Volume 24 Number 1
New technologies for tissue replacement
Franco Bassetto, Jean-Pierre Becquemin, Edwin den Braber, Luca Dalla Paola, Alexandra Marques, Ilaria Palla, Alberto Piaggesi, Katherine Raspovic, Carlotta Scarpa, Luc Téot, Isotta Triulzi, Giuseppe Turchetti
For referencing Piaggesi A, Bassetto F, den Braber E, Dalla Paola L, Marques A, Palla I, Raspovic K, Scarpa C, Téot L, Triulzi I, Turchetti G. New technologies for tissue replacement; J Wound Management, 2023;24(1 Sup1):S1-S130
DOI https://doi.org/10.35279/jowm2023.24.01.sup01
1. Introduction
In 2018, EWMA released a document titled ‘Advanced Therapies in Wound Management’ (1). This document focused on the latest progress in the field of medical technologies for use in wound management, since the recent period has been very innovative within this field.
The aim of the EWMA document (1) was not only to revise and comment upon the most interesting news documented in the literature, but also to provide an overview of the evidence available for each of the technologies described and, whenever possible, to connect the new technologies with their clinical indications. By doing this, we aimed to bridge the need for new technical tools and skills among the professionals involved in wound management with the new products that were made available by the industry.
The document, which has frequently been downloaded and cited, was conceived to provide some considerations concerning the regulatory and economic aspects of the technologies applied to wound management. This was included to support an understanding of the complexity of this field.
The document was concluded with a so-called ‘wish list’: Several issues that would need to be addressed from the political side, rather than from a technical perspective, to help reduce the gap between patients’ needs and new technical solutions introduced across the European Union (EU).
After only two years, by 2020, many new technological resources had been released and proposed for clinical use. These were mainly technologies for the surgical management of wounds, based on suggestions and input from the clinical and technical fields. This is why, we decided to publish a new document covering these interesting innovations, which in some cases constitute real breakthroughs. This new document focuses on tissue replacement, as most of the new technologies are related to this field.
The new document is entitled New Technologies for Tissue Replacement. The structure and organisation of the content follows that of the previous document, including the same presentation and evaluation of evidence in tables for each section.
The group of authors, all well-known opinion-leaders within their fields, has been challenged to provide an updated overview of the new technologies and their possible influence on the area of tissue replacement in the 2020s. The technologies reviewed for this document range from physical tools to new materials, and from cellular and tissue-based therapies to surgical devices. Several innovative technologies have been evaluated, including a thorough assessment of the supporting evidence, and their possible role in the available catalogue of tools for tissue replacement is reported. The evaluation of technologies will also rely on the authors’ own experiences, going beyond the published evidence, whenever relevant.
As in the previous document, we have included a section on the regulatory and economic aspects of the new technologies. Special attention will be paid to the new European rules for medical devices, which have been in effect across the EU for all new devices since May 2021 and will soon be extended to all medical devices, irrespective of their release date.
Although the sections of the document have been developed and initially written by one or several specific members of the author group, the final document is the result of a collective process, and it should therefore be considered a joint publication with the scientific responsibility shared by the group of authors.
As for other EWMA documents, this one has been made possible by the unconditional contributions of industry sponsors, and they have been recognised for their generosity in the acknowledgements section. Their commitment has been exclusively related to sustaining the production of the document, without any other direct or indirect involvement. The author group would like to express their gratitude for their neutrality and correctness in the process.
1.1 Methodology
The search strategy presented in Table 1 was used to identify the relevant literature. A literature search was performed in PubMed and Embase for each topic included in the document. The search covered the period of 2011–2021. The authors responsible for the included topics were asked to evaluate the search results and to select rele-vant literature based on the agreed definition of ‘advanced therapies’ defined for this document. Additional literature is included by the authors, if relevant, to describe theories and concepts behind each identified technology. This additional literature may fall outside the period covered in the search. The literature was evaluated with reference to the GRADE methodology (2).
Tables providing an overview of the evaluation of evidence supporting the technologies are inserted at the end of each document section.
1.2 Structure of the document
This document is organised into eight sections. Six of them deal with the different technologies for tissue replacement and are, in order of position in the document, dedicated to: physical technologies and delivery systems, materials, skin substitutes, surgical off-loading, bone substitutes with local antibacterial activity and vascular- and endovascular related technologies. Each of these sections includes: 1) A text describing and summarising the status and possible evolutions within the field; 2) Tables outlining available relevant studies (indicating the number of subjects, main findings, etc.) and 3) A table outlining the available evidence and the strength of recommendations for using the different therapies with the related indications. The document also includes two sections dedicated to economic and organisational aspects, as well as a status update on the regulatory issues related to the availability and use of new technologies for tissue replacement. The aim of these sections is to provide a different perspective on this complex and fast-evolving field that bridges the gap between the technologies and their inception in the real world of wound healing. The authors hope that reading this document will not only be interesting for scientists and clinicians, but also helpful for other stakeholders in the field of wound management by supporting better care for patients with wounds.
Table 1: Literature search strategy
All searches were performed in titles and abstracts

2. Tissue replacement - physical/delivery system
2.1 Introduction
The inception of physical means into the management of chronic ulceration was a game-changer, since it opened the possibility for a brand-new philosophy behind the diagnosis and treatment of these complex conditions based on the interactions between physical forces and the biology of the lesions, rather than on chemical and biochemical reactions.
This was, in a way, a revolution, because the ease of delivering, the re-usability of technologies, the lack of direct contact and the wide range of solutions – from electric and electro-magnetic fields to light and lasers and ionic plasma to fluorescence – made it possible to re-shape the diagnostic and therapeutic strategies in many complex situations. This has improved our potential to cure patients.
More recently, in addition to physical technologies in a strict sense, delivery systems and materials have come into play, opening new possibilities for patients suffering from chronic wounds.
In this section, we focus on some of the newest and most promising technologies and delivery systems based on physical principles and forces, as applied to the diagnosis and treatment of tissue defects as consequences of chronic pathologies or surgical interventions.
While a previous EWMA document, Advanced Therapies in Wound Management, covered all the advanced therapies related to treatment of chronic and acute wounds (1), this document will only cover the technologies that have specific indications for supporting, promoting or sustaining tissue replacement for post-surgical defects and/or loss of substance.
For the sake of the exposition, we will group the different technologies according to their basic physical principles and describe the documented interactions and integrations of these methods at the end of the document, if any exist.
2.2 Auto-fluorescence
The presence of infection, or critical contamination, represents one of the key factors for the non-progression of wounds towards healing, especially in post-surgical wound types.
A diagnosis of infection is still based on the presence and recognition of clinical signs. The most frequent signs include pain, erythema oedema, secretion odour and necrosis. Unfortunately, sub-clinical infections are very common occurrences, especially in diabetic, elderly and/or post-surgical patients, in whom the poor reactivity of the immune system makes it difficult to detect and quantify the presence and extent of infection.
The late or absent diagnosis of an underlying infection is typically associated with a poor prognosis and delays tissue replacement in post-surgical patients, who frequently need to be re-operated on to make a surgical revision because of an
under-evaluation of local infections. Local detection and the identification of bacterial strains are also tricky and somehow misleading, since both the technique and the site of sampling may condition the outcomes. Improving the ability to detect and characterise subclinical infections in wounds is a new area of technology based on the possibility of detecting bacteria. Tissue auto-fluorescence has been set up and validated in different kinds of chronic wounds and tissue defects. The technology is based on the possibility of detecting the auto-fluorescence induced by irradiation with violet light at a wavelength of 405 nm. While normal tissue is coloured in green, bacteria results in red, because of pophyrins produced by their metabolism. Pseudomonas aeruginosa are coloured in cyan, because of the pyoverdine reacting to illumination (3).
The possibility of identifying bacteria inside and around the lesions has been tested in some pivotal studies in different wound models, and many bacterial strains responsible for wound infection have been characterised, even when in a biofilm-producing form (4).
The utility of this technology is intuitive, since it can be used not only as a detector of infection, but also as guidance for sampling debridement, and as a follow-up tool to test the efficacy of the treatment.
Moreover, imaging with auto-fluorescence can be compared to images taken with the same device in natural light, to precisely locate the bacterial load in and around the lesion and to follow up adequately on its clinical course.
Auto-fluorescence has gained a positive reputation among clinicians and is now widely accepted as a point-of-care tool for those who manage chronic wounds and tissue defects. This has been a process, starting from the first description of the technology and its first application in humans in 2015, through the evaluation of its ability to reduce the consumption of antibiotics and its cost-effectiveness in 2020. Finally, a Delphi-based consensus was published on its correct use and applications in 2021(5).
2.3 Hyperspectral imaging
For at least 20 years, the possibility of splitting visible and near-infrared light into its spectral components and then detecting these has made it possible to characterise images with details that would otherwise not be visible (6).
This technology, known as hyperspectral imaging (HSI), is based on the possibility of analysing the spectra of an incident light beam after it has been refracted in the tissues, mainly by haemoglobin, cytochromes, melanin and other chromophores, at a depth that is dependent on the wavelength of the incident light (7).
The basic concepts of HIS lies in the capacity to develop integrated imaging systems for analysing the spectrum of each pixel of a bi-dimensional (x, y) image by adding a new dimension. This dimension is related to the spectrum of the refracted light of the pixel, thereby creating a hyperspectral cube that carries information on its spatial and spectral dimensions (8).
By selecting the incident wavelength and focusing on different spectra, it is possible to produce not only morphological but also functional images of a region of interest (ROI). Figure 1 shows a schematic illustration of HSI (9).
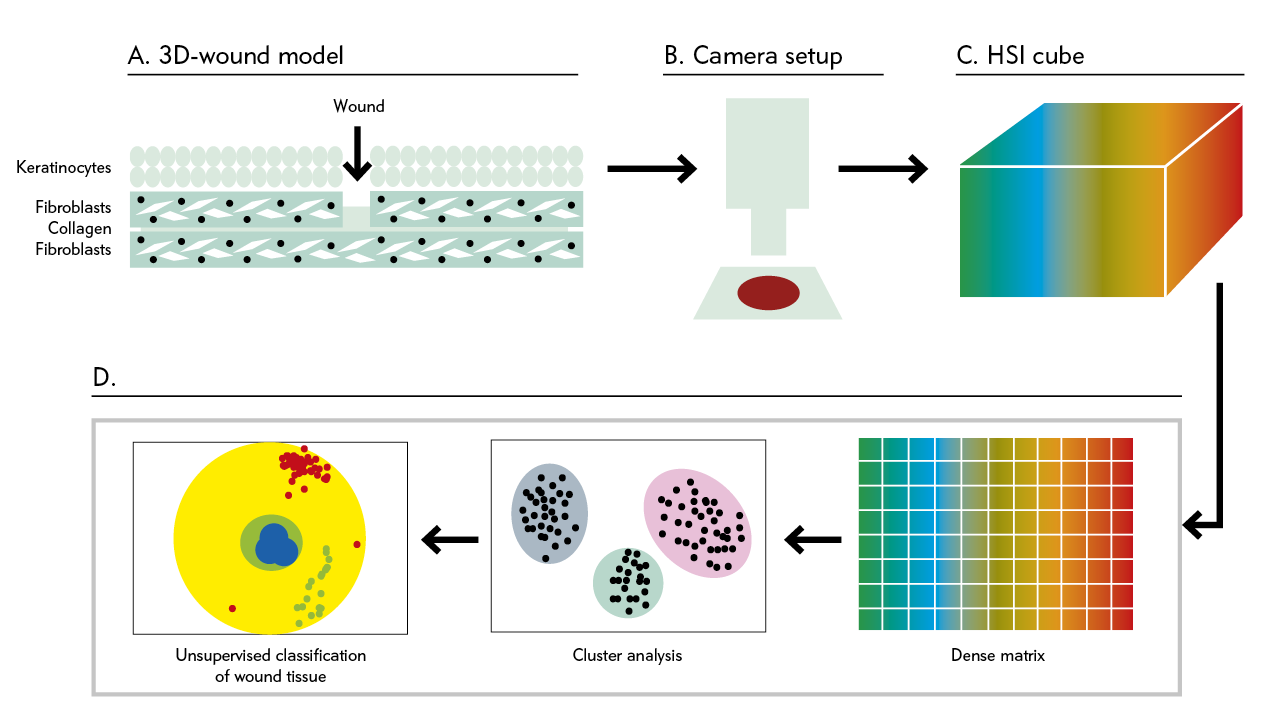
Figure 1: Automated and efficient interpretation of 3D wound models using non-invasive in vitro hyperspectral imaging.
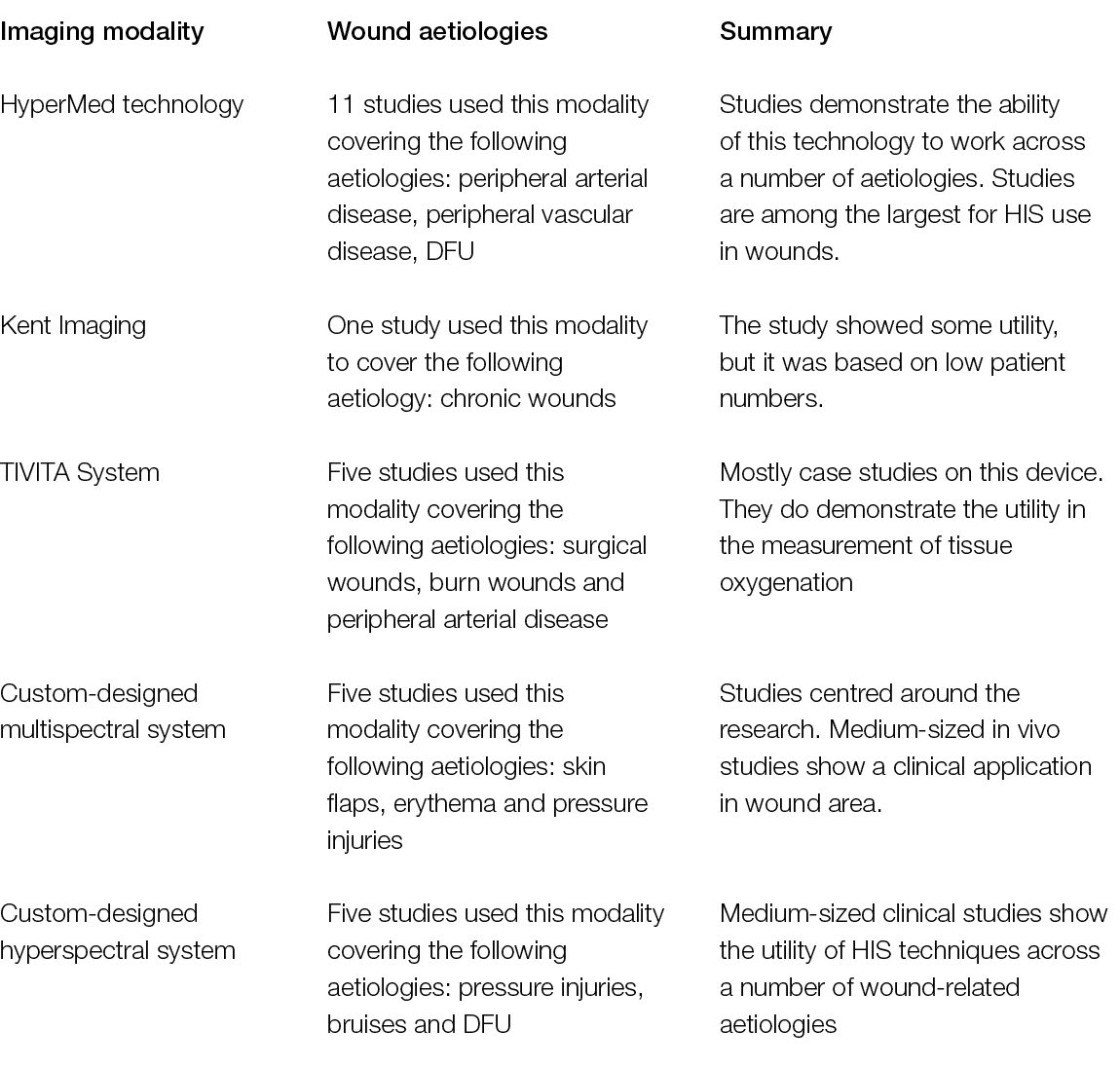
Relatively recently, HSI has moved from the lab to the bench, and some custom and commercial devices have been developed by scientists and manufacturers who have validated them in several clinical conditions, ranging from cancer to eye diseases, including diabetic foot ulceration (DFU) and other chronic ulcers (Table 2).
Table 2: HIS systems developed to date. Custom systems refer to those developed in a scientific setting and validated with experimental and/or clinical studies but which are not yet commercially available (9)
The focus in wound management has been on the vascular supply to the wound, since this is one of the most important predictors of healing/non-healing in many clinical wound-related syndromes (8).
The ability of HSI to detect oxy- and deoxy-haemoglobin and quantify their content in an ROI has been applied to the diagnosis and treatment of limb ischemia. This was done to stratify it according to its severity, and to monitor the effect of the treatment (i.e., revascularising procedures) (8).
HSI demonstrated how we may discriminate between ischemic and non-ischemic angiosomes in the foot when peripheral arterial disease (PAD) is present. This feature correlates with Doppler waveforms and the ankle brachial pressure index (ABPI), although it cannot predict the presence and severity of PAD (10).
When correlated with TcPO2, the most frequent standard in the assessment of critical limb ischemia comes into play; high-definition imaging was shown to correlate with both TcPO2 and the severity of PAD, according to Chiang et al. (10). However, as Lopez-Moral et al. have recently shown, TcPO2 was superior to HSI in predicting DFU healing in ischemic patients (11). Using the same target, HSI has been challenged against the possibility of characterising the biology of chronic lesions, eventually associating other sensors and devices based on different technologies (12).
Although still pioneering, an interesting clinical application of HSI is in the characterisation of the biofilm in chronic wounds. In a pilot trial, Poosapadari et al. demonstrated how HSI was able to discriminate between S. Aureus and E. Coli in DFUs with 100% sensitivity and 75% specificity, with a 100% predicting value in excluding infection in these wound types (13).
The interest in this technology in the field of tissue replacement consists of the possibility of establishing the viability of tissues without a direct contact between the source and the sensors, overlapping and conjugating morphological and physiological information in an integrated dataset able to guide and assist with surgical planning (14).
The limitations lie in the bidimensional charac-teristics of the method, which is only able to investigate a few millimetres of depth beyond the surface exposed to light. This significantly limits its applicability in a surgical context, apart from superficial debridement purposes. In addition, the costs are still high enough to strongly limit the accessibility of the technology for a large number of potential users (15).
Despite its great potential in wound management, the evidence behind HSI is still insufficient to promote its adoption as a first-line diagnostic tool, at least in tissue replacement. However, new studies and the possibly of developing a new generation of more accessible devices with a more favourable cost/benefit ratio will most likely lead to the implementation of HSI in wound management.
2.4 Cold atmospheric plasma
Cold atmospheric plasma (CAP) is a type of plasma containing different reactive species produced at near normal (<40°C) temperature from gases, by means of high-energy electric or electro-magnetic discharge. CAP has been applied to many clinical fields, including haemostasis, the treatment of cancer and wound management (16).
Plasma is a peculiar form of matter that is constituted by a gas of ions containing a wide range of reactive species, from OH to O3, to O- and NO. It can be produced via the application of high energy power to air, nitrogen, helium, argon and other gasses, at both high and low temperatures.
While high temperature plasma has long been commonly used in industrial sterilisation processes or in chemistry, so-called ‘cold’ plasma has more recently been applied as a therapeutic means for the management of various pathologies, including chronic wounds.
The interaction between plasma and the wounds exerts a range of different effects, all demonstrated in vitro and in vivo, mostly in animal models, with some pivotal experience in clinical protocols. Beyond the obvious bactericidal action, anti-inflammatory, neo-angiogenetic and pro-proliferative effects have been associated with plasma application (17).
CAP has proven effective for eradicating MRSA and MDR colonisation and infections in both animal and human wound models, promoted angiogenesis and boosted microcirculation, reduced inflammatory markers and stimulated the proliferation and migration of fibroblasts and keratinocytes.
Even though clinical studies are still too few and presently limited to a small number of patients, thus precluding a definitive evaluation, the safety profile of CAP is fair. No reported local or systemic side effects when the dose and timing of application (20–180” daily; 7–14 days of treatment) are respected. Despite the fact that one study demonstrates the production of ultraviolet light (UV) radiation and of NO2, and their dispersion in the environment is a consequence of CAP production, no pathologic sequelae were reported by the authors of the paper (18).
Recently, some commercial devices that use the CAP technology have been produced and proposed by manufacturers for use in a variety of conditions, such as chronic ulceration, venous leg ulcers, pressure ulcers and, in particular, DFU. The devices are made by a plasma generator associated with a nozzle from which a plasma jet can be directed to the wound surface from a distance of approximately 10–12 cm (19).
As noted above, we are still at the beginning of the clinical application of CAP, and there remains a need for more evidence, but the technology is promising, especially in view of the potential reduction of the use of antibiotics for the management of infected ulcers (20).
2.5 Light
A variety of experiences with the application of light (UV, visible, infrared) have, in recent years, led to the emergence of photo-biomodulation. Photo-biomodulation is defined as the result of the interaction of light with the biology of wounds, including all the modifications in the biology and physiology of the lesions produced by this interaction.
Blue light (410–430 nm) has been the focus of several studies targeted to test its efficacy and safety in three aspects related to tissue replacement: haemostasis, inflammation and tissue proliferation.
These experiences were possible because a light-emitting diode (LED) emitting blue light for medical applications was recently manufactured and introduced in the field as a Class IIA medical device (EmoLED) (Figure 2).
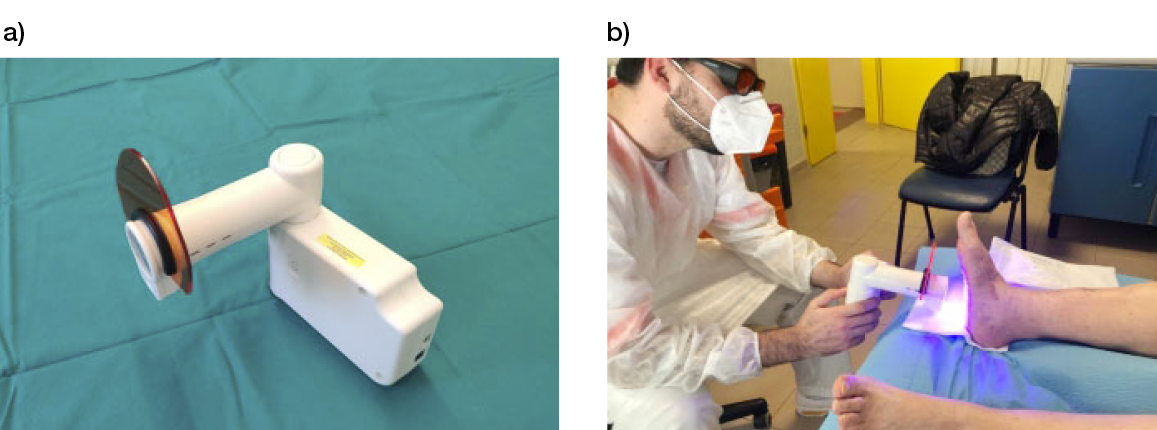
Figure 2: a) the blue LED light-emitting device (EmoLED, Florence, IL). b) The application of blue light therapy to a patient with DFU.
Unlike other light-emitting devices, which require the application of a photosensitising gel on the wound surface as a medium for the biological interactions, EmoLED directly transfers energy to tissue by interacting with haemoglobin, cytochromes and protoporphirines, activating cells’ metabolism and functions, both in leukocytes and in fibroblasts.
The technique has been proven in in vitro settings, and animal and human studies show how the haemostatic effect of blue light is mediated by its interaction with intra-erythrocyte haemoglobin, and possibly secondary to the local increase in temperature. This leads to the denaturation of proteins, which in turn activate the coagulating process.
The links with pro-regenerative aspects are more controversial, but they are most likely exerted via the interaction with cytochromes, transferring energy that can be used by the cell to activate or deactivate genes that, in turn, change the behaviour of the cells involved in the repair process.
Among the many observed changes, the anti-inflammatory effects, the increase in collagen synthesis and deposition, the neo-angiogenesis and the modulation of fibroblasts’ activity are the changes that are more directly involved in the repair of tissue defects (21).
It has been demonstrated, both in animal and in vitro models, how blue light is able to reduce the concentration of several pro-inflammatory cytokines and mediators, increasing and promoting, in turn, the production of a series of growth factors that characterise the proliferative phases of tissue repair. These findings have been confirmed in pivotal studies that, in different clinical settings varying from venous leg ulcers (VLU) and pressure ulcers to inflammatory lesions and burns, showed the positive effects of blue light in terms of decreased inflammation and tissue regeneration (22).
In a pivotal prospective comparative non-ran-domized study of patients with chronic wounds of mixed origin on the lower limbs, blue light was applied in addition to standard of care (SoC) on half of each lesion, while the other half of the lesion was used as matching control. The authors found a greater reduction in the residual area of the part that received light-treatment, compared to SoC (residual area 42.1% vs 63.4%; p=0.029). The difference was particularly clear when the analysis was limited to venous leg ulcers only (33.3% vs 60.1%; p=0.007). A highly significant (p=2x10-7) reduction of pain was also observed (23).
Due to the novelty of the approach, we still do not have prospective RCTs to sustain its application as a first-line treatment in tissue replacement. To fill this gap, a prospective controlled trial has recently been designed in diabetic foot (DF) patients, in collaboration between a hospital-based DF clinic and community nurses, comparing blue light with standard care in tissue replacement. The study, for which the design was recently published, is ongoing, and the results will be available by the end of 2024 (24).
Another interesting technology that has been tested in chronic leg ulcers is a light-activated nanofiber textile with antimicrobial characteristics. This material is made by a special polyurethane (Teicophilic™) electrospun nanofiber textile doped with a tetra-phenylporphyrin photosensitiser. It is activated by visible light, producing short-lived, highly reactive oxygen singlet that can exert an antibacterial effect without interfering with tissue repair processes. In 162 chronic VLU cases, the application of this material reduced pain in 71% of cases. It sterilised 98 lesions, reducing the lesioned area by 35% during the study (25).
If translated into dressing materials and products for clinical application, this approach could be very interesting for use in the management of large post-surgical tissue defects, protecting them from re-infection during long repair phases.
2.6 Electricity and magnetism
Despite the fact that this particular physical approach was covered extensively in the previous EWMA document Advanced Therapies in Wound Management (1), a new development has recently been made in this field concerning large defects, pressure ulcers (PU). We find it relevant to refer to this study, since it is these new developments that are interesting relative to tissue replacement (26).
Electric stimulation (ES) was challenged against (SoC) in 61 patients with PUs; their blood flow and area reduction rates were evaluated after a period of 8 weeks.
Patients received either anodal or cathodal high-voltage monophasic voltage current (HVMPC = 154 μs 100 Hz; 360 μC/s; 1.08 C/day) for 50 minutes per day, 5 days a week. They were evaluated with Doppler flowmetry and computer-assisted image analysis. Patients who received HVMPC, irrespective of whether it was anodal or cathodal, showed a higher rate of peri-wound blood flow and a greater area reduction, compared to the control groups.
These results, when transferred to patients with large tissue defects because of surgical debridement, are extremely interesting, since they may justify the inclusion of ES into the post-surgical wound management strategy. The justification is based on the extremely positive safety profile and the low cost, when compared to other approaches (i.e., NPWT).
Unfortunately, despite promising results, these findings do not yet allow the indication of ES as a first-line treatment option, as we lack prospective RCTs to support the evidence base for this treatment choice.
2.7 Combined approach
One of the positive characteristics of physical therapies is their possibility for use in combination for the same patients, either in sequence or simultaneously (i.e., NPWT with instillation). This can result in the added value of combining the positive effects of different treatments, if they are mediated by different biological mechanisms and pathways that do not compete.
Recently, some researchers have gained further experience by testing the combination of topical O2 with magneto-therapy and light in VLU against topical O2 therapy alone. They demonstrated how the reduction of the ulcer areas was more significant when the methods were used in combination; that pain perception was significantly reduced; and that the quality of life, as described by the patients, improved in the group treated with the combined methods, compared to the oxygen therapy alone group (27).
Although this was based on a single-centre study and a limited number of patients with one type of chronic wound, and thereby not generalisable to other pathologies, these results are interesting and promising, since they support opportunities related to combining different physical treatments in the treatment of one pathology. When large tissue defects are the target of our treatments, this strategy can reduce time and complication rates in a clinical course that usually lasts for months (28).
Further prospective RCTs in other pathologic settings would be beneficial for building the evidence behind the effectiveness of such a promising therapeutic option.
2.8 Delivery systems
In an era during which miniaturisation and robotics allow us to pursue solutions that would not even have been conceivable a few years ago, wound management still lacks technologies to overcome the barriers related to repair. These barriers are often related to the complexities of physiological processes and the variety of the involved pathologies. Examples of recent breakthroughs in related fields include the patenting of a miniaturised, implantable robotic insulin pump that delivers insulin to the peritoneal cavity and can be refilled per os by robotic insulin-containing ingestible capsules, freeing Type 1 diabetic patients from the need for multiple injections per day (29).
Recently, new and interesting perspectives on a delivery system suitable for wound management were opened by the possibility of making cross-linked hydrogels that are responsive to various environmental stimuli and capable of delivering drugs according to changes in the local conditions (30).
Among the different options in this vast and fast-evolving field, two are particularly interesting, from a tissue-replacement point of view. Watarai et al. have described a gel responsive to the concentration of tissue metal-proteases and based on star-PEG-heparin loaded with transforming growth factor-beta (TGF-Beta), a cytokine essential for the proliferation and differentiation of fibroblasts. When the concentrations of MMP rise in the wound, the gel is partially hydrolysed by them and releases TGF-Beta in a dose-dependent way. Fibroblasts are attracted and attach to the peptides exposed in the gel by the actions of the MMP, and then TGF-Beta promotes the transformation of fibroblasts into myo-fibroblasts (31). Prokoph et al. used the same hydrogels and loaded a chemokine with SDF-1a, thereby promoting the migration of endothelial progenitor cells (EPC). They were able to demonstrate how the increase of MMP in the presence of the hydrogel was associated with a more intense and sustained migration of EPC, the initial step for neo-angiogenesis (32).
Similarly, Chwalek et al., using an MMP-degradable star-PEG-heparin hydrogel (Figure 3), provided reversible binding and sustained delivery of proangiogenic growth factors via the electrostatic interaction between the growth factors and heparin (33).
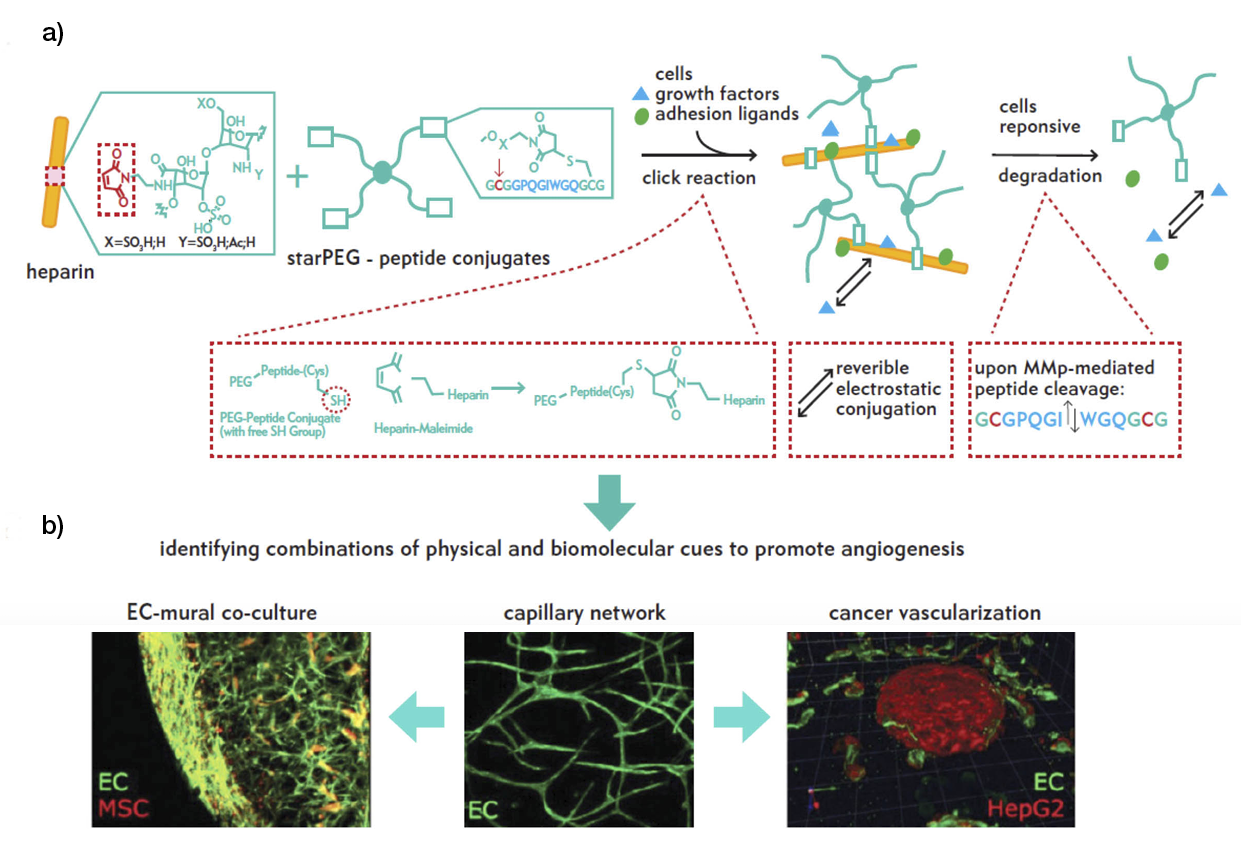
Figure 3: a) In situ hydrogel formation via the reaction of maleimide-functionalized heparin units with terminal thiol groups of starPEG peptide through Michael-type addition. b) Hydrogels enable heterocellular cell–cell interactions during vascularisation.
Although these technologies are in the very early stage of development, they lead to imagining a near future in which they could be injected into wounds. They could, in this case, act as ‘transformation agents’ that can restore the progress to chronic ulcerations frozen in a chronic inflammatory state or speed up the closure of vast tissue defects in tissue replacement.
2.9 Conclusion
Several new technologies have come to the stage in recent years in the field of physical approaches to tissue replacement. They are all extremely interesting, and the pivotal experiences made thus far are very promising, both as stand-alone options and in combination with others.
New well-dimensioned and designed prospective trials in the clinical fields will possibly confirm the effectiveness of these proposals and, eventually, define the indications for their use in clinical practice for tissue replacement.
Table 3: Studies on physical technologies for tissue replacement
3. Materials
3.1 Introduction
Tissue replacement relies on natural or artificial three-dimensional (3D) matrices that provide a temporary template for the invasion of host cells that gradually deposit their own matrix and neo tissue. Naturally, a successful interaction with host cells is expected to be reached if they encounter a support that resembles their own extracellular matrix (ECM), maximising their response. In fact, ECM-derived structures to which cells were removed while preserving (not completely) native structure and composition can be considered the gold standard of dermal templates. Additionally, ECM has been the source of components that are combined in various formulations and then processed/manufactured as porous 3D structures to form scaffolds that tend to provide the elements that stand out in the native tissue to achieve improved clinical performance.
ECM has been the source of inspiration for the development of artificial (bio)materials, but it is not evident if these have superior performance compared to ECM-derived structures, or if this depends on the application/tissue to be healed. The properties of artificial materials are highly controlled, in opposition to the variability associated with natural sources, allowing the use of a greater number of processing methodologies to generate 3D structures that can act as tissue templates. Nonetheless, this is also directly linked to their bioactivity, as the coupling of biomolecules/cues to those materials narrows that window. Therefore, a well-balanced compromise between bioactivity/ECM resemblance and processing conditions is required for the development of tissue templates with a maximised potential for tissue replacement.
3.2 Non-living tissue-derived matrices – Skin wounds
Non-living tissue-derived matrices are among the most procured tissue replacement options for skin wounds; therefore, they are the ones with the most documented performance. These products comprise 1) acellular matrices that are obtained by decellularisation of the dermis; acellular dermal matrices (ADMs); or other tissues, such as placental membrane, urinary bladder or small intestinal submucosa (SIS); acellular matrices (AMs), both from human and non-human origin; and 2) artificial matrices that are prepared in porous 3D structures using chemical processes from ECM components such as collagens, elastin and glycosaminoglycans that were also extracted from non-human-origin tissues.
3.2.1 Decellularised matrices
Despite the numerous clinical trials with decellularised matrices, randomised controlled trials (RCTs) have mostly focused on DFUs. Moreover, while the performance of ADMs as replacement approaches has only been compared with SoC, ADMs have been tested in parallel with cellular products.
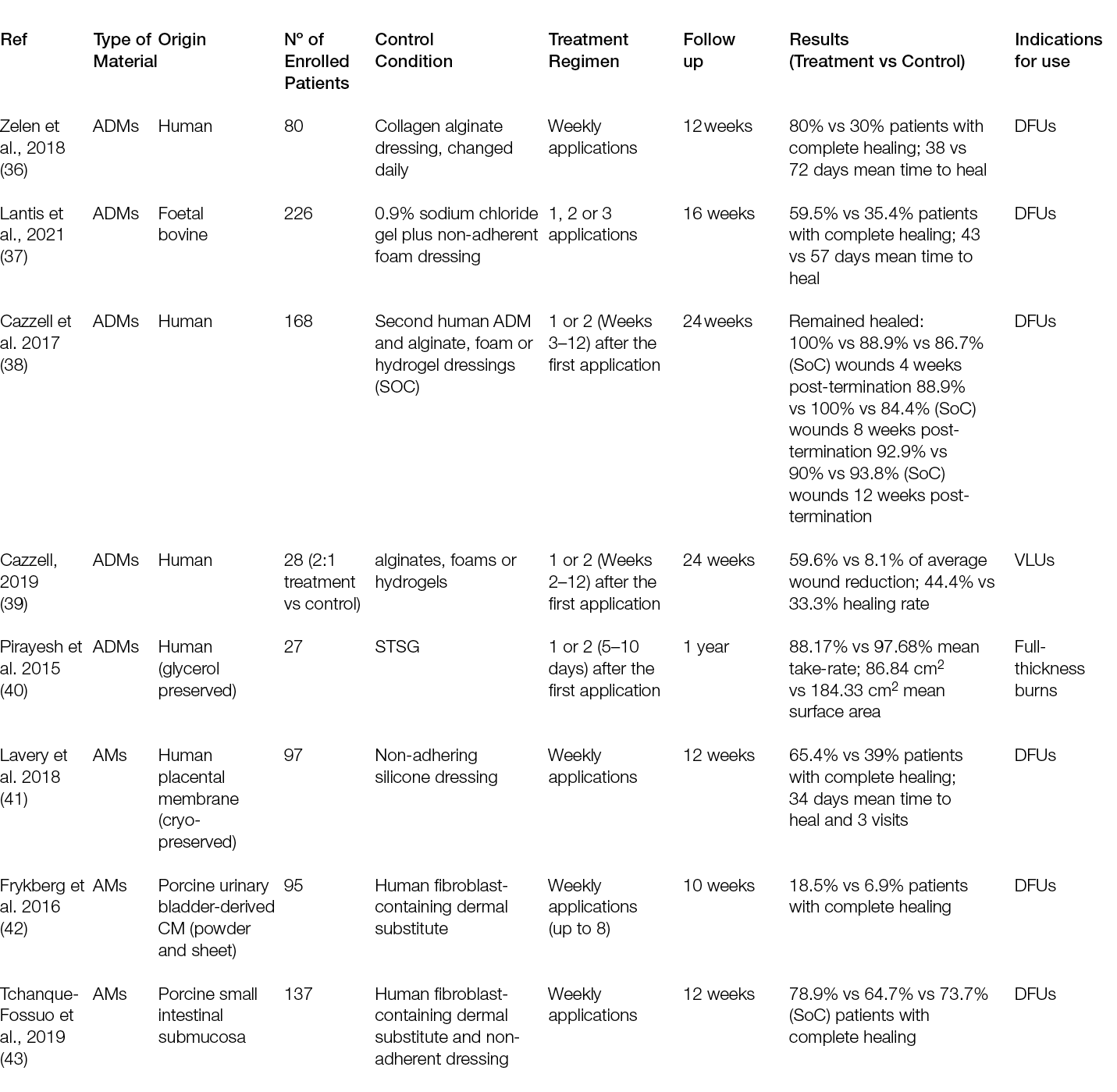
A randomised controlled multi-centre trial with 80 patients showed that 27 (68%) patients who received the ADM healed completely after 6 weeks, in contrast to only 6 (15%) in the SoC group. After 12 weeks, those numbers increased to 80% and 30%, respectively, leading to a mean time to heal of 38 days for the ADMs and 72 days for the SoC group (36). A more recent trial conducted in 21 sites in the US included 226 patients; in its first analysis, it showed that 45.6% of the patients treated with a foetal bovine ADM achieved complete wound closure, compared to only 27.9% in the SoC group (37). Interestingly, an earlier trial with two different human ADMs, which enrolled 168 subjects in 13 centres in the US, showed that the healing rate compared to SoC was significantly higher for one of the ADMs, but not for the other. Moreover, in the ADM group with faster healing, 100% of the wounds remained healed 4 weeks after termination, while in the SoC (which was not significantly different from the second ADM group), this percentage was 86.7% (38).
Overall, ADMs are an efficacious treatment for chronic, non-healing DFUs, but this clinically superior performance compared with SoC is not as evident for VLUs. An RCT in which 18 patients were included in the ADM arm and 10 patients in the control arm showed higher healing rates and rates of percent wound closure for the ADM group. At 24 weeks, ADM led to an average wound reduction of 59.6%, compared to 8.1% in the control group, but the healing rate was not significantly different (44.4% vs. 33.3%, respectively). In addition, the wound area increased in size by more than 100% for one-third (3/9) of patients in the SoC arm (39).
Similarly, the superior performance of ADMs over SoC in burn wounds is not evident. A Phase III randomised, controlled, paired, intra-individual study compared the performance of a human glycerol preserved ADM plus split-thickness skin graft (STSG) and STSG alone in full-thickness skin wounds (burns or after radial forearm flap harvest) and showed that the mean take-rate and mean surface area for ADM, 88.17%. and 186.84 cm2 respectively, were comparable to the STSG group. The skin treated with the ADM was significantly more elastic than the one treated with STSG, although not as much as native skin (40).
Regarding the performance of acellular matrices obtained from tissues other than dermis, the results seem to confirm a superior outcome in relation to SoC in the treatment of DFUs. The results of an extension phase of a multi-centre, blinded RCT showed that 65.4% (17/26) of the patients treated with a cryopreserved human placental membrane achieved complete wound re-epithelialisation in a median of 34 days and 3 visits. These patients were enrolled in the control group at the beginning of the project, and wounds did not heal after 12 weeks. During the initial 12 weeks of SoC, the average wound size reduction was 39% (41).
Acellular matrices have also shown comparable performances with cellular dermal substitutes.
At the end of the treatment phase (Day 56) of a randomised study conducted with 56 subjects at 13 centres throughout the US, 8.5% (5/27) and 6.9% (2/29) of the subjects reached complete wound closure in the acellular and cellular groups, respectively. The results at the end of the post-treatment SoC phase (Day 70 – treatment plus 2 weeks of SoC) showed that 7/27 subjects (25.9%) had complete wound closure in the acellular arm and 9/29 subjects (31.0%) in the cellular arm. From these 16 subjects with complete wound closure, 3/5 who returned for follow-up showed ulcer recurrence, one from the acellular arm and two subjects from the cellular arm (42). Similarly, no differences were observed in a randomised controlled and single-blinded trial regarding complete wound closure by 12 and 28 weeks of treatment, with an SIS and a cellular product. Further, the percentage of area reduction from treatment weeks 1 to 12, and from treatment weeks 1 to 28, was 73.7% (14/19) for SoC, which was not statistically different from the other groups. Respectively, these were 78.9% (15/19) and 64.7% (11/17) for the acellular and cellular groups (43).
3.2.2 Artificial matrices
Despite RCTs with artificial matrices for DFUs, they have been looked at from a management, rather than a replacement, perspective. Different dressings, such as pig atelocollagen, poloxamer and hyaluronic acid matrix (44), porcine type I collagen sheets (45) and chitosan/collagen hydrogel (46) have, in fact, shown superior performance in comparison to SoC approaches, but the dressings were not used as a template for neo-tissue deposition.
Recently, artificial matrices were considered as regenerative templates in burns. The performance of a porcine type I collagen artificial dermis prospective cohort study was evaluated in 95 patients when used in combination with STSG, compared to STSG alone. The average take rates were 94.55 ± 3.02% and 97.40 ± 2.57% at 7 and 14 days, respectively. When these artificial matrices were compared with the results of another study, in which burns were covered with an artificial dermis composed of bovine dermal collagen and bovine nuchal ligament elastin, no significant differences were detected (47).
Another study, which did not include hard to heal wounds, compared two bovine type I collagen-based artificial dermises in 30 patients with post-traumatic wounds localised on the inferior limbs. This study revealed that healing time, pain and self-estimation were not statistically significant among groups after 35, 42, and 49 days and at 1-year follow up. However, the wounds treated with the bovine collagen matrix revealed improved epidermal proliferation, angiogenesis and dermal renewal, compared to those treated with the other collagen matrix that contained shark chondroitin sulphate (Figure 4) (48).
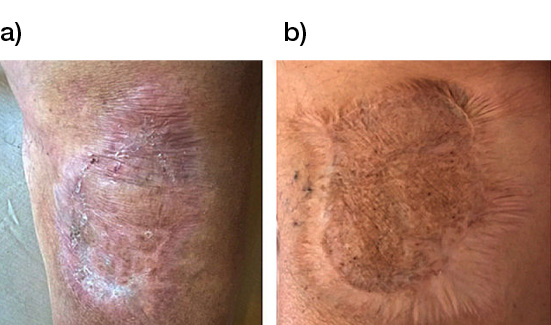
Figure 4: Long-term follow up at 3 years of post-traumatic wounds treated with collagen matrices a) without and b) with shark chondroitin sulphate (48).
Overall, the evidence regarding the use of artificial matrices for tissue replacement in hard to heal skin wounds remains sparse, but clinical studies with other ECM materials have recently revealed relevant results. For example, a heparan sulphate mimetic designed to replace the destroyed heparan sulphate in the extracellular matrix of wound cells led to the complete healing of the wound in 3 out of 5 patients, while the remaining two showed significant improvements in size and quality (49). Also, a single-arm, open-label, multi-centre trial with lyophilised tobacco plant-purified type I recombinant human collagen and hydroxy propyl methyl cellulose matrices showed that 15 patients (out of 20) with chronic lower limb ulcers exhibited ≥ 70% wound closure, and 9 achieved complete closure (50). Therefore, ECM still represents a source/inspiration of materials to be used in tissue replacement products for chronic skin wounds.
3.3 Non-living tissue-derived matrices - Complex wounds
Wounds involving exposed vital structures represent a reconstructive challenge to which acellular and artificial matrices can contribute. Retrospective studies looking at ovine forestomach extracellular (51) and biodegradable polyurethane (52) matrices outcomes in complex soft-tissue defects with exposed structures (bone or tendon) support their use as an alternative to flap reconstruction in complex wounds. Although there are no RCTs assessing the efficacy of tissue-derived matrices in these types of wounds, recent studies further confirm these results. A prospective, single-arm, multi-centre, open-label trial evaluated the safety and efficacy of human ADM in healing large, complex DFUs with exposed bone or tendon on the lower extremities. The ulcers were deep, with 59 of 61 probing to the bone, and an average wound area of 29.0 ± 21.0 cm2 (maximum, 113.6 cm2). The mean percent wound area reduction was 80.3% at 16 weeks, and wounds with 15 cm2 or smaller had a 14 times better chance of closure compared to those with 29 cm2 or larger (53). A series of three cases of exposed osteo-tendinous wounds treated with a non-commercial human cadaveric ADM followed by the application of an autologous graft skin showed a clear reduction of granulation tissue in the damaged area in which ADM was applied alone after the first 14 days. In the patient with an exposed bone and Achilles’ tendon, the gap between the tendon and the remaining damaged area in one of the legs was totally covered by viable and well-vascularised tissue. Similar results were observed for the third clinical case, with osteo-tendinous exposure on the malleolar region of the left lower limb due to a car accident; that patient had an initial engraftment of ADM/autologous skin evident after three days. The 1-year follow up confirmed a well-organised/oriented connective neo-tissue (Figure 5) (54).
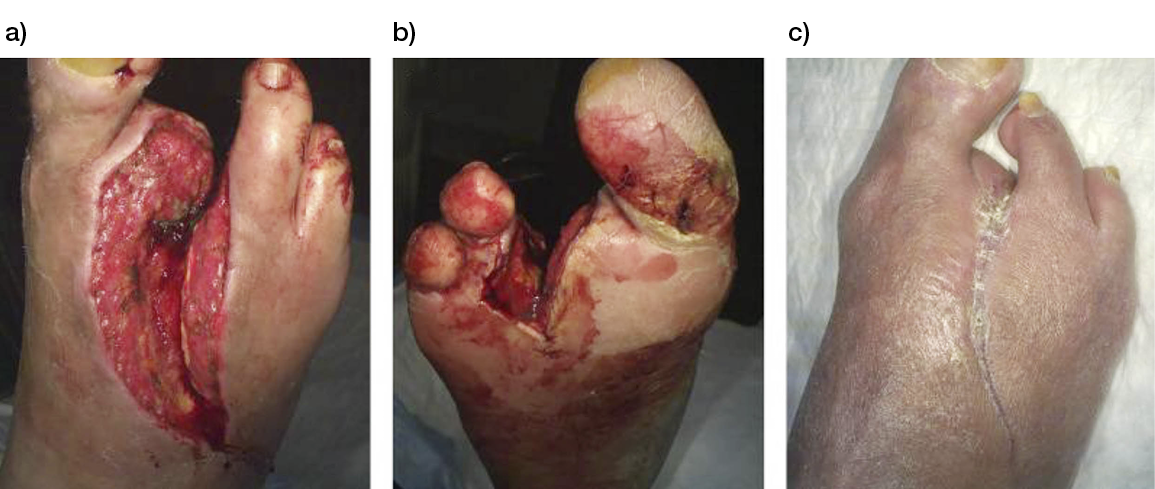
Figure 5: Diabetic foot ulcer with exposed bone a), b) before and c) after the treatment with ADM (53).
As many complex wounds also affect the bone, materials that are not tissue-derived, but consist solely of elements that exist naturally in the human body and have osteoconductive and osteoinductive properties after reacting with body fluids, have also been proven relevant in their treatment. This is the case of bioactive glass, which has different rates of bioactivity and resorption depending on its chemical composition. This material bears unique properties in comparison with other synthetic bioresorbable bioactive ceramics, inducing high local turnover of bone formation and resorption (55). Moreover, bioactive glass is antibacterial against anaerobic (56) and aerobic bacteria (57). Importantly, some formulations also inhibit bacterial biofilm formation on prosthetic material by methicillin-resistant Staphylococcus aureus and multi-drug-resistant Pseudomonas aeruginosa (58). Therefore, in addition to osteoconductive and osteoinductive advantages, bioactive glass can be considered an adjuvant in the treatment of infections, as detailed in Section 6.5. Recently, the safety and efficacy of bioactive glass was assessed for the management of DFUs with osteomyelitis (OM) after surgical procedures. Of the 10 patients enrolled, 7 were subjected to revascularisation procedures before treatment with bioactive glass and controlled weekly for 6 months, or until complete healing. A healing rate of 80% with a mean time of 34 ± 2 days, with only 1 patient in need of a second surgical look, was observed (59). A case report with a similar clinical presentation and pathogenesis of chronic hindfoot-infected ulceration in a demyelinated patient with Guillain-Barré syndrome also reported that bioactive glass was effective for the replacement of infected bone without recurrence after 24 months of follow-up (60).
3.4 New biomaterials
Following the original rationale that led to the development of tissue-derived wound replacement products, new biomaterials are either based on new sources of ECM materials or new combinations of their different components. A collagen-rich acellular swim bladder matrix from Rohu fish, which is expected to overcome any ethnocultural stigma associated with other animal-related products, showed its ability to support re-epithelialisation, improved neovascularisation and dermal matrix deposition in full-thickness skin wounds in rabbits (Figure 6). Interestingly, although the total IgG in the serum of the animals was significantly lower for the crosslinked matrix than for the non-crosslinked one between Days 20 and 40, it was significantly higher than the sham group, which might raise concerns regarding the immunogenicity of the materials. (61) Recently, a newly designed non-woven animal-derived collagen and gelatine matrix was compared with a commercial matrix made of collagen type I/chondroitin-6-sulphate glycosaminoglycan. A significantly shorter time to complete wound closure was attained for the commercial matrix, in comparison with the new matrix, even when this was applied multiple times in full-thickness wounds in pigs (62).
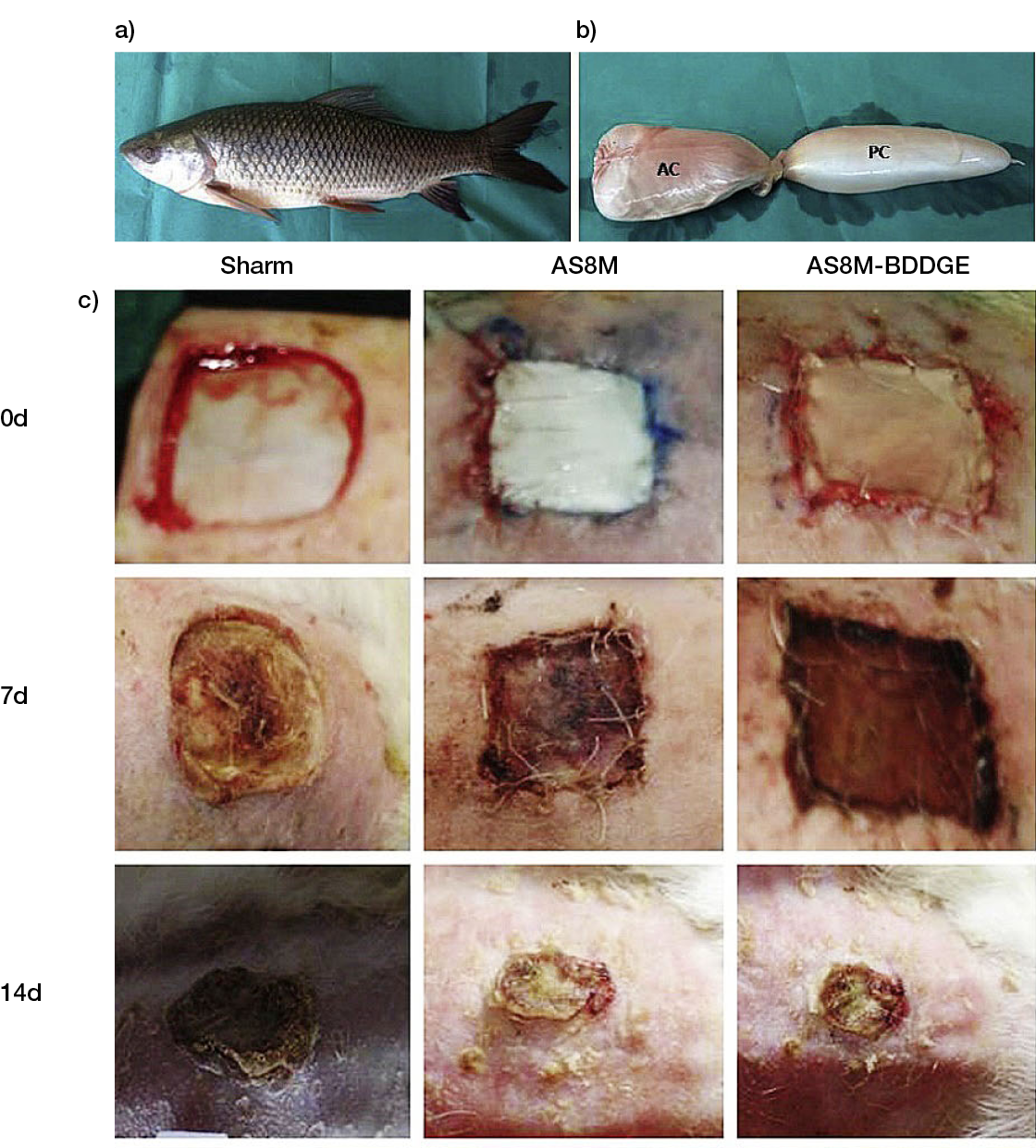
Figure 6: a) Image of Rohu fish (Labeo rohita), b) native swim bladder used to obtain the acellular swim bladder matrix (ASBM). c) Representative images of skin wounds in rabbits on Days 0, 7, 14, 21 and 28. ASBM-BDDGE refers to the crosslinked ASBM (61).
