Volume 28 Number 3
Save our skin: a pressure injury reduction project targeting pressure injuries acquired in the intensive care unit
Matthew Tinker, Veronica Roach and Rosalind Elliott
Keywords quality improvement, prevention, pressure injury, critical care
For referencing Tinker M et al. Save our skin: a pressure injury reduction project targeting pressure injuries acquired in the intensive care unit. Wound Practice and Research 2020; 28(3):106-114.
DOI https://doi.org/10.33235/wpr.28.3.106-114
Abstract
Background Despite a process of rigorous investigation of the cause of severe pressure injuries (PIs) and implementing specific interventions, the number did not decrease in our adult intensive care unit (ICU). The aim of this project was to reduce the incidence of stage III and IV PIs acquired in the ICU and associated costs.
Methods A Plan-Do-Study-Act (PDSA) quality improvement approach incorporating thorough ICU-wide multicomponent broad skin care/hygiene focused intervention and a multifaceted communication strategy (Save Our Skin: SOS) was used. The project was conducted in a 600-bed tertiary referral hospital in Sydney, Australia from 2015 to 2019. The main outcomes were the number of ICU-acquired stage III and IV PIs and costs associated with treating PIs per annum, and the number of severe PIs per 1,000 ICU bed days.
Results There was a sustained decrease in the incidence of PIs (52 during 2014 and four during 2018). The number per 1,000 ICU bed days decreased; 2.57 during 2014 and 0.29 during 2018, with lower costs associated with the treatment of stage III and IV PIs.
Conclusions This pragmatic multicomponent ICU-wide PI prevention project resulted in sustained outcomes in our ICU and cost savings.
Key points
What is already known
- Prevalence of PIs in ICUs ranges from 10–41%.
- PIs result in increased mortality, costs and discomfort.
What this quality improvement project contributes
There was a sustained decrease in the incidence of PIs over the 4 year period after the implementation of the project; 52 during 2015 and four during 2018. In addition, there were also a number of beneficial spin-offs such as improved management of moisture-associated skin damage and development of a PI extreme risk assessment tool, and significant cost savings.
Background
Pressure injuries (PIs) are relatively common healthcare-associated complications which are financially costly and negatively impact on patient well-being. The prevalence of PIs is highest in intensive care units (ICUs) and are reported to range from 10–41%1–4. It is widely accepted that PIs, particularly severe injuries, are a significant potential problem for critically ill patients.
The discomfort for patients, increased risk of mortality, and additional costs resulting from severe PIs is evident globally in healthcare settings. In Australia the treatment cost across all states and all PI severity in 2012–13 was estimated to be A$983 (US$683, £544 and €604) million per annum, representing approximately 1.9% of all public hospital expenditure or 0.6% of the public recurrent health expenditure5. The severity of injury had a significant impact on costs. While severe PIs (stage III and IV) accounted for 12% of cases, they were responsible for 30% of the total cost in Australian hospitals5. The adverse effect of severe PIs is recognised by policy makers and reflected in key performance indicators and may even attract financial penalties. In Queensland the Statement of Health (Australia) has already instigated disincentives for PIs acquired in healthcare settings6.
Locally our ICU has a long history of PI prevention and management programs7. Our initiatives for PI prevention have been recognised and clinicians have received local (state) awards for them. Despite the many successes, the results have been challenging to sustain. Some of the factors that contributed to difficulties with sustainability include over reliance on labour and resource-intensive monthly point prevalence surveys and academic detailing (the practice of intense one-to-one information giving)8 to fill gaps in clinicians’ knowledge. These factors were compounded by increasing patient numbers, competing demands as a result of increased patient case load complexity, and environmental changes when the ICU moved from a multi-occupancy room configuration to a new single-bed roomed unit in November 2012. The number of severe PIs (stage III and IV) acquired in the ICU rose to a peak of 52 during 2013.
This concerning trend resulted in an increase in the number of mandatory (Severity Assessment Code [SAC]9) reports to the hospital governance and New South Wales Ministry of Health (NSW Health) and investigations using the London Protocol (LP) investigation and analysis methodology for root cause analysis10. The London Protocol is a highly resource-intensive process which incurs a significant burden for clinicians. Despite rigorous completion of this thorough process of investigation and implementation of specific interventions, the number of PIs did not decrease.
