Volume 28 Number 1
A pilot integrated clinic using a biopsychosocial model to treat incontinence and prolapse
Alice Beban, Samantha Newman and Bernadette Nolan
Keywords prolapse, urinary incontinence, biopsychosocial healthcare, integrated healthcare, women's health
For referencing Beban A et al. A pilot integrated clinic using a biopsychosocial model to treat incontinence and prolapse. Australian and New Zealand Continence Journal 2021; 27(4):84-92
DOI https://doi.org/10.33235/anzcj.27.4.84-92
Abstract
This study aim was to evaluate women’s experiences of a biopsychosocial approach to incontinence and pelvic organ prolapse care through an integrated clinic with a general practitioner and physiotherapist providing care. A prospective observational study was undertaken with 36 women, prior to treatment and upon discharge from the clinic, using the International Consultation on Incontinence Questionnaires, Female Lower Urinary Tract Symptoms and
Vaginal Symptoms. Qualitative interviews were also undertaken with 20 of the 36 study participants. Participants reported significant reduction in biological symptoms, including nocturia, urgency, frequency, urge incontinence, frequency of incontinence, and vaginal symptoms of dragging and soreness (p<0.05). Index scores for vaginal symptoms, filling and incontinence decreased significantly (p<0.05). Interviews revealed that participants also experienced improvements in related conditions, including constipation and pelvic pain. Psychosocial improvements included significant decrease in bother associated with urinary and vaginal symptoms, and fewer negative effects of symptoms on relationships, sex life and quality of life (p<0.05). During interviews, participants reported improved confidence and sense of control, and better sexual and social relationships. This study concludes that an integrated general practitioner/physiotherapy clinic taking a biopsychosocial approach to healthcare can significantly reduce physical symptoms and improve psychological and social dimensions of health for women with POP and incontinence.
Introduction
In New Zealand urinary incontinence is reported to affect 34% of women, and pelvic organ prolapse (POP) affects an estimated 30–50% of women1,2. Pelvic organ prolapse is associated with faecal and urinary incontinence3, pain4, poorer quality of life5, and a reduced ability to socialise and maintain intimate relationships6. Surgical repair is effective, but can be associated with complications, has significant cost implications, and is not desired by all women7. Physiotherapy-based interventions8 and conservative treatment such as the fitting of pessaries, topical oestrogen cream, dietary advice and lifestyle modifications are recommended as first line treatment, and are reported to be effective in helping women regain control over their symptoms and life7,9.
Despite effective treatment being available, most women who experience urinary incontinence or symptoms related to POP do not seek help; embarrassment, discomfort and fear of surgical interventions discourage women from consulting their general practitioner (GP)9. Even when women do seek help, they report feeling that their concerns are dismissed10 and they feel they have little power over their healthcare9. Women seeking assistance through the New Zealand public health system face challenges in long wait times for specialist referrals and disconnects between different health providers. Women often see multiple providers, requiring them to report symptoms to their GP, see a physiotherapist, return to their GP for medication, and then see a gynaecologist for pessary fitting and further treatment. This leads to severe inequity in health services, with women either enduring long waits and multiple referrals for publicly funded treatment, or paying for private treatment11. Lack of access and cost barriers are linked to poorer health outcomes for low-income women, particularly those in rural areas12, and for Māori women who also face cultural barriers in the Western medical model13.
A proposed solution to these issues of inequity, disempowerment and disconnected services is integrated care that aims to improve patient experience through coordinated, holistic approaches to healthcare14. In the Hawke’s Bay region of New Zealand, a pilot, publicly-funded clinic was established with the aim to provide women with timely, integrated care for POP and incontinence. The clinic adopted a biopsychosocial approach to healthcare which recognises that biological, psychological and sociocultural factors all affect health, and these factors interact with each other in a dynamic system15. There is growing interest in adopting this model for pelvic health, recognising that pelvic floor dysfunction is complex and affects “many areas of the woman’s life including social, psychological, occupational, domestic, physical and sexual”16. A summarised definition of the biopsychosocial model is shown in Box 1, adapted from Moseley and Butler17.
