Volume 25 Number 4
Haemodialysis central venous catheter exit site dressings in the tropics: a crossover randomised controlled trial
Joleen McArdle, Wendy Smyth, Kristin Wicking and Anne Gardner
Keywords haemodialysis, Central venous catheters, intravascular device dressings, tropical climate.
Abstract
Aim: The trial aimed to identify the most effective dressing for haemodialysis central venous catheter exit sites in a tropical region.
Background: Central venous catheters, often used to deliver haemodialysis, require meticulous exit site care. Staff in a tropical renal service were concerned a transparent dressing could increase the likelihood of dressings not remaining intact or of infection of the exit site.
Design/Methods: Patients (n=26) with central venous catheter access attending this Australian renal service for haemodialysis consented to participate in this population-based, prospective, randomised crossover trial. Participants were randomly assigned to a specific sequence of transparent (IV3000) and non-transparent (Primapore™) dressings. The primary outcome measure of effectiveness was intactness of dressings between haemodialysis episodes.
Results: The majority of participants were Aboriginal and Torres Strait Islander people (n=21). There were no statistical differences between intactness of the IV3000 and Primapore™ dressing types. No laboratory-confirmed catheter-related infections occurred while patients were wearing the non-transparent dressing.
Conclusions: Nurses in tropical settings can safely select either a non-transparent or transparent dressing until the study is replicated in other geographical locations with a larger sample size.
Introduction
Central venous catheters (CVCs) are a commonly used access route for haemodialysis and the type of dressing used on a CVC exit site is an important first line of defence to prevent infection. Infection of the CVC is a serious and common complication1 with infectious complications relating to CVCs one of the leading causes of death for patients receiving haemodialysis2.
At the time of this study’s conceptualisation, the Queensland Government’s Centre for Health Related Infection Surveillance and Prevention (CHRISP) guidelines3 recommended that a transparent dressing be applied to CVC exit sites. The CHRISP guidelines recommended transparent dressings because they allow for the continual observation of the exit site and they assist in the protection, stabilisation and securement of the CVC. More recent, updated guidelines recommend that patient and environmental factors be considered when deciding upon the dressing of choice4-7.
The northern Australian renal service in this study manages approximately 150 patients undergoing haemodialysis, mostly on an outpatient basis. The patients come from the renal service’s extensive catchment area of approximately 500,000 km2, including many remote communities, rural towns and a regional city. The population of Aboriginal and Torres Strait Islander peoples within this area is larger than the state’s average. Many patients need to relocate to the regional city for their haemodialysis.
The service identified that there was inconsistency in the type of dressing used, compared with the existing guidelines at the time. The renal service continued to use a non-transparent, gauze-like, adhesive dressing because the staff postulated that the transparent dressing led to the accumulation of excess moisture as a result of an increase in perspiration due to the warm climate. Staff thought that this excess moisture increased the bacterial count, which, in turn, enhanced the risk of infection. The non-transparent dressing was determined to be a more appropriate dressing in this context because the staff perceived it to have better breathability, hence decreasing the amount of moisture accumulation beneath the dressing. However, an audit of the intactness of the non-transparent dressings8 identified that dressings were not fully intact at almost 40% of the haemodialysis sessions.
A preliminary literature review9 failed to identify any supporting evidence for choice of dressing in a tropical climate and found no articles that compared the specific dressing types used in this renal service. In studies contrasting types of dressings for CVC exit sites, the outcome most commonly examined was infection. Four primary studies conducted between 1985 and 2013 showed a clinical trend of an increased risk of catheter tip infection at CVC sites when using transparent polyurethane dressings compared to "gauze plus tape" dressings10-13. Conly et al.11 found statistically significantly higher infection rates for transparent polyurethane dressings. An increase in infection rates with a gauze-type dressing has been reported in two individual studies14,15. Of the 11 systematic and narrative literature reviews examined pertaining to CVC care and infection, only one did not support the use of a transparent dressing compared to a gauze dressing16. Theaker found more evidence to support the use of a gauze dressing, arguing that the transparent dressing increased the risk of infection due to the promotion of moisture and bacterial proliferation beneath the dressing16. A recent Cochrane Systematic Review17 concluded there was insufficient evidence to support either type of dressing (polyurethane or gauze plus tape) as being better than the other at preventing infection. This review did provide some evidence that chlorhexidine-impregnated dressings may reduce the incidence of catheter-related infections when compared to other dressing types. At the time of the current study these dressings were not readily available to this renal service.