Thus, in 2014, the nurses in the ICU, led by the clinical nurse consultant (CNC), committed to addressing the situation. It was decided a context-specific multicomponent ICU-wide intervention was required to address the unacceptably high number of severe (stage III and IV) PIs acquired in the ICU. The rationale for focusing on prevention of severe PIs was that there are more serious consequences for patient outcomes. This paper reports the Save Our Skin (SOS) quality improvement program to reduce PIs, namely stage 3 and 4 PIs, acquired in the ICU.
Methods
Setting
This quality improvement project was conducted in the adult ICU of a 600-bed tertiary referral hospital in Sydney, Australia. The ICU provides specialty services such as neuroscience, renal, spinal, burns and cardiac. An intensive care staff specialist is responsible for the management of all patients (closed unit model). Ward rounds are conducted by the intensivist twice a day when treatment plans are reviewed. The registered nurse (RN) to patient ratio is 1:1 for mechanically ventilated patients and 1:2 for patients requiring high dependency care. There is no separate area for the care of high dependency level patients; mechanically ventilated patients are cared for alongside patients of lower acuity. The RN performs all the nursing care for the patient.
The geographical configuration and number of beds of the ICU changed after relocation to a new hospital building in 2013. Prior to this time the ICU comprised 36 beds in predominately multi-occupancy areas (six beds) with single rooms for patients with infectious diseases or burn injuries. After 2013 the ICU now comprises 58 beds which are accommodated in four areas – two general ICUs, a cardiothoracic ICU and a neurological ICU. All rooms are single occupancy only, each with sliding glass door access and closed windows to adjoining rooms. The approximate annual activity level of the 58-bed ICU during the period reported in this paper was 3800 patient admissions and 14,000 bed days.
Design
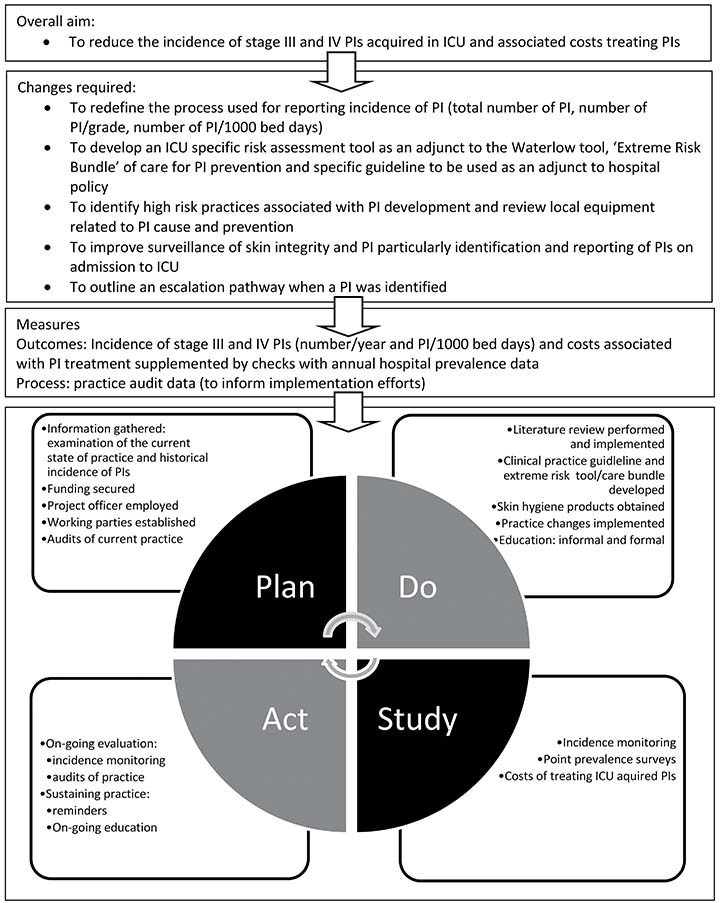
The Plan-Do-Study-Act (PDSA)11 quality improvement approach was selected for this problem. The aims were determined, appropriate measures to track success were established, and key changes were decided. Figure 1 provides an overview of the overall project aim, measures and activities in relation to one PDSA cycle. The intention is to continue the cycles to ensure sustainability every 2–3 years. The project is ongoing; the methods and results (process and outcomes) reported in the current paper pertain to the period July 2014 to December 2018. The Standards for QUality Improvement Reporting Excellence (SQUIRE) guidelines12 were used to report the project to date.