Box 1. Defining the biopsychosocial model used for the pilot clinic

The biopsychosocial approach used at this clinic aimed to improve pelvic floor dysfunction by addressing not just gynaecological health but the factors that influence it. This approach included listening to each woman’s story about how their pelvic health affected their quality of life and creating individual treatment plans based on the priorities each woman identified. An aim was to offer women attending the clinic choices about their health, to empower them to manage their health issues themselves, and to ensure that they felt believed, respected and understood.
The integrated clinic
The pilot integrated clinic was held in Napier, Aotearoa NZ from June to November 2020. The regional gynaecological specialist who reviews GP referrals for women with incontinence and/or POP symptoms assigned those he thought appropriate for GP/physiotherapy management to the clinic. Face-to-face appointments were held at three adjoining rooms in a medical centre which allowed the GP and physiotherapist to hold separate consultations simultaneously with a middle room for handover and administration. The patient stayed in the same room throughout their clinic experience. This model enabled the clinicians to spend more time with patients who needed their specific expertise.
Initial consultation
Initial consultations were 60 minutes. Upon arriving at the clinic, each patient saw either the GP or physiotherapist first and, following their consultation, the clinicians handed over the patient using the SBAR18 approach (Situation, Background, Assessment, and Recommendations) to ensure comprehensive, effective communication. Patients completed a proforma that was used to guide initial discussion with the clinicians and to enable effective handover. A healthcare assistant with experience in sexual health assessment was present to offer support and to ensure smooth patient flow.
The GP (a female GP with special interest in women’s health) performed, with the woman’s specified consent: abdominal examination; external genitalia examination; internal gentle bimanual examination to assess masses, tissue movement, and fullness of ovaries, uterus and bowel; and speculum examination of anterior/middle/posterior compartments with valsava manoeuvre to assess pelvic organ descent. The physiotherapist (a female pelvic health physio) conducted, with the women’s specified consent: observation of the woman’s gait and movement; palpation of the abdominal fascia, viscera and musculature to improve fascial mobility; mobilisation of bony structures; breathing mechanics; guidance with pelvic floor exercises; and energetic techniques to help with identification of muscles and affirm correct movements. Women who were not comfortable with a genital or internal examination were externally examined, provided with education materials and supported.
An individual treatment pathway was designed which included: modifying behavioural risk factors; physiotherapy recommendations; pessary use if required; referral for gynaecological surgery or other specialties if required and as needed (in combination with physiotherapy). Lifestyle modification and specific nutrition advice relating to bowel health was provided to each woman and personalised to address modifiable risk factors for POP and incontinence, and factors that may exacerbate risk such as bowel regimen, nutrition, skin health, hormonal health, techniques for lifting. Each woman was provided written information from the website yourpelvicfloor.org which included pamphlets on POP, stress urinary incontinence (SUI), pelvic floor exercises and other conditions as indicated on examination. This information was provided in English and, in one case, in Spanish. Women were asked if they would like a translator present for their appointment; none of the patients requested this service. The physiotherapist read through and explained the information and provided time for each woman to raise questions and concerns.
Follow-up
Follow-up appointments of 30–60 minutes were held with each woman according to need. A majority of women completed two to three sessions before discharge. At follow-up appointments the physiotherapist provided ongoing guidance on pelvic floor exercises and education on pelvic floor and core function, bladder and bowel habits, gait re-education, lifestyle modification and pressure management. If examination indicated pessary treatment, the GP selected an appropriate pessary for fitting from a sterilisable sizing kit. If required, each woman was offered a weekly visit with the GP until correct fit was achieved; ‘correct fit’ referred to what the participant said felt right for them. Pessary compliance and outcomes are shown to improve when women can self-manage their pessary19; therefore, the clinic supported the women in self-management if they felt comfortable. Vaginal oestrogen was prescribed if indicated on examination, including estriol cream or a non-hormonal option, hyaluronic acid.
Patient adherence was facilitated by phone contact with each participant after pessary fittings and via written communication with each participant’s GP to enable ongoing care. In line with the patient-centred approach, the success of the intervention was based on self-reported improvements in physical symptoms and psychosocial domains.