These outcomes, together with the lack or reduced effectiveness of commercial products, reinforce the need to further advance these approaches, as they support higher bioactivity. Various strategies, including naturally bioactive molecules and growth factors, have been used to this end. Human amniotic membrane-derived gels containing aloe vera (AV) extract were proposed for the healing of second-degree burns. The AV control group showed faster healing of full-thickness burns in rats, potentially due to higher contraction. No other significant differences were observed between the treatment and control groups (63). The ability of three different commercial artificial dermal matrices, 1) bovine tendon type I collagen and shark chondroitin-6-sulfate glycosaminoglycan structure, 2) bovine atelocollagen crosslinked sponge and 3) porcine tendon atelocollagen and porcine dermal gelatine, impregnated with b fibroblast growth factor (bFGF) to provide its sustained release and accelerate the healing of full-thickness wounds in diabetic mice, were compared (Figure 7). Long epithelium and wide granulation tissue were formed after treatment with Matrix 3. However, within each matrix group, the impregnation with bFGF did not add a significant effect, except for the granulation tissue formation on Day 7. Wounds treated with Matrices 2 and 3 had more capillaries than the ones treated with Matrix 1, particularly after longer time periods, which might be attributed to the release of the bFGF to the matrix, which was higher for Matrix 2, followed by Matrices 3 and 1. (64) From a different perspective, a poloxamer thermo-sensitive polymer hydrogel containing Lactococcus lactis was designed as an in situ lactic acid delivery system capable of modulating wound healing. After 12 days, diabetic mice full-thickness wounds treated with the L. lactis thermo-sensitive hydrogel presented thicker granulation tissue compared with the control groups (sham and thermo-sensitive hydrogel alone), and significantly lower amounts of inducible nitric oxide synthase positive cells and higher number of CD206-positive cells. It seems that the proposed system can produce and deliver lactic acid in situ, promoting the polarisation of macrophages from M1 to M2. However, in this study, the hydrogel was replaced every day, which does not allow a direct translation to a replacement approach (65).
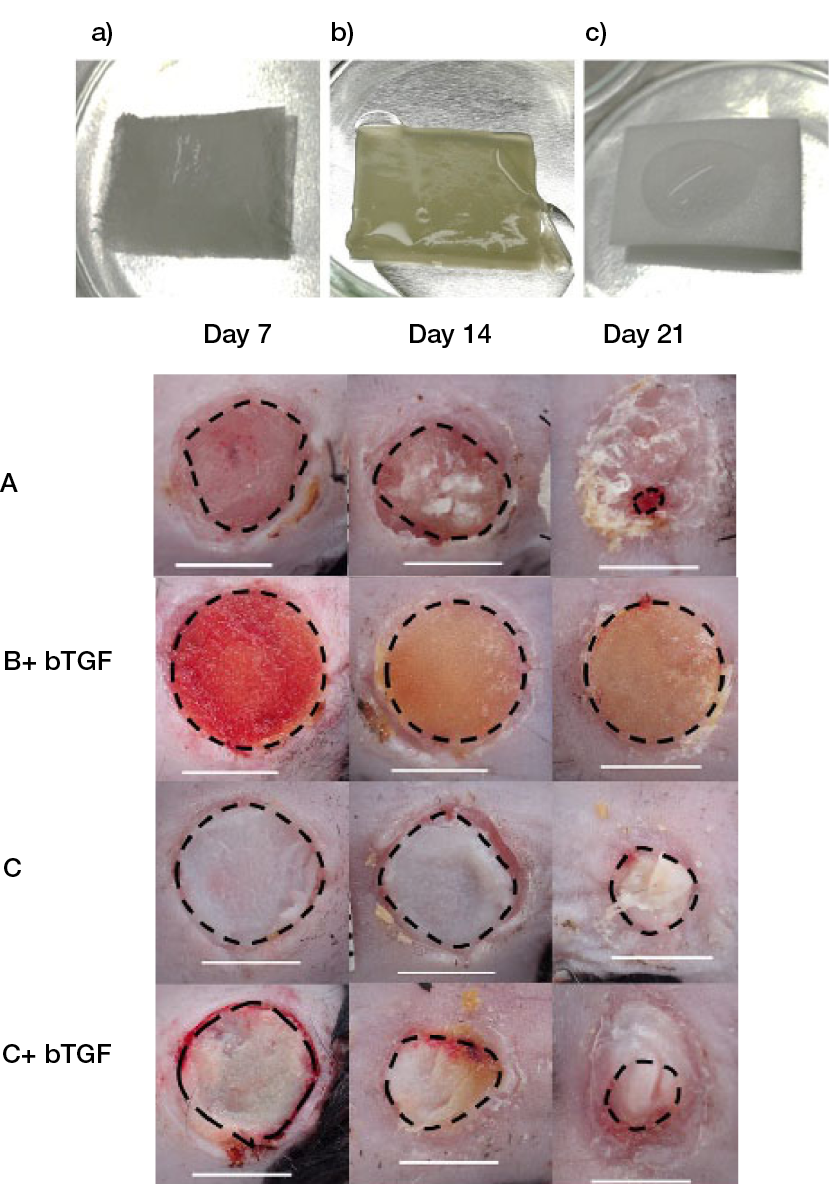
Figure 7: Macroscopic view of a) bovine tendon type I collagen and shark chondroitin-6-sulfate glycosaminoglycan structure, b) bovine atelocollagen crosslinked sponge, c) porcine tendon atelocollagen and porcine dermal gelatine impregnated with FGF, and of the wounds on Days 7, 14 and 21 after surgery. (64)
Recently, new approaches have involved attempts to change the way dermal replacement therapies have been considered. For example, in addition to the composition, structures that target specific needs of the wounds, such as deficient vascularisation, have been developed. A hydrogel with an innovative microarchitecture that is composed of dense type I collagen microspheres suspended in a less-dense collagen bulk drives cell invasion (including vascular cells) into the scaffold solely by mechanical cues inherent to this differential density interface. This leads to higher vascularisation of the structure, compared to the commercial artificial matrix composed of bovine tendon type I collagen and shark chondroitin-6-sulfate glycosaminoglycan (66). Another work proposes simplifying the access to donor tissues by suggesting the decellularisation of adipose-derived stem cell sheets, which are easy to culture in the laboratory. When compared with porcine SIS, the homogeneous decellularised cell sheets had less monocyte–macrophage infiltrating and induced higher production of IL-4/IL-10 than the SIS (67). While these innovative approaches have not been tested in cutaneous wounds, they represent relevant options and, more importantly, support the relevance of looking beyond the composition of current dermal replacement templates.
3.5 3D Printing
3D printing is a fast-emerging manufacturing technology that uses data from computer-aided designs to form 3D matrices with high spatial resolution and reproducibility. This manufacturing technique encompasses different types of printing that, among other aspects, define and limit the type of materials that can be used and the resolution that can be achieved (68). Despite this, 3D printing has the enormous advantage of allowing precise control of internal architectures and topologies that are hard or impossible to achieve with other methods of fabricating scaffolds. Additionally, when the materials used for 3D printing are combined with cells (bioprinting), it is expected that this will make it possible to accurately control the internal organisation of the structure, thereby allowing the generation of complex tissue-like structures for transplantation.
3D printing has also been extensively explored in the context of cutaneous wound healing, but this work has not always taken advantage of the possibility of controlling 3D structures’ architecture, valorising the cues that can be provided to the wound. This is the case in several studies that have used 3D printing to manufacture matrices with antimicrobial properties. The rapid switching between the sol and gel states of a cytidine, B(OH)(3) and AgNO(3) supramolecular hydrogel in response to shear stress enabled the 3D printing of a flexible patch with high water content. It was hypothesised to maintain tissue hydration, thereby facilitating the autolytic debridement of burn wounds while releasing silver ions (69). In another silver-based system, polydimethylsiloxane containing silver nanoparticles and oil infusion was printed into a porous structure with anti-adherence, non-fouling and antibacterial capacity, confirmed in infected (Staphylococcus aureus and Escherichia coli) full-thickness mice wounds (70). Similarly, a super-porous polyacrylamide/hydroxypropyl methylcellulose printed hydrogel cross-linked with silver nanoparticles demonstrated antibacterial properties in infected (Staphylococcus aureus) full-thickness rat wounds (71). Other works have also explored the antibacterial properties of other molecules. A polyvinyl alcohol/carbon quantum dot/silica nanoparticles (Si NP)/silk fibroin structure prepared by spray printing and electrospinning, took advantage of Si NP release (72), while a highly porous 3D-printed core/shell scaffold fabricated using poly-lactic acid, hyaluronic acid, copper carbon dots (Cu-CDs), Rosmarinic acid and chitosan relies on the Cu-CDs (73).
The use of 3D printing in which the material (ink) composition and the 3D organisation are complementary has also been explored, for example, with the objective of developing flexible electronics using an electrically conductive ink composed of poly(glycerol-co-sebacate) (PGS)-based polymer and zinc particles (74). Also, an electroceutical dressing was printed using Ag/AgCl ink onto silk substrates and confirmed to inhibit biofilms in non-healing and chronically infected wounds in dogs. This dressing was integrated with a Bluetooth®-enabled circuit, allowing remote monitoring of the current flow within the wound bed (75). The application of 3D printing to generate bio-integrated electronics for electronic skin still needs to overcome some limitations related to the mechanical, biological and manufacturing parameters, but it will certainly play a key role in the future.
The benefit of being able to design and customise, with high reproducibility, innovative 3D matrices using 3D printing has been addressed. However, their validation as dermal templates for skin repair/regeneration have not yet been achieved. A hydrocolloid ink consisting of an aqueous solution of poly-(ethylene glycol)-diacrylate emulsified with mineral oil was used to fabricate 3D-printed hydrogels with hierarchical porosity conferring self-tuning hydration due to the dual porosity. Moreover, this allowed tuning the release of gallium maltolate used in as a model molecule in this work (76).
A micro- and macro-structured 3D-printed chitosan and bioglass 3D matrix has been shown to enhance wound closure, neovascularisation and collagen deposition in rats’ full-thickness wounds, in comparison with a freeze- dried foam. This allowed for speculation that the 3D structure created by the 3D printing might also influence the observed response, thereby benefiting cell proliferation and migration (77). A bi-layer 3D-printed structure consisting of an outer poly (lactic-co-glycolic acid) nanofibrous membrane and a lower alginate hydrogel layer has also been proposed, aiming at preventing bacterial invasion and maintaining wound bed moisture, respectively. Implantation in rats’ full-thickness wounds showed the benefit of the alginate hydrogel (in both the control and bilayer groups) to support faster wound closure and promote neovascularisation and collagen I/III deposition in relation to sham and poly (lactic-co-glycolic acid) membrane groups. However, the benefit of the upper layer and ultimately of using 3D printing was not demonstrated (78). Solvent exchange deposition modelling was combined with electrospinning technology to manufacture another poly(lactide-co-glycolide) bi-layered scaffold with nano-/microstructure. The printed part acted as a sub-layer for cell and tissue ingrowth, and the densely packed electrospun nanofibers served as an upper layer, improving the sub-layer’s tensile strength and acting as a physical barrier. Additionally, the printed scaffolds were loaded with epidermal growth factor (EGF), which promoted a faster closure of the rats’ full-thickness wounds. Nonetheless, the degradation kinetics of the material and its connection with the released EGF and observed response need further analyses (79). A 3D-printed halofuginone-laden keratin scaffold was specifically optimised to slowly degrade in the wound, providing a moist environment, absorbing exudate and delivering halofuginone, a collagen synthesis inhibitor that has been shown to decrease collagen synthesis in fibrosis cases to reduce scarring. The outcome in the healing of partial-thickness porcine wounds was assessed between 30 and 70 days post-treatment, which, as for the work described above, does not allow us to establish a direct effect between the release of halofuginone and the observed response (80).
3.5.1 Bioprinting
In addition to the requirements associated with the 3D printing technology that, as mentioned above, does not allow for the use of indiscriminate materials, bioprinting further requires that the printing conditions do no harm to the cells and that the materials themselves provide adequate biological cues that support their survival and functionality.
Using as a basis skin substitutes already employed in the clinic, researchers have started using bioprinting technology to somehow reproduce them in terms of cellular content (epidermal or dermal-epidermal) in an automated and highly reproductive manner, one that can even be used on-site for extensive wounds (Figure 8) (81).
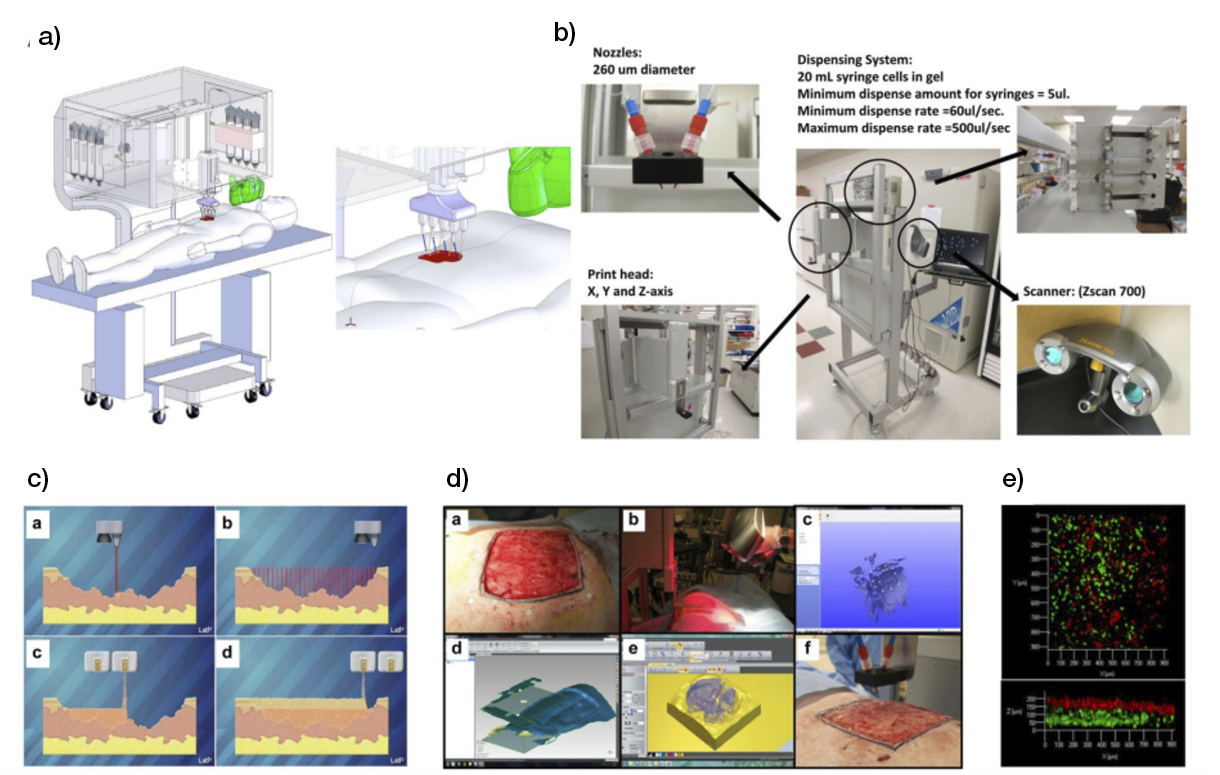
Figure 8: Skin bioprinter prototype and on-site bioprinting concept. (81)
The use of bioprinting has, however, been explored beyond this to attain successively more complex skin substitutes. A melt electro written technology was employed to 3D print a fibrous 3D polycaprolactone network (Figure 9), mimicking the wavy pattern of collagen fibres that displayed nonlinear stress/strain response in both radial and circumferential directions, recapitulating the mechanical behaviour of native rat dorsal skin. These structures were able to reduce scar tissue formation in mice full-thickness excisional wounds, but only when combined with human gingival mesenchymal stem cells. Additionally, this effect was more pronounced when these cells were transplanted into the wounds after in-vitro culture in the scaffolds, in opposition to equivalent cryopreserved constructs (82). A different approach was followed to create a dermal substitute to target neovascularisation from bio-inks composed of gelatine methacrylate, N-(2-aminoethyl)-4-(4-(hydroxymethyl)-2-methoxy-5-nitrosophenoxy) butanamide-linked hyaluronic acid and human skin fibroblasts, or human umbilical vein endothelial cells. Digital light processing-based 3D printing technology provided a rapid method for precisely positioning clusters of fibroblasts and endothelial cells with high cell viability. This was done to form a graft with microchannels that facilitates host cell migration and neo-tissue formation, covered by a dense epidermal-like acellular layer. Studies in small (rats) and large (pigs) animals confirmed the superior performance of cellular grafts in the healing of full-thickness wounds, accelerating wound closure and promoting neovascularisation and the regeneration of some skin appendages (83).
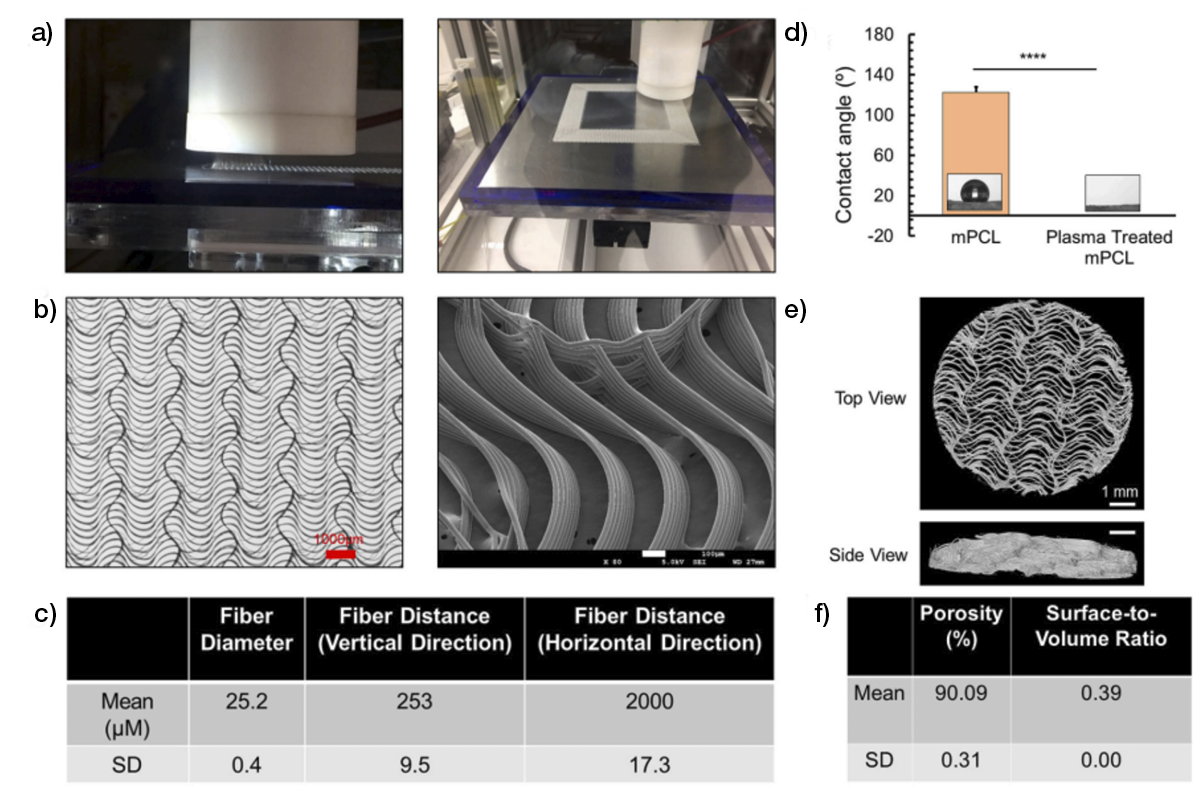
Figure 9: a) Melt electro writing technology used for the 3D printing of anisotropic fibrous polycaprolactone structures mimicking the microscopic architecture of collagen fibres. b)–f) Detailed structures and properties of the manufactured 3D structure (82).
Dermal–epidermal grafts were prepared with gelatine/sodium alginate inks containing human dermal fibroblasts mixed with human dermal microvascular endothelial cells (1:1) and human epidermal keratinocytes, respectively, as dermal and epidermal parts. Their transplantation into full-thickness wounds in mice led to higher vascularisation of the wound site that ultimately seemed to have contributed to lower wound contraction, in comparison to the grafts lacking endothelial cells (84). More complex skin substitutes, in terms of cellular components but not regarding 3D organisation, were also proposed. A rat tail type I collagen ink containing human foreskin dermal fibroblasts, human endothelial cells derived from cord blood, human endothelial colony-forming cells and human placental pericytes was used to form a dermis. This was followed (4 days after in vitro culture) by the printing of a second ink containing human foreskin keratinocytes to form an epidermis. The transplanted vascular cells were shown to participate in the vasculature of the neo tissue resulting from the healing of full-thickness mice wounds and seemed to improve the quality of the neoepidermis (85). Ultimately, a tri-layer skin structure (epidermis-dermis-hypodermis) was printed using a fibrinogen ink using cell type, other than keratinocytes, fibroblasts and endothelial cells, to promote pigmentation (melanocytes), hair follicle formation (follicle dermal papillary cells) and immunomodulation (adipocytes). When compared with an acellular fibrinogen hydrogel after implantation in full-thickness excisional mice wounds, complete wound closure was achieved with the skin substitute, but the contribution of the transplanted cells presents in the neodermis at Day 21 is still to be understood (86).
3.5.2 3D printing in the clinic
One of the most immediate clinical applications of 3D printing technology refers to reconstructive surgery for the fabrication of custom-made materials, such as maxillary (87), mandible (88) and temporomandibular joint (TMJ) (89) prosthesis, and sacral endoprostheses to reconstruct the pelvic ring and re-establish spinopelvic stability after total en bloc sacrectomy (TES) (90). Maxillary and dental reconstruction was successful using a custom-made titanium mesh plate and the particulate cancellous bone and marrow graft from a patient’s iliac bone, followed by the insertion of three dental implants in the graft after 10 months (87). An aesthetic defect of the unilateral hypoplastic mandible after completion of the orthognathic surgery was also treated with a 2-piece titanium implant designed and printed to restore the osseous frame of the basal border of the mandible. Due to its split design, the implant could be placed anatomically exactly at the mandibular margin via intraoral access. This also prevented damage of the mental nerve, leading to a fully resilient jaw (88). In another study, the use of a customised TMJ prostheses consisting of three components, including the fossa, condylar head and mandibular handle units, led to significant improvements on patients’ pain, diet, mandibular function and maximal interincisal opening. However, the lateral movement was limited to the non-operated side, and the mandible deviated towards the operated side upon opening mouth following surgery (89). A retrospective analysis showed that the 3D-printed endoprosthesis after TES provided reliable spinopelvic stability and implant survival by facilitating osseointegration at the bone-implant interfaces, with acceptable levels of haemorrhage and complications (90).
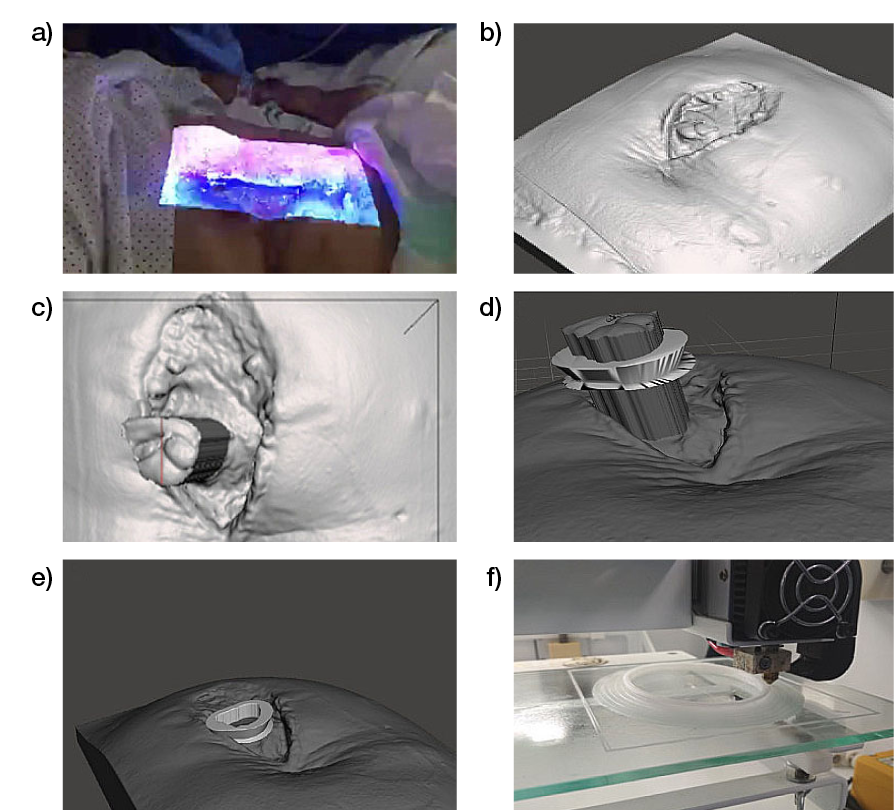
Figure 10: Process of using bioscanning and 3D printing. a) Process of taking pictures with the bioscanner. b) Images obtained with the bioscanner. c) Measurement of the exposed intestinal surface dimensions for device design. d) Verification of the suitability of the prosthesis by extrusion of the fistulous surface. e) Placement of the device on the image of the bioscanned wound to determine the correct adaptation to the patient. f) 3D printing of the bioprosthesis (91).
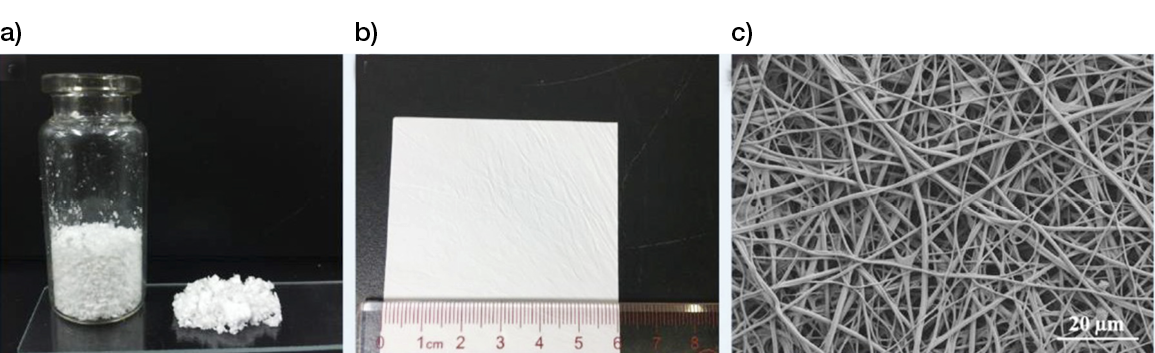
Figure 11: 3D-printed scaffolds, a) powder from and b) membrane, made from poly (L-lactide acid) (PLLA) and gelatine by a modified nanofibers-additive manufacturing method. (92)
3D printing has also shown clinical relevance for wound-healing. From one perspective, it was used to manufacture a custom device for use with NPWT in the management of an enteroatmospheric fistula, allowing a good adaptation to the anatomical characteristics of each patient and a control of the spillage of intestinal effluvium from the wound. The personalised polycaprolactone device was designed for each patient by 3D printing the shape of a prism and a hollow base, considering the dimensions of the fistulous area, to perform a floating ostomy to isolate the wound from the debit enteric. This proof of concept confirms the feasibility of the approach and offers promising results; nevertheless, the use of other materials that allow the perfect adhesion to the NPWT system would facilitate the overcoming of some technical limitations (91).
From a different point of view, 3D-printed scaffolds (membrane or powder from) made from poly-(L-lactide) acid (PLLA) and gelatine by a modified nano-fibres additive manufacturing method (Process 11), were proposed for the treatment of pressure ulcers and DFUs. When the patient was treated with the 3D-printed scaffold membrane (n=1), their PU healed in 28 days, and for patients treated with the 3D-printed scaffold powder (n=2), their PUs healed in 54 days. For the patients treated with the 3D printing powder mixed with platelet-rich fibrinogen (PRF) (n=2), the PU healed in 11 days, and the DFU healed in 14 days (92). Despite these results, the limited number of patients and the use of PRF and NPWT before treatment restricts the conclusions that can be drawn based on the effectiveness of the 3D-printed scaffold compared to SoC.
3.6 Conclusions
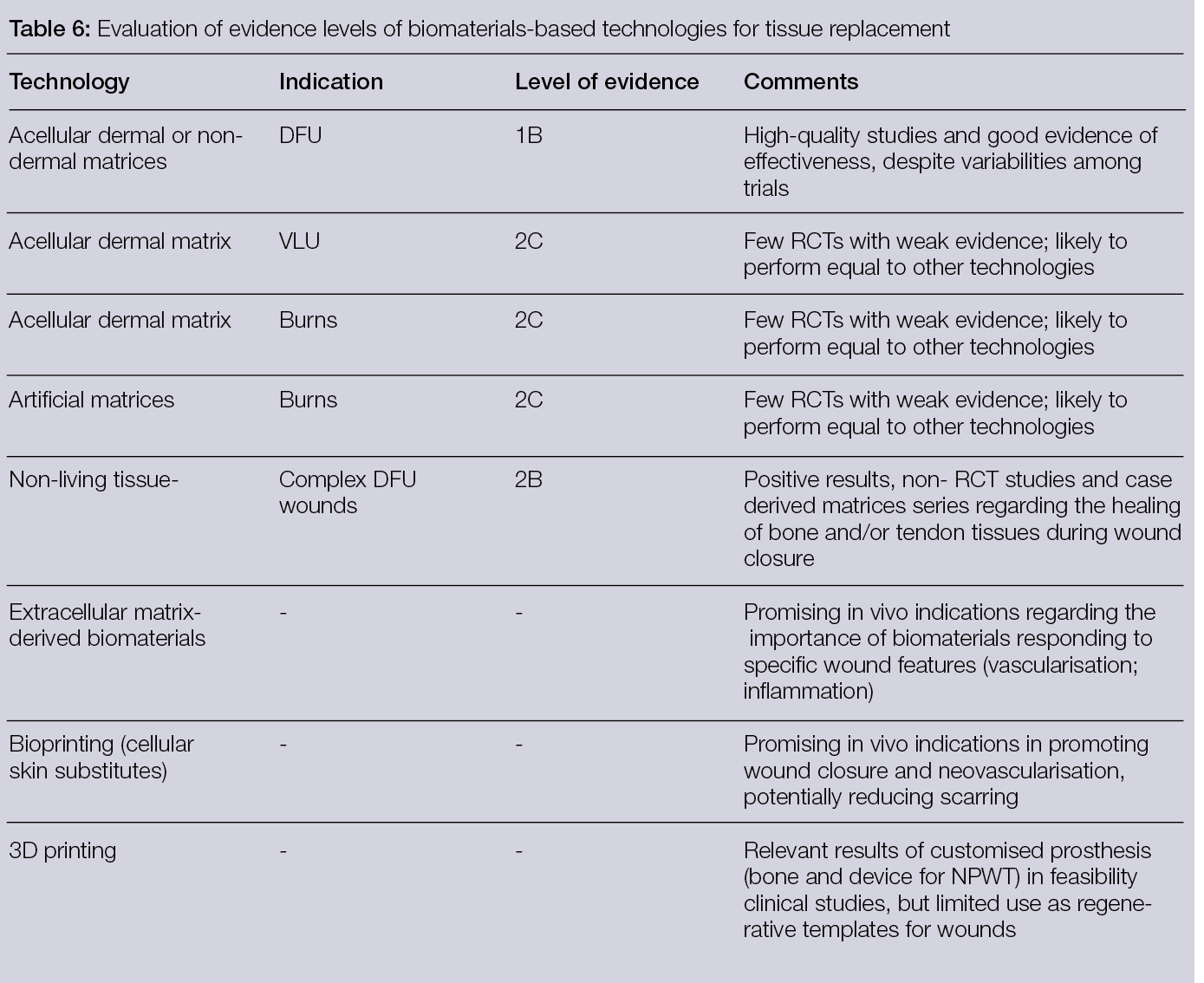
Clinical evidence regarding the efficacy of decellularised matrices for tissue replacement in hard to heal skin wounds is still sparse. A superior outcome seems to be evident, but only in the treatment of DFUs and in relation to SoC. Additionally, AMs have shown comparable performance with cellular dermal substitutes; however, much still has to be done regarding artificial matrices, as they have been used mostly in wound management, rather than as a dermal replacement.
Although ECM represents a valid source/inspiration of materials to be used in tissue replacement products for chronic skin wounds, there is a need to look deeper into their bioactivity and to adjust the composition of the tissue/dermal templates to the needs of the wounds. This is also valid for complex wounds in which replacement matrices are used as an alternative to flap reconstruction, since each tissue (bone/cartilage/tendon/skin) has specific healing requirements. Ultimately, these requests will also have to be addressed together with the processing methodologies, to avoid the loss of essential bioactivity. This might further narrow the applicability of 3D printing to design complex acellular structures that meet spatial and temporal healing specificities. However, if biomaterials cannot meet those, it has the potential to manufacture living substitutes that can act as in situ factories of bioactive healing factors.
Overall, the evidence shows that both materials and processing methodologies still have room for improvement with respect to the generation of tissue-healing templates or substitutes.
Table 5: Randomised controlled trials evaluating non-living tissue-derived matrices’ efficiency in skin wounds
4. Skin substitutes (dermal and epidermal)
4.1 Introduction
Skin substitutes can be classified as epidermal, dermal or composite, and then split into different categories depending on their composition and source material (xenograft, acellular allograft, cellular allograft, autograft, synthetic skin substitutes), contraction capacity, pore size and shape (93). Because there is no ideal option for skin substitutes, a lot of research goes into evaluating and developing different skin substitute options.
Over the last three decades, acellular dermal substitutes have changed the concept of skin reconstruction. The neodermal component forming the dermal substitute limits the secondary retraction of the thin autologous skin graft used to cover it. Many products, both with or without elastin, have been proposed; their collagen can come from different animals, such as cows, sharks or pigs, and different combinations with elastin. They can be covered by a protective film in silicone and secondarily skin grafted after three weeks. This period is essential for the dermal component to adhere to the underlying granulation tissue, to be penetrated by factors allowing the covering partial thickness skin graft to take place.
Other medical devices help with collagen matrix formation and enhance the formation of granulation tissue, improving global wound healing.
The heterogeneity of the different dermal substitutes and their different indications make the global perception of these medical devices somehow confusing, beginning with their classification.
In light of the recent literature on the topic, the authors describe here the different devices that are currently present in the market, discuss their clinical indications and define new proposals.
4.2 Dermal substitutes: Principles and requirements
Since their introduction in the 1950s, bioengineered tissues and dermal substitutes have become one of the most-used treatments for both acute and chronic wounds. Both dermo-inductivity and dermo-conductivity have been proposed, featuring great mechanical stability and a special ability to modulate pathological scar formation.
Technological evolutions and improvements in clinical research (94) have permitted the combination of the best dermal substitute with a specific lesion, and now wounds such as burns, post-traumatic lesions, diabetic and vascular ulcers, post-oncology wounds and lesions with high risk of infection (Figure 12,13) can be treated using dermal substitutes. However, to optimise the outcomes, some prerequisites must be satisfied prior to their use, particularly the need for a non-infected and non-ischemic wound bed on which the dermal substitute will be applied. Therefore, wound bed preparation using debridement and the promotion of granulation tissue should be performed meticulously.
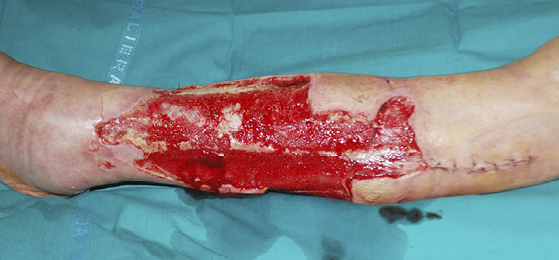
Figure 12: Post-traumatic wound after debridement.
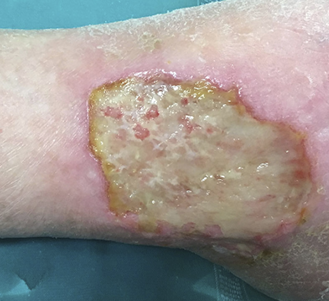
Figure 13: Vascular ulcer with important slough.
4.2.1 Debridement
Indeed, it is mandatory to achieve an optimal ‘wound bed preparation’, removing all devitalised tissues (eschar, necrotic, slough, fibrin) and/or infected tissues or biofilm through a proper surgical debridement (95) that can be performed with a sharp instrument (Figure 14) and/or Volkmann curette and/or with devices, as for example (see Figure 15, 16):
- •Hydrodebrider: Featuring a handpiece connected with an irrigation system, this device can be very useful for controlling the depth of debridement more precisely. It is suitable for reducing bioburden.
- Ultrasonic debrider: Featuring an association between an irrigation system and ultrasound technology, this device uses low frequency ultrasound to provoke a cavitation effect which consequently removes the undesired tissue through gas-filled bubbles. This type of debridement is very safe and supports a precise debridement that saves healthy tissue (96)
- Coblation debrider: Featuring an association between a surgical debrider and a radiofrequency generator, this device permits the application of focused plasma that chemically disrupts the devitalised tissue and/or biofilm. This kind of debridement can be performed in a more precise way, thereby permitting the surgeon to save more healthytissue (97).
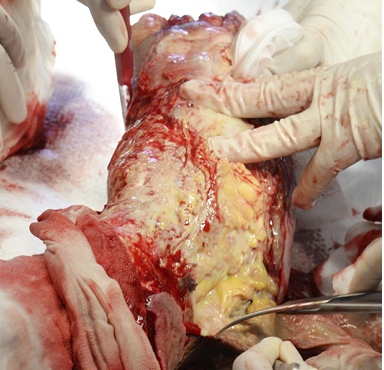
Figure 14: Surgical debridement with sharp instruments in necrotising fasciitis.
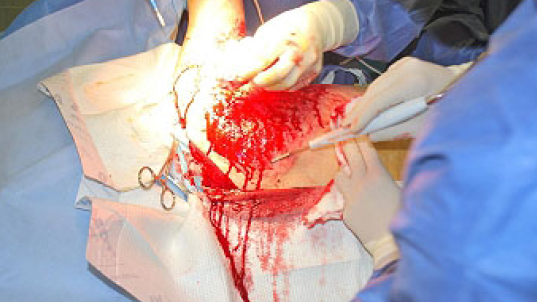
Figure 15: Hydrodebridement in a burned patient.
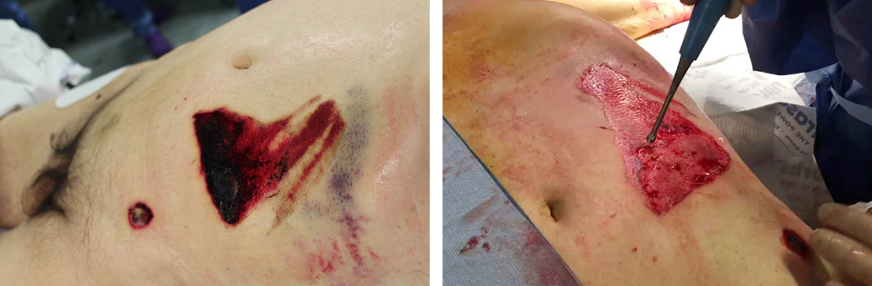
Figure 16: Ultrasound debridement in a burned patient.
4.2.2 Granulation tissue formation: Negative pressure wound therapy (NPWT)
Once surgical debridement has been performed, the second step must provide stimulation of the granulation tissue, to lead to the final closure. Even though there are many possible techniques, the most-used treatments today are:
− The application of negative pressure wound therapy (NPWT), with or without instillation (98, 99): This technique can be used if the debridement has not been sufficient to obtain good granulation tissue. It exploits micro and macro mechanical forces to stimulate granulation tissue and achieve an optimal wound bed after a period of one to three weeks, as a result of the depression applied by the device (mechanically or by means of a battery/electric network). This causes the collapse of a polyurethane sponge interface. These processes stimulate the lesion bed both macro- and micro-mechanically. Specifically, the macro-mechanical stimulation allows the contraction of the margins of the lesion, with a consequent reduction of the wound area. Unlike the micro-mechanical stimulation, which has an effect on the wound bed at a microscopic level, this allows, through the application of tensile, compressive, shear and hydrostatic forces (100):
1) The activation of the cytoskeleton, with the onset of proliferation and cell migration
2) The draining of interstitial fluids and a reduction in hydrostatic and osmotic pressure, and consequently the amount of exudate and oedema
3) A stabilisation of the microenvironment via the removal of inflammatory mediators, including matrix metalloproteases (MMP) 2 and 9, which are often responsible, when hyper-produced, for the wound becoming chronic
The vacuum effect also causes local hypoxia with the activation and increase of vascular endothelial growth factor (VEGF) and, subsequently, neo-angiogenesis (101). Tissue perfusion and oxygenation of the area are therefore increased, and a better preparation of the wound bed and the formation of an active granulation tissue are obtained. Furthermore, it has been shown that tissue hypoxia caused by negative pressure stimulates not only the cell proliferation of fibroblasts and keratinocytes, but also has a marked adipogenic effect, with proliferation present in the preadipocytes and their maturation in adipocytes (102-104).
These mechanisms of action and biological effects have led the scientific community to consider negative pressure therapy from the outset to be a ‘suitable/ideal’ treatment in cases of acute and chronic injuries where there was a need to: 1) promote the formation of granulation tissue; 2) prepare the wound bed to re-epithelise and/or be treated with advanced medications, or to be subject to definitive repair intervention with a dermo epidermic graft or flap; 3) control oedema and exudate; 4) stabilise the lesion; 5) stabilise the patient with complex trauma and a significant loss of substance; and 6) prepare the tissue to receive an autologous adipose tissue graft.
- Immediate application of the dermal substitute to improve the final closure. In this case, it is possible to choose a one-step, single-layer substitute that can be immediately covered by, for example, a skin graft. Or, if a better dermal tissue is needed, we can use a two-step, double-layer substitute; these are characterised by the presence of an outer silicon layer that is removed after three weeks. During this time, the dermal substitute improves the granulation tissue to receive the final closure. Once an optimal wound bed is achieved, a surgical closure will be performed. Even in this case, the surgeon can match the best technique with the specific characteristics of each lesion, starting from a ‘simple’ skin graft, which can be considered if we have to treat a superficial lesion (Figure 17), and continuing to local or distant flaps (Figure 18) for deep surfaces that expose noble structures, such as tendons. Finally, to improve the intake, it may be possible to combine NPWT with a dermal substitute and skin graft. Indeed, Diehm et al. (2021) (103) presented a retrospective non-blinded, non-randomised comparative study of 86 patients treated with artificial dermis skin substitute with or without NPWT. They noted a better intake in patients treated with NPWT after dermal substitute plus skin graft.
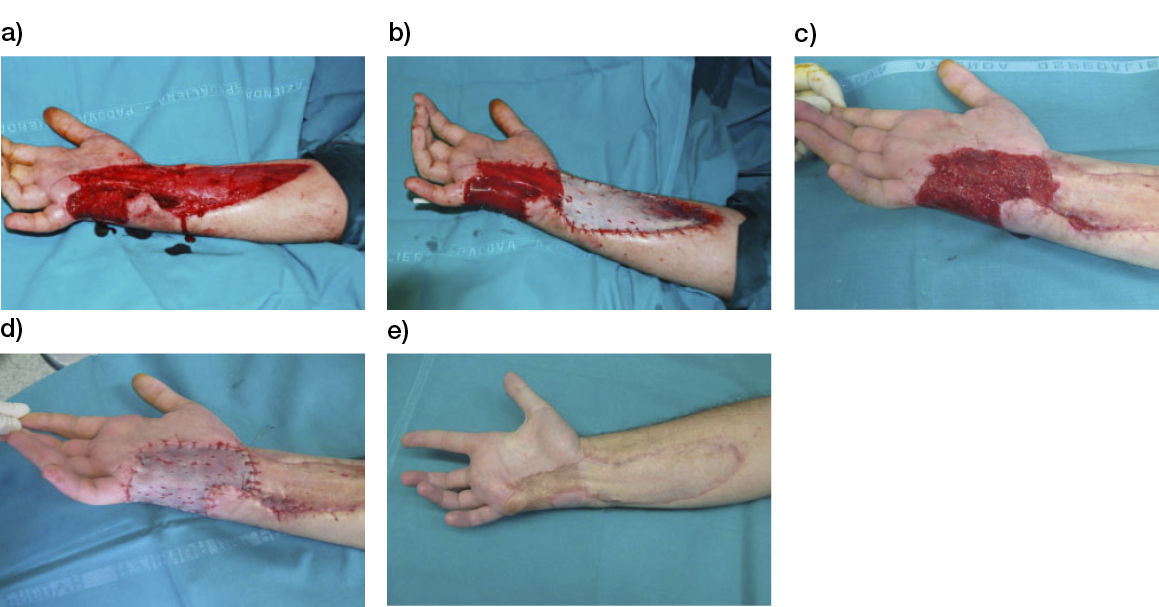
Figure 17: Post-traumatic lesion: a) optimal granulation tissue, b) skin graft and dual layer dermal sub-stitute, c) wound bed after silicone layer removal, d) reconstruction with skin graft, e) at 1 year follow-up.
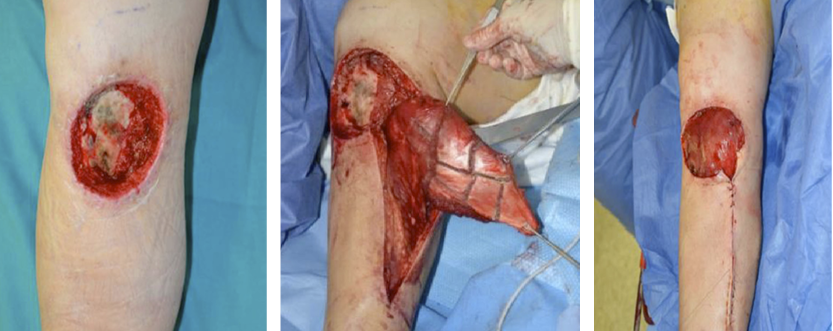
Figure 18: Knee ulcer: Reconstruction with a locoregional muscular flap.
4.3 Acellular dermal substitutes
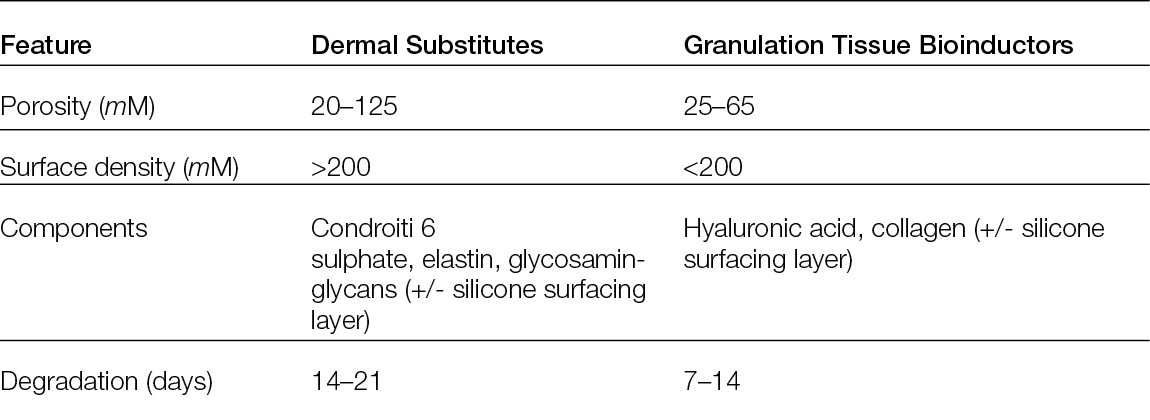
Skin substitutes are dermal constructs fabricated to either temporarily or permanently replace dermal defects. They can also protect against microorganisms, reduce pain, promote wound healing and assist in recreating the skin’s barrier function. To improve skin regeneration, reduce scar contracture (105), improve scar quality and elasticity (40) and minimise donor site morbidity, this method has been considered for the treatment of open or chronic wounds, burns and deep tissue donor sites. Today, a wide range of skin substitutes with different characteristics, such as mono- or bilayered compositions, temporary or permanent fixture and cellular or acellular skin substitutes, are present in the European market (106). Many classifications are also available, depending on their different impacts on tissue regeneration. As reported earlier, pore size, composition and degradation time are key features when describing the products, but these characteristics are also essential for differentiating them, depending on their different impacts on tissue regeneration, in: 1) permanent dermal substitute or 2) granulation tissue bio-inductor.
Permanent dermal substitutes: These feature an average porosity between 20–125 μM, the presence of chondroitin 6 sulphate or elastin, a surface chemistry of the scaffold with ligand densities exceeding 200 μM for both α1β1 or α2β1 ligands and a degradation time of 14 ± 7 days. This type of substitute has been considered active for tissue regeneration. In 1989, Yannas et al. (107) demonstrated that the diameter of the pores could influence both the ability to modulate the contraction of the wound and regenerative activity, suggesting that an average porosity ranging from 20 to 125 μm could be the ideal compromise for reducing contraction and maintaining regenerative activity. To improve these outcomes, Soller and Tzeranis (108, 109) described the other mentioned features some years later. They showed that the presence of macromolecules, such as chondroitin 6 sulphate, could stabilise the scaffold, thereby improving its binding with the cells and the extracellular matrix.
Granulation tissue bioinductors: These are inactive scaffolds for both the absence of macromolecules as chondroitin 6 sulphate and for their fast degradation composed of hyaluronic acid, porcine or bovine collagen or fully synthetic scaffolds. Due to their faster degradation, they can stimulate the formation of granulation tissue, proving suitable to cover the wound bed while waiting for an autologous skin graft or a flap. Therefore, many studies have been published in recent years to better understand both the clinical indication and biological effects (Table 7) of different products, such as dehydrated human amion/chorion membrane allograft.
Table 7: Differential features between dermal substitutes and granulation tissue bioinductors
4.4 Permanent dermal substitutes
Integra® Dermal Regeneration Template (IDRT): The IDRT was developed in the early 1980s and was the first dermal substitute. Its goal was to minimise fluid loss and bacterial contamination and to promote cell migration into the wound bed (110). It is supposed to do this through its two-layered composition and a two-stage procedure. The deeper layer of IDRT is made of a combination of bovine collagen and glycosaminoglycan chondroitin-6-sulphate, whereas the top is made with a 0.2-mm-thick polysiloxamine polymer membrane with vapor-transmitting characteristics. This membrane can be placed on the full-thickness wound, so the outer silicone membrane functions as a temporary epidermal replacement. This feature requires replacement by a split-skin graft after 2–3 weeks. Due to the combination with a silicone layer as temporary epidermal coverage, IDRT can immediately function as a barrier while providing the required extracellular scaffolding for cell ingrowth and the proliferation of fibroblasts and endothelial cells. After 2–3 weeks, when vascular ingrowth is complete, the silicone layer is replaced by a thin split-skin graft. In recent years, many studies have demonstrated this approach’s different indications (skin ulcers, burned areas, to fill spaces or to improve scar quality) (Figure 19, 20, 21). In 2015, Driver et al. (111) published the results of an RCT on 307 patients affected by diabetic foot ulcers (154 cases and 153 controls) and reported faster healing in patients treated with IDRT. Dalla Paola et al. (112) and Hicks et al. (113) reported the same results in 2020. In the first paper, Dalla Paola et al. (112) reported a case–control study on patients affected by critical limb ischemia post-revascularisation and noted that those treated with IDRT were characterised by faster healing (83 days vs 139). In the second paper, Hicks et al. (113) described a prospective case series of 107 diabetic foot cases treated with IDRT. In this case series, they noted that, after 18 months, 93+/-3.3% of patients were healed. Meanwhile, in 2018, Reynolds et al. (114) reported a 92% rate of healing and hand function restoration after 6 months of follow up in 14 patients who had undergone hand reconstruction. Similar results were also reported in 2020 by Choughri et al. (115), who described the possibility of using IDRT as a good alternative to flap reconstruction in 14 patients affected by hand lesions after 36 months. In the same year, other studies reported on burned patients, scalp reconstruction, elderly patients, etc. Bernstein et al. (116) noted complete healing in 86% of 14 patients treated with IDRT plus skin graft, and Rudnicki et al. (117) used IDRT on 13 burned patients immediately after escharectomy with good results. In addition, Shakir et al. (118) published a retrospective case control study on 191 wounds, demonstrating 70% healed cases after 180 days, while Chaiyasate et al. (119) reported their experience with 13 patients in which they demonstrated a good scalp reconstruction after 3 months of follow up. A year later, Romano et al. (120) reported the same good results after 68 days of follow up on 20 patients affected by scalp lesion, but, most importantly, also by comorbidities such as aggressive or relapsing tumours. In the meantime, Scalise et al. (121) reported their experience on 111 patients affected by different wounds. In that study, they sorted patients into two categories according to their complications, but reported no differences in complications and the possibility of using IDRT only without a skin graft for elderly patients and people with multimorbidities, thereby confirming the recommendation published in 2019 by Magnoni et al. (122), who recommended the use of IDRT in elderly people with comorbidities, large and complex bone exposure, radiation and recurrent and/or aggressive tumours. In 2020, Gonzalez et al. (123) focused on possible complications and associated IDRT with infections in a systematic review of the literature reporting on 212 infections in 602 patients and 1254 treated areas (16.9%). They consequently suggested that the application of IDRT was not suitable for sites at risk of infection. Finally, in the same year, Vana et al. (124) published a prospective study on 24 patients comparing Matriderm® and Integra®. According to this paper, Integra® had a better retraction and skin quality that was still present at 12 months.
