Volume 29 Number 4
Direct use of autologous fat graft on the treatment of lower extremity wounds
Umut Tuncel, Alper Kurt and Osman Demir
Keywords diabetic foot, lower extremity, fat graft, needle, non-centrifuge
For referencing Tuncel U et al. Direct use of autologous fat graft on the treatment of lower extremity ulcers. Wound Practice and Research 2021; 29(4):238-244.
DOI
https://doi.org/10.33235/wpr.29.4.238-244
Submitted 19 August 2021
Accepted Accepted 20 August 2021
Abstract
Background This preliminary study aims to present our experience regarding the efficacy of non‑centrifuged autologous fat graft in the treatment of lower extremity ulcers.
Methods A total of 16 ulcers from 16 cases were retrospectively analysed. VAC (vacuum-assisted therapy) or silver-impregnated dressings were used to achieve a healthy wound bed before fat grafting. Autologous fat graft was harvested from the patients’ abdominal or gluteal regions and injected into the wound edge, wound bed and surrounding environment. Clinical observation and photographic records were used to analyse the ulcers.
Results The mean age was 55.88±9.71 years old; 52 years old (range: 44–65.5) for venous ulcers, 57 years old (range: 51–63) for diabetic ulcers and 49.5 years old (range: 45–54) for traumatic ulcers. Of the cases, nine were male. Twelve ulcers needed a skin graft or flap surgery, and four had secondary healing. At the beginning, the mean wound surface area was 92.69±62.74cm2; 125cm2 (range: 52–175) for venous ulcers, 100cm2 (range: 25–112) for diabetic ulcers and 81cm2 (range: 42–120) for traumatic ulcers. The mean number of fat injection procedures was 1.63±0.89, and the mean fat volume used was 26.56±15.33cc. The mean healing time was 32.56±12.03 days. The ulcers were deemed uneventful in the following 12-month period.
Conclusion It can be determined that non‑centrifuged autologous fat graft is a quite effective and useful method in reducing the treatment time for lower extremity ulcers.
Introduction
Fat grafting has been a commonly used method in both cosmetic and reconstructive surgery for a long time. Autologous fat graft is easily obtainable, and its injection is a minimally invasive procedure1–4. The procedure has also received a great deal of attention in the field of tissue engineering in recent years due to its differing stem cell content which has the capability to heal different tissues under appropriate conditions5,6.
Diabetic foot ulcers are one of the chronic complications of diabetes and the main risk factor leading to non‑traumatic amputation in diabetic patients. The prevalence of peripheral vascular disease in diabetic patients is 15–30%7,8. Diabetes and other chronic conditions negatively affect all wound healing processes and the duration and degree of hyperglycemia play a major role in terms of complications9,10. The deterioration of the metabolic system leads to reduced resistance to infections which often results in amputation. A healthy wound healing process needs a well-orchestrated integration of cell migration, cell proliferation and extracellular matrix deposition, whereas chronic conditions such as diabetes, venous and/or arterial insufficiency, lead to disregulation of the cellular and molecular signals during this process; this can result in inadequate wound healing and a rise in chronic ulcers.
In the literature there have been lots of reports relating to the chemical and physical properties of pre-manipulated adipose (fat) tissue which was generally used in the treatment of chronic scars, burn scars or cosmetic conditions. However, there have only been two papers about the direct use of non‑centrifuged adipose tissue in human subjects11,12. In our previous experimental study using various surgical methods for the healing of nerves, only centrifuged autologous adipose tissue was used – again directly applied to the wound edge, wound bed and surrounding environment after achieving a healthy and suitable wound bed – for which we obtained considerably beneficial results5. Thus, the present study aimed to investigate the efficacy of non‑centrifuged autologous fat graft on the treatment of various chronic challenging ulcers on the lower extremity.
Methods
Demographics
In this preliminary study, 16 lower extremity ulcers treated using non‑centrifuged autologous fat graft between 2016–2019 were retrospectively reviewed using data collected from patients’ files and photographs (Table 1). The study was approved by ClinicalTrials.gov (NTC identification number: NCT04896437).
