Volume 24 Number 4
Biofilm update
Gregory S Schultz, Daniel J Gibson, John Lantis and Gojiro Nakagami
Abstract
Bacterial biofilms remain a topic of critical importance for wound care clinicians. This brief update will describe important new research being done in laboratories and in clinical studies that is increasing our understanding of the roles that biofilms play in preventing wounds from healing. Improved detection and treatments of biofilms will ultimately lead to better and faster healing of chronic wounds.
Introduction
For centuries, bacteria have been known to cause impediments to wound healing. With the advent of antibiotics, it was believed that the bacterial menace was subdued. Two problems have since been identified that have been found to explain why bacterial infections are still a problem: resistance and tolerance1. Bacterial resistance is generally defined as the inherited ability of a microorganism (for example, bacteria) to grow at high concentrations of an antibiotic, irrespective of the duration of treatment. Antibiotic resistance generally arises through gene mutation or through transfer of plasmids and molecular mechanisms of resistance typically include mutations in the bacterial target protein that prevent (reduce) the interaction between the antibiotic and the bacterial protein, acquiring an enzymatic activity that directly inactivates the antibiotic, and the activation of efflux pumps that pump out the antibiotic. These genes have been termed the “resistome”2. In contrast, bacterial tolerance is more generally used to describe the ability, whether inherited or not, of microorganisms to survive transient exposure to high concentrations of an antibiotic, which is often achieved by slowing down an essential bacterial process like energy generation (metabolism), protein or DNA synthesis. One of the most common mechanisms for bacteria to develop transient tolerance to antibiotics is for the bacteria to shift from a rapidly proliferating planktonic state that is sensitive to an antibiotic into a biofilm community of bacteria that contains a subpopulation of metabolically dormant cells that persist even for long periods of exposure to the antibiotic. Importantly, when persistent bacterial cells are isolated, regrown, and re-exposed to the antibiotic, almost all will be effectively killed — no change in the minimum inhibitor concentration (MIC) of the planktonic bacteria.
In chronic wounds, the formation of biofilms is believed to be the primary form of antibiotic and antimicrobial tolerance. Research on the microbiology of biofilms and the effectiveness of treatments and management strategies has been vigorous and continues to proceed. This brief article we will update you on recent strategies to identify biofilms on wound beds, physically remove (debride) biofilms from wound beds, and to disrupt and kill biofilms.
Rapid Detection of Biofilms on Wound Bed Surfaces
A major challenge for clinicians is to accurately assess if a bacterial biofilm is present in a chronic wound bed and, even more importantly, where the biofilm is located. A survey of 81 clinicians’ perceptions of wound biofilms indicated that wound biofilms were acknowledged as existing in a majority of chronic wounds, but the impact on wound recalcitrance and infection was less well understood by many clinicians3. Four common characteristics were associated with the presumed presence of biofilm, namely, a recalcitrant wound, film of slime on the surface of a wound, signs of infection/inflammation/increased exudate and ineffective antimicrobials. A clinical algorithm for identification of wound biofilm was proposed based on visual indicators, including the ease of removal of substances on the surface of wound beds, how rapidly surface substances reform, poor healing response to topical or systemic antibiotics or antimicrobial dressings, and improved healing using multiple approaches of debridement, cleansing and topical antimicrobial agents and dressings4.
Recently, a simple, rapid, inexpensive technique was to visually detect bacterial biofilms on the surface of wound beds5. This ‘biofilm wound map’ technology uses a high binding capacity membrane that is briefly pressed onto a wound bed to non-specifically adsorb biological molecules, such as free bacterial DNA, polysaccharides, proteins, and lipids that typically constitute the exopolymeric matrix of bacterial biofilms present on a wound bed6. The membrane is then briefly submerged in a concentrated solution of a cationic dye, such as ruthenium red then the membrane is rinsed for a few minutes in saline or dilute acetic acid (vinegar), and the dye-stained areas correspond to the areas of the wound bed that contain a biofilm. As shown in Figure 1, biofilm exopolymeric matrix can be detected on the wound bed surface of some chronic pressure ulcers, but not detected on other pressure ulcers. Using this technique to assess biofilms in 70 separate wound measurements made on 23 pressure ulcers after debridement in 16 patients, biofilm was detected in 61% of the wound beds. More importantly, wound beds that had positive staining for biofilm had minimal reduction in wound slough (~3% reduction in slough area) during the following week of standard care compared to wounds that were negative for biofilm staining which had an average reduction of 45% slough area (p=0.03) Thus, this technique may be useful in identifying regions of wound beds that contain biofilm before, and especially after debridement, and successful debridement of areas with biofilm may predict a reduction in wound slough and inflammation, which usually correlates with improved healing.