Figure 1. Project overview (Plan-Do-Study-Act)
Procedures
Planning was comprehensive and included acquisition of funding (A$16,724, £9266, US$11,581 or €10,275). This supported the appointment of a part-time project officer who was a RN seconded from the ICU. The program was co-lead by the project officer and the intensive care CNC who oversaw the work of several small working parties (e.g. Escalation pathway for PIs and Research: association between severity of illness and PI severity/risk) who worked on PI initiatives including obtaining and adapting the best available evidence for the clinical practice guideline (Pressure injury prevention and management guideline for intensive care) and areas for improvement in PI prevention identified during audits of practice. All nurses working in the ICU were invited to join the working parties. Many working party members (n=28, 10% nurses working in ICU) were informal or formal leaders. The project officer received remuneration but working party members conducted the majority of the work as part of their requirements for continuing professional development. Specific tasks for completion in achievable timeframes were provided to working parties by the project officer. Frequent meetings for the working parties were convened when progress with tasks was discussed, and regular communication was provided via the SOS dedicated closed Facebook group.
Interventions
The interventions for this program were all outlined in the clinical practice guideline (a local context-specific adjunct to related organisation policies). The interventions were comprehensive and addressed all aspects of PI prevention and management (Figure 2). They included an early detection, escalation, management and documentation pathway for PI which clearly outlined ICU-specific processes not only for reporting and referral but also immediate treatment including evidence-based wound care. Another intervention was an additional risk assessment tool developed for patients who were at severe risk of PI to complement the Waterlow scale. This risk tool included factors considered to increase the likelihood of PI including serum albumin level. The specific skin care bundle was another important intervention which comprised replacement of soap with Dermalux soft towel lotion wash (Whitely Corporation, Australia) and use of specific moisturiser and prophylactic silicone dressings.
Figure 2. List of interventions, strategies and initiatives

Implementation
Once all interventions were established and resources available, a comprehensive multifaceted implementation strategy was used to disseminate the changes in practice; this was the Do part of PDSA. The clinical practice guideline was placed on the ICU intranet with other ICU guidelines and manuals. The implementation strategies and initiatives included informal and formal education, the use of academic detailing (each working party member provided one-to-one sessions for at least four colleagues), announcements via social media (the ICU Facebook group), and items in the ICU newsletter. Progress with the project was also discussed at the ICU Quality Forum, an ICU-based open group in which all ICU staff contribute to quality initiatives. Formal teaching sessions were provided during mandatory training and orientation of all newly employed nurses. Posters containing reminders and information were placed in areas frequented by staff such as near the arterial blood gas analysis machine.
Measures
Incidence data
The main measures (Study part of PDSA) were the number of stage III and IV PIs acquired over 12 months in the ICU and the number per 1,000 ICU bed days. PI data were acquired from the NSW Health Incident Information Management System (IIMS). Reporting by clinicians of PIs through this system has been consistently reliable. The number of ICU patient bed days was obtained from the ICU electronic database. This enabled costs to be calculated for treatment associated with stage III and IV PIs and a more accurate reflection of the rate according to the ICU activity level.
The limitations associated with conventional methods of presenting the rate as the number of PIs per 1,000 completed inpatient stays were considered at the beginning of the project. This method of reporting suggests that each patient episode has equal denominator weighting. Arguably the primary problem with this method of reporting is the use of a patient number denominator does not give a true reflection of activity-based incidence that is sensitive to risk and length of stay. As a patient’s length of stay increases, their risk of developing PI also increases. Increased length of stay also increases true activity measured as bed days. The number of PIs per 1,000 bed days (rather than per 1,000 inpatient stays) addresses this and is consistent with reporting for other hospital-acquired complications such as infections and falls. In addition, in order to compare results with other studies, we have also provided annual incidence rates as reported by others, i.e. number of PIs divided by the number of patients treated in the ICU multiplied by 100.
Costs
Costs were estimated for treatment associated with stage III and IV PIs. These data were calculated for the 3 years prior to initiation of the SOS project and during the PDSA.