Table 1. Patient demographics
To be included in the study, the ulcers had to have been present for at least 3 months, have high to moderate exudate, and require open management of secondary intervention. The aetiologies of the wounds were venous, diabetic or traumatic ulcers. An informed consent form was taken from all patients. The mean age of the patients was 55.88±9.71 years. Exclusion criteria were malignant disease, a life expectancy of <1 year, radiation or chemical exposure.
Intervention
The ulcers were debrided, and vacuum-assisted therapy (VAC) or silver-impregnated dressing alone (Silverlon; Cura Surgical, Geneva, Illinois) was applied to achieve a healthy wound bed in preparation for the fat graft. Wound dressing changes were made at 24–48 hourly intervals, and VAC therapy was set at the intermittent mode at –80mmHg. If the wound was smaller than 10cm2 and/or had less exudate at the beginning, the silver-impregnated dressing was used alone to prepare the wound bed for fat grafting; this was the case in four cases. When wound exudation decreased and a healthy granulation tissue started to observe clinically, VAC therapy or silver-impregnated dressing was stopped, and fat grafting was planned.
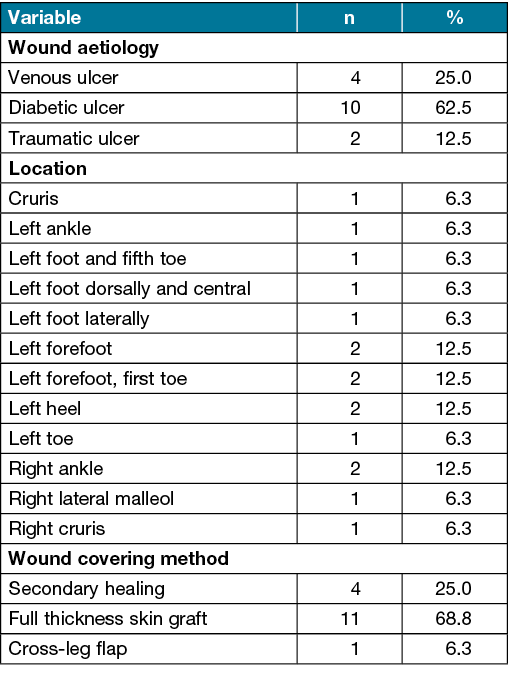
The autologous fat graft was harvested from the patients’ abdominal or gluteal regions. After the infiltration of the standard tumescence solution, tunnelling was carried out with a liposuction cannula (3–5mm). The cannula was attached to a syringe to harvest the fat graft from the deep subcutaneous tissue; the volume of fat graft depended on the wound size. After the procedure, the patient wore an abdominal garment for 2–4 weeks to minimise swelling and allow for smooth, controlled adaptation of the elevated pockets of subcutaneous tissue to the underlying fascia. The fat graft was filtered under sterile conditions using a metal sieve and then washed with 0.9% saline solution to concentrate the fat particles and separate them from fluids and debris. The purified fat was then collected using a sterile spoon and placed in a 10cc syringe (Figure 1A). After debridement and wound care, the fat graft was then injected around the wound bed and surrounding areas approximately 1cm from the wound edge by using a 4G blunt single hole cannula or a 2.5cc syringe to stay in contact with the wound site (Figure 1B).
Care was taken not to tighten the skin edges so as to not cause any skin necrosis. The amount and frequency of the fat graft was determined on the clinical observation of the ulcers. Ointment dressing and a plaster cast were used for the stabilisation of the fat graft. The ulcers were monitored closely for 5–7 days. Patients were given perioperative and postoperative antibiotics guided by their positive culture results and any sensitivities. Dressing changes were made on Days 10, 14 and 21. When wound shrinkage was observed, the wound was left to secondary healing, whereas the wounds not showing any shrinkage required a skin graft or flap.
