed healing of DFU will be discussed. The key obstacle to the management of chronic wounds must overcome the factors that delay healing as a part of a holistic approach to wound care.
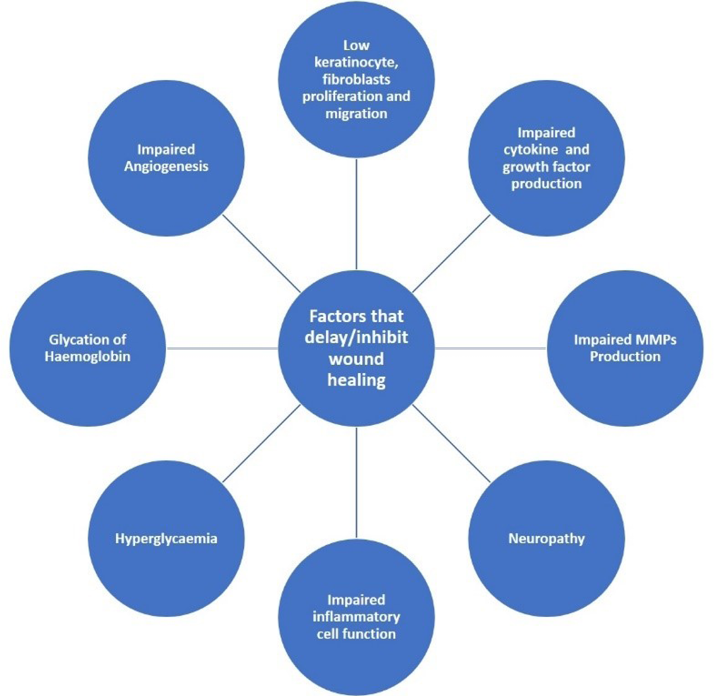
Figure 2. Factors that delay or inhibit wound healing
Molecular Mechanisms
Inflammation
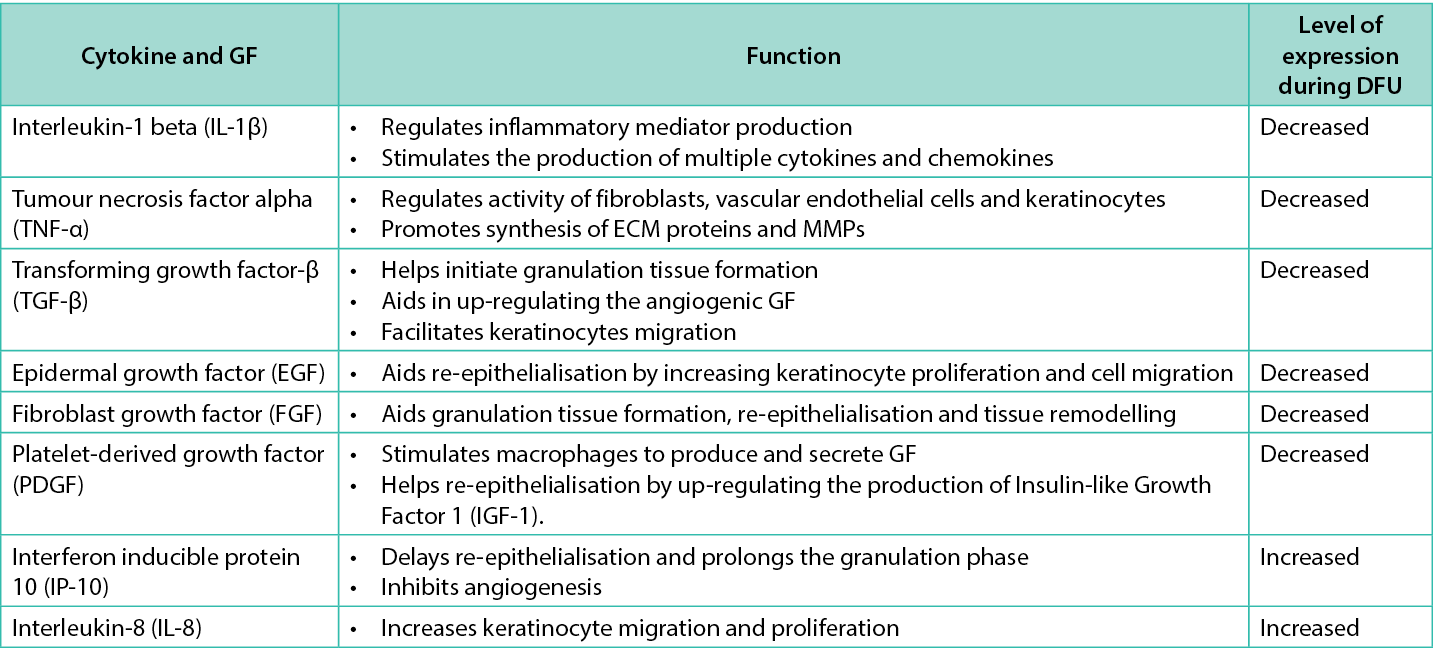
DFU have a chronic pro-inflammatory phenotype, with elevated inflammatory cytokine production. It has been observed that interleukin‑1 beta (IL‑1β) expression is increased in DFU in both human and mouse tissue samples23. Furthermore, high levels of tumour necrosis factor alpha (TNF‑α) and monocyte chemoattractant protein‑1 (MCP‑1), interleukin‑1 (IL‑1) and interleukin-6 (IL-6) are associated with delayed wound healing in humans24. Forkhead box protein M1 (FOXM1), which is involved in the activation and recruitment of inflammatory cells, was also found to be downregulated in DM patients25. The roles of various cytokines and GFs involved in wound healing are highlighted in Table 1.
Table 1. Cytokines and GF involved in wound healing with their expression level
Neutrophils are the first inflammatory cell recruited to the wound site; they function to clear dead cells and infectious microorganisms. Recent research has shown that an increased inflammatory response by neutrophils can have a negative impact on DFU healing25. Neutrophil extracellular traps (NETs) are secreted when neutrophils infiltrate a wound to neutralise microorganisms in the decondensed chromatin form by peptidyl arginine deiminase 4 (PAD4)-mediated histone citrullination in a process known as NETosis26,27. Wong et al discovered that