Prevalence data
In order to check the reliability of our approach, PI data for the hospital annual 1-day point prevalence survey were also examined for the years 2016–2018. The 1-day point prevalence survey is facilitated by skin integrity experts in the organisation who verify the results from each department. A standardised skin assessment is conducted for every patient present in the hospital on that day by trained nurses. Survey data were used to check for congruence with our incidence data and to monitor the distribution of PIs across the ICU areas and location of all stages of PIs on patients.
Process data
The audits of practice (process data) were performed at various frequencies for 6 months (during 2014) by the working party responsible. These audits were performed without the prior knowledge of other clinicians working in the ICU and included opportunities to reposition patients versus actual repositioning, spot checks of documentation (integumentary and PI risk assessment) and appropriate use of support surfaces for high risk patients. Results of the audits performed by working parties were used to monitor uptake of interventions and target implementation efforts, in other words the next phase of education and academic detailing (these data are not reported).
Data analysis
Data were stored and analysed using Excel (Microsoft, CA, USA) software. Simple descriptive analyses were performed (e.g. raw PI frequencies). The number of PIs per 1,000 ICU bed days were calculated. Costs associated with treating PIs acquired in the ICU were calculated using the estimated mean cost per PI by Nguyen et al.5 who included both the direct health costs (dressing, nursing time to dress wounds, and other treatments) and indirect health costs related to the extended length of hospital associated with PIs. Trends in the data were examined and presented in simple graphs.
Ethical and governance approval
The executive of the local health district human research and ethics committee (HREC) categorised the project as a quality improvement activity and therefore HREC review and approval was not required. Approval to conduct the project was provided by the hospital director of nursing and midwifery, medical director of the ICU, and nurse manager of the ICU. Signed informed consent for the collection of patient data was not required. All data were unidentifiable and reported in aggregate.
Results
Pressure injuries
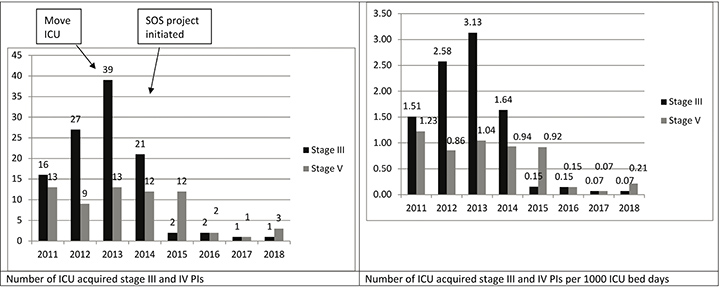
There was a sustained decrease in the incidence of PIs over the 4 year period after the implementation of SOS; 39 stage III (annual incidence: 1.26%) and 13 stage IV (annual incidence: 0.41%) PIs during 2013 and one stage III (annual incidence: 0.03%) and three stage IV (annual incidence: 0.07%) PIs during 2018 (annual incidence: 0.10%). The total number per year and number per 1,000 ICU bed days are shown in Figure 3. Also shown are data pertaining to the 2 years before (2013) the move from the open plan multiple bed occupancy ICU to the single room occupancy ICU.
Figure 3. The total number of ICU-acquired stage III and IV PIs, number (IIMS data) per 1,000 ICU bed days, number of PIs per patient (x 100%)
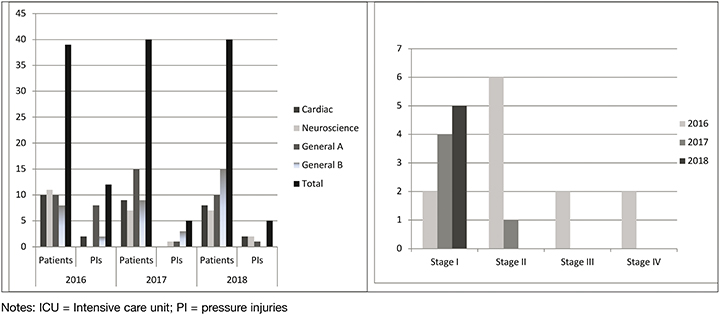
Point prevalence data for all stages of PIs for all areas of the ICU also decreased for the period 2016–2018 (Figure 4). For the 2016 survey there were 12 PIs located on eight patients (prevalence rate: 30.5%), and for the 2017 and 2018 surveys there were five PIs located on four patients (prevalence rate: 12.5%). There were five device-related PIs identified for the 2016 survey, three for 2017 and one for 2018. Devices implicated included faecal collection bag, endotracheal tube, and an oxygen saturation probe (adhesive for use on temple). For 2016 PIs were found on the patients in the following locations: ear (n=2); sacrum (n=2); heel (n=1); leg (n=1); occiput (n=1); toe (n=1); back (n=1); anus (n=2); and neck (n=1). For 2017 PIs were found on the: nare (n=2); foot (n=1); and leg (n=2). The stage I PIs found in the 2018 survey were located on the heel, back, lip, elbow and leg.
