Volume 26 Number 2
Advanced wound therapies
Parinaz Ahangar, Michael Woodward and Allison J Cowin
Keywords Chronic wounds, Antibodies, Wound therapy, skin substitute, oxygen therapy, growth factors, negative pressure wound therapy.
Introduction
Wound management is a significant and growing issue not only in Australia but worldwide. This increase can be attributed to the rise in prevalence of diabetes and obesity as well as the increasing geriatric population; leading to large numbers of pressure ulcers (PUs), venous leg ulcers (VLUs) and other chronic wounds. The cost to the Australian health care system has been estimated at A$2.85 billion and globally it is expected to reach US$13.07 billion by 20221. The costs attributable to a new foot ulcer are estimated at $17,5002. Lower extremity amputation is a common complication, affecting some 15% of diabetics with foot ulcers, with direct costs escalating to up to US$60,000 per patient2. In the US alone, some 80,000 lower extremity amputations are performed on diabetic patients each year. Furthermore, it costs approximately US$60,000 for lower limb amputation, and hospitalisation can cost a further US$16,000–20,000 for patients with diabetic ulcers so effective non-surgical treatments that improve healing is providing an incentive for the biopharmaceutical industry to focus on this area of unmet medical need2. This has led to the development of a multitude of new approaches and products undergoing trials or in clinics and whilst moist wound healing with topical dressings remains the core of wound healing therapeutics, there is increasing interest and need for more advanced wound therapies.
This review has focussed on some of the new approaches and therapies which are currently under development or in clinical use. Some of these new approaches have only been assessed in animal studies, while others have had more rigorous assessment in human clinical trials and are currently on the market. This is not an exhaustive list but provides an insight into the breadth of research and development currently being performed to improve healing of people with wounds.
Skin substitutes
Skin substitutes were first developed as xenografts in 1871 by Reverdin3. They are bioengineered products which are able to replace the function and form of skin. Skin substitutes have been used traditionally for burns and wounds which require large areas of skin replacement. Currently, advanced skin substitutes are utilised universally for deep and chronic wounds as they are able to reduce infection, decrease body fluid loss, improve cytokine and growth factor production as well as acting as a covering to help protect the healing wound4. Several types of skin substitutes that are currently available and are used for wound management are shown in Figure 15.
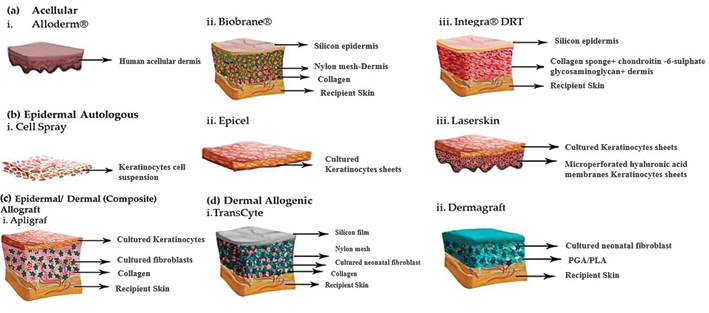
Figure 1: The structure of some common skin substitutes. Modified from reference 5.
Vig K et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci 2017; 18(4):789.
Acellular skin substitutes
Acellular or synthetic skin substitutes are composed of different matrices without the inclusion of any cells in order to minimise any foreign body response. These substitutes can be designed for specific purposes by changing the composition of the scaffold. They mainly prevent body fluid loss and microbial infection.
Biobrane consists of porcine collagen type 1 on a silicone membrane6 and has been shown to decrease the pain, length of hospital stay and wound healing time of partial thickness burns in several prospective randomised controlled trials (RCTs)7-9.
Integra, a bilayer skin equivalent, consists of an epidermal substitute layer on a chondroitin-6 sulphate and collagen-based dermis10. Integra facilitates the migration of macrophages to the wound and promotes angiogenesis and granulation tissue formation11. RCT studies have shown that burn patients treated with Integra healed faster and have elevated amounts of serum constitutive protein and lower resting energy compared to patients treated with standard grafts12-14. Moreover, patients with a diabetic foot ulcer (DFU) demonstrated greater complete wound closure with no infection and recurrent ulcer during the 12-week study15.
AlloDerm is an allograft skin in which all the epidermal and dermal cells are removed and the human dermal matrix acts as a template for soft tissue regeneration16 and promotes the functional performance in burn patients17. Use of AlloDerm on patients with major burns resulted in reduction in post-burn joint contracture18.
Graftjacket is a micro-ionised human dermal collagen matrix, which has been shown to increase the number of healed ulcers in a treated DFU group while decreasing the healing time and ulcer size19. Moreover, treatment of lower extremity wounds with Graftjacket in diabetic patients led to a significant increase in the rate of healing20.
Omnigraft is composed of a matrix layer made of collagen and the glycosaminoglycan chondroitin-6 sulphate with a layer of silicone. Recently, the Food and Drug Administration (FDA) has approved Omnigraft, which previously had approval for burn therapy, for the treatment of DFUs21.
Cellular skin substitutes
Cellular or natural skin substitutes contain living cells which are predominantly skin cells contained within a matrix. Incorporation of cells within a matrix makes them more effective than acellular substitutes; however, they are more expensive, require specific storage conditions and are more difficult to use. Cellular skin substitutes are classified in two groups: Autologous substitutes which contain the patient’s own cells and allogeneic substitutes in which cells are obtained from donors.
TransCyte is a temporary skin substitute that consists of fibroblasts, from neonatal foreskins, seeded over a nylon mesh scaffold16. Several clinical studies have shown that TransCyte improves the rate of re-epithelialisation and reduces healing times of partial and full-thickness burns. It also decreased the daily wound care time, length of stay and need for autografts in burn patients.
Dermagraft is made from viable neonatal foreskin fibroblasts with a collagen mesh made from polyglycolic acid11. Administration of Dermagraft has been shown to result in faster wound closure, fewer ulcers and reduced infection in chronic DFU, suggesting that this is a safe and effective treatment22. The healing of VLUs has also been shown to be improved when treated with Dermagraft and compression therapy23-25.
Apligraf, a bilayered skin equivalent, contains allogenic keratinocytes on a type 1 bovine collagen gel and has been studied clinically in chronic wounds26. Patients with VLUs treated with apligraf were significantly more effective in reaching complete wound healing27. Apligraf was also found to improve vascularity, pigmentation, wound height and scar scores in burn patients28 as well as shortening the healing time of donor site wounds29.