Data collection
This study used a mixed methodology approach, combining a prospective observational cohort study using a validated survey tool with semi-structured qualitative interviews to evaluate the effectiveness of the clinic at treating POP and incontinence, including biological symptoms and psychosocial dimensions of health. Self-reported symptomatic and quality of life changes in participants of the pilot clinic study were measured using the validated International Consultation on Incontinence Questionnaire-Vaginal Symptoms (ICIQ-VS) and Female Lower Urinary Tract Symptoms questionnaire (ICIQ-FLUTS) questionnaires20. The ICIQ questionnaires are shown to measure clinically relevant improvements21,22 with high internal consistency and reliability23. Each symptom question is followed by a question asking “How much does this bother you?” using a scale of 0–10, where 0 is ‘not at all’ and 10 is ‘a great deal’. Pre-treatment surveys were mailed out prior to treatment. Post-treatment surveys were mailed to patients after they were discharged, with a follow-up phone call to encourage survey completion. Comparisons of pre- and post-treatment variables were made using two-tailed paired t-tests. Statistical significance was set at p 0.05 or less. R statistical software was used for data analysis.
A purposive sample was used to select candidates for in-depth interviews from amongst the 36 participants; 21 women were approached via email or telephone for interviews after they had been discharged, and 20 participants were interviewed between October and December 2020. The sample was selected to include a range of demographics, including different ages, ethnicities and urban and rural participants, as well as a range of pathways following the clinic, including women who used conservative physical therapy treatment, pessaries and referrals for surgical intervention. The rationale for this selection was to reduce bias toward ‘success’ cases (i.e., only interviewing those who had successful conservative treatment), and to ensure that a range of women’s voices was included.
Interviews took 1–2 hours and were carried out at the women’s homes with only the interviewee and researcher present. Interviews were conducted by the first author, a female sociologist who was not affiliated with the clinic and did not have a prior relationship with participants. Her interest in the topic stems from her professional desire to raise awareness of common health issues experienced by women, and her personal experience with POP. These interests were disclosed to participants at the start of the interview to build rapport with interviewees.
The interviews explored five key areas in a semi-structured format:
- Previous experiences of living with incontinence and POP.
- Previous interactions with the health system.
- Interactions with the pilot clinic.
- Biological, psychological and social changes experienced during and after clinic treatment.
- Suggestions for service improvement.
Interviews were audio-recorded and transcribed by the first author. Thematic analysis was used to identify, analyse and report patterns within the dataset for each of these key areas using NVivo 13 analysis programme. The first author performed coding and analysis, with themes derived inductively from the data.
Ethics and consent
The study received ethics approval after full review from Massey University Human Ethics Committee (SOA 20/36). The study aims were explained to participants, and participants gave verbal consent to participate.
Results
Participants were 36 women who completed pre- and post-treatment surveys; the average age was 64 years (range 24–86 years). A total of 41 women were recruited to the study, with 36 women (88%) completing a post-treatment survey. No significant difference was found in pre-treatment reported symptoms between those who completed follow-up surveys and those who did not. Only the 36 women who completed both pre- and post-treatment surveys are included in analysis.
Change in biological symptoms
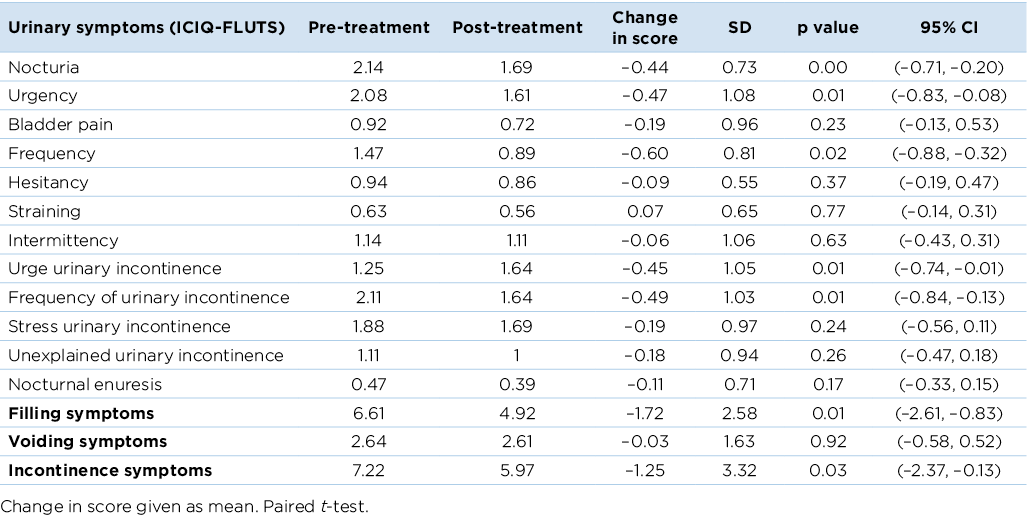
Prior to treatment, the most common urinary symptoms reported were nocturia (92%), urgency (92%), SUI (89%), urge incontinence (81%), and frequency of incontinence (81%). Urinary symptoms reduced in severity following treatment (Table 1). A statically significant decrease was evident in nocturia, urgency, frequency, urge incontinence and frequency of incontinence (p<0.05). Index scores for filling symptoms decreased significantly from 6.61 to 4.92 (on a scale of 0–15) and incontinence symptoms also showed a significant decrease from 7.22 to 5.97 (on a scale of 0–20) (p<0.05).