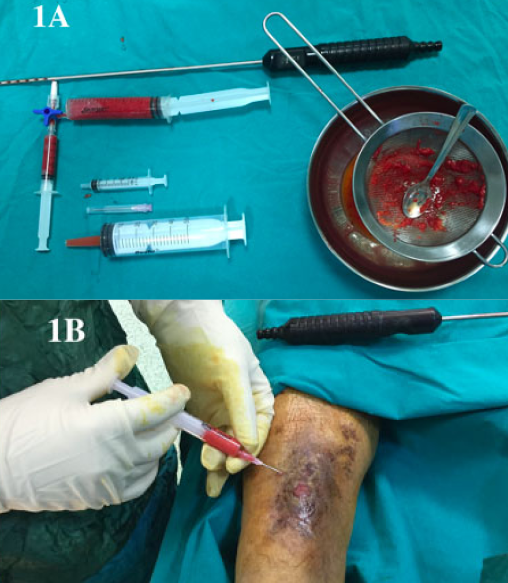
Figure 1. Autologous fat graft preparation and delivery
Case 1: diabetic foot ulcer
The patient was admitted to our clinic with chronic, infected abscesses and non-healing diabetic foot ulcers on the left lateral of his left foot; they were large, over 3 months old, and had a high exudate considered to be Wagner Grade 3–4 (Figure 2A). The ulcer was immediately debrided under general anaesthesia, followed by a 13-day period of VAC therapy and debridement (Figure 2B). When debridement and VAC therapy were completed, autologous fat grafting was planned. The method was performed under general anaesthesia and 19cc of fat graft was injected into the wound site. The ulcer was then dressed using a closed dressing for 7 days and was found to be suitable for a skin graft (Figure 2C). However, the patient did not agree to this procedure so a routine of wet-to-dry secondary wound healing was followed; the ulcer was completely healed at 55 days (Figure 2D).
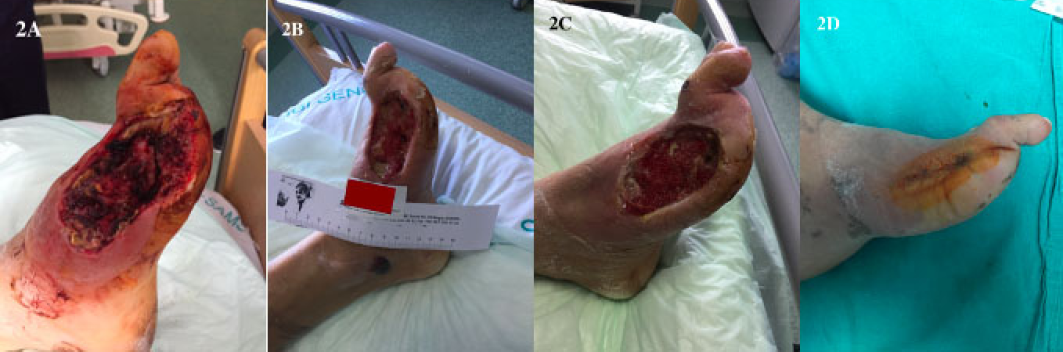
Figure 2. Case 1: a diabetic ulcer treated with an autologous fat graft
Case 2: venous foot ulcer
A 45-year-old male patient was admitted to our clinic because of a venous ulcer on his right cruris (Figure 3A). Wound debridement was carried out under spinal anaesthesia; the ulcer was then covered with a silver-impregnated dressing in order to decrease exudate and attain a healthy wound bed. After 3 days of wound care, fat grafting was performed under general anaesthesia; 10cc of fat graft was transferred to the wound site. 10 days after the application, considerable wound shrinkage was observed, and this ulcer was left to secondary healing. The total treatment time was 10 days and the ulcer completely healed in 16 days (Figure 3B).
Figure 3. Case 2: a venous ulcer treated with an autologous fat graft
Case 3: traumatic foot ulcer
A 51-year-old male patient presented with large foot ulcer due to trauma with signs of odour, necrosis and moderate exudation (Figure 4A). After serial debridement, the ulcer underwent VAC therapy for 7 days. After this, the ulcer was found suitable for fat grafting and 40cc was administered. The procedure was performed twice; exudation decreased and necrotic tissues were properly cleaned (Figure 4B). VAC therapy was continued for 12 more days until the ulcer became suitable for a skin graft. The total treatment period was 53 days (Figure 4C).