hyperglycaemia increases neutrophil PAD4 expression, and that the resulting NETs formed in skin wounds are harmful to wound healing28. The inflammatory period is also prolonged due to activation of Nod-like receptor protein (NLRP3) inflammasomes in macrophages which stimulate greater production of IL‑1β and other cytokines, thus delaying the formation of granulation tissue29.
Following neutrophils, macrophages are the next cells to migrate to the injury site. Wound macrophages generally transit from a pro-inflammatory (M1) phenotype (CD14+CD16–cells in humans) to an anti-inflammatory (M2) phenotype (CD14+CD16+ cells in humans) during the normal inflammatory stage of wound healing. In the wound bed, this anti-inflammatory transformation stimulates keratinocytes and fibroblasts which proliferate and contribute to the healing process30. In DM, however, this phenotypic transition does not occur as easily, and macrophages remain primarily pro-inflammatory, resulting in chronic inflammation31. Furthermore, experiments in diabetic mice have shown that pro-inflammatory macrophages’ defective efferocytosis of apoptotic neutrophils results in apoptotic cell burden that induces persistent inflammation, preventing macrophages from transitioning to an anti-inflammatory state32. Another study in mice demonstrated that a reduction of M2 macrophages with surgical wounds exhibited increased neutrophil count and M1 macrophage infiltration, which helps in extending the duration of inflammatory phase and results in less collagen deposition at the wound bed33. These findings indicate that sustained immune activation is an important contributor to delayed wound healing.