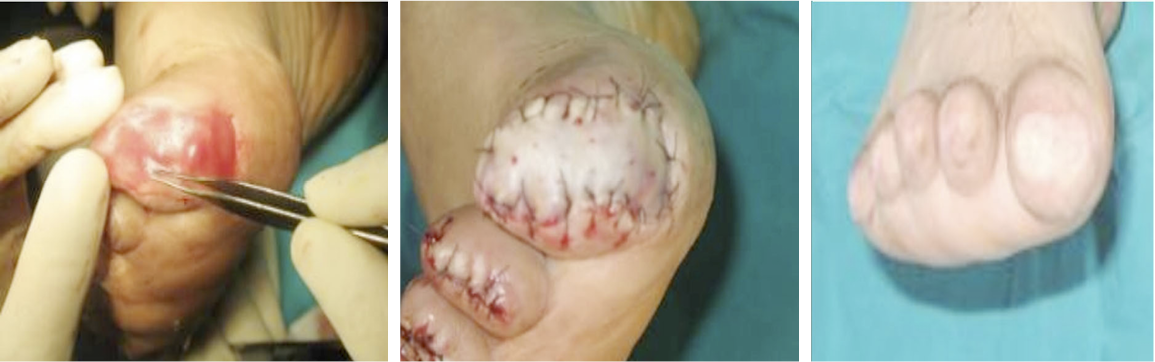
Figure 19: IDRT, case 1. In this case, we treated a diabetic foot ulcer on the big toe with a single-layer, one-step procedure; the dermal substitute was immediately covered by a skin graft.
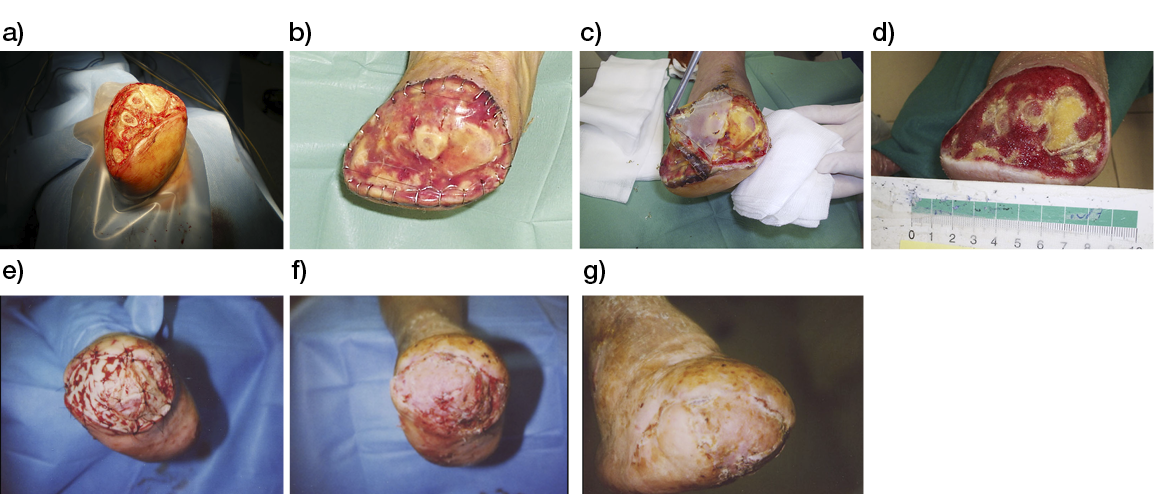
Figure. 20: a)–g) An open trans-metatarsal amputation because of a forefoot gangrene in a diabetic patient (a); managed with the application of Integra Bilayer® (b); after 21 days, the external silicone sheet is removed (c); after 30 days, the metatarsal stumps are covered with granulation tissue (d); and the autologous skin graft is applied (e). The take at 3 weeks from the grafting (f) and after one year (g).
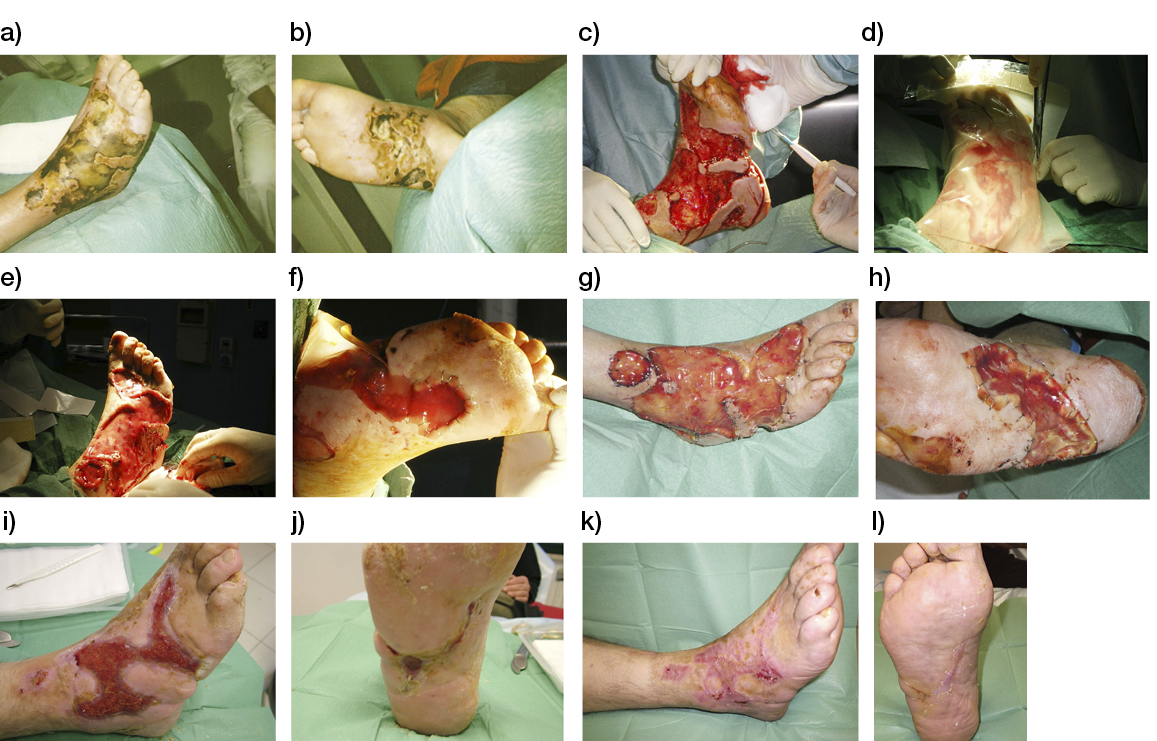
Figure 21: a)–l) A necrotising fasciitis in a diabetic foot (a–b) aggressively debrided (c) and managed with the application of Integra Bilayer© (d–f). After 21 days from the application, at the removal of the external silicone sheet (g–h); after 15 days from the removal of silicone sheet; at the moment of autologous skin grafting (i–j). After one month from grafting (k–l).
Recently, Lantis et al. (37), in a prospective RCT conducted in 21 centres in the U.S. on foetal bovine acellular dermal matrix (FBADM), demonstrated 45.6% complete wound closure at 12 weeks in the FBADM-treated group, versus 27.9% in the group treated with SoC (p=0.008). Despite the trial being terminated early due to the Covid-19 pandemic, the figures are based on a modified intention-to-treat analysis and refer to 103 patients in the FBADM group and 104 in the SoC group. FBADM (PriMatrix™ – Integra Life Sciences, Princeton US), is an acellular dermal tissue matrix derived from foetal bovine dermis and is rich in type I and III collagen that is processed in a way so that it maintains its native three-dimensional structure, without potentially immunogenic contaminants such as lipids or non-collagen proteins.
Matriderm®: This is an extracellular matrix scaffold made out of purified, freeze-dried bovine collagen mixed with 3% elastin hydrolysate; it has a degradation time equal to 6 weeks. It is usually applied in a one-step procedure, as this dermal scaffold allows immediate coverage with an autologous thin split-skin graft. This one-step procedure showed a slower take of the graft, due to the interpositioning of the unvascularised scaffold between the wound bed and the split-skin graft. Nevertheless, the outcome in terms of scar quality was shown to be superior to split-skin graft treatment, even after a 12-year follow-up (125). It promotes neo-angiogenesis and the building of a new, stable and very elastic tissue. Even if it presents many advantages, unfortunately only a few studies have been published about it in recent years. In 2017, Watfa et al. (126) reported a retrospective controlled study on 37 patients (29 cases and 8 controls) in which the authors applied Matriderm® and a skin graft on a free flap radial forearm donor site. In this study, Matriderm® was used to preserve sensory function and decrease morbidity of the donor site. In 2020, Maitz et al. (127) described a comparison between crosslinked vs non-crosslinked Matriderm®. The authors demonstrated that the crosslinked version had a better tensile strength, increased fibroblast proliferation and migration in in-vitro experiments. Finally, in an in-vivo mouse model, the crosslinked Matriderm®, as opposed to non-crosslinked Matriderm®, remained in place after 14 days.
Figure 22: Matriderm® case 1: In this case, we treated a vascular ulcer with Matriderm® and immediate coverage with a skin graft.
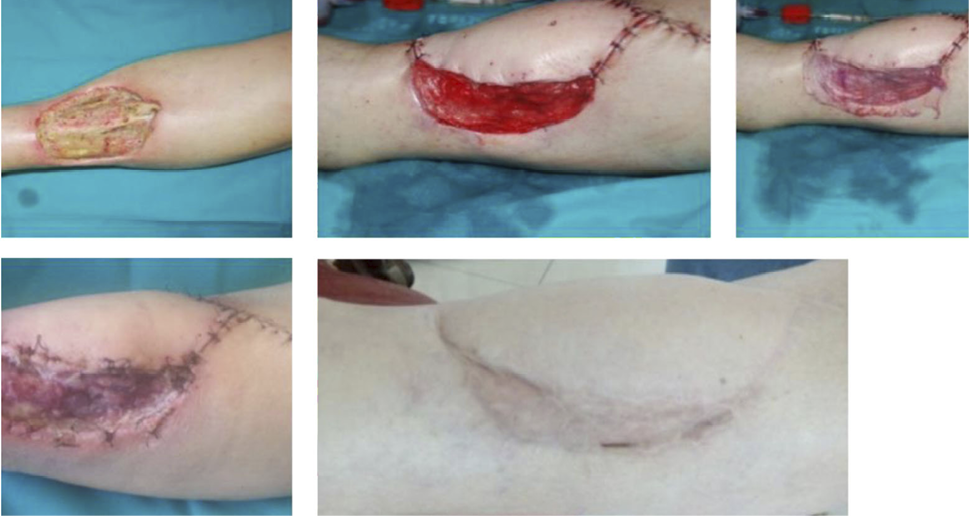
Figure 23: Matriderm® case 2: In this case, we treated a postoncological wound reconstructed with an anterolateral free flap with Matriderm® and an immediate skin graft.
Pelnac®: A porcine origin matrix, this dermal substitute is available both with and without a silicone layer and in fenestrated type. These features make it suitable both for one- and two-step procedures (Figure 30, 31). It is mostly used in Asia, but has recently also become available in Europe. It promotes fibroblast infiltration and neoangiogenesis, and it can be used especially for very thick defects, wounds at high risk of infection and wounds that are expected to shrink (e.g., fingertips). In 2019, Lv et al. (128) reported their experience in a prospective case series of 13 patients affected by bone and tendon exposure in the forearm and hand injuries. In these cases, Pelnac® was applied with a skin graft, and the authors reported a 100% Pelnac® intake and an 84.6% of skin graft take. In 2020, Lisa et al. (129) reported a retrospective study on 12 patients (9 tumour resections and 3 chronic ulcers) that demonstrated a complete intake after 21.3 days in 11 of 12 patients (91.6%). Meanwhile, De Francesco et al. (130) proposed a comparison between Pelnac® and Integra® in 71 patients in a randomised prospective observational paired study. Pelnac® demonstrated a better epidermal proliferation at 2 weeks and a better contracture at 2 and 4 weeks. Integra was suitable for wounds deeper than 1.5 cm. Finally, in the same year, Lv et al. (131) demonstrated a 100% graft taking after 16.5 months in 16 patients affected by wounds with underlying bone and/or tendon exposure.
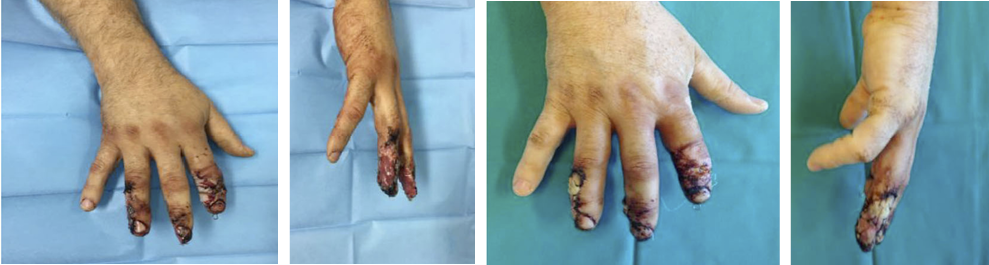
Figure 24: Pelnac® case 1. In this first case, you can see its application as a two-step procedure in post-traumatic wounds of fingertips. After 2 weeks, the external layer was removed to perform a skin graft.
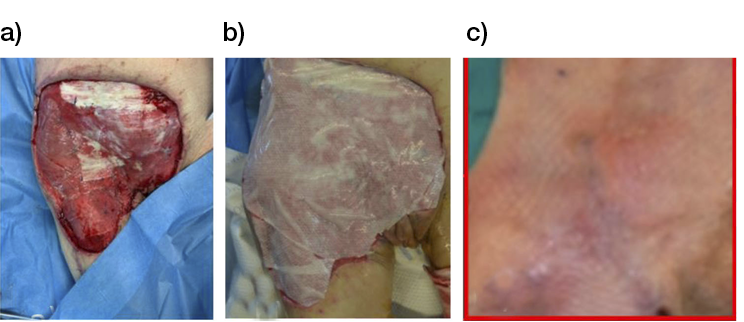
Figure 25: Pelnac® case 2. In this second case, you can see a reconstruction with two-step porcine origin matrix and, after 2 weeks, the coverage with meshed skin graft in a post-oncological abdominal wound.
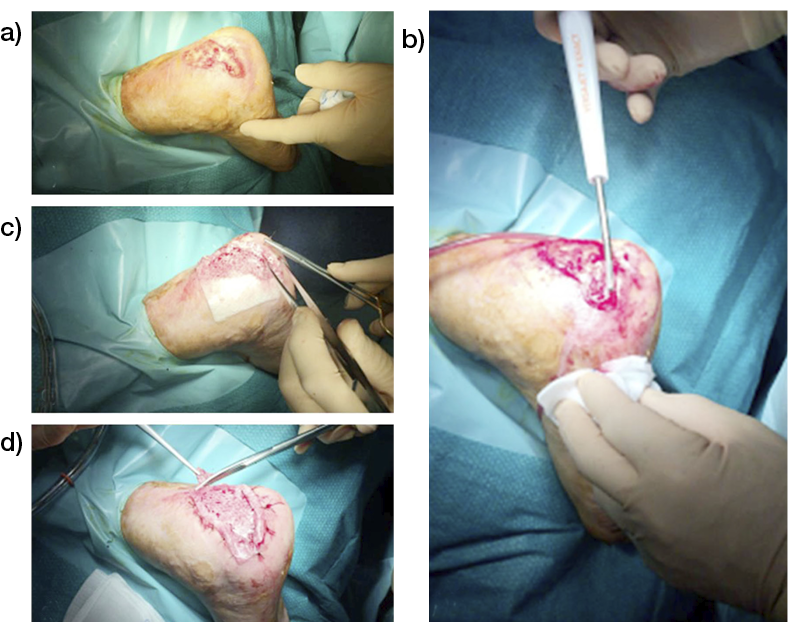
Figure 26: Use of Pelnac® in a diabetic foot ulceration in the heel a). The lesion is debrided via hydrosurgery b) and then an opprefenestrated sheet of Pelnac® is positioned and sutured c) and then tailored according to the actual shape of the lesion d).
Nevelia®: This dermal substitute was recently introduced to the market. It features a bi-layered, three-dimensional porous matrix of stabilised bovine origin type I collagen and a degradation time equal to 14+/- 7 days. It can be considered a bio-inductor. It seems to optimise colonisation, as fibroblasts recognise collagen fibres. It can be used in a two-step procedure for the treatment of deep grade II or grade III burns (Figure 27), skin ulcers, post-oncologic or post-traumatic wounds. In 2019, Yiğitbaş et al. (132) reported their experience of 20 burned patients affected by BSA 50%. They noted a short hospitalisation time for 10 patients treated with Nevelia® and skin graft. In the same year, De Angelis et al. (133) reported the histological features of 35 chronic vascular ulcers treated with Nevelia®, after 28 days. In this analysis, the regenerated skin presented a reacted epidermal hyperplasia and dermal granulation tissue after 3 weeks. In the same period, a new tissue architecture was present and it was analogous to normal skin. One year later, in 2020, Montanaro et al. (134) presented a randomised case control study on 15 patients affected by chronic diabetic ulcers (10 cases and 5 controls) in which they demonstrated Nevelia’s® ability to activate macrophage and M2 cells to a reparative polarisation. In the same year, Gurbuz et al. (135) reported their experience with 24 wounds in 12 patients affected by major burns and six months of follow-up. Graft-taking was achieved in 92% of patients, while 87.5% of patients reported a good/excellent aesthetic and functional results. Finally, Uccioli et al. (136) described a cross-sectional study of 41 diabetic patients after 1 year of follow up. In this study, 21 patients (51%) were healed, but 10 (24%) did not heal after 1 year. Of these 10 patients, all had a size reduction of >50%; 7 patients (17%) were amputees and 3 patients (7.3%) died.
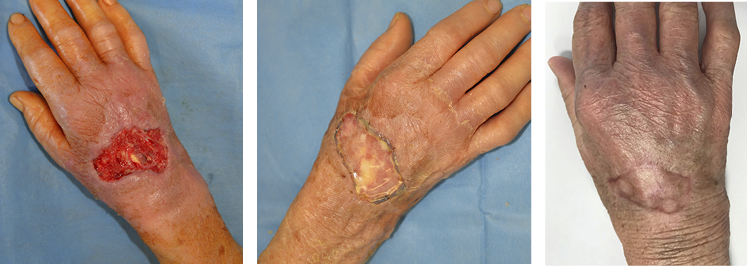
Figure 27: Nevelia®, in this case, you can see its application after II deep degree burn of the hand in an 82-year-old patient. Note the quality of scarring after 3 months from final closure with a skin graft.
In 2021, Cottone et al. (137) published a retrospective comparison of Integra®, Nevelia® and Pelnac® and observed different ‘outcomes’ depending on the dermal substitute. In particular, Integra® had the highest rate both of skin graft take and viability, and Nevelia® had a low secondary healing induction rate, but its graft take was superior compared to Pelnac®. Pelnac® showed the fastest healing times in acute wounds (137).
4.5 Granulation tissue bioinductors
Epifix®: This homologous amniotic membrane is derived from human placenta, after a dehydration and sterilisation process. Available for the treatment of chronic ulcers, the amniotic membrane is also widely used to minimise the possibility of developing important scar adhesions in cases of second- and third-degree skin burns (Figure 28, 29), ocular burns, general and gynaecological surgery andepidermolysis bullosa. It has been used since the beginning of the 1900s, and in recent years many studies have been published about its efficacy. In 2018, Garoufalis et al. (138) published a retrospective analysis on 117 patients affected by different wounds. They noted that 91.1% of the treated patients reported complete healing, with a mean +/- SD number of weekly applications/wounds of 5.1 +/- 4.2. One year later, Tettlebach et al. (139) reported their experience in a prospective randomised controlled multi-centre study of 98 patients affected withs DFUs (47 cases and 51 controls). After 16 weeks, 95% the patients treated with an amniotic membrane were healed in comparison to 86% of the control group.
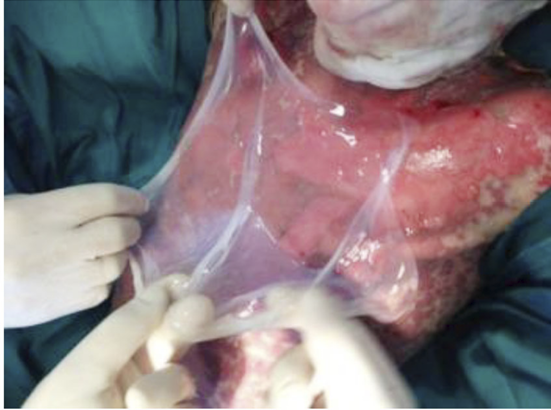
Figure 28: Amniotic membrane. 10-year-old child affected by epidermolysis bullosa. The amniotic membrane has been positioned on the thorax, to both stimulate wound healing and prevent keloid development.
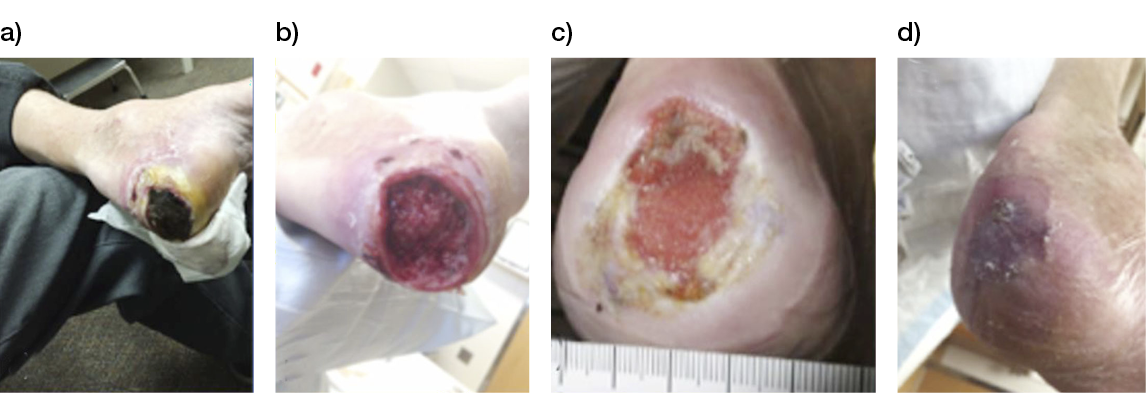
Figure 29: Patient with DM and neuropathy: a) Patient presented with osteomyelitis and underlying purulent tissue; b) Post- debridement + NPWT initiated; c) Week 8: 1st Epifix® application after insurance coverage was received; d) Week 15: Closed, s/p 4 total Epifix® applications.
A Markov analysis performed by Guest et al. (140) estimated that the inception of Epifix™ on top of SoC in the management of DFU, would translate into a 90% increase in the probability of healing and a 6% increase in the probability of avoiding amputation, with an 8% improvement of QALYs.
Kerecis®: This substitute is composed of an acellular fish skin and has a micro-structural composition extremely like human skin, which is rich in omega-3 poly-unsaturated fatty acid (Figure 30, 31). Thanks to this last feature, described in 2020 by Kotronoulas et al. (141), Kerecis® seems to be suitable for obtain a very ‘natural’ looking skin and reducing pain. The analgesic effect, plus a 100% reepithelisation rate, was reported in 2019 by Alam et al. (142) on 10 donor split-thickness sites on burned patients. In the same year, Michael et al. (143) described a retrospective case series of 58 diabetic ulcers in which they obtained both a surface reduction of 87.57% and complete healing in 60.34% of cases. In the same year, Woodrow et al. (144) presented a prospective study on 8 postoperative diabetic feet. This study included 6 weeks of follow up and a weekly dressing change. They noted wound area reductions primarily in recent lesions (<3 months). Kirsner et al. (145), in an RCT, showed that fish skin was superior to porcine dermis in 162 full-thickness punch biopsy wounds in 81 healthy volunteers; fish skin demonstrated accelerated would healing with significantly (p<0.05) more wounds fully healed on Days 14, 21 and 25, compared to a porcine graft. Meanwhile, Badois et al. (146) demonstrated that fish skin-treated donor wounds healed twice as fast as those treated with SoC dressing (32 and 68 days, respectively). Patients also reported a significant decrease in pain (p=0.0034), and local infection rates dropped from 60% to 0% (p=0.0039). Finally, in 2019, Kirsner et al. (145) showed that fish skin was superior to dehydrated human amnion/chorion membrane (dHACM) in the healing rates of 170 full-thickness punch biopsy wounds in another RCT. Wounds in the fish skin-treated group healed significantly faster (HR 2.37, p=0.001) compared to dHACM after 28 days.
Figure 30: Scanning electron microscope images of Kerecis® fish-skin graft (left) and human skin (right), showing similarities in their 3D structures.
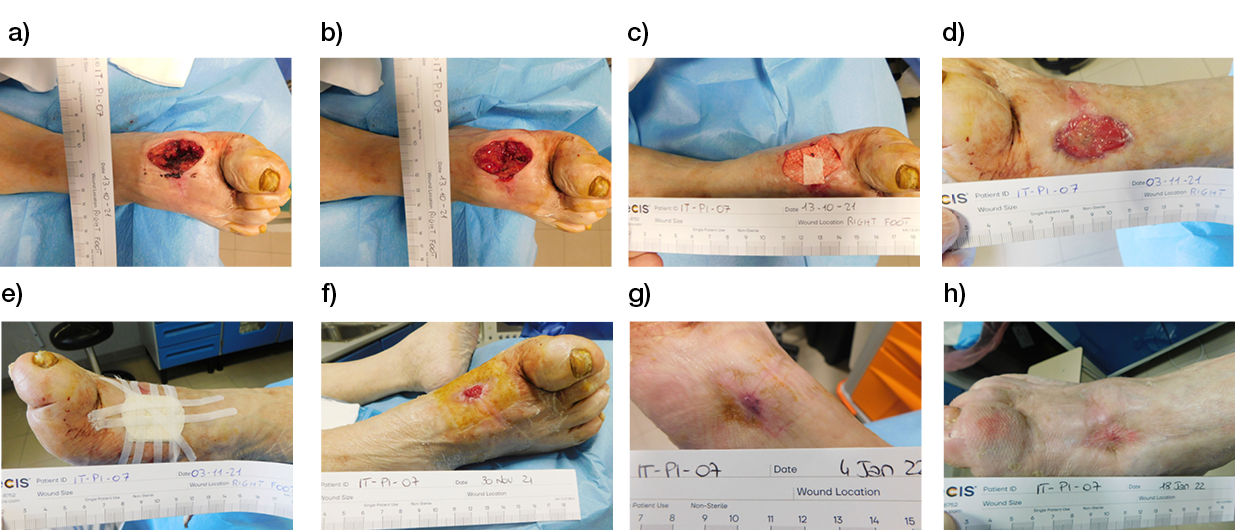
Figure 31: Fish-skin graft case study: a) Post-surgical lesion of the dorsum of a right foot after the drainage of a deep abscess; b) The lesion after surgical debridement; c) Application of Kerecis®. The dressing was applied weekly for six consecutive weeks; d) The lesion after three weeks of treatment with Kerecis®. Granulation tissue has grown and filled the tissue gap created by the drainage and debridement procedures; e) New application of Kerecis®; f) The lesion after five weeks of treatment. The area of the lesion is reduced by 75%, and the bottom is fully covered by healthy granulation tissue; g) The lesion is completely and durably healed after 9 weeks of treatment; h) The lesion at 10 weeks. Despite the application of Kerecis® being interrupted at Week 8, the evolution of the lesion’s healing further progressed, improving the quality of the tissue replacement.
Oasis®: This product is derived from pig small intestine submucosa. It is 0.10–0.15 mm thick and features a collagen-based extracellular matrix plus other components, such as proteoglycans, TGF beta, basic fibroblast growth factors, glycosaminoglycans and so on. (Figure 32). First reported in 1989 (147) in an animal model, this matrix has been used for the treatment of chronic ulcers, including pressure sores, vascular peripheral ulcers and diabetic ulcers, and for post traumatic lesions.
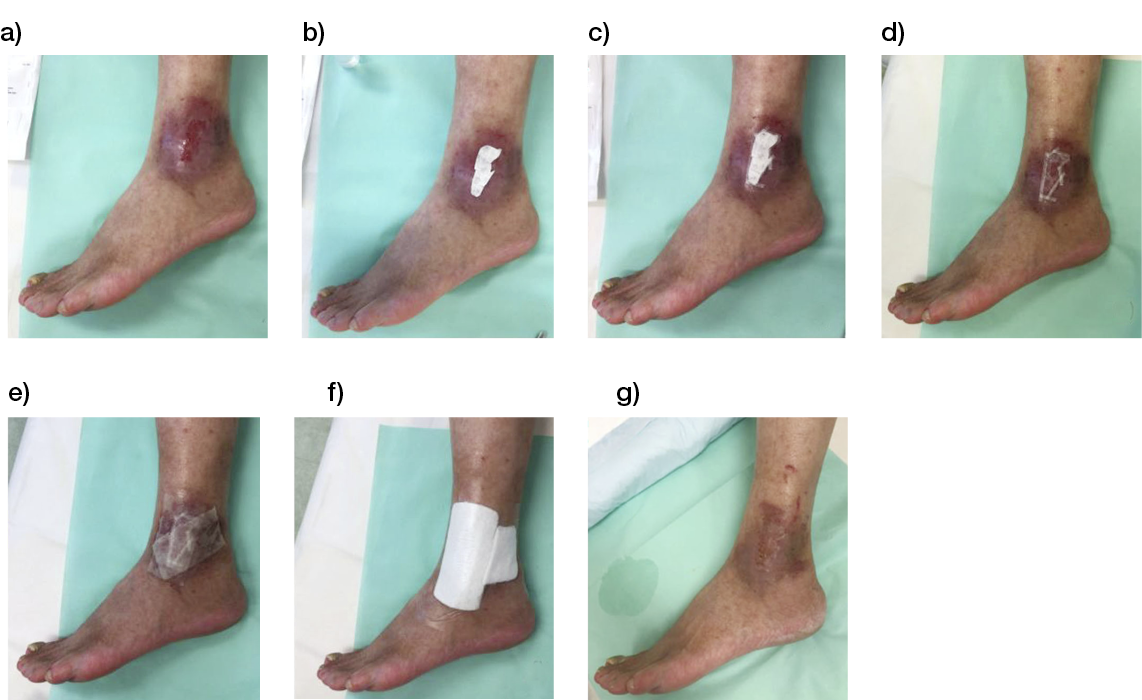
Figure 32: A case of venous leg ulceration a) managed with Oasis®. After initial debridement, Oasis® was trimmed according to the shape of the lesion b) and secured with steri-strips c). A secondary dressing of non-adherent gauze was interposed d), re-hydrated with saline e) and covered with a protective layer of polyurethane foam f). The last picture shows the lesion after one week g).
In 2019, Brown-Etris et al. (148) described their experiences with III and IV pressure sores in a randomised clinical trial in which they compared 67 patients treated with Oasis® to 63 patients treated with the SoC . They reported complete healing in 40% of patients treated with the matrix, versus 29% of patients treated with the SoC. 55 % of the ‘Oasis® patients’ presented a wound area reduction of 90%, while only 38% of the ‘standard patients’ presented a 90% reduction (p=0.037).
4.6 Cellularised dermal substitutes and human dermal fibroblasts therapy
Today, dermal substitutes are composed of either autologous or allogeneic dermal fibroblasts. Biomaterials, as benzyl-esterified derivatives of hyaluronic acid (Hyaff-11), polyglycolic acid or polyglactin, are used as scaffold for the cells. Dermagraft® is a very interesting and successful product, especially for the diabetic foot. It has an allogeneic cell culture using neonatal dermal fibroblast grown on a biodegradable scaffold and has the capacity to secrete several growth factors to stimulate neo-angiogenesis and re-epithelialisation. Finally, its efficacy is maintained even after cryopreservation and thawing. In 2019, Tchanque-Fossuo et al. (43) reported an interim analysis result of a RCT in which Dermagraft® was compared with Oasis® ,a naturally derived scaffold of ECM composed of porcine small intestinal submucosa. They treated 56 diabetic patients and analysed the differences between the two substitutes after 28 weeks. They found no differences between the two.
4.7 Full-skin substitutes
Human cadaver skin is surely the first and most used skin substitute reported. Used as a biological dressing, this homologous tissue is notable for its early pseudo-grafting and a reject phase after 2–3 weeks (Figure 33). During the rejection phase, it stimulates a physiological debridement through macrophages with a consequently physiological wound bed preparation. In our experience, this treatment is now mostly applied in burned patients, to both debride the wounds and temporarily cover the burned areas, and in ulcers that cannot be treated with NPWT.
Figure 33: Cadaver skin applied after a post-traumatic scalp lesion.
Alloderm® is based on ‘traditional cadaver skin’, one of the first developed acellular allogeneic skin substitutes; thus, real human tissue is harvested for skin grafts from cadaver donors. Alloderm® is washed with hypertonic saline to remove cell remnants and then deepithelialised. The remaining dermal layer is treated with inactivate viruses and then freeze-dried to be used on-demand. This provides a nonantigenic dermal scaffold with basement membrane proteins. After rehydration of the Alloderm®, coverage with an ultrathin split skin is sufficient as a full coverage treatment option. Recently, Alloderm® has been used more and more often for breast reconstruction surgery (149), whereas its use on wounds and burns remains limited. Glyaderm® is a similar product based on allogeneic dermis preserved in glycerol, instead of being freeze-dried. Initial studies on burned patients have been performed with favourable results (40), demonstrating a better elasticity and scar quality.
Full biological skin substitutes comprising both dermis and epidermis that contain both allogeneic fibroblasts and keratinocytes are present on the market. Apligraf® was the first to become commercially available. It consists of cultured allogenic human foreskin-derived neonatal fibroblasts in a bovine type I collagen matrix over which allogenic human foreskin-derived neonatal epidermal keratinocytes are then cultured and stratified. These features can promote the rejection of the keratinocyte, requiring an autologous split-skin coverage for definite would closure. Therefore, Apligraf® can be used in the treatment of chronic wounds. In 2017, Stone et al. (150) reported their experience in a RCT on 24 patients affected by VLUs (15 cases and 9 controls). They observed an acute inflammatory response in patients treated with Apligraf® and a restoring of wound healing. Other studies describing a full-skin substitute have focussed on the TissueTech Autograft System™ (151) and the combination of a dermal skin substitute (Hyalograft 3D®) with Laserskin®, a cultured epithelial autograft (CEA) (152) (Figure 34). One major advantage of autologous composite skin substitutes is the one-stage procedure. Except for the initial skin biopsy from which the autologous cells are harvested and cultured, no secondary procedures, such as split-skin transplantations, are required. These newly developed skin constructs are commercially available at present. The limitations to the further expansion of autologous composite skin substitutes are their high production costs, long preparation times and the need for a well-organised production-to-clinic transfer.
Figure 34: Cultured epithelial autograft (CEA) a) the harvesting of skin samples b) The preparation and culturing of skin cellular components in the laboratory.
DenovoSkinTM: DenovoSkinTM perhaps represents the most advanced double layer autologous cell culture among the dermo-epidermal skin substitutes, as it contains both dermal and epidermal skin layers. In 2019, Meuli et al. (153) used it in a phase I clinical trial at the University Children’s Hospital Zurich for the treatment of 10 patients affected by deep partial- or full-thickness skin defects. They performed skin grafts of 49 cm that were bioengineered with autologous keratinocytes and fibroblasts incorporated in a collagen hydrogel. At 21 days post-operation, they noted a median graft take of 78%. Interestingly, at 3 months post-operation, a histological examination demonstrated a well-stratified epidermis and a dermal compartment comparable to native skin.
4.8 Regulatory/Safety Issues
Based on the most recent regulations, cell-based therapies have been classified as advanced therapy medicinal products (ATMPs). In Europe, these therapies are controlled by the European Medicines Agency (EMA), a monitoring institute of the EU, which is dedicated to the scientific evaluation and supervision of market access of medicinal products. In the US, the Office of Cellular, Tissue, and Gene Therapies, a branch of the Food and Drug Administration (FDA), evaluates and supervises market access of ATMP products. European Regulation (EC) No. 1394/2007 provides the overall framework for the production and use of ATMPs within the EU. According to this regulation, an ATMP is a ‘medicine for human use that is based on genes, cells or tissue engineering’. With the aim of regenerating, repairing or replacing human tissue, some products may contain or consist of engineered cells or tissues, and they can contain viable or nonviable cells or tissues of human or animal origin. This means that most of the skin substitutes with living cells (both autologous and allogeneic), adipose tissue and matrices containing human and/or xenogeneic material must be considered according to these regulations. Scaffolds made of isolated and/or purified animal- or human-derived proteins, however, are classified as medical devices. One of the main features of these regulations is that the production of ATMPs for human use must take place under Good Manufacturing Practice conditions, requiring both high standards for production facilities and an important administrative burden for importing and exporting ATMPs across international borders.
These strict regulatory and production require-ments imply intensive collaboration among centres to develop new ATMPs and promote their commercial exploitation.
4.9 Bioprinting for skin substitutes
To fabricate living tissue, cells are mixed with biomaterial, such as a skin decellularised extracellular matrix (dECM), which is called ‘bio-ink’. The biomaterial is derived from a chemical and enzymatic treatment of the dermis. After preserving the structural and functional ECM’s protein, the biomaterial is repopulated by autologous fibroblasts, to prevent rejection.
The different components are mixed into a 3D bioprinter to create the 3D construct. In this new technique, Won et al. observed 90% cell viability and proliferation, as confirmed by the gene expression pattern (154).
Despite its extremely recent introduction to the market, this technology has gained the attention and interest of clinicians and scientists in many areas of tissue repair because it is extremely promising. Its potential has led to the testing of almost all types of chronic wounds as targets for this therapeutic approach, and many clinical studies are presently ongoing.
4.10 Conclusions
Thanks to the ability to improve skin quality over a standard split-skin graft treatment, acellular skin substitutes are considered very important today in the treatment of acute and chronic wounds, and for scar revisions. Autologous cellular skin substitutes and tissue repair and regeneration using autologous cells can promote better healing, compared to traditional methods. Drawbacks to the production and application of tissue-engineered skin constructs are, however, still present, and further studies are needed to bring us closer to techniques that have been adapted to the population suffering from chronic wounds.
Table 8: Dermal substitutes
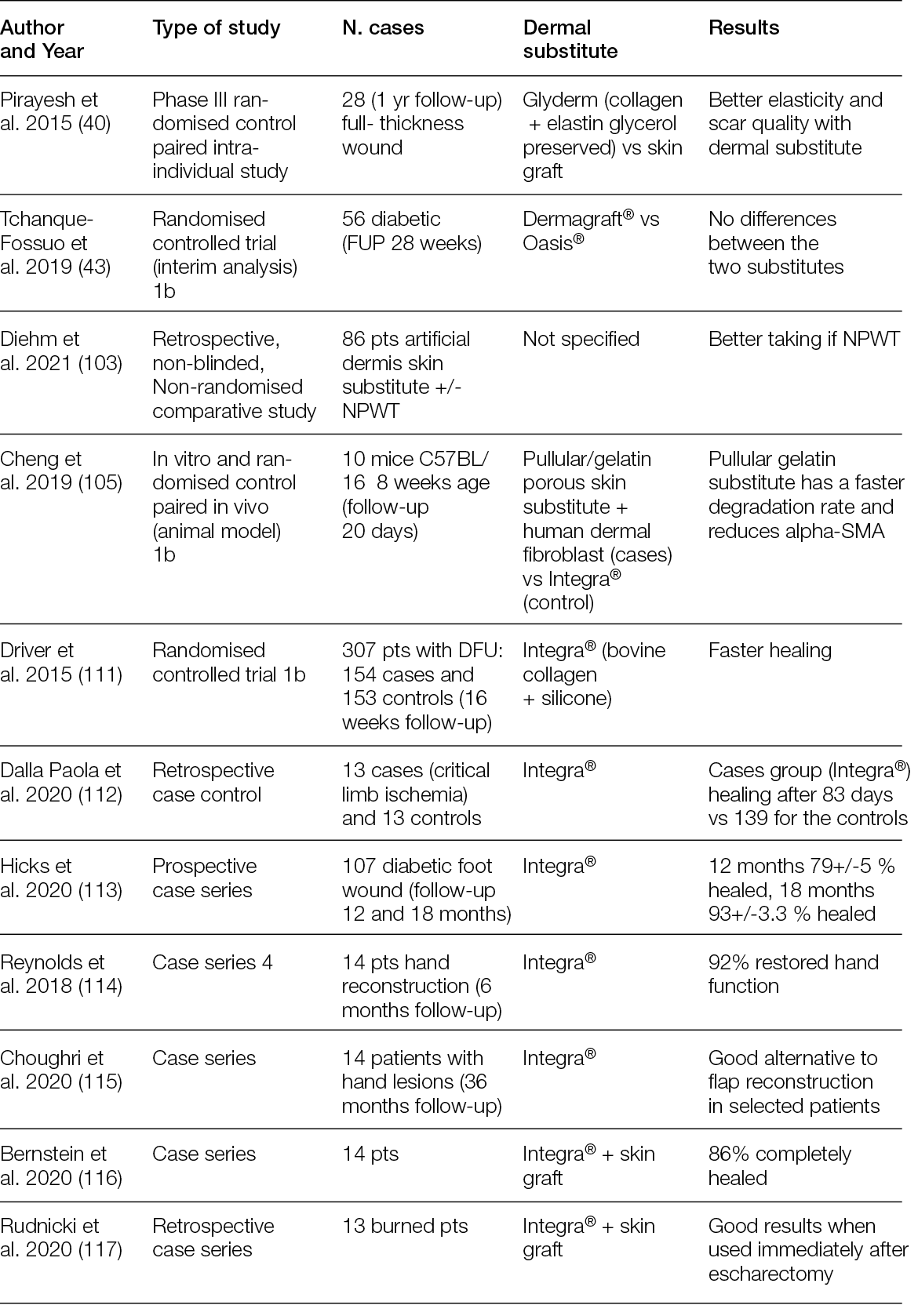
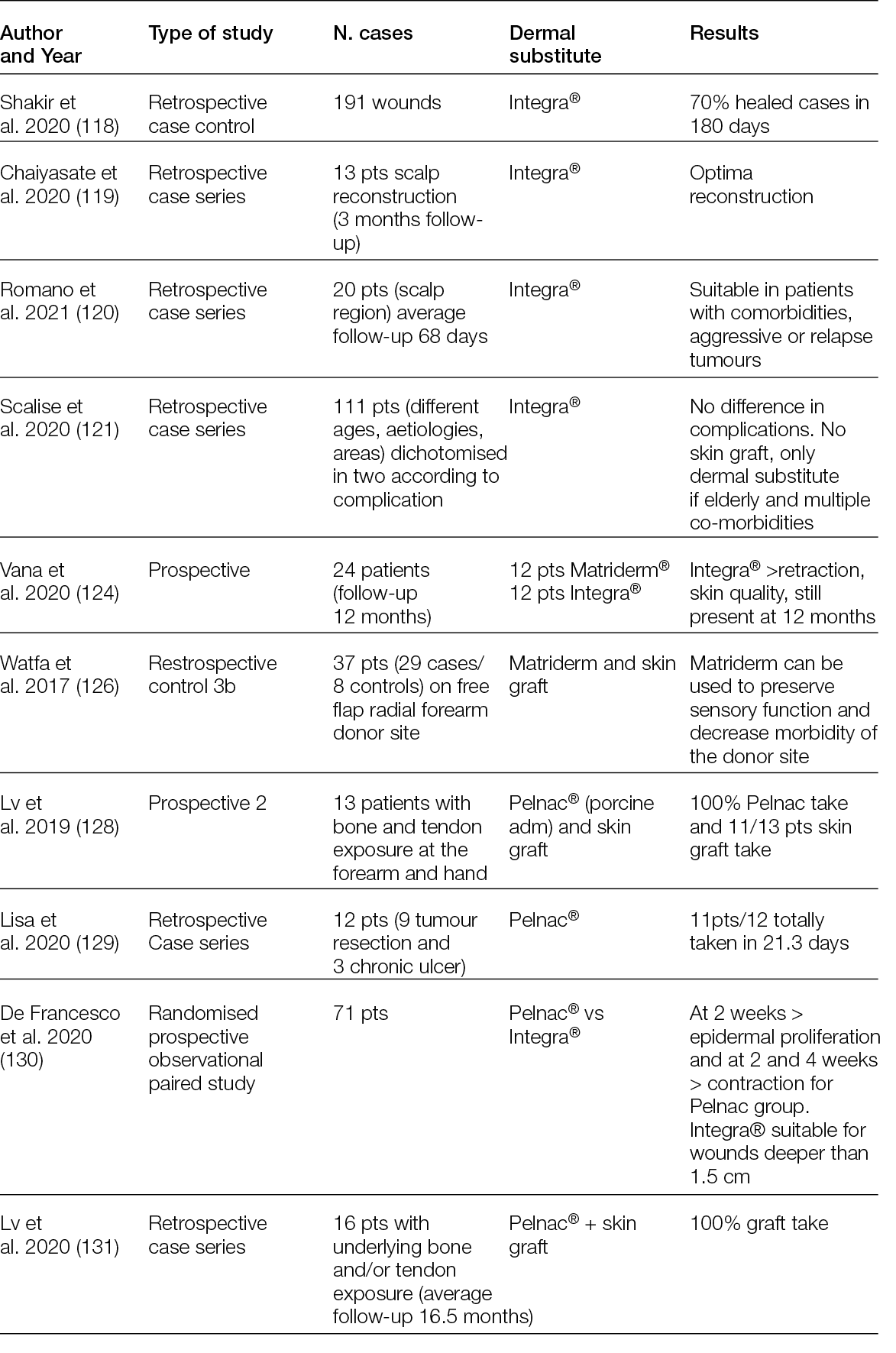
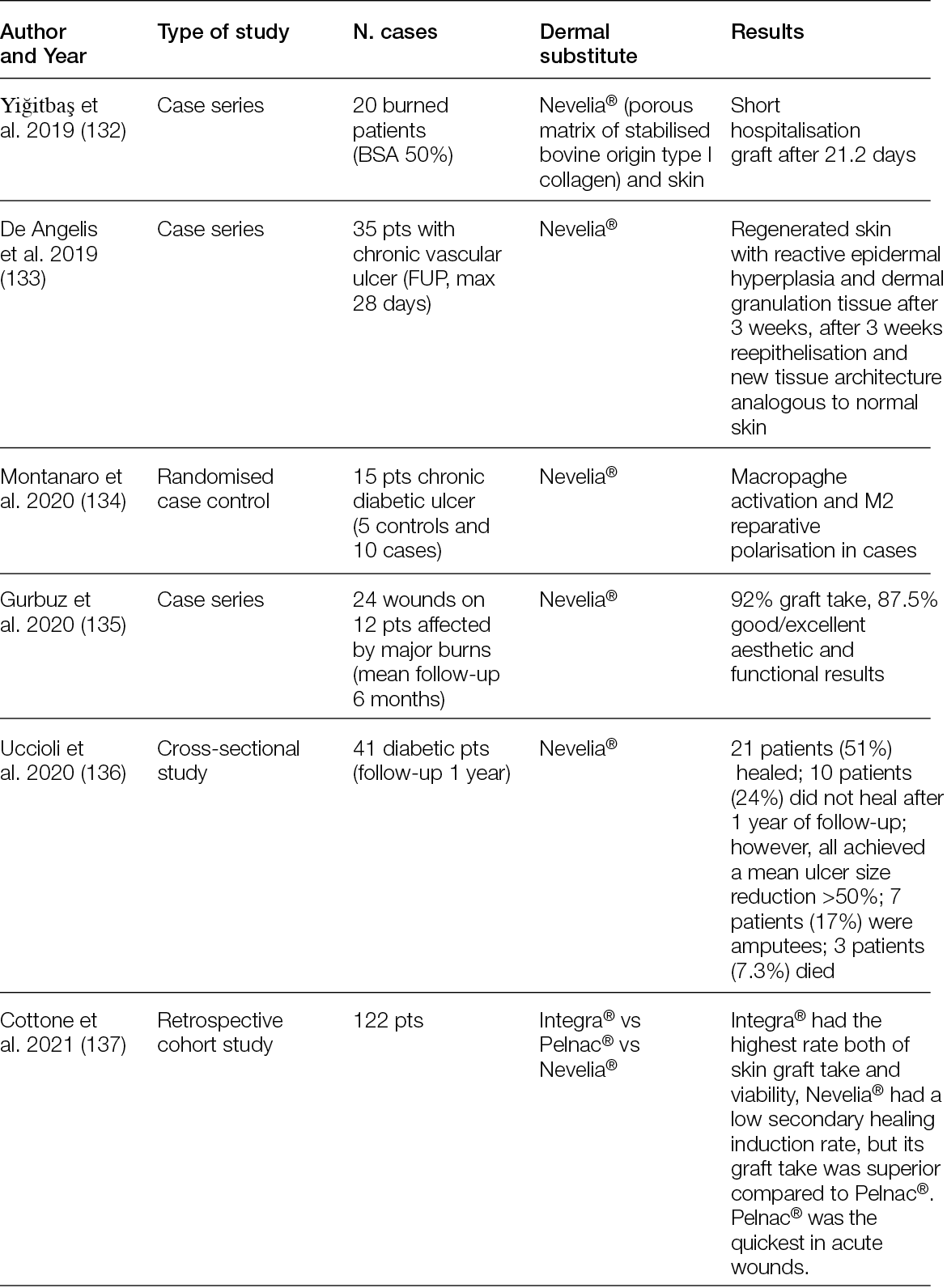
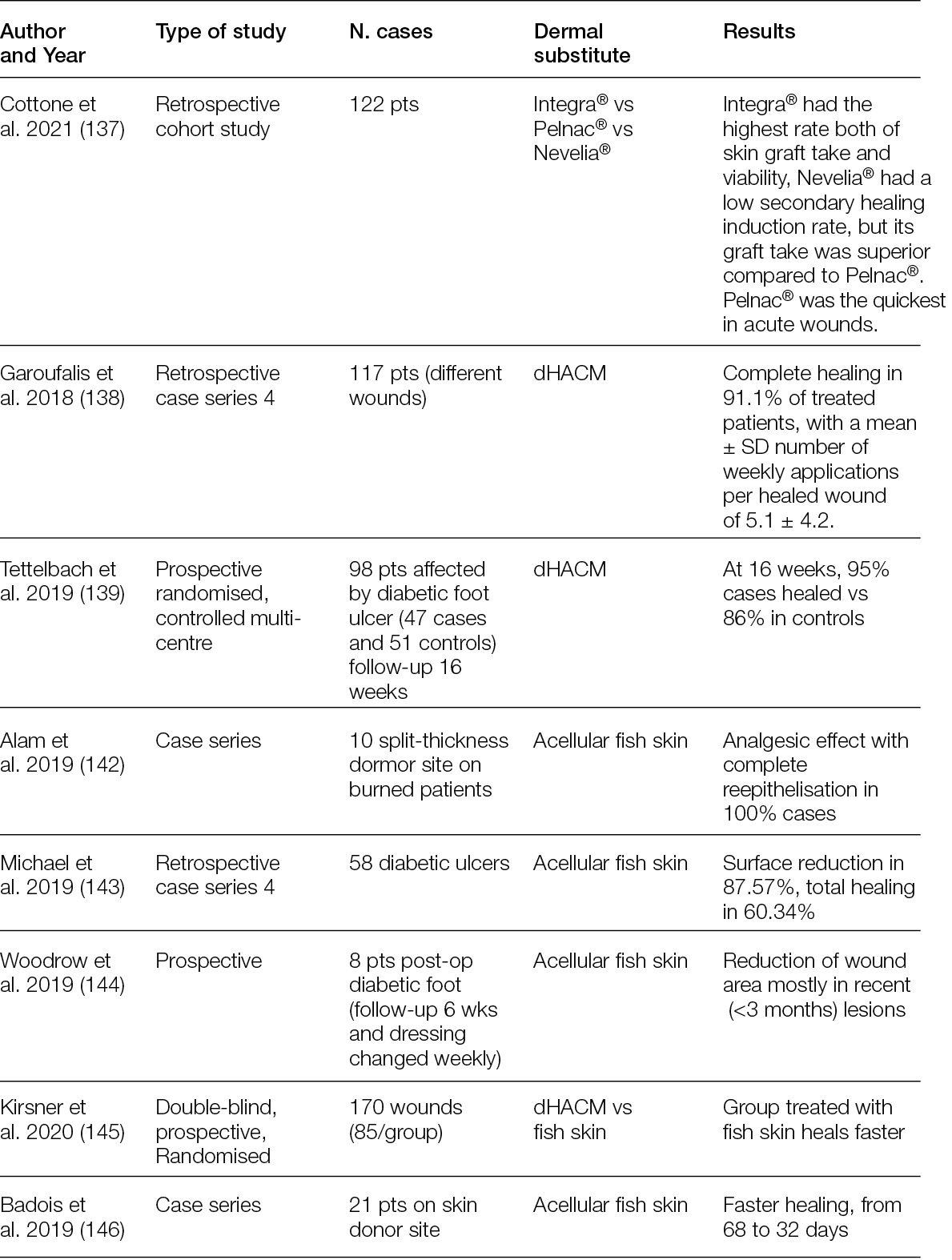
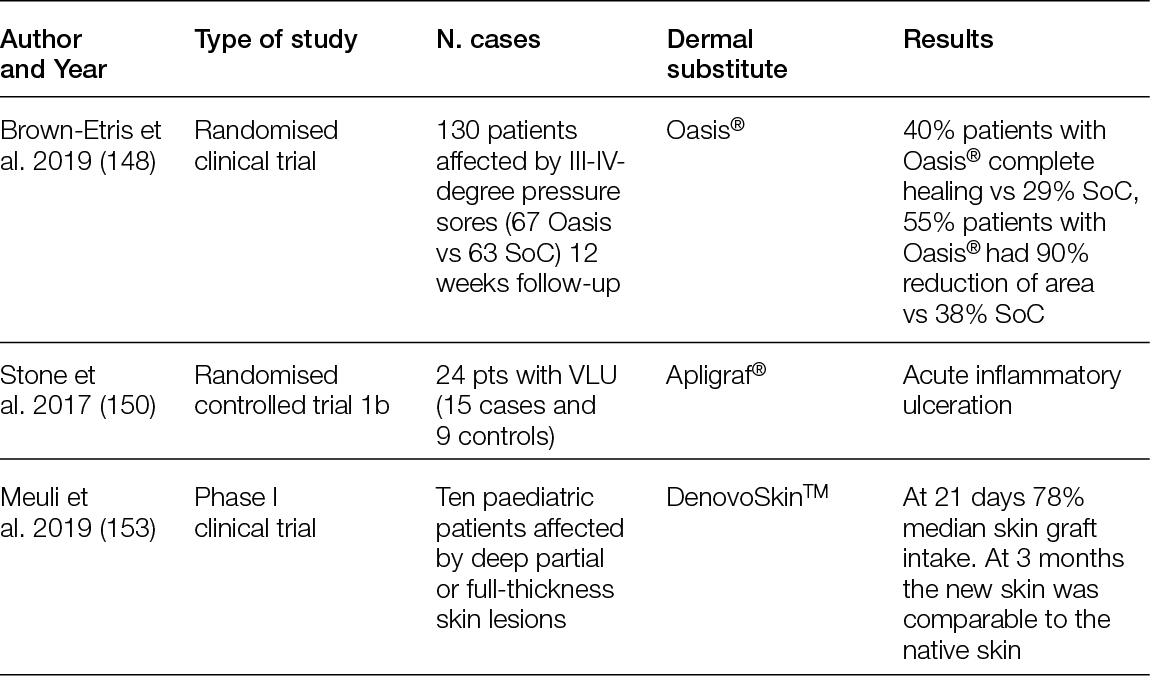
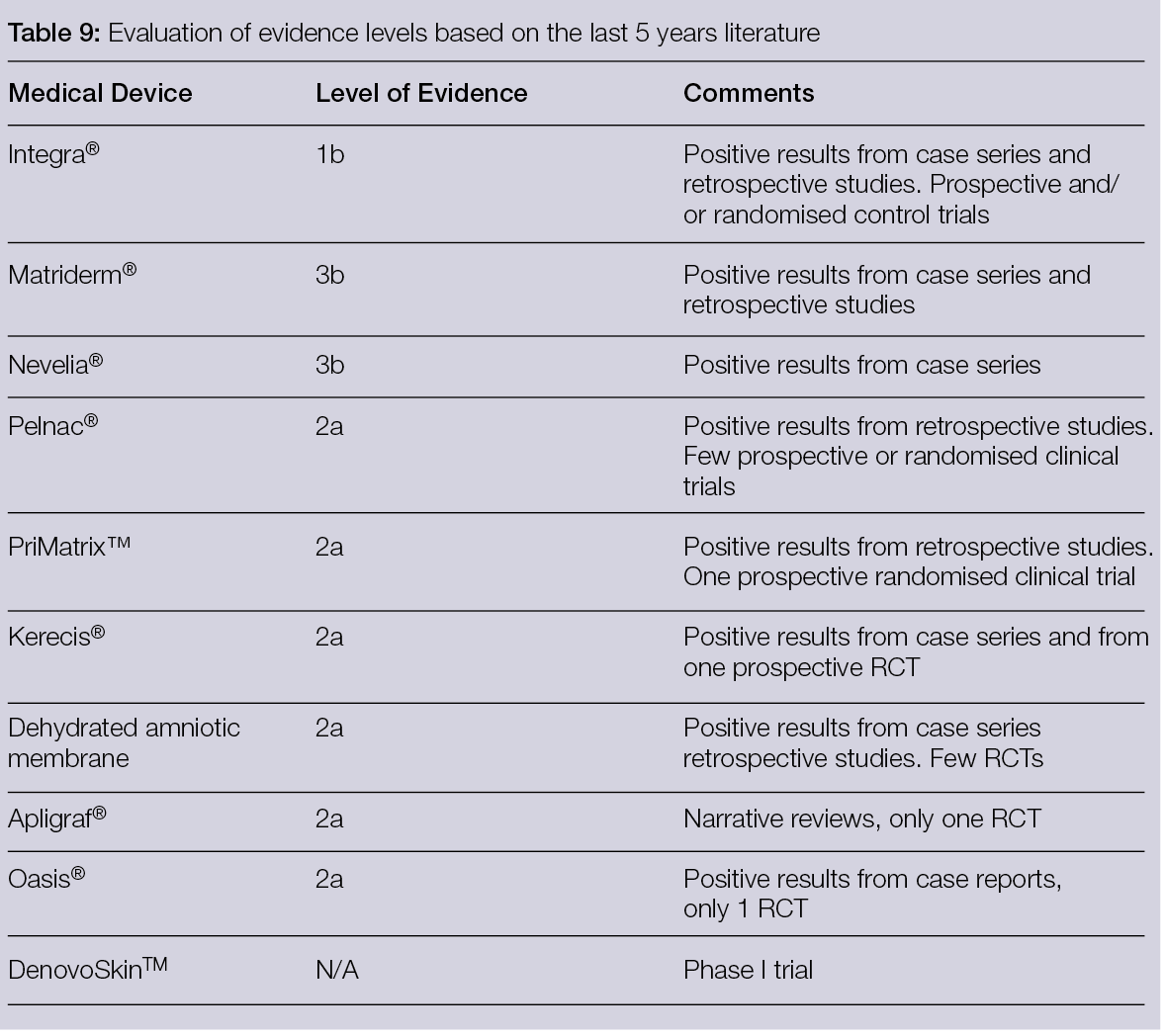
5. Surgical off loading of the diabetic foot
5.1 Introduction
The diabetic foot, with its clinical manifestations (biomechanical) that may be associated with chronic critical ischemia, constitutes a challenge for healthcare systems around the world.