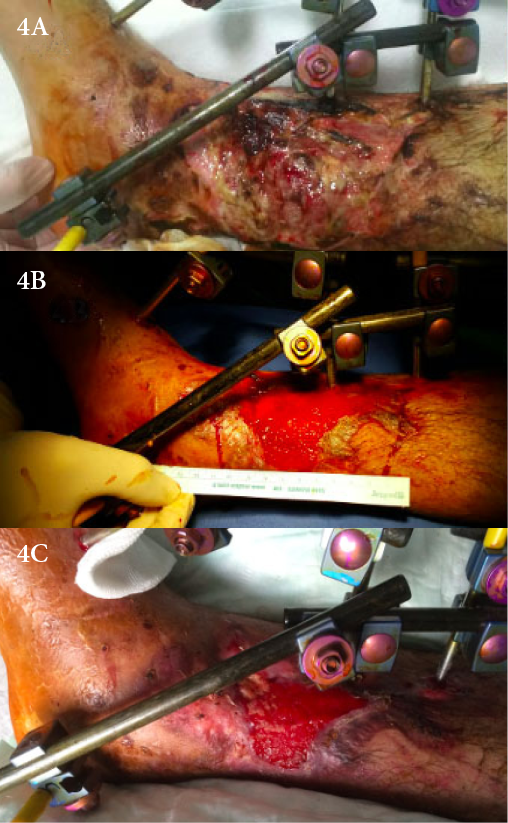
Figure 4. Case 3: a traumatic ulcer treated with an autologous fat graft
Case 4: diabetic foot ulcer
A 55-year-old female diabetic patient was admitted to our clinic with signs and symptoms of a large diabetic foot ulcer on her left foot at the first toe to the level of the metatarsophalangeal joint. There were signs of odour, necrosis and moderate exudation. Wound debridement was done under spinal anesthesia and VAC therapy was applied (Figure 5A). VAC therapy was continued for 10 days and, when the wound exudation was clinically observed to have reduced a fat graft was planned. A single fat application of 10cc was made and the ulcer was then covered by a skin graft (Figure 5B). The total healing time was 28 days.
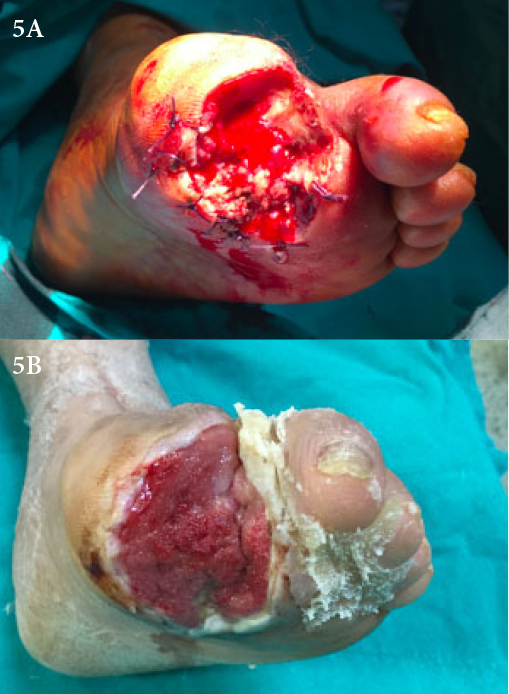
Figure 5. Case 4: a diabetic ulcer treated with an autologous fat graft
Statistical analysis
Quantitative data were obtained regarding the arithmetic mean, standard deviation or frequency, and percentage. A one-way analysis of variance (ANOVA) test was used to compare quantitative variables among groups. A p value <0.05 was considered significant. Analyses were performed using SPSS 19 (IBM SPSS Statistics 19, SPSS Inc, an IBM Co, Somers, NY).