Figure 4. Prevalence data: the number of patients surveyed and total PIs identified in each area of the ICU and number of PIs according to stage of PI
Costs associated with PI treatment
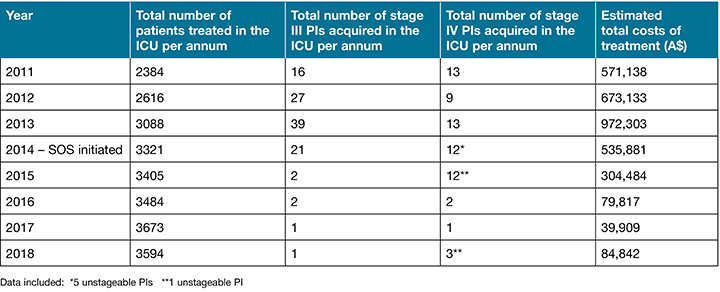
Results presented in Table 1 show lower costs associated with the treatment of fewer stage III and IV PIs were incurred after the initiation of the SOS project. A sharp decrease was realised in the year 2016 and was sustained for years 2017 and 2018.
Table 1. Estimated costs (A$) incurred in association with treating stage III and IV PIs acquired in the ICU before and after the initiation of SOS
Discussion
This pragmatic comprehensive quality improvement project resulted in a dramatic and sustained decrease in the incidence of PIs in ICU. Furthermore, significant costs associated with treating PIs were avoided as a result. These results were achieved at a small one-off cost (project officer: A$16,724) and as a result of many factors including the style of project management, widespread collaboration, and multicomponent intervention. The expertise and knowledge of a significant proportion of the ICU nursing team (SOS working party) was deployed.
Our results for the incidence of ICU-acquired stage III and IV PIs were lower than the mean incidence reported in a systematic review for PI incidence and prevalence in hospital settings (stage III: 8.98±10.26% and stage IV: 7.66±8.11%), consistent with the low range for this review (0.6%)13 and lower than ICU prevalence and incidence rates reported in another recent meta-analysis1. It is notoriously difficult to compare PI incidence as methods of measurement vary considerably, for example health record audit, skin assessment and incident monitoring were all used in the studies included in the review1. Point prevalence rates for all stages of PIs were low compared to findings of Al Mutairi’s13 review. However, ICU prevalence for stage III and IV PIs in the current project were comparable to rates reported by Coyer et al.14 in an analysis of state-wide Queensland bedside audits (stage III: 2% and stage IV: 2%).
With regards to improvement in rates of PIs the project realised similar results to others. For example a pre/post-interventional study incorporating a PI prevention bundle and nursing rounds provided by a wound expert nurse conducted in the ICUs of a trauma centre resulted in a PI reduction from 15.5% to 2.1%15. Notably there were no stage III/IV PIs acquired in the ICU in the post-intervention phase of this study. Unlike the results of other PI quality initiatives, the number of device-associated PIs was relatively low for the current project15,16.
Overall the results of the current project reflect the findings of other pre/post-intervention studies in which similar multicomponent PI prevention bundle interventions and multifaceted implementation strategies were used17,18. For example, in the study conducted in a tertiary referral ICU there were no stage III/IV PIs in the post-intervention group, compared with two in the pre-intervention group17. Likewise, in a study involving the implementation of a comprehensive PI prevention intervention bundle in an entire tertiary facility, the incidence for stage II and above PIs was much lower in the post-intervention group (1.7%) compared to the pre-intervention group (6.4%)18. These intervention bundles contained many of the components used in the current project such as clear direction about skin assessment and escalation, evidence-based skin hygiene practices, and instructions for selection of support surfaces and a multifaceted implementation program including thorough inclusive communication.