OrCel is a product produced by culturing human allogeneic keratinocytes and dermal fibroblasts in two separate layers onto the different sides of a sponge made of type I collagen30. A prospective, randomised, multicentre study comparing OrCel with Biobrane for the treatment of donor site injuries revealed shorter healing times and less scarring in wounds that were treated with OrCel31.
Epicel is made from human keratinocytes and murine fibroblasts that are placed onto petroleum gauze32 and has been investigated for the treatment of patients with a large burn area (37% ± 17% TBSA) in which about 90% of patients survived33.
Vivoderm or laser skin is composed of cultured autologous keratinocytes on laser-perforated hyaluronic acid34. A number of studies have confirmed the safety and effectiveness of this dressing for the treatment of deep leg ulcers35 and DFUs36-38.
Keratinocyte dressings
Keratinocytes are specialised epidermal cells and are the main cells that participate in the re-epithelialisation process and contribute to wound healing by secreting several cytokines and growth factors39. These cells were first cultured successfully in 1975 and were first used as grafts for burns treatment five years later40. Keratinocytes have been used widely as allografts and autografts for the treatment of various wounds including acute, chronic and burn wounds41. Treatment of burns with autologous keratinocytes minimised the morbidity for patients with severe burns. Several clinical studies (a case study and a study on 55 patients) which assessed the effects of autologous keratinocytes on burns and chronic wounds demonstrated that these cells have remarkable therapeutic value42,43. Moustafa et al. used an autologous keratinocyte dressing as a therapy for non-healing diabetic neuropathic foot ulcers which showed promising results44. Treatment with cultured autologous keratinocytes resulted in the complete healing in 6 of 9 ulcers and one improved ulcer in 6 weeks45. Treatment of 51 chronic VLUs with allogenic keratinocyte sheets for a month also resulted in accelerated wound healing46. A pilot study on patients with DFUs indicated that all patients treated with allogenic keratinocyte sheets achieved complete wound healing in 12 weeks47.
Keratin-derived dressings
Keratinocytes enhance wound healing by facilitating re-epithelialisation through the up-regulated expression of keratins48. It has been shown that keratin-based dressings are able to improve the function of keratinocytes in terms of proliferation and migration49. An RCT confirmed that treatment of partial-thickness wounds with keratin-derived dresses caused an increase in re-epithelialisation after seven days50. In a case study conducted on three patients with mixed venous and arterial leg ulcers, faster healing rates in were also observed in ulcers51.
Amniotic membrane
Amniotic membrane (AM) is a widely used and cost-effective wound dressing which contains cytokines, growth factors and progenitor cells. AM has unique properties, including being antibacterial, anti-inflammatory, anti-scarring, providing pain relief, as well as low immunogenicity52,53. AM grafts have been trialled clinically for diabetic neurovascular ulcers, VLUs, and many other types of wounds54,55. For example, in a pilot study, AM graft treatment of patients with VLUs caused a significant increase in granulation tissue formation and led to a reduction in ulcer size and pain level56. Evaluating the effects of dehydrated human amnion/chorion membrane (EpiFix) on the treatment of VLUs showed a significant improvement in time of the healing57. In another RCT on patients with diabetes, lower extremity ulcers treated with EpiFix demonstrated complete healing and a rapid reduction in wound size, compared to wounds treated with other grafts58. All the clinical studies revealed that the use of AM for covering wounds contributed to reduced scarring, infection and pain and accelerated wound closure.
Oxygen
Oxygen is a vital component of the wound healing process and is necessary for modulation of cell responses and angiogenesis59. Providing additional external oxygen has been shown to contribute to improved wound healing in chronic wounds60 and has been demonstrated to be valuable to wound healing since the 1960s61.
In topical oxygen therapy, oxygen is provided to the wound area using portable devices which create external pressure and increase the oxygen concentration. A clinical trial on 20 patients with DFUs receiving topical oxygen revealed an increase in healing rate compared to those receiving standard care62. Currently, commercially available systems based on pressurised oxygen have been reported to be effective and safe dressings for severe DFUs and VLUs in several case reports and clinical studies63-65. Topical oxygen therapy is a cheap and flexible method without any marked side effects related to systemic oxygen therapy, which makes it preferable to hyperbaric oxygen therapy (HBOT)60,66,67. HBOT is a procedure in which patients are placed in a chamber with greater oxygen pressure — two to three times greater than normal atmospheric pressure — that results in an elevated amount of pure oxygen in wound tissues68. While local hypoxia results in impaired wound healing, the growth in oxygen concentration has a number of physiologic and cellular benefits related to collagen deposition, angiogenesis and wound closure rate. A number of clinical trials on patients with a DFU demonstrated a rise in the rate of ulcer healing at six weeks but not at longer term. In another trial conducted on VLUs, the wound size reduction was shown by week 6. To the best of our knowledge, there are no clinical studies investigating HBOT and arterial ulcers.
However, there are some adverse effects of systemic oxygen including central nervous toxicity, ear barotrauma, pulmonary barotraumas and seizures69,70. A recent sponsored consensus round table meeting supported the use of topical oxygen therapy to promote wound healing, particularly with DFUs71.
Topical negative pressure
Topical negative pressure (TNP) or vacuum-assisted closure (VAC) has been used classically for open wound care for over two decades. In this therapy, a polyurethane foam is applied to the wound surface and is sealed with an adhesive dressing72. The negative pressure given by a vacuum pump results in the promotion of wound healing, which is probably caused by an increase in local body fluid and new tissue formation along with a reduction in infection, mechanical stress and pain73. In the treatment of DFUs, TNP has promoted wound healing and granulation tissue formation and decreased wound size and bacterial infections74. Patients with PUs treated by TNP, in several RCTs, had a significant reduction in the wound size, depth, healing time and length of hospital stay75,76. A number of other clinical studies have demonstrated the beneficial effects of TNP on non-healing chronic wounds including necrotic ulcers, arterial ulcer, PUs, DFUs and VLUs77-82. However, the dressing should be replaced every two or three days to prevent tissue ingrowth, infection and pain. Recently, an extra polymeric membrane dressing has been used to overcome this replacement barrier. This RCT study indicated that the use of additional polymeric membrane dressing in patients with chronic wounds was a cost-effective, safe and efficient method which decreases the need for dressing changes83.