Fewer studies have investigated intactness of dressings as their outcome measure, even though an intact dressing is a first line of defence against infection and this process outcome is likely to be more common and much easier to measure. Three articles supported the use of a transparent type dressing as the dressing more likely to remain intact18-20. Conversely, one article found that a gauze and tape dressing was the better choice to improve dressing intactness11. No studies that compared the types of dressings used in this renal service could be located.
Although no evidence could be found of studies that investigated dressing types and infection rates in tropical climates, several authors mentioned related factors such as level of diaphoresis, and factors related to climatic variation19,21-25. A dated study, specifically targeting peripheral line dressings, found that the rate of infection for those using a transparent dressing was higher in summer months than in cooler months26. More recently, in a study conducted in sub-tropical Brazil that compared transparent dressings with gauze and tape, the transparent dressing type was claimed to not be “feasible for patients with abundant sweating”10,p.485. The authors did not elaborate on that statement, nor provide any evidence to support the claim.
Aim of this study
Against this background of limited evidence to support the choice of dressings in a practice setting, a study was undertaken which aimed to identify the most effective and safe dressing for haemodialysis CVC exit sites in a tropical region.
Methods
Study design, research hypothesis
The study used a crossover, randomised controlled trial (RCT) design. The null hypothesis was: There is no difference in effectiveness between transparent and non-transparent dressings on CVC exit sites for patients undergoing haemodialysis in the tropics. The crossover design maximised the sample size in the small population of potential participants. It was not feasible in this study to have a washout period between treatments because, if a washout period had been used, then the CVC exit site would have been left uncovered for some days, which was deemed clinically inappropriate.
Primary outcome measure of effectiveness
The primary outcome measure was intactness, defined as “all four edges of the dressing remaining adhered to the skin”8. Intactness was assessed by the nurses at each dialysis session, on the audit tool described later in the "Study procedure and measurements" subsection.
Secondary outcome measure of effectiveness
The secondary outcome measure was infection, either clinical signs of infection or laboratory-confirmed infections. It was not practical to include infection as a statistically significant outcome measure, due to the requirement for very large samples needed to demonstrate statistically significant results in intravenous line-related studies. However, it was important to include this as a descriptive measure. Clinical signs of infection were the presence or absence of crust at the exit site, and the colour of the exit site, assessed as per the Twardowski Scale at each dialysis session27 (Table 1). The Twardowski Scale is a validated tool28 that is used for assessing peritoneal dialysis exit sites and has been used in other studies to assess CVC exit sites8,14. Laboratory-confirmed infections were classified as local (either an exit site infection or a tunnel infection) or systemic. Exit site infections, also assessed as per the Twardowski Scale, were defined as local infection of the skin and soft tissue around the exit site, with erythema and purulent discharge with tenderness typically present. Tunnel infections were defined as “an invasive painful soft tissue infection along the catheter tunnel superior to the cuff”29; diagnosis of confirmed tunnel infection required a positive wound swab. A systemic infection was diagnosed as a growth of >10² colony forming units (cfu) from a catheter by quantification of broth culture and/or a growth of >15 cfu from a 5 cm segment of catheter tip30. Laboratory confirmation of suspected systemic infection is sought as part of the routine practice of this renal service, consistent with state guidelines4.
