Volume 30 Number 4
Topical anaesthesia for treating pain in chronic wounds: a WHAM evidence summary
Sharon MacLean and Emily Haesler
Keywords debridement, topical anaesthesia, lignocaine, wound pain
For referencing MacLean S & Haesler E. Topical anaesthesia for treating pain in chronic wounds: a WHAM evidence summary. Wound Practice and Research 2022; 30(4):246-248.
DOI https://doi.org/10.33235/wpr.30.4.246-248
Clinical question
What is the best available evidence for the use of topical anaesthesia for pain in chronic wounds as a primary dressing, and for managing procedural pain?
Summary
In patients with chronic wounds, wound related pain (WRP) is a significant and a major contributor impacting wound healing and quality of life1,2. Level 1 evidence1, 3, 4, 5 reports effectiveness of lignocaine/prilocaine 5% cream and lignocaine hydrochloride 5-10% in reducing WRP. Level 1 and 3 evidence1,2 showed a statistically significant improvement in pain relief when applying lignocaine/prilocaine cream or lignocaine hydrochloride cream as a primary dressing, with or without wound procedures being carried out; however, the need for identified larger randomised controlled trials (RCTs) to provide further evidence was identified. Several studies4, 5, 6, 7 provided Level 1, 2 and 3 evidence that a significant reduction in WRP can be achieved with the use of lignocaine/prilocaine cream or lignocaine hydrochloride spray/cream during debridement. All studies showed improvement in pain relief, giving practitioners another treatment option for WRP.
Clinical practice recommendations
All recommendations should be applied with consideration to the wound, the person, the health professional, and the clinical context.
Topical anaesthetics could be used pre and post wound procedures (e.g., debridement) and as a primary dressing to reduce pain associated with chronic wounds. (Grade B).
Sources of evidence
This summary was conducted using methods published by the Joanna Briggs Institute.8-10 The summary is based on a systematic literature search combining search terms related to topical anaesthetic, lignocaine, prilocaine, pain, debridement and wound dressing. Searches were conducted in Medline, PubMed, the Cochrane Library and Google Scholar for evidence published up to July 2022 in English. Levels of evidence for intervention studies are reported in the table below.
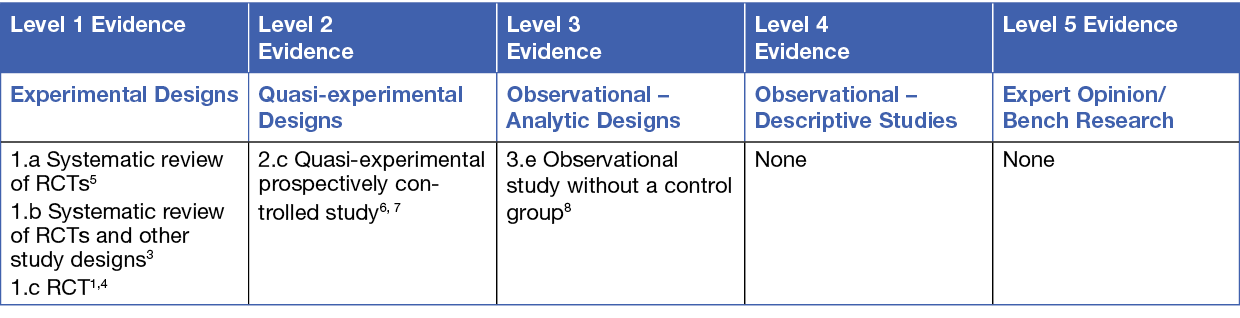
Background
Wound related pain is a common experience for people living with chronic wounds and can have a negative impact on quality of life5. The cause of WRP is often complex and poorly defined. Wound related pain can result from a normal physiological response to wounding (nociceptive pain) and can also be generated by damaged nerves (neuroreceptive pain)1. The most significant WRP generally occurs during procedures (i.e., procedural pain) such as wound dressing application and/or removal, wound cleansing and debridement. Various methods of debridement (e.g., mechanical, enzymatic, chemical, autolytic, biological, etc.) that are used to remove dead and devitalised tissue, reduce infection risk of infection and prepare the wound bed for healing5 can be very painful, sometimes influencing the ability to fully implement the procedure to achieve an optimal healing environment6. Other procedural-related factors (e.g., change in temperature, application of cleansing agents and the process of wound edge refashioning) can exacerbate WRP. Pain associated with wound dressing application or removal can also be exacerbated by inappropriate dressing selection1.