Electrical stimulation
Human intact skin has an electrical potential due to the ATPase pumps present in the epidermal cells. Following damage to the skin, an additional electrical current, referred to as the “current of injury”, is generated to facilitate wound healing84. It has been shown that administration of external electrical currents leads to an enhancement in the immunity of cells, a reduction in infection and improvement of wound healing85. Therefore, electrical stimulation (ES) therapy for wounds, particularly chronic wounds, has gained interest in clinical trials. ES devices vary due to the different voltages, length of application time and currents. Three types of ES including alternating current (AC), direct current (DC), and pulsed current (PC) have been used for wound healing86. The majority of clinical trials using ES for wound healing have used PC. In an RCT, patients with chronic dermal ulcers treated using PC electric stimulation showed a significant reduction in wound area87. Gentzkow et al. in a prospective study has also shown complete healing in 43% of patients with PUs88. Several RCTs using PC ES on PUs have resulted in accelerated wound healing rate89,90. In terms of DFUs, treatment with PC ES has led to enhanced wound healing91. Recently, in a pilot RCT, positive effects of PC ES on reduction of VLUs size was observed92 and a much larger RCT is in progress with three arms: ES, ES + compression and compression alone. If shown to be effective, it will be an attractive therapy for those who cannot tolerate compression or where compression is contra-indicated.
Extracorporeal shock wave therapy (ESWT)
Extracorporeal shock wave therapy (ESWT) is based on shock waves which are transient acoustic pulses and are applied with high pressure to the wound site. ESWT has been used as an efficient therapy method for treatment of urinary and kidney stones for about three decades93. Several clinical trials investigating the effects of ESWT on wound healing have achieved very encouraging outcomes. One study conducted on 208 patients with acute and chronic wounds demonstrated safety and potential efficacy for ESWT applied to the wounds; in which 75% of patients showed complete wound healing (with 100% epithelialisation) in a mean time of 44 days after therapy94. A prospective, single-blind, placebo-controlled study was conducted to assess the effects of ESWT on scar pain which confirmed the scar pain reducing effect of ESWT95. A number of clinical studies have also evaluated the use of ESWT on deep second- and third-degree burns. The results revealed an increase in complete wound healing rate and a decrease in burn-pruritus96,97. Another randomised, single-blind, controlled study compared the effects of HOBT and ESWT on DFUs. The results revealed that the number of patients with complete wound healing or improved wound healing was greater in the ESWT-treated group after daily treatment for 20 times98. A case series in VLUs was also supportive of a benefit of ESWT99.
Growth factor therapy
Wound healing processes rely on cytokines and growth factors to regulate cellular signalling pathways to alter the proliferation and migration of cells in a wound bed. These proteins contribute to every step of the wound healing process and have been suggested as effective therapies for acute and chronic wounds.
Granulocyte-macrophage colony stimulating factor
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a multipotent cytokine which plays a vital role in wound healing. In vivo studies using GM-CSF have suggested its effects on skin cell proliferation and migration and recruitment of immune cells to the wound bed100,101. A number of in vivo and clinical studies have shown significant effects of GM-CSF treatment on the healing of chronic wounds and burns. In one study of chronic VLUs, 50% of patients treated with GM-CSF completely healed by two months as compared to 11% of the control, non-treated group102. In another double-blind, randomised, placebo-controlled study, chronic VLUs patients receiving GM-CSF demonstrated significant improvement in the healing of wounds in the treatment group103. Two randomised controlled studies confirmed the therapeutic effects of a GM-CSF hydrogel on second-degree burns and indicated that early application achieved the best outcome104,105. However, studies of GM-CSF on PUs have yielded diverse results. Topical GM-CSF did not have a marked effect on PUs, compared to the placebo group in a study by Robson et al.106; while other case studies have demonstrated enhanced healing rate and increased granulation tissue formation in PUs107. One randomised controlled study on diabetic patients with infected foot ulcers did, however, show improved outcomes on receiving GM-CSF along with insulin therapy108.
Platelet-derived growth factor
Platelet-derived growth factor (PDGF) that is released from platelets following injury has important functions in all phases of the wound healing process. In vitro and in vivo animal studies have confirmed the positive effects of this growth factor on inflammation initiation, fibroblast proliferation, re-epithelialisation and recruitment of other cells to the wound site109-111. The amount of PDGF has been shown to decrease in chronic wounds, which implicates it as a therapeutic factor. Promoted wound healing was observed in patients with chronic PUs treated with Becaplermin, an FDA-approved human PDGF, in RCTs112. A phase II study conducted on patients with advanced PUs demonstrated a reduction in wound size in the PDGF-treated group compared to the control group113. DFUs treated have shown accelerated closure in an RCT114. Although the clinical trials are promising, the use of PDGF is limited owing to the need for dressing replacement. Moreover, applying Becaplermin to the wound site caused erythematous rashes and a burning sensation at the site of application115 and using three tubes or more increased the risk of malignancy in a 20-month follow-up study from two RCTs116,117.
Opal A
Opal A is an alkalinised compound which is derived from paw paw fruit. In a case study conducted on patients with PUs, treatment with Opal A cream promoted wound healing118. Treatment of arterial ulcers in a woman with arteriopathy resulted in improved wound healing and quicker wound closure. Furthermore, application of Opal A to a patient with an arterial leg ulcer decreased the pain and time of healing to half. Overall, arterial ulcers are resistant to common standard therapies but have shown improvement after Opal A treatment, which suggests that this compound may be an effective and safe method for wound healing119.
Monoclonal antibody therapy
Monoclonal antibodies are mono-specific antibodies which are able to bind to target proteins and neutralise their activity. Some proteins has been shown to be elevated in acute and chronic wounds; targeting these proteins with monoclonal antibodies may contribute to improved wound healing.
Transforming growth factor ß CAT-152
A number of studies have suggested that transforming growth factors contribute to hypertrophic scarring120. CAT-152 is a human monoclonal antibody that neutralises the activity of transforming growth factor ß2. Several in vitro and in vivo animal studies have shown the anti-scarring effects of CAT-152 on post-surgery wounds121. However, not enough success was observed in an RCT conducted on the effectiveness and safety of CAT-152 on people undergoing trabeculectomy and other surgeries122.
TNF-α
Several studies implicated tumour necrosis factor-α (TNF-α) as a pivotal contributing factor in impaired healing in human and animal models123. The levels of TNFα elevate in chronic wounds, suggesting its association with different chronic ulcers124.
Infliximab is a therapeutic monoclonal antibody that is able to neutralise the activity of TNF-α. In a number of case studies, Infliximab was applied topically to the patients with chronic ulcers for 16 weeks. A significant reduction of ulcer surface area and improvement in healing rates was observed125.
