Volume 28 Number 1
Overflow effect of high intensity exercise in the treatment of urinary incontinence in spinal stroke: a case study
Christopher Myers and Moira Smith
Keywords exercise, urinary incontinence, pelvic floor, spinal cord, stroke rehabilitation
For referencing Myers C and Smith M. Overflow effect of high intensity exercise in the treatment of urinary incontinence in spinal stroke: a case study. Australian and New Zealand Continence Journal 2022; 28(1):9-14
DOI
https://doi.org/10.33235/anzcj.28.1.9-14
Submitted 8 November 2021
Accepted 7 February 2022
Abstract
Spinal cord infarction is rare, often with a sudden onset of symptoms including bladder and bowel dysfunction, paralysis and sensory disturbances.
This case reports the efficacy of a therapeutic exercise program as a non-invasive functional treatment for urinary incontinence and reports its results. It describes a 67-year-old woman who experienced a T10‑L1 anterior spinal cord infarction living with residual urinary incontinence and lower limb sensory and strength deficits.
This paper explains the overflow effect of high volume exercise prescribed to address urinary incontinence symptoms resulting from spinal cord infarction. It highlights the potential use of co‑contraction techniques in the conservative management of neurogenic lower urinary tract symptoms.
Introduction
Spinal cord infarction (SCI) is rare, often with a sudden onset of symptoms including bladder and bowel dysfunction, paralysis and sensory disturbances. This case report identifies the outcome of an 11‑week therapeutic exercise program as a non-invasive functional treatment for urinary incontinence (UI) and reports its results. It describes the care of a 67-year-old woman who experienced a T10‑L1 anterior SCI and who was living with residual UI and lower limb sensory and strength deficits. This case describes a common symptom of SCI, and the overflow effect of prescribing high volume exercise in addressing UI. It highlights the potential use of co‑contraction techniques in the conservative management of neurogenic lower urinary tract symptoms.
Background
Spinal cord infarct is rare, with an age-adjusted female incidence of 4.6 (95% CI 2.2–8.7) per 100,000 person-years, accounting for less than 1% of all stroke types1. Symptoms and impairment severity are dependent on the spinal cord level affected, with commonly reported symptoms including bladder and bowel dysfunction, paralysis and sensory disturbances2. Long-term prognosis varies, but is linked closely with initial impairment severity3. On discharge from hospital, individuals often experience ongoing bladder and bowel dysfunction2.
Bladder dysfunction significantly impacts quality of life, with rehabilitation of neurogenic lower urinary tract dysfunction often requiring comprehensive multi-disciplinary input4. Addressing UI may include bladder retraining, education, pelvic floor muscle training (PFMT), pharmacological prescription, and even surgical intervention4. Preferred management options within this demographic include timed voiding and self-catheterisation, with varying degrees of efficacy and time-cost benefits5. A growing body of literature suggests that PFMT is a non-invasive and less costly first-line intervention in the conservative management of UI6. However, protocol variation exists with respect to overall frequency, intensity, contraction type and length, alongside use of supplementary biofeedback and electrical stimulation7. In addition, there is considerable variability in methods used to facilitate pelvic floor muscle (PFM) contraction8. This case study describes the overflow effect of an intensive gluteal strengthening program in the rehabilitation of an individual with a chronic SCI who is experiencing UI. The purpose of this case report is to highlight the importance of volume and intensity in exercise prescription for a novel approach in improving UI in a person with a motor incomplete SCI.
The following case study was developed in accordance with the CARE guidelines9. Ethical approval from the James Cook University Human Research Ethics Committee (H7636) was gained prior to initiating the case report. The patient, de-identified in this case report, provided informed written consent before taking part. All patient identifiers were removed for the purpose of this case report.
Patient information
The patient (R), a 67-year-old woman, was diagnosed with a T10‑L1 anterior SCI 3 years prior to self-referral to a community-based physiotherapist for improvement of her balance and mobility issues. Residual symptoms from her infarct included reduced balance and (left) lower limb sensory and strength deficits, and insensible UI. She reported the need to wear two or more incontinence pull‑ups daily to contain UI episodes and reported changing her pull‑ups during timed voids when she would notice the worn pull‑ups were wet. She did not weigh the pads or measure her leakage. She received monthly visits from a community-based continence nurse for provision of continence products. Her daily routine was organised around toileting habits, employing a timed toileting schedule (reported voids every 90–120 minutes) with double voiding techniques. Regular use of a bisacodyl suppository for bowel movements was reported. She reported no concerns with faecal continence except when she made significant changes to her diet or fluid intake. Her goal was to return to maximal functional independence.