Physical parameters
Tissue hypoxia
Hypoxia is a condition in which an adequate level of oxygen is not available at the tissue level. In typical wound healing situations, local hypoxia stimulates hypoxia inducible factor‑1 (HIF‑1) which stimulates numerous cellular processes including erythropoiesis, angiogenesis, proliferation and cell survival intended to help adaptive cellular reactions and wound healing21. Even with the hypoxia found in diabetic wounds, the amount of HIF-alpha and HIF‑1 focused genes are decreased in the wounds of diabetic animal models compared with non-diabetic littermates, causing weakened reactions to cellular hypoxia and prolonging the rate of healing34. Prolonged hypoxia, along with hyperglycaemia, is harmful since it exaggerates these early physiological events and causes reperfusion damage as well the production of oxygen free radicals30. Hypoxia impairs neutrophil and macrophage activity when combined with hyperglycaemia and other metabolic perturbations35. Hyperglycaemia is also linked to the formation of advanced glycation end product (AGE), inactivates HIF‑1, and inhibits synthesis of vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS), causing delayed healing. Reactive oxygen species such as hydroxyl radical, superoxide and hydrogen peroxide, as well as reactive nitrogen species, cause increased oxidative stress which causes endothelial damage and slows the rate of healing36.
Pressure foot-loading
DM patients frequently have increased plantar pressures due to peripheral neuropathy and structural deformities which contribute to the onset of plantar foot ulcers. Increased plantar pressure is an established risk factor for foot ulceration37. Subcallus ulcers are caused by the deterioration of the underlying skin and soft tissues caused by persistently high pressures. These ulcers gradually deepen, causing localised deep tissue abscess or osteomyelitis due to prolonged repetitive trauma38. Unrelieved pressure, particularly in an insensate foot, results in ongoing mechanical stress and contributes to chronic inflammation in the tissues that, unless addressed, delay wound healing and tissue restoration39.
pH
In natural conditions, an acidic milieu is found on the surface of skin which is an important feature of the barrier function of skin. A human pathogenic bacterium needs a pH value above 6 to grow which is inhibited by lower pH values of skin. For example, the pathogenic microorganism Candida albicans favours increased skin pH, and a more alkaline environment accelerates its overgrowth. Therefore, maintaining an acidic skin pH could help in reducing the microbial overgrowth on the body surface given the fact that they are less resistant to antibiotics. An alteration in the pH value in infected wounds can also change the efficacy of antibiotics. A study has shown that the toxicity of new glycopeptide antibiotic (Oritavancin, LY333328) towards vancomycin-resistant Enterobacter species decreases significantly in an acidic milieu with a pH value of 6.4 compared to pH value of 7.4 and 8.440. It has been advocated that the matrix metalloproteinases (MMPs) similar to most other enzymes in the body are very delicate to changes in their instantaneous pH atmosphere41. A study by Hart elucidates that creating slightly acidic wound environments would decrease the level of MMPs which, in turn, decreases the inflammatory response42.
Temperature
The diabetic foot is more prone to ulceration which may in part be due to elevated skin temperature caused by excessive microvasculature blood flow. Using infrared thermal imaging, Long et al discovered that Streptozotocin-induced (STZ) mice had higher wound temperatures which corresponded to slower wound closure43.
Extracellular matrix
The ECM, which acts as an interactive scaffold for cells and promotes growth and regeneration in wound tissue, is a significant environmental factor in the healing of DFU. In diabetic patients an imbalance between the synthesis and degradation of the ECM causes a delay in wound healing.
Protease levels in DFU surpass those of their antagonists, resulting in ECM destruction and GF and receptor degradation. The proteolytic degradation of ECM not only stops the wound from progressing into the proliferative stage but also draws in more inflammatory cells, hence accelerating the inflammatory cycle44.