Lower PI rates appear to be more consistent in the current project unlike some QI projects in which PI prevention interventions were not multicomponent and bundled19. There is no evidence that bundles comprising more interventions than others are more effective20 but, given that many factors affect the health and integrity of skin, arguably a comprehensive bundle addressing many factors simultaneously is probably more robust than single interventions. The estimated lower costs associated with the treatment of fewer PIs based on previously published Australian methods5 reflects the calculations of other PI researchers21,22. They are significant and importantly do not include the financial penalties which have not been implemented yet in the state of New South Wales.
There have been many benefits associated with conducting the SOS project aside from improved patient outcomes, cost savings, and improved patient care. For example, better metrics for reporting PI (that is per 1,000 ICU bed day) allowing greater accuracy in monitoring trends in PI incidence, development of more relevant extreme PI risk assessment and management, and cultivation of collaborative working relationships across the organisation.
The key to the success of SOS was the inclusive nursing leadership style in which working party members were encouraged to be autonomous, take responsibility, and be accountable for dedicated aspects of the work23. Working party members were nurses already highly regarded by their colleagues for their expertise and opinion24. Thus ICU nurses required little ‘persuasion’ to adopt the new PI prevention and management practices. Although we did not purposively seek to enrol opinion leaders for this study, a large (10% of the ICU nursing workforce) and highly influential cohort of clinical nurses serendipitously self-selected to be involved in the project. The practice of using local opinion leaders, particularly in conjunction with other implementation interventions such as audit and feedback, academic detailing and educational materials, is known to increase the uptake of evidence-based practice24.
The adaption of the best available evidence by local opinion leaders and the use of creative and multifaceted communication to maximise implementation efforts, a broad approach to prevention e.g. skin microclimate management, together with the emphasis on the avoidance of sustained pressure and shear forces was successful in our ICU. This holistic multifaceted approach incorporating risk assessment, prevention, monitoring and management has previously been shown to reduce PIs in healthcare settings25,26 and improve skin health for the critically ill27. It is well known that translation of evidence into practice is facilitated by context-specific adaption and ownership by local clinicians8 and this was a feature of our approach. Further, a cycle of developing, feasibility testing, evaluation, reporting and implementation is a highly effective method of implementing complex interventions28 and is likely the key to the sustainability of PI prevention and management in this case.
Future directions and research
The future directions of this ongoing work include preventing device-related PIs, better treatments for intertriginous dermatitis (ITD) – a rare but particularly severe form of skin inflammation often associated with moisture collection in skin folds29 – and exploration of more accurate methods of classifying unstageable PIs and mandatory reporting for unstageable PIs. Although rare, device-related PIs and ITD tend to be serious and affect the most severely ill patients. With this in mind, the multidisciplinary team is devising a regimen to reposition patients receiving extra corporeal membrane oxygenation which can be implemented routinely and safely. A potentially effective intervention for the prevention of ITD is silver moisture-wicking textile30; however, the effectiveness of this intervention has not yet been thoroughly investigated. We plan to explore this potential prophylactic treatment.
With regard to classifying unstageable PIs, the organisation in which the project was conducted currently mandates an immediate investigation (London Protocol) on discovery of an unstageable PI (considered full thickness). However, evidence suggests that the full extent of tissue damage is not evident for 7–10 days31. The plan is to advocate a ‘watch and act alert’ with intervention and mandatory investigation and reporting if damage is found to be full thickness after 7 days. This was and is our continuing practice when examining IIMS (incident data); each PI is treated and monitored by the ICU CNC who confirms the staging. In addition, we continue to explore the availability of ICU-specific PI risk assessment tools such as the Risk Assessment Pressure Ulcer Scale – ICU32 with a view to testing the tool we currently use for patients at ‘extreme risk’ of PIs.
Limitations
The breadth and scope of the project was both a strength and limitation. Even though the scope of SOS was consciously holistic, there may have been benefit in concentrating on specific aspects of the work at a time. Thus more detailed analysis of some aspects of the project could have been performed. For example, data from practice audits was useful at the time for guiding implementation but had limited use for examining trends in specific risk factors for our patient cohort.
Another potential limitation was the use of the organisation’s IIMS to obtain the incident rate. As with all reporting systems, incident rates are dependent on accurate reporting and any omission is likely to artificially decrease the rate. However, this potential problem was and is mitigated by the ICU CNC who attends the clinically setting to ensure all PIs are reported. We are completely confident that this process has ensured complete PI reporting as a skin assessment is performed within 24 hours of the patient’s transfer to the hospital ward. No PIs have been ‘missed’ since 2013.