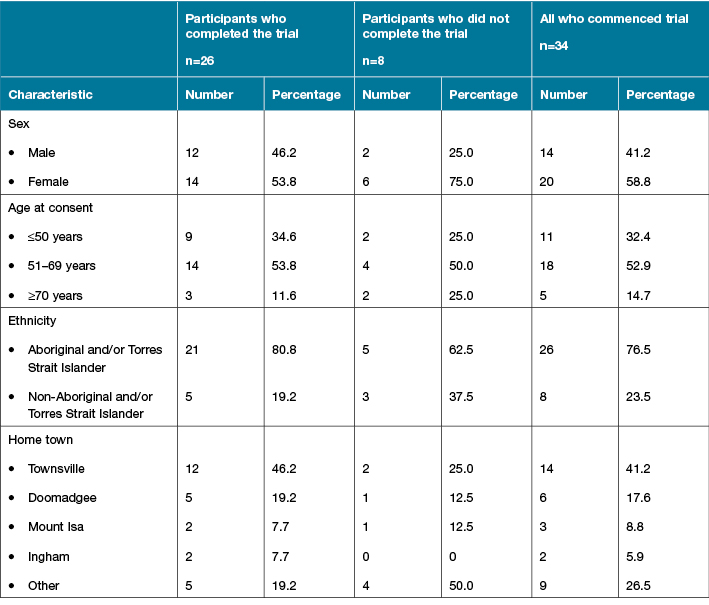
Table 1: Participant details, comparing those who completed the trial, those who did not complete the trial
Setting and target population
The study took place in two facilities (one in-hospital and one satellite unit) of a renal service in a regional city in northern Queensland, Australia. The trial took place over the ‘wet’ season, where the monthly rainfall ranged from 178.6 mm to 696.2 mm, and the highest daily temperature ranged from 31.1°C to 33.9°C. The target population comprised patients whose access to haemodialysis was via a CVC. Patients were excluded from participation if they had an exit site or CVC-related infection at the time of recruitment.
Interventions
Each participant trialled the two dressings, each for six weeks. The dressing routinely used in the renal service for CVC site coverage was a non-transparent dressing, Primapore™ (Smith & Nephew). Primapore™ was described on the manufacturer’s website as “a conformable adhesive dressing consisting of a breathable non-woven top layer and a low-adherent absorbent pad … The low allergy acrylic adhesive is spread evenly onto the non-woven backing layer of the dressing, providing safe and secure fixation over the wound site” (http://www.smith-nephew.com/key-products/advanced-wound-management/primapore/ accessed 1 June, 2017).
The comparison dressing, IV3000™ (also by Smith & Nephew), was a transparent dressing, “consisting of a thin polyurethane membrane coated with a layer of low allergy adhesive” with a high moisture vapour transmission rate (http://www.smith-nephew.com/australia/healthcare/products/product-types/iv-care/iv3000--product-range/ accessed 1 June, 2017).
Sample size, recruitment and randomisation
The target population comprised patients undergoing haemodialysis via a CVC, and was estimated to be approximately 35, and that between 80% and 85% would agree to participate. No sample size calculations were possible since the target population was limited (most patients undergo haemodialysis with access other than via a CVC).
Recruitment commenced two weeks prior to the commencement of the trial in mid-November 2010 and continued for four weeks after the commencement of the trial. Research nurses and several senior nurses working in the renal service undertook the recruitment, after receiving training about the principles of recruitment. After assessing patients for eligibility, the recruiting nurses explained to potential participants the nature of the trial of different dressings, that they would not be able to choose the order of the dressings, and that their consent was voluntary. All potential participants were provided a participant information sheet to complement the verbal explanation. During the recruitment process, patients were encouraged to discuss their participation with a family member or an Indigenous liaison officer before signing the consent form.
There were two strata of participants according to the CVC type — tunnelled or non-tunnelled. Participants were assigned their strata, and then randomly allocated to the sequence of dressing types using www.randomization.com; varying block sizes were used during the randomisation. Researchers and research assistants, who were neither involved in the recruitment process nor in the clinical care of the patients, carried out the randomisation. Thus the recruiting nurses and nurses who would be changing the participants’ dressings were blinded to the dressing sequence generation; however, it was not possible to blind either the treating nurses or the participants to the dressings used because of their obvious different properties.
Study procedure and measurements
Prior to the commencement of the trial, an existing work practice instruction about care of CVC exit sites was reviewed and education was provided about the trial, data collection tools and the necessity for consistency of dressing techniques and exit site management. Senior nurses in the haemodialysis unit were trained by the principal investigators to assist in data collection and monitor nurses’ compliance with the dressing techniques throughout the trial. A folder containing all necessary tools and information about the conduct of the trial was prepared for each of these senior nurses.