Adalimumab is another human monoclonal antibody that inhibits the TNF-α function and is clinically used for the treatment of autoimmune diseases126. VLUs have higher TNF-α levels compared with normal skin. Treatment of 4 patients with VLUs for 4 weeks reduced the level of TNF-α in the wound site and decreased the ulcer size in a clinical pilot study127.
These case and pilot studies indicated that future RCTs of TNF-α antibodies are viable and may further reveal the safety and efficacy of these new therapies on wound healing.
Flightless I protein
Flightless I protein (Flii) is an inflammatory modulatory protein128. Several studies have demonstrated that Flii is elevated in human chronic wounds and the action of Flii negatively regulates wound healing by affecting process such as inflammation, collagen I expression, migration of keratinocytes during re-epithelialisation and migration of fibroblasts129-131. In preclinical models, Flii neutralising antibodies (FnAbs) have been shown to promote healing of acute and chronic wounds with wounds becoming smaller and more contracted in murine and porcine models132-134. Clinical studies need to be conducted to further investigate the effects of FnAbs on human wounds.
Protease inhibitors
The activity of proteases and their inhibitors plays a critical role in maintaining the balance between extracellular matrix construction and deconstruction135. Upregulation of proteases in chronic wounds contributes to abnormal degradation of extracellular matrix and proteins such as cytokines and growth factors136. These findings implicate protease inhibitors as a potential therapeutic approach for improving wound healing. In an ex vivo study, the administration of protease inhibitors to wound fluid taken from patients with DFUs caused a marked decrease in the protease activity including matrix metalloproteinase, plasmin and elastase137. Several dressings have been developed to address this imbalance in proteolytic activity.
Promogran is a dressing that is made of collagen I and oxidised regenerated cellulose. This dressing reduces the activity of proteases such as elastin and matrix metalloproteinase. In several case studies Promogran dressing has been shown to be effective in chronic wound healing138. Applying this dressing to VLU resulted in improved wound healing139,140. An RCT conducted to evaluate the treatment with Promogran on DFUs showed that this treatment led to a safe and faster wound healing in a 6-month study141.
UrgoStart is a dressing that contains a layer of polyester mesh, hydrocolloid polymers and nano-oligosaccharide factor, which is a matrix metalloproteinase inhibitor factor142. UrgoStart has been shown to reduce the ulcer size and time of healing compared with foam dressings in two RCTs143,144.
Doxycycline is an antibiotic and matrix metalloproteinase inhibitor which has been shown to improve the healing of chronic diabetic ulcers mainly based on its protease inhibitor activity in a small pilot RCT145. Evaluating the effects of doxycycline as an adjunct to compression therapy on patients with chronic VLUs demonstrated the positive effects of this inhibitor on reduction of ulcer size146.
Connexin inhibitor
Connexin 43 (Cx43) is a protein in gap junction channels which has a role in inflammatory and fibrotic processes. The expression of Cx43 quickly decreases after wounding147 but is elevated in chronic wound fluids148, suggesting this protein may be a potential target for wound healing therapies. An in vivo study investigating the effect of Cx43 inhibition on a corneal wound confirmed the promotion of wound healing in superficial epidermal wounds in murine model149. Another animal study conducted by Qiu et al. revealed that application of Cx43 antisense oligonucleotides led to accelerated wound healing150. In a prospective, multicentre clinical trial conducted on patients with VLUs, connexin expression was inhibited by alpha connexin carboxyl-terminal 1 (ACT1) peptide gel. The treatment resulted in an increase in skin cell proliferation, migration and re-epithelialisation. A significant reduction in ulcer area was observed at 12 weeks, as compared with standard care, which indicates the safety and efficacy of using ACT1 gel on healing of skin wounds151. In another prospective, randomised, multicentre clinical trial the efficacy of ACT1 was evaluated on DFUs and also showed greater re-epithelialisation rate and accelerated healing when treated with ACT1, as compared with standard care152.
Platelet-rich plasma
Platelet-rich plasma (PRP) is a concentrated plasma which is derived from whole blood and contains platelets and elevated amounts of growth factors, cytokines and fibrin153. PRP has been used for treating inflammatory diseases, including osteoarthritis and diabetes for three decades. Several clinical studies demonstrated that PRP treatment promotes wound healing by affecting the proliferation, angiogenesis and cell recruitment in the wound site. In a case study, the autologous PRP treatment of a diabetic patient with unhealed chronic lower extremity wounds was successful154. PRP treatment applied using a spray gave rise to an improved rate of healing and less antibiotic consumption and pain on surgery wounds after one week in a prospective cohort study155. Driver et al. have conducted RCTs to evaluate the effects of autologous PRP gel on DFUs in a 12-week study. The results showed an improvement in complete wound healing rate and shorter healing time in treated groups156. Furthermore, it has been shown in a clinical study with 56 patients that autologous PRP gel increased the healing rate and decreased infection in diabetic ulcers157.
Medications
A number of medications have been utilised in wound healing. Where there is an inflammatory or auto-immune dimension to the wound aetiology, agents targeting these mechanisms have been successfully utilised. Such wounds include vasculitis ulcers, pyoderma gangrenosum and necrobiosis lipoidica. The agents have variously been utilised topically, orally and systemically, in widely varying doses.
Immunosuppressive drugs: Drugs with immunomodulatory roles including Cyclosporine, Mycophenolate, Tacrolimus, Pimicrolimus, Corticosteroids and Azathioprine have been shown to have positive effects on pyoderma gangrenosum158,159.
Other medications, including complementary and alternative medicines, have been used for specific wound conditions — mostly orally but in some cases topically. Examples include:
Rutosides (oxerutins): These drugs have been examined on wounds associated with lymphoedema. In two different RCTs, they increased the freedom, movement and decreased the pain in patients with arm and leg ulcers160,161.
Horse chestnut: This drug has been found effective for chronic venous insufficiency and lower limb oedema from various causes162,163. There is little evidence, however, to support its use in wound healing.
Phenytoin: Topical phenytoin has been used to promote chronic wound healing such as leg ulcers, PUs, and DFUs but there is a paucity of recent evidence164,165.
Pentoxifylline: This agent improves oxygen delivery to ischaemic areas and has been successfully used for symptoms of peripheral vascular disease. It has also been utilised for arterial and DFUs166. A Cochrane review demonstrated its efficacy when used in addition to compression, or where compression cannot be used, in VLUs167.