Noteworthy medical history included a cholecystectomy, depression, irritable bowel syndrome, mild heart failure, recurrent urinary tract infections, and restless leg syndrome. Recurrent urinary tract infections occurred post-ischaemic spinal stroke. Her obstetric history included one caesarean section carried to term in the early 1970s and a hysterectomy in 1990. Medications prescribed included amitriptyline, aspirin, bisacodyl, fludrocortisone, magnesium and rosuvastatin. Family history included breast cancer, glaucoma, macular degeneration and ischaemic heart disease. She identified as an ex-smoker and did not consume alcohol.
At the time of self-referral, R was living alone in a high set house with five steps up to the entry. She required the use of a rail for ascending and descending stairs. She was functionally independent, and regularly performed housework and gardening, and socialised with friends. Outpatient multi-disciplinary rehabilitation to date had consisted of lower limb strengthening, balance and gait retraining.
Physical exam
Functionally, R had distinct kinematic gait deviations, ambulating independently with a left-sided ankle foot orthosis (AFO), and a single point stick (SPS) in her right hand. Her average speed for her timed 10m walk test (T10MWT) was 18.2s (0.55m/s), considerably slower than the age-related norm (M = 1.0m/s, SD = 0.23m/s). Her Berg Balance Score (BBS) was recorded at 46/56, indicating a low risk of falls (M = 55/56, SD = 2, CI 95%).
Upon examination, R reported intact sensation of her right lower limb, with impaired sensation in an L4‑S2 distribution of her left lower limb. She reported no bladder-filling sensation. The muscular strength of her left lower limb was assessed using the Oxford Grading scale (a 6-point grading system with 0 being no movement and 5 full range of movement). Muscle strength varied between grades 3/5 to 4/5, with the exception of her left tibialis anterior (dorsiflexion: grade one). No tonal abnormalities were identified. Selective activation of transversus abdominis (TA) activation was trialled unsuccessfully in several positions. TA activation occurred only when beginning in a crook lie position and moving into a gluteal bridge position. She had difficulty in her ability to maintain TA contraction for more than a few seconds in this position. No internal examination was conducted as the treating therapist was male, unchaperoned and it was not the key focus of the patient’s self-referral. Verbal instructions were trialled to cue a voluntary PFM contraction but she reported no change in sensation or ability to recognise a PFM contraction.
Therapeutic focus and assessment
The goal was for R to return to maximal functional independence. Initially, a graded walking program was prescribed with functional lower limb strengthening. A flexible approach to exercise volume was prescribed with recommendations that she continue all activities of daily living, and to not substitute them for her prescribed rehabilitation program. Exercise was progressed at week 3 during her first follow-up appointment (Table 1).
Table 1. Week 3: Progression of the home exercise program (HEP)
Follow-up and outcomes
The first review between R and the clinician sought clarification of her home exercise program (HEP) with focus on TA co‑contraction during a gluteal bridge. She began attending a once weekly balance program, supervised by the same physiotherapist. By her second review 2 weeks later, she reported bladder and sensory changes. Upon further investigation, she reported misreading her updated HEP prescribed at week 3, performing five sets of 15 (30s holds), equating to 37.5 minutes of gluteal bridges. She had also increased the frequency of exercise to daily.