Table 1. Change of symptoms from baseline to end of treatment using the ICIQ-FLUTS
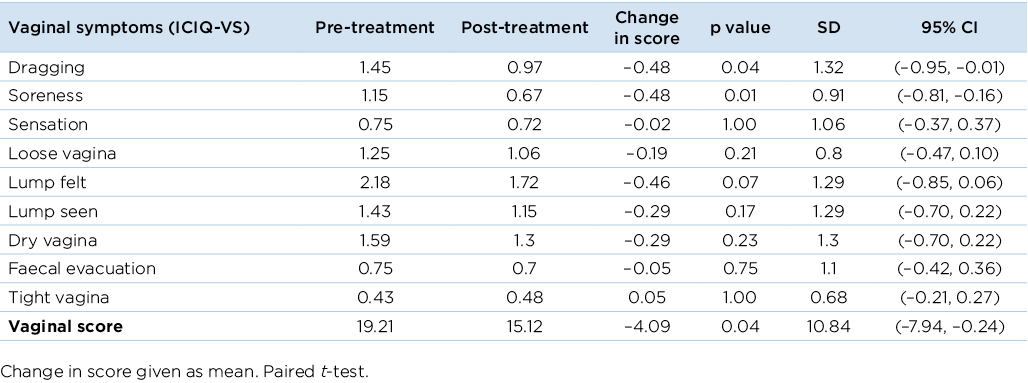
The ICIQ-VS questionnaire complemented the ICIQ-FLUTS questionnaire by eliciting information on POP symptoms. Prior to treatment, 70% (25/36) of participants reported feeling a lump at least some of the time, and 52% (19/36) of women reported feeling ‘dragging’ and seeing a lump at least some of the time. In the post-treatment survey, vaginal symptoms of dragging and soreness decreased significantly (p<0.05) (Table 2). The vaginal index score also showed a significant decrease from 19.21 to 15.21 (on a scale of 0–53) (p<0.05).
Table 2. Change of symptoms from baseline to end of treatment using the ICIQ-VS
The interviews provide a broader picture of the improvement in physical symptoms related to incontinence and POP. Of the participants interviewed, 18/20 responded that their physical symptoms had improved, including a reduction in urine leaks and POP symptoms. For example:
Within a couple of weeks, I noticed that when I went to the toilet the flow had improved… it’s much better (Respondent #10).
I don’t get the heaviness now, the bulging of my bowel. I used to have to manually push up when I pooed, but now it’s good 90 % of the time, I can go normally and more often, and emptying properly. It’s amazing (Respondent #6).
Improvements in related physical symptoms beyond incontinence and POP, including constipation, pelvic pain, coughing, vaginal dryness, haemorrhoids and other menopausal symptoms, were noted by 14/20 women. For example:
It’s great. Now I don’t have to wear pads. This is the first time I’ve not had to wear pads in five years… the exercises are different and they’ve release[ed] on the muscles and that’s helped a lot, found points where I’ve got pain (Respondent #2).
I’ve been multiple times to the doctors, and it’s not that they laugh me off, but they tell me it’s not bad enough for surgery. But with haemorrhoids, it made me internally sick, I couldn’t focus until they calm down… What she said is that we need to take all that pelvic region into consideration and work with the whole. So the fact that I have a weak bladder is related to the haemorrhoids. That’s why I’ve found it beneficial. The first person that has seen me for me. My individual issues, and not just a problem (Respondent #3).