There were two data collection tools, each completed by the nurses caring for the patients: the demographic form was completed at the commencement of the trial and the audit tool was completed at each dialysis session. Limited history, such as when the participants began dialysis and whether they had diabetes, was included in the demographic form. The audit tool had been trialled previously in the haemodialysis unit and included information about the patient and catheter details on the day of dialysis, nurses’ observations of the dressing and assessments of the catheter exit site as per the Twardowski Scale8. Given the known higher incidence of skin fragility in patients with chronic kidney disease, scrutiny of the skin for breakdown at each dressing change was included on the audit tool31.
Audit tools, colour-coded according to the dressing type, together with the corresponding dressing were prepared and placed in an easily-accessible location for use by the nurses caring for the patient. The principal investigator placed the appropriately coloured audit tool in the participant’s chart when the participant was due to rotate to the different dressing. Laminated copies of the Twardowski Scale placed on the front of the medical records reminded nurses that the patient was participating in the trial. Using an aseptic technique and cleansing the exit site with chlorhexidine, the dressings were changed at each dialysis session (usually three times per week); the exit site assessment was recorded on the audit tools by the attending nurse. These audit tools were collected weekly by the investigators.
Ethical considerations
The study was approved by the health service and university human research ethics committees (approval numbers HREC/09/QTHS/121 and HS3851, respectively). Many patients undergoing haemodialysis in this service are Aboriginal and/or Torres Strait Islander. During the planning phase, an Indigenous health professional was consulted to ensure that the values underlying ethical conduct in these cultures were integrated into the study.
Data analysis
Quantitative data were coded numerically and entered into the computerised Statistical Package for Social Sciences (SPSS Version 19.0 for Windows, Armonk, NY: IBM Corp). Choice of data analysis methods was guided by a statistician. The main outcome measure of mean percentage of intactness was calculated for each participant for each dressing type. The numerator was the number of times the dressing was deemed intact; the denominator was the total number of dialysis episodes for that participant. Wilcoxon signed rank tests were performed where the data were not normally distributed. For some comparisons, the small sample size precluded inferential statistical testing. Data were analysed "per protocol" rather than "intention to treat"32.
Results
The participants
All 37 eligible patients agreed to participate, but three withdrew before commencing the trial. Twenty-six participants completed the trial (Figure 1). Participants’ ages ranged from 36 to 86 years; mean age was 56.41 years (SD = 10.55). The majority (n=21) were Aboriginal and/or Torres Strait Islander; 15 were female; and more than half (n=14) had relocated to the regional city from more rural or remote locations for haemodialysis. Only one participant did not have diabetes; 20 participants used medications to control their blood glucose. All but one of the CVCs had been in place for less than one year, although 12 of the participants had been undergoing haemodialysis for longer than one year. Twenty-four of the CVCs were tunnelled; most commonly CVCs were inserted into either the jugular or subclavian vein.
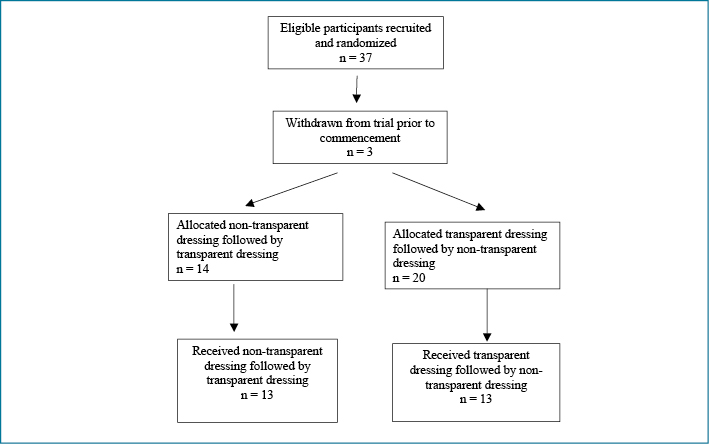
Figure 1: CONSORT diagram
Primary outcome — intactness
The mean percentage of intact non-transparent dressings was 68.15% (Md = 73.86); the mean percentage of intact transparent dressings was 68.84% (Md = 75.96). A Wilcoxon signed rank test indicated no statistically significant difference between dressings (z = 0.386, p = 0.700).
Secondary outcome — infection
Clinical signs of infection — colour and crust — were assessed using the Twardowski Scale. A Wilcoxon signed rank test indicated no statistically significant differences between the two dressings in either colour of the CVC exit sites (z = –0.454, p = 0.650) or crust (z = 1.650, p = 0.099 respectively).