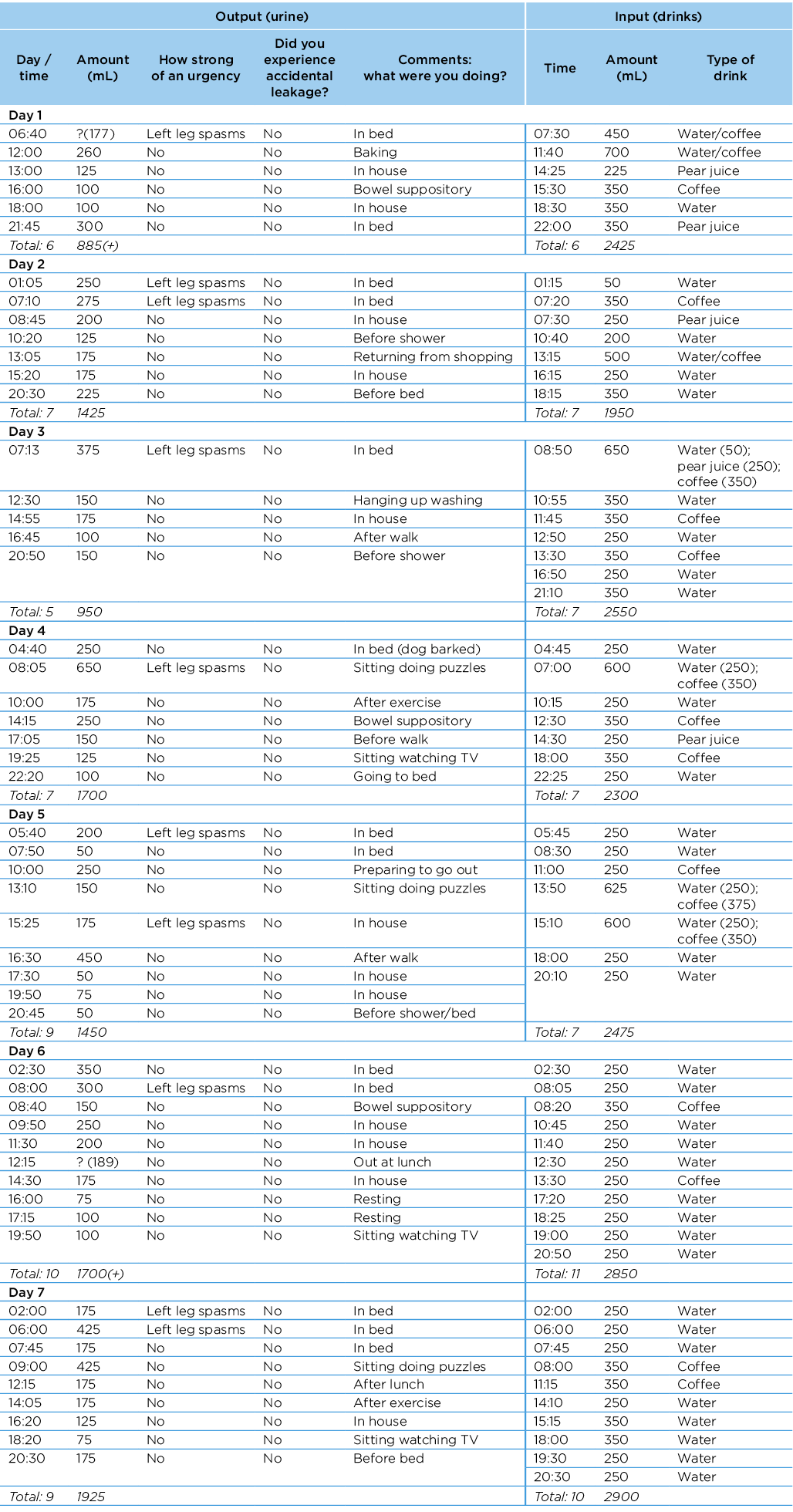
She was instructed to complete a confirmatory 7-day bladder diary at week 5 which indicated a moderate fluid imbalance (Appendix 1). Figure 1 shows the timeline of care episodes. She reported reduced need for continence pull‑ups, using one pull‑up per day for added security. Her pull‑up was consistently dry at the end of each day; she experienced no leakage as recorded on her confirmatory bladder diary. She also reported the initiation of left leg spasms that coincided with an "urge sensation" to urinate. The spasms typically occurred early morning and, despite being inconsistent, almost always occurred with an urge sensation.