Modern diagnostic and therapeutic achievements have made it possible to increase limb salvage rates, reserving major amputation surgery for an ever-smaller number of patients (155-160). Dia-betic foot surgeons should assimilate and consolidate their knowledge with the surgical techniques from orthopaedics and plastic surgery. The emerging specialty, called ortho-plastic surgery, described by Levin, is therefore well-suited to the treatment of the diabetic foot (159).
The coexistence of peripheral vascular disease in this patient population greatly impacts their reconstructive choices and sometimes limits potential reconstructive options. Even in the case of effective revascularisation, it is necessary to understand what the best conservative or ablative case treatment option is. In addition to peripheral arterial disease, neuropathy is another challenge affecting diabetic patients, as it involves a reduction in sensitivity or complete anaesthesia, which can increase the risk of post-operative complications and reduced compliance with off-loading in the post-operative period.
In the last 10 years, a real evolution of new approaches has developed with the application of lower extremity plastic surgery, wound care, fascio-cutaneous rotational flaps and advancements in muscle flaps for the surgical treatment of the diabetic foot requiring resection of osteomyelitis or deformity correction. Knowledge of limb salvage has increased exponentially, and as a result, the number of limbs saved has increased significantly. The holistic approach to complete treatment of the diabetic foot takes into consideration not only the treatment of bone structures and soft tissues, but also the off-loading of the surgical reconstruction site (161, 162).
The main objectives of ortho-plastic surgery applied to the diabetic foot take into consideration the following points:
- Structural bone deformities are linked to peripheral neuropathy, which creates instability and pathological hyper-pressure points, leading to the development of ulceration. Surgical treatment of bone deformities may be indicated if it is not possible to control the risk of ulcer development with a conservative approach.
- Skin lesions related to bone deformities can lead to the infection of deep structures (septic arthritis and/or osteomyelitis). These infections can easily spread from the ulceration and peri ulceration site and lead to phlegmons or abscesses and compartmental syndromes.
- The bone infection foci (osteomyelitis): Usually following an identification of the extent of the osteomyelitis, surgical planning provides for the simultaneous or staged treatment of the deformity, foci of bone infection and coverage with an appropriate amount of soft tissue.
The peri-wound skin quality and the surgical site area are of critical importance and are linked to various factors, such as the arterial and/or venous vascular condition, the state of sensory-motor neuropathy and the possible presence of oedema.
In general, the ortho-plastic approach for diabetic foot treatment involves a stepladder approach (complexity stepladder) beginning with the simplest surgical approaches (tendon releases and simple exostectomy), through more complex fusions and arthrodesis and microsurgical flap surgeries.
5.1.1 Tenotomy
Digital flexor tenotomy is effective as a decompression treatment for dorsal and acral toe ulcerations. The Achilles tendon delivers a strong deforming thrust in the sagittal plane with a significant increase in plantar pressure on the forefoot and midfoot. This pressure carries a high risk of developing ulcers, especially in Charcot’s osteoarthropathy cases. Furthermore, valgus and varus deformities are the cause of a high ulcer risk in the medial and lateral portions of midfoot amputations, respectively. A release of the tendons responsible for the deformities may mitigate this predicament (163).
5.1.2 Exostectomy
Charcot midfoot plantar bone prominence is a common indication for exostectomy. The resection of plantar bony protrusions of a chronic ulcer lesion helps to reduce a source of extreme pressure. After an exostectomy, primary closure of the surgical site should be the surgeon’s key objective. This approach allows for healing time reduction and, secondarily, a reduction in healthcare costs. However, if this is not possible, it can be managed by leaving the wound open to heal by secondary intention, particularly if it is small and there is concern about an ongoing infection. Larger wound coverage techniques include dermal substitutes or split-skin grafts, local flaps (advancement or rotation) and local muscle transposition or rotation flaps.
5.1.3 Bone deformity corrections
In the event of failure or non-applicability of the simplest surgical approaches (i.e., a simple exostectomy), you can move on to more complex procedures, such as arthrodesis/fusion both at the midfoot and hindfoot or ankle levels. The choice of fixation options (internal or external) is linked to the presence or absence, as well as the location, of the bone infection, the quality of the bone, any associated comorbidities and, ultimately, the surgeon’s experience.
In cases of major soft tissue defects, adjuvant therapies such as growth factors and NPWT associated with or without antiseptic agents should be considered to accelerate the healing process, followed by lesion coverage with skin grafting techniques. In recent years, it has been proven that a viable neo-dermis can be formed on top of deep tissue, such as bone, by applying a three-dimensional collagen scaffold or a similar bioengineered tissue product (164-167). In this case, it is critical to immobilise the foot and ankle until the reconstruction site is completely healed.
The indication for a simple exostectomy is the area of deformity affecting the midfoot, indicative of Charcot’s osteoarthropathy. Removing areas of bony protrusions at a chronic ulcerative lesion help by reducing a source of pathological pressure. This surgical technique is relatively simple and reasonable for surgeons of different specialties.
Following an exostectomy, the residual ulcerative lesion can be treated in different ways (Table 10).
Table 10: Treatment options - residual ulcerative lesion
5.2 External fixation
One of the most crucial issues that determines surgical outcomes in the diabetic foot is post-operative off-loading. Historically, the most common methods for patients’ weight-bearing mobilisation after healing and reconstructive surgery of the diabetic foot have been fiberglass casts, healing shoes, walkers, wheelchairs and crutches (164, 165). These methods, in the presence of poor patient compliance – a very common condition in diabetic foot patients – can lead to a failure of the reconstructive and limb salvage stages. The most common methods of mobilisation and protection of surgical sites carry a significant risk to the results of the reconstructive techniques (flaps or skin grafts).
There are potential risks of complications when total contact casting (TCC) is applied in neuroischemic diabetic patients. Furthermore, an incorrect TCC construction does not allow for a suitable immobilisation and, potentially, can be a source of further injuries and infection (166-168).
The presence of joint instability, infected ulcerative lesions, oedema and peripheral vascular disease may contraindicate the use of a cast. The surgeon should create a protective environment around the reconstruction site (169, 170).
A complication related to the failure to protect the reconstruction site in the context of the diabetic foot involves not only a longer hospitalisation time and an increase in healthcare costs, but also a high risk of amputation.
External fixation plays a major role in the treatment of exposed bone and osteomyelitis. In these clinical applications, this device makes it possible to stabilise osseous structures that, in the past, would have required a major amputation. The fixator maintains the alignment of the bone structure through a rigid external frame, although large bone excision procedures are often required for osteomyelitis. External fixation can achieve stabilisation objectives that are usually achieved with internal fixation (rods, nails, plates and screws), but this is contraindicated in cases of infection.
5.2.1 Circular frames
It has been shown that the Ilizarov technique (Figure 35) allows healing to be obtained in a shorter time, reducing the risk of pin track infection and allowing early loading. Historically, the application of external fixation techniques has been aimed at the treatment of Charcot neuroarthropathy (CN). CN patients have poor bone quality and localised osteoporosis. Internal means of synthesis in patients with diabetic neuropathy do not allow normal bone turnover and have been shown to have a decrease in pull-out strength (171).
Figure 35: Circular frame.
The indications for the surgical treatment of CN are deformities that cannot be managed with conservative techniques, severe ankle instability, ulcerative lesions localised in areas of deformity and hyper-pressure and infectious progression with the involvement of bone structures.
Treatment of an infection component with or without bone involvement is the first goal of salvage surgery, and the correction of the deformities should only subsequently be considered through procedures of increasing difficulty, such as decompressive exostectomies, osteotomies and arthrodesis (172-180).
Once the correction of the deformities has been achieved, temporary stabilisation with pins can be obtained. Latt et al. showed that the stability and compression possible with external fixation is almost double compared to the use of screws (181). External fixators can facilitate multiplanar correction (distraction, compression, angulation, stabilisation and translation) (182-186). The first description of the use of external fixation techniques as an adjuvant therapy in reconstructive podoplastic surgery dates to 2003 (169).
In 2007, Bibbo et al. emphasised the usefulness of this technique in the off-loading of a plantar medial artery flap to cover a chronic lesion of the hindfoot after debridement of an osteomyelitis of the heel (187). Subsequently, the use of surgical off-loading with an external fixator has been proposed in every surgical treatment of ortho-plastic surgery in cases of plantar wounds. Protection of the surgical site with the external fixator helps prevent non-healing/dehiscence. In addition, circular external fixation allows for a deformity correction approach through simple decompressive ostectomies, osteotomies and fusions (Figure 36) (188-190).
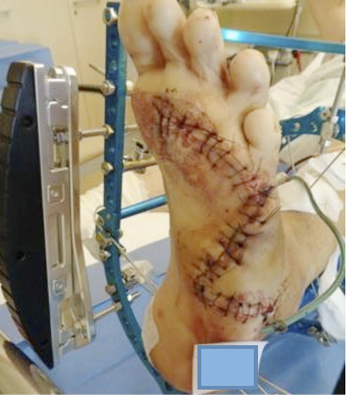
Figure 36: Optimal offloading of a surgical site.
Patients undergoing extensive reconstructive surgery are at risk for wound dehiscence or relapse. Cast immobilisation can lead to difficulties in following the progress of the surgical wound and/or lead to pressure on the wound. Patient compliance and adherence to the indications for post-operative off-loading have been described as the cause of failure in 20% of reconstructive diabetic foot treatments. The option of a circular external fixator, instead of casting, allows for, on one hand, immobilisation of the surgical site and, on the other hand, excellent access to the wound for post-operative observation and dressing changes. The fixator can be removed as soon as the wound has healed. Premature loading on the reconstructed site carries a high risk of re-ulceration. In the case of plantar soft tissue reconstruction, a healing time of at least 6 weeks is recommended.
The objectives of surgical off-loading are to provide protection to the surgical site, reduce the risk of complications (dehiscence) at the surgical site and fixation for achieving a plantigrade and stable foot.
Many works published in the last 10 years have shown a significant success rate in the use of external fixation for the protection of podoplastic surgery treatments in the lower limb (170, 191-196) (Figure 37).
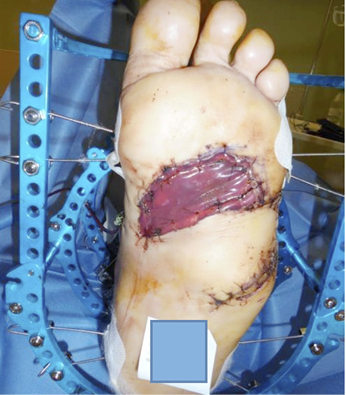
Figure 37: Plantar fascio-cutaneous flap and dermal substitute for donor sitecoverage.
The protective action takes place through both joint immobilisation (usually the ankle), preventing tension in the tissues undergoing reconstruction, and use of multi-planar circular fixators that allow complete off-loading of the plantar surface and the hindfoot.
Clemens et al. reported on the application of a multiplanar circular external fixator in 12 patients who had a clinical failure in healing after an average period of 285 days of conservative treatment with off-loading measures. The overall limb salvage rate was 83%, and mean time to healing was 128 days after frame application. The reconstructive techniques were 1 delayed primary closure, 5 split-thickness skin grafts, 4 local or pedicle flaps and 2 free flaps. During the post-operative follow-up, 6 complications (50%, 4 pin site infections) and 2 rescue treatment failures that required a major amputation (13%) were described (193).
In a prospective study, Dalla Paola et al. reported the results using a circular external fixator (Figure 38) designed for the off-loading treatment of hindfoot lesions complicated by osteomyelitis in a cohort of 18 consecutive diabetic patients admitted to the diabetic foot department. Revascularisation procedures were performed where necessary, as were subtotal calcanectomy, application of dermal substitute and final coverage procedures with autologous split-thickness skin graft; 18 patients were enrolled. The mean follow-up was 212 days. Healing was achieved in all 18 patients (100%). The average time elapsed between surgery and healing was 69 days. No major amputation was performed in the follow-up period (170).
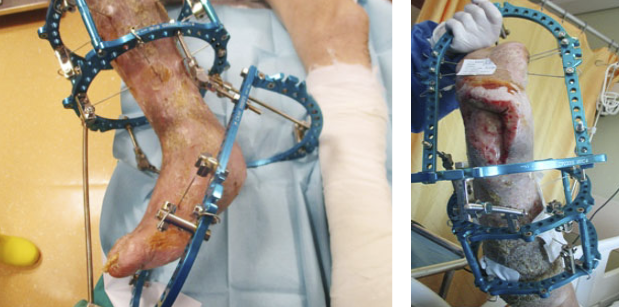
Figure 38: External fixator ideated for hindfoot reconstruction.
5.2.2 Technical considerations for surgical off-loading
The advantages of using external fixation for ortho-plastic surgery of the diabetic foot consist of osteo-articular stabilisation, the ability to monitor the condition of soft tissues and the option to initiate advanced therapies for wound closure.
External fixation can be applied in situations where other traditional methods are contraindicated or insufficient, especially when its role is to protect reconstructed soft tissues. The construct allows for soft tissue management in a strain-protected environment (192, 197).
An off-loading external fixation is a temporary, non-weightbearing device. Several components, such as wires, pins, circular rings and bars, allow a complex construction that protects the foot and ankle to be used. The application of an external fixator is an aggressive surgical procedure that requires knowledge of the lower limb’s anatomy for the correct positioning of the pins. It is essential not to compromise the skin and the vascularity of the flap.
Application of external fixation techniques applied to podoplastic surgery must consider, in the planning phase, the relationship between the positioning of the k-wires or pins and the site and the type of reconstruction. If the positioning of an external fixator will prevent access to the soft tissue reconstruction site, then it must be applied after the soft tissue reconstruction/wound closure has been performed.
The surgical technique for positioning and assembling the external fixator depends on the anatomical location of the surgical site, the type of tissue loss and the presence or absence of osteomyelitis. The presence of hindfoot lesions associated with osteomyelitis is a difficult and challenging treatment site. As is often the case with pressure lesions, deep tissue involvement is extensive and requires extensive excisions. The presence of bone involvement of the hindfoot makes it necessary to perform a partial or total calcanectomy (Figure 39).
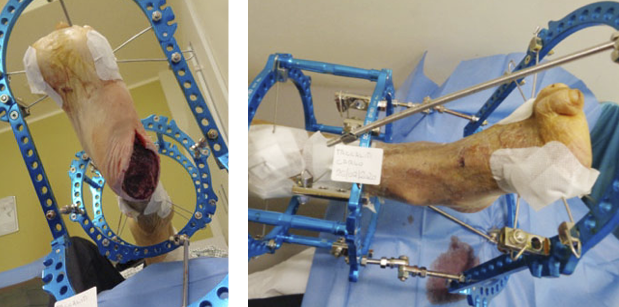
Figure 39: Open partial calcanectomy.
The debridement site can be closed by primary intention, be kept open for a secondary intention healing treatment or by using plastic reconstructive techniques. In any case, the positioning of a circular external fixator allows for the following objectives:
- Maintaining an equinus position of the ankle, which reduces the tissue tension of the hindfoot
- Off-loading of the hindfoot, allowing protection of the surgical site
- Maintaining access to the surgical site for reconstructive procedures and local treatment
The frame is composed of a foot plate (parallel to the sole of the foot) stabilised by three or four 1.8-mm Kirshner wires (on the forefoot and mid-tarsal line) and two rings for the tibia. The foot plate and tibial rings are connected by 2–4 threaded rods. The equinus position is maintained by inclining the foot plate with a centre of rotation wire through the ankle axis (the tip of medial malleolus to the tip of the lateral malleolus). Plantar flexion is gradually reduced over the course of follow-up. The large posterior opening allows for local medication of the wound site and dressing changes. With this device, it is possible to eliminate the use of a postoperative cast and its related potential complications (170).
For midfoot lesions, the theory of a complete excision of non-viable and infected tissue associated with the debridement of the bone structures involved in the infection progression is always valid. The deformity is treated with a resection of the bony prominences, which also leads to correcting the pathological overload. The treatment of midfoot plantar lesions, which involve the bone structures in relation to Charcot osteoarthropathy, often requires a multistage approach that primarily aims at the debridement of the infection involving soft tissue. The surgical site is kept open and cleansed with NPWT and instillation with antiseptic agents (198, 199).
Once the infection is controlled, the curative/corrective surgical step associated with stabilisation using a circular external fixator is planned. The stabilisation of the foot plate takes place by positioning 2–3 Kirshner wires in the forefoot and hindfoot while keeping the midfoot free from metal hardware. Any medial or lateral compression is obtained by placing olive K-wires. The treatment duration with an external fixator depends on whether the fixation objective is to obtain off-loading, or if it is used as a fusion/stabilisation tool.
In the first case, the construct can be removed when the surgical site is healed (usually within 4–8 weeks). In the case of a Charcot osteoarthropathy fusion complicated by infection and osteomyelitis, the external fixator use varies from 3 to 6 months.
5.2.3 Hybrid constructs
Hybrid constructs in a delta or box configuration are useful for obtaining total off-loading. The rods are oriented in a triangular position with the base oriented perpendicular to the axis of the leg (166) (Figure 40).

Figure 40: Box/Hybrid constructions.
Circular frames allow a complete off-loading position using a foot plate (one or multiple) and tibial rings. Variations of the construct could be made for differing locations of the ulcer/surgical site (forefoot, midfoot, hindfoot).
Skin grafts positioned on the forefoot, midfoot or rearfoot may not take if the graft site is not immobilised. The same outcome can affect local random flaps on the plantar aspect of the foot, if there are no factors that control an active range of motion at the metatarsal–phalangeal joints or ankle joint. Immobilisation optimises complex flaps’ viability by minimising intracompartment pressure in the reconstructed limb (192, 200, 201). The external fixator cancels direct pressure on the foot compartments and stabilises the muscle groups adjacent to the flap.
In conclusion, the use of an external fixator for 4–6 weeks following a reconstructive treatment allows the creation of a favourable healing environment by reducing pressure on the compartments, off-loading the flap and immobilising the adjacent joints. External fixation techniques also make it possible to avoid all the complications of strict immobilisation, such as heel decubitus and contracture, which leads to equinus of the foot. Off-loading of the hindfoot is particularly critical and difficult to achieve with standard unloading techniques, but the use of external fixation allows this to be achieved easily (170).
The general contraindications for the use of external fixation techniques are also valid in the case of surgical off-loading. A lack of compliance, blindness or neurological diseases, severe peripheral arterial ischemia, morbid obesity, social or psychiatric problems are absolute contraindications for the use of external fixation.
5.3 Conclusions
Surgical offloading is a promising adjuvant support for the conservative/minimally demolitive or reconstructive approach to diabetic foot surgery. All the surgeons using this approach recognise its usefulness. One of the problems with its extensive use, however, is the need for specific training. The second issue is the evaluation of scientific data from literature. Table 11 highlights that this approach has weak evidence, and well-designed randomised trials are needed to investigate indications and timing when applying these techniques.
Table 11: Studies on surgical offloading for DF ulcers
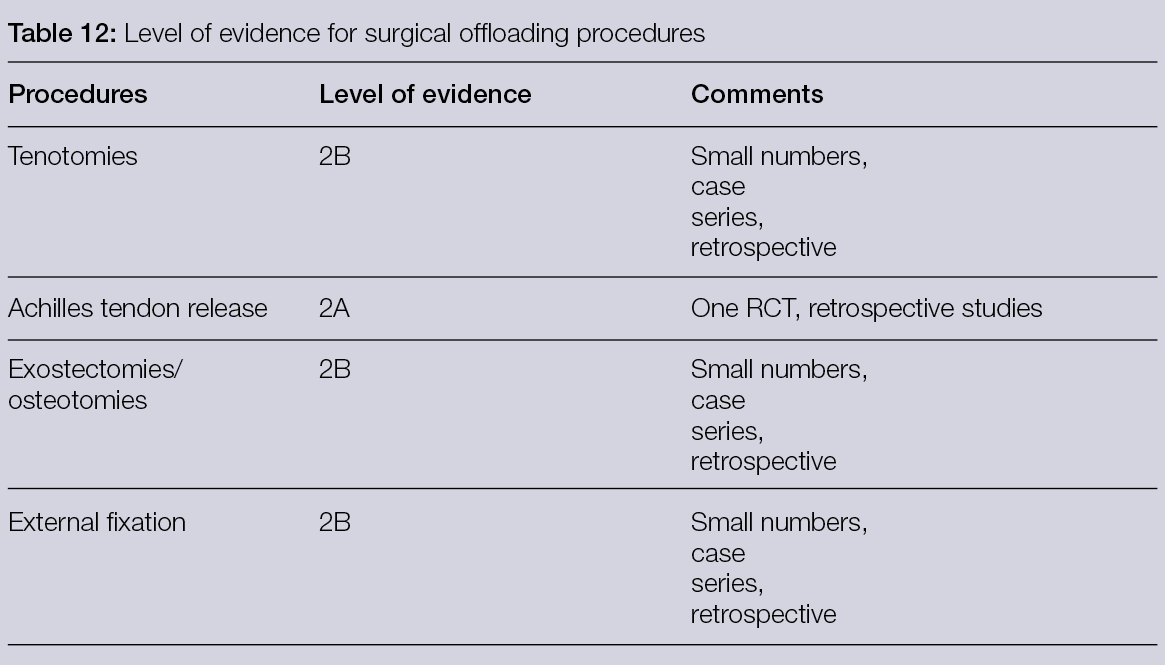
6. Bone substitutes with local antibacterial activity in the treatment of diabetic foot osteomyelitis
6.1 Introduction
It has been estimated that 60% of DFUs are infected at the time of initial evaluation (202). In the setting of osteomyelitis and/or soft tissue infection, antibiotic therapy, most often in conjunction with surgical debridement of infected, non-viable tissue and bone, is the usual initial course of treatment. In addition to debridement and systemic antibiotic therapy, local antibiotic delivery via non-absorbable/non-resorbable bone cement polymethyl-methacrylate (PMMA) and absorbable/resorbable bone graft substitutes may be a beneficial adjunct to surgical treatment of osteomyelitis in patients with infected diabetic foot ulceration. Antibiotic-impregnated cement has been used for many years, and recently resorbable bone graft substitutes have been used in the treatment of diabetic foot osteomyelitis (DFO). The addition of this method for the local delivery of antibiotics during the surgical treatment of DFO may help improve outcomes and reduce amputation rates.
There are several challenges that can occur with the surgical resection of infected tissue/bone and systemic antibiotic therapy. The use of local antibiotic delivery via non-resorbable and resorbable carriers may help mitigate these potential issues. The decision to surgically resect infected bone is dependent on several variables, including the location of osteomyelitis, the specialty of the provider and available resources. The extent of debridement is also an area of debate, as some advocate for total resection and clean margins, while others perform limited bone resections. Surgical excision of infected bone can result in dead space and/or bone defects, which can impact skeletal stability. In addition, despite surgical debridement, residual microorganisms may remain at the site of infection (i.e., positive margins). It is also thought that biofilm related to long-standing DFU may have a role in the development of chronic DFO, serving as a barrier to systemic antibiotics (203, 204). Systemic antibiotics can lead to complications such as renal toxicity, bacterial resistance and gastrointestinal dysfunction. In theory, the local delivery of antibiotics may provide several benefits, compared to oral and intravenous antibiotic therapies. Local delivery can lower the risk for systemic complications and can result in an increased concentration of antibiotics at the infection site, which is especially beneficial in the setting of peripheral arterial disease. Local antibiotic delivery systems can elude a higher concentration of local antibiotics to a site of infection, as much as 10 to 100 times greater than the minimum inhibitory concentration (205). When used as a bone substitute, they can also fill a void or dead space left by the resection of bone and tissue.
Local antibiotic delivery systems in the form of cement and bone graft substitutes have been widely used and described in the orthopaedic literature, but few studies have been published regarding their role in the surgical treatment of DFO. The purpose of this section is to review the recent evidence for local antibiotic delivery systems, with a focus on the role of the newer resorbable bone graft substitutes and their potential benefits in the surgical management of DFO.
6.2 Non-resorbable bone cement and resorbable bone graft substitutes for local antibiotic delivery
The terms absorbable/resorbable/biodegradable and non-absorbable/non-resorbable/non- biodegradable are used interchangeably in the literature. For the purposes of this document, the terms resorbable and non-resorbable will be used for consistency.
Once non-viable tissue and bone have been surgically resected during the treatment of a diabetic foot infection/DFO, it may be necessary to fill the remaining void for stability, or to fill a dead space to prevent bacterial colonisation. In some cases, stability is not needed after bone resection. However, a local antibiotic delivery system can be used for adjunctive treatment of infection. These antibiotic delivery systems/carriers can be categorised as either non-absorbable/non-resorbable/non-biodegradable (i.e., PMMA) or absorbable/resorbable/biodegradable bone graft substitutes (i.e., biodegradable ceramics; calcium phosphate based, or calcium sulphate based).
General Antibiotic Concepts: Aminoglycosides and Vancomycin are most commonly used in local antibiotic delivery vehicles because of their thermostability and broad coverage. Aminoglycosides, such as tobramycin and gentamycin, are effective in treating aerobic gram-negative bacilli (206). Vancomycin is effective against gram-positive bacteria and is commonly used to treat methicillin-resistant Staphylococcus aureus (206). Antibiotic elution is affected by multiple factors, such as the delivery device, antibiotic selected and amount used, surface area of the delivery device and the local environment (207). PMMA antibiotic release takes place via diffusion from its surface (208). When using PMMA, the rate of antibiotic released depends on the concentration gradient between the surface and the surrounding tissues and the size/surface area of the cement (207). During the first several days, the antibiotic concentration release is high, but subsequently drops to subtherapeutic levels. After a prolonged period of time, biofilm can form on the cement, thus necessitating removal (205, 207, 209). Conversely, resorbable bone graft substitute antibiotic delivery systems dissolve completely over time (207).
6.3 Non-resorbable bone cement for local antibiotic delivery
PMMA is the most commonly described and used non-resorbable carrier for local antibiotic delivery (206) and has been extensively studied in the orthopaedic literature. It is an acrylic polymer that can be used as a bone cement and has several benefits. When combined with antibiotics (most often Vancomycin, Tobramycin and Gentamycin), PMMA can be used as a structural bone void filler for osseous defects after the surgical resection of bone. This not only provides stability but can also manage dead space left after bone removal. PMMA can be easily moulded into the desired shape/size intra-operatively, to essentially provide a customised bone void filler. Liu et al. (210) retrospectively analysed the use of PMMA and antibiotics in patients with infected DFUs and peripheral arterial disease (210). Patients were categorised into two groups: a PMMA group (debridement of non-viable bone/tissue, defect filled with PMMA impregnated with antibiotic, N = 28) and a conventional treatment group (debridement of non-viable bone/tissue, N = 22). Patients in the PMMA group had significantly fewer debridements (P ≤ 0.001) and shorter healing times (P = 0.016), compared to the conventional treatment group (210).
The main disadvantage of PMMA is that it is non-resorbable, therefore further surgical intervention is usually required for removal. Interestingly, a few centres have reported retaining PMMA on a permanent basis with good results. Elmarsafi et al. (211) investigated the long-term outcomes of patients undergoing the application of a permanent PMMA antibiotic-eluting spacer for foot infection. They reported on 30 patients with a minimum 12 months follow up. Twenty-seven of the patients had diabetes. Twenty patients permanently retained their spacer; the longest retained spacer was 76 months. The need for additional surgery to remove non-resorbable antibiotic delivery systems has prompted the development of biodegradable antibiotic carrier systems.
6.4 Resorbable bone graft substitutes for local antibiotic delivery
Resorbable bone graft substitutes: Resorbable bone graft substitutes (also known as ceramic biocomposites, biodegradable ceramics or synthetic bone graft substitutes) have, more recently, been developed and can be used for local antibiotic delivery. A main benefit of their use is the avoidance of a secondary surgical procedure for removal by resorbing over time. The resorption time is variable and depends on the composition. Bone graft substitutes are most commonly calcium sulphate-based, calcium phosphate-based or combinations (Figure 41). Calcium sulphate and calcium phosphate bone graft substitutes are osteoconductive, but they are not osteogenic or osteoinductive. Osteogenic grafts can make new bone by the differentiation of osteoprogenitor cells. Osteoinduction is the ability of a graft to induce formation of bone-forming cells via the differentiation of mesenchymal stem cells. Osteoconduction is the ability of a graft to provide a scaffold or mechanical support for the growth of new bone. Autogenous bone is the ideal graft, because it is osteogenic, osteoinductive and osteoconductive (212).
Figure 41: Resorbable bone graft substitute impregnated with antibiotic is being prepared. It is an injectable form that will harden once it dries. This product contains both calcium sulphate and calcium phosphate.
Calcium sulphate: Calcium sulphate (also known as plaster of Paris) is an osteoconductive, biodegradable ceramic that has been used as a bone graft material since the late 1800s (207, 213). It can be used in different forms, such as an injectable that hardens (Figure 42) or beads can be created (213). Biomechanically, the compressive strength of calcium phosphate is similar to cancellous bone; however, resorption is relatively quick (3–6 weeks in soft tissue and 6–12 weeks in bone) (207, 213). Consequently, calcium sulphate is not an option for providing structural bone support. However, its ability to rapidly dissolve permits effective and high levels of local antibiotic delivery (207). Another disadvantage of calcium sulphate is prolonged aseptic drainage from wounds. This is a particular problem in wounds that have been closed over calcium sulphate bone void fillers.
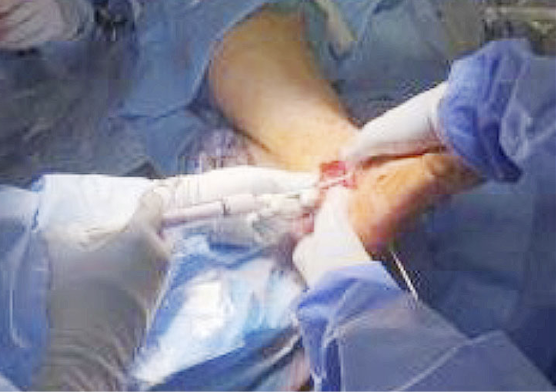
Figure 42: A resorbable bone graft substitute impregnated with antibiotic is injected into an arthrodesis site of a patient with a history of previous infection during a diabetic Charcot foot reconstruction revision surgery.
Calcium phosphate: Calcium phosphate is the main mineral found in bone (207). There are different forms of calcium phosphate, but tricalcium phosphate and hydroxyapatite are the two main types. Calcium phosphate ceramics take longer to resorb compared to calcium sulphate ceramics. Tricalcium phosphate resorbs over the course of 6–18 months, and hydroxyapatite can resorb over the course of 6 months to even 10 years. Calcium phosphate has a crystalline surface compatible with osteoconduction, with hydroxyapatite being the most osteoconductive. Given its longer time for resorption, there is more time for bone repair and regeneration, compared to calcium sulphate. Calcium phosphate products have less associated aseptic drainage than calcium sulphate products.
Polyphasic bioceramics: Combining both calcium sulphate and calcium phosphate ceramics can make use of the properties of both, such as faster resorption/earlier antibiotic release from the calcium sulphate ceramic and the benefits of structural support of the calcium phosphate ceramic.
Several minor complications have been described after the use of calcium sulphate and calcium phosphate bone graft substitutes. Wound drainage appears to be a common complication with the use of calcium sulphate, due to its faster resorption. Inflammatory reactions from the release of calcium have also been described. Local antibiotic release using calcium sulphate and calcium phosphate and systemic toxicity does appear not to be an issue (207).
6.4.1 Resorbable bone graft substitute/local antibiotic delivery and treatment of diabetic foot osteomyelitis
Most recently, several studies have investigated the use of resorbable bone graft substitutes and their role in the treatment of DFO (Table 13). Over the past five years, several studies have evaluated the use of both antibiotic-impregnated calcium sulphate and calcium sulphate-hydroxyapatite bone graft substitutes. Because the uses of these are more recent, the number of available studies is limited. Despite the limited number of studies, the overall findings appear promising in regard to enhancing the surgical treatment of DFO.
Table 13: Observational studies: Bone substitutes for local antibiotic delivery in the treatment of DFO
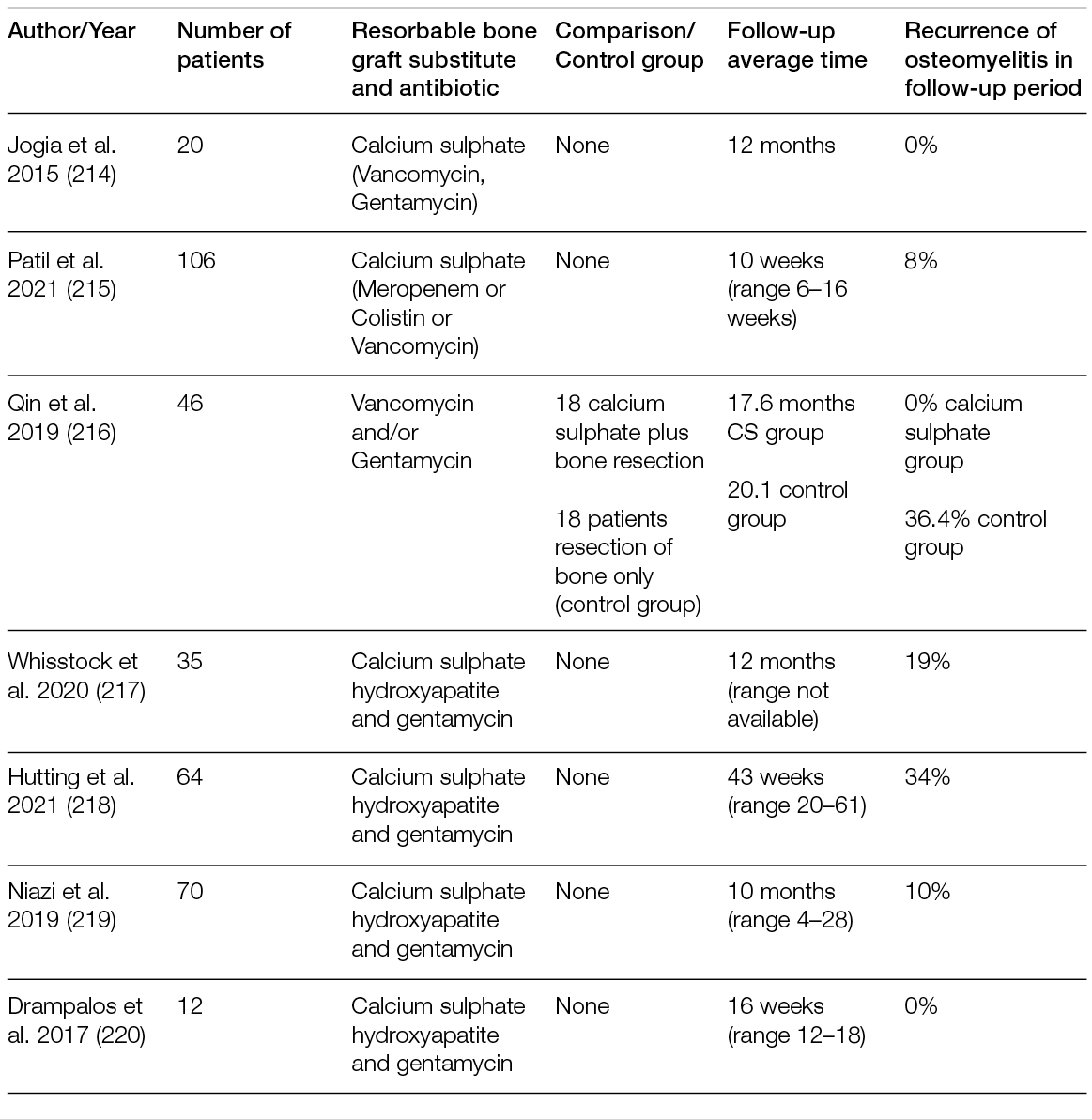
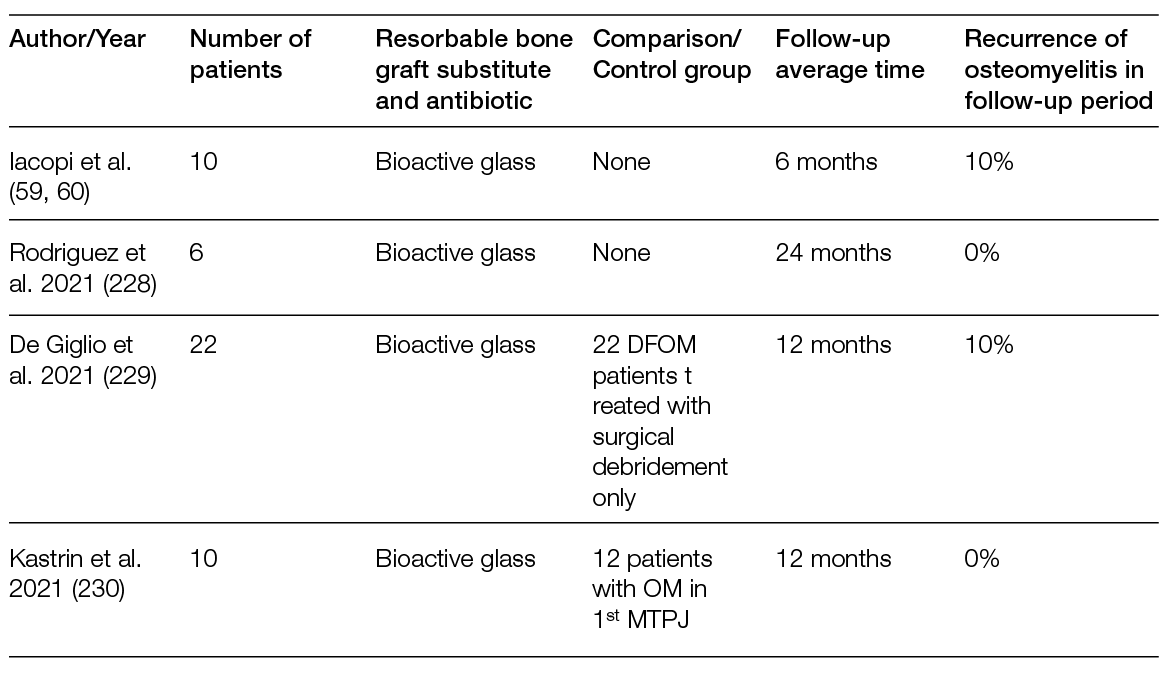
Calcium Sulphate DFO studies: Jogia et al. (214) evaluated 20 patients with DFO who were treated with surgical intervention in a multidisciplinary foot clinic. The protocol included the resection of infected bone and placement of calcium sulphate pellets impregnated with vancomycin and gentamycin with primary closure. Treatment success was defined as no recurrent ulceration over the course of one year. None of the 20 patients experienced a recurrence, and the authors achieved a successful outcome in 100% of patients at 12 months. No adverse events were reported. The average duration of systemic antibiotic therapy was two weeks (0–9 week range), and the average healing time was five weeks. Another retrospective study by Patil et al. (215) reviewed the outcomes of 106 patients undergoing surgical treatment of DFO in conjunction with antibiotic-impregnated calcium sulphate beads. There was a range of interventions, including debridement, toe amputation, forefoot amputation and below knee amputation. The choice of antibiotic selected (vancomycin, meropenem or colistin) was based on culture susceptibility results. At final follow up, 92% of patients had not experienced a recurrence. There were no systemic or local adverse events. Although the reported outcomes are promising, the limitations of these two studies include their small patient numbers, retrospective nature and lack of control groups. Qin et al. (216) investigated the outcomes of 46 patients (48 limbs) treated for forefoot DFO. Their study group was comprised of 18 patients (20 limbs) who were treated with bone resection plus antibiotic-impregnated calcium sulphate. The control group was comprised of 20 patients (28 limbs) treated with bone resection without the application of antibiotic-impregnated calcium sulphate. Vancomycin and/or gentamycin were used in the calcium sulphate group. Wounds were closed primarily after surgical intervention in both groups. At a mean follow up of one year, 90.0% of the patients in the calcium sulphate group healed, compared to 78.6% in the control group (p > 0.05). The authors felt that the study was likely underpowered for determining statistical significance. There was no significant difference in wound healing duration of time (average 13.3 weeks in the calcium sulphate group and 11.2 weeks in the control group). In patients with healed wounds, the study group had a significantly lower rate of recurrence than the control group (0.0% recurrence in the calcium sulphate group, versus 36.4% in the control group, p = 0.014). The most common post-operative adverse event was aseptic persistent drainage from the surgical site in the calcium sulphate group. The average duration of persistent drainage was 8.5 weeks. However, this drainage did not result in any complications and was managed with dressing changes. Overall, the investigators found that use of antibiotic-impregnated calcium sulphate decreased the risk recurrence of forefoot DFO. To the best of our knowledge, this was first controlled study to compare outcomes of DFO resection with and without antibiotic-impregnated calcium sulphate.
Calcium sulphate-hydroxyapatite (CaS-HA) DFO studies: Several recent studies have investigated the use of CaS-HA in DFO surgical treatment. The benefit of CaS-HA is that it is ‘biphasic’, meaning calcium-sulphate resorbs faster, while the calcium hydroxyapatite resorbs more slowly. The slower resorbing hydroxyapatite provides an osteoconductive framework (217). Although the number of studies is limited, CaS-HA biocomposite bone graft substitute has shown promise in several reports. In 2021, Hutting et al. (218) reported on a multi-centre retrospective study of patients undergoing treatment of forefoot, midfoot or hindfoot DFO with surgical resection and placement of a gentamycin-loaded calcium sulphate-hydroxyapatite bio-composite. The gentamycin-loaded CaS-HA bio-composite was used in paste form or as pellets and placed into the remaining void after resection. Wounds were closed by either primary closure or soft tissue reconstruction (such as a flap). Thirteen centres and 64 patients were included. Most patients had DFO of the forefoot. The average follow up was 43 weeks. Wound healing was observed in 84%, and treatment success (defined as wound healing without ulcer recurrent) in 66%. Niazi et al. (219) performed a retrospective review of 70 patients with DFO treated with surgical resection and antibiotic-impregnated calcium sulphate hydroxyapatite bio-composite. Like the previous study, forefoot, midfoot and hindfoot DFOs were included, and the majority of DFO cases involved the forefoot. The surgical site was either closed primarily, or negative pressure therapy was used. Patients were followed until infection eradication or ulcer healing. The average follow up was 10 months (range 4–28 months). Infection was eradicated in 90%, with an average ulcer healing time of 12 weeks. Higher failure rates were noted with treatment of hindfoot DFO. There were no systemic antibiotic related complications. Whisstock et al. (217) studied the use of gentamycin-impregnated calcium sulphate hydroxyapatite bone graft substitute over the course of three years in 35 patients with DFO. The overall success rate was 81.3%, and the best results were found in treatment of DFO of the forefoot/metatarsals. There were no systemic complications. Drampalos et al. (220) also investigated gentamycin-impregnated calcium sulphate hydroxyapatite bone graft substitute in 12 patients with DFO undergoing surgical management of calcaneal osteomyelitis. All patients underwent a single-stage (i.e., only a single surgical intervention) procedure with infection eradication in all patients at follow up. The average healing time was 16 weeks (range 12–18 weeks).
One recent study did not find a benefit in using local antibiotic delivery as part of the surgical treatment of DFO. Chatzipapas et al. (221) treated 25 patients with DFO. They were divided into three groups: surgical debridement and systemic antibiotics (N=8), surgical debridement/systemic antibiotics plus PMMA antibiotic beads (N=9) and surgical debridement/systemic antibiotics plus calcium sulphate hydroxyapatite beads loaded with antibiotic (N=8). When comparing the three groups, there was no difference in the average time to healing (P = 0.094), healing rate (P = 0.543), amputation rate (P = 0.331) or recurrence rate (P= 0.543). Ideally, prospective, randomised studies with adequate strength are needed to document treatment efficacy.
Limitations of Studies: Although the outcomes appear promising in the studies reviewed, providers should recognise the limitations that included relatively small numbers, retrospective nature and the lack of control groups. These were acknowledged by all investigators.
6.5 Bioactive glass
In relatively recent years, the development of a glass with a peculiar composition, provided in granules or paste which makes it biocompatible, Known as S53P4 Bioactive Glass – (S53P4BG), has opened a range of new opportunities in the management of osteomyelitis, including those associated with DFU (Figure 43) (222).
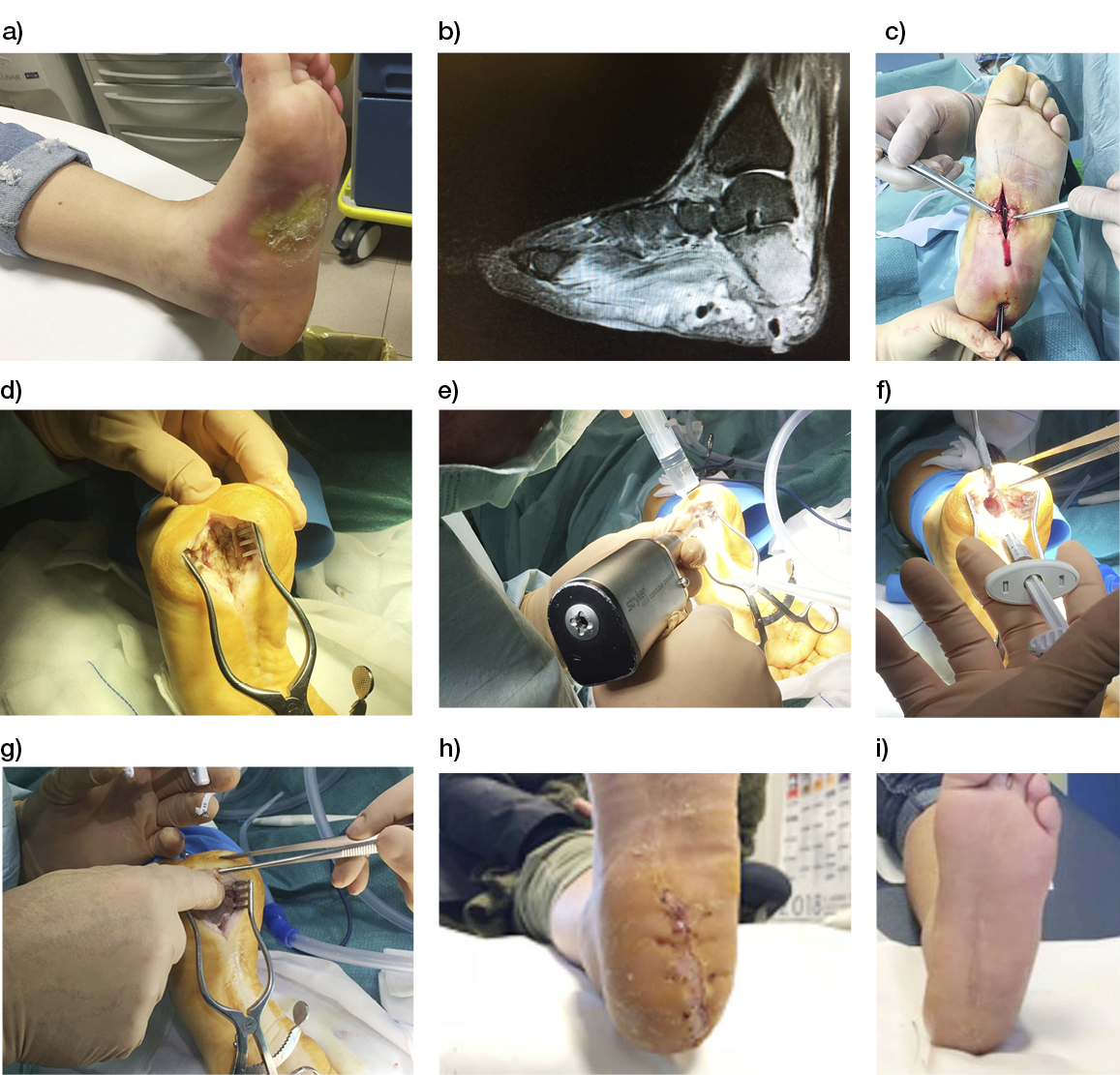
Figure 43: Bioactive glass: a) Heel lesion in a neuropathic patient previously submitted to Achilles tendon lengthening, complicated with a plantar sub-fascial abscess; b) The magnetic resonance imaging shows a gross involvement of plantar intermediate compartment, with gas, oedema and infection of the subcutaneous plantar pad, fascia and muscles. The osteomyelitis of the calcaneal bone is also evident, with a bone marrow oedema involving almost all the posterior process of the calcaneum; c) Surgical drainage of the sub-plantar abscess and opening of the intermediate plantar compartment in urgency; d) In a second step, after two weeks of Parenteral antibiotics and NPWT with instillation, the patient underwent to revision of the OM of the calcaneum; e) The malacic bone involved in the OM was removed by drilling a cavity inside the posterior process of the calcaneum; f) The cavity was then filled with S53P4BG (Bonalive granules™) in order to both contrast bacterial survival and to stimulate new bone formation; g) The bioactive glass granules were pressed into the cavity and sealed with BG paste (Bonalive putty paste®); h) The foot after three weeks, at the removal of the stitches; i) The foot two years after the intervention.
The new S53P4BG has proven not only osteoinductive and osteoconductive, but also bactericidal, with a demonstrated activity against all the most prevalent strains of bacteria, including MRSA and Pseudomonas aeruginosa MDR, by means of the sharp and sustained increase of pH and increased osmotic pressure, which is induced locally. This makes the environment unsuitable for bacterial survival and multiplication. Hence, the antibacterial mechanism of action is based on a physical–chemical reaction and not on locally delivered antibiotics (223-225).
The first successful clinical application of BG was in the management of long bones OM, in orthopaedic surgery, but more recently there has been growing interest from the DF-related OM that has led to several clinical pilot studies, followed by more structured studies (226, 227).
In a small series of six patients with DFOM treated with S53P4BG, Rodriguez et al. (228) reported healing at 24 months in 2/3 of the cases. There was no re-infection or need for intervention. In a series of 10 consecutive DF patients, all affected by OM and treated with S54P4 BG on top of standard surgical debridement and systemic antibiotic therapy, Iacopi et al (59) observed a healing rate of 80% at 6-month follow up.
In a retrospective observational study comparing 22 consecutive DFOM treated with S53P4 BG with 22 superimposable controls treated with SoC, De Giglio et al. (229) found a significant (p=0.03) increase in the healing rates in patients treated with S53P4BG (90% vs 62%). Patients treated with BG had a likelihood of resolving OM that was five times higher than controls. The probability of additional antibiotic therapy was also 81% lower in the group treated with S53P4BG.
In an observational comparative study, Kastrin et al. (230) found no differences in healing rates at 1-year follow up between 10 DF patients with OM of the first metatarsal-phalangeal joint (MPJ), treated with joint resection, external fixation and S53P4BG, compared with 12 similar cases treated with antibiotic-loaded beads.
The interest in using S53P4BG in the management of OM in DF is not only related to its filling and osteogenic properties, but also to its antibacterial activity, which allows for controlling bone infections without inducing bacterial resistance (222), as this approach is not based on bio-molecular, but rather on bio-physical, interactions.
This feature is extremely relevant in view of the increase of multiple-resistance strains that have occurred because of the inappropriate administration of antibiotics, especially in hospitalised patients with chronic conditions, such as patients with DFOM (231, 232).
The generally positive results, univocal in all the experiments reported to date, position BG as a very interesting therapy in the surgical management of DFOM. This is especially true in connection with the management of ulcers on locations such as 1st-MPJ or the heel, when there is a need to replace the bone and joint tissues destroyed by infections, with the objective of avoiding partial amputations that may decrease the biomechanical performance of the foot (233).
Despite the promising results in a repeated series of observational and comparative studies, a prospective RCT on BG in DFOM is still missing, and this does not support the idea that the therapy should be considered a first option in the management of this difficult pathology. Solid prospective data in this field, which could prove the basis for eventually modifying this position, would be welcome.