In addition, although the overall aims of the project were set a priori, the methods adopted to achieve them evolved as the work progressed. This is a known limitation but also a strength of quality improvement approaches which are more flexible and iterative. Arguably, a priori selection of a framework or underlying philosophy and process may have assisted us to report the processes and outcomes more objectively and allow replication. However, as with any quality improvement project, clinicians wishing to repeat the methods must exercise caution; we advise at the very least careful consideration and adaptation for their context.
Conclusion
This pragmatic context-specific multicomponent ICU-wide PI prevention project resulted in both sustainable solutions and sustained outcomes in our ICU at little cost. The decrease in severe PIs resulted in a significant reduction in costs incurred as a result of the treatment of PIs and many other incidental benefits. The success of the project was a function of the holistic focus on skin care and hygiene, multicomponent intervention, collaborative team work, nursing leadership, and a multifaceted communication approach. We will continue to maintain the momentum and champion the message that ‘one pressure injury is too many’.
Acknowledgements
The authors wish to sincerely thank the NSW Ministry of Health, project officer, Royal North Shore Hospital ICU SOS working party members and the hospital and local health district executive.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This work was supported by New South Wales Ministry of Health, Nursing and Midwifery Office, [Nursing and Midwifery Strategy Reserve Funding 2014/15]. The funding organisation did not play any part in the conduct of the project nor this publication.
Author(s)
Matthew Tinker
RN, MN
Department of Intensive Care, Royal North Shore Hospital, Northern Sydney Local Health District,
St Leonards NSW, Australia
Veronica Roach
RN
Department of Intensive Care, Royal North Shore Hospital, Northern Sydney Local Health District,
St Leonards NSW, Australia
Rosalind Elliott*
RN, PhD
Department of Intensive Care, Royal North Shore Hospital, Northern Sydney Local Health District,
St Leonards NSW, Australia
Nursing and Midwifery Research Centre, Nursing and Midwifery Directorate, Northern Sydney Local Health District, St Leonards NSW, Australia
Email Rosalind.Elliott@health.nsw.gov.au and Rosalind.Elliott@uts.edu.au
* Corresponding author
References
- Chaboyer WP, Thalib L, Harbeck EL, Coyer FM, Blot S, Bull CF, et al. Incidence and prevalence of pressure injuries in adult intensive care patients: a systematic review and meta-analysis. Crit Care Med; 2018;46(11):e1074–e81.
- Nijs N, Toppets A, Defloor T, Bernaerts K, Milisen K, Van Den Berghe G. Incidence and risk factors for pressure ulcers in the intensive care unit. J Clin Nurs 2009;18(9):1258–66.
- Tubaishat A, Papanikolaou P, Anthony D, Habiballah L. Pressure ulcers prevalence in the acute care setting: a systematic review, 2000–2015. Clin Nurs Res 2017:1054773817705541.
- Yarad E, O’Connor A, Meyer J, Tinker M, Knowles S, Li Y, et al. Prevalence of pressure injuries and the management of support surfaces (mattresses) in adult intensive care patients: a multicentre point prevalence study in Australia and New Zealand. Aust Crit Care 2020.
- Nguyen KH, Chaboyer W, Whitty JA. Pressure injury in Australian public hospitals: a cost-of-illness study. Aust Health Rev 2015;39(3):329–36.
- Queensland Health. Health funding principles and guidelines 2017–18 financial year. In: Healthcare Purchasing and Funding Branch QH, editor. Brisbane, Australia: Healthcare Purchasing and System Performance Division; 2018.
- Elliott R, McKinley S, Fox V. Quality improvement program to reduce the prevalence of pressure ulcers in an intensive care unit. Am J Crit Care 2008;17(4):328–334; quiz 335; discussion 336–327. doi: 17/4/328 [pii]
- Rogers EM. Diffusion of innovations. Simon and Schuster; 2010.
- NSW Health. Incident management policy. In: Commission CE, editor. North Sydney, Australia: Clinical Excellence Commission, NSW Health; 2019.
- Taylor-Adams S, Vincent C. Systems analysis of clinical incidents: the London protocol. Clinical Risk 2004;10(6):211–20.
- Deming WE. The new economics for industry, government, education. MIT press; 2018.