There were four laboratory-confirmed catheter-related infections during this trial, all whilst participants were wearing the transparent dressing. There were no laboratory-confirmed infections associated with the non-transparent dressing; hence no statistical analysis was possible. All infections occurred with a tunnelled catheter; two catheters were inserted in the right jugular vein, and one each in the left femoral and right femoral vein. The causative organisms were:
1. Staphylococcus aureus (exit site)
1. Achromobacter xylosoxidans (exit site)
2. Staphylococcus aureus (exit and tip)
3. Klebsilla pneumoniae (blood culture) and Aeromonas hydrophilia (present in tip of CVC).
Discussion
This randomised crossover population study tested the null hypothesis that a non-transparent dressing would be equally effective as a transparent dressing for the outcome measures of intactness, and of infection (at the CVC exit site, of the CVC catheter tip and systemically). Most studies located during the initial literature review found no statistically significant difference in rates of intactness between transparent dressings and the most commonly used comparator at the time they were conducted, that of gauze and tape dressings. No studies were found which specifically compared transparent dressing to non-transparent adherent dressings. In addition, none were found that compared dressing types within a tropical climate, hence underlining the need for this present study.
Consistent with the limited previous evidence, intactness was similar for both dressing types. The rates of intactness for both types of dressings were disturbingly low, and the rates were midway between those reported in other studies. For instance, Trotter et al.20 found even lower rates of intactness (23%) for their gauze and tape dressing, while Chu, Adams and Crawford33 reported 80% intactness prior to implementing an educational intervention and 85% afterwards. Similarly, an earlier clinical audit completed in the same context as this present study8 found lower rates (57%) of intactness than in this RCT. The moderate increase in intactness in the current study may be explained partially by the rigorous process of staff education undertaken in the present RCT that served to tighten up variability in dressing change technique. Possible reasons for dressing non-intactness included perspiration, showering and increased intervals between dialysis sessions owing to patients’ non-attendance at all scheduled sessions.
Although many other variables besides dressing type may influence intactness, the use of a crossover RCT design in this present study, with its resultant high levels of participation of nearly all eligible patients, served to control for many of these known and unknown confounding variables and begins to address gaps identified in the most recent Cochrane review available at the time of the study design34. A later Cochrane review by Ullman et al.17 did review clinical trials with a focus on the outcomes of CVC catheter securement and infection, rather than intactness of the dressing itself; but none of the reviewed trials used the PrimaporeTM dressing (or a similar product) examined in this present study as a comparator. Later recommendations coming out of the Ullman et al.17 review encourage a consideration of multiple factors when selecting a dressing, using a clinical decision tree35.
Similarly, the majority of the studies reviewed when designing this study also did not form a clear picture of superiority of a transparent dressing over gauze and tape dressings, in regard to the outcome measure of infection, and again they did not address the climatic variable. Conley et al.11 did find a higher infection incidence with transparent dressings versus gauze and tape, in their older study, but otherwise the evidence does not clearly favour one dressing type over another. In this RCT, the only infections occurred during the transparent phase (four infections), with zero infections noted during the non-transparent phase. Therefore, the findings for the outcome measure of infection were unable to reach the threshold of statistical significance due to the mathematical inability to solve for a p value when the incidence is zero for one arm of the trial. However, an incidence of zero CVC site infections associated with non-transparent and four infections for the transparent dressing phase is clinically intriguing.
Three publications were located after the completion of this study, which did discuss dressing types in a tropical climate. Two were primary research studies conducted in Brazil, contemporaneously with this RCT10,19 and one in Indonesia36. Their recommendations echo the clinical intuition of the renal service’s nurses, and those of a meta-analysis on CVC dressings for any purpose25, that transparent dressings did not perform well in climates where perspiration was frequent and profuse. While not reaching statistical significance in this study, the trend was congruent with the three tropical studies, in that infection happened more often with a transparent dressing than with a non-transparent dressing. A review by Thomas et al.37 proposed that accumulation of fluid beneath semipermeable dressings over catheter sites may promote bacterial growth, and recommended that moisture vapour transmission rates (MVTR) be considered in dressing selection. Manufacturers are now beginning to disclose a MVTR specification in their product information, a laudable trend that should become a standard practice, and will thus enable MVTR to be reported in future clinical trials, and considered by clinicians when selecting a dressing. However, MVTR can be affected by temperature and humidity, there are different means of assessing MVTR, and the laboratory assessment methods may not reflect the clinical situation. Thus, it is not possible to categorically state whether a high, medium or low MVTR on its own should determine dressing selection37. The manufacturer of the transparent dressing used in this trial indicated that whilst moist wound healing is generally beneficial, it is important to keep CVC exit sites dry to minimise the risk of bacterial proliferation (http://www.smith-nephew.com/australia/healthcare/products/product-types/iv-care/iv3000--product-range/ accessed 9 November, 2017).