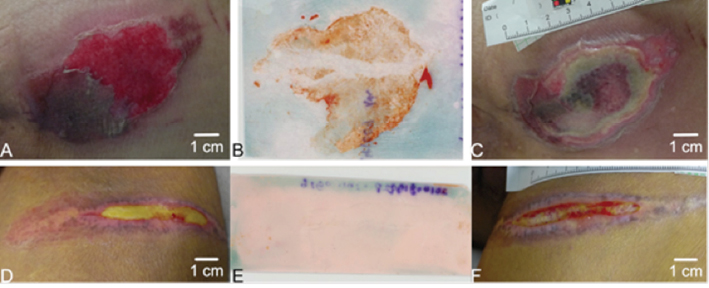
Figure 1: A rapid, biofilm “wound map” detects components that are common in bacterial biofilms (free bacterial DNA, acid polysaccharides, proteins, lipids) that are stained with a cationic red dye. Panel A is a 78-year-old male with a stage II chronic pressure ulcer on his sacral region that stained for biofilm (Panel B), and developed slough during the following week (Panel C) with standard care. Panel D is a 57-year-old female with a stage II chronic pressure ulcer on her thigh region that did not stain for biofilm (Panel E) and after one week the slough was dramatically reduced (Panel F).
Debridement of Bacterial Biofilms
One of the two most important core principles of “biofilm-based wound care” during the initial period of treatment of a chronic wound is effective debridement of biofilms. With the commercial availability of medical-grade larvae from Lucilia sericata (green bottle fly), use of larval debridement therapy (LDT) has been increasing steadily. As summarised in the Evidence Summary for Wound Management with Larval Therapy7 a multicentre, blinded, randomised controlled trial (RCT) of patients with chronic, sloughy wounds compared LDT (n=51) with conventional treatment (surgical debridement three times a week) (n=54) over a two-week period8. On day 8, there was a significant difference (p=0.04) in the percentage of slough between the LDT group (54%) and the control group (66%). However, there was no difference by day 15. Two other RCTs found LDT significantly reduced the time to debridement compared to hydrocolloid dressings — autolytic debridement9,10.
Based on these encouraging clinical results with LDT, we asked the question: what effect does LDT have on removing established bacterial biofilms on wounds? We used the pig skin explant biofilm model that closely replicates several important components of bacterial biofilms in chronic skin wounds, especially the growth of biofilms on the most relevant material, which is dermal extracellular matrix11. As shown in Figure 2, LDT of mature biofilms of Pseudomonas aeruginosa (PA01) or Staphylococcus aureus (SA35556) totally eliminated both planktonic and biofilm bacteria to undetectable levels after two days of LDT12. These laboratory data are the basis for an ongoing clinical study that is assessing the effects of LDT on biofilm colony-forming units (CFUs) in chronic ulcer patients.
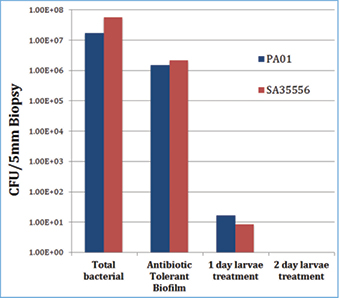
Figure 2: Larval debridement therapy (LDT) of mature biofilms of P. aeruginosa (PA01) or S. aureus (SA35556) totally eliminated both planktonic and biofilm bacteria to undetectable levels after two days of LDT.
Negative pressure wound therapy (NPWT) combined with periodic instillation of solutions is becoming another option to treat inflammated chronic wounds, and using micorbicidal wound wash solutions significantly reduced CFU of mature P. aeruginosa biofilm grown on pig skin explants. (our citation P.L. Phillips, Q. Yang, G.S. Schultz. The Effect of Negative Pressure Wound Therapy with Periodic Instillation Using Antimicrobial Solutions on Pseudomonas Aeruginosa Biofilm on Porcine Skin Explants. International Wound J, 10 (suppl. 1) 48-55, 2013.) Both contact and non-contact ultrasonic debridement techniques have been reported in laboratory models to reduce CFU of bacteria in mature biofilm grown on pig skin explants. In addtion, substituting microbicidal wound wash solutions for the standard saline solutions used to transmit the ultrasonic energy further reduced the levels of bacteria in mature biofilms compared to using saline solution.(SAWC abstract Yang QP, Cowan LJ, Phillips PL, Schultz GS. Assessment of Contact and Non-Contact Ultrasonic Treatments on Pseudomonas aeruginosa Biofilms Grown on Porcine Skin Explants, SAWC,2012 ).