Sodium thiosulphate: Administered intravenously, this has been shown to improve healing of ulcers caused by calciphylaxis168.
Arginine: This amino acid is required for wound healing but the body has only limited capacity to produce it. Several trials have supported its use in assisting healing of stage 2 and above pressure injuries, in doses from 3 to 9 gm/day169-171.
Other nutrients: Whilst extensively administered, there is little to support the role of vitamin C or zinc to promote wound healing, despite their importance to collagen formation and wound healing. If a clear deficiency is demonstrated, they should be administered in replacement doses.
Herbal: Many herbs have been promoted as assisting wound healing, but there is little to no evidence to support their benefit.
Conclusions
Advanced wound therapies offer the appropriately trained clinician a wide range of options to promote wound healing, particularly for certain wound types or where more standard therapies are failing. Some of these therapies have been investigated in animal trials and case studies and are not approved for use due to the lack of clinical evidence. More RCT studies need to be done to prove the efficiency and safety of these methods. They are often more expensive than standard therapies and have limited availability, but where they can be sourced and afforded, they should be considered. As in all therapeutic areas, they should only be used where cheaper therapies have been tried and failed, or are unlikely when used alone to assist wound healing and should be used with a full awareness of possible adverse effects. Due to space restrictions, it has not been possible to review all new advances in wound technologies. Others include stem cells, gene therapies, and honey and silver dressings, which also show promise as treatments for wounds. However, given the magnitude of interest at the moment by companies keen to enter the wound care market, the development of advanced wound therapies is on the rise and only time will tell what the next breakthrough will be for the treatment of chronic non-healing wounds.
Author(s)
Parinaz Ahangar
MSc
Future Industries Institute
University of South Australia
SA, Australia
Michael Woodward
OAM
Director, Wound Management Service and Director Aged Care Research,
Heidelberg Repatriation Hospital
Austin Health
VIC, Australia
Allison J Cowin*
PhD
Professor Regenerative Medicine
Future Industries Institute
University of South Australia
SA, Australia
Email Allison.Cowin@unisa.edu.au
* Corresponding author
References
1. Graves N, Zheng H. Modelling the direct health care costs of chronic wounds in Australia. Wound Practice & Research 2014;22(01):20–24,26–33.
2. Elder M. The market for wound care technology. 2007: BCC Research Report ID PHMO11E.
3. Horch RE et al. Tissue engineering of cultured skin substitutes. J Cell Mol Med 2005;9(3):592–608.
4. Kroner E et al. Bioinspired polymeric surface patterns for medical applications. J Appl Biomater Funct Mater 2012;10(3):287–92.
5. Vig K et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci 2017;18(4):789.
6. Whitaker IS, Prowse S, Potokar TS. A critical evaluation of the use of Biobrane as a biologic skin substitute: a versatile tool for the plastic and reconstructive surgeon. Ann Plast Surg 2008;60(3):333–7.
7. Lesher AP et al. Effectiveness of Biobrane for treatment of partial-thickness burns in children. J Pediatr Surg 2011;46(9):1759–63.
8. Barret JP et al. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast Reconstr Surg 2000;105(1):62–5.
9. Feldman DL, Rogers A, Karpinski RH. A prospective trial comparing Biobrane, Duoderm and xeroform for skin graft donor sites. Surg Gynecol Obstet 1991;173(1):1–5.
10. Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res 1980;14(1):65–81.
11. Hansen SL et al. Using skin replacement products to treat burns and wounds. Adv Skin Wound Care 2001;14(1):37–44; quiz 45–6.
12. Heimbach DM et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil 2003;24(1):42–8.
13. Heimbach D et al. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg 1988;208.
14. Branski LK et al. Longitudinal assessment of Integra in primary burn management: a randomized pediatric clinical trial. Crit Care Med 2007;35(11):2615–23.
15. Yao M et al. Ease of use, safety, and efficacy of integra bilayer wound matrix in the treatment of diabetic foot ulcers in an outpatient clinical setting: a prospective pilot study. J Am Podiatr Med Assoc 2013;103(4):274–80.
16. Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol 2001;2(5):305–13.
17. Lattari V et al. The use of a permanent dermal allograft in full-thickness burns of the hand and foot: a report of three cases. J Burn Care Rehabil 1997;18(2):147–55.
18. Yim H et al. The use of AlloDerm on major burn patients: AlloDerm prevents post-burn joint contracture. Burns 2010;36(3):322–8.
19. Reyzelman A et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J 2009;6(3):196–208.
20. Winters CL et al. A multicenter study involving the use of a human acellular dermal regenerative tissue matrix for the treatment of diabetic lower extremity wounds. Adv Skin Wound Care 2008;21(8):375–81.
21. Driver VR et al. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen 2015;23(6):891–900.
22. Hart CE, Loewen-Rodriguez A, Lessem J. Dermagraft: Use in the treatment of chronic wounds. Adv Wound Care 2012;1(3):138–141.
23. Omar AA et al. Treatment of venous leg ulcers with Dermagraft. Eur J Vasc Endovasc Surg 2004;27(6):666–72.
24. Gentzkow GD et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care 1996;19(4):350–4.
25. Warriner RA, 3rd, Cardinal M. Human fibroblast-derived dermal substitute: results from a treatment investigational device exemption (TIDE) study in diabetic foot ulcers. Adv Skin Wound Care 2011;24(7):306–11.
26. Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg 1995;21(10):839–43.
27. Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen 1999;7(4):201–7.
28. Waymack P, Duff RG, Sabolinski M. The effect of a tissue engineered bilayered living skin analog, over meshed split-thickness autografts on the healing of excised burn wounds. The Apligraf Burn Study Group. Burns 2000;26(7):609–19.
29. Hu S et al. Evaluation of Apligraf persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen 2006;14(4):427–33.
30. Martin LK, Kirsner RS. Use of a meshed bilayered cellular matrix to treat a venous ulcer. Adv Skin Wound Care 2002;15(6):260,262,264.
31. Still J et al. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns 2003;29(8):837–41.
32. Vacher D. Autologous epidermal sheets production for skin cellular therapy. Ann Pharm Fr 2003;61(3):203–6.
33. Carsin H et al. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns 2000;26(4):379–87.
34. Ramos-e-Silva M, Ribeiro de Castro MC. New dressings, including tissue-engineered living skin. Clin Dermatol 2002;20(6):715–23.