Although CHRISP guidelines3 at the commencement of the study recommended the transparent dressing as a superior choice for CVC sites, the updated guidelines both for dialysis sites4, and for CVC sites in general5, now refrain from recommending transparent over non-transparent and instead defer to clinician judgement of situational variables, including climate, for the final selection of a dressing. This study provided locally generated evidence to support nursing clinical judgement in this north Queensland context.
Strengths and limitations
Meticulous adherence to study protocol, even in the face of a major cyclone, which required cancellation and rescheduling of all clients in the haemodialysis service38, was a clear strength of the study and resulted in no missing data during the 72-hour time period around the cyclone. Clinicians’ input was solicited and accommodated in both study design and conduct, with resources and education provided to ensure that all data collection was completed. All invited eligible participants consented to be included in the study, strengthening confidence in using the findings to inform local policy; with potential further applicability to the many other services in tropical climates which also provide dialysis for a predominantly Australian Aboriginal and Torres Strait Islander clientele, and potential applicability in other tropical climes. An Indigenous liaison officer was involved in the project from the planning phase onward, ensuring that principles of cultural safety were considered and applied.
Limitations include the relatively small sample size, despite it being a population study; and the lack of a funded full-time research assistant position to track missing data on a daily rather than weekly basis. The small difference found in rates of intactness unfortunately precluded the ability to calculate a meaningful effect size from which to determine sample size for a later larger study. It was not practical to use infection as the primary outcome measure in this study, because of the rareness of infections. A much larger study would be required to be adequately powered to detect a difference in infection rates related to the different dressing types. The study also included qualitative data collection via focus groups of the dialysis nurses’ attitudes and clinical judgement, which is reported elsewhere7.
Recommendations
Local practice and policy have been informed by the dissemination of the findings across the renal service, with the non-transparent dressing continuing to be preferentially selected for haemodialysis clients in the local tropical climate7. Moisture vapour transmission rates (MVTR) as a specification of the product by its manufacturers, were unavailable at the commencement of this RCT, but later literature23,37 included awareness and discussion of this issue, thus echoing the clinicians’ intuition that breathability of the dressing is a critical factor. Therefore, it is recommended that MVTR be consistently disclosed by all manufacturers in dressing specifications, and then be considered in dressing selection by clinicians and health services. In addition, these findings underscore issues of skin fragility in clients with chronic kidney disease, so it is recommended that meticulous attention be paid to any skin cleansing, skin preparation or skin barrier cream recommendations that are now increasingly being made by manufacturers, both to minimise skin reactions and to maximise dressing adherence over time. In-service education to staff proved to be vital in the success of the study and may also constitute an effective intervention on its own, to ensure appropriate and improved dressing techniques in a rapidly mobile workforce. Multi-site RCTs should be conducted in varying climates to further delineate optimum choices that suit environmental conditions, with larger sample sizes and dedicated research assistant support, but replicating many of the procedural strengths noted in this RCT regarding methods for training and prompting staff to achieve high levels of protocol adherence.
Conclusion
On the primary outcome measure of intactness, neither dressing showed a statistically significant superiority over the other dressing. No infections were reported during the non-transparent dressing arm of this randomised crossover population study that was conducted in the context of a tropical climate and a very high prevalence of diabetes population. Conversely, four site infections were observed during the transparent dressing arm, in keeping with the clinicians’ expertise in the dialysis service, that transparent dressings with less breathability might thus trap moisture under the dressing and promote bacterial growth. The results of this population crossover trial have informed local policy and practice to remain with the clinicians’ existing preference of a non-transparent dressing rather than the transparent recommended by the CHRISP guidelines at the time. Multi-site RCTs should be conducted in varying climates to further delineate optimum choices that suit environmental conditions, with larger sample sizes and dedicated research assistant support.