Dispersal/Disruption of Biofilms
Another active area of research for removing or reducing biofilms has centred on the disruption/killing of biofilms using physical chemistry methods including treatment of biofilms with a poloxamer-based surfactant. Pluronic poloxamer polymers are unique in the world of synthetic polymer surfactants because, unlike most synthetic polymer solutions that get ‘runny’ when they are warmed and become viscous when they are cooled, pluronic polymers behave exactly the opposite. Solutions of pluronic polymers gel when they are warmed and become liquid when they are cooled. Recently, we assessed the effects of a high-concentration, non-ionic, surfactant, poloxamer pluronic gel (PluroGel®) on reducing mature biofilms formed by P. aeruginosa on pig skin explants13. As shown in Figure 3, the CFU of total bacteria (planktonic and biofilm bacteria) was ~5x108 CFUs, of which ~5x104 CFUs were biofilm bacteria. When the pig skin explants were wiped with a gauze dressing, the levels of total and biofilm bacteria were both reduced ~2-logs (99%). However, the levels of total and biofilm bacteria began to recover even with daily wiping of the explants for the next three days, with the level of biofilm bacteria fully recovered on day 3. In marked contrast, daily wiping with gauze combined with daily treatment with the pluronic surfactant gel progressively reduced the levels of both total and biofilms, with no viable bacteria recovered in biopsies taken after three days of treatment. These in vitro laboratory data agree in general with the positive results of a very recent multicentre study conducted in Europe on 1036 patients treated with the pluronic surfactant gel containing silver sulfadiazine that showed impressive closure rates in chronic wounds14.
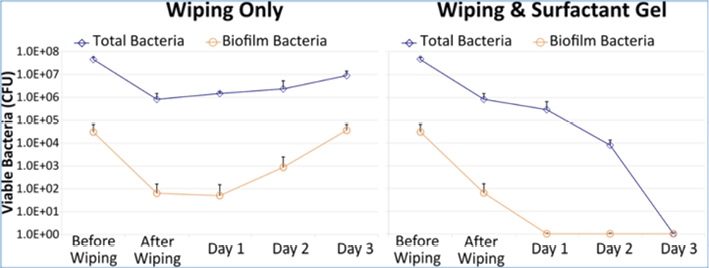
Figure 3: Effects of wiping alone or wiping plus daily treatment with a high concentration of a pluronic surfactant gel on biofilm bacteria grown on pig skin explants.
Effects of Cadexomer Iodine Dressings on Bacterial Biofilms in Chronic Wounds
A second major principle of biofilm-based wound care is to combine debridement with the use of microbicidal dressings that prevents growth of bacteria in the dressing and also is an effective barrier to penetration of bacterial through the wound dressing to the wound bed. We previously reported that a cadexomer iodine gel (CIG) (Iodosorb®) that provides sustained release of iodine for several days was the only microbicidal wound dressing that completely eliminated CFUs of P. aeruginosa or S. aureus grown on pig skin explants15. While the laboratory findings were exciting, the clinical efficacy of the CIG on reducing planktonic and biofilm bacteria can only be accurately assessed through a controlled clinical trial. A recent open-label, pilot, clinical study was completed that assessed the effects of the CIG on reduction of total and biofilm bacteria in patients with chronic ulcers16. Nineteen subjects with chronic diabetic foot ulcers (DFU grade 1 or 2, ≥3cm diameter) and suspected biofilms were randomly assigned to CIG or hydrogel, both covered with secondary non-adhesive foam dressing secured with adhesive tape. Dressings were changed daily for the hydrogel and at least twice between weekly clinic visits for CIG. Debridement and collection of tissue samples for bacterial analysis occurred immediately prior to initial treatment and at weeks 2 and 4. The primary outcome of interest was change in (log10CFU/gram tissue) biofilm-protected bacteria from baseline to weeks 2 and 4. As shown in Figure 4, biofilm counts were reduced by 1 log CFU/g for CIG versus an increase of 0.3 log CFU/g for hydrogel (95% CI for difference –0.3 to 4.5; p=0.063). At 4 weeks the CIG-treated patients maintained the 1-log reduction in biofilm bacteria while the hydrogel achieved a 0.8-log reduction (p=0.34).