These responses show broader biological symptom improvement beyond the information captured in the surveys.
Change in psychosocial symptoms
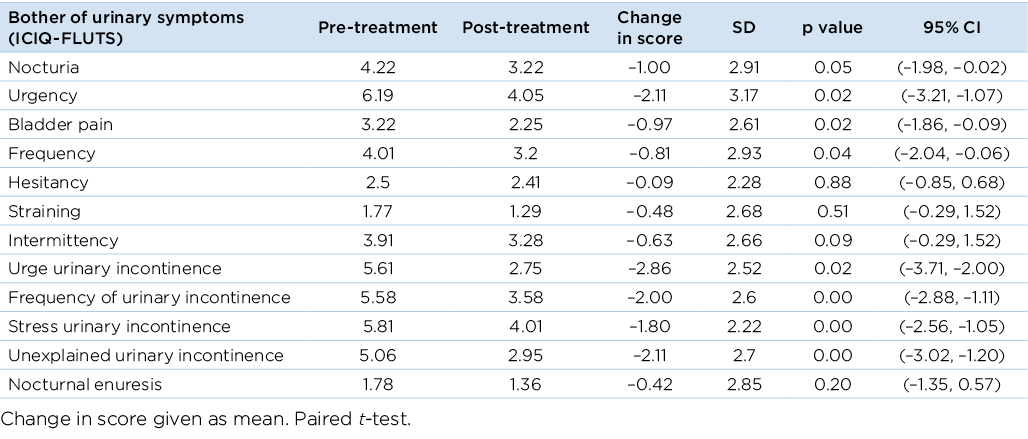
Psychosocial changes were assessed in the survey by analysing change in self-reported ‘bother’ caused by the symptoms. In the ICIQ-FLUTS scores, the bother associated with urinary symptoms decreased across categories, with a significant decrease in the bother associated with nocturia, urgency, bladder pain, frequency and urge urinary incontinence (p<0.05) (Table 3). Significant decrease was also reported in bother associated with frequency of urinary incontinence, SUI and unexplained incontinence (p<0.01).
Table 3. Change in bother experienced from baseline to end of treatment using the ICIQ-FLUTS
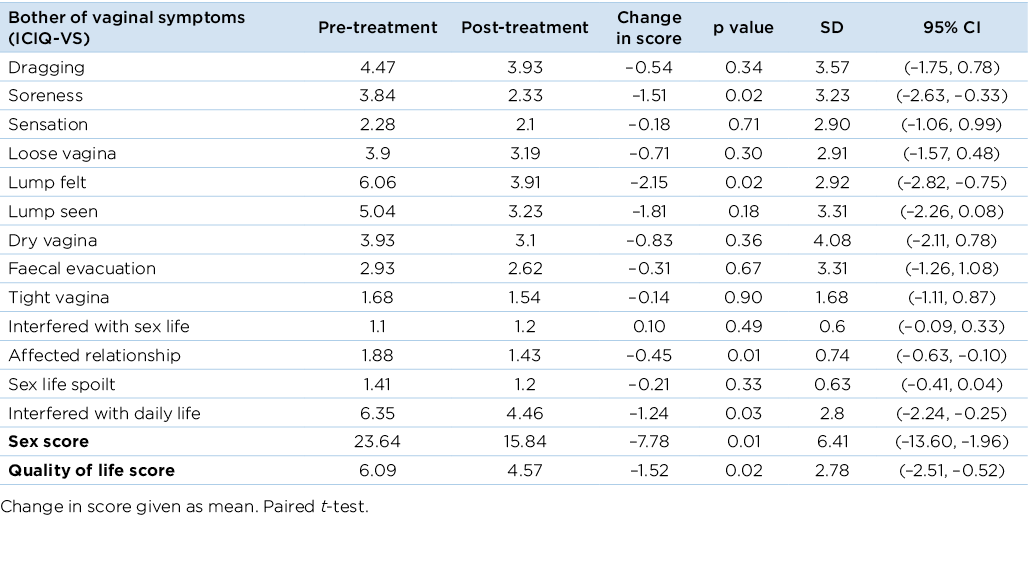
The ICIQ-VS questionnaire scores showed a significant decrease in bother associated with soreness and feeling a lump, and in the effects of vaginal symptoms on relationships and interference with daily life (p<0.05) (Table 4). The ICIQ-VS also elicits information on the effects of POP on sex life and quality of life. The sexual domain score decreased significantly from 23.6 to 15.8 (on a scale of 0–58) (p<0.01), indicating that participants reported fewer negative effects of their vaginal symptoms on their relationships and sex life. Scores showed a significant decrease in negative effects of vaginal symptoms on quality of life, from a score of 6.09 to 4.57 (on a scale of 0–10) (p<0.05).