35. Pajardi G et al. Skin substitutes based on allogenic fibroblasts or keratinocytes for chronic wounds not responding to conventional therapy: a retrospective observational study. Int Wound J 2016;13(1):44–52.
36. Uccioli L et al. Two-step autologous grafting using HYAFF scaffolds in treating difficult diabetic foot ulcers: results of a multicenter, randomized controlled clinical trial with long-term follow-up. Int J Low Extrem Wounds 2011;10(2):80–5.
37. Monami M et al. Autologous skin fibroblast and keratinocyte grafts in the treatment of chronic foot ulcers in aging type 2 diabetic patients. J Am Podiatr Med Assoc 2011;101(1):55–8.
38. Lobmann R et al. Autologous human keratinocytes cultured on membranes composed of benzyl ester of hyaluronic acid for grafting in nonhealing diabetic foot lesions: a pilot study. J Diabet Complications 2003;17(4):199–204.
39. Pastar I et al. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care 2014;3(7):445–464.
40. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975;6(3):331–43.
41. You H-J et al. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes—A pilot study. Wound Repair Regen 2012;20(4):491–499.
42. Yanaga H et al. Cryopreserved cultured epidermal allografts achieved early closure of wounds and reduced scar formation in deep partial-thickness burn wounds (DDB) and split-thickness skin donor sites of pediatric patients. Burns 2001;27(7):689–98.
43. Gallego L et al. Use of cultured human epithelium for coverage: a defect of radial forearm free flap donor site. Med Oral Patol Oral Cir Bucal 2010;15(1):e58–60.
44. Moustafa M et al. A new autologous keratinocyte dressing treatment for non-healing diabetic neuropathic foot ulcers. Diabet Med 2004;21(7):786–9.
45. Moustafa M et al. Randomized, controlled, single-blind study on use of autologous keratinocytes on a transfer dressing to treat nonhealing diabetic ulcers. Regen Med 2007;2(6):887–902.
46. Leigh IM et al. Treatment of chronic venous ulcers with sheets of cultured allogenic keratinocytes. Br J Dermatol 1987;117(5):591–597.
47. You HJ et al. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes — a pilot study. Wound Repair Regen 2012;20(4):491–9.
48. Omary MB, Ku N-O. Skin care by keratins. Nature 2006;441:296.
49. Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 2006;441(7091):362–5.
50. Pechter PM et al. Keratin dressings speed epithelialization of deep partial-thickness wounds. Wound Repair Regen 2012;20(2):236–42.
51. Than MP et al. Keratin-based wound care products for treatment of resistant vascular wounds. J Clin Aesthet Dermatol 2012;5(12):31–35.
52. John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am 2003;16(1):43–65,vi.
53. Niknejad H et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99.
54. Bianchi C et al. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix®) allograft for the treatment of venous leg ulcers. Int Wound J 2018;15(1):114–122.
55. Castellanos G et al. Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta 2017;59:146–153.
56. Mermet I et al. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen 2007;15(4):459–464.
57. Haugh AM et al. Amnion membrane in diabetic foot wounds: A meta-analysis. Plast Reconstr Surg Glob Open 2017;5(4):e1302.
58. Zelen CM et al. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J 2015;12(6):724–732.
59. Bishop A. Role of oxygen in wound healing. J Wound Care 2008;17(9):399–402.
60. Dissemond J et al. Topical oxygen wound therapies for chronic wounds: a review. J Wound Care 2015;24(2):53–63.
61. Wada J, Ikeda T Kamata, K. Oxygen hyperbaric treatment for carbon monoxide poisoning and severe burns in coal mine gas explosion. Igakunoayumi (Japan) 1965;54(1):68.
62. Yu J et al. Topical oxygen therapy results in complete wound healing in diabetic foot ulcers. Wound Repair Regen 2016;24(6):1066–1072.
63. Tawfick WA, Sultan S. Technical and clinical outcome of topical wound oxygen in comparison to conventional compression dressings in the management of refractory nonhealing venous ulcers. Vasc Endovascular Surg 2013;47(1):30–7.
64. Davis SC et al. Topical oxygen emulsion: a novel wound therapy. Arch Dermatol 2007;143(10):1252–6.
65. Gordillo GM et al. Topical oxygen therapy induces vascular endothelial growth factor expression and improves closure of clinically presented chronic wounds. Clin Exp Pharmacol Physiol 2008;35(8):957–64.
66. Hayes PD, Alzuhir N, Curran G, Loftus IM. Topical oxygen therapy promotes the healing of chronic diabetic foot ulcers: a pilot study. J Wound Care 2017;26(11):652–660.
67. Curran G, Fisher C, Hayes P, Loftus I, Sequeira L. Case series: The impact of NATROX® Oxygen Wound Therapy System on patients with diabetic foot ulcers. The Diabetic Foot Journal 2017;20(3):193–197.
68. Tibbles PM, Edelsberg JS. Hyperbaric-Oxygen Therapy. New Engl J Med 1996;334(25):1642–1648.
69. Camporesi EM. Side effects of hyperbaric oxygen therapy. Undersea Hyperb Med 2014;41(3):253–7.
70. Hampson N, Atik D. Central nervous system oxygen toxicity during routine hyperbaric oxygen therapy. Undersea Hyperb Med 2003;30(2):147–53.
71. Wounds UK Expert Panel Report. Consensus round table meeting: Clinical pathway for using topical oxygen therapy in practice. London: Wounds UK, 2017. Available to download from: www.wounds-uk.com.
72. Banwell PE, Musgrave M. Topical negative pressure therapy: mechanisms and indications. Int Wound J 2004;1(2):95–106.
73. Huang C et al. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg 2014;51(7):301–331.
74. Nather A et al. Effectiveness of bridge V.A.C. dressings in the treatment of diabetic foot ulcers. Diabet Foot Ankle 2011;2(1):5893.
75. Schwien T, Gilbert J, Lang C. Pressure ulcer prevalence and the role of negative pressure wound therapy in home health quality outcomes. Ostomy Wound Manage 2005;51(9):47–60.
76. Nakayama M. Applying negative pressure therapy to deep pressure ulcers covered by soft necrotic tissue. Int Wound J 2010;7(3):160–166.
77. Ford CN et al. Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg 2002;49(1):55–61; discussion 61.
78. Wanner MB et al. Vacuum-assisted wound closure for cheaper and more comfortable healing of pressure sores: a prospective study. Scand J Plast Reconstr Surg Hand Surg 2003;37(1):28–33.
79. Eginton MT et al. A prospective randomized evaluation of negative-pressure wound dressings for diabetic foot wounds. Ann Vasc Surg 2003;17(6):645–9.