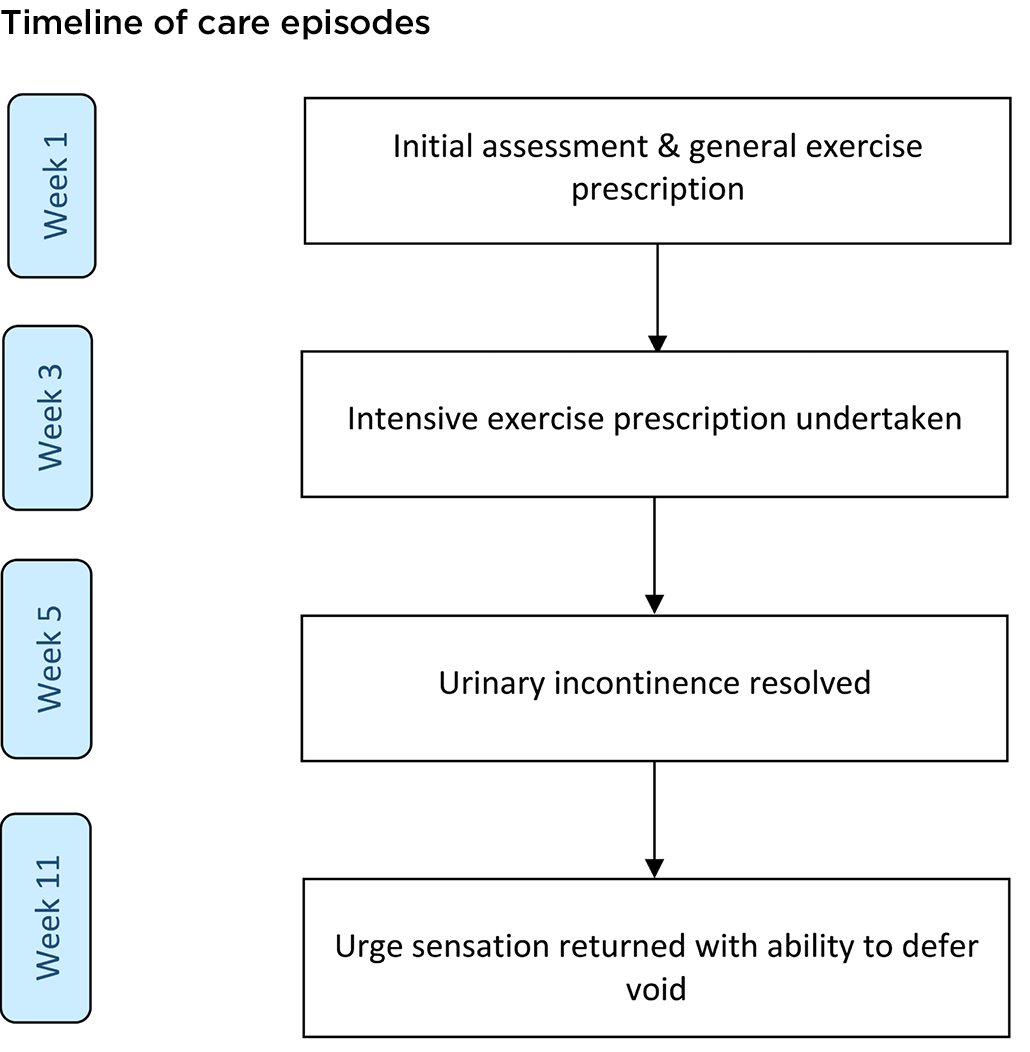
Figure 1. Timeline of events
Discussion
This case demonstrates a potential motor overflow effect of the PFM occurring after performing high volume lower limb strengthening exercises over a 2‑week period. The participant experienced a return of an urge sensation to void and no involuntary leakage at follow-up. This motor overflow effect occurred despite her inability to selectively contract her TA or PFM. An inability to discriminate and voluntarily contract these muscles was likely negated through gross contraction achieved from performing frequent high intensity gluteal bridges.
Recent literature has identified successful co‑contraction of the PFM during TA and other lumbopelvic muscular activation, with lumbopelvic muscle irradiation potentially assisting in recruiting neighbouring musculature and amplifying existing PFM and TA contraction10,11. The intensive nature of exercise likely induced local neural adaptations, promoting greater motor unit recruitment and frequency of firing. This may have resulted in increased urethral closure pressure, with increased PFM firing allowing for an extension to the guarding reflex from parasympathetic motor neuron inhibition12,13.
Isolated PFM training is preferred to gross or lumbopelvic co‑contraction as it limits intra-abdominal pressure, reducing the risk of subsequent stretching and weakening of the muscle due to "bearing down"11. However, isolated contraction requires the ability to discriminate between, and voluntarily contract, the lumbopelvic musculature. In an incomplete SCI population this is difficult as many may lack sensory awareness of either periurethral or perivaginal pressures11. This sensory awareness is important as it is required for voluntary PFM strength. However, the return of an urge sensation through lower limb spasm suggests improved afferent pathways from the bladder to the spinal cord, coinciding with larger morning voids (Appendix 1)12,13. Consistent small voids without this urge sensation suggests existing voiding dysfunction with possible moderate post-void residual volumes (Appendix 1). In this case, exercise of an intense nature, and specific to the lumbopelvic region, may have contributed to this sensory return and improvement in some of her urogenital symptoms14.