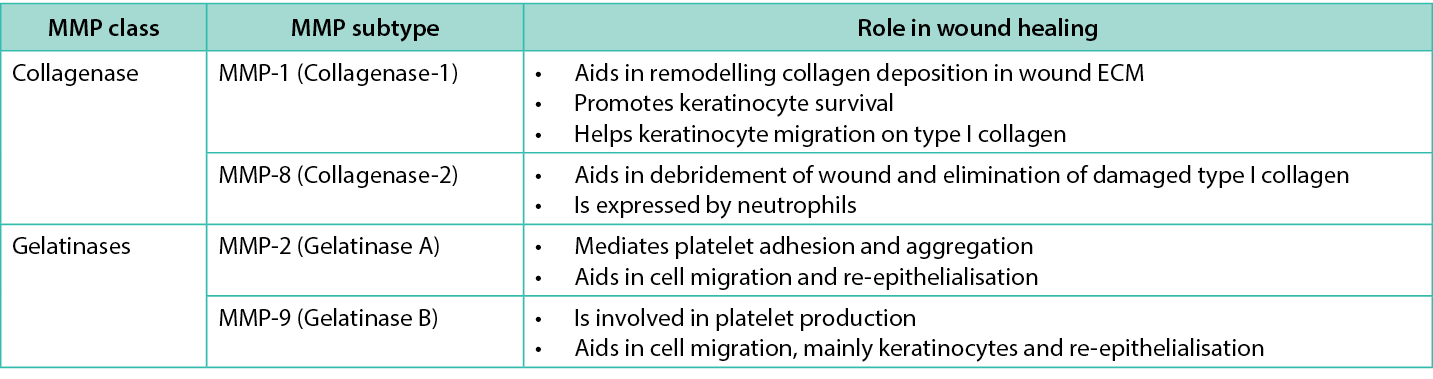
DM disrupts the equilibrium of MMP concentrations and proteolytic activity. MMPs are a family of zinc-dependent endopeptidases that degrade ECM components involved in tissue remodelling. MMPs digest all matrix proteins, including collagens, elastin, proteoglycans and fibronectin. Although there are 24 different MMP categories, only collagenase (MMP‑1 and MMP‑8) and gelatinases (MMP‑2 and MMP‑9) have a role in wound healing45 (Table 2). The gelatinases (MMP‑2 and MMP‑9) are the two proteinases that mainly break down type IV collagen from the basic matrix. MMP activity is regulated by tissue inhibitor of MMPs (TIMP) as MMPs are present in an inactive state and need activation to become functional. Thus, it is vital to have a balance between MMPs and TIMPs46. Reserved MMPs obstruct wound healing when MMPs are produced in excess during NET formation and cannot be digested to uphold cellular balance47.
Table 2. Role of metalloproteinases in wound healing
The level of MMPs is 60 times higher in chronic wounds than acute wounds48. The increased levels of MMP‑1, activated MMP‑2, MMP‑8, MMP‑9, and decreased level of TIMP‑1 were found in DFU patients when compared to a wound in non-diabetic patients. Furthermore, high MMP‑1 expression is essential for wound healing, but surplus MMP‑8 and MMP‑9 may slow wound healing in DFU patients, while the MMP‑1/TIMP‑1 ratio may represent the wound’s proteolytic environment49,50. Higher MMP‑9 expression is associated with poor DFU healing due to poor balance between ECM synthesis and degradation51.
Hyperglycaemia is linked to lower levels of urokinase plasminogen activator and higher levels of tissue plasminogen activator inhibitor, which may decrease fibrinolysis and impair matrix deposition24. Furthermore, in diabetic ulcers, some of the resident cells such as smooth muscle cells and fibroblasts undergo apoptosis due to mitochondrial damage, causing up-regulation of pro-apoptotic proteins and down-regulation of anti-apoptotic proteins, including B-cell lymphoma‑229. Fibroblasts isolated from DFU display increased apoptosis, decreased migration ability and reduced proliferative response to GFs such as TGF-β1, platelet-derived growth factor (PDGF) as they become senescent31. Fibroblasts are unable to remodel the ECM, causing MMPs, collagenase, serine protease and elastase levels to rise52. Type 2 TGF receptor expression is reduced in chronic wound fibroblasts followed by phosphorylation of transduction signals such as Smad2, Smad3 and mitogen-activated protein kinase53.
Biofilms
The study of how unregulated host-pathogen interactions impact healing processes is gaining in popularity. For example, local infection with high levels of replicating bacteria plays a major role in delayed healing and the development of non-healing ulcers54. Healing can also be hampered by a high bacterial burden without the classic symptoms of infection55.