Table 4. Change in bother experienced from baseline to end of treatment using the ICIQ-VS
Interviews provided in-depth information on how the treatment improved psychosocial dimensions of health. Positive psychological changes were noted by 17/20 interview participants and included improved mental health, positive life outlook, confidence and sense of control. Several participants said the clinic was “life changing”, and they were now encouraging their family and friends to talk about their symptoms so they could also be helped. Participants reported the interventions provided them with a sense of having power over their own healthcare, and confidence that they could manage their symptoms. For example:
I feel a lot happier than before. Now I know what the problem is. The physio came in and explained it to me, so I know what’s going on, so that makes me feel better (Respondent #2).
Going to them has given me tools so I can feel when I need to go to the toilet, I can do the exercises properly… It is so helpful, not having to run my baby to the car because I’ve had an accident or go home and change for the third time. It’s been so good for my confidence (Respondent #7).
Among the responses improved confidence was related to decreased anxiety. Respondents reported that prior to attending the clinic they were anxious they could not be helped or would need surgery but, following treatment, their anxiety had decreased. Five women said they did not want surgery due to risk or underlying conditions; now this anxiety had reduced. For example:
Now I’m in control, I haven’t had accidents since I went to the clinic… I’m happy. I’m not worried about it… It’s taken away the anxiety that I need surgery. It’s great (Respondent #12).
Improved mental health was reported by 7/20 respondents, and reasons reported include ability to exercise and be more social, increased knowledge and awareness of their condition, and having control of their body. For example:
It’s been a life changer for me. I think now, looking back, I was depressed. I can go really badly into it… I realise now that I was going down further. And now, it’s not an issue… I know that if I take the drugs and I do my exercises regularly. And now, I can do ordinary exercise too, walking more, using my tummy muscles (Respondent #13).
We were brought up that your whare tangata [uterus] is sacred, my grandmother would say that, ‘it’s sacred to you, you don’t talk about it’… So we never knew about what can happen. After [my baby was born] I developed depression and anxiety, because it’s like, I can’t even go toilet, I can’t even walk through town… what’s wrong with me… It brought me down. Now it’s a lot better. Since I started going to the clinic, I went to the pub with friends, and I got up and went to the toilet and I was so happy that I could distinguish when I needed to go, I came back and announced it to everyone. I was so happy! And they got it, they all high fived me (Respondent #4).
Interview respondents discussed improvements in social dimensions of health. A theme that emerged in 15/20 interviews was improvements in intimate relationships following treatment. Respondents described the effects shame and embarrassment of incontinence previously had on their quality of life, including being unable to date or be intimate with partners, and the subsequent loneliness, low self-esteem and tensions in their relationships. Improvements in this area were mentioned particularly by younger women, For example:
I haven’t even been on a date for a few years… now I feel like maybe I’ll try again, now things have improved… the improvement I’ve had so far is massive (Respondent #6).
It had got to the point where I stopped having sex, I was just so ashamed of the smell, I couldn’t do it. And [the clinicians] gave me something for that, and it’s fixed it. Now I can have sex, I can be intimate with my partner and not always thinking about it (Respondent #14).
Prior to the clinic treatment, 3/20 respondents reported that they had stopped going out in public or exercising because of the discomfort caused by their symptoms. This strategy of avoiding activities that aggravated symptoms was described as “living a shadow life” (Respondent #11). Improvements in social life following clinic treatment included 4/20 respondents reporting the ability to play with their children, 4/20 reporting they were confident to exercise in public, 4/20 reported they were confident to go out with friends, and 4/20 reported they were able to work longer hours or engage in different work due to a reduction in symptoms. Another psychosocial benefit reported by 5/20 was financial as they reported saving money on incontinence pads and laundry.