Results
Tables 2 and 3 show the distribution of quantitative and qualitative variables according to wound aetiologies. The mean age was 55.88±9.71 years old; 52 years old (range: 44–65.5) for venous ulcers, 57 years old (range: 51–63) for diabetic ulcers and 49.5 years old (range: 45–54) for traumatic ulcers. Of the cases, nine were male. Four cases were venous ulcers, 10 were diabetic ulcers and two were traumatic ulcers. All wounds were applied with either VAC or silver-impregnated dressing alone in order to reduce exudation and provide a healthy wound bed before fat grafting. A healthy granulation tissue formation was generally started to be observed by Day 5.
Table 2. Distribution of quantitative variables
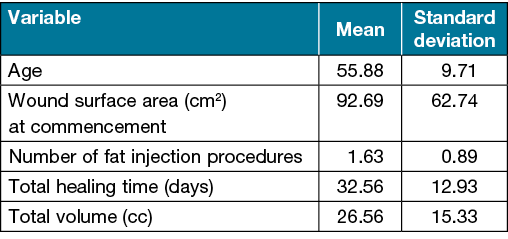
Table 3. Distribution of qualitative variables by wound aetiology
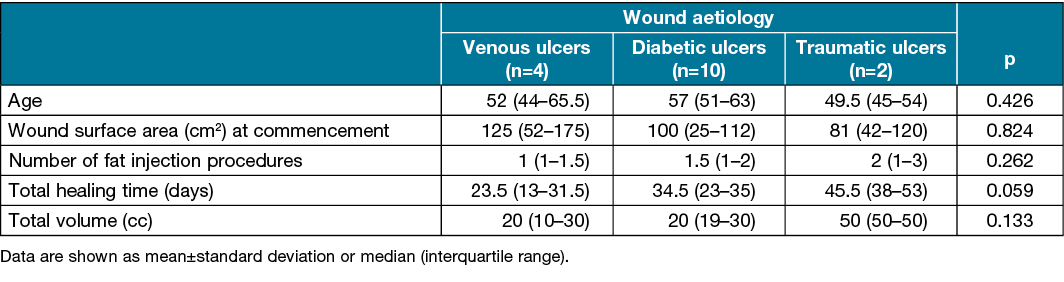
At commencement, the mean wound surface area was 92.69±62.74cm2; 125cm2 (range: 52–175) for venous ulcers, 100cm2 (range: 25–112) for diabetic ulcers and 81cm2 (range: 42–120) for traumatic ulcers. The mean healing time was 32.56±12.03 days; 23.5 days (range: 13–31.5), 34.5 days (range: 23–35), and 45.5 days (range: 38–53), respectively. The mean number of fat injection procedures was 1.63±0.89; one time (range: 1–1.5) for venous ulcers, 1.5 times (range: 1–2) for diabetic ulcers and two times (range: 1–3) for traumatic ulcers. The mean fat volume used was 26.56±15.33cc; 20cc (range: 10–30), 20cc (range: 19–30), and 50cc (range: 50–50), respectively. Seven wounds required a fat graft application one more time.
According to the number of fat injection procedures, the mean fat volume and the mean treatment time, a statistically significant difference was not found between the measurements. A total of 12 wounds required a skin graft or flap surgery and four were left to secondary healing. A cross-leg flap was used for one diabetic heel ulcer because these wounds were at the weight-bearing area. The ulcers were deemed uneventful in the following 12-month period.
Discussion
In chronic wounds, the use of adipose tissue has become very popular due to its regenerative capacity and its stem cell content; as such, it has shown great promise in tissue engineering and reconstructive surgery in recent years5. The stem cells secrete various growth factors and cytokines named paracrine function. Experimental and previous clinical studies have shown that transplantation of autologous mesenchymal stem cells into ischaemic limbs could promote collateral vessel formation and angiogenesis13–18. Stem cells have also enormous potential for skin tissue regeneration as the cells can both regenerate lost tissue and promote wound repair through paracrine coordination of their actions5.