Biofilms can be defined as a complex microbial colony including bacteria and fungi covered in a polysaccharide matrix that can attach to the surface of wounds56. This microbial burden is generally polymicrobial and it appears to obstruct host healing. Gram-positive bacteria Streptococcus agalactiae, Staphylococcus aureus, Enterococcus faecalis and Staphylococcus epidermidis, as well as gram-negative Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii and Stenotrophomonas maltophilia, were the most commonly identified pathogens30. A report by Trostrup et al showed that VEGF, antimicrobial peptide and neutrophil effector cytokine production is inhibited by P. aeruginosa57. In addition, the immune system is often ineffective in fighting biofilm-related infections and impairs wound epithelialisation and granulation tissue formation30.
Polymicrobial biofilms comprising Bacillus subtilis, S. aureus, P. aeruginosa and E. faecalis were found to increase necrosis, delay inflammation and granulation, and hinder ECM production in a porcine model. Upregulation of inflammatory mediators like arginase‑1 (ARG‑1), IL‑8 and chemokine ligand 13 (CXCL13), as well as genes involved in the oxidative stress response like angiopoietin-like 4 (ANGPTL-4) and superoxide dismutase 2 (SOD2), was observed during gene expression analysis58.
Quorum sensing (bacterial communication mechanism) occurs frequently in biofilms, which influences the chemotaxis towards the surface, availability of key nutrients for biofilm formation, presence of surfactants, bacteria mobility, and surface adhesion59. Antimicrobials are unsuccessful at penetrating biofilms, lowering the concentration acting on the bacterial cells within the biofilms and, as a result, biofilms provide a physical barrier to bacteria60. These bacterial colonies are frequently multispecies and coated in glycocalyx matrix, making them immune to antibiotics used in topical, parental or oral forms. Within 10 hours of debridement, biofilms can reform. DFU wound bioburden is a notable potentially universal barrier to the healing of chronic wounds due to the diversity of biofilms and their inherent resistance to antibiotics, biocides and host immunity61,62. A recent study by Caruso et al indicated a nearly three-fold increase in the risk of antibiotic-resistant infections relative to 201963.
Angiogenesis
The inability to rebuild the microvasculature through the process of angiogenesis is a major feature of non-healing wounds in DM and it mainly affects the proliferative phase52. Macrophages are necessary for wound healing because they coordinate the angiogenic response and produce VEGF and other pro-angiogenic mediators in wounds that regulate new blood vessel formation64,65. The failure of transition of macrophages during the inflammatory phase adversely affects angiogenesis. In the context of diabetic wound healing, the synthesis of the anti-angiogenic factor, pigment epithelium derived factor (PEDF) was investigated and it has been suggested that increased levels of PEDF could have a deleterious impact on wound healing outcomes66. Moreover, the key pathways for maintenance and angiogenesis are angiopoietin‑1 (Ang1) and angiopoietin‑2 (Ang2), and, in diabetic wounds, Ang2 is dramatically upregulated and the Ang2/Ang1 ratio is dysregulated, disrupting the angiogenesis process67.
MicroRNAs, often called miRNAs, are another type of molecule that can affect wound healing and the angiogenic process. MiR26‑b is highly expressed in diabetic endothelial cells, and neutralisation of this miRNA causes enhanced wound closure and granulation tissue development in diabetes wound models68. In diabetic mouse models, restoration of miR27‑b regulates angiogenesis in vivo and in vitro in experiments employing local miR27-b,which is thought to impact levels of the anti-angiogenic protein thrombospondin 1 (TSP1) in the wound bed69.
Conclusion
Diabetes frequently affects the healing of wounds which can cause poor outcomes in terms of non-healing wounds, limb threatening infections and amputations. Whilst DFU are complicated to treat, an understanding of the fundamental pathophysiology and a focus on controlling these disturbances may lead to successful wound healing. The main obstacle in the management of chronic wounds is overcoming the factors that lead to delayed healing; these interventions should occur as part of a holistic approach to wound care. Due to severe infection, irreversible ischaemia, imbalance in cytokine, and GF production in the wound bed, patients with DM are more likely to need to have tissue resection. Intensive therapy is required as early as feasible after the development of an ulcer to minimise its chronicity, resultant morbidity and associated mortality.