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Quality & Safety 2016;25(12):986–92.
- Al Mutairi KB, Hendrie D. Global incidence and prevalence of pressure injuries in public hospitals: a systematic review. Wound Med 2018;22:23–31.
- Coyer F, Miles S, Gosley S, Fulbrook P, Sketcher-Baker K, Cook JL, et al. Pressure injury prevalence in intensive care versus non-intensive care patients: a state-wide comparison. Aust Crit Care 2017;30(5):244–50.
- Anderson M, Finch Guthrie P, Kraft W, Reicks P, Skay C, Beal AL. Universal pressure ulcer prevention bundle with WOC nurse support. J WOCN 2015;42(3):217–25.
- Padula CA, Paradis H, Goodwin R, Lynch J, Hegerich-Bartula D. Prevention of medical device–related pressure injuries associated with respiratory equipment use in a critical care unit: a quality improvement project. J WOCN 2017;44(2):138–41.
- Coyer F, Gardner A, Doubrovsky A, Cole R, Ryan FM, Allen C, et al. Reducing pressure injuries in critically ill patients by using a patient skin integrity care bundle (Inspire). Am J Crit Care 2015;24(3):199–209.
- Gupta P, Shiju S, Chacko G, Thomas M, Abas A, Savarimuthu I, et al. A quality improvement programme to reduce hospital-acquired pressure injuries. BMJ Open Qual 2020;9(3).
- Kelleher AD, Moorer A, Makic MF. Peer-to-peer nursing rounds and hospital-acquired pressure ulcer prevalence in a surgical intensive care unit: a quality improvement project. J WOCN 2012;39(2):152–7.
- Lin F, Wu Z, Song B, Coyer F, Chaboyer W. The effectiveness of multicomponent pressure injury prevention programs in adult intensive care patients: a systematic review. Int J Nurs Stud 2020;102:103483.
- Polancich S, Miltner R, Poe T, Williamson J, Vander Noot R, Shirey M. Cost of quality pilot: a systematic methodology for examining the cost of pressure injury. JHQ 2020;42(2):72–82.
- Tchouaket E, Dubois C-A, D’Amour D. The economic burden of nurse-sensitive adverse events in 22 medical-surgical units: retrospective and matching analysis. J Adv Nurs 2017;73(7):1696–711.
- Kueny A, Shever LL, Lehan Mackin M, Titler MG. Facilitating the implementation of evidence-based practice through contextual support and nursing leadership. J Healthc Leadersh 2015;7:29–39.
- Flodgren G, Parmelli E, Doumit G, Gattellari M, O’Brien MA, Grimshaw J, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database System Rev 2011(8):Cd000125.
- Tayyib N, Coyer F. Translating pressure ulcer prevention into intensive care nursing practice: overlaying a care bundle approach with a model for research implementation. J Nurs Care Qual 2017;32(1):6–14.
- Martin D, Albensi L, Van Haute S, Froese M, Montgomery M, Lam M, et al. Healthy skin wins: a glowing pressure ulcer prevention program that can guide evidence-based practice. Worldviews Evid Based Nurs 2017;14(6):473–83.
- Coyer F, Gardner A, Doubrovsky A. An interventional skin care protocol (InSPiRE) to reduce incontinence-associated dermatitis in critically ill patients in the intensive care unit: a before and after study. Intensive Crit Care Nurs 2017;40:1–10.
- Craig P, Dieppe P, Mcintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions. London, England: Medical Research Council and National Institute of Health Research; 2019.
- Black JM, Gray M, Bliss DZ, Kennedy-Evans KL, Logan S, Baharestani MM, et al. MASD part 2: incontinence-associated dermatitis and intertriginous dermatitis: a consensus. J WOCN 2011;38(4):359–70; quiz 71–2.
- Montazer M, Keshvari A, Kahali P. Tragacanth gum/nano silver hydrogel on cotton fabric: in-situ synthesis and antibacterial properties. Carbohydr Polym 2016;154:257–66.
- Farid K. Applying observations from forensic science to understanding the development of pressure ulcers. Ostomy Wound Manag 2007;53(4):26–44.
- Wåhlin I, Ek A-C, Lindgren M, Geijer S, Årestedt K. Development and validation of an ICU-specific pressure injury risk assessment scale. Scand J Caring Sci.