Respondents related improvements in psychosocial health to the holistic care provided by the clinic. Notably, all 20/20 reported that an important dimension of the care provided was that they felt listened to. This was even the case for 2/20 respondents who said they did not perceive a reduction in physical symptoms following treatment. Feeling listened to was defined by 20/20 respondents as clinicians providing time for them to tell their story and clinicians understanding their experience of their condition and their point of view. For example:
Before I wasn’t given advice, I was treated as a number and swept away. Now I’m not treated as a number, I’m treated as a human being (Respondent #1).
What I loved the most about [the doctor at the clinic] was she listened to me… I thought this was going to be my life now. I felt listened to. And at ease. Now, it’s better… I feel like I don’t need any more interventions, I don’t need surgery (Respondent #13).
A majority (19/20) of interviewees reported feeling at ease and comfortable at the clinic. The clinicians’ friendly manner, clear communication and smooth handover between physiotherapist and GP was noted as contributing to participants’ comfort. The importance for women to have access to women clinicians was noted and respondents described that their comfort increased over time as the relationship with the clinician was built over multiple clinic visits. For example:
They explain things really well. When I got all emotional and started crying, they were really nice about it. They are holistic. They know that it’s not just physical, that there will be psychological, emotional aspects (Respondent #6).
It is worth noting that the interviewer did not use the language of ‘holistic’ care during the interview, but several interviewees, like the woman quoted above, used this term to describe why they felt the clinic treatment enabled improvements in their biological and psychosocial health.
Areas for service improvement and expansion
Consultation follow-up
Some women experienced discomfort using the pessary, and two of the women interviewed discontinued use. These women said they had experienced discomfort prior to having the pessary removed, but they had not contacted the clinic immediately because they didn’t know if they could do so outside their scheduled appointments. The clinicians did provide follow-up phone calls and appointments. However, because the clinic was a pilot running 1 day per week and there were no permanent staff, it was difficult for patients to contact the clinicians outside the clinic’s scheduled days. Flexibility in the system to enable rapid follow-up appointments if the pessary is not appropriate, and permanent nursing staff available to respond quickly to patient concerns, could enable women to more easily report pain and complications.
Reach of service to Māori communities
Two Māori women in the interview cohort described the benefits of the clinic model and how engagement with Māori could be improved. Suggestions for improving access included working with Māori health practitioners to run holistic clinics at Marae (Māori meeting ground) and in rural areas. For example:
We need education and publicity. Within Māoridom, there are cultural barriers. That area is your whare tangata and it’s sacred. I know Māori women who have really suffered badly rather than having anything done… I’d like to see the clinic grow. And to take it out to Marae, to get to women because they will not say anything… if it could be mobile and out there and people could just go and not have to go with the GP first (Respondent #13).
Rural access
Rural women in the interview cohort reported barriers to care, including lack of access to female GPs they trust in their local area, and the financial and time costs of coming into the city for the clinic. Respondents from rural areas, 5/20, expressed concern about pessary management and monitoring if the clinic closed as they were not aware of local services. For example:
What do I do after this? Because this thing is going to have to be taken out and checked at some stage. Can the GP do that? I’m not sure here [in the countryside]… They get GPs to come in here for a while, but it’s never the same people, I don’t know if they are trained in pessaries, or if there is a woman GP to do it (Respondent #11).
Discussion
The current study finds that a GP/physiotherapist clinic rooted in an integrated, biopsychosocial model of healthcare can be effective at reducing biological symptoms associated with incontinence and POP, and can improve psychosocial dimensions of health. Integrating healthcare provides co-ordinated person-centred care that improves patient outcomes and reduces inefficiencies in the system24. There is a lack of research focusing on the various combinations and interventions for treating POP and incontinence25; however, studies suggest that collaborative integration can improve quality of life and symptoms for women with POP24, as well as increasing adherence to pelvic floor physical therapy26, improving patient satisfaction with public health services and reducing wait times27.