Acknowledgements
This paper is part of a project undertaken with the financial assistance of a Novice Nurse Researcher Grant 2009, Office of the Chief Nursing Officer, Queensland Health ($9,678), and a Townsville Hospital and Health Service Private Practice Research and Education Trust Fund Grant 2010 ($24,237). It was the topic of Joleen McArdle’s Master of Nursing Science (Research).
Author(s)
Joleen McArdle*
BNSc, PGCertNSc(RenalN), MNsgSci(Research)
Nurse Unit Manager
Renal Satellite Units
Townsville Renal Service, Townsville, QLD, Australia
Email joleen.mcardle@ealth.qld.gov.au
Wendy Smyth
RN, BA, MAppSc, GradDipQuality, MBus, PhD
Nurse Researcher
Nursing and Midwifery Research
Townsville Hospital and Health Service, Townsville QLD, Australia; and
Adjunct Senior Research Fellow
College of Healthcare Sciences
James Cook University, Townsville, QLD, Australia
Kristin Wicking
RN, BSN, MSN, PhD, GradCertEd (Tertiary Teaching)
Senior Lecturer
Nursing, Midwifery and Nutrition
James Cook University, Townsville, QLD, Australia
Anne Gardner
RN, CritCareCert, BA, MPH, PhD
Professor of Nursing, Australian Catholic University Canberra, ACT, Australia (retired); and
Adjunct Professor, College of Healthcare Sciences James Cook University, Townsville, QLD, Australia
* Corresponding author
References
- Besarab A, Raja R. Vascular access for hemodialysis. In: Daugardis J, Blake P, Ing T, editors. Handbook of dialysis. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
- ANZDATA Registry. The thirty fifth annual report: Australia and New Zealand Dialysis and Transplant Registry. Adelaide, South Australia: Australia and New Zealand Dialysis and Transplant Registry; 2012.
- Centre for Healthcare Related Infection Surveillance and Prevention (CHRISP). Queensland Government’s Centre for Health Related Infections Surveillance and Prevention Guidelines. CHRISP QH, editor. Brisbane, Australia: Queensland Health; 2007.
- Centre for Health Care Related Infection Surveillance and Prevention & Tuberculosis Control. Guideline. Haemodialysis Catheters (Version 2). Brisbane, Australia: Queensland Government Department of Health; 2013.
- Centre for Health Care Related Infection Surveillance and Prevention & Tuberculosis Control. Guideline. Tunnelled central venous catheters (Version 2). Brisbane, Australia: Queensland Government Department of Health; 2013.
- NHMRC. Australian Guidelines for the Prevention and Control of Infection in Healthcare. Canberra, Australia: Commonwealth of Australia; 2010.
- Smyth W, McArdle J, Gardner A. Central venous catheter exit site dressings: Balancing patients’ needs, nurses’ experiences and the research evidence. Wound Practice and Research 2016;24(1):41–6.
- Hughes K, Gardner A, McArdle J. Audit of factors associated with the intactness of central venous catheter exit site dressings for northern Australian haemodialysis patients. Renal Soc Australas J 2011;7(3):108–14.
- McArdle J, Gardner A. A literature review of central venous catheter dressings: Implications for haemodialysis in the tropics. Healthcare Infection 2009;14(4):139–46.
- de Barros LdFNM, Arênas VG, Bettencourt ARdC, Diccini S, Fram DS, Belasco AGS et al. Evaluation of two types of dressings used on central venous catheters for hemodialysis [English translation]. Acta Paulista de Enfermagem 2009;22:481–6.
- Conly JM, Grieves K, Peters B. A prospective, randomized study comparing transparent and dry gauze dressings for central venous catheters. J Infect Dis 1989;159(2):310–9.
- Dickerson N, Horton P, Smith S, Rose RC, 3rd. Clinically significant central venous catheter infections in a community hospital: association with type of dressing. J Infect Dis 1989; 160(4):720–2.
- Petrosino B, Becker H, Christian B. Infection rates in central venous catheter dressings. Oncol Nurs Forum 1988;15(6):709–17.