80. Braakenburg A et al. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006;118(2):390–7; discussion 398–400.
81. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366(9498):1704–10.
82. Khashram M et al. Effect of TNP on the microbiology of venous leg ulcers: a pilot study. J Wound Care 2009;18(4):164–7.
83. Skrinjar E et al. Randomized controlled trial comparing the combination of a polymeric membrane dressing plus negative pressure wound therapy against negative pressure wound therapy alone: The WICVAC study. Wound Repair Regen 2016;24(5):928–935.
84. Becker RO. Search for evidence of axial current flow in peripheral nerves of salamander. Science 1961;134(3472):101–2.
85. Szuminsky NJ et al. Effect of narrow, pulsed high voltages on bacterial viability. Phys Ther 1994;74(7):660–7.
86. Stillings D. A survey of the history of electrical stimulation for pain to 1900. Med Instrum 1975;9(6):255–9.
87. Feedar JA, Kloth LC, Gentzkow GD. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther 1991;71(9):639–49.
88. Gentzkow GD et al. Improved healing of pressure ulcers using dermapulse, a new electrical stimulation device. Wounds 1991;3(5):158–170.
89. Franek A et al. Using high-voltage electrical stimulation in the treatment of recalcitrant pressure ulcers: results of a randomized, controlled clinical study. Ostomy Wound Manage 2012;58(3):30–44.
90. Houghton PE et al. Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal cord injury. Arch Phys Med Rehabil 2010;91(5):669–78.
91. Baker LL et al. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care 1997;20(3):405–12.
92. Miller C et al. Venous leg ulcer healing with electric stimulation therapy: a pilot randomised controlled trial. Journal of Wound Care 2017;26(3):88–98.
93. Mittermayr R et al. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair Regen 2012;20(4):456–465.
94. Schaden W et al. Shock wave therapy for acute and chronic soft tissue wounds: a feasibility study. J Surg Res 2007;143(1):1–12.
95. Cho YS et al. Effect of extracorporeal shock wave therapy on scar pain in burn patients: A prospective, randomized, single-blind, placebo-controlled study. Medicine 2016;95(32):e4575.
96. Joo SY, Cho YS, Seo CH. The clinical utility of extracorporeal shock wave therapy for burn pruritus: A prospective, randomized, single-blind study. Burns 2017.
97. Arno A et al. Extracorporeal shock waves, a new non-surgical method to treat severe burns. Burns 2010;36.
98. Wang CJ, Wu RW, Yang YJ. Treatment of diabetic foot ulcers: a comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy. Diabetes Res Clin Pract 2011;92(2):187–93.
99. Miller C, Kapp S, Green J, McGuiness W, Woodward M. Treating venous leg ulcers with extracorporeal shockwave technology (ESWT). Wounds Int 2017;8(3):22–28.
100. Mann A et al. Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing: Stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J Invest Dermatol 2001;117(6):1382–90.
101. Rubbia-Brandt L, Sappino AP, Gabbiani G. Locally applied GM-CSF induces the accumulation of alpha-smooth muscle actin containing myofibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol 1991;60(2):73–82.
102. Da Costa RM et al. Randomized, double-blind, placebo-controlled, dose-ranging study of granulocyte-macrophage colony stimulating factor in patients with chronic venous leg ulcers. Wound Repair Regen 1999;7(1):17–25.
103. Marques da Costa R et al. Double-blind randomized placebo-controlled trial of the use of granulocyte-macrophage colony-stimulating factor in chronic leg ulcers. Am J Surg 1997;173(3):165–8.
104. Zhang L, Chen J, Han C. A multicenter clinical trial of recombinant human GM-CSF hydrogel for the treatment of deep second-degree burns. Wound Repair Regen 2009;17(5):685–9.
105. Wang ZY et al. [Effect of recombinant human granulocyte-macrophage colony stimulating factor on wound healing in patients with deep partial thickness burn]. Zhonghua Shao Shang Za Zhi 2008;24(2):107–10.
106. Robson MC et al. Sequential cytokine therapy for pressure ulcers: clinical and mechanistic response. Ann Surg 2000;231(4):600–11.
107. El Saghir NS et al. Pressure ulcer accelerated healing with local injections of granulocyte macrophage-colony stimulating factor. J Infect 1997;35(2):179–182.
108. Gough A et al. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet 1997;350(9081):855–9.
109. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 1999;79(4):1283–316.
110. Lin H et al. The effect of collagen-targeting platelet-derived growth factor on cellularization and vascularization of collagen scaffolds. Biomaterials 2006;27(33):5708–14.
111. Krishnaswami S et al. Thrombospondin-1 promotes proliferative healing through stabilization of PDGF. J Surg Res 2002;107(1):124–30.
112. Rees RS et al. Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo-controlled study. Wound Repair Regen 1999;7(3):141–7.
113. Mustoe TA et al. A phase II study to evaluate recombinant platelet-derived growth factor-BB in the treatment of stage 3 and 4 pressure ulcers. Arch Surg 1994;129(2):213–9.
114. Margolis DJ et al. Effectiveness of recombinant human platelet-derived growth factor for the treatment of diabetic neuropathic foot ulcers. Wound Repair Regen 2005;13(6):531–6.
115. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 1998;21(5):822–7.
116. Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996;4(4):411–20.
117. Papanas N, Maltezos E. Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Saf 2010;33(6):455–61.
118. Graves N, Ashby AE. The use of OPAL001 filtrate and cream in the treatment of chronic pressure ulcers. Wound Practice & Research 2008;16(2):22–29.
119. Mitchell G. Clinical observations supporting a vasodilatory effect of the modified papaya extract OPAL001. Wound Practice & Research 2011;19(4):190–195.
120. Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma 2012;2(1):18–28.
121. Lu L et al. The temporal effects of Anti-TGF-beta 1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J Am Coll Surg 2005;201(3):391–397.
122. Siriwardena D et al. Human antitransforming growth factor beta(2) monoclonal antibody — a new modulator of wound healing in trabeculectomy. Ophthalmology 2002;109(3):427–431.
123. Ashcroft GS et al. Tumor necrosis factor-alpha (TNF-alpha) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen 2012;20(1):38–49.
124. Ashcroft GS et al. TNFα is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen 2012;20(1):38–49.
125. Streit M, Beleznay Z, Braathen LR. Topical application of the tumour necrosis factor-α antibody infliximab improves healing of chronic wounds. Int Wound J 2006;3(3):171–179.