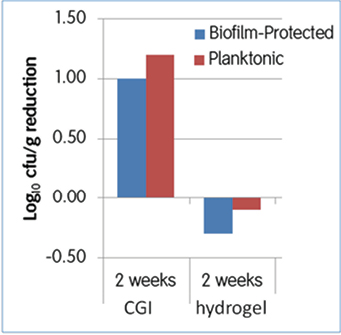
Figure 4: Effects of cadexomer iodine gel or hydrogel dressing on reducing bacterial biofilms in chronic wounds after two weeks of treatment.
Conclusion
Bacterial biofilms have been implicated as a major factor in promoting chronic inflammation that chacterized multiple disease states, including chronic otitis media, cystic fibrosis, osteomyelitis, and catheter associated chronic infections.( del Pozo,J.L., Patel,R.The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol.Ther., 82:204-209, 2007). Substantial evidence is accumulating that supports that concept that bacterial biofilms also play a key role in promoting chronic inflammation that leads to elected proteases and ROS that destroy proteins that are essential for healing, which leads to the failure of skin wounds to heal. New diagnost tools for detecting bacterila biofilms in wound beds, better understanding of how to effectively debride biofilms, and use of dressing that are more effective at preventing refomation of biofilms has led to the concept of biofilm-based wound care, which builds on the core principles of wound bed preparation. Ultimately, a better understanding by clinicians of the distinct properties of bacterial biofilms and how to select and use different treatment options will result in more effective healing of chronic wounds.
Author(s)
Gregory S Schultz
PhD
Institute for Wound Research, University of Florida, Gainesville, Florida, USA
Daniel J Gibson
PhD
Institute for Wound Research, University of Florida, Gainesville, Florida, USA
John Lantis
MD
Department of Surgery, St Luke’s & Roosevelt Hospital, New York, NY, USA
Gojiro Nakagami
PhD, RN
Department of Gerontological Nursing, University of Tokyo, Tokyo, Japan
References
- Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 2016;14(5):320–30.
- D’Costa VM, McGrann KM, Hughes DW, Wright GD. Sampling the antibiotic resistome. Science 2006;311(5759):374–7.
- Metcalf DG, Bowler PG. Clinician perceptions of wound biofilm. Int Wound J 2014.
- Metcalf DG, Bowler PG, Hurlow J. A clinical algorithm for wound biofilm identification. J Wound Care 2014;23(3):137–2.
- Nakagami G, Schultz G, Gibson D et al. Biofilm detection by wound blotting can predict slough development in pressure ulcers: a prospective observational study. Wound Repair and Regeneration 2016; in press.
- Schultz GS, Phillips PL, Sampson EM. Materials and Methods for Assessing and Mapping Microbes and Microbial Biofilms on Wounds. University of Florida Research Foundation. [US 9,145,574,B2], 1–20. 9-29-2015. Florida/USA.
- Wound Healing and Management Node Group – Watts R. Evidence Summary: Wound management: larval therapy. Wound Practice and Research 2016; 24(3):180-182.
- Opletalova K, Blaizot X, Mourgeon B et al. Maggot therapy for wound debridement: a randomized multicenter trial. Arch Dermatol 2012;148(4):432–8.
- Dumville JC, Worthy G, Bland JM et al. Larval therapy for leg ulcers (VenUS II): randomised controlled trial. BMJ 2009;338:b773.
- Wayman J, Nirojogi V, Walker A, Sowinski A, Walker MA. The cost effectiveness of larval therapy in venous ulcers. J Tissue Viability 2000;10(3):91–4.
- Yang Q, Phillips PL, Sampson EM et al. Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen 2013;21(5):704–14.
- Cowan LJ, Stechmiller JK, Phillips P, Yang Q, Schultz G. Chronic wounds, biofilms and use of medicinal larvae. Ulcers 2013; 2013(http://dx.doi.org/10.1155/2013/487024):1–7.
- Yang Q, Larose C, Della Porta AC, Schultz GS, Gibson DJ. A surfactant-based wound dressing can reduce bacterial biofilms in a porcine skin explant model. Int Wound J 2016.
- Palumbo FP, Harding KG, Abbritti F et al. New surfactant-based dressing product to improve wound closure rates of nonhealing wounds: A European multicenter study including 1036 patients. Wounds 2016;28(7):233–40.
- Phillips PL, Yang Q, Sampson E, Schultz G. Effects of antimicrobial agents on an in vitro biofilm model of skin wounds. Advances Wound Care 2010;1:299–304.
- Lantis J, Schultz G, Edmondson-Jones M et al. Effects of Cadexomer Iodine on biofilm in diabetic foot ulcers: A pilot study. World Union of Wound Healing Societies Congress; 2016.