- Harwood L, Wilson B, Thompson B, Brown E, Young D. Predictors of hemodialysis central venous catheter exit-site infections. Cannt J 2008;18(2):26–35.
- Treston-Aurand J, Olmsted R, Allen-Bridson K, Craig C. Impact of dressing materials on central venous catheter infection rates. J Intraven Nurs 1997;20(4):201–6.
- Theaker C. Infection control issues in central venous catheter care. Intensive Crit Care Nurs 2005;21(2):99–109.
- Ullman AJ, Cooke ML, Mitchell M, Lin F, New K, Long DA et al. Dressings and securement devices for central venous catheters (CVC) (Review). Cochrane Database Syst Rev Issue 9, Art No: CD010367. 2015.
- Shivnan JC, McGuire D, Freedman S, Sharkazy E, Bosserman G, Larson E et al. A comparison of transparent adherent and dry sterile gauze dressings for long-term central catheters in patients undergoing bone marrow transplant. Oncol Nurs Forum 1991;18(8):1349–56.
- Silveira R, Braga F, Garbin L, Galvao C. The use of polyurethane transparent film in central venous catheters. Rev Lat Am Enfermagem 2010;18(6):1212–20.
- Trotter B, Brock J, Schwaner S, Conaway M, Burns S. Central venous catheter dressings put to the test. Am Nurse Today 2008;3(4):43–4.
- Jezova L, Ziakova K, Serfelova R. Comparison of transparent polyurethane film and sterile gauze dressing materials for central venous access. Journal of Nursing, Social Studies, Public Health and Rehabilitation 2012;1(2):72–8.
- Jones A. Dressings for the management of catheter exit sites. JAVA 2004;9(1):26–33.
- Lin Y, Chen J, Li Q, Pan K. Moisture vapor transmission rates of various transparent dressings at different temperatures and humidities. Chin Med J 2009;122(8):927–30.
- O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG et al. Guidelines for the prevention of intravascular catheter-related infections. Pediatrics 2002;110(5):e51.
- Safdar N, Kluger DM, Maki DG. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, non-cuffed central venous catheters. Medicine 2002;81(6):466–79.
- Craven DE, Lichtenberg DA, Kunches LM, McDonough AT, Gonzalez MI, Heeren TC et al. A randomized study comparing a transparent polyurethane dressing to a dry gauze dressing for peripheral intravenous catheter sites. Infect Control 1985;6(9):361–6.
- Twardowski ZJ, Prowant BF. Classification of normal and diseased exit sites. Perit Dial Int 1996;16:32–50.
- Twardowski ZJ, Prowant BF. Exit-site study methods and results. Perit Dial Int 1996;16:Suppl 3.
- National Kidney Foundation. Updates, clinical practice guidelines and recommendations. Boston, MA: National Kidney Foundation; 2006.
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. [Erratum appears in Clin Infect Dis 2010 Feb 1;50(3):457], [Erratum appears in Clin Infect Dis. 2010 Apr 1;50(7):1079 Note: Dosage error in article text]. Clin Infect Dis 2009;49(1):1–45.
- Maroz N, Simman R. Wound healing in patients with impaired kidney function. J Am Coll Clin Wound Spec 2013;5(1):2–7.
- Büttner P, Muller R. Epidemiology. South Melbourne, Victoria, Australia: Oxford University Press; 2011.
- Chu G, Adams K, Crawford S. Improving catheter-related blood stream infection in haemodialysis patients using a practice development framework. Renal Soc Australas J 2012;9(1):16–21.
- Webster J, Gillies D, O’Riordan E, Sherriff K, Rickard C. Gauze and tape and transparent polyurethane dressings for central venous catheters (Review). The Cochrane Collaboration; 2011.
- Rolls K, Ullman A. Central venous access device-associated skin impairment (CASI). Inscope 2017;Winter:44-5.
- Callaghan S, Copnell B, Johnston L. Comparison of two methods of peripheral intravenous cannula securement in the pediatric setting. J Infus Nurs 2002;25(4):256–64.
- Thomas S. The effect of weather and other environmental factors on the performance of surgical dressings. Wounds 2012;24(12):335–8.
- McArdle J. Cyclone Yasi: dialysis mission impossible. Renal Soc Australas J 2011;7(3):76–8.