6.6 Conclusions
The use of non-resorbable and resorbable local antibiotic delivery systems via the use of non-resorbable PMMA cement and resorbable bone graft substitutes has been extensively studied in the orthopaedic literature. Over the past several years, interest in and the use of antibiotic-impregnated resorbable bone graft substitutes has emerged in the treatment of DFO. Although studies show promising results using these modalities as a part of the surgical treatment of DFO, the patient numbers are small, study designs are retrospective and there is lack of a standardised protocol. Further investigations are needed in the form of larger, prospective and randomised trials. Despite these limitations, local antibiotic delivery systems are a potentially promising adjunctive tool for the surgical management of DFO.
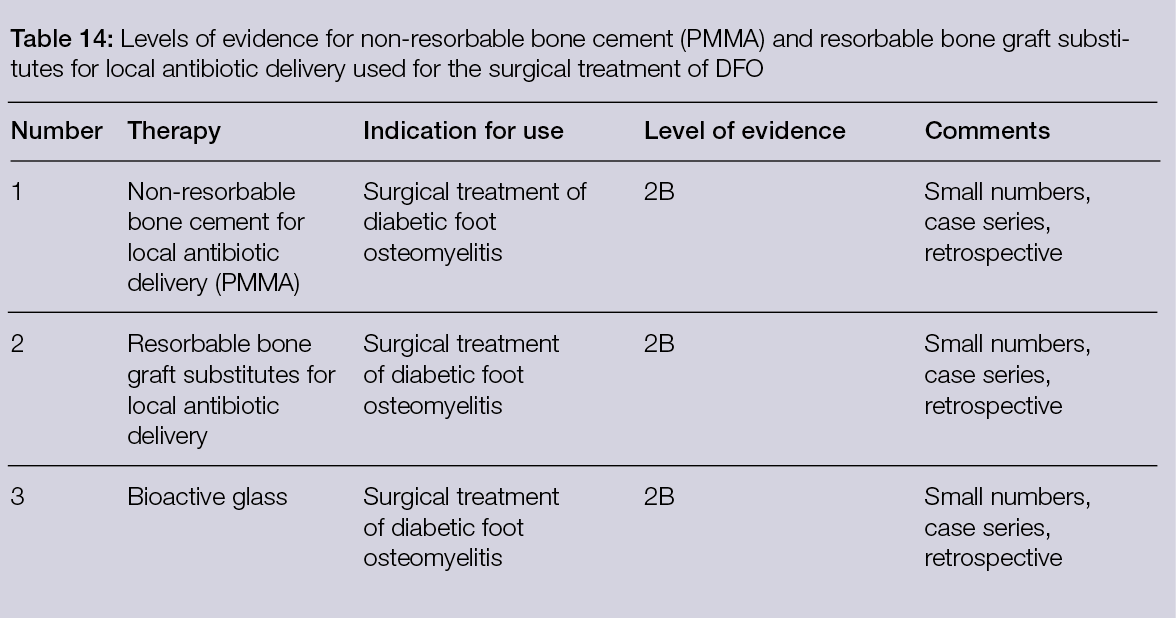
7. Vascular surgery for chronic limb-threatening ischema
7.1 Introduction
In 2015, occlusive PAD was diagnosed in 236.62 million adults over the age of 25 world-wide (234). The prevalence, estimated between 4–20% of the population, varies according to age, smoking habits, diabetes, high blood pressure, hypercholesterolemia and social status. Of these patients, 5–10% will develop chronic limb-threatening ischemia (CLTI) within five years (235).
CLTI patients are at high risk of amputation and death. PAD is often underdiagnosed. In a retrospective German study based on the statutory health scheme, 81% of patients received a vascular diagnostic measure, and only 50% had a vascular procedure before amputation (236). To improve limb salvage and life, there is a consensus concerning the pursuit of revascularisation whenever feasible (237), which has proven be effective in the short and long term (238).
The strategies of CLTI management have been reviewed thoroughly by international societies, which have issued recommendations concerning the grading of the diseases, prognosis, explorations, treatments and follow-up. It is not in the scope of this chapter to detail these recommendations, but they may be found in the following references: TASC (Trans-Atlantic Inter-Society Consensus) (235); GLASS (Global Limb Anatomic Staging System) (239, 240); Wound, Ischemia, and Foot Infection (WIFI) (241, 242); and Diabetic Foot Management (243).
In the following section, we focus on the most recent advances in vascular repair, as they have improved preoperative evaluation, intra operative imaging, techniques, materials and devices.
The Duplex scan remains the first and routine examination for detecting PAD and indicating more specific investigations. This examination is inexpensive, non-invasive and highly reliable in well-trained hands. Recent portable machines make it easy to perform the exam at the patient’s bedside (244). The ankle-brachial index is simple and widely applicable, the limits of which are incompressible leg arteries, which are frequently seen in diabetic or renal insufficiency patients.
The latest generation of CT scans with injections of iodine contrast medium visualise the lesions thanks to curvilinear and orthogonal 2D MPR 2 D computer reconstructions, 3D MPR, 3D MIP, 3D volume rendering and 3D endoscopic reconstruction drawn from the slice imaging acquisition. This allows, with excellent precision, the identification of the locations of lesions, their nature (calcic or thrombotic), the percentage of stenosis or the presence of occlusion (Figs. 44, 45). Finally, determining the lengths of the lesions and the internal and external diameters of the vessels to be treated makes it possible to choose the necessary tools, the types of balloons and appropriate stents ahead of the procedure. These preoperative assessments facilitate the management of materials stocks by healthcare institutions. Severe renal failure or allergy to iodine are relative contraindications that can be overcome with recommended precautions (hyperhydration, antihistamine, corticosteroids). Unfortunately, lesions of the arteries of the legs are sometimes difficult to analyse, especially in cases of severe calcification. The combined data from a Duplex scan and CT scan are very helpful.
Figure 44: Reconstructions obtained from an injected CT scan. Software allows the reconstruction of all tissues, from the skin to the muscles, bones and vessels.
Figure 45: 3D CT scanner reconstruction of the aortoiliac segment showing an occluded right common iliac artery.
Angio–magnetic resonance imaging (MRI) is requested mainly in the event of contraindication to CT angiography. Even though 2D and 3D computer reconstructions are similar, the images can be more difficult to analyse, especially with distal lesions, calcification, previous stents or metallic materials that induce major artifacts (Figure 46, 47).
Figure 46: MRI of the aortoiliac and legs arteries showing an occlusion of the left superficial femoral artery.
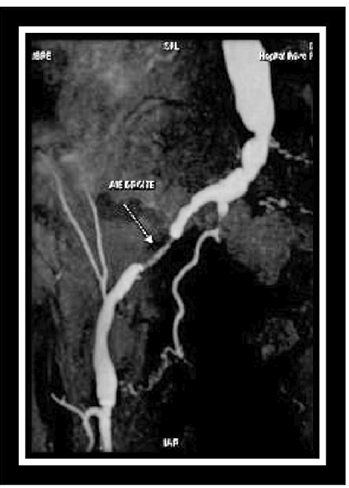
Figure 47: MRI of the right iliac artery showing an artefact due to a previous stent mimicking a severe stenosis.
7.2 Operating Rooms
Radiological equipment present in operating rooms has evolved considerably, particularly with the recent advent of mobile C-arms and hybrid rooms equipped with sophisticated software. Mobile C-arms are less expensive when compared to traditional settings, but still provide excellent 3D imaging with navigation-enhanced tools such as EndoNaut software (245). One drawback is the relatively small size of the screen.
Hybrid rooms combine the environment of an operating theatre and high-performance imaging comprising a radiology bar, a mobile and transparent X-ray operating table and a large display screen (Figure 48, 49). The leaders in these fields are Siemens, GE and Philips. This equipment allows the surgeon to manipulate the radiological equipment; to choose and modify the best viewing angles, as he or she deems appropriate; and to initiate the injection of contrast medium. The equipment is loaded with computer software that makes it possible to fuse the images of a preoperative scan or MRI with the ‘scanner-like’ images acquired from the rotation of the arch around the patient at the beginning of the operation. The physicians can then work on anatomical reconstructions in three dimensions, which are adjustable at will. Images obtained by fusion allow for the accurate assessment of lesions and greatly facilitate endovascular surgeries, reducing patients’ and staff members’ exposure to ionising radiation, and allowing for a reduced dose of contrast agent (246, 247). On the screen, the reconstructions can visualise the whole injected vessel, or only the internal and external contours of the arteries, bifurcations and important collateral branches (Figure 49), avoiding the reinjection of contrast products. Specific software calculates the number of pixels during injections, providing information about the arterial flow in the plain organs, in the arteries of the lower limbs and the efficiency of revascularisation.
Figure 48: Photograph of a Siemens hybrid room.
Figure 49: Large display screen with a 3D reconstruction of the aortoiliac segment.
An intraoperative Doppler ultrasound machine placed in a sterile bag can be connected to the monitor. The puncture under ultrasound is useful for choosing the site of the puncture according to the state of the arterial wall (calcification) and for following the advances of the guide. Finally, after removing the material, the Doppler ultrasound checks for the absence of hematomas and the quality of the arterial flow.
Due to the size of the screen, different images can all be viewed in real time. In the near future, the development of augmented reality devices and individual masks will make it possible to review images, patient files and other useful data without an intermediary screen and will facilitate the practice of endovascular surgery (248).
7.3 Pathology and patients
The patterns of patients with CTLI are well described (237). The decision regarding the type of treatment is based on multiple factors, among these are age, comorbidities, ASA classification (classification by the American Society of Anesthesiologists, ASA), life expectancy, nursing home stay, socioeconomic status, vessel anatomy and location of the disease (249). To help the decision-making process, multiple predictive models of survival and limb salvage have been constructed, but none has a widespread use. A recent study (250) from the Netherlands included 449 patients treated by surgery or endovascular techniques and followed for up to two years. Among them, 90 patients had died at 6 months (death probability 20%), 130 at 12 months (death probability 29%) and 165 at two years (death probability 38%). A predictive probability model including 15 variables was established. The authors provide two examples demonstrating that a 65-year-old ASA 3 patient not living in a nursing home and without physical impairment has a survival probability at 6 months of 86%, while an 82-year-old ASA 4 patient in a nursing home with physical impairment has a survival probability of 67% at 6 months. Limb salvage depends partially on the feasibility of revascularisation. In non-revascularisable CTLI, amputation-free survival was 43% at 5 years (251). A meta-analysis of 27 randomised control studies assessing conservative treatment found a 12-month mortality of 18% and amputation rate of 27% (252).
Results of revascularisation in a real-world setting are mitigated. A German national study (238) included 15,314 patients with CTLI. Of these, 7,651 were revascularised (R+) and 7,663 were given a conservative treatment (R-). At four years, mortality and amputation rates were, respectively, 55.1% for R+ and 40.6% for R+, and 59.5% and 48.2% for R-. In a multivariate Cox regression model, Rx− status was associated with increased death and the increased cumulative endpoint of death and amputation (each P < 0.001).
7.4 Revascularisation in CTLI patients
The GLASS staging system is a useful method for assessing options and treatment results (239). A majority of CTLI patients have infrainguinal lesions located in the femoro-popliteal and infra popliteal segment, and some have associated inflow impairment due to aorto-ilio-femoral stenosis or occlusion. Those proximal lesions must be treated first-hand by endo vascular approaches, as shown in Figure 50 and 51, or by aorto-iliac bypasses.
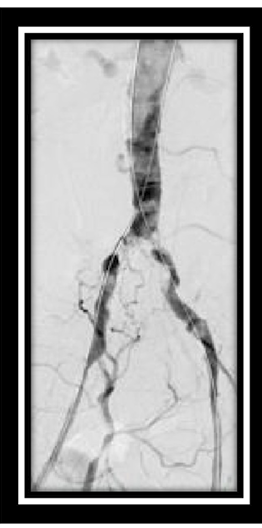
Figure 50: Intraoperative imaging of a successful aortoiliac reconstruction using the kissing technique with two balloons and stents.
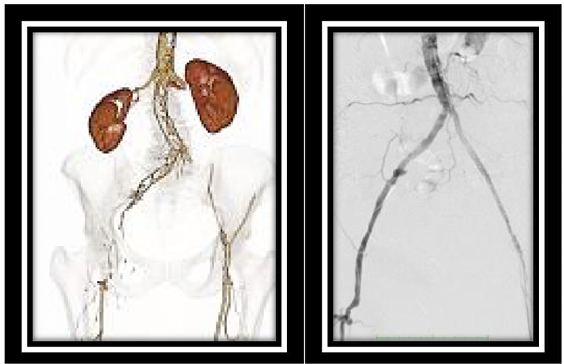
Figure 51: Preoperative CT scan and intraoperative angiography showing severe aorto-iliac lesions successfully recanalised.
At the femoro-popliteal levels, despite the increasing use of endovascular tools, reversed saphenous vein bypass remains the first choice. A retrospective study of 2869 CTLI patients comparing primary bypass with primary angioplasty stenting found wound healing at 6 months, a higher freedom from restenosis, improved patency rates, significantly fewer reinterventions and higher survival than percutaneous trans-luminal angioplasties within 3 years. However, a bypass-first approach was associated with an increased total hospital length of stay and wound infection. Perioperative mortality and amputation rates were similar between procedure types (253). At the level of leg arteries, peroneal bypasses offered a better patency rate at 24 months, but similar wound healing and limb salvage (47% vs 23%) and a higher complications rate (254). The authors of these two studies concluded that endovascular intervention is low risk and may be sufficient to heal ischemic foot wounds. One drawback of the endovascular approach is the frequent need for reintervention (255). Companies have extensive research and development programmes to improve the feasibility and results of endovascular treatment.
Balloons are not only used to mechanically fracture the plague, but also to deliver in situ drugs such as Paclitaxel and Sirolimus. The aim of these drugs is to reduce the stenotic cascade following the inflammation of the arterial wall. A comparison of plain balloons and different coated balloons (DCBs) (256-258) shows better vessel-targeting and overall patency with DCBs. Figure 53 shows a recanalisation of a long superficial artery lesion, and Figure 54 shows the use of the Jetstream device to debulk calcified plaque before balloon angioplasty and stenting.
Various stents are available. Figure 52 shows the characteristics of the different stents. They are routinely used in the femoro-popliteal segment, generally as a bailout procedure when the results of plain balloon angioplasty are suboptimal or in the case of dissection. A metanalysis of studies (259) comparing systematic versus selective stenting has confirmed that this practice is well-founded.

Figure 52: Stents’ descriptions and characteristics.
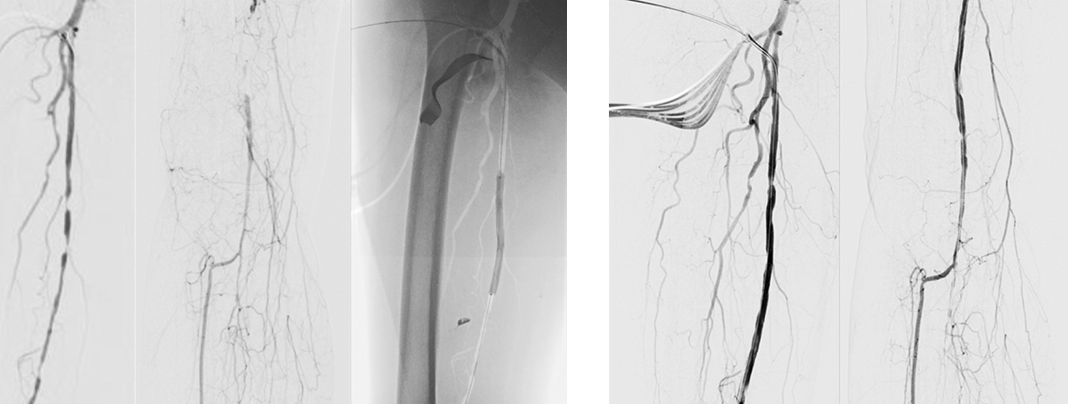
Figure 53: Recanalisation of a long superficial femoral artery and popliteal occlusion with a drug coated balloon.
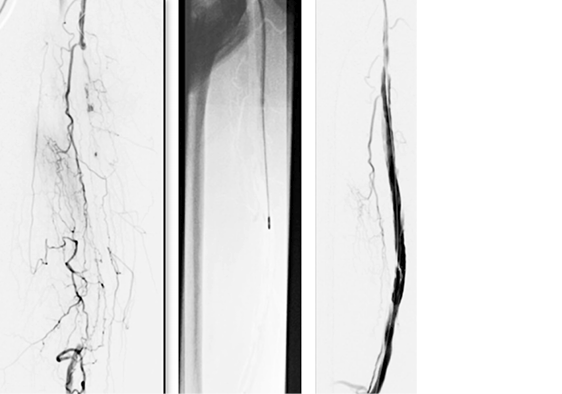
Figure 54: Recanalisation of the extensive and calcified occlusion of the superficial femoral artery using the Jetstream device.
Drug-eluting stents (DES) with Paclitaxel are available on the market for peripheral atherosclerotic diseases. The fixation of the drug on the metal frame, the dose and the time release are different from one stent to another. The Zilver PTX®, from Cook Medical, is made of Nitinol, directly coated with 3 microg/mm2 of Paclitaxel. The Eluvia® stent, from Boston Scientific, is also made of Nitinol, with the Paclitaxel 0.167 microg/ mm2 linked to a polymer layer. With this later stent, the Paclitaxel is still present on the arterial wall after six months, compared to only three months with the former. Clinically, DES has shown good efficacy. An RCT of the Zilver PTX® versus bare stents (260) has shown a better five-year patency rate with the DES. Similarly, a cohort study with the Eluvia® stent has shown excellent 24-month patency (261). An international study comparing these two stents did not find any differences, in terms of patency and major adverse effects (262).
Are DES better than DCB? Two RCTs comparing DCB and DES failed to show a difference of patency at one year (79% vs 80%) (263), and at three years, there was a statistically non-significant difference in favour of DES (54% vs 38% (264).
Self-expandable covered stents (PolyTetra Fluoro Ethanol (PTFe) + heparin) are a valuable option for long superficial femoral artery (SFA) lesions (Figure 55). The long-term patency is superior to those obtained with bare stents (265).

Figure 55: Covered stent graft from Gore Medical.
A meta-analysis of 45 RCTs, including a total of 5,565 patients (264), found a better 24-month patency with covered stents, compared to DES, bare stents, cutting balloons, atherectomy and DCB.
In patients with a long SFA occlusion, the crossings through the artery are sometimes impossible. The Detour system, which comprises a crossing device, a specifically designed snare and a covered self-expandable stent made of PTFe, enables the placement of the stent within the superficial femoral vein while the extremities of the stent are in the proximal and distal patent stump of the SFA, or the popliteal artery. At one year, a prospective study of 81 patients (266) showed 96.3% clinical success, with four stents occluded. At one year, the Kaplan Meyer primary patency and the secondary patency were, respectively, 81% +/- 4% and 90% +/- 3%. Surgeons are developing similar techniques using less expensive available tools. (Figure 56)
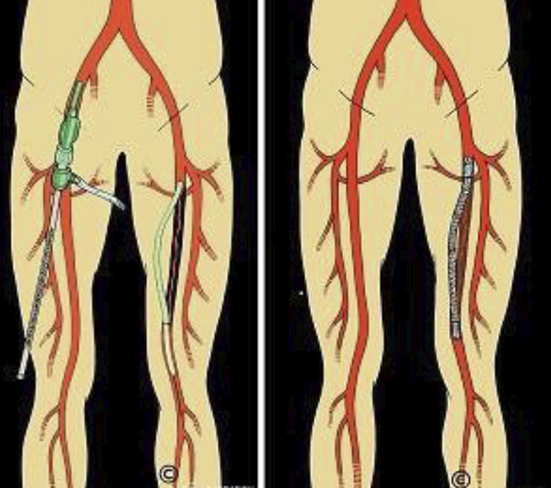
Figure 56: Drawing of a totally percutaneous femoropopliteal bypass technique (courtesy of Dr A. Sarradon).
Popliteal and leg arteries diseases are predominant in patients with CTLI, as physicians are reluctant to use stents in these locations. DCBs have been specifically designed for this purpose. Unfortunately, they have not met expectations. A review of 21 RCTs with 3,760 lower limbs concluded that the risk of major amputation was increased with Paclitaxel-coated balloons (267). This higher risk was not found with DES. A higher Paclitaxel dose, drug and excipient migration (up to 90% of the original dose) and release and fixation for several months in the ischemic tissue of the leg may account for the poorer outcomes of DCBs.
Paclitaxel-impregnated devices have been thoroughly scrutinised after the publication of a meta-analysis (268) showing a relative excess of mortality of 38% at 5 years. By contrast, conflicting data were drawn from the RCTs and did not find any difference of mortality (269-271). This uncertainty led the FDA and the French health care administration (Haute Autorité de Santé; HAS) to issue a warning and recommendations on using Paclitaxel devices only in patients with a high risk of restenosis, who had been informed about the potential risk and to report any adverse effects to the authorities.
Arterial lesions of the leg and foot have benefited from new developments. Micro catheters, micro guides and micro balloons that can be introduced from the pedal or tibial posterior arteries (272, 273), are improving the access to and feasibility of leg artery repair. From a technical point of view, the rendezvous technique (274) enlarges the feasibility of recanalisation. Finally, to deal with post angioplasty dissection, which is a frequent cause of re-occlusion, the Tack Endovascular system has shown excellent results at 6 months, with 92% patency and 95% limb salvage (275).
7.5 Cellular therapies
Since the discovery that blood cells contribute to postnatal angiogenesis (276), there has been an increasing number of clinical studies to test the efficacy of autologous cell therapies for the treatment of CLTI, ranging from case reports to small series, uncontrolled trials and RCTs reported in numerous meta-analyses (277-285). The main goal of cell therapy is the induction of therapeutic angiogenesis with the formation of collaterals leading to increased blood flow in the ischemic limb and tissue regeneration in non-healing wounds. The first clinical trial of therapeutic angiogenesis in the treatment of critical limb ischemia, which used the transplantation of bone marrow cells and peripheral blood origin, was performed in Japan in 2002 (286). Since then, an increasing number of studies and several meta-analyses have suggested that autologous cell therapy is more effective than conventional treatment for non- revascularisable critical limb ischemia, suggesting that implants of autologous cell therapy might promote the wound-healing process (277, 287-289). In a comprehensive data pooling analysis conducted on different databases, Dong et al. recently searched for a comparison between cell therapy and regular therapy. They observed that fewer patients underwent major amputation in the cell therapy group, compared with the standard therapy group. Moreover, those in the cell therapy group were characterised by a smaller ulcer area, and there was a significant difference in the wound-healing rate between the intervention and control groups (277).
Benoit et al. assessed 45 clinical trials and 1,272 patients showing a significant reduction in amputations in patients treated with both peripheral blood mononuclear cells (PB‑MNC) and bone marrow mononuclear cells (BM‑MNC), compared to patients treated with medical therapy (290). In a meta‑analysis of 16 RCTs for a total of 774 patients, Liew et al. reported a significant reduction in major amputations and complete healing of the wounds (288). Interestingly, both PB‑MNCs and BM‑MNCs significantly reduced the risk of major amputation, but only PB‑MNCs significantly improved wound healing (288). PB‑MNCs were significantly associated with improved wound healing and were not associated with any increased risk for side effects in 12 clinical studies of 290 patients (287). Another recent meta-analysis by Rigato et al. (291) was conducted on RCTs of 837 patients, 7 non-randomised trials of 338 patients and 41 non-controlled trials of 1177 patients. In this meta-analysis, the authors observed that autologous cell therapy reduced the risk of amputation by 37%, improved amputation-free survival by 18%, and improved wound healing by 59%. They also observed that PB-MNCs, but not BM-MNCs or bone marrow mesenchymal cells, were effective in significantly reducing amputations (291).
PB-MNCs, which consist of a heterogeneous population of lymphocytes and monocytes, CD34+ hematopoietic stem cells and EPCs, seem to be a promising autologous cell therapy. The angiogenic and arteriogenic potency of PB-MNCs has been extensively demonstrated (292-300). Moreover, blood vessels control macrophage differentiation and maturation from recruited monocytes, promoting arteriogenesis and tissue repair in ischemic tissue (301). Monocytes and macrophages also maintain angiogenic potency in diabetic patients, while hematopoietic stem cells showed a reduction of the angiogenic ability (302).
Autologous PB-MNC cell concentrate can be produced with Cook Regentec’s HemaTrate® Blood Filtration System (Figure 57), a class IIB, point-of-care medical device for intra-operative use, for the rapid preparation of TNC/PB-MNC concentrate from 20 to 120 mL of anticoagulated blood for use in human cell therapy applications (303). Characteristic of the HemaTrate® Blood Filtration System is the gravity filtration separation technology that separates cell populations across the membrane potential. This is useful for the concentration of total autologous nucleated cells from low volumes of peripheral blood (20 to 120 mL). The system is user-friendly, non-operator-dependent, single-use and requires neither dedicated instrumentation nor centrifugation. PB-MNCs are produced in approximately 10 minutes in three simple steps: load, filter and recover (Figure 58, original IFU). Filtration takes place in 8–12 min, and PB-MNCs remain trapped in the filter. After a backwashing of the filter with 10 ml of physiological saline, which allows for the collection of the cells in an empty syringe, the cells are ready to be implanted (Figure 59). Cells are not further manipulated, stored or frozen. Implantation takes place immediately after filtration in a single surgical procedure. PB-MNC are resuspended in saline solution, and the product does not contain any plasma, serum or a physiological concentration of platelets. The cell concentrate produced with this system has been extensively characterised by Spaltro et al. (303). Total nuclear cells are concentrated 2.9-fold (16.24+/-3.97 X103 /ul) with an average implanted dose of 2.08 +/-0.53 X108, while MNCs (monocytes and lymphocytes) are concentrated 4.2-fold (8.3+/-2.31 X103 /ul) with an average implanted dose of 1.06 +/-0.28 X108). The cellular concentrate contains neither plasma nor serum, and it does not concentrate platelets (from 226,000 platelets/µl in peripheral blood to 292,000/µl in the cellular concentrate). Finally, it cannot be traced in any way to platelet-rich plasma (PRP). Moreover, the system concentrates CD34+ stem cells, which are enriched by 5.6% ± 4.2%, compared to peripheral blood with an average implanted CD34+ cell count of 1.37 × 106, which corresponds to 0.7%–1% of total implanted cells. Importantly, the CD34+ haematopoietic stem cell enrichment efficiency of this selective filtration system is comparable with the CD34+ concentration obtained from the use of the point-of-care device for bone marrow cells (BMAC 2) (304). PB-MNCs isolated by this filtration system have also been shown to secrete a panel of angiogenic factors and are able to migrate in response to a gradient of VEGF and stromal-derived factor 1, SDF-1 (303).
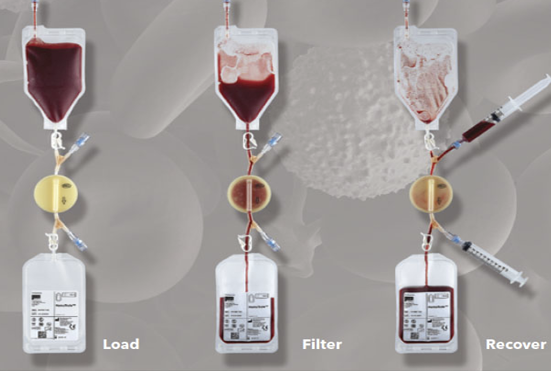
Figure 57: HemaTrate® blood filtration system.
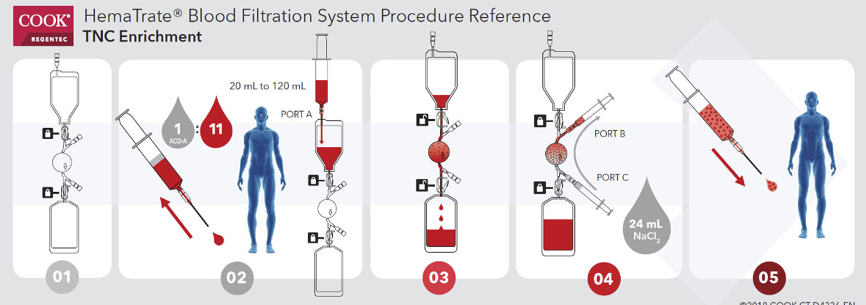
Figure 58: HemaTrate® blood filtration system procedure- 1, 2 Load anticoagulated peripheral blood; 3 Filter; 4 recover P-BMNC by backwash with saline; 5 PB-MNC ready to be implanted.
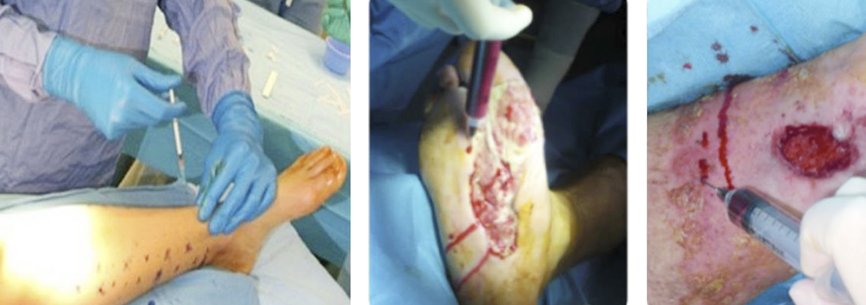
Figure 59: PB-MNC implants.
Interestingly, filtration preserves and optimises the release of paracrine factors, which is significantly reduced when the cell concentrate is produced by centrifugation (303). In addition, after injection into a mouse model of hind limb ischaemia, PB-MNCs produced by this system from healthy donors induce neo-vascularisation by increasing the number of capillaries, arterioles and regenerative fibres (303). It is thereby suggested that this filtration system represents a new, effective and reliable point-of-care device for obtaining an autologous cell product from peripheral blood with adequate potency for therapeutic angiogenesis in no-option CLTI patients.
7.6 Conclusions
In CTLI patients, the reestablishment of a direct pulsatile flow to the foot prevents the loss of the limb and improves the healing of wounds. Multidisciplinary approaches are recommended; however, open surgery with a saphenous conduit, whenever feasible, is the most durable option.
Endovascular tools have improved the feasibility of vascular repair, especially in frail patients. They are associated with a lower mortality and shorter length of hospital stay. They offer the same limb salvage rate and wound healing at the cost of more reinterventions. It is worth noting that the more proximal the lesions (aorto-iliac, versus femoropopliteal, versus foot and tibial arteries), the better the results.
In no-option patients, cellular therapies can represent a valid therapeutic choice, if patients are carefully selected and adequately managed in a multidisciplinary setting with a dedicated programme.
Table 15: Endovascular devices and studies
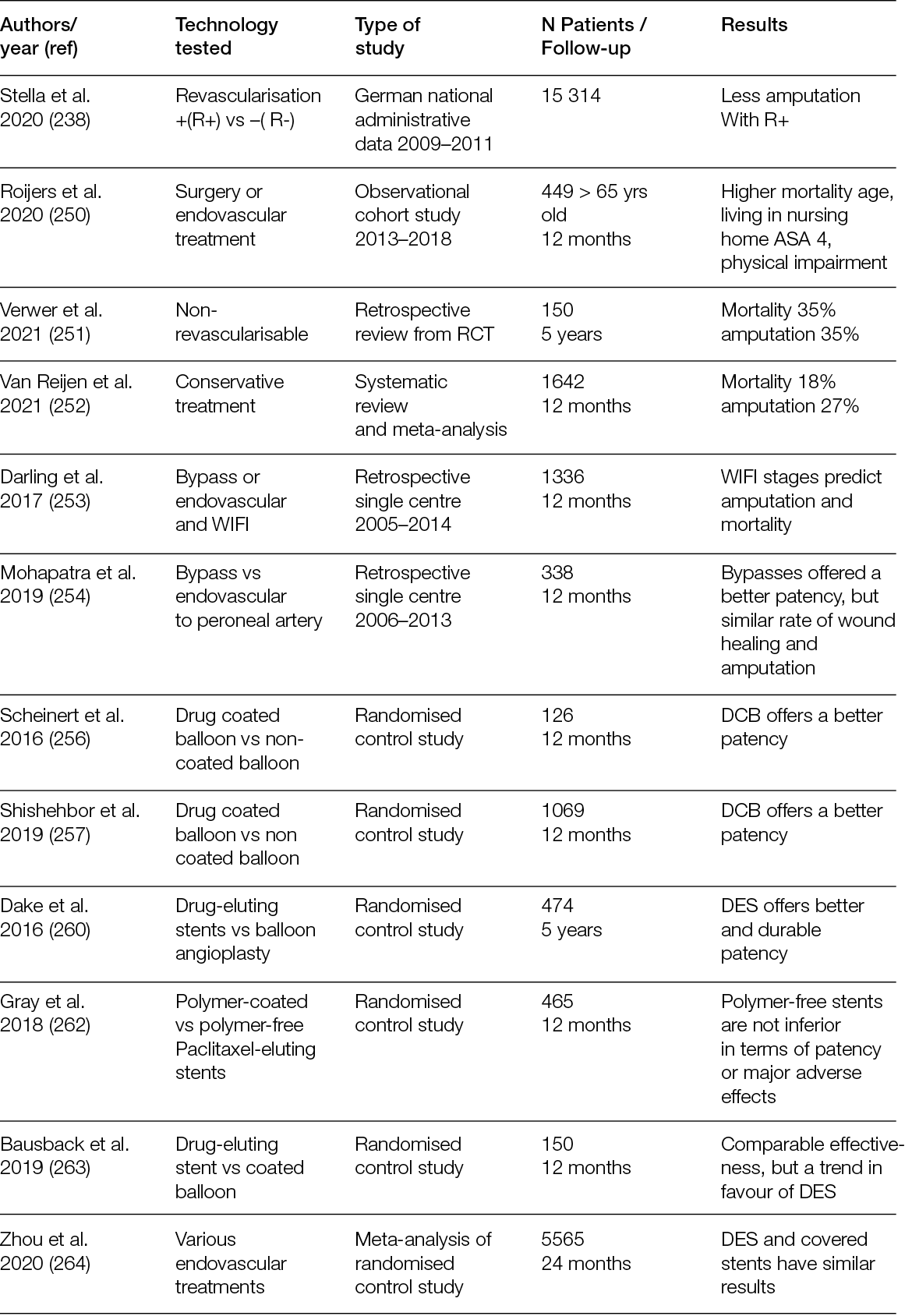
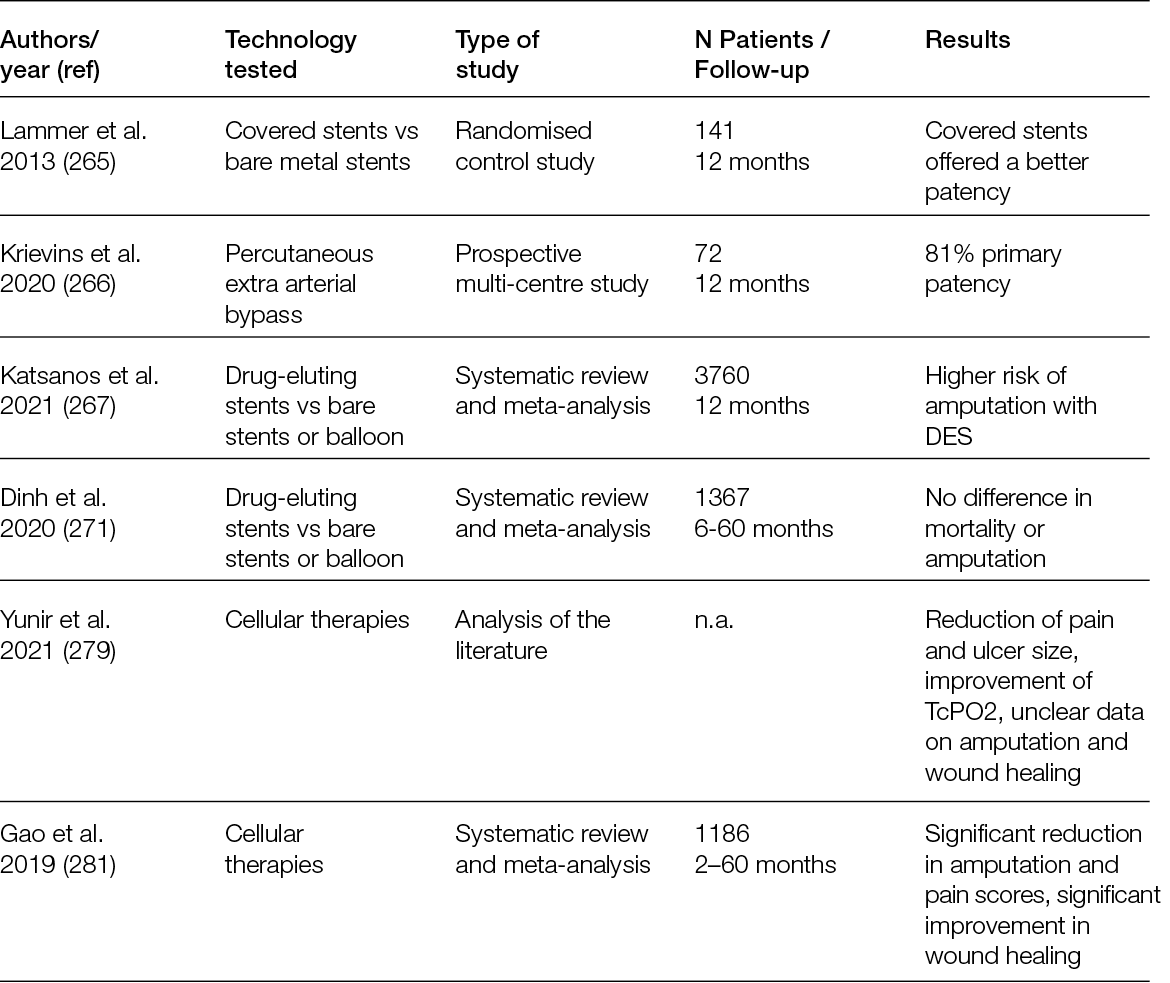
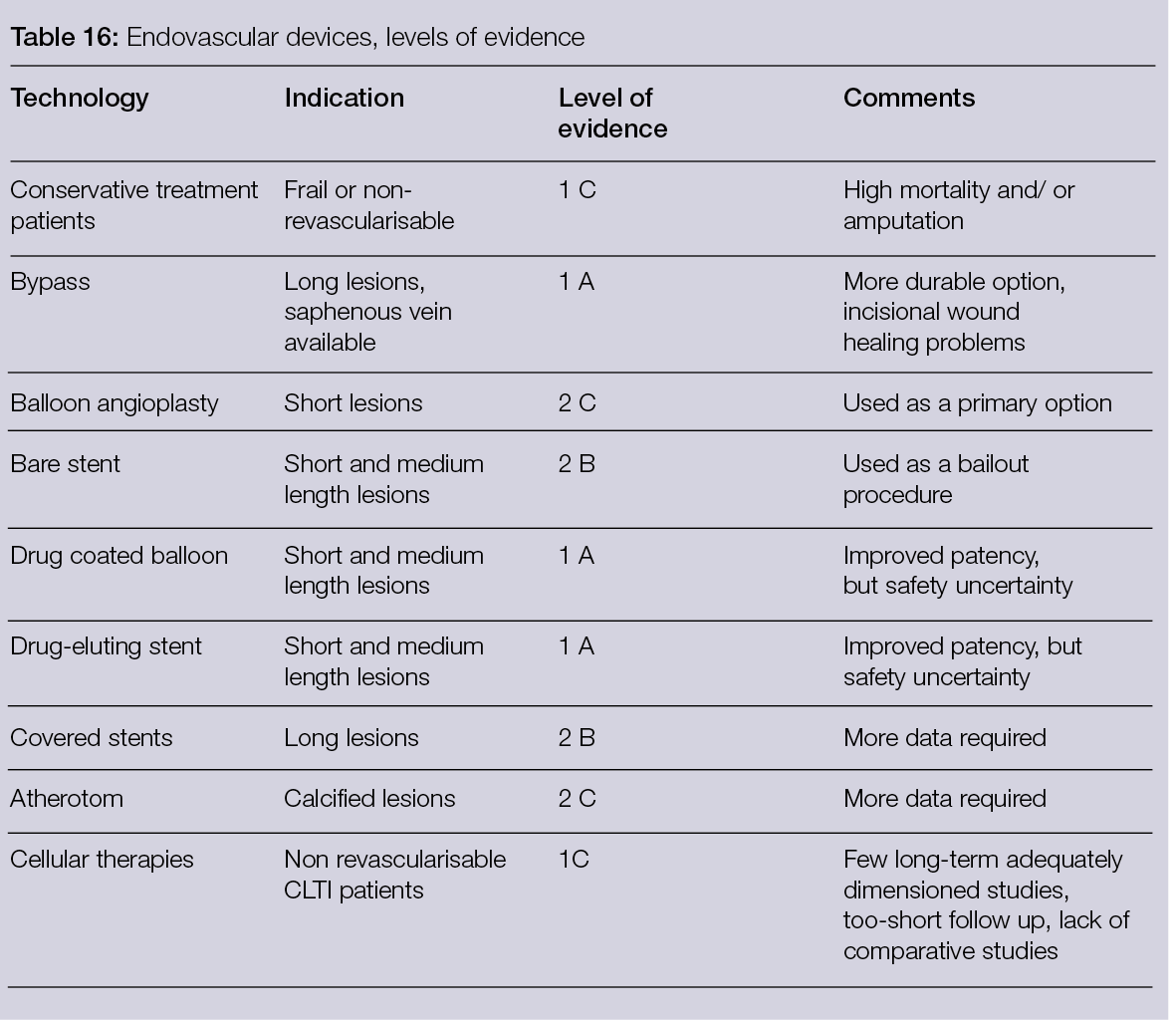
8. The economic perspective
8.1 Introduction
Chronic wounds are generally recognised as ‘wounds that have not proceeded through an orderly and timely reparation to produce anatomic and functional integrity after an amount of time that normally should be sufficient for healing’. There is no established consensus on the time horizon within the wound that would be considered ‘chronic’ (305, 306). Patients affected by chronic wounds often have a simultaneous presence of non-communicable diseases, such as diabetes and obesity, and they are mostly older. Since the non-communicable disease prevalence is increasing and the population is growing older, more people are at risk of developing chronic wounds, and healthcare resources are increasingly required to treat these conditions (305).
In Europe, 2–4% of all healthcare expenditures are devoted to wound care (307). In the U.S, a retrospective analysis of Medicare users showed that about 8.2 million people suffered from at least one type of wound or related infection in 2014 (308), and it demonstrated that the cost of illness is higher than expected. A value ranging from $28.1 billion to $96.8 billion was estimated to be spent every year for acute and chronic wound care in this analysis. Surgical wounds have the most significant impact on estimated expenditures, followed by DFUs, with double costs associated with outpatient care compared to inpatient (308). A recent and comprehensive systematic review on chronic ulcers’ cost of illness (309) confirmed the highest mean cost of DFUs ($44,200 per year) from a healthcare perspective, followed by pressure ulcers ($15,400) and leg ulcers ($11,000). The cost of chronic ulcers ranged from $1,000 out of pocket per year to $35,000 per episode from the healthcare public payer perspective (309). The main drivers of costs that affect the high burden of wound care are extensively described and discussed in the economics chapter of the previous report by Piaggesi et al. (1), where hospital costs were identified as the main cost component (309); followed by nursing time, for dressing changes in the hospital or at home; and finally materials. Alongside the economic burden associated with direct medical costs (attributable to patient care, such as the cost of medical visits, nursing services, surgeries, diagnostics, treatments and hospitalisation) and non-medical costs (expenditures not directly involved in medical services, such as transportation costs and paid caregiver time), wounds have a significant humanistic burden, as assessed based on the patient’s quality of life after developing the disease. A recently published review on the burden of chronic wounds highlighted the low health-related quality of life in patients (especially pain and limited mobility) with chronic wounds and their higher costs, mainly due to diabetes- related amputations (307, 310). This indicates the importance of preventing the progression of mild forms of disease into more severe wounds, both in terms of the economic and humanistic burdens. Beyond direct and quality of life-related costs, another cost category is the indirect costs related to the loss of productivity of patients and caregivers. Very few studies to date have addressed this aspect (310).
Since the demand for technologies to treat wounds will rise in the coming years, and their economic burden is substantial, policymakers and healthcare experts must be aware of the costs of new technologies available for the treatment of these patients.
In the future, one important issue will be represented by the economic impact related to pressure ulcers, due to the prolonged hospitalisation in intensive care units following COVID-19 infection. Pressure ulcers can prolong hospitalisation and increase healthcare costs. According a 2022 study by Ziede et al., the total cost for the treatment of the pressure ulcers can reach 561 USD per day (311).
Despite the growing number of technological resources that have entered or approached the market and clinical practice in recent years, the literature review on economic studies concerning the technologies used in wound management produced a limited number of results. The 14 papers selected and included in this section were divided in two categories: ulcers and trauma. In the ulcers (chronic wound) category, eight papers discuss the economic aspects of wound care in patients with DFUs (two papers), VLUs (three papers) and PUs (three papers). Beyond chronic wounds, we also included in this review technologies adopted to treat wounds that were generated during a trauma or injury. In this category, four articles discussed wound care management after different types of traumas, and two discussed burns. We have organised this chapter following these criteria, rather than based on technology, due to the heterogeneity of the studies in terms of their objectives, methods and technologies.
8.2 Diabetic foot ulcers
Two studies discussed the economic dimension concerning the treatments for patients affected by DFUs. Zelen et al. (312) compared three approaches: bioengineered skin substitutes (BSS), dHACM allograft and standard wound therapy, whereas Carter (313) compared dehydrated human amnion and chorion allograft (dHACA) plus SoC versus SoC alone.
The aim of the study performed by Zelen et al. was to compare time-to-heal among three groups, rates of complete healing and costs of advanced wound therapies. The study was realised in four outpatient wound care centres and included patients with Type 1 and Type 2 diabetes and lower extremity ulcers. Patients were randomised to one of the three groups: BSS, dHACM allograft and standard wound therapy (1:1:1). Patients were seen at least once every day for up to 12 weeks, or until one week after complete healing. In all, one hundred patients were enrolled. The median cost of graft material was 83% lower in the dHACM group, compared to the BSS group, while the median graft cost was $8,918 in the BSS group, versus $1,517 in the dHACM group. In the dHACM group, wounds healed faster, respective to the other groups, and presented the highest rates of complete healing.
A study by Carter in 2020 estimated the cost-utility of dHACA plus SoC versus SoC alone in patients with DFUs. Data came from a trial aimed at comparing the intervention group (weekly application of dHACA for up to 12 weeks + SoC, Group 1) and SoC alone (Group 2); 40 patients were included in each group. A Markov model simulated the health states with a one-year time horizon. At four weeks, 38.7% of the ulcers had healed in Group 1, versus 13.9% in Group 2. At 12 weeks, 74.6% and 36.0% of the ulcers were healed, respectively, in the groups receiving dHACA + SoC versus SoC alone. The rate of amputations was higher in Group 2 compared to Group 1 (15% versus 5.4%) at the end of one year. After one year, the Incremental cost-effectiveness ratio (ICER) was $-4,373 per QALY, with Group 1 dominant over Group 2. According to a willingness to pay (WTP) analysis, 70% of interventions were cost-effective for Group 1, compared to 30% in Group 2. dHACA added to SoC was cost-effective, compared to SoC alone.
8.3 Venous leg ulcers
Three RCTs containing an economic evaluation or considering patient-reported outcomes were conducted among patients affected by VLUs. These studies compared three techniques: electroceutical device (EAE) with SoC (314), an autologous skin suspension with SoC (315) and two different full skin substitutes available in the US (Apligraf® and Theraskin®) (316).
In 2018, Guest at al. conducted a cost-effectiveness analysis of EAE that found it to be less expensive than SoC by 24 weeks, with no significant difference in patient-reported outcomes (pain and health related quality of life) and healthcare resources used. These were calculated based on number of dressings per patient, debridements performed, visits/tests and prescribed drugs. The incremental cost per QALY gained was £4,480 at 8 weeks, £2,265 at 16 weeks and £-2,388 at 24 weeks.
In 2018, Towler et al. (316) performed an analysis of costs (31 subjects) based only on the cost of materials, since further costs were equal between the two groups. They found that the human, living, split-thickness allograft (Theraskin®, $2,495.33/subject) was cheaper than the living, bi-layered skin substitute (Apligraf®, $4,316.67/subject) in the US.
An RCT on RECELL (315) was conducted in six centres in England and one in France (52 patients). It found that the application of autologous skin cell suspension, produced by the RECELL Autologous Cell Harvesting Device, accelerated healing, decreased pain and increased health-related quality of life, compared with standard compression. Disease-specific quality of life was measured at each visit through a questionnaire exploring cosmesis, domestic activity, social interaction and emotional status. Ulcer pain was assessed via a 10-point scale. The detailed results of these studies are provided in Table 17 below.
Table 17: Synthesis of the studies: Ulcers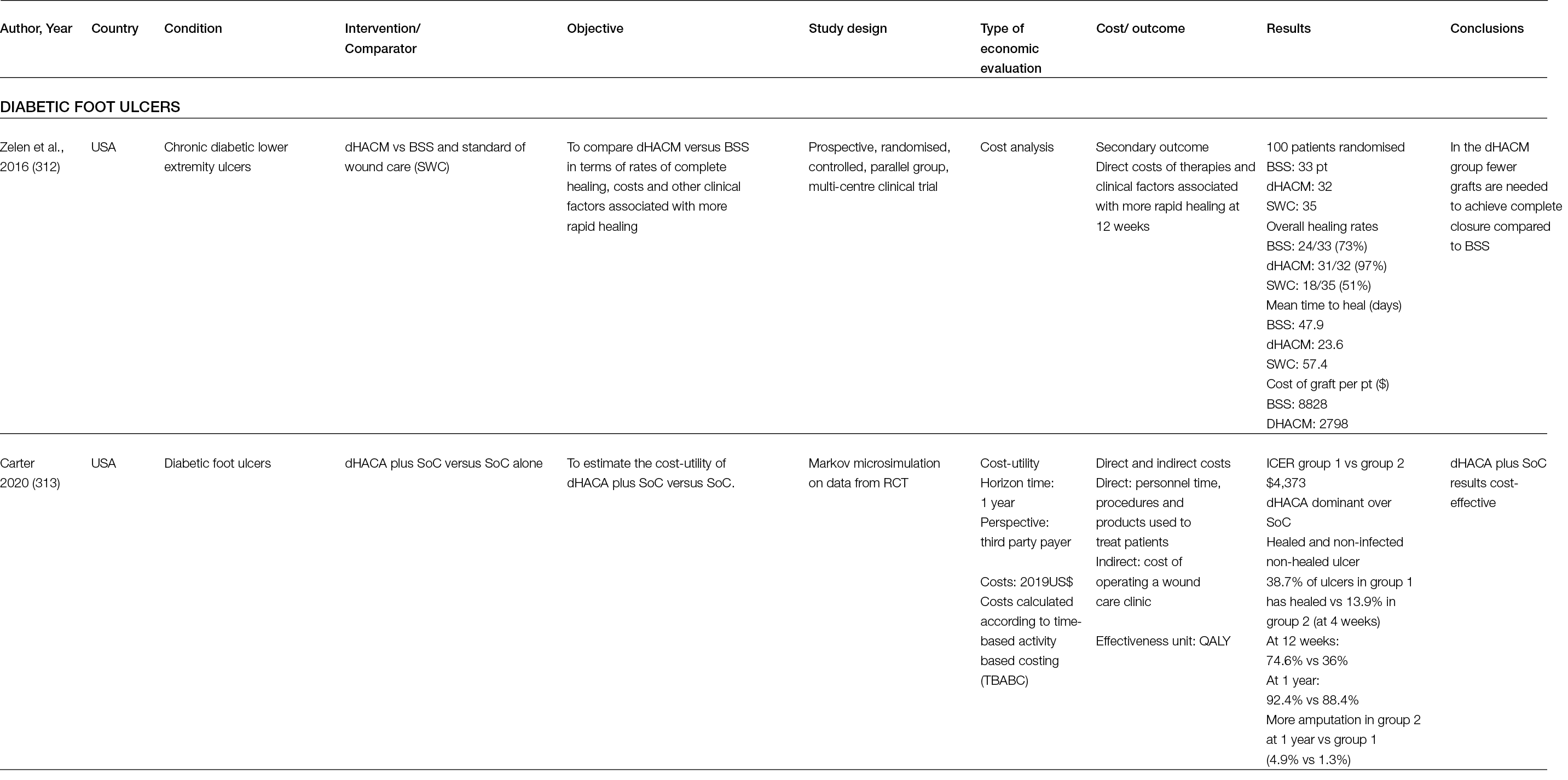
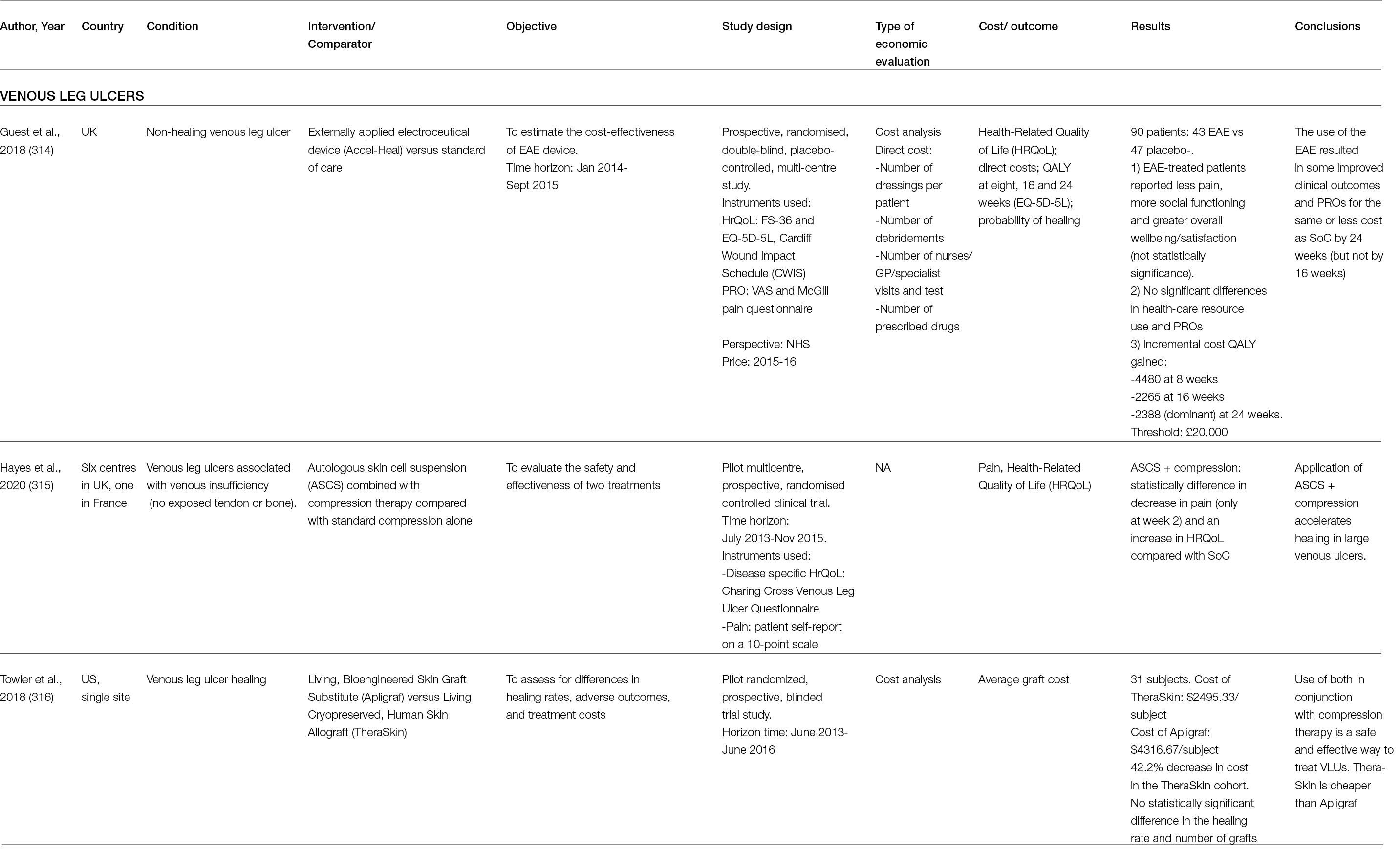
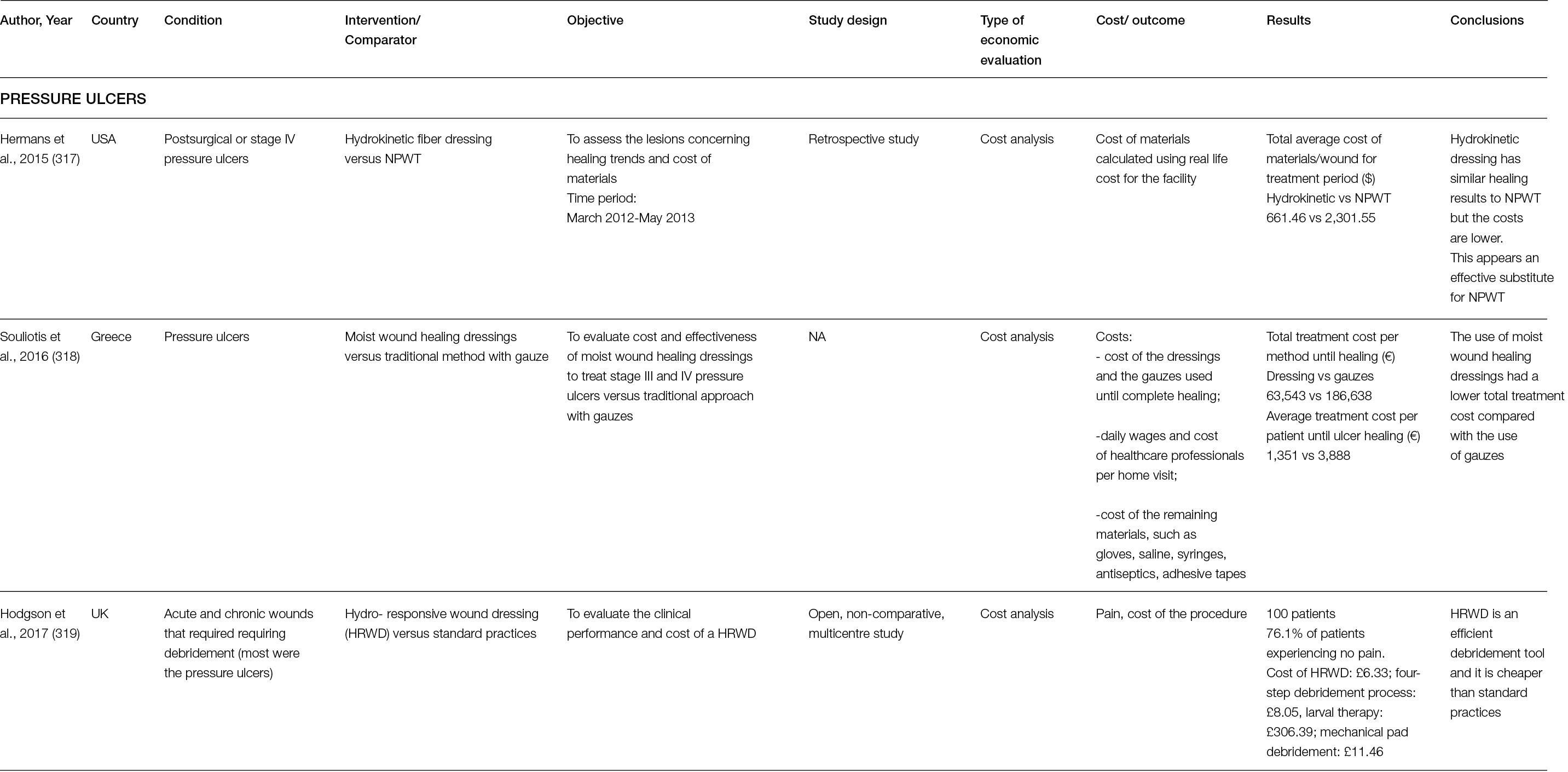
8.4 Pressure ulcers
Three studies were focused on pressure ulcers: Hermans et al. compared hydrokinetic fibre dressing versus negative pressure wound therapy (317), Souliotis et al. compared plain gauze with a moist wound healing dressing (318) and Hodgson et al. assessed the cost of the hydro-responsive wound dressing without comparison (319).