Thus, the rationale of the present study was to investigate whether adipose tissue could aid the cutaneous wound healing process owing to its rich stem cell content in various acute and chronic wounds. This study describes our clinical outcomes in the treatment of lower extremity ulcers using a non‑centrifuged fat graft. In a similar study11, non‑centrifuged autologous fat graft was used for oncologic, traumatic and congenital defects in the craniofacial region in nine cases; no clinical differences were found between centrifuged and non‑centrifuged grafts. Furthermore, in our previous study5, we used the same grafting method as this present study after closed releasing the Dupuytren contracture bands in 17 patients; satisfactory clinical results were also achieved with the use of non‑centrifuged autologous fat graft.
In clinical practice, fat graft centrifugation is routinely carried out at 3000rpm for 3 minutes. Centrifugation is introduced to concentrate adipocytes and separate them from substances that may degrade them such as blood cells, proteases and lipases, and to establish a better environment for tissue viability, although there is no consensus on the latter19. A recent study by Condé-Green et al.20 compared the influence of the three most used fat processing techniques – decantation, washing and centrifugation – on the viability and number of adipocytes and mesenchymal stem cells in the aspirated fat. They conclude that washing is the best processing method for adipose tissue grafting as it maintains adipocyte integrity and number, clears the fat with most blood contaminations, and has a greater number of endothelial cells and mesenchymal stem cells. However, in a study by Asilian et al.21, no significant difference was found between the two fat processing methods. Similarly, in a different study, the transplantation of non‑centrifuged adipose tissue was found to have more active pre‑adipocytes which could possibly lead to better potential chances of survival and even de novo development of fat22. In the present study, the non‑centrifuged autologous fat graft was applied to the wound edge, wound bed and surrounding environment; better results were observed compared to our previous observation and experiments for similar wounds using centrifuging.
In addition, in the majority of the ulcers in this study, VAC therapy was used because these wounds were larger and had more exudate than others. In other wounds, silver-impregnated dressing alone was used to prepare them for fat grafting. A healthy granulation tissue formation was generally started to be observed by Day 5. In a similar study, Stasch et al.9 used autologous fat graft without centrifuging in chronic lower extremity ulcers; they obtained complete healing in 22 of 25 wounds. However, the authors used a fat graft for relatively smaller sized wounds compared to those of our study and, parallel to that, they transferred a smaller amount of fat graft. Also, our mean healing time was shorter. VAC therapy was also used after fat grafting in that study rather than before as in our study. It can therefore be speculated that VAC therapy could have positively affected their results, although they suggest that a VAC therapy time of 4–5 days was probably not responsible for the good outcome. In the present study, while VAC therapy was used before applying the fat graft, in one case we had to use VAC with fat grafting. This was because tissue necrosis was ongoing, and the wound was relatively large and more highly exudated than the others. We also used silver-impregnated dressings in four cases which were relatively smaller than others and less exudated. The use of silver-impregnated dressings or VAC therapy has several theoretical advantages, including antimicrobial inhibition and the enhancement of soft tissue granulation7; this can therefore explain the difference in the mean healing time between the two comparative studies.
Various intrinsic factors such as microcirculation and chronic diseases, diabetes and venous insufficiency determine the wound response to the fat graft used. After non‑vascularised fat grafting, the connective tissue or extracellular matrix may be preserved as a scaffold and, depending on the microenvironment, all differentiated cells usually die and are replaced by those of the next generation derived from tissue-specific stem cells5,6. In addition to these intrinsic factors, the fat graft volume is mainly determined according to the wound surface area. Therefore, while making a decision about any therapeutic fat graft dose or frequency of application, wound aetiology and localisation should be taken into consideration. A hypoxic or diabetic environment can affect fat graft survival, increasing the mesenchymal and endothelial progenitor cell responses and enhancing the formation of blood vessels23,24. In our study, we generally observed that the skin surrounding the ulcers rapidly started to turn into normal skin colour, especially in venous ulcers. It is known that these pathologic changes in chronic wounds can arise due to the inflammatory process and that extensive inflammation plays a major role in the disruption of the normal healing cascade25,26. It can therefore be speculated that our clinical observations are due to the anti-inflammatory and immunoregulatory function of the stem cell content of adipose tissue graft26–28. This is one of the clinical signs indicating the efficacy of the use of fat graft on the treatment of challenging wounds.