To enhance the management of foot disease in diabetic patients, primary care professionals must be made aware of the significance of early referral to a specialised unit. Combination approaches involving advanced wound therapies and MMP inhibitors, ECM stimulator, GF, cells combinations or angiogenesis stimulator can be used at different phases of wound healing. Ulcer resolution and ulcer recurrence can be aided by biomechanical examination and treatment planning. When DFU do not heal adequately after 4 weeks of standard treatment, the underlying pathology should be re-evaluated, and the need for advanced therapies that can address molecular and/or physical disturbances should be considered.
Implications for clinical practice or future research
- Patients with DM exhibit delayed, impaired and uncoordinated wound healing.
- An understanding of the fundamental pathophysiology and a focus on controlling these disturbances may facilitate successful healing.
- When DFU do not heal adequately after 4 weeks of standard treatment, the need for advanced therapy, taking into account the pathophysiological deficits, should be considered.
- Future research to better understand the role of molecular and physical parameters in impaired wound healing in diabetes is needed.
- There is a need for the development of advanced wound products that specifically aim to address the pathophysiological components of impaired healing in DFU, and that are shown through definitive trial designs to improve clinical and patient outcomes.
Author contributions
All authors meet the criteria for authorship. All authors made substantial contributions to this literature review including conception and design, literature searching, drafting of the manuscript, critical revision of the manuscript and final approval of the manuscript.
Conflict of interest
Prof. Timothy O’Brien is a founder, director and equity holder in Orbsen Therapeutics.
Funding
This work was supported by Health Research Board (HRB) Ireland through the HRB Collaborative Doctoral Awards under the grant CDA-PA-2019-011 and grant from Science Foundation Ireland (SFI) number 13/RC/2073_P2.
Author(s)
Isha Sikri* MSc1,2, Timothy O’Brien MD, PhD1,2, Caroline McIntosh PhD2,3
1The Regenerative Medicine Institute (REMEDI), School of Medicine, CURAM, University of Galway, Galway, Ireland
2Alliance for Research and Innovation in Wounds, College of Medicine, Nursing & Health Sciences, University of Galway, Galway, Ireland
3Discipline of Podiatric Medicine, School of Health Sciences, University of Galway, Galway, Ireland
*Corresponding author email I.SIKRI1@universityofgalway.ie
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: International Diabetes Federation, 2021.
- National Clinical Programme for Diabetes Clinical Design and Innovation, HSE. Diabetic foot model of care; 2021. Available from: https://www.hse.ie/eng/about/who/cspd/ncps/diabetes/moc/diabetic-foot-model-of-care-2021.pdf
- van Netten JJ, Bus SA, Apelqvist J, Lipsky BA, Hinchliffe RJ, Game F, et al. Definitions and criteria for diabetic foot disease. Diabetes Metab Res Rev 2020;36 Suppl 1:e3268.
- Guest JF, Fuller GW, Vowden P. Diabetic foot ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J 2018;15(1):43–52.
- Banik PC, Barua L, Moniruzzaman M, Mondal R, Zaman F, Ali L. Risk of diabetic foot ulcer and its associated factors among Bangladeshi subjects: a multicentric cross-sectional study. BMJ Open 2020;10(2):e034058.
- Fortune Business Insights. Chronic wound care market; 2022. Available from: https://www.fortunebusinessinsights.com/industry-reports/chronic-wound-care-market-100222.
- Bowling FL, Rashid ST, Boulton AJ. Preventing and treating foot complications associated with diabetes mellitus. Nat Rev Endocrinol 2015;11(10):606–16.
- Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann Med 2017;49(2):106–16.
- Bandyk DF. The diabetic foot: pathophysiology, evaluation, and treatment. Semin Vasc Surg 2018;31(2–4):43–8.
- International Diabetes Federation. International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: International Diabetes Federation, 2019.
- Sobhani S, Asayesh H, Sharifi F, Djalalinia S, Baradaran HR, Arzaghi SM, et al. Prevalence of diabetic peripheral neuropathy in Iran: a systematic review and meta-analysis. J Diabetes Metab Disord 2014;13(1):97.
- Carey O, Smith M, McIntosh C. The relationship between clinician observed anhidrosis, Neuropad® detected anhidrosis and MNSI findings in a community sample of participants with diabetes. Diabetic Foot Journal 2021;24(3):1–9.
- Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29(6):1288–93.
- Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep 2019;19(10):86.
- Yang SL, Zhu LY, Han R, Sun LL, Li JX, Dou JT. Pathophysiology of peripheral arterial disease in diabetes mellitus. J Diabetes 2017;9(2):133–40.
- Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med 2017;110(3):104–9.
- Bekele F, Chelkeba L, Fekadu G, Bekele K. Risk factors and outcomes of diabetic foot ulcer among diabetes mellitus patients admitted to Nekemte referral hospital, western Ethiopia: prospective observational study. Ann Med Surg 2020;51:17–23.
- Sinwar PD. The diabetic foot management – recent advance. Int J Surg 2015;15:27–30.
- Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res 2020;13(1):16.
- McGuire J, Thomson A, Kennedy PG. The biomechanics of diabetic foot amputation. Wounds 2021;33(9):231-236.
- Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr Diab Rep 2018;18(1):2.
- Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 2012;25(7):304–14.
- Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013;62(7):2579–87.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366(9498):1736–43.
- Sawaya AP, Stone RC, Brooks SR, Pastar I, Jozic I, Hasneen K, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun 2020;11(1):4678.
- Fadini GP, Menegazzo L, Rigato M, Scattolini V, Poncina N, Bruttocao A, et al. NETosis delays diabetic wound healing in mice and humans. Diabetes 2016;65(4):1061–71.
- Yang S, Gu Z, Lu C, Zhang T, Guo X, Xue G, et al. Neutrophil extracellular traps are markers of wound healing impairment in patients with diabetic foot ulcers treated in a multidisciplinary setting. Adv Wound Care (New Rochelle) 2020;9(1):16–27.
- Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015;21(7):815–9.
- Liu D, Yang P, Gao M, Yu T, Shi Y, Zhang M, et al. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin Sci (Lond) 2019;133(4):565–82.
- Versey Z, da Cruz Nizer WS, Russell E, Zigic S, DeZeeuw KG, Marek JE, et al. Biofilm-innate immune interface: contribution to chronic wound formation. Front Immunol 2021;12:648554.
- Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther 2014;31(8):817–36.
- Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 2010;5(3):e9539.
- Klinkert K, Whelan D, Clover AJP, Leblond AL, Kumar AHS, Caplice NM. Selective M2 macrophage depletion leads to prolonged inflammation in surgical wounds. Eur Surg Res 2017;58(3–4):109–20.
- Catrina SB, Zheng X. Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes Metab Res Rev 2016;32 Suppl 1:179–85.
- Patel V, Chivukula IV, Roy S, Khanna S, He G, Ojha N, et al. Oxygen: from the benefits of inducing VEGF expression to managing the risk of hyperbaric stress. Antioxid Redox Signal 2005;7(9–10):1377–87.
- Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact 2014;219:101–12.
- Bus SA. The role of pressure offloading on diabetic foot ulcer healing and prevention of recurrence. Plast Reconstr Surg 2016;138(3 Suppl):179S–87S.
- Sabapathy SR, Periasamy M. Healing ulcers and preventing their recurrences in the diabetic foot. Indian J Plast Surg 2016;49(3):302–13.
- Piaggesi A, Viacava P, Rizzo L, Naccarato G, Baccetti F, Romanelli M, et al. Semiquantitative analysis of the histopathological features of the neuropathic foot ulcer: effects of pressure relief. Diabetes Care 2003;26(11):3123–8.
- Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res 2007;298(9):413–20.
- McArdle C, Lagan KM, McDowell DA. The pH of wound fluid in diabetic foot ulcers – the way forward in detecting clinical infection? Curr Diabetes Rev 2014;10(3):177–81.
- Hart J. Inflammation. 2: its role in the healing of chronic wounds. J Wound Care 2002;11(7):245–9.
- Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R, et al. An essential role of NRF2 in diabetic wound healing. Diabetes 2016;65(3):780–93.
- Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4(9):560–82.
- Trung T, Nguyen SMaMC. Roles of matrix metalloproteinases in cutaneous wound healing. IntechOpenBook; 2016. https://www.intechopen.com/chapters/51825
- Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother 2019;112:108615.