The retrospective observational study by Hermans et al., from 2015, compared hydrokinetic fibre dressing with NPWT to treat wounds in patients with serious morbidity. The fibre dressing was used to manage 23 patients with 26 lesions, and NPWT was used to treat 15 patients with 16 lesions. The pain level, measured using the visual analog scale (VAS) (0= no pain/10= excruciating pain) at the start of the study, was 3.7 in the test dressing group and 0.7 in the NPWT group. At the end of the study, it was 0.6 for the test dressing group and 0.2 for NPWT. A cost analysis was performed considering two subsets: the first subset included all lesions in both groups treated with noncontact low frequency ultrasound adjunct (NLFU), and the second subset addressed all lesions in both groups without NLFU. The total average cost of materials/wound amounted to $661.46 in hydrokinetic fibre dressing (26 patients) and $2,301.55 in NPWT (16 patients). In Subset 1, the total cost of materials/wound amounted to $132.38 in hydrokinetic fibre dressing (5 patients) and $2,374.6 in NPWT (14 patients); in Subset 2, the total costs amounted to $799.62 in Group 1 (21 patients) and $1,504.62 in Group 2 (2 patients). It emerged from the study that all lesions in both groups showed progress in terms of healing, whereas the cost of materials was lower in the hydrokinetic fibre dressing treatment.
The study by Souliotis et al., from 2018, aimed to analyse the cost and clinical effectiveness of two treatments for managing the homecare setting of patients with stage III or IV pressure ulcers; 100 patients were included, with 50 in each group. One patient in the plain gauze group and two in the moist wound healing dressings group withdrew from the study. In addition, one patient in each group died during the study. All patients had full-thickness pressure ulcers in stage III or IV. There was no significant difference between the groups, in terms of initial ulcer surface size. Eight patients in the moist wound healing dressing group (n = 47) presented 12 cases of local infection in the ulcer; in the pain gauze group (n = 48), 14 patients manifested 21 cases of local infection. The average time to complete healing was lower with moist healing dressings (p=0.0001). The mean cost per patient until ulcer healing was lower in the dressing group, compared to the gauze group, at €1,351 versus €3,888, respectively.
Hodgson et al. evaluated the effectiveness and cost of the hydro-responsive wound dressing (HRWD) in the debridement and wound bed preparation of a variety of wounds, a majority of which were pressure ulcers. Among 100 patients in the Glasgow area (UK), the wound area was reduced by 40%, and dressing-related pain (measured by the VAS scale) was reduced in 50% of the patients (their pain level was the highest at the beginning of the study), showing that HRWD is a good technique for the management of these wounds. A cost comparison of methods adopted before the use of HRWD for patients requiring debridement was conducted and showed that HRWD is a cost-saving technique. Its application cost was £6.33, which was less expensive than the four-step standard process (£8.05), larval therapy (£306.39) and debridement pad (£11.46). However, the study had several limitations: it did not compare different methods and did not include a control arm or randomisation. Moreover, the authors did not take into consideration the frequency of dressing changes.
8.5 Trauma
Four economic evaluations on wound treatment due to trauma were considered in this section. In 2013, Guest et al. performed a cost-effectiveness analysis of Polyheal® compared to surgery (320). In 2015, Kempton et al. compared two approaches (negative pressure dressings (NPDs) and conventional compressive dressings (CDs) (321). In 2020, Jiang et al. compared post-traumatic osteomyelitis versus non-post-traumatic OM (322), and Png et al. compared standard dressing with incisional negative pressure wound therapy (iNPWT) (323).
Concerning patients affected by chronic wounds with exposed bones and/or tendons, Guest et al. assessed the cost-effectiveness of Polyheal® compared with surgery in three European countries. They developed a decision model based on data concerning: 1) clinical outcomes of surgery (from a systematic review); 2) healing rate with Polyheal® (from three previous studies); and 3) healthcare resources, assessed from interviews conducted by the authors with clinicians from France, Germany and the UK. In terms of resource use and costs calculated over one year, Polyheal® led to a total healthcare cost of €7,984, €7,517 and €8,860 per patient, while surgery led to €12,300, €18,137 and €11,330 per patient in France, Germany and the UK, respectively. The main cost drivers were nurse visits in France (36% of costs) and the UK (42% of costs), while surgery and hospitalisation were the primary cost drivers in Germany (50%) in the Polyheal® group; 18–22% of the costs were attributed to the cost of Polyheal®. The main cost driver in the surgery group accounted for surgical procedures and hospitalisation: 72% of the total cost in France, 67% in Germany and 69% in the UK.
The cost-effectiveness assessment showed a decrease in healthcare costs when using Polyheal®, compared to surgery, and a 5% improvement in the probability of healing.
In 2015, Kempton et al. performed a retrospective study to determine the cost difference between NPDs and CDs for the treatment of traumatic wounds (treated with split-thickness skin grafts). Their clinical study did not show an improvement in clinical outcomes associated with NPD, compared with conventional therapy. Regarding costs, the mean cost of NPD ($4,959.22) was higher than the conventional dressing cost ($2,654.17). These costs considered the length of postoperative hospital stay, duration of NPD, cost of admission and occurrence of early readmission to the hospital before the first dressing change. Data on postoperative care, such as dressing supplies and follow-up visits, were not calculated. The mean cost of NPD was $2,302.05 per patient higher than CDs. These findings suggested that clinical outcomes and costs did not justify the use of NPDs, but several relevant families of cost were not included in the analysis.
The study by Jiang et al., from 2020, aimed to analyse the direct healthcare costs for inpatients with extremity post-traumatic osteomyelitis, in comparison with non-post traumatic OM patients. The retrospective observational study was realised in a tertiary medical centre in Southern China. The survey included data related to 278 post-traumatic OM inpatients (228 males and 50 females). Total costs for overall post-traumatic patients amounted to $3,524,668. The main cost drivers were materials (61% of the total), pharmaceuticals (12%) and treatment (11%). Inpatients using an external fixator had a significantly higher number of hospital admissions, longer lengths of stay and higher costs, compared to inpatients without a fixator. Healthcare costs were influenced by the use of an external fixator, the type of fixator and the infection site.
The cost-utility analysis performed by Png et al. in 2020 (323) compared standard dressing with incisional iNPWT in patients with closed surgical wounds associated with major trauma to the lower limbs. Data for economic evaluation came from a multi-centre randomised clinical trial comparing standard dressing (759 patients) and iNPWT (781 patients). The mean costs of initial intervention, including the cost of the dressings, hospitalisation, antibiotics and dressing changes, showed that iNPWT was more costly compared to SoC (+£647). In terms of EQ-5D VAS and mean QALYs, no statistical differences between the two groups were observed. In the base case, ICER was £396,531 per QALY, as gained from NHS and personal social services perspective. The probability of iNPWT being cost-effective was lower compared to standard dressing. This is due to higher costs related to iNPWT per patient and the lack of difference in QALYs between the two groups.
8.6 Burns
Two cohort studies were conducted on patients with burns to evaluate the costs of 1) two temporary wound coverage (biosynthetic temporary skin substitute, Biobrane™, versus cadaveric allograft, Allograft®) (324), and 2) various nano-crystalline silver formulations (325).
The retrospective 5-year study by Austin et al. (324) conducted in a regional burn unit on 45 patients with upper extremity burns compared the cost of materials and the time consumption for the procedure of two wound coverages, Biobrane™ and Allograft®. Results showed that Biobrane™ is less expensive ($1.30 for Biobrane™ versus $2.35 for Allograft®, per minute per % time for burn surgical Allograft p=0.002). The time consumption related to application is lower compared to Allograft® (21.12 minutes for Biobrane™, versus 54.78 minutes for Allograft, per %TBSA, p=0.02).
In 2019, Erring et al. (325) evaluated the efficacy, tolerance, safety and cost effectiveness of silver nanoparticle gel (SG), nanosilver foam (SF) and collagen in a retrospective single-centre study in India. Twenty patients were enrolled in this prospective study that showed a higher re-epithelisation in the SF groups (on Days 10 and 14), as well as less time taken for changing the dressing and quicker reduction of pain by the fifth day and after two weeks, compared with other treatments. Pain rates were evaluated using the VAS scale. Although SF may be more efficacious and tolerated, the cost of dressing per %TBSA was comparable (p=0.09).
8.7 Conclusions
This section on the economic aspects related to advanced wound management techniques/products showed that economic evaluations are very scarce within this field. Most of the studies compared technologies included in the previous publication by Piaggesi et al., from 2018 (1). Our analysis shows that the studies employed varied approaches, such as cost utility and cost effectiveness analyses. The main data sources were clinical trials, administrative data and data from questionnaires, and most of the patient cohorts originated from the hospital/healthcare setting. It was not possible to perform a comparison among the studies, as they were heterogeneous in terms of objective, technology, methods for cost calculation, study design, data collection methodology and included cost dimensions. The studies mainly considered the cost of materials and other direct medical costs, such as the cost of procedures, cost of hospitalisation and cost of healthcare professionals’ time. None considered direct non-medical costs. Very few studies included the indirect costs (cost of absenteeism) in their analysis. However, 5 out of 14 studies calculated the humanistic burden in terms of pain and HRQoL. Since wounds have an important humanistic burden, engaging patients in their care pathway could enhance patients’ healing progress and outcomes (305).
Further economic studies related to wound treatment are needed to inform clinicians, policy makers and healthcare experts on the sustainability of these new techniques and technologies (326).
Table 18: Synthesis of studies: Trauma
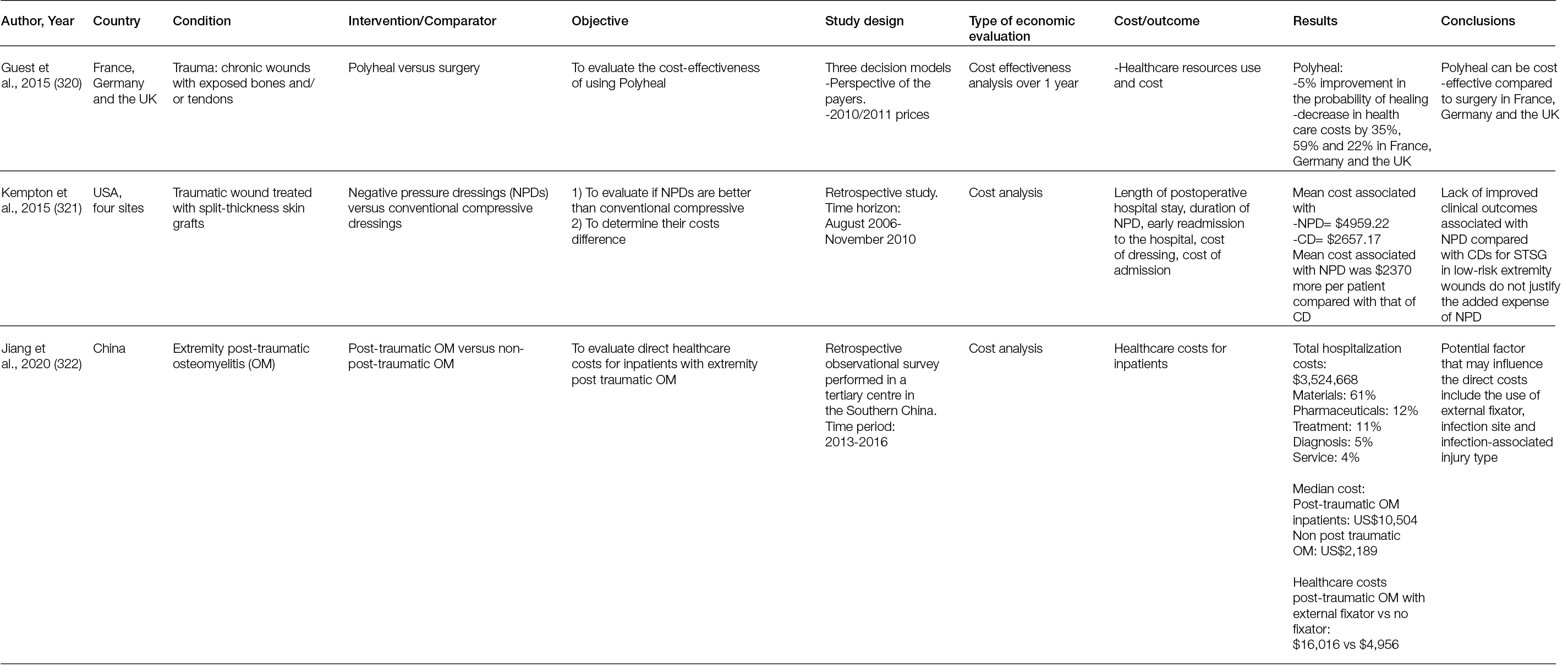
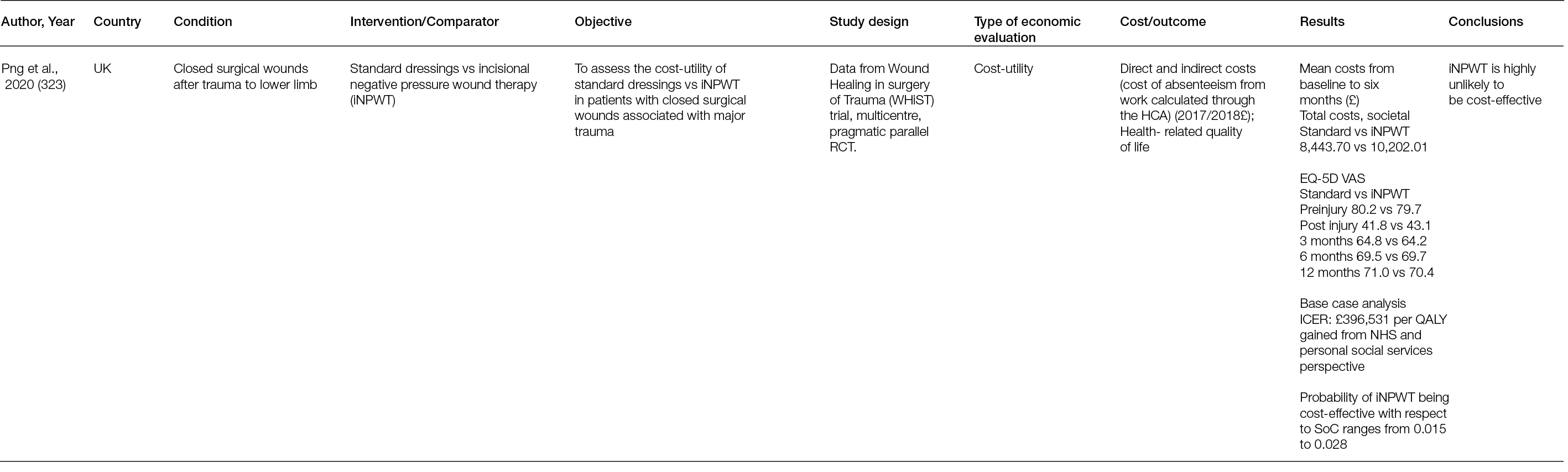
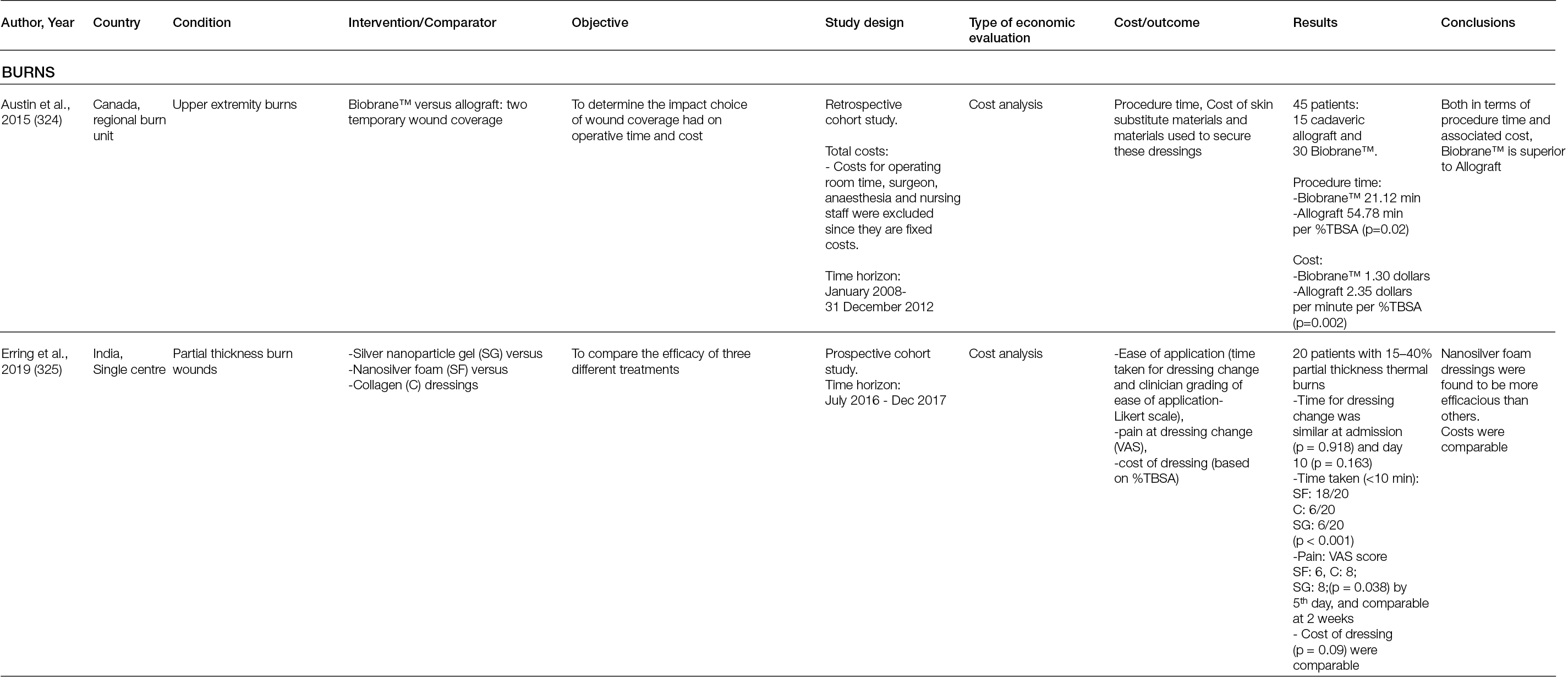
9. Regenerative medicine and regulatory product approval: Do patients really have access to optimal care?
9.1 Introduction
For several decades, the term ‘translational medicine’ has been used, and not seldom misused, to define different things. In 2010, Rubio et al. summarised translational research as ‘multidirectional and multidisciplinary integration of basic research, patient-oriented research, and population-based research, with the long-term aim of improving the health of the public’ (327). Some years later, in an open discussion documented by Wang, John Hutton quoted the Translational Research Working Group of the National Cancer Advisory Board, who defined it as ‘scientific discoveries arising from laboratory, clinical or population studies into new clinical tools and applications that improve human health by reducing disease incidence, morbidity and mortality’ (328).
Perhaps the most comprehensive definition of translational medicine is the one by the European Society for Translational Medicine from 2015, which defined it as the ‘interdisciplinary branch of the biomedical field supported by three main pillars: bench side, bedside and community’, and its goal as ‘to combine disciplines, resources, expertise, and techniques within these pillars to promote enhancements in prevention, diagnosis, and therapies’ (329). However, when contemplating the pathway of new, enhanced (wound healing) patient therapies, it is imperative to recognise a critical milestone on this journey from ‘bench to bedside’, which is not captured in any of these definitions: its review and subsequent approval by the appropriate regulatory authorities.
9.2 Relevant legaslative overview
Wound healing therapies available to healthcare professionals comprise a large variety of products that, due to their characteristics, composition and approaches, are governed by different, partly overlapping pieces of regulatory legislation. In sum, three groups of wound healing products can be identified (i.e., medical devices, combination products and advanced therapy medicinal products (ATMPs)).
The legislation for medical devices in the EU and European Free Trade Association countries has gone through considerable changes recently. Previously, this group of wound healing products was governed by the Medical Device Directive (MDD 92/42/EC). From May 2021 however, the Medical Devices Regulation (MDR) 2017/745 has come into force, replacing the previous MDD (330). Although a transition period for products approved under the MDD is still in effect, all products available from May 2024 on are expected to possess a CE certificate according to the MDR (331). Reviewing the differences between the two pieces of legislation, it is possible to recognise a stronger emphasis on clinical performance and benefits with the MDR. Furthermore, an increased focus on safety is apparent, as illustrated, for example, by the introduction of Post Market Clinical Follow up (PMCF), Periodic Safety Update Reports (PSUR) and specific risk management and assessment obligations for manufacturers, the added responsibilities for the newly defined group of ‘economic operators’, and the limited, five-year CE Marking certificate validity (332). The transition from MDD to MDR, and the connected recertification of products, has been welcomed by healthcare professionals for its increased focus on patient safety, but also triggered concerns regarding reduced product availability, caused by increased withdrawals of existing devices and a decrease in new medical device innovation and EU specific new device regulations (333).
Combination products are, as the name indicates, the combination of a medical device and ‘a substance which, if used separately, can be considered to be a medicinal product, as defined in point 2 of Article of Directive 2001/83/EC (332). Following the potential risk assessment principle of medical device classification, such combination products are always placed in the highest potential risk category (i.e., class III) (332). Critical for the classification of combination devices is the determination of which component performs its ‘primary’ and ‘secondary’ or ‘supportive’ action, since its primary action is decisive for its classification as, indeed, a medical device, or as a pharmaceutical product, governed by Directive 2001/83/EC. For example, a wound dressing containing an antimicrobial agent will be regarded as a medical device if its primary action is regarded to be wound coverage, protection and absorption. However, if its primary action is judged to be the delivery of antimicrobial agents for the treatment of wound infection, the device will be considered a medicinal product, and thus regulated accordingly. This deliberation becomes even more opaque when more novel therapeutic designs and approaches are implemented. An example of this is the totally artificial implantable heart, developed by French manufacturer Carmat, which significantly increases survival and mobility of patients with terminal heart failure awaiting donor availability. To prevent coagulative complications, the implant is supplemented with living bovine cells as a precursor for autologous cells to ‘coat’ the blood-contacting surfaces of the implant (334). Although the use of living cells is a determining regulatory factor, no doubt based on the primary and secondary action principle, this implant is classified in Europe as an active implantable medical device (CE0344) (335).
Some would argue that truly regenerative medicine products are human cell therapies with gene-based methods, biomaterials and molecular medicines aimed at promoting the regeneration of tissues or replacing failing or malfunctioning organs (1, 336). Regenerative medicine therapeutical products are, however, not classified as medical devices or combination products, but as Advanced Therapy Medicinal Products (ATMP) under the Advanced Therapy Medicinal Product Regulation (EC) No 1394/2007, which has been under revision since November 2020 (337, 338). An example of an ATMP would be autologous chondrocytes seeded onto a collagen membrane to repair cartilage. Since the autologous chondrocytes represent an integral part of the product, the whole product falls under the ATMP Regulation. An ATMP will, however, not be regarded a medicine, when the cells that compose it have the same essential function in the donor as in the recipient, and when the cells are not subject to any substantial manipulation. (336) These ‘non-medicine’ ATMPs are regulated in Europe by Directives 2004/23/EC, 2006/17/EC and 2006/86/EC. These describe the quality and safety standards for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells (339-341). Overall, ATMPs can be categorised in four distinct groups: gene therapy medicinal products (GTMP), somatic cell therapy medicinal products (sCTMP), tissue engineered products (TEP) and combined advanced therapy products (CATP) (342). An overview and short characterisation of these ATMP categories can be found in Table 19. Their authorisation and commercialisation are regulated by Directive 2001/83/EC and Regulation (EC) No. 726/2004, amended by EU Regulation No. 1235/2010. In practice, this means that ATMPs have to fulfil the same high regulatory standards as other pharmaceuticals, which are overseen by the EMA (343-345).
Table 19: Overview and concise description of ATMP categories (1, 386).
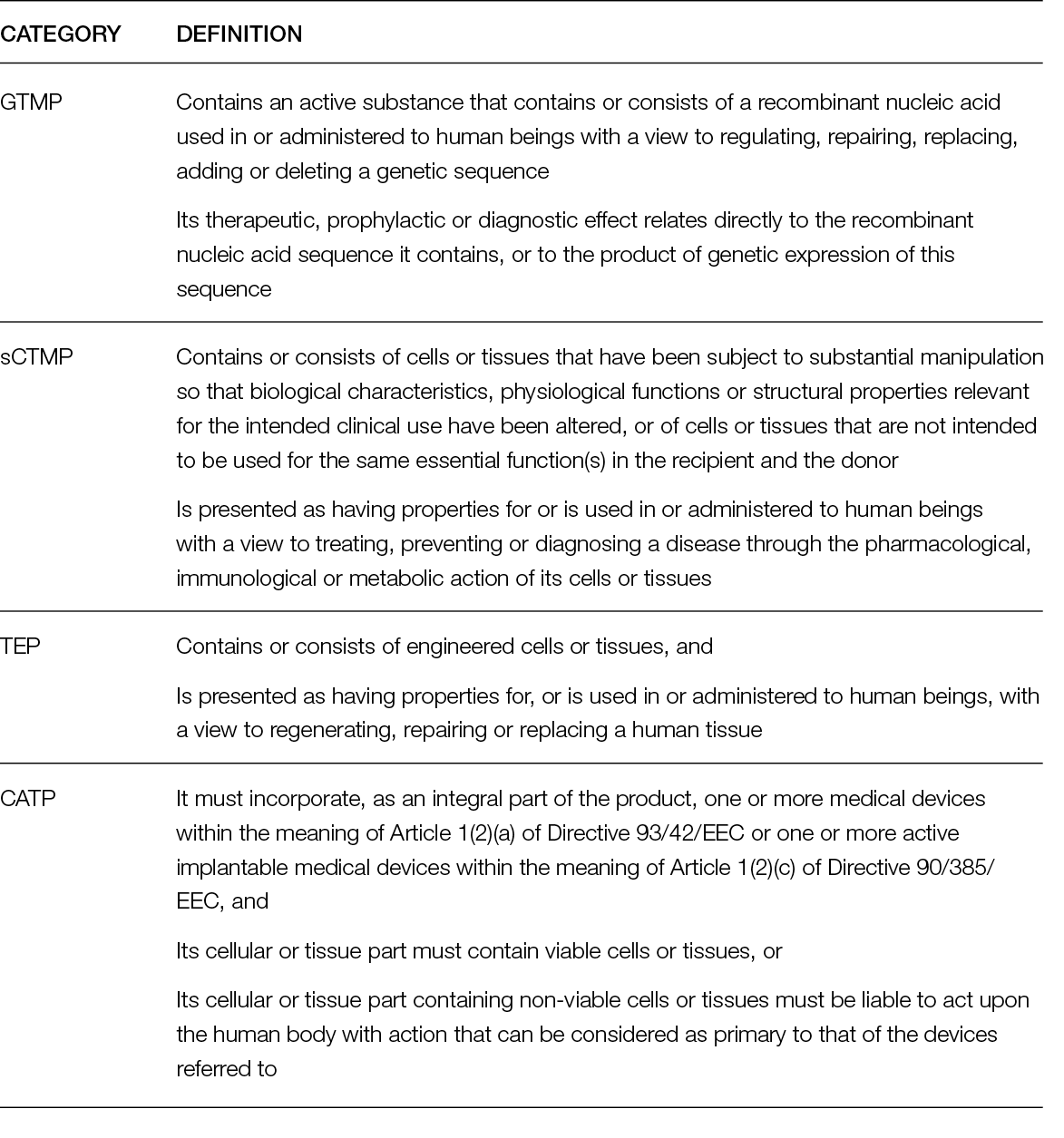
9.3 ATMPs available to European patients today
An overview of registered ATMPs in Europe can be found in Table 20. A review of the currently approved cell and gene therapies in this table shows that only a very limited number of therapies has become available to European patients since the Advanced Therapy Medicinal Product Regulation came into force in 2007.
Table 20: Approved ATMPs in Europe (as of October 2022) (347, 348, 387).
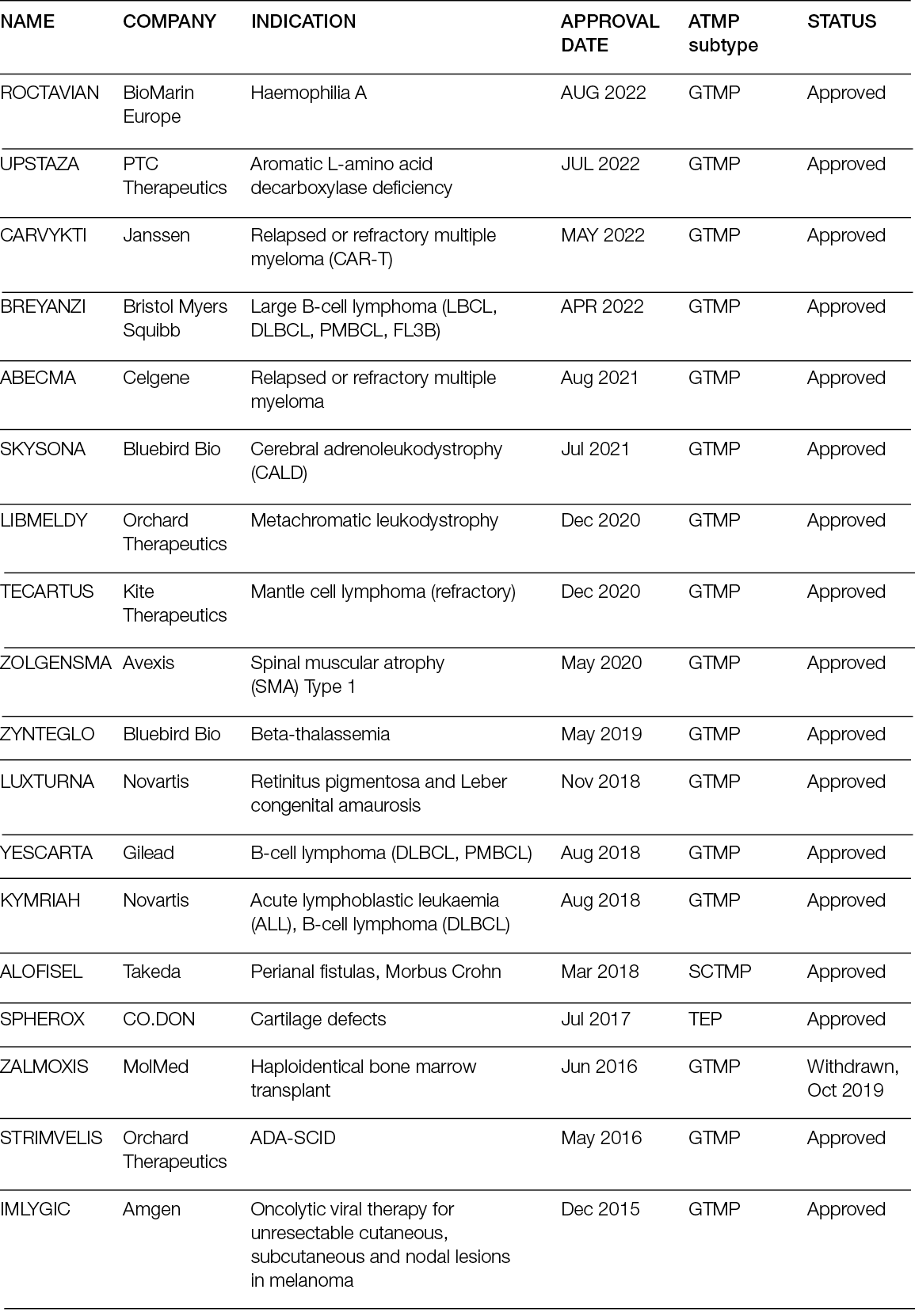
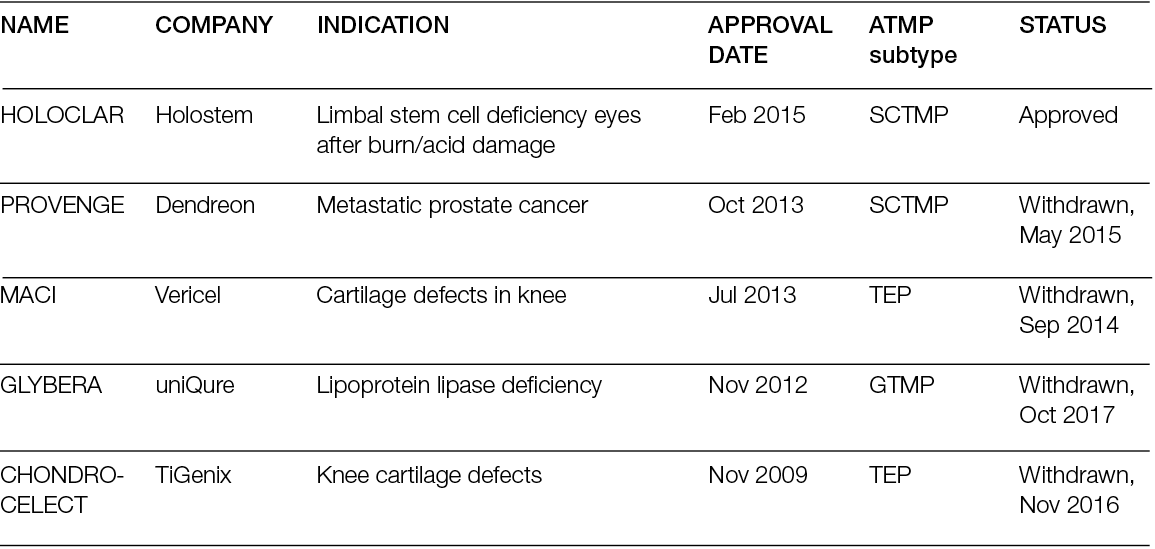
Focussing on therapies applicable specifically to (wound) healing indications in their broadest definition leads to an even more discouraging conclusion: most of the currently approved ATMPs are gene therapies, which would bring the total to a mere two cell and tissue-engineered products in 14 years. During this period, the same number of TEP therapies were withdrawn. This limited availability of ATMPs is multifactorial. First, since these high potential therapies are, indeed, on the cutting edge of medical science, their development pathways are long, investment-intensive and filled with uncertainties. For example, Professor Graziella Pellegrini, currently at the Centre for Regenerative Medicine Stefano Ferrari at the University of Modena and Reggio Emilia, mentioned that the development work of Holoclar® was started 25 years before its eventual regulatory approval in 2015 (346, 347). Second, the regulatory approval process of ATMPs is detailed and long; the approval of Chondrocelect® in 2009 was reported to have taken nine years, according to its developer, TiGenix. Third, products are withdrawn frequently after a certain period of time, either by the EMA, due to observed safety concerns in the real world, or by the manufacturer, who may discontinue the market approval due to disappointing commercial returns on investment. Of the five withdrawn products in Table 20, four were discontinued by the manufacturer due to commercial reasons and/or bankruptcy (348). The latter is not seldom instigated by the discrepancy between the manufacturer’s price expectation and the willingness of European healthcare insurers to reimburse (349).
Whether or not the limited access of European patients to these kinds of therapies is the cause for the emergence of ‘cell therapy tourism’ is unclear. No doubt triggered by the emergence of this phenomenon, the EMA’s Committee for Advanced Therapies (CAT) advised the public, including patients, in April 2020 to be beware of unproven, unregulated, cell-based therapies, following the appearance of advertisements across the EU for cell therapies as cures for serious conditions (Figure 60).

Figure 60: Info card advice for patients considering treatment with a non EMA CAT approved cell-based therapy (381). Reproduced with kind permission of EMA.
In its statement CAT writes: ‘EMA’s Committee for Advanced Therapies (CAT) is advising patients and the public against using unregulated cell-based therapies which may not be safe or effective. The CAT’s advice is in response to individuals, companies and hospitals promoting unproven cell-based therapies as cures for a broad range of conditions including cancer, cardiovascular diseases, autism, cerebral palsy, muscular dystrophy, and vision loss. These treatments can pose serious risks to patients for little or no benefit. Patients using unproven or unregulated cell-based therapies have reportedly suffered serious, sometimes fatal, side effects including infections, unwanted immune reactions, tumor formation, loss of vision and bleeding in the brain. [...] Healthcare providers should explain the benefits and risks of the cell- based therapies that they are providing to patients, as well as confirming that regulatory authorities have approved their use’ (350).
Although the CAT’s warning is certainly justified, it should also be acknowledged that many ATMPs exist that are not available in Europe, but are approved by regulatory authorities elsewhere (Table 21).
Table 21: Cell therapies available outside Europe (selection) (348, 388).
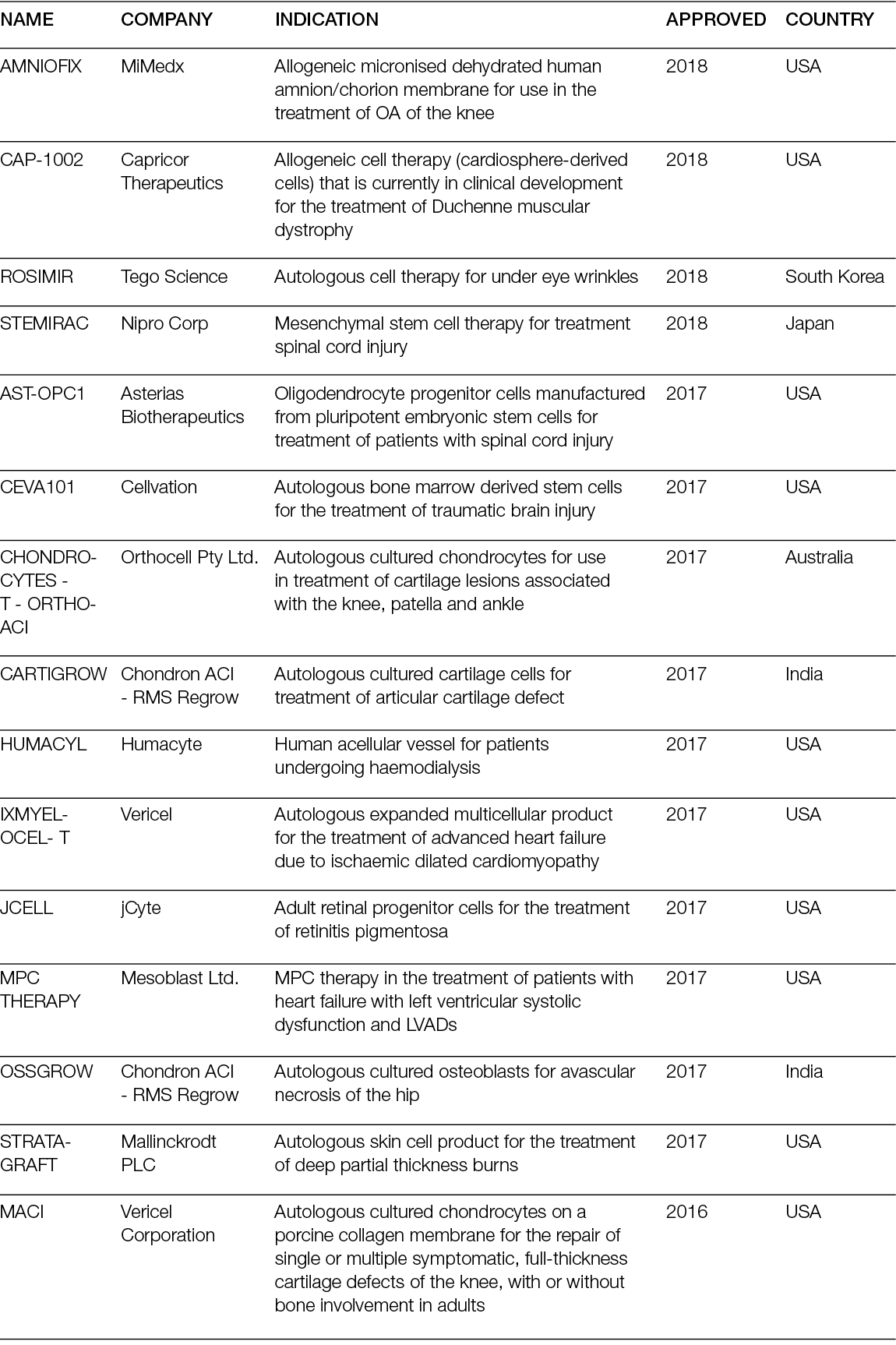
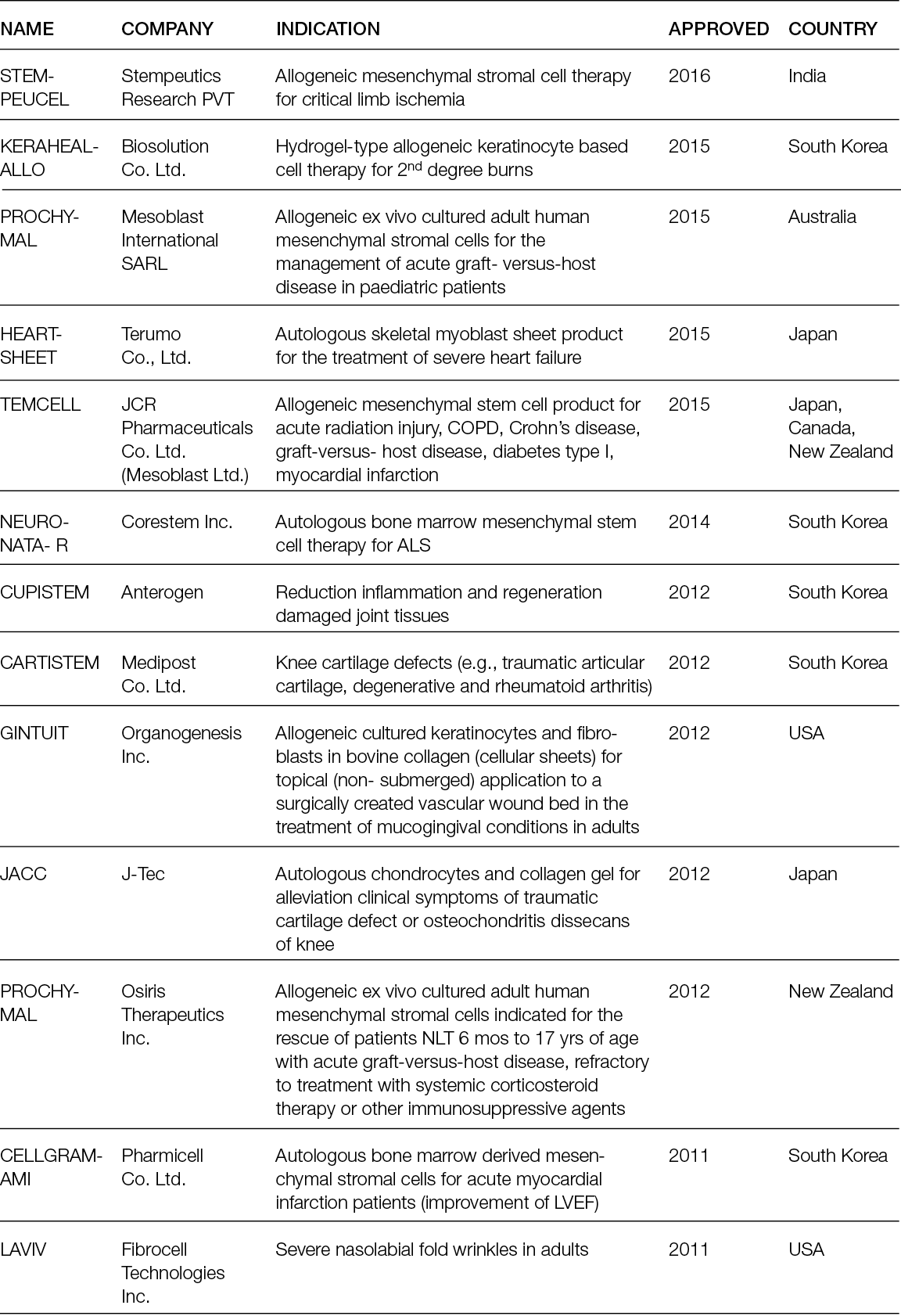
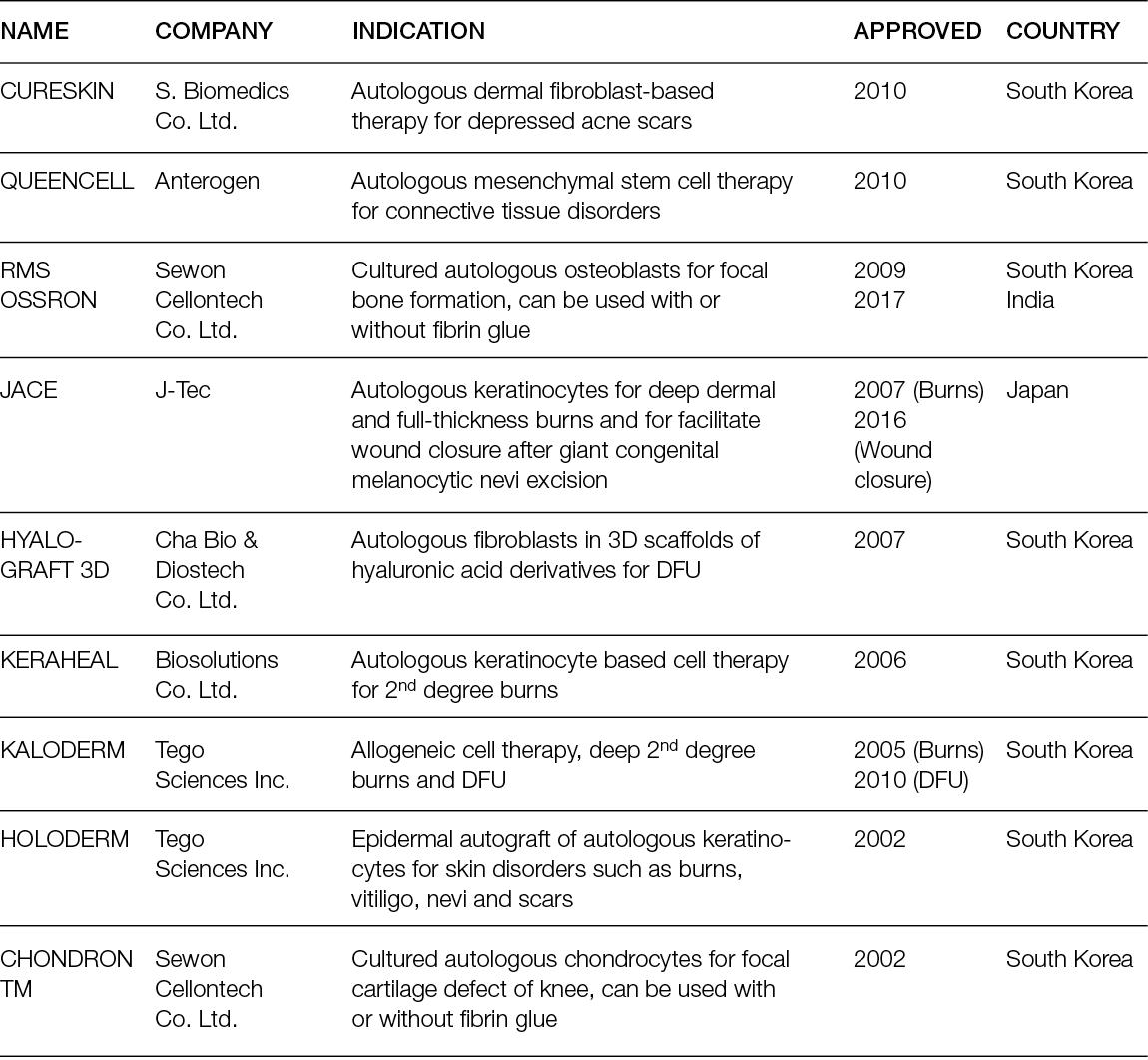
9.4 Different regulatory environments: Comparison of the EU and US
As outlined by Piaggesi et al. in their earlier overview of advanced therapies in wound management, many challenges during the non-clinical and clinical development of ATMPs should be considered. For example, an effective application of various regulatory tools can benefit enormously development times and the cost of bringing a product to market (1). Later, the excellent review by Detela and Lodge summarised the standard, accelerated and adaptive EU regulatory pathways for ATMPs to market authorisation, supplementing the EMA’s base documentation on ATMPs in a very detailed and effective manner (347). Nonetheless, as the Regulatory Affairs Professionals Society (RAPS) concluded:
‘The regulatory environment for ATMPs is evolving and advancing, often retrospectively to product development. Thus, it is essential to have strong, continuing regulatory intelligence efforts as well as frequent interaction with regulatory agency officials. In order to ride the crest of the wave rather than have it swamp development efforts, increasing education and gaining better understanding about the nature of ATMPs—as well as how they are regulated—is imperative. This effort includes staying tuned to current EMA, FDA and global regulatory development incentives and framework as ATMP evolution continues well into the 21st century (351).
Having said this, it is imperative to acknowledge that, as with any fast-developing, broad, versatile and cutting-edge research field, regenerative medicine encompasses more than one therapeutic approach or development. Hence, ATMPs represent just one facet of the broad palette of potential wound healing tools for healthcare professionals. This was underlined in 2020, for example, when Snyder et al. published an update of their 2012 Technical Brief on skin substitutes for treating chronic wounds (352, 353). Both versions of these studies are based on research conducted by the ECRI Institute-Penn Medicine Evidence-Based Practice Center, under contract to the US Agency for Healthcare Research and Quality (AHRQ), and describe skin substitute products commercially available in the United States. A determination of the European regulatory status of these skin substitute products (Table 22) was conducted in four phases, reflecting the common path of enquiry by practicing health care professionals (i.e., consultation of publicly available product information, by using the contact information form on the company’s web site (when available), by telephone through the company’s published contact number(s) and through direct telephone and email contact with known company representatives). Complete non-response to a total of 10 physician requests for regulatory product information over a 6-month period (25.8%) was interpreted as an indication for potential ‘off-label use’, and therefore classified, by default, as not regulatory-approved for use in Europe. Reviewing the data compiled by Snyder et al. from an European perspective, it becomes apparent that only 18% of these therapies, in some cases used and evaluated in real life clinical settings for decades, are also available to European patients. This percentage increases to 23.6% if regulatory clearance is evaluated as approved for use in some, but not all, European countries (Table 22). Upon enquiry, these differences in therapy availability are caused largely by differences in regulatory reviews and classifications between the EU and US. For example, in 1998, the FDA’s Center for Devices and Radiological Health (CDRH) approved Apligraf®, a regenerative medicine living cell and scaffold therapy for wound healing, as a medical device (354). However, regulatory authorities in Europe classify such therapeutic products in a significantly different way (355), thus confronting investigators and manufacturers with significant regulatory and financial investment, which often leads to the executive decision not to submit the product for European market authorisation. In 2015, almost 20 years after its original introduction in the US, Apligraf® was approved in Switzerland, while in 2022 it remains unavailable to patients within the EU (356). Reviewing the listing by Snyder et al. in Table 22, it should also be noted that not all products have the same regulatory classification, even in the US. This, too, can be seen as an indication of the real-life conundrum to fit new, innovative therapeutic approaches within a less-agile and not frequently updated regulatory framework (357). Although it should be acknowledged and appreciated that the different regulatory authorities are committed to working towards the facilitation and harmonisation of regulatory standards, the question remains whether this will effectively enhance access of healthcare professionals and their patients to innovative regenerative medicine solutions in the short term (351).