Limitation
In the present study, although traumatic ulcers were relatively smaller than the other wounds, the total fat volume, application frequency and total healing time were found to be longer in traumatic ulcers. However, it is proposed that the limited number of cases did not allow for sufficient comparison and could therefore not provide a significant conclusion. Also, the reason for the longer treatment time and the increased fat volume needed in traumatic ulcers might have been due to these ulcers being more complicated wounds. In addition, the fat graft volume used in wounds caused by different aetiologies was always not correlated with the degree of clinical signs and improvement.
Conclusions
There are a limited numbers of papers regarding the direct use of adipose tissue graft in the literature, and these are generally related to burn scars. In the majority of these reports, a centrifuged fat graft was used. In this preliminary study, we found that the presence of a non‑centrifuged fat graft during wound healing on the wound site could have beneficial effects. However, more clinical and experimental studies are needed to provide more comparable, clarifying and therefore optimal results.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Umut Tuncel* MD
Assistant Professor, Bahcesehir University, Medical Faculty, Department of Plastic Reconstructive and Aesthetic Surgery, and Samsun Liv Hospital
55100, Samsun,Turkey
Email drumuttuncel@gmail.com
Alper Kurt MD
Department of Plastic Reconstructive and Aesthetic Surgery, Samsun Gazi State Hospital, 55100
Samsun,Turkey
Osman Demir PhD
Assistant of Professor; Gaziosmanpaşa University
Faculty of Medicine, Department of Biostatistics
60250, Tokat, Turkey
* Corresponding author
References
1. Scotto di Santolo M, Sagnelli M, Tortora G, Santoro MA, Canta PL, Molea G, Schonauer F, et al. The utility of the high-resolution ultrasound technique in the evaluation of autologous adipose tissue lipofilling, used for the correction of post-surgical, post-traumatic and post-burn scars. Radiol Med 2016;121(6):521–527.
2. Hivernaud V, Lefourn B, Robard M, Guicheux J, Weiss P. Autologous fat grafting: a comparative study of four current commercial protocols. J Plast Reconstr Aesthet Surg 2017;70(2):248–256.
3. Gonçalves AI, Gershovich PM, Rodrigues MT, Reis RL, Gomes ME. Human adipose tissue-derived tenomodulin positive subpopulation of stem cells: a promising source of tendon progenitor cells. J Tissue Eng Regen Med 2017 Jun 7,12(3).
4. Condé-Green A, Marano AA, Lee ES, Reisler T, Price LA, Milner SM, Granick MS. Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring: a systematic review of the literature. Plast Reconstr Surg 2016:137(1):302–312.
5. Tuncel U, Kostakoglu N, Turan A, Cevik B, Cayli S, Demir O, Elmas C. The effect of autologous fat graft with different surgical repair methods on nerve regeneration in a rat sciatic nerve defect model. Plast Reconstr Surg 2015;136(6):1181–1191.
6. Hur W, Lee HY, Min HS, Wufuer M, Lee CW, Hur JA, Kim SH, et al. Regeneration of full-thickness skin defects by differentiated adipose-derived stem cells into fibroblast-like cells by fibroblast-conditioned medium. Stem Cell Res Ther 2017;20;8(1):92.
7. Günal Ö, Tuncel U, Turan A, Barut S, Kostakoglu N. The use of vacuum-assisted closure and GranuFoam silver® dressing in the management of diabetic foot ulcer. Surg Infect (Larchmt) 2015;16(5):558–565.
8. Tchero H, Herlin C, Bekara F, Kangambega P, Sergiu F, Teot L. Failure rates of artificial dermis products in treatment of diabetic foot ulcer: a systematic review and network meta-analysis. Wound Repair Regen 2017 Jun 8, 25(4)
9. Stasch T, Hoehne J, Huynh T, De Baerdemaeker R, Grandel S, Herold C. Débridement and autologous lipotransfer for chronic ulceration of the diabetic foot and lower limb improves wound healing. Plast Reconstr Surg 2015;136(6):1357–1366.