- Zhu S, Yu Y, Ren Y, Xu L, Wang H, Ling X, et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis 2021;12(11):984.
- Sibbald RG, Woo KY. The biology of chronic foot ulcers in persons with diabetes. Diabetes Metab Res Rev 2008;24 Suppl 1:S25–30.
- Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP‑1 to TIMP‑1 is a predictor of wound healing. Diabet Med 2008;25(4):419–26.
- Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002;45(7):1011–6.
- Wang W, Yang C, Wang XY, Zhou LY, Lao GJ, Liu D, et al. MicroRNA‑129 and -335 promote diabetic wound healing by inhibiting Sp1-mediated MMP-9 expression. Diabetes 2018;67(8):1627–38.
- Liu Y, Liu Y, Deng J, Li W, Nie X. Fibroblast growth factor in diabetic foot ulcer: progress and therapeutic prospects. Front Endocrinol (Lausanne) 2021;12:744868.
- Kim BC, Kim HT, Park SH, Cha JS, Yufit T, Kim SJ, et al. Fibroblasts from chronic wounds show altered TGF-beta-signaling and decreased TGF-beta type II receptor expression. J Cell Physiol 2003;195(3):331–6.
- Percival SL, Thomas JG, Williams DW. Biofilms and bacterial imbalances in chronic wounds: anti-Koch. Int Wound J 2010;7(3):169–75.
- Maheswary T, Nurul AA, Fauzi MB. The insights of microbes’ roles in wound healing: a comprehensive review. Pharmaceutics 2021;13(7).
- Rajpaul K. Biofilm in wound care. Br J Community Nurs 2015;Suppl Wound Care:S6, S8, S10–1.
- Trostrup H, Lerche CJ, Christophersen LJ, Thomsen K, Jensen PO, Hougen HP, et al. Chronic Pseudomonas aeruginosa biofilm infection impairs murine S100A8/A9 and neutrophil effector cytokines-implications for delayed wound closure? Pathog Dis 2017;75(7).
- Klein P, Sojka M, Kucera J, Matonohova J, Pavlik V, Nemec J, et al. A porcine model of skin wound infected with a polybacterial biofilm. Biofouling 2018;34(2):226–36.
- Moore-Ott JA, Chiu S, Amchin DB, Bhattacharjee T, Datta SS. A biophysical threshold for biofilm formation. Elife 2022;11.
- Ahmad J. The diabetic foot. Diabetes Metab Syndr 2016;10(1):48–60.
- Ghotaslou R, Memar MY, Alizadeh N. Classification, microbiology and treatment of diabetic foot infections. J Wound Care 2018;27(7):434–41.
- Wolcott RD, Cox SB, Dowd SE. Healing and healing rates of chronic wounds in the age of molecular pathogen diagnostics. J Wound Care 2010;19(7):272–8, 80–1.
- Caruso P, Maiorino MI, Macera M, Signoriello G, Castellano L, Scappaticcio L, et al. Antibiotic resistance in diabetic foot infection: how it changed with COVID‑19 pandemic in a tertiary care center. Diabetes Res Clin Pract 2021;175:108797.
- Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev 2019;146:97–125.
- Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci 2017;18(7).
- Qi W, Yang C, Dai Z, Che D, Feng J, Mao Y, et al. High levels of pigment epithelium-derived factor in diabetes impair wound healing through suppression of Wnt signaling. Diabetes 2015;64(4):1407–19.
- Isidori AM, Venneri MA, Fiore D. Angiopoietin‑1 and Angiopoietin‑2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes. J Endocrinol Invest 2016;39(11):1235–46.
- Icli B, Nabzdyk CS, Lujan-Hernandez J, Cahill M, Auster ME, Wara AK, et al. Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a. J Mol Cell Cardiol 2016;91:151–9.
- Wang JM, Tao J, Chen DD, Cai JJ, Irani K, Wang Q, et al. MicroRNA miR-27b rescues bone marrow-derived angiogenic cell function and accelerates wound healing in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2014;34(1):99–109.