Table 22: Skin substitute products commercially available in the US, compared to the EU (352). When only approved in some European countries, country code according ISO3166-1 is given.
[NR] = no reply from manufacturer
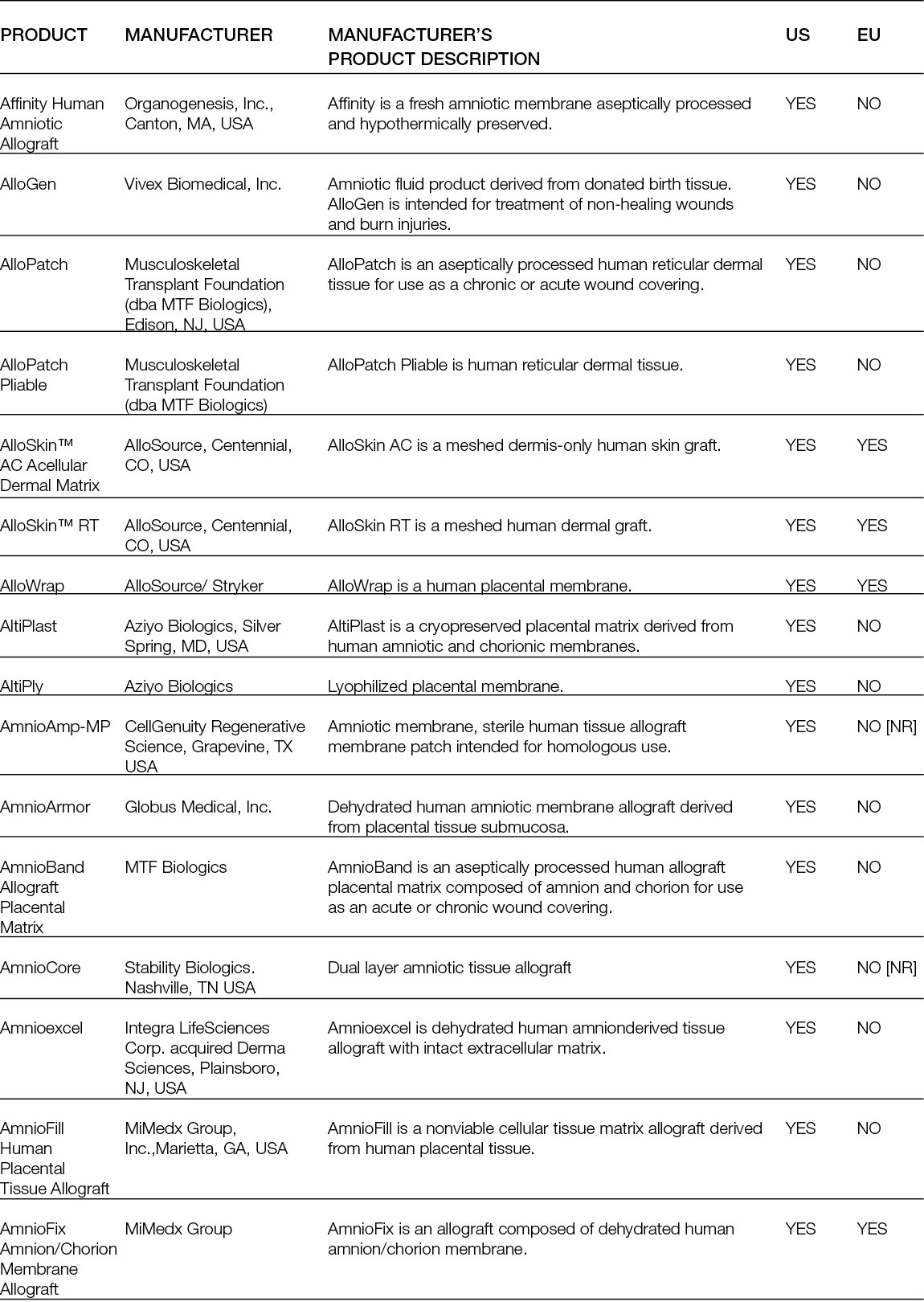
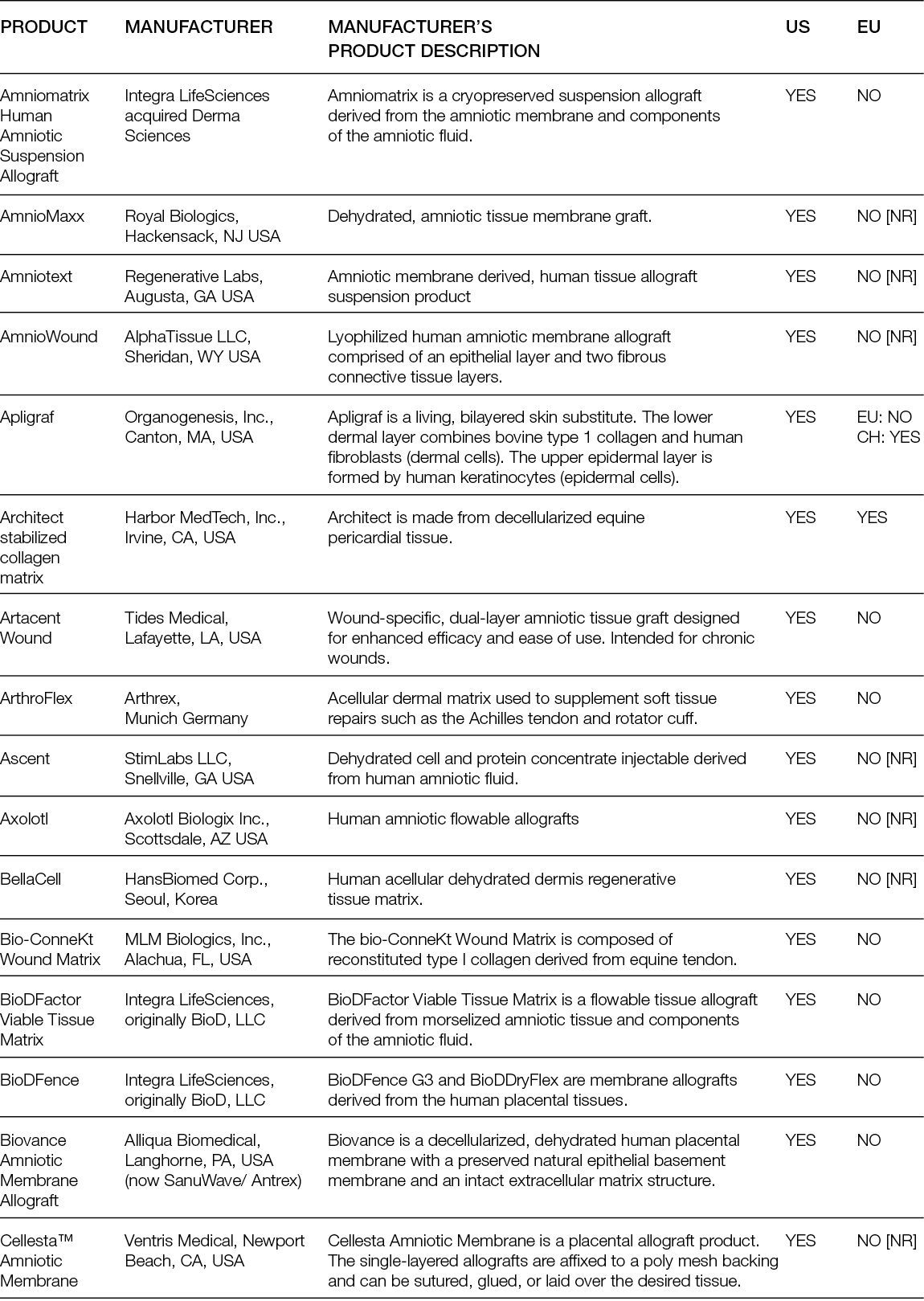
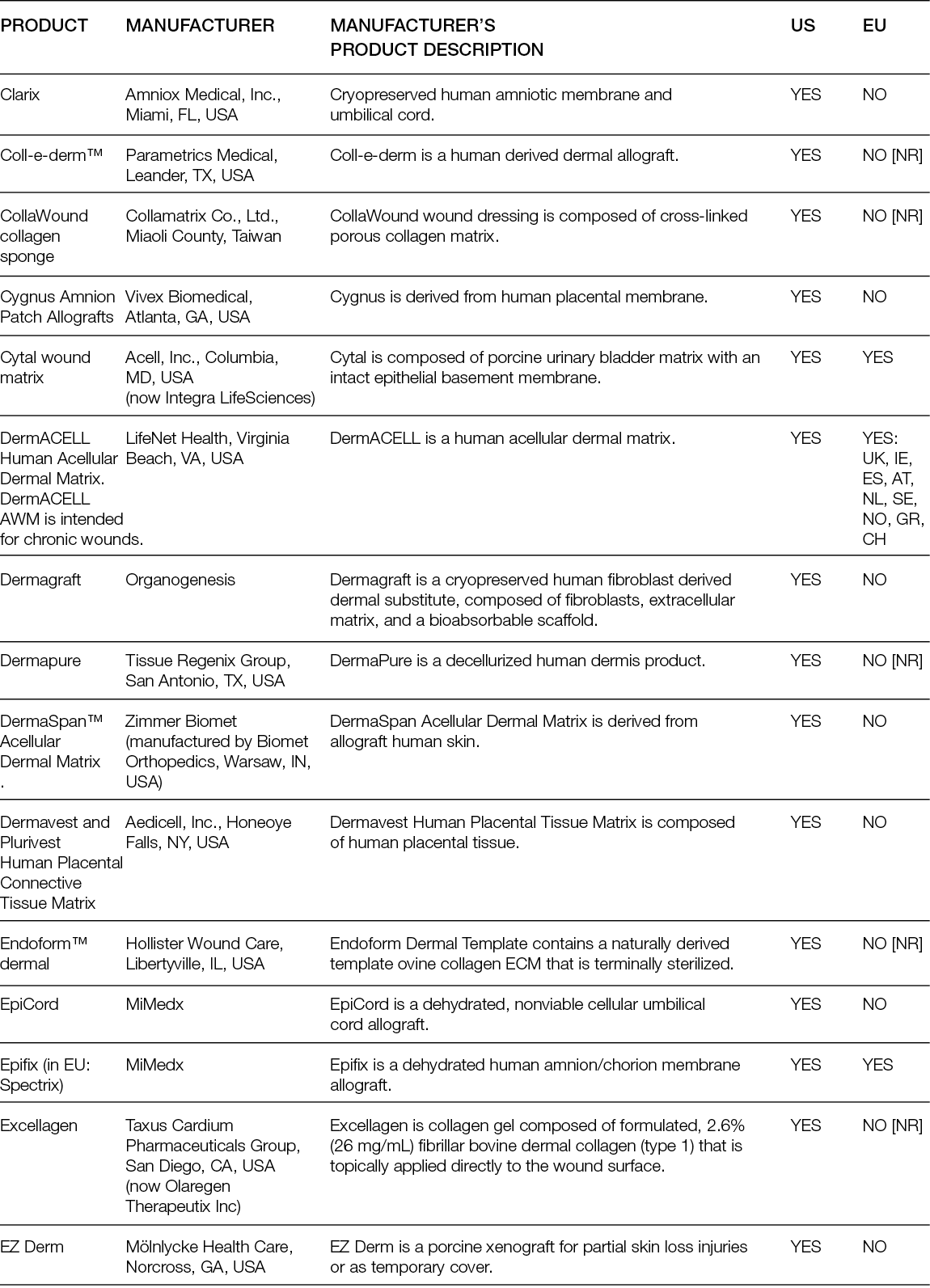
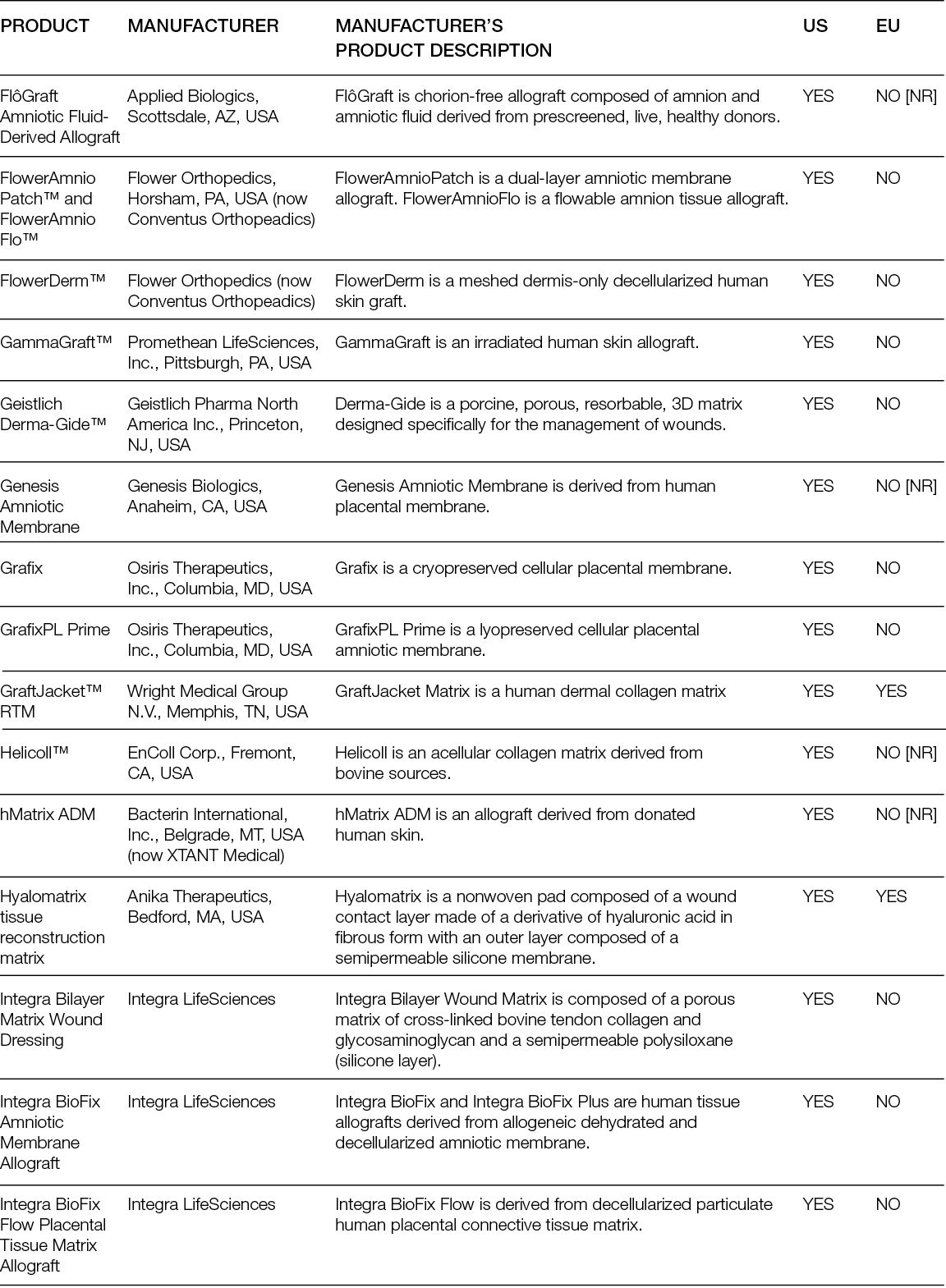
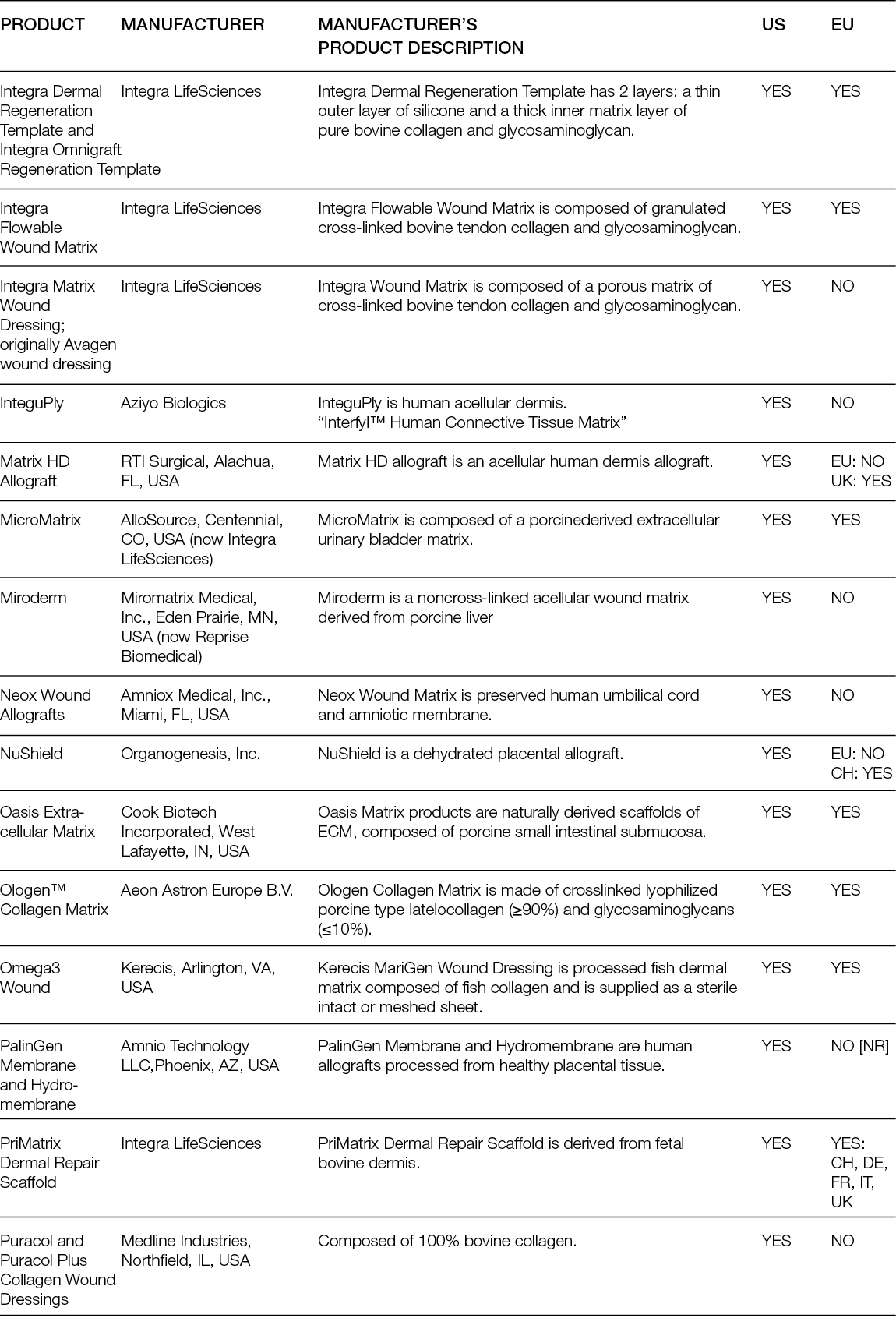
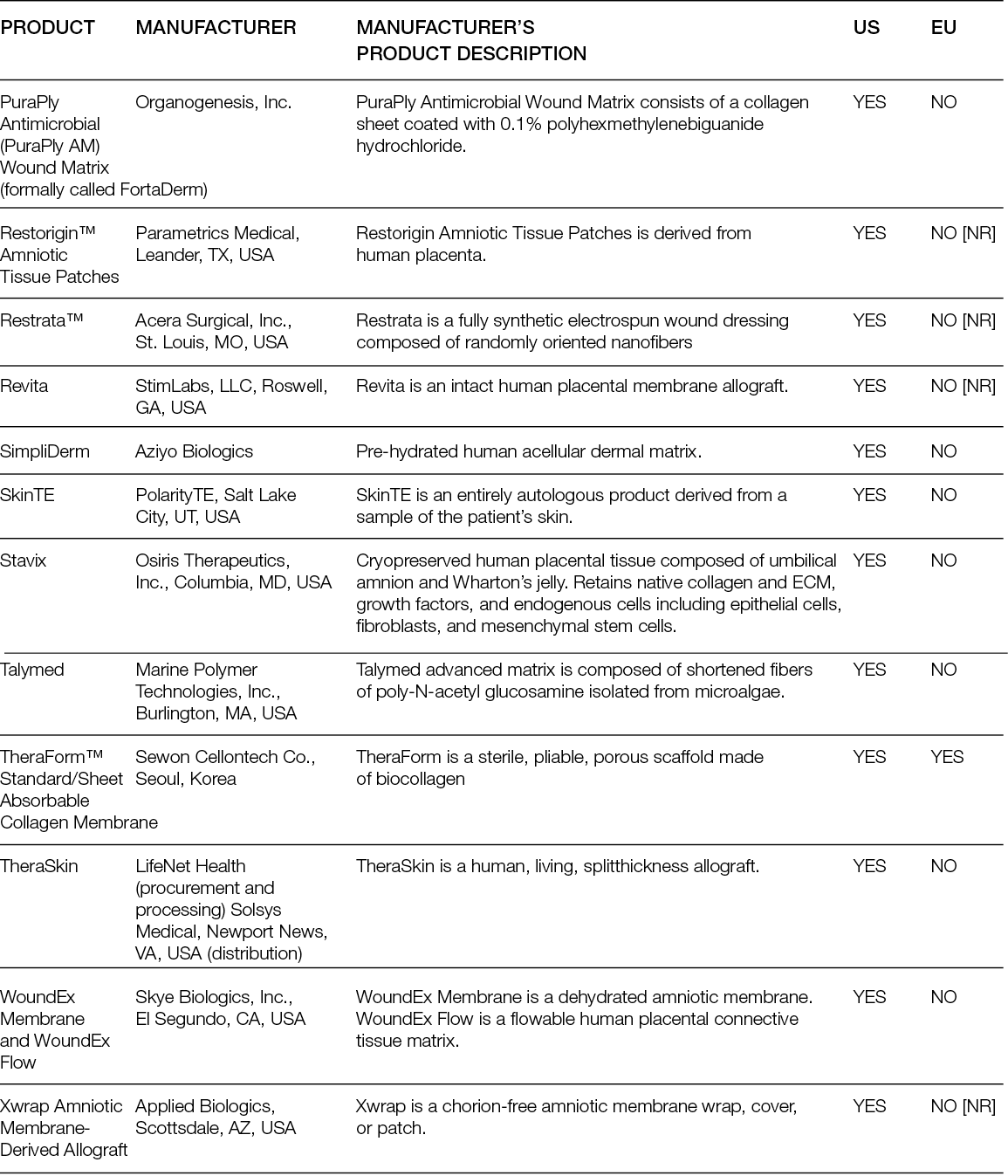
9.5 The future for new innovative regenerative medicine therapies in Europe
Despite the above, research on new and innovative regenerative medicine therapies is becoming ever more versatile. The emerging interdisciplinary field of skin tissue bioengineering is an example. Here, extensive deep tissue injuries, such as large burns and other major skin loss conditions, are investigated, aiming at developing therapies to reduce the time required to accomplish stable closure of wounds with minimal scarring in patients with insufficient donor sites for autologous split-thickness skin grafts. Regarding the composition of such tissue engineered skin, which may include cells, biopolymer scaffolds and drugs, it is not difficult to recognise the challenges these therapeutic approaches will encounter in their transition from clinical research to achieving regulatory market approval (358). This becomes even more apparent when reviewing the exciting and encouraging research into the application of 3D bioprinting in skin wound healing (359-368). Although 3D bioprinting research of skin seems to have advanced furthest towards a potential everyday clinical application, the opportunities of this technique do not stop there. Good examples here are the 3D (re)construction of cartilage and osseous structures (369-374); the bioprinting of cardiovascular structures like cardiac tissue, arteries and vessels (375-378); or even a combination of the above (i.e., a complete pre-vascularised implant for the repair of critically sized bone defects) (379). Even further reaching, unimaginable regenerative therapeutic potential is demonstrated by the work of Koffler et al., who investigated 3D-printed scaffolds loaded with neural progenitor cells that supported successful axon regeneration and the formation of new neural relays across sites of complete spinal cord injury in vivo in rodents (380). Recognising the impressive potential of these therapeutic approaches, it cannot be surprising that, during the recent COVID-19 pandemic and its aftermath, many investigators and clinicians looked towards regenerative medicine for potential solutions (381-385).
Acknowledging this, and focusing on the regenerative wound healing therapies available to European healthcare professionals, some questions remain for contemplation: Will the European regulatory framework be able to keep up? Considering the differences in regenerative medicine therapies available to patients in and outside Europe, might a recalibration of the EU’s regulatory framework be called for, as has happened elsewhere in response to new developments? (357) Or, should regulatory frameworks and authorities first and foremost safeguard a more stable, albeit less flexible, base where new products just need to comply with the applicable standards and policies? Then again, for healthcare professionals and providers, the remaining, and perhaps most important, question might be: Will it, in everyday practice within the EU, currently and in the future, be possible to provide and guarantee patients optimal (wound) care, as obligated?
Author(s)
Franco Bassetto MD
Clinic of Plastic Surgery, Padova
University Hospital, Padova, Italy
Jean-Pierre Becquemin MD
Professor of Vascular Surgery, University of Paris XII, Hopital Privé Paul d Egine, Champigny, Ramsay Group France
Edwin den Braber PhD
Independent Consultant,
EWMA Innovation Forum, Netherlands
Luca Dalla Paola MD
Diabetic Foot Unit, Maria Cecilia
Hospital, Cotignola, Italy
Alexandra Marques PhD
University of Minho, 3B’s Research Group in Biomaterials, Biodegradables and Biomimetics, Portugal
Ilaria Palla MA, MBA
Institute of Management, Sant´Anna School of Advanced Studies, Pisa, Italy
Alberto Piaggesi MD (Editor)
Diabetic Foot Section, Pisa University Hospital, Department of Endocrinology and Metabolism,
University of Pisa, Italy
Katherine Raspovic DPM
Department of Plastic Surgery,
Division of Wound Healing, Georgetown University School of Medicine, Washington DC, USA
Carlotta Scarpa MD PhD
Plastic and Reconstructive Surgery Clinic, Padova University Hospital, Padova, Italy
Luc Téot MD
Department of Burns, Wound Healing and Reconstructive Surgery,
Montpellier University Hospital, Montpellier, France
Isotta Triulzi PharmD
Institute of Management, Sant´Anna School of Advanced Studies, Pisa, Italy
Giuseppe Turchetti Prof, PhD
Institute of Management, Sant´Anna School of Advanced Studies, Pisa, Italy
Corresponding author:
Alberto Piaggesi, MD
Email alberto.piaggesi@med.unipi.it
Editorial support and coordination:
Julie Bjerregaard, EWMA Secretariat
Laura Pohl, EWMA Secretariat
This publication was supported by Bonalive, COOK Biotech, Emoled, Gunze, Integra, Kerecis and MiMedx
The supporting companies did not have any influence on the content of the publication.
References
- Piaggesi A, Låuchli S, Bassetto F, Biedermann T, Marques A, Najafi B, et al.: Advanced therapies in wound management: Cell and tissue based therapies, physical and bio-physical therapies smart and IT based technologies. J Wound Care 2018; 27(Sup6a):1-137.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al.: GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336(7650):924-6.
- DaCosta RS, Kulbatski I, Lindvere-Teene L, Starr D, Blackmore K, Silver JI, et al.: Point-of-care autofluorescence imaging for real-time sampling and treatment guidance of bioburden in chronic wounds: First-in-human results. PLoS One 2015; 10(3):e0116623.
- Price N.: Routine fluorescence imaging to detect wound bacteria reduces antibiotic use and antimicrobial dressing expenditure while improving healing rates: Retrospective analysis of 229 foot ulcers. Diagnostics (Basel) 2020; 10(11):927.
- Oropallo AR, Andersen C, Abdo R, Hurlow J, Kelso M, Melin M, et al.: Guidelines for point-of-care fluorescence imaging for detection of wound bacterial burden based on Delphi consensus. Diagnostics (Basel) 2021; 11(7):1219.
- Lu G, Fei B.: Medical hyperspectral imaging: A review. J Biomed Opt 2014; 19(1):10901.
- Han XH, Sun Y, Wang J, Shi B, Zheng Y, Chen YW.: Spectral representation vis data-guided sparsity for hyperspectral image super-resolution(†). Sensors (Basel) 2019; 19(24):5401.
- Saiko G, Lombardi P, Au Y, Queen D, Armstrong D, Harding K.: Hyperspectral imaging in wound care: A systematic review. Int Wound J 2020; 17(6):1840-1856.
- Wahabzada M, Besser M, Khosravani M, Kuska MT, Kersting K, Mahlein AK, et al.: Monitoring wound healing in a 3D wound model by hyperspectral imaging and efficient clustering. PLoS One 2017; 12(12):e0186425.
- Chiang N, Jain JK, Sleigh J, Vasudevan T.: Evaluation of hyperspectral imaging technology in patients with peripheral vascular disease. J Vasc Surg 2017; 66(4):1192-1201.
- López-Moral M, García-Álvarez Y, Molines-Barroso RJ, Tardáguila-García A, García-Madrid M, Lázaro-Martínez JL.: A comparison of hyperspectral imaging with routine vascular noninvasive techniques to assess the healing prognosis in patients with diabetic foot ulcers. J Vasc Surg 2022; 75(1):255-261.
- Basiri A, Nabili M, Mathews S, Libin A, Groah S, Noordmans HJ, et al.: Use of a multi-spectral camera in the characterization of skin wounds. Opt Express 2010; 18(4):3244-3257.
- Poosapadi Arjunan S, Tint AN, Aliahmad B, Kumar DK, Shukla R, Miller J, et al.: High-resolution spectral analysis accurately identifies the bacterial signature in infected chronic foot ulcers in people with diabetes. Int J Low Extrem Wounds 2018; 17(2):78-86.
- Zhang S, Gnyawali S, Huang J, Ren W, Gordillo G, Sen CK, et al.: Multimodal imaging of cutaneous wound tissue. J Biomed Opt 2015; 20(1):016016.
- Calin MA, Parasca SV, Savastru D, Manea D.: Hyperspectral imaging in the medical field: Present and future. Applied Spectroscopy Rev 2014; 49(6):435-447.
- Lou BS, Hsieh JH, Chen CM, Hou CW, Wu HY, Chou PY, et al.: Helium/argon-generated cold atmospheric plasma facilitates cutaneous wound healing. Front Bioeng Biotechnol 2020; 8:683.
- Kisch T, Schleusser S, Helmke A, Mauss KL, Wenzel ET, Hasemann B, et al.: The repetitive use of non-thermal dielectric barrier discharge plasma boosts cutaneous microcirculatory effects. Microvasc Res. 2016; 106:8-13.
- Kletschkus K, Haralambiev L, Nitsch A, Pfister F, Klinkmann G, Kramer A, et al.: The application of a low-temperature physical plasma device operating under atmospheric pressure leads to the production of toxic NO2. Anticancer Res. 2020; 40(5):2591-2599.
- Wiegand C, Fink S, Beier O, Horn K, Pfuch A, Schimanski A, et al.: Dose- and time-dependent cellular effects of cold atmospheric pressure plasma evaluated in 3D skin models. Skin Pharmacol Physiol 2016; 29(5):257-265.
- He R, Li Q, Shen W, Wang T, Lu H, Lu J, et al.: The efficacy and safety of cold atmospheric plasma as a novel therapy for diabetic wound in vitro and in vivo. Int Wound J 2020; 17(3):851-863.
- Cicchi R, Rossi F, Alfieri D, Bacci S, Tatini F, De Siena G, et al.: Observation of an improved healing process in superficial skin wounds after irradiation with a blue-LED haemostatic device. J Biophotonics 2016; 9(6):645-55.
- Magni G, Tatini F, Bacci S, Paroli G, De Siena G, Cicchi R, et al.: Blue LED light modulates inflammatory infiltrate and improves the healing of superficial wounds. Photodermatol Photoimmunol Photomed 2020; 36(2):166-168.
- Fraccalvieri M, Amadeo G, Bortolotti P, Ciliberti M, Garrubba A, Mosti G, et al. Effectiveness of Blue light photobiomodulation therapy in the treatment of chronic wounds. Results of the Blue Light for Ulcer Reduction (B.L.U.R.) Study. Ital J Dermatol Venerol. 2022 Apr;157(2):187-194. doi: 10.23736/S2784-8671.21.07067-5. Epub 2021 Sep
- Piaggesi A SA, Sandroni S, Gasperini S.: The HERMES study – blue light photobiomodulation therapy on neuroischemic patients – Experimental design and study protocol. J Wound Manage 2021; 22(2):55-64.
- Arenbergerova M, Arenberger P, Bednar M, Kubat P, Mosinger J.: Light-activated nanofibre textiles exert antibacterial effects in the setting of chronic wound healing. Exp Dermatol 2012; 21(8):619-624.
- Polak A, Kucio C, Kloth LC, Paczula M, Hordynska E, Ickowicz T, et al.: A randomized, controlled clinical study to assess the effect of anodal and cathodal electrical stimulation on periwound skin blood flow and pressure ulcer size reduction in persons with neurological injuries. Ostomy Wound Manage 2018; 64(2):10-29.
- Pasek J, Szajkowski S, Pietrzak M, Cieślar G.: Comparison of the efficacy of topical hyperbaric oxygen therapy alone vs a combination of physical methods including topical hyperbaric oxygen therapy, magnetotherapy, and low-energy light therapy in the treatment of venous leg ulcers. Dermatol Ther 2020; 33(6):e14474.
- Pasek J, Szajkowski S, Cieślar G.: Therapeutic efficacy of physical combined therapy in the treatment of venous crural ulcers. Phlebology 2021; 36(6):481-488.
- Iacovacci V, Tamadon I, Kauffmann EF, Pane S, Simoni V, Marziale L, et al.: A fully implantable device for intraperitoneal drug delivery refilled by ingestible capsules. Sci Robot 2021;6(57):eabh3328.
- Rizzo F, Kehr NS.: Recent advances in injectable hydrogels for controlled and local drug delivery. Adv Healthc Mater 2021; 10(1):e2001341.
- Watarai A, Schirmer L, Thönes S, Freudenberg U, Werner C, Simon JC, et al.: TGF- functionalized starPEG-heparin hydrogels modulate human dermal fibroblast growth and differentiation. Acta Biomater 2015; 25:65-75.
- Prokoph S, Chavakis E, Levental KR, Zieris A, Freudenberg U, Dimmeler S, et al.: Sustained delivery of SDF-1- from heparin-based hydrogels to attract circulating pro-angiogenic cells. Biomaterials 2012; 33(19):4792-4800.
- Chwalek K, Tsurkan MV, Freudenberg U, Werner C.: Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Sci Rep 2014; 4:4414.
- Ubbink DT, Koopman B.: Near-infrared spectroscopy in the routine diagnostic work-up of patients with leg ischaemia. Eur J Vasc Endovasc Surg 2006; 31(4):394-400.
- He H-P, Weng J-C, Zhao Y, Cai S-H, Zhang X-l, Yin H-H.: Impact of plaque calcification and stent oversizing on clinical outcomes of atherosclerotic femoropopliteal arterial occlusive disease following stent angioplasty. Eur J Vasc Endovasc Surg 2019; 58(2):215-222.
- Zelen CM, Orgill DP, Serena TE, Galiano RE, Carter MJ, DiDomenico LA, et al.: An aseptically processed, acellular, reticular, allogenic human dermis improves healing in diabetic foot ulcers: A prospective, randomised, controlled, multicentre follow-up trial. Int Wound J 2018; 15(5):731-739.
- Lantis JC, Snyder R, Reyzelman AM, Van Gils CC, Sigal F, Vayser D, et al.: Fetal bovine acellular dermal matrix for the closure of diabetic foot ulcers: A prospective randomised controlled trial. J Wound Care 2021; 30(Sup7):S18-s27.
- Cazzell S, Vayser D, Pham H, Walters J, Reyzelman A, Samsell B, et al.: A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Repair Regen 2017; 25(3):483-497.
- Cazzell S.: A randomized controlled trial comparing a human acellular dermal matrix versus conventional care for the treatment of venous leg ulcers. Wounds 2019; 31(3): 68-74.
- Pirayesh A, Hoeksema H, Richters C, Verbelen J, Monstrey S.: Glyaderm(®) dermal substitute: Clinical application and long-term results in 55 patients. Burns 2015; 41(1):132-144.
- Lavery L, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, et al.: Open-label extension phase of a chronic diabetic foot ulcer multicenter, controlled, randomized clinical trial using cryopreserved placental membrane. Wounds 2018; 30(9):283-289.
- Frykberg RG, Cazzell SM, Arroyo-Rivera J, Tallis A, Reyzelman AM, Saba F, et al.: Evaluation of tissue engineering products for the management of neuropathic diabetic foot ulcers: An interim analysis. J Wound Care 2016; 25(Sup7):S18-S25.
- Tchanque-Fossuo CN, Dahle SE, Lev-Tov H, West KIM, Li CS, Rocke DM, et al.: Cellular versus acellular matrix devices in the treatment of diabetic foot ulcers: Interim results of a comparative efficacy randomized controlled trial. J Tissue Eng Regen Med 2019; 13(8):1430-1437.
- Lee M, Han SH, Choi WJ, Chung KH, Lee JW.: Hyaluronic acid dressing (Healoderm) in the treatment of diabetic foot ulcer: A prospective, randomized, placebo-controlled, single-center study. Wound Repair Regen 2016; 24(3):581-8.
- Park KH, Kwon JB, Park KH, Shin JC, Han SH, Lee JW.: Collagen dressing in the treatment of diabetic foot ulcer: A prospective, randomized, placebo-controlled, single-center study. Diabetes Res Clin Pract 2019; 156:107861.
- Djavid GE, Tabaie SM, Tajali SB, Totounchi M, Farhoud A, Fateh M, et al.: Application of a collagen matrix dressing on a neuropathic diabetic foot ulcer: A randomised control trial. J Wound Care 2020; 29(Sup3):S13-S18.
- Yoon D, Cho YS, Joo SY, Seo CH, Cho YS.: A clinical trial with a novel collagen dermal substitute for wound healing in burn patients. Biomater Sci 2020;8(3):823-829.
- De Angelis B, Orlandi F, Fernandes Lopes, Morais D’Autilio M, Scioli MG, Orlandi A, Cervelli V, et al.: Long-term follow-up comparison of two different bi-layer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings. Int Wound J 2018; 15(5):695-706.
- Irving S.: Managing chronic, nonhealing wounds stalled in the inflammatory phase: A case series using a novel matrix therapy, CACIPLIQ20. Br J Community Nurs 2019; 24(Sup9):S33-S37.
- Wiser I, Tamir E, Kaufman H, Keren E, Avshalom S, Klein D, et al.: A novel recombinant human collagen-based flowable matrix for chronic lower limb wound management: First results of a clinical trial. Wounds 2019; 31(4):103-107.
- Bohn GA, Chaffin AE.: Extracellular matrix graft for reconstruction over exposed structures: A pilot case series. J Wound Care 2020; 29(12):742-749.
- Wu-Fienberg Y, Wu SS, Gatherwright J, Chepla KJ.: An alternative dermal template for reconstruction of complex upper extremity wounds. Plast Reconstr Surg - Glob Open 2021; 9(7):e3674.
- Cazzell S, Moyer PM, Samsell B, Dorsch K, McLean J, Moore MA.: A prospective, multicenter, single-arm clinical trial for treatment of complex diabetic foot ulcers with deep exposure using acellular dermal matrix. Adv Skin Wound Care 2019; 32(9):409-415.
- Bondioli E, Purpura V, Orlandi C, Carboni A, Minghetti P, Cenacchi G, et al.: The use of an acellular matrix derived from human dermis for the treatment of full-thickness skin wounds. Cell Tissue Bank 2019; 20(2):183-192.
- Välimäki VV, Aro HT.: Molecular basis for action of bioactive glasses as bone graft substitute. Scand J Surg 2006; 95(2):95-102.
- Leppäranta O, Vaahtio M, Peltola T, Zhang D, Hupa L, Hupa M, et al. Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J Mater Sci Mater Med 2008; 19(2):547-551.
- Munukka E, Leppäranta O, Korkeamäki M, Vaahtio M, Peltola T, Zhang D, et al.: Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. J Mater Sci Mater Med 2008; 19(1):27-32.
- Drago L, Vassena C, Fenu S, De Vecchi E, Signori V, De Francesco R, et al.: In vitro antibiofilm activity of bioactive glass S53P4. Future Microbiol 2014; 9(5):593-601.
- Lacopi E, Pieruzzi L, Goretti C, Piaggesi A.: Pilot experience on the use of S53P4 bioactive glass in the surgical management of diabetic foot osteomyelitis. Int J Low Extrem Wounds 2022; 21(1):57-64.
- Lacopi E, Ferranti S, Riitano N, Abbruzzese L, Pieruzzi L, Goretti C, et al.: Bioactive glass in a rare case of osteomyelitis of the heel in a Guillain-Barré Syndrome: A case report. Int J Low Extrem Wounds 2021;20(1):60-66.
- Kumar V, Kumar N, Gangwar AK, Singh H, Singh R.: Comparative histologic and immunologic evaluation of 1,4-butanediol diglycidyl ether crosslinked versus noncrosslinked acellular swim bladder matrix for healing of full-thickness skin wounds in rabbits. J Surg Res 2015; 197(2):436-446.
- Baur J-O, Rahmanian-Schwarz A, Held M, Schiefer J, Daigeler A, Eisler W.: Evaluation of a cross-linked versus non-cross-linked collagen matrix in full-thickness skin defects. Burns 2021; 47(1):150-156.
- Rahman MS, Islam R, Rana MM, Spitzhorn L-S, Rahman MS, Adjaye J, et al.: Characterization of burn wound healing gel prepared from human amniotic membrane and Aloe vera extract. BMC Complement Altern Med 2019; 19(1):115.
- Notodihardjo SC, Morimoto N, Munisso MC, Le TM, Mitsui T, Kakudo N, et al.: A comparison of the wound healing process after the application of three dermal substitutes with or without basic fibroblast growth factor impregnation in diabetic mice. J Plast Reconstr Aesthet Surg 2020; 73(8):1547-1555.
- Lu YF, Deng J, Wang J, Luo GX.: Effects and mechanism of Lactococcus lactis thermo-sensitive hydrogel on the wound healing of full-thickness skin defects in diabetic mice. Zhonghua shao shang za zhi 2020; 36(12):1117-1129.
- Celie K-B, Toyoda Y, Dong X, Morrison KA, Zhang P, Asanbe O, et al.: Microstructured hydrogel scaffolds containing differential density interfaces promote rapid cellular invasion and vascularization. Acta Biomater 2019; 91:144-158.
- Zhou L, Ramezani H, Sun M, Xie M, Nie J, Lv S, et al.: 3D printing of high-strength chitosan hydrogel scaffolds without any organic solvents. Biomater Sci 2020; 8(18):5020-5028.
- Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S.: 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol 2016; 40:103-112.
- Tang Q, Plank TN, Zhu T, Yu H, Ge Z, Li Q, et al.: Self-assembly of metallo-nucleoside hydrogels for injectable materials that promote wound closure. ACS Appl Mater Interfaces 2019; 11(22):19743-19750.
- Shi G, Wang Y, Derakhshanfar S, Xu K, Zhong W, Luo G, et al.: Biomimicry of oil infused layer on 3D printed poly(dimethylsiloxane): Non-fouling, antibacterial and promoting infected wound healing. Mater Sci Eng C Mater Biol Appl. 2019; 100:915-927.
- Wu Z, Hong Y.: Combination of the silver-ethylene interaction and 3D printing to develop antibacterial superporous hydrogels for wound management. ACS Appl Mater Interfaces 2019; 11(37):33734-33747.
- Abolghasemzade S, Pourmadadi M, Rashedi H, Yazdian F, Kianbakht S, Navaei-Nigjeh M.: PVA based nanofiber containing CQDs modified with silica NPs and silk fibroin accelerates wound healing in a rat model. J Mater Chem B. 2021; 9(3):658-676.
- Azadmanesh F, Pourmadadi M, Reza JZ, Yazdian F, Omidi M, Haghitosadat BF.: Synthesis of a novel nanocomposite containing chitosan as a 3D printed wound dressing technique: Emphasis on gene expression. Biotechnol Prog 2021; 37(4): e3132.
- Farizhandi AAK, Khalajabadi SZ, Krishnadoss V, Noshadi I.: Synthesized biocompatible and conductive ink for 3D printing of flexible electronics. J Mech Behav Biomed Mater 2020; 110:103960.
- Heald R, Bennett M, Subramaniam VV, Dusane D, Lochab V, Sundaram PM, et al.: Printed electroceutical dressings for the inhibition of biofilms and treatment of chronic wounds. J Microelectromech Syst 2020; 29(5):918-923.
- Cereceres S, Lan Z, Bryan L, Whitely M, Wilems T, Greer H, et al.: Bactericidal activity of 3D-printed hydrogel dressing loaded with gallium maltolate. APL Bioeng 2019; 3(2):026102.
- Wu C, Yu Z, Li Y, Zhou K, Cao C, Zhang P, et al.: Cryogenically printed flexible chitosan/bioglass scaffolds with stable and hierarchical porous structures for wound healing. Biomed Mater 2020; 16(1):15004.
- Wang S, Xiong Y, Chen J, Ghanem A, Wang Y, Yang J, et al.: Three dimensional printing bilayer membrane scaffold promotes wound healing. Front Bioeng Biotechnol 2019;7:348.
- Gao D, Wang Z, Wu Z, Guo M, Wang Y, Gao Z, et al.: 3D-printing of solvent exchange deposition modeling (SEDM) for a bilayered flexible skin substitute of poly (lactide-co-glycolide) with bioorthogonally engineered EGF. Mater Sci Eng C Mater Biol Appl. 2020; 112:110942.
- Navarro J, Clohessy RM, Holder RC, Gabard AR, Herendeen GJ, Christy RJ, et al.: In vivo evaluation of three-dimensional printed, keratin-based hydrogels in a porcine thermal burn model. Tissue Eng Part A 2020; 26(5-6):265-278.
- Albanna M, Binder KW, Murphy SV, Kim J, Qasem SA, Zhao W, et al.: In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep 2019; 9(1):1856.
- Shafiee A, Cavalcanti AS, Saidy NT, Schneidereit D, Friedrich O, Ravichandran A, et al.: Convergence of 3D printed biomimetic wound dressings and adult stem cell therapy. Biomaterials 2021; 268:120558.
- Zhou F, Hong Y, Liang R, Zhang X, Liao Y, Jiang D, et al.: Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 2020; 258:120287.
- Huyan Y, Lian Q, Zhao T, Li D, He J.: Pilot study of the biological properties and vascularization of 3D printed bilayer skin grafts. Int J Bioprinting 2020; 6(1):246.
- Baltazar T, Merola J, Catarino C, Xie CB, Kirkiles-Smith NC, Lee V, et al.: Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Eng Part A 2020; 26(5-6):227-238.
- Jorgensen AM, Varkey M, Gorkun A, Clouse C, Xu L, Chou Z, et al.: Bioprinted skin recapitulates normal collagen remodeling in full-thickness wounds. Tissue Eng Part A 2020; 26(9-10):512-526.
- Takano M, Sugahara K, Koyachi M, Odaka K, Matsunaga S, Homma S, et al.: Maxillary reconstruction using tunneling flap technique with 3D custom-made titanium mesh plate and particulate cancellous bone and marrow graft: A case report. Maxillofac Plast Reconstr Surg 2019; 41(1):43.
- Igelbrink S, Zanettini LMS, Bohner L, Kleinheinz J, Jung S.: Three-dimensional planning of the mandibular margin in hemifacial microsomia using a printed patient-specific implant. J Craniofac Surg 2020; 31(8):2297-2301.
- Zheng J, Chen X, Jiang W, Zhang S, Chen M, Yang C.: An innovative total temporomandibular joint prosthesis with customized design and 3D printing additive fabrication: A prospective clinical study. J Transl Med 2019; 17(1):4.
- Wei R, Guo W, Yang R, Tang X, Yang Y, Ji T, et al.: Reconstruction of the pelvic ring after total en bloc sacrectomy using a 3D-printed sacral endoprosthesis with re-establishment of spinopelvic stability: A retrospective comparative study. Bone Joint J 2019; 101-B(7):880-888.
- Muñoz-Cruzado VD, Castro FJC, Eguía AP, Aguilar LT, González JT, Puyana JC, et al.: Using a bio-scanner and 3D printing to create an innovative custom made approach for the management of complex entero-atmospheric fistulas. Sci Rep 2020; 10(1):19862.
- Sun H, Lv H, Qiu F, Sun D, Gao Y, Chen N, et al.: Clinical application of a 3D-printed scaffold in chronic wound treatment: a case series. J Wound Care 2018; 27(5):262-271.
- Hori K, Osada A, Isago T, Sakurai H.: Comparison of contraction among three dermal substitutes: Morphological differences in scaffolds. Burns 2017; 43(4):846-851.
- Oualla-Bachiri W, Fernández-González A, Quiñones-Vico MI, Arias-Santiago S.: From grafts to human bioengineered vascularized skin substitutes. Int J Mol Sci 2020; 21(21):8197.
- Schwartz JA, Goss SG, Facchin F, Avdagic E, Lantis JC.: Surgical debridement alone does not adequately reduce planktonic bioburden in chronic lower extremity wounds. J Wound Care 2014; 23(9):S4, S6, S8 passim.
- Chang YJR, Perry J, Cross K.: Low-frequency ultrasound debridement in chronic wound healing: A systematic review of current evidence. Plast Surg (Oakv) 2017; 25(1):21-26.
- Trial C, Brancati A, Marnet O, Téot L.: Coblation technology for surgical wound debridement: Principle, experimental data, and technical data. Int J Low Extrem Wounds 2012; 11(4):286-92.
- Scarpa C, de Antoni E, Vindigni V, Bassetto F.: Efficacy of negative pressure wound therapy with instillation and dwell time for the treatment of a complex chronic venous leg ulcer. Wounds 2020; 32(12):372-374.
- Téot L, Boissiere F, Fluieraru S.: Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int Wound J 2017; 14(5):842-848.
- Bassetto F, Lancerotto L, Salmaso R, Pandis L, Pajardi G, Schiavon M, et al.: Histological evolution of chronic wounds under negative pressure therapy. J Plast Reconstr Aesthet Surg 2012; 65(1):91-9.
- Labler L, Rancan M, Mica L, Härter L, Mihic-Probst D, Keel M.: Vacuum-assisted closure therapy increases local interleukin-8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma 2009; 66(3):749-57.
- Anghel EL, Kim PJ, Attinger CE.: A solution for complex wounds: The evidence for negative pressure wound therapy with instillation. Int Wound J 2016; 13(Suppl 3):19-24.
- Diehm YF, Loew J, Will PA, Fischer S, Hundeshagen G, Ziegler B, et al.: Negative pressure wound therapy with instillation and dwell time (NPWTi-d) with V. A. C. VeraFlo in traumatic, surgical, and chronic wounds-A helpful tool for decontamination and to prepare successful reconstruction. Int Wound J 2020;17(6):1740-1749.
- Hurd T, Kirsner RS, Sancho-Insenser JJ, Fumarola S, Garten A, Patel M, et al.: International consensus panel recommendations for the optimization of traditional and single-use negative pressure wound therapy in the treatment of acute and chronic wounds. Wounds 2021; 33(suppl 2):S1-s11.
- Cheng N, Jeschke MG, Sheikholeslam M, Datu AK, Oh HH, Amini-Nik S.: Promotion of dermal regeneration using pullulan/gelatin porous skin substitute. J Tissue Eng Regen Med 2019; 13(11):1965-1977.
- Dai C, Shih S, Khachemoune A. Skin substitutes for acute and chronic wound healing: An updated review. J Dermatolog Treat 2020; 31(6):639-648.
- Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF.: Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA 1989; 86(3):933-7.
- Soller EC, Tzeranis DS, Miu K, So PTC, Yannas IV.: Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials 2012; 33(19):4783-91.
- Tzeranis DS, Soller EC, Buydash MC, So PTC, Yannas IV.: In situ quantification of surface chemistry in porous collagen biomaterials. Ann Biomed Eng 2016; 44(3):803-15.
- Yannas IV, Burke JF, Orgill DP, Skrabut EM.: Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 1982; 215(4529):174-6.
- Driver VR, Lavery LA, Reyzelman AM, Dutra TG, Dove CR, Kotsis SV, et al.: A clinical trial of Integra template for diabetic foot ulcer treatment. Wound Repair Regen 2015; 23(6):891-900.
- Dalla Paola L, Cimaglia P, Carone A, Boscarino G, Scavone G.: Use of Integra dermal regeneration template for limb salvage in diabetic patients with no-option critical limb ischemia. Int J Low Extrem Wounds 2021; 20(2):128-134.
- Hicks CW, Zhang GQ, Canner JK, Mathioudakis N, Coon D, Sherman RL, et al.: Outcomes and predictors of wound healing among patients with complex diabetic foot wounds treated with a dermal regeneration template (Integra). Plast Reconstr Surg 2020; 146(4):893-902.
- Reynolds M, Kelly DA, Walker NJ, Crantford C, Defranzo AJ.: Use of Integra in the management of complex hand wounds from cancer resection and nonburn trauma. Hand (N Y). 2018; 13(1):74-79.
- Choughri H, Weigert R, Heron A, Dahmam A, Abi-Chahla ML, Delgove A.: Indications and functional outcome of the use of integra(®) dermal regeneration template for the management of traumatic soft tissue defects on dorsal hand, fingers and thumb. Arch Orthop Trauma Surg 2020; 140(12):2115-2127.
- Bernstein JL, Premaratne ID, Levy AS, Kuhel WI, Kutler DI, Spector JA.: Reconstruction of Full thickness scalp defects in extremely elderly patients using dermal regeneration templates. J Craniofac Surg 2020; 31(5):e511-e514.
- Rudnicki PA, Purt B, True D, Siordia H, Lohmeier S, Chan RK.: Single-stage composite skin reconstruction using a dermal regeneration template. Plast Reconstr Surg Glob Open 2020; 8(2):e2622.
- Shakir S, Messa CA, Broach RB, Rhemtulla IA, Chatman B, D’Angelantonio A, et al.: Indications and limitations of bilayer wound matrix-based lower extremity reconstruction: A multidisciplinary case-control study of 191 wounds. Plast Reconstr Surg 2020; 145(3):813-822.
- Chaiyasate K, Oliver LN, Kreitzberg SA, Lyons M, Goldman J, Lu SM, et al.: Use of pericranial flaps with dermal substitute for scalp reconstruction: A case series. Plast Reconstr Surg Glob Open 2020; 8(8):e3011.