Protocols for PFMT have significant variance, with many recommending supervised PFMT be performed for a minimum of 12 weeks15. PFMT adherence over these extended timeframes have been linked to high dropout rates and less successful outcomes14. Mixed literature exists regarding the optimal volume of exercise prescription, with no guidelines making recommendations of high intensity, high frequency, co‑contractile methods, nor their potential effect on improving sensory awareness. Given the susceptibility to infection and significant impact UI has on quality of life in an SCI population, further investigation into intensive PFMT for incomplete SCI populations should be considered4,6,11.
Limitations
Evaluation of the female PFM typically requires strength assessment via the Oxford Grading Scale, manometry, or dynamometry, with PFM activation assessed with real time ultrasound or surface electromyography. The assessment of our case included only verbal cueing and external palpation of TA with no investigation of the PFM. Furthermore, the effect of the intervention was unexpected, so more appropriate baseline measurements were unable to be assessed. This limits the ability to accurately determine the true effect of the intervention without further investigation. Lastly, the natural course of healing is often unpredictable and may still be occurring, but is unlikely given the time since R’s injury.
Conclusion
This case describes a common symptom of SCI and highlights the possible application of using high volume co‑contractile techniques over a short intervention period for improving urinary continence in an individual with an incomplete motor SCI. Further investigation into both the effects of PFM co‑contractile techniques and high frequency PFMT for populations lacking sensory input is required.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Christopher Myers*
Associate Lecturer, Discipline of Physiotherapy,
James Cook University, Townsville, QLD, Australia
Email chris.myers@jcu.edu.au
Moira Smith
Lecturer, Discipline of Physiotherapy,
James Cook University, Townsville, QLD, Australia
*Corresponding author
References
- Qureshi AI, Afzal MR, Suri MFK. A population-based study of the incidence of acute spinal cord infarction. J Vasc Interv Neurol 2017;9(4):44–48.
- Nedeltchev K, Loher TJ, Stepper F, et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke 2004;35(2):560–565.
- Robertson CE, Brown RD, Wijdicks EFM, et al. Recovery after spinal cord infarcts. Neurol 2012;78(2):114.
- Dorsher PT, McIntosh PM. Neurogenic bladder. Adv Urol 2012;2012:816274–816274.
- Romo PGB, Smith CP, Cox A, et al. Non-surgical urologic management of neurogenic bladder after spinal cord injury. World J Urol 2018;36(10):1555–1568.
- Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database System Rev 2018(10).
- Ferreira M, Santos P. Pelvic floor muscle training programmes: a systematic review. Acta Med Port 2011;24(2):309–318.
- Mateus-Vasconcelos ECL, Ribeiro AM, Antônio FI, et al. Physiotherapy methods to facilitate pelvic floor muscle contraction: a systematic review. Physiother Theory Pract 2018;34(6):420–432.
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2013;2(5):38–43.
- Burke RE. Sir Charles Sherrington’s the integrative action of the nervous system: a centenary appreciation. Brain 2007;130(4):887–894.
- Bø K, Mørkved S, Frawley H, et al. Evidence for benefit of transversus abdominis training alone or in combination with pelvic floor muscle training to treat female urinary incontinence: a systematic review. Neurourol Urodyn 2009;28(5):368–373.
- Chancellor MB, Yosimura N. Neurophysiology of stress urinary incontinence. Rev Urol 2004;6(Suppl 3):S19-S28.
- Chermansky CJ, Moalli PA. Role of pelvic floor in lower urinary tract function. Auton Neurosci 2016;(200):43–48.
- Zhou X, Williams AMM, Lam T. Effects of exercise-based interventions on urogenital outcomes in persons with spinal cord injury: a systematic review and meta-analysis. J Neurotrauma 2021;38:1225–1241.
- Garcia-Sanchez E, Avila-Gandia V, Lopez-Roman J, et al. What pelvic floor muscle training load is optimal in minimizing urine loss in women with stress urinary incontinence? A systematic review and meta-analysis. Int J Environ Res Public Hlth 2019;16(22):4358.
Appendix
Appendix 1. Patient’s 7-day confirmatory bladder diary