Wound related pain can be treated using a range of pharmaceutical options (e.g., systemic analgesics, topical analgesia, and topical anaesthesia) as well as non-pharmacological interventions. Although systemic analgesics are widely available and utilised, these are sometimes ineffective and may result in a high consumption of opiates, systemic side effects and misuse2, 3. Topical anaesthesia provides another option for treating pain1 that can allow the practitioner more time to complete wound procedures, consequently improving healing outcomes6.
Topical anaesthetics are available in a variety of formulations and are often used with a protective film dressing to help the treatment stay in position during therapy1,2,3,4. Two commonly used topical anaesthetics are lignocaine/prilocaine cream and lignocaine/hydrochloride preparations. These topical anaesthetics have shown to be effective in treating pain associated with pathological skin conditions, herpetic infections, and systemic sclerosis, as well as WRP associated with procedures such as mechanical debridement4. More recently, topical analgesics have been employed as a primary dressing for ongoing pain relief1,2.
Clinical evidence
Evidence on topical anaesthetic preparations used to reduce procedural pain
A meta-analysis5 at moderate risk of bias included six studies reporting the use of lignocaine/prilocaine cream to manage pain experienced by people (n = 343) with venous leg ulcers. The results demonstrated that when lignocaine/prilocaine cream was applied there was a significant reduction in pain scores measured on a 100 mm visual analogue scale (VAS) both during and after surgical sharp debridement compared to placebo/no anaesthesia (mean difference – 20.65; 95% confidence interval [CI] –29.11 to –12.19, p<0.0001)5. A second, more recent systematic review3 at moderate risk of bias reported the same evidence base in narrative form and concurred with the findings of the meta-analysis5 (Level 1).
Two small quasi-experimental studies6,7 (n = 25 and n = 21, respectively) at moderate risk of bias reported use of lignocaine/prilocaine cream applied pre and post sharp debridement to manage pain in chronic leg ulcers. In people with moderate to severe WRP, daily application of lignocaine/prilocaine cream prior to performing sharp debridement was well tolerated and reduced the VAS score from 75 to 21 by day ten of treatment.6 One of the studies7 evaluated the use of lignocaine/prilocaine cream versus a nitrous oxide/oxygen (N2O/O2) inhaled gas premix during a sharp debridement procedure. Approximately 90% of participants reported overall more comfort and pain relief with lignocaine/prilocaine cream compared with the inhaled gas. Both studies6, 7 support the use of lignocaine/prilocaine cream for debridement wound pain; however, a combination of both inhaled N2O/O2 and lignocaine/prilocaine cream could be an effective strategy if one treatment is not sufficient7 (Level 2).
One RCT4 (n = 50) at moderate risk of bias compared lignocaine/prilocaine cream used with a film occlusion to a topical lignocaine hydrochloride anaesthetic spray. Both products worked effectively to reduce WRP. Lignocaine/prilocaine cream required a longer wait time to achieve an anaesthetic effect, but also provided the practitioner a longer time to complete the debridement procedure. Lignocaine hydrochloride spray worked quickly; however, a more superficial degree of anaesthesia is achieved4 (Level 1).
Evidence on topical anaesthetic preparations used as a pain relief dressing
One RCT3 (n = 60) at moderate risk of bias compared the pain experience of an intervention group receiving lignocaine/prilocaine cream applied topically as a primary dressing to the experience of a control group receiving standard wound care. The mean pain scores at baseline were similar, however, after a 4-week treatment period there were significantly lower pain scores for the intervention group (intervention group: mean, 2.71; control group: mean, 3.92, p = 0.03). The intervention group had a significant reduction in pain during both the wound dressing change and after the wound dressing change for up to 24 hours. The application of lignocaine/prilocaine cream maintained sufficient anaesthetic effect to reduce anticipated pain before the wound dressing change the following day. This pilot study showed that using lignocaine/prilocaine cream has a significant effect in reducing WRP when used as a primary dressing (Level 1).
A small observational study 2 (n = 78) at moderate risk of bias investigated the use of lignocaine hydrochloride 5% cream for management of painful wounds. The pain intensity was reduced from baseline levels (mean score 6.71) at the beginning of treatment to (3.02) at the end of treatment (14 days treatment). The study participants reported reduced pain intensity and high safety and tolerability were demonstrated (Level 3).
The findings from these two studies2,3 showed evidence of pain relief when preparations were applied as a topical primary dressing; however, both studies had a small sample size and only one study compared the results with a control group. This highlights the need for further RCTs to examine the effectiveness of using topical anaesthetics as a primary dressing.