10. Suckow MA, Gobbett TA, Peterson RG. Wound healing delay in the ZDSD rat. In Vivo 2017:2;31(1):55–60.
11. Guijarro-Martínez R, Miragall Alba L, Marqués Mateo M, Puche Torres M, Pascual Gil JV. Autologous fat transfer to the cranio-maxillofacial region: updates and controversies. J Craniomaxillofac Surg 2011 Jul;39(5):359–63.
12. Tuncel U, Kurt A, Gumus M, Aydogdu O, Güzel N, Demir O. Preliminary results with non-centrifuged autologous fat graft and percutaneous aponeurotomy for treating Dupuytren’s disease. Hand Surg Rehabil 2017 Oct;36(5):350–354.
13. O’Loughlin A, Kulkarni M, Vaughan EE, Creane M, Liew A, Dockery P, Pandit A, et al. Autologous circulating angiogenic cells treated with osteopontin and delivered via a collagen scaffold enhance wound healing in the alloxan-induced diabetic rabbit ear ulcer model. Stem Cell Res Ther 2013;4(6):158.
14. Deldar Y, Pilehvar-Soltanahmadi Y, Dadashpour M, Montazer Saheb S, Rahmati-Yamchi M, Zarghami N. An in vitro examination of the antioxidant, cytoprotective and anti-inflammatory properties of chrysin-loaded nanofibrous mats for potential wound healing applications. Artif Cells Nanomed Biotechnol 2017;9:1–11.
15. Hyldig K, Riis S, Pennisi CP, Zachar V, Fink T. Implications of extracellular matrix production by adipose tissue-derived stem cells for development of wound healing therapies. Int J Mol Sci 2017:31;18(6).
16. van de Vyver M. Intrinsic mesenchymal stem cell dysfunction in diabetes mellitus: implications for autologous celltherapy. Stem Cells Dev 2017 May 18, 26(14).
17. Wan J, Xia L, Liang W, Liu Y, Cai Q. Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. J Diabetes Res 2013;2013:647107.
18. Hou C, Shen L, Huang Q, Mi J, Wu Y, Yang M, Zeng W, et al. The effect of heme oxygenase-1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials 2013;34(1):112–20.
19. Kurita M, Matsumoto D, Shigeura T, Sato K, Gonda K, Harii K, Yoshimura K. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg 2008;121:1033–1041.
20. Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg 2010;63:1375–1381.
21. Asilian A, Siadat AH, Iraji R. Comparison of fat maintenance in the face with centrifuge versus filtered and washed fat. J Res Med Sci 2014;19(6):556–561.
22. Khater R, Atanassova P, Anastassov Y, Pellerin P, Martinot-Duquennoy V. Clinical and experimental study of autologous fat grafting after processing by centrifugation and serum lavage. Aesthetic Plast Surg 2009;33(1):37–43.
23. Moyer HR, Namnoum JD. Autologous fat transfer: the progenitor cell response to different recipient environments. Aesthet Surg J 2014;34(6):932–940.
24. Jung JA, Kim YW, Cheon YW, Kang SR. Effects of the diabetic condition on grafted fat survival: an experimental study using streptozotocin-induced diabetic rats. Arch Plast Surg 2014;41(3):241–247.
25. Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol 2017;18(3):383–390.
26. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci 2016:11;17(12).
27. Lu X, Wang X, Nian H, Yang D, Wei R. Mesenchymal stem cells for treating autoimmune dacryoadenitis. Stem Cell Res Ther 2017:5;8(1):126.
28. Yang Y, Song HL, Zhang W, Wu BJ, Fu NN, Dong C, Shen ZY. Heme oxygenase-1-transduced bone marrow mesenchymal stem cells in reducing acute rejection and improving small bowel transplantation outcomes in rats. Stem Cell Res Ther 2016:20;7(1):164.