- Romano G, Bouaoud J, Moya-Plana A, Benmoussa N, Honart JF, Leymarie N.: Integra® dermal regeneration template for full thickness carcinologic scalp defects: Our 6 years’ experience retrospective cohort and literature review. J Stomatol Oral Maxillofac Surg 2021; 122(3):256-262.
- Scalise A, Torresetti M, Di Benedetto G.: Reconstruction of full-thickness soft tissue defects with Integra: Risk factors and treatment algorithm. Plast Reconstr Surg Glob Open 2020; 8(9):e3099.
- Magnoni C, De Santis G, Fraccalvieri M, Bellini P, Portincasa A, Giacomelli L, et al.: Integra in scalp reconstruction after tumor excision: Recommendations from a multidisciplinary advisory board. J Craniofac Surg 2019; 30(8):2416-2420.
- Gonzalez SR, Wolter KG, Yuen JC.: Infectious complications associated with the use of Integra: A systematic review of the literature. Plast Reconstr Surg Glob Open 2020; 8(7):e2869.
- Vana LPM, Battlehner CN, Ferreira MA, Caldini EG, Gemperli R, Alonso N.: Comparative long-term study between two dermal regeneration templates for the reconstruction of burn scar contractures in humans: Clinical and histological results. Burns 2020; 46(3):596-608.
- Bloemen MC, van Leeuwen MC, van Vucht NE, van Zuijlen PP, Middelkoop E Dermal substitution in acute burns and reconstructive surgery: a 12-year follow-up. Plast Reconstr Surg. 2010; 125:1450
- Watfa W, di Summa PG, Meuli J, Raffoul W, Bauquis O.: MatriDerm decreases donor site morbidity after radial forearm free flap harvest in transgender surgery. J Sex Med 2017; 14(10):1277-1284.
- Maitz J, Wang Y, Fathi A, Escobar FX, Parungao R, van Zuijlen P, et al.: The effects of cross-linking a collagen-elastin dermal template on scaffold bio-stability and degradation. J Tissue Eng Regen Med 2020; 14(9):1189-1200.
- Lv Z, Yu L, Wang Q, Jia R, Ding W, Shen Y. The use of dermal regeneration template for treatment of complex wound with bone/tendon exposed at the forearm and hand, a prospective cohort study. Medicine (Baltimore). 2019 Nov;98(44):e17726. doi: 10.1097/MD.0000000000017726. PMID: 31689814; PMCID: PMC6946402.
- Lisa AVE, Galtelli L, Vinci V, Veronesi A, Cozzaglio L, Cananzi FCM, et al.: Adoption of a newly introduced dermal matrix: Preliminary experience and future directions. Biomed Res Int 2020; 2020:3261318.
- De Francesco F, Busato A, Mannucci S, Zingaretti N, Cottone G, Amendola F, et al.: Artificial dermal substitutes for tissue regeneration: Comparison of the clinical outcomes and histological findings of two templates. J Int Med Res 2020; 48(8):300060520945508.
- Lv Z, Wang Q, Jia R, Ding W, Shen Y.: Pelnac® artificial dermis assisted by VSD for treatment of complex wound with bone/tendon exposed at the foot and ankle, A prospective study. J Invest Surg 2020; 33(7):636-641.
- Yiğitbaş H, Yavuz E, Özdemir EB, Onen O, Pençe HH, Meriç S, et al.: Our experience with dermal substitute Nevelia® in the treatment of severely burned patients. Ulus Travma Acil Cerrahi Derg 2019; 25(5):520-526.
- De Angelis B, Orlandi F, D’Autilio MFLM, Di Segni C, Scioli MG, Orlandi A, et al.: Vasculogenic chronic ulcer: Tissue regeneration with an innovative dermal substitute. J Clin Med. 2019; 8(4):525.
- Montanaro M, Meloni M, Anemona L, Giurato L, Scimeca M, Izzo V, et al.: Macrophage activation and M2 polarization in wound bed of diabetic patients treated by dermal/epidermal substitute Nevelia. Int J Low Extrem Wounds 2020; 1534734620945559.
- Gurbuz K, Demir M, Das K.: The use of dermal substitute in deep burns of functional/mobile anatomic areas at acute phase after early excision and subsequent skin autografting: Dermal substitute prevents functional limitations. J Burn Care Res 2020; 41(5):1079-1083.
- Uccioli L, Meloni M, Izzo V, Giurato L.: Use of Nevelia dermal-epidermal regenerative template in the management of ischemic diabetic foot postsurgical wounds. Int J Low Extrem Wounds 2020; 19(3):282-288.
- Cottone G, Amendola F, Strada C, Bagnato MC, Brambilla R, De Francesco F, et al.: Comparison of efficacy among three dermal substitutes in the management of critical lower-limb wounds: The largest biases-reduced single-center retrospective cohort study in literature. Medicina (Kaunas). 2021; 57(12):1367.
- Garoufalis M, Nagesh D, Sanchez PJ, Lenz R, Park SJ, Ruff JG, et al.: Use of dehydrated human amnion/chorion membrane allografts in more than 100 patients with six major types of refractory nonhealing wounds. J Am Podiatr Med Assoc 2018; 108(2):84-89.
- Tettelbach W, Cazzell S, Reyzelman AM, Sigal F, Caporusso JM, Agnew PS.: A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: A prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J 2019; 16(1):19-29.
- Guest JF, Atkin L, Aitkins C.: Potential cost-effectiveness of using adjunctive dehydrated human amnion/chorion membrane allograft in the management of non-healing diabetic foot ulcers in the United Kingdom. Int Wound J 2021; 18(6):889-901.
- Kotronoulas A, Jónasdóttir HS, Sigurðardóttir RS, Halldórsson S, Haraldsson GG, Rolfsson Ó.: Wound healing grafts: Omega-3 fatty acid lipid content differentiates the lipid profiles of acellular Atlantic cod skin from traditional dermal substitutes. J Tissue Eng Regen Med 2020; 14(3):441-451.
- Alam K, Jeffery SLA.: Acellular fish skin grafts for management of split thickness donor sites and partial thickness burns: A case series. Mil Med 2019; 184(Suppl 1):16-20.
- Michael S, Winters C, Khan M.: Acellular fish skin graft use for diabetic lower extremity wound healing: A retrospective study of 58 ulcerations and a literature review. Wounds 2019; 31(10):262-268.
- Woodrow T, Chant T, Chant H.: Treatment of diabetic foot wounds with acellular fish skin graft rich in omega-3: A prospective evaluation. J Wound Care 2019; 28(2):76-80.
- Kirsner R, Dove C, Reyzelman A, Vayser D, Jaimes H.: A prospective, randomized, controlled clinical trial on the efficacy of a single-use negative pressure wound therapy system, compared to traditional negative pressure wound therapy in the treatment of chronic ulcers of the lower extremities. Wound Repair Regen 2019; 27(5):519-529.
- Badois N, Bauër P, Cheron M, Hoffmann C, Nicodeme M, Choussy O, et al.: Acellular fish skin matrix on thin-skin graft donor sites: A preliminary study. J Wound Care 2019; 28(9):624-628.
- Badylak SF, Lantz GC, Coffey A, Geddes LA.: Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res 1989; 47(1):74-80.
- Brown-Etris M, Milne CT, Hodde JP.: An extracellular matrix graft (Oasis(®) wound matrix) for treating full-thickness pressure ulcers: A randomized clinical trial. J Tissue Viability 2019; 28(1):21-26.
- Gabriel A, Maxwell GP.: AlloDerm RTU integration and clinical outcomes when used for reconstructive breast surgery. Plast Reconstr Surg Glob Open 2018; 6(5):e1744.
- Stone RC, Stojadinovic O, Rosa AM, Ramirez HA, Badiavas E, Blumenberg M, et al:. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci Transl Med 2017; 9(371)eaaf8611.
- Téot L, Mustoe TA, Middelkoop E, Gauglitz GG.: Textbook on scar management - State of the art management and emerging technologies. Springer Cham; 2020. ISBN 978-3-030-44765-6
- Brockmann I, Ehrenpfordt J, Sturmheit T, Brandenburger M, Kruse C, Zille M, et al.: Skin-derived stem cells for wound treatment using cultured epidermal autografts: Clinical applications and challenges. Stem Cells Int 2018; 2018:4623615.
- Meuli M, Hartmann-Fritsch F, Hüging M, Marino D, Saglini M, Hynes S, et al.: A cultured autologous dermo-epidermal skin substitute for full-thickness skin defects: A phase I, open, prospective clinical trial in children. Plast Reconstr Surg 2019; 144(1):188-198.
- Won JY, Lee MH, Kim MJ, Min KH, Ahn G, Han JS, et al.: A potential dermal substitute using decellularized dermis extracellular matrix derived bio-ink. Artif Cells Nanomed Biotechnol 2019; 47(1):644-649.
- Dalla Paola L, Faglia E.: Treatment of diabetic foot ulcer: An overview strategies for clinical approach. Curr Diabetes Rev 2006; 2(4):431-47.
- Uçkay I, Aragón-Sánchez J, Lew D, Lipsky BA.: Diabetic foot infections: What have we learned in the last 30 years?. Int J Infect Dis 2015; 40:81-91.
- Senneville E, Robineau O.: Treatment options for diabetic foot osteomyelitis. Expert Opin Pharmacother 2017; 18(8):759-765.
- Blume P, Wu S.: Updating the diabetic foot treatment algorithm: Recommendations on treatment using advanced medicine and therapies. Wounds 2018; 30(2):29-35.
- Levin LS.: The reconstructive ladder. An orthoplastic approach. Orthop Clin North Am 1993; 24(3):393-409.
- Apelqvist J.: Diagnostics and treatment of the diabetic foot. Endocrine 2012; 41(3):384-97.
- Pehde CE, Bennett J, Kingston M.: Orthoplastic approach for surgical treatment of diabetic foot ulcers. Clin Podiatr Med Surg 2020; 37(2):215-230.
- Azoury SC, Stranix JT, Kovach SJ, Levin LS.: Principles of orthoplastic surgery for lower extremity reconstruction: Why is this important? J Reconstr Microsurg 2021; 37(1):42-50.
- Liette MD, Crisologo PA, Johnson LJ, Henning JA, Rodriguez-Collazo ER, Masadeh S.: A surgical approach to location-specific neuropathic foot ulceration. Clin Podiatr Med Surg 2021; 38(1):31-53.
- de Oliveira AL, Moore Z.: Treatment of the diabetic foot by offloading: A systematic review. J Wound Care 2015; 24(12):560, 562-70.
- Bus SA.: The role of pressure offloading on diabetic foot ulcer healing and prevention of recurrence. Plast Reconstr Surg 2016; 138(3 Suppl):179s-187s.
- Castro-Aragon OE, Rapley JH, Trevino SG.: The use of a kickstand modification for the prevention of heel decubitus ulcers in trauma patients with lower extremity external fixation. J Orthop Trauma 2009; 23(2):145-7.
- Wukich DK, Motko J.: Safety of total contact casting in high-risk patients with neuropathic foot ulcers. Foot Ankle Int 2004; 25(8):556-60.
- McHenry T, Simmons S, Alitz C, Holcomb J.: Forward surgical stabilization of penetrating lower extremity fractures: Circular casting versus external fixation. Mil Med 2001; 166(9):791-5.
- Buford GA, Trzeciak MA.: A novel method for lower-extremity immobilization after free-flap reconstruction of posterior heel defects. Plast Reconstr Surg 2003; 111(2):821-4.
- Dalla Paola L, Carone A, Boscarino G, Scavone G, Vasilache L.: Combination of open subtotal calcanectomy and stabilization with external fixation as limb salvage procedure in hindfoot-infected diabetic foot ulcers. Int J Low Extrem Wounds 2016; 15(4):332-337.
- Grant WP, Rubin LG, Pupp GR, Vito G, Jacobus D, Jerlin EA, et al.: Mechanical testing of seven fixation methods for generation of compression across a midtarsal osteotomy: A comparison of internal and external fixation devices. J Foot Ankle Surg 2007; 46(5):325-35.
- Saltzman CL.: Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J 2005; 25:47-52.
- Frykberg RG, Sage RA, Wukich DK, Pinzur MS, Schuberth JM.: Charcot arthropathy. Foot Ankle Spec 2012; 5(4):262-71.
- Myerson MS, Henderson MR, Saxby T, Short KW.: Management of midfoot diabetic neuroarthropathy. Foot Ankle Int 1994; 15(5):233-41.
- Pinzur MS, Sage R, Stuck R, Kaminsky S, Zmuda A.: A treatment algorithm for neuropathic (Charcot) midfoot deformity. Foot Ankle 1993; 14(4):189-97.
- Pinzur MS.: Neutral ring fixation for high-risk nonplantigrade Charcot midfoot deformity. Foot Ankle Int 2007; 28(9):961-6.
- Dalla Paola L, Brocco E, Ceccacci T, Ninkovic S, Sorgentone S, Marinescu MG, et al.: Limb salvage in Charcot foot and ankle osteomyelitis: Combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int 2009; 30(11):1065-70.
- Pinzur MS, Gil J, Belmares J.: Treatment of osteomyelitis in Charcot foot with single-stage resection of infection, correction of deformity, and maintenance with ring fixation. Foot Ankle Int 2012; 33(12):1069-74.
- Dalla Paola L.: Confronting a dramatic situation: the Charcot foot complicated by osteomyelitis. Int J Low Extrem Wounds 2014; 13(4):247-62.
- Frykberg RG, Wukich DK, Kavarthapu V, Zgonis T, Dalla Paola L.: Surgery for the diabetic foot: A key component of care. Diabetes Metab Res Rev 2020; 36 (Suppl 1):e3251.
- Latt LD, Glisson RR, Adams Jr. SB, Schuh R, Narron JA, Easley ME.: Biomechanical comparison of external fixation and compression screws for transverse tarsal joint arthrodesis. Foot Ankle Int 2015; 36(10):1235-42.
- Cooper PS.: Application of external fixators for management of Charcot deformities of the foot and ankle. Foot Ankle Clin 2002; 7(1):207-54.
- Jolly GP, Zgonis T, Polyzois V.: External fixation in the management of Charcot neuroarthropathy. Clin Podiatr Med Surg 2003; 20(4):741-56.
- Baravarian B, Van Gils CC.: Arthrodesis of the Charcot foot and ankle. Clin Podiatr Med Surg 2004; 21(2):271-89.
- Farber DC, Juliano PJ, Cavanagh PR, Ulbrecht J, Caputo G.: Single stage correction with external fixation of the ulcerated foot in individuals with Charcot neuroarthropathy. Foot Ankle Int 2002; 23(2):130-4.
- Roukis TS, Zgonis T.: The management of acute Charcot fracture-dislocations with the Taylor’s spatial external fixation system. Clin Podiatr Med Surg 2006; 23(2):467-83, viii.
- Bibbo C, Stough JD.: Reduction calcaneoplasty and local muscle rotation flap as a salvage option for calcaneal osteomyelitis with soft tissue defect. J Foot Ankle Surg 2012; 51(3):375-8.
- Oznur A.: Management of large soft-tissue defects in a diabetic patient. Foot Ankle Int 2003; 24(1):79-82.
- Oznur A, Tokgözoğlu M.: Closure of central defects of the forefoot with external fixation: A case report. J Foot Ankle Surg 2004; 43(1):56-9.
- Roukis TS, Zgonis T.: Skin grafting techniques for soft-tissue coverage of diabetic foot and ankle wounds. J Wound Care 2005; 14(4):173-6.
- Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB.: Off-loading the diabetic foot wound: A randomized clinical trial. Diabetes Care 2001; 24(6):1019-22.
- Sagebien CA, Rodriguez ED, Turen CH.: The soft-tissue frame. Plast Reconstr Surg 2007; 119(7):2137-2140.
- Clemens MW, Parikh P, Hall MM, Attinger CE.: External fixators as an adjunct to wound healing. Foot Ankle Clin 2008; 13(1):145-56, vi-vii.
- Ramanujam CL, Facaros Z, Zgonis T.: External fixation for surgical off-loading of diabetic soft tissue reconstruction. Clin Podiatr Med Surg 2011; 28(1):211-6.
- Lowenberg DW, Sadeghi C, Brooks D, Buncke GM, Buntic RF.: Use of circular external fixation to maintain foot position during free tissue transfer to the foot and ankle. Microsurgery 2008; 28(8):623-7.
- Oznur A, Zgonis T.: Closure of major diabetic foot wounds and defects with external fixation. Clin Podiatr Med Surg 2007; 24(3):519-28, ix.
- Klaue K.: The role of external fixation in acute foot trauma. Foot Ankle Clin 2004; 9(3):583-94, x.
- Kim PJ, Attinger CE, Crist BD, Gabriel A, Galiano RD, Gupta S, et al.: negative pressure wound therapy with instillation: Review of evidence and recommendations. Wounds 2015; 27(12):S2-s19.
- Dalla Paola L.: Diabetic foot wounds: The value of negative pressure wound therapy with instillation. Int Wound J 2013; 10 Suppl 1(Suppl 1):25-31.
- Jerosch J.: Intracompartmental pressure of the anterior tibial compartment as a function of body and joint position. Biomed Tech (Berl) 1989; 34(9):202-6.
- Willy C, Gerngross H, Sterk J.: Measurement of intracompartmental pressure with use of a new electronic transducer-tipped catheter system. J Bone Joint Surg Am 1999; 81(2):158-68.
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al.: High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007; 50(1):18-25.
- Johani K, Fritz BG, Bjarnsholt T, Lipsky BA, Jensen SO, Yang M, et al.: Understanding the microbiome of diabetic foot osteomyelitis: Insights from molecular and microscopic approaches. Clin Microbiol Infect 2019; 25(3):332-339.
- Howlin RP, Brayford MJ, Webb JS, Cooper JJ, Aiken SS, Stoodley P.: Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother 2015; 59(1):111-20.
- Markakis K, Faris AR, Sharaf H, Faris B, Rees S, Bowling FL.: Local antibiotic delivery systems: Current and future applications for diabetic foot infections. Int J Low Extrem Wounds 2018; 17(1):14-21.
- Hake ME, Young H, Hak DJ, Stahel PF, Hammerberg EM, Mauffrey C.: Local antibiotic therapy strategies in orthopaedic trauma: Practical tips and tricks and review of the literature. Injury 2015; 46(8):1447-56.
- Ferguson J, Diefenbeck M, McNally M.: Ceramic biocomposites as biodegradable antibiotic carriers in the treatment of bone infections. J Bone Jt Infect 2017; 2(1):38-51.
- Stravinskas M, Horstmann P, Ferguson J, Hettwer W, Nilsson M, Tarasevicius S, et al.: Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: In vitro and clinical release studies. Bone Joint Res 2016; 5(9):427-35.
- Neut D, van de Belt H, Stokroos I, van Horn JR, van der Mei HC, Busscher HJ.: Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother 2001; 47(6):885-91.
- Liu C, You JX, Chen YX, Zhu WF, Wang Y, Lv PP, et al.: Effect of induced membrane formation Followed by Polymethylmethacrylate Implantation on Diabetic foot ulcer healing when revascularization is not feasible. J Diabetes Res 2019; 2019:2429136.
- Elmarsafi T, Oliver NG, Steinberg JS, Evans KK, Attinger CE, Kim PJ.: Long-term outcomes of permanent cement spacers in the infected foot. J Foot Ankle Surg 2017; 56(2):287-290.
- Oryan A, Alidadi S, Moshiri A, Maffulli N.: Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 2014; 9(1):18.
- Wang W, Yeung KWK.: Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater 2017; 2(4):224-247.
- Jogia RM, Modha DE, Nisal K, Berrington R, Kong MF.: Use of highly purified synthetic calcium sulfate impregnated with antibiotics for the management of diabetic foot ulcers complicated by osteomyelitis. Diabetes Care 2015; 38(5):e79-80.
- Patil P, Singh R, Agarwal A, Wadhwa R, Bal A, Vaidya S.: Diabetic foot ulcers and osteomyelitis: Use of biodegradable calcium sulfate beads impregnated with antibiotics for treatment of multidrug-resistant organisms. Wounds 2021; 33(3):70-76.
- Qin CH, Zhou CH, Song HJ, Cheng GY, Zhang HA, Fang J, et al.: Infected bone resection plus adjuvant antibiotic-impregnated calcium sulfate versus infected bone resection alone in the treatment of diabetic forefoot osteomyelitis. BMC Musculoskelet Disord 2019; 20(1):246.
- Whisstock C, Volpe A, Ninkovic S, Marin M, Meloni M, Bruseghin M, et al.: Multidisciplinary approach for the management and treatment of diabetic foot infections with a resorbable, gentamicin-loaded bone graft substitute. J Clin Med 2020; 9(11):3586.
- Hutting KH, de Stegge WBA, van Netten JJ, Cate WAT, Smeets L, Welten GMJM, et al.: Surgical treatment of diabetic foot ulcers complicated by osteomyelitis with gentamicin-loaded calcium sulphate-hydroxyapatite biocomposite. J Clin Med 2021; 10(2):371.
- Niazi NS, Drampalos E, Morrissey N, Jahangir N, Wee A, Pillai A.: Adjuvant antibiotic loaded bio composite in the management of diabetic foot osteomyelitis - A multicentre study. Foot 2019; 39:22-27.
- Drampalos E, Mohammad HR, Kosmidis C, Balal M, Wong J, Pillai A.: Single stage treatment of diabetic calcaneal osteomyelitis with an absorbable gentamicin-loaded calcium sulphate/hydroxyapatite biocomposite: The silo technique. Foot 2018; 34:40-44.
- Chatzipapas C, Kougioumtzis IE, Karaglani M, Panagopoulos P, Panopoulou M, Papazoglou D, et al.: Local antibiotic delivery systems in the surgical treatment of diabetic foot osteomyelitis: Again, no benefit? Int J Low Extrem Wounds 2020; 1534734620973961.
- Lindfors NC, Hyvönen P, Nyyssönen M, Kirjavainen M, Kankare J, Gullichsen E, et al.: Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010; 47(2):212-8.
- Lindfors N, Geurts J, Drago L, Arts JJ, Juutilainen V, Hyvönen P, et al.: Antibacterial bioactive glass, S53P4, for chronic bone infections - A multinational study. Adv Exp Med Biol 2017; 971:81-92.
- van Gestel NAP, Geurts J, Hulsen DJW, van Rietbergen B, Hofmann S, Arts JJ.: Clinical applications of S53P4 bioactive glass in bone healing and osteomyelitic treatment: A literature review. Biomed Res Int 2015; 2015:684826.
- Romanò CL, Logoluso N, Meani E, Romanò D, De Vecchi E, Vassena C, et al.: A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis: A retrospective comparative study. Bone Joint J 2014; 96-B(6):845-50.
- Godoy-Santos AL, Rosemberg LA, de Cesar-Netto C, Armstrong DG.: The use of bioactive glass S53P4 in the treatment of an infected Charcot foot: A case report. J Wound Care 2019; 28(Sup1):S14-s17.
- De Giglio R, Stefani I, Mondello T, De Filippis G, Mazzone A.: Bioactive glass S53P4: A new opportunity for the treatment in the diabetic foot osteomyelitis. Eur J Intern Med 2018; 54:e15-e16.
- Rodríguez Á, Parra G, Cuervas-Mons M.: Bioactive glass, a new tool for the treatment in the diabetic foot recalcitrant osteomyelitis: A case series with 24-month follow-up. Foot 2021; 48:101831.
- De Giglio R, Di Vieste G, Mondello T, Balduzzi G, Masserini B, Formenti I, et al.: Efficacy and safety of bioactive glass S53P4 as a treatment for diabetic foot osteomyelitis. J Foot Ankle Surg 2021; 60(2):292-296.
- Kastrin M, Rovan VU, Frangež I.: Possible advantages of S53P4 bioactive glass in the treatment of septic osteoarthritis of the first metatarsophalangeal joint in the diabetic foot. J Clin Med 2021; 10(6):1208.
- Dörr S, Holland-Letz AK, Weisser G, Chatzitomaris A, Lobmann R.: Bacterial diversity, antibiotic resistance, and the risk of lower limb amputation in younger and older individuals with diabetic foot infection. Int J Low Extrem Wounds 2021: 1534734621992290.
- Hicks CW, Selvarajah S, Mathioudakis N, Sherman RE, Hines KF, Black 3rd JH, et al.: Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg 2016; 33:149-58.
- McGuire J, Thomson A, Kennedy PG.: The biomechanics of diabetic foot amputation. Wounds. 2021; WNDS20210414-2.
- Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, et al.: Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob Health 2019; 7(8):e1020-e1030.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et. al.: Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007; 45(Suppl) S:S5-67.
- Hagenström K, Garbe C, Debus ES, Augustin M.: Vascular diagnostic and surgical treatments before lower limb amputations in patients with arterial vascular diseases: A population based study from 2013 to 2015 in Germany. Eur J Vasc Endovasc Surg 2021; 62(3):469-475.
- Aboyans V, Ricco JB, Bartelink MLEL, Björck M, Brodmann M, Cohnert T, et al:. Editor’s choice - 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55(3):305-368.
- Stella J, Engelbertz C, Gebauer K, Hassu J, Meyborg M, Freisinger E, et al.: Outcome of patients with chronic limb-threatening ischemia with and without revascularization. Vasa 2020; 49(2):121-127.
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al.: Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019; 58(1S):S1-S109.e33.
- 240. Liang P, Marcaccio CL, Darling JD, Kong D, Rao V, St John E, et al.: Validation of the Global Limb Anatomic Staging System in first-time lower extremity revascularization. J Vasc Surg 2021; 73(5):1683-1691.e1.
- Mills JL, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al.: The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014; 59(1):220-34.e1-2.
- Zhan LX, Branco BC, Armstrong DG, Mills Sr. JL.: The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System based on wound, ischemia, and foot infection (WIfI) correlates with risk of major amputation and time to wound healing. J Vasc Surg 2015; 61(4):939-44.
- Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al.: The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 2016; 63(2 Suppl):3S-21S.
- Normahani P, Epstein DM, Gaggero A, Davies AH, Sounderajah V, Jaffer U.: Cost-effectiveness of diagnostic tools to establish the presence of peripheral arterial disease in people with diabetes. Ann Surg 2021; doi: 10.1097
- Caradu C, Stenson K, Houmaïda H, Ny JL, Lalys F, Ducasse E, et al.: EndoNaut 2D fusion-imaging with a mobile c-arm for endovascular treatment of occlusive peripheral arterial disease. J Vasc Surg 2021; 75(2):651-659.
- Tacher V, Lin M, Desgranges P, Deux JF, Grünhagen T, Becquemin JP, et al.: Image guidance for endovascular repair of complex aortic aneurysms: Comparison of two-dimensional and three-dimensional angiography and image fusion. J Vasc Interv Radiol 2013; 24(11):1698-706.
- Mougin J, Louis N, Maupas E, Goueffic Y, Fabre D, Stéphan H.: Fusion imaging guidance for endovascular recanalization of peripheral occlusive disease. J Vasc Surg 2022; 75(2):610-617.
- Mialhe C, Raffort J, Lareyre F.: Holographic imaging with the HoloLens head mounted system to enhance angio suite ergonomics during an endovascular procedure. Eur J Vasc Endovasc Surg 2021; 61(5):849-850.
- Hughes K, Olufajo OA, White K, Roby DH, Fryer CS, Wright JL, et al.: The influence of socioeconomic status on outcomes of lower extremity arterial reconstruction. J Vasc Surg 2022; 75(1):168-176.
- Roijers JP, Rakké YS, Hopmans CJ, Buimer MG, Ho GH, Groot HGWd, et al.: A mortality prediction model for elderly patients with critical limb ischemia. J Vasc Surg 2020; 71(6):2065-2072.e2.
- Verwer MC, Wijnand JGJ, Teraa M, Verhaar MC, Borst GJd.: Long term survival and limb salvage in patients with non-revascularisable chronic limb threatening ischaemia. Eur J Vas Endovasc Surg 2021; 62(2):225-232.
- van Reijen NS, Hensing T, Santema TKB, Ubbink DT, Koelemay MJW.: Outcomes of conservative treatment in patients with chronic limb threatening ischaemia: A systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2021; 62(2):214-224.
- Darling JD, McCallum JC, Soden PA, Korepta L, Guzman RJ, Wyers MC, et al.: Results for primary bypass versus primary angioplasty/stent for lower extremity chronic limb-threatening ischemia. J Vasc Surg 2017; 66(2):466-475.
- Mohapatra A, Boitet A, Malak O, Henry JC, Avgerinos ED, Makaroun MS, et al.: Peroneal bypass versus endovascular peroneal intervention for critical limb ischemia. J Vasc Surg 2019; 69(1):148-155.
- Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, et al.: Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication. J Vasc Surg 2015; 61(3 Suppl):2S-41S.
- Scheinert D, Schmidt A, Zeller T, Müller-Hülsbeck S, Sixt S, Schröder H, et al.: German center subanalysis of the LEVANT 2 global randomized study of the lutonix drug-coated balloon in the treatment of femoropopliteal occlusive disease. J Endovasc Ther 2016; 23(3):409-16.
- Shishehbor MH, Schneider PA, Zeller T, Razavi MK, Laird JR, Wang H, et al.: Total IN.PACT drug-coated balloon initiative reporting pooled imaging and propensity-matched cohorts. J Vasc Surg 2019; 70(4):1177-1191.e9.
- Shishehbor MH, Hammad TA, Zeller T, Baumgartner I, Scheinert D, Rocha-Singh KJ.: An analysis of IN.PACT DEEP randomized trial on the limitations of the societal guidelines-recommended hemodynamic parameters to diagnose critical limb ischemia. J Vasc Surg 2016; 63(5):1311-7.
- Kasapis C, Henke PK, Chetcuti SJ, Koenig GC, Rectenwald JE, Krishnamurthy VN, et al.: Routine stent implantation vs. percutaneous transluminal angioplasty in femoropopliteal artery disease: A meta-analysis of randomized controlled trials. Eur Heart J 2009; 30(1):44-55.
- Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, et al.: Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation 2016; 133(15):1472-83; discussion 1483.
- Müller-Hülsbeck S, Keirse K, Zeller T, Schroë H, Diaz-Cartelle J.: Long-term results from the MAJESTIC trial of the Eluvia paclitaxel-eluting stent for femoropopliteal treatment: 3-year follow-up. Cardiovasc Intervent Radiol 2017; 40(12):1832-1838.
- Gray WA, Keirse K, Soga Y, Benko A, Babaev A, Yokoi Y, et al.: A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): A randomised, non-inferiority trial. Lancet 2018; 392(10157):1541-1551.
- Bausback Y, Wittig T, Schmidt A, Zeller T, Bosiers M, Peeters P, et al.: Drug-eluting stent versus drug-coated balloon revascularization in patients with femoropopliteal arterial disease. J Am Coll Cardiol 2019; 73(6):667-679.
- Zhou Y, Zhang Z, Lin S, Xiao J, Ai W, Wang J, et al.: Comparative effectiveness of endovascular treatment modalities for de novo femoropopliteal lesions: A network meta-analysis of randomized controlled trials. J Endovasc Ther 2020; 27(1):42-59.
- Lammer J, Zeller T, Hausegger KA, Schaefer PJ, Gschwendtner M, Mueller-Huelsbeck S, et al.: Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: The randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease). J Am Coll Cardiol 2013; 62(15):1320-7.
- Krievins DK, Halena G, Scheinert D, Savlovskis J, Szopiński P, Krämer A, et al.: One-year results from the DETOUR I trial of the PQ bypass DETOUR system for percutaneous femoropopliteal bypass. J Vasc Surg 2020; 72(5):1648-1658.e2.
- Katsanos K, Spiliopoulos S, Teichgräber U, Kitrou P, Del Giudice C, Björkman P, et al.: Risk of major amputation following application of paclitaxel coated balloons in the lower limb arteries: A systematic review and meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg 2022; 63(1):60-71.
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D.: Risk of death following application of Paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018; 7(24):e011245.
- Schneider PA, Laird JR, Doros G, Gao Q, Ansel G, Brodmann M, et al.: Mortality not correlated with Paclitaxel Exposure: An independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol 2019; 73(20):2550-2563.
- Nordanstig J, James S, Andersson M, Andersson M, Danielsson P, Gillgren P, et al.: Mortality with Paclitaxel-coated devices in peripheral artery disease. N Engl J Med 2020; 383(26):2538-2546.
- Dinh K, Gomes ML, Thomas SD, Paravastu SCV, Holden A, Schneider PA, et al.: Mortality after Paclitaxel-coated device use in patients with chronic limb-threatening ischemia: A systematic review and meta-analysis of randomized controlled trials. J Endovasc Ther 2020; 27(2):175-185.
- Bob-Manuel T, Obi K, N’Dandu Z.: Successful revascularization of infrapopliteal chronic Total occlusions using the plantar arch as a conduit and retrograde pedal access. Ochsner J. 2021; 21(2):209-213.
- Silvestro M, Palena LM, Manzi M, Gómez-Jabalera E, Vishwanath D, Casini A, et al.: Anterolateral retrograde access to the distal popliteal artery and to the tibioperoneal trunk for recanalization of femoropopliteal chronic total occlusions. J Vasc Surg 2018; 68(6):1824-1832.
- Gahide G, Bui BT, Beland M.: The Cupidon’s strike technique: How to never miss a rendezvous while going on SAFARI. Ann Vasc Surg 2021; 70:555-558.
- Geraghty PJ, Adams G, Schmidt A, Investigators TIB.: Six-month pivotal results of tack optimized balloon angioplasty using the Tack Endovascular System in below-the-knee arteries. J Vasc Surg 2021; 73(3):918-929.e5.
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al.: Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85(3):221-8.
- Dong Y, Yang Q, Sun X.: Comprehensive analysis of cell therapy on chronic skin wound healing: A meta-analysis. Hum Gene Ther 2021; 32(15-16):787-795.
- Beltrán-Camacho L, Rojas-Torres M, Durán-Ruiz MC.: Current status of angiogenic cell therapy and related strategies applied in critical limb ischemia. Int J Mol Sci 2021; 22(5):2335.
- Yunir E, Kurniawan F, Rezaprasga E, Wijaya IP, Suroyo I, Matondang S, et al.: Autologous bone-marrow vs. peripheral blood mononuclear cells therapy for peripheral artery disease in diabetic patients. Int J Stem Cells 2021; 14(1):21-32.
- Jaluvka F, Ihnat P, Madaric J, Vrtkova A, Janosek J, Prochazka V.: Current status of cell-based therapy in patients with critical limb ischemia. Int J Mol Sci 2020; 21(23):8999.
- Gao W, Chen D, Liu G, Ran X.: Autologous stem cell therapy for peripheral arterial disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther 2019; 10(1):140.
- Soria-Juan B, Escacena N, Capilla-González V, Aguilera Y, Llanos L, Tejedo JR, et al.: Cost-effective, safe, and personalized cell therapy for critical limb ischemia in type 2 diabetes mellitus. Front Immunol 2019; 10:1151.
- Xie B, Luo H, Zhang Y, Wang Q, Zhou C, Xu D.: Autologous stem cell therapy in critical limb ischemia: A meta-analysis of randomized controlled trials. Stem Cells Int 2018; 2018:7528464.
- Wahid SFA, Ismail NA, Jamaludin WFW, Muhamad NA, Hamid MKAA, Harunarashid H, et al.: Autologous cells derived from different sources and administered using different regimens for ‘no-option’ critical lower limb ischaemia patients. Cochrane Database Syst Rev 2018; 8(8):CD010747.
- Shu X, Shu S, Tang S, Yang L, Liu D, Li K, et al.: Efficiency of stem cell based therapy in the treatment of diabetic foot ulcer: a meta-analysis. Endocr J 2018; 65(4):403-413.
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al.: Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002; 360(9331):427-35.
- Jiang X, Zhang H, Teng M.: Effectiveness of autologous stem cell therapy for the treatment of lower extremity ulcers: A systematic review and meta-analysis. Medicine (Baltimore) 2016; 95(11):e2716.
- Liew A, Bhattacharya V, Shaw J, Stansby G.: Cell therapy for critical limb ischemia: A meta-analysis of randomized controlled trials. Angiology 2016; 67(5):444-55.
- Guo J, Dardik A, Fang K, Huang R, Gu Y.: Meta-analysis on the treatment of diabetic foot ulcers with autologous stem cells. Stem Cell Res Ther 2017; 8(1):228.
- Benoit E, O’Donnell TF, Patel AN.: Safety and efficacy of autologous cell therapy in critical limb ischemia: a systematic review. Cell Transplant 2013; 22(3):545-62.
- Rigato M, Monami M, Fadini GP.: Autologous cell therapy for peripheral arterial disease: Systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res 2017; 120(8):1326-1340.
- Capoccia BJ, Gregory AD, Link DC.: Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J Leukoc Biol 2008; 84(3):760-8.
- Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, et al.: CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012; 120(3):613-25.
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al.: Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010; 116(5):829-40.
- Baer C, Squadrito ML, Iruela-Arispe ML, De Palma M.: Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp Cell Res 2013; 319(11):1626-34.
- Vågesjö E, Parv K, Ahl D, Seignez C, Hidalgo CH, Giraud A, et al.: Perivascular macrophages regulate blood flow following tissue damage. Circ Res 2021; 128(11):1694-1707.
- Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, et al.: Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. Embo J 2018; 37(13):e97786.
- Fung E, Helisch A.: Macrophages in collateral arteriogenesis. Front Physiol 2012; 3:353.
- Barnett FH, Rosenfeld M, Wood M, Kiosses WB, Usui Y, Marchetti V, et al.: Macrophages form functional vascular mimicry channels in vivo. Sci Rep 2016; 6:36659.
- Martin KE, García AJ.: Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater 2021; 133:4-16.
- Krishnasamy K, Limbourg A, Kapanadze T, Gamrekelashvili J, Beger C, Häger C, et al.: Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat Commun 2017; 8(1):952.
- Awad O, Dedkov EI, Jiao C, Bloomer S, Tomanek RJ, Schatteman GC.: Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol 2006; 26(4):758-64.
- Spaltro G, Straino S, Gambini E, Bassetti B, Persico L, Zoli S, et al.: Characterization of the Pall Celeris system as a point-of-care device for therapeutic angiogenesis. Cytotherapy 2015; 17(9):1302-13.
- Procházka V, Gumulec J, Jalvvka F, Salounová D, Jonszta T, Czerný D, et al.: Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant 2010; 19(11):1413-24.
- Sen CK.: Human wounds and its burden: An updated compendium of estimates. Adv Wound Care (New Rochelle) 2019; 8(2):39-48.
- Kyaw BM, Järbrink K, Martinengo L, Car J, Harding K, Schmidtchen A.: Need for improved definition of “chronic wounds” in clinical studies. Acta Derm Venereol 2018; 98(1):157-158.
- Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, et al.: The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev 2017; 6(1):15.
- Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, et al.: An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health 2018; 21(1):27-32.
- Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M.: Cost-of-illness studies in chronic ulcers: A systematic review. J Wound Care 2017; 26(sup4):S4-s14.
- Olsson M, Järbrink K, Divakar U, Bajpai R, Upton Z, Schmidtchen A, et al.: The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen 2019; 27(1):114-125.
- Ziede E, Orellana E, Tejos R, Olivera M, Searle S. Pressure ulcers: the last mile in the COVID-19 road. Arch Plast Surg. 2022 Jan;49(1):137-138. doi: 10.5999/aps.2021.01354. Epub 2022 Jan 15. PMID: 35086325; PMCID: PMC8795641.
- Zelen CM, Serena TE, Gould L, Le L, Carter MJ, Keller J, et al.: Treatment of chronic diabetic lower extremity ulcers with advanced therapies: A prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int Wound J 2016; 13(2):272-82.
- Carter MJ.: Dehydrated human amnion and chorion allograft versus standard of care alone in treatment of Wagner 1 diabetic foot ulcers: A trial-based health economics study. J Med Econ 2020; 23(11):1273-1283.
- Guest JF, Singh H, Rana K, Vowden P.: Cost-effectiveness of an electroceutical device in treating non-healing venous leg ulcers: Results of an RCT. J Wound Care 2018; 27(4):230-243.
- Hayes PD, Harding KG, Johnson SM, McCollum C, Téot L, Mercer K, et al.: A pilot multi-centre prospective randomised controlled trial of RECELL for the treatment of venous leg ulcers. Int Wound J 2020; 17(3):742-752.
- Towler MA, Rush EW, Richardson MK, Williams CL.: Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg 2018; 35(3):357-365.
- Hermans MHE, Lee SK, Ragan MR, Laudi P.: Results of a retrospective comparative study: Material cost for managing a series of large wounds in subjects with serious morbidity with a hydrokinetic fiber dressing or negative pressure wound therapy. Wounds 2015; 27(3):73-82.
- Souliotis K, Kalemikerakis I, Saridi M, Papageorgiou M, Kalokerinou A.: A cost and clinical effectiveness analysis among moist wound healing dressings versus traditional methods in home care patients with pressure ulcers. Wound Repair Regen 2016; 24(3):596-601.
- Hodgson H, Davidson D, Duncan A, Guthrie J, Henderson E, MacDiarmid M, et al.: A multicentre, clinical evaluation of a hydro-responsive wound dressing: The Glasgow experience. J Wound Care 2017; 26(11):642-650.
- Guest JF, Sladkevicius E, Panca M.: Cost-effectiveness of using Polyheal compared with surgery in the management of chronic wounds with exposed bones and/or tendons due to trauma in France, Germany and the UK. Int Wound J 2015; 12(1):70-82.
- Kempton LB, Larson TB, Montijo HE, Seymour RB, Getz SB, Bosse MJ.: Increased cost of negative pressure dressings is not justified for split-thickness skin grafting of low-risk wounds. J Orthop Trauma 2015; 29(7):301-6.
- Jiang N, Wu HT, Lin QR, Hu YJ, Yu B.: Health care costs of post-traumatic osteomyelitis in China: Current situation and influencing factors. J Surg Res 2020; 247:356-363.
- Png ME, Madan JJ, Dritsaki M, Achten J, Parsons N, Fernandez M, et al.: Cost-utility analysis of standard dressing compared with incisional negative-pressure wound therapy among patients with closed surgical wounds following major trauma to the lower limb. Bone Joint J 2020; 102-B(8):1072-1081.
- Austin RE, Merchant N, Shahrokhi S, Jeschke MG.: A comparison of Biobrane™ and cadaveric allograft for temporizing the acute burn wound: Cost and procedural time. Burns 2015; 41(4):749-53.
- Erring M, Gaba S, Mohsina S, Tripathy S, Sharma RK.: Comparison of efficacy of silver-nanoparticle gel, nano-silver-foam and collagen dressings in treatment of partial thickness burn wounds. Burns 2019; 45(8):1888-1894.
- Song YP, Chen HL.: More economic evaluations are needed in calculating the burden of wound care. Int Wound J 2020; 17(5):1541-1542.
- Rubio DM, Schoenbaum EE, Lee LS, Schteingart DE, Marantz PR, Anderson KE, et al.: Defining translational research: Implications for training. Acad Med 2010; 85(3):470-5.
- Wang X.: A new vision of definition, commentary, and understanding in clinical and translational medicine. Clin Transl Med 2012; 1(1):5.
- Cohrs RJ, Martin T, Ghahramani P, Bidaut L, Higgins PJ, Shahzad A. Translational medicine definition by the European Society for Translational Medicine. European Journal of Molecular & Clinical Medicine, 2015; 2(3): 86-88
- European Commission, New EU rules on medical devices to enhance patient safety and modernise public health, (2017). https://ec.europa.eu/commission/presscorner/detail/en/IP_17_847
- European Commission, Transition Timelines from the Directives to the Medical Revices Regulation, (2020). https://ec.europa.eu/health/sites/health/files/md_newregulations/docs/timeline_mdr_en.pdf (accessed October 14, 2022).
- European Union Law.: Regulation (EU) 2017/745 of the European Parliament and the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Official Journal of the European Union – EUR-Lex, 2017. 5 of April 2017. Available at URL: https://eur-lex.europa.eu/legal content/EN/TXT/?uri=CELEX%3A32017R0745
- Vasiljeva K, van Duren BH, Pandit H.: Changing device regulations in the European Union: Impact on research, innovation and clinical practice. Indian J Orthop 2020; 54(2):123-129.
- Guyonnet L, Detriché G, Gendron N, Philippe A, Latremouille C, Soret L, et al.: Elevated circulating stem cells level is observed one month after implantation of Carmat bioprosthetic total artificial heart. Stem Cell Rev Rep 2021; 17(6):2332-2337.
- Carmat. Our Product: How Does Aeson® work?. Carmat 2022. Available at URL: https://www.carmatsa.com/en/our_product/
- Mason C, Dunnill P.: A brief definition of regenerative medicine. Regen Med. 2008; 3(1):1-5.
- European Union Law.: Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007: on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Official Journal of the European Union Union – EUR-Lex, 2007. 13 of November 2007. Available at URL: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32007R1394&qid=1666268995659
- European Medicines Agency.: Advanced therapy classification. European Medicines Agency; 2018. Available at URL: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/advanced-therapies/advanced-therapy-classification
- European Union Law.:. Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Official Journal of the European Union – EUR-Lex, 2004. 31 of March 2004. Available at URL: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32004L0023&qid=1666269329283
- European Union Law.:. Commission Directive 2006/17/EC of 8 February 2006 Implementing Directive 2004/23/EC of the European Parliament and of the Council as Regards Certain Technical Requirements for the Donation, Procurement and Testing of Human Tissues and Cells. Official Journal of the European Union – EUR-Lex, 2006. 8 of February 2006. Available at URL: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006L0017&qid=1666269771956
- European Union Law.:. Commission Directive 2006/86/EC of 24 October 2006 Implementing Directive 2004/23/EC of the European Parliament and of the Council as Regards Traceability Requirements, Notification of Serious Adverse Reactions and Events and Certain Technical Requirements for the Coding, Processing, Preservation, Storage and Distribution of Human Tissues and Cells. Official Journal of the European Union - EUR-Lex, 2006. 24 of October 2006. Available at URL: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006L0086&qid=1666269944424
- Gálvez P, Clares B, Hmadcha A, Ruiz A, Soria B.: Development of a cell-based medicinal product: regulatory structures in the European Union. Br Med Bull 2013; 105:85-105.
- European Union Law.:. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Official Journal of the European Union - EUR-Lex, 2001. 6 of November, 2001. Available at URL: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32001L0083&qid=1666270077024
- European Union Law.:. Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 Laying Down Community Procedures for the Authorisation and Supervision of Medicinal Products for Human and Veterinary Use and Establishing a European Medicines Agency. Official Journal of the European Union Union - EUR-Lex, 2004. 31 of March 2004. Available at URL: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32004R0726&qid=1666270225921
- European Union Law.:. Regulation (EU) No 1235/2010 of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No 726/2004 laying down Community procedures for the authorisation and supervision of medicina products for human and veterinary use and establishing a European Medicines Agency, and Regulation (EC) No 1394/2007 on advanced therapy medicinal products. Official Journal of the European Union - EUR-Lex, 2010. 15 of December 2010. Available at URL: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010R1235&qid=1666272427933
- EuroStemCell.: Interview with Graziella Pellegrini – Using stem cells to cure blindness. EuroStemCell; 2015. Available at URL: https://www.eurostemcell.org/interview-graziella-pellegrini-using-stem-cells-cure-blindness
- Detela G, Lodge A.: EU regulatory pathways for ATMPs: Standard, accelerated and adaptive pathways to marketing authorisation. Mol Ther Methods Clin Dev 2019; 13:205-232.
- Cuende N, Rasko JEJ, Koh MBC, Dominici M, Ikonomou L.: Cell, tissue and gene products with marketing authorization in 2018 worldwide. Cytotherapy 2018; 20(11):1401-1413.
- Regalado A.: The world’s most expensive medicine is a bust. MIT Technology Review; 2016 May 4. Available at URL: https://www.technologyreview.com/2016/05/04/245988/the-worlds-most-expensive-medicine-is-a-bust/
- Committee for advanced therapies.: EMA warns against using unproven cell-based therapies. European Medicines Agency; 2020. Available at URL: www.ema.europa.eu/en/documents/public-statement/ema-warns-against-using-unproven-cell-based-therapies_en.pdf
- Hurley P, Jurmeister S, Parsley K.: Advanced therapy medicinal products - An evolving regulatory landscape. Regulatory Focus; 2017.
- Snyder D, Sullivan N, Margolis D, Schoelles K.: Skin substitutes for treating chronic wounds. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020.
- Snyder DL, Sullivan N, Schoelles KM.: Skin substitutes for treating chronic wounds. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012.
- Schmidt C.: Gintuit cell therapy approval signals shift at US regulator. Nat Biotechnol 2012; 30(6):479.
- European Medicines Agency, EU/3/15/1596: Orphan designation for the treatment of partial deep dermal and full-thickness burns, (2015). https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu-3-15-1596 (accessed October 14, 2022).
- Organogenesis, Apligraf Wound Healing Technology Becomes First Transplant Product to Receive Marketing Approval in Switzerland, (2015). https://www.prnewswire.co.uk/news-releases/apligraf-wound-healing-technology-becomes-first-transplant-product-to-receive-marketing-approval-in-switzerland-539442491.html (accessed October 19, 2021).
- Marks P, Gottlieb S.: Balancing safety and innovation for cell-based regenerative medicine. N Engl J Med 2018; 378(10):954-959.
- Dearman BL, Boyce ST, Greenwood JE.: Advances in skin tissue bioengineering and the challenges of clinical translation. Front Surg 2021; 8:640879.
- Zhu J, Wang Y, Zhong L, Pan F, Wang J.: Advances in tissue engineering of vasculature through three-dimensional bioprinting. Dev Dyn 2021; 250(12)1717-1738.
- Weng T, Zhang W, Xia Y, Wu P, Yang M, Jin R, et al.: 3D bioprinting for skin tissue engineering: Current status and perspectives. J Tissue Eng 2021; 12: 20417314211028574.
- Roshangar L, Rad JS, Kheirjou R, Khosroshahi AF.: Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J Tissue Eng Regen Med 2021; 15(6):546-555.
- Jin R, Cui Y, Chen H, Zhang Z, Weng T, Xia S, et al.: Three-dimensional bioprinting of a full-thickness functional skin model using acellular dermal matrix and gelatin methacrylamide bioink. Acta Biomater 2021;131:248-261.
- Jamee R, Araf Y, Naser IB, Promon SK.: The promising rise of bioprinting in revolutionizing medical science: Advances and possibilities. Regen Ther 2021; 18:133-145.
- Turnbull G, Clarke J, Picard F, Zhang W, Riches P, Li B, et al.: 3D biofabrication for soft tissue and cartilage engineering. Med Eng Phys 2020; 82:13-39.
- Wang R, Wang Y, Yao B, Hu T, Li Z, Liu Y, et al.: Redirecting differentiation of mammary progenitor cells by 3D bioprinted sweat gland microenvironment. Burns & Trauma 2019; 7:29.
- Varkey M, Visscher DO, van Zuijlen PPM, Atala A, Yoo JJ.: Skin bioprinting: The future of burn wound reconstruction? Burns & Trauma 2019; 7:4.
- van Kogelenberg S, Yue Z, Dinoro JN, Baker CS, Wallace GG.: Three-dimensional printing and cell therapy for wound repair. Adv Wound Care 2018; 7(5):145-155.
- Tarassoli SP, Jessop ZM, Al-Sabah A, Gao N, Whitaker S, Doak S, et al.: Skin tissue engineering using 3D bioprinting: An evolving research field. J Plast Reconstr Aesthet Surg 2018; 71(5):615-623.
- Korkmaz HI, Visscher DO, Helder MN, van Zuijlen PPM.: What is the stat of art in 3D bioprinting of cartilage? Challenges concerning the reconstruction of a burned ear. Ned Tijdschr Geneeskd 2021; 165:D5676.
- Su X, Wang T, Guo S.: Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen Ther 2021; 16:63-72.
- Pedrero SG, Llamas-Sillero P, Serrano-López J.: A multidisciplinary journey towards bone tissue engineering. Materials (Basel) 2021; 14(17):4896.
- Huang J, Xiong J, Wang D, Zhang J, Yang L, Sun S, et al.: 3D bioprinting of hydrogels for cartilage tissue engineering. Gels 2021; 7(3):144.
- Choe R, Devoy E, Jabari E, Packer JD, Fisher JP.: Biomechanical aspects of osteochondral regeneration: Implications and strategies for 3d bioprinting. Tissue Eng Part B Rev 2022; 28(4):766-788.
- Alcala-Orozco CR, Mutreja I, Cui X, Hooper GJ, Lim KS, Woodfield TBF.: Hybrid biofabrication of 3D osteoconductive constructs comprising Mg-based nanocomposites and cell-laden bioinks for bone repair. Bone 2022; 154:116198.
- M. Alonzo, S. AnilKumar, B. Roman, N. Tasnim, B. Joddar, 3D Bioprinting of cardiac tissue and cardiac stem cell therapy, Transl Res. 211 (2019) 64–83. https://doi.org/10.1016/j.trsl.2019.04.004.
- Sedlakova V, Ahumada M, Suuronen EJ, Alarcon EI.: Building new cardiac vasculature and myocardium: Where are we at? Curr Opin Cardiol 2021; 36(6):728-734.
- Kim KS, Joo HJ, Choi SC, Kim JH, Park CY, Song MH, et al.: Transplantation of 3D bio-printed cardiac mesh improves cardiac function and vessel formation via ANGPT1/Tie2 pathway in rats with acute myocardial infarction. Biofabrication 2021;13(4).
- Fazal F, Raghav S, Callanan A, Koutsos V, Radacsi N.: Recent advancements in the bioprinting of vascular grafts. Biofabrication 2021; 13(3).
- Nulty J, Freeman FE, Browe DC, Burdis R, Ahern DP, Pitacco P, et al.: 3D bioprinting of prevascularised implants for the repair of critically-sized bone defects. Acta Biomater 2021; 126:154-169.
- Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, et al:. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med 2019; 25(2):263-269.
- Asgharzade S, Alizadeh A, Arab S.: Regenerative medicine approaches in COVID-19 pneumonia. Curr Stem Cell Res Ther 2021; 16(6):647-655.
- Ramezankhani R, Solhi R, Memarnejadian A, Nami F, Hashemian SMR, Tricot T, et al.: Therapeutic modalities and novel approaches in regenerative medicine for COVID-19. Int J Antimicrob Agents 2020; 56(6):106208.
- Kumar A, Ghosh SB.: Emerging treatment options of regenerative medicine in severe corona virus/COVID 19 infections. Int J Stem Cells 2020; 13(3):305-311.
- Park YJ, Farooq J, Cho J, Sadanandan N, Cozene B, Gonzales-Portillo B, et al.: Fighting the war against COVID-19 via cell-based regenerative medicine: Lessons learned from 1918 Spanish flu and other previous pandemics. Stem Cell Rev Rep 2021; 17(1):9-32.
- Basiri A, Pazhouhnia Z, Beheshtizadeh N, Hoseinpour M, Saghazadeh A, Rezaei N.: Regenerative medicine in COVID-19 treatment: Real opportunities and range of promises. Stem Cell Rev Rep 2021; 17(1):163-175.
- European Medicines Agency.: Advanced therapy medicinal products: Overview. European Medicines Agency; 2018. Available at URL: https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview
- Verband Forschender Arzneimittelhersteller.: Übersicht über zentralisiert in der EU zugelassene ATMP. Die forschenden Pharma-Unternehmen; 2022. Available at URL: https://www.vfa.de/de/arzneimittel-forschung/datenbanken-zu-arzneimitteln/atmp
- Alliance for Regenerative Medicine.: Available Products. 2021. Available at URL: https://alliancerm.org/available-products/