126. Feldmann M, Maini RN. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nature Medicine 2003;9:1245.
127. Fox JD et al. Adalimumab treatment leads to reduction of tissue tumor necrosis factor-alpha correlated with venous leg ulcer improvement: a pilot study. Int Wound J 2016;13(5):963–6.
128. Archer SK et al. The flightless I protein and the gelsolin family in nuclear hormone receptor-mediated signalling. Biochem Soc Trans 2004;32(6):940–942.
129. Kopecki Z, Cowin AJ. Flightless I: An actin-remodelling protein and an important negative regulator of wound repair. Int J Biochem Cell Biol 2008;40(8):1415–1419.
130. Lin C-H et al. Decreased expression of Flightless I, a gelsolin family member and developmental regulator, in early-gestation fetal wounds improves healing. Mammalian Genome 2011;22(5):341–352.
131. Cowin AJ et al. Flightless I deficiency enhances wound repair by increasing cell migration and proliferation. J Pathol 2007;211(5):572–581.
132. Turner CT et al. Delivery of flightless I neutralizing antibody from porous silicon nanoparticles improves wound healing in diabetic mice. Adv Healthc Mater 2017;6(2):1600707–n/a.
133. Jackson JE et al. Flii neutralizing antibodies improve wound healing in porcine preclinical studies. Wound Repair Regen 2012;20(4):523–36.
134. Kopecki Z et al. Topically applied flightless I neutralizing antibodies improve healing of blistered skin in a murine model of epidermolysis bullosa acquisita. J Invest Dermatol 2013;133(4):1008–16.
135. Caley MP, Martins VLC, O’Toole EA. Metalloproteinases and wound healing. Adv Wound Care 2015;4(4):225–234.
136. McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care 2013;2(8):438–447.
137. Cullen B et al. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 2002;10(1):16–25.
138. Romanelli M, Dini V, Romanelli P. Hydroxyurea-induced leg ulcers treated with a protease-modulating matrix. Arch Dermatol 2007;143(10):1310–1313.
139. Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care 2002;11(9):335–41.
140. Barrett SA, Moore K. Use of Promogran to treat venous leg ulcers. J Wound Care 2004;13(1):suppl 2–7.
141. Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137(7):822–7.
142. Meaume S et al. Urgotul®: a novel non-adherent lipidocolloid dressing. Br J Nurs 2002;11(Sup3):S42–S50.
143. Meaume S et al. A randomized, controlled, double-blind prospective trial with a lipido-colloid technology-nano-oligosaccharide factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen 2012;20(4):500–11.
144. Schmutz J-L et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. Int Wound J 2008;5(2):172–182.
145. Chin G et al. Treatment of chronic ulcers in diabetic patients with a topical metalloproteinase inhibitor, doxycycline. Wounds 2003;15(10):315–323.
146. Sadler GM, Wallace HJ, Stacey MC. Oral doxycycline for the treatment of chronic leg ulceration. Arch Dermatol Res 2012;304(6):487–93.
147. Mori R et al. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci 2006;119(Pt 24):5193–203.
148. Mendoza-Naranjo A et al. Targeting Cx43 and N-cadherin, which are abnormally upregulated in venous leg ulcers, influences migration, adhesion and activation of Rho GTPases. PLoS One 2012;7(5):e37374.
149. Elbadawy HM et al. Effect of connexin 43 inhibition by the mimetic peptide Gap27 on corneal wound healing, inflammation and neovascularization. Br J Pharmacol 2016;173(19):2880–93.
150. Qiu C et al. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol 2003;13(19):1697–703.
151. Ghatnekar GS et al. The effect of a Connexin43-based peptide on the healing of chronic venous leg ulcers: A multicenter, randomized trial. J Invest Dermatol 2015;135(1):289–298.
152. Grek CL et al. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound Repair Regen 2015;23(2):203–12.
153. Marx RE et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85(6):638–646.
154. McAleer JP et al. Use of autologous platelet concentrate in a nonhealing lower extremity wound. Adv Skin Wound Care 2006;19(7):354–63.
155. Everts PA et al. Platelet gel and fibrin sealant reduce allogeneic blood transfusions in total knee arthroplasty. Acta Anaesthesiol Scand 2006;50(5):593–9.
156. Driver VR et al. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage 2006;52(6):68–70, 72, 74 passim.
157. Ahmed M et al. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg 2017;38:206–211.
158. Gameiro A et al. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015;8:285–293.
159. Brooklyn T, Dunnill G, Probert C. Diagnosis and treatment of pyoderma gangrenosum. Br Med J 2006;333(7560):181–184.
160. Piller NB, Morgan RG, Casley-Smith JR. A double-blind, cross-over trial of O-(beta-hydroxyethyl)-rutosides (benzo-pyrones) in the treatment of lymphoedema of the arms and legs. Br J Plast Surg 1988;41(1):20–7.
161. Mortimer PS et al. A double-blind, randomized, parallel-group, placebo-controlled trial of O-(β-Hydroxyetnyl)-rutosides in chronic arm oedema resulting from breast cancer treatment. Phlebology 1995;10(2):51–55.
162. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev 2012;11.
163. Suter A, Bommer S, Rechner J. Treatment of patients with venous insufficiency with fresh plant horse chestnut seed extract: a review of 5 clinical studies. Adv Ther 2006;23(1):179–90.
164. Sinha S, Amarasena I. Does phenytoin have a role in the treatment of pressure ulcers? Wound Practice & Research 2008;16:37–41.
165. Shaw J et al. The clinical effect of topical phenytoin on wound healing: a systematic review. Br J Dermatol 2007;157(5):997–1004.
166. Campbell RK. Clinical update on pentoxifylline therapy for diabetes-induced peripheral vascular disease. Ann Pharmacother 1993;27(9):1099–105.
167. Jull AB et al. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev 2012;12:Cd001733.
168. Meissner M, Kaufmann R, Gille J. Sodium thiosulphate: A new way of treatment for calciphylaxis? Dermatology 2007;214(4):278–82.
169. Desneves KJ et al. Treatment with supplementary arginine, vitamin C and zinc in patients with pressure ulcers: a randomised controlled trial. Clin Nutr 2005;24(6):979–87.
170. Barbul A, Lazarou SA, Efron, DT, Wasserkrug HL, Efron G. Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery 1990;108(2):331–6; discussion 336–7.
171. Armstrong DG et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabet Med 2014;31(9):1069–1077.