In this study, participants scored significant reduction in physical symptoms associated with incontinence. Index scores for filling symptoms (which is calculated from four symptom scores) and incontinence symptoms (calculated from five symptom scores) also showed a significant decrease. Voiding symptoms, which affected fewer women upon presentation to the clinic, reduced but not significantly. Vaginal symptoms of dragging and soreness decreased significantly, and the vaginal index score (calculated from eight symptom scores) also showed a significant decrease.
The study also found improvements in psychosocial dimensions of health. The 'bother' associated with symptoms reduced significantly in both urinary and vaginal symptoms. The bother scores are important to assess in order to understand participants’ perception of the degree to which their symptoms affect their everyday life. For example, a marked improvement in the bother score where there is no or only slight improvement in the symptomatic score may suggest that women are learning to manage and feeling more in control of their symptoms, even if the condition is not completely resolved. This is reiterated in the qualitative interviews, in which a majority of respondents said the clinic provided them with a sense of having power over their own healthcare, and confidence to manage their symptoms. Psychological improvements reported by respondents including decreased anxiety and a more positive life outlook, and improvements in social life, including improved intimate and social relationships, ability to exercise, ability to work, and financial savings.
The mixed method approach to this study revealed findings that would not be visible through a survey approach alone. The qualitative data suggests that the benefits of the biopsychosocial clinic approach surpassed gynaecological and urological symptom reduction. Respondents reported the clinic helped them with a variety of related symptoms, including constipation, coughing, vaginal dryness, haemorrhoids and other menopausal symptoms, that improved their quality of life. Respondents emphasised the benefits of clinicians listening to them; one respondent described that previous interactions with health professionals made them feel like they were "a number", but now they were treated "as a human being".
It is likely that a number of factors associated with the clinic structure, the specific clinicians involved, and the biopsychosocial treatment approach all enabled this positive perception. It is important for clinicians in an integrated health setting to have excellent communication skills so that their patients feel the providers are working as a team, and trust is built rapidly28. Coordinated care approaches to pelvic healthcare has been shown to increase patient satisfaction27. In this clinic, the physiotherapist and GP worked closely as a team in order to provide coordinated, empathetic care. The structure of the clinic, whereby women were able to have longer appointments and visit the clinic multiple times, also facilitated the building of a trusting relationship with clinicians.
However, barriers to access treatment remain for rural women and for Māori women. Interview participants noted several ideas that could be pursued, including a mobile clinic providing outreach to rural areas and Marae which could reduce costs and time/distance barriers associated with accessing services that are usually in city locations; this could further support the creation of a comfortable atmosphere for women.
This study is limited as it drew on a relatively small sample of 36 women; this must be taken into consideration when interpreting the results. As a pilot study, the sample did not include a control group. The quantitative data also did not capture the long-term effects of the treatment, nor the various treatment pathways (including continued physical therapy, pessary use or surgery) that women continued beyond the clinic.
Conclusion
This study found that an integrated GP/physiotherapy clinic taking a biopsychosocial approach to healthcare can significantly reduce physical symptoms and improve psychological and social dimensions of health for women with POP and incontinence. This model of clinical care could decrease the need for gynaecological surgery, empower women to manage their care, and improve access for Māori and women in rural areas.
Author(s)
Alice Beban*
Senior Lecturer in Sociology, School of People, Environment and Planning, Massey University, Manawatu Campus, Tennent Drive, Palmerston North 4442, New Zealand
Email A.beban@massey.ac.nz
Samantha Newman
General Practitioner with Special Interest in Women’s Health, FemaleGP, Napier, New Zealand
Bernadette Nolan
Pelvic Health Physiotherapist, Hawke’s Bay Pelvic Health and Women’s Physiotherapy, Napier, New Zealand
*Corresponding author
References
- New Zealand Continence Association & New Zealand Carers Alliance. Continence services in New Zealand: history, services, costs and impacts. A call for action paper. 2009. Available from: https://www.continence.org.nz/user_files/Continence_Services_in_New_Zealand_FINAL_Oct_2009.pdf
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 2013;4. Art. No. CD004014.
- Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in US women. Obstet Gynecol 2014;123(1):141–148.
- Faubion SS, Shuster LT, Bharucha AE. Recognition and management of nonrelaxing pelvic floor dysfunction. Mayo Clin Proc 2012;87(2):187–193.
- Fritel X, Varnoux N, Zins M, et al. Symptomatic pelvic