Considerations for use
- The clinical decision to use a topical anaesthetic to treat WRP should be made with consideration to the patient’s goals of care, which may differ depending on the wound aetiology, pain profile and type of wound procedure being perofrmed1,2,3,4.
- Topical anaesthetic use for treating WRP should be consistent with the manufacturer’s directions and local guidelines.
- Topical anaesthetics can be applied directly to the wound using the dose recommended by the manufacturer, and usually requires a wait time before an anaesthetic effect takes place4. A protective film dressing can be used secure the application in position during therapy1,2,3,4.
- Burning and itching, particularly on removal, have been reported as adverse events associated with anaesthetic creams, but a small body of evidence suggests they are not experienced statistically significantly more than when a placebo or no anaesthetic cream is applied1.
Funding
The development of WHAM evidence summaries is supported by a grant from The Western Australian Nurses Memorial Charitable Trust.
Conflicts of interest
The authors declare no conflicts of interest in accordance with International Committee of Medical Journal Editors (ICMJE) standards.
About wham collaborative evidence summaries
WHAM Collaborative evidence summaries are consistent with methodology published in
Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: A streamlined rapid review approach, Worldviews Evidence Based Nursing. 2015;12(3):131-8.
Methods are provided in detail in resources published by the Joanna Briggs Institute as cited in this evidence summary. WHAM Collaborative evidence summaries undergo peer-review by an international multidisciplinary Expert Reference Group. WHAM Collaborative evidence summaries provide a summary of the best available evidence addressing specific clinical questions and make suggestions that can be used to inform clinical practice. Evidence contained within this summary should be evaluated by appropriately trained professionals with expertise in wound prevention and management, and the evidence should be considered in the context of the individual, the professional, the clinical setting and other relevant clinical information. More information is available at the WHAM Collaborative website: http://WHAMwounds.com. Copyright © 2022 Wound Healing and Management Collaborative, Curtin University.
Author(s)
Sharon MacLean
Sharon MacLean, PhD, RN RM, Post Grad Dip (Midwifery), Masters Midwifery, Post Grad Dip (Wound, Ostomy and Continence)
Emily Haesler, PhD, B Nurs, Post Grad Dip Adv Nurs (Gerontics)
References
- Purcell A, Buckley T, King J, Moyle W, Marshall AP. Topical Analgesic and Local Anaesthetic Agents for Pain Associated with Chronic Leg Ulcers: A Systematic Review. Adv Skin Wound Care, 2020;33(5):240–51.
- Janowska A, Papa G, Romanelli M, Davini G, Oranges T, Stocco C, et al. 5% lidocaine hydrochloride cream for wound pain relief: A multicentre observational study. J Invest Surg,2020:1–4.
- Purcell A, Buckley T, Fethney J, King J, Moyle W, Marshall AP. the effectiveness of EMLA as a primary dressing on painful chronic leg ulcers: A pilot randomized controlled trial. Adv Skin Wound Care,2017;30(8):354–63.
- Cuomo R, D’Aniello C, Grimaldi L, Nisi G, Botteri G, Zerini I, et al. EMLA and lidocaine spray: A comparison for surgical debridement in venous leg ulcers. Adv Wound Care, 2015;4(6):358–61.
- Briggs M, Nelson EA, Martyn-St James M. Topical agents or dressings for pain in venous leg ulcers. Cochrane Sys Rev, 2012. Issue 11. Art. No.: CD001177
- Effendy I, Gelber A, Lehmann P, Huledal G, Lillieborg S. Plasma concentrations and analgesic efficacy of lidocaine and prilocaine in leg ulcer-related pain during daily application of lidocaine–prilocaine cream (EMLATM) for 10 days. British J Derm, 2015;173(1):259–62.
- Traber J, Held U, Signer M, Huebner T, Arndt S, Neff TA. Analgesic efficacy of equimolar 50% nitrous oxide/oxygen gas premix (Kalinox®) as compared with a 5% eutectic mixture of lidocaine/prilocaine (EMLA®) in chronic leg ulcer debridement. Int Wound J,2016;14(4):606–15.
- Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer’s Manual, 2017. The Joanna Briggs Institute: https://reviewersmanual.joannabriggs.org/.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party, 2013 New JBI Grades of Recommendation. JBI: Adelaide.
- The Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. Supporting Document for the Joanna Briggs Institute, 2014. Levels of Evidence and Grades of Recommendation. JBI: Adelaide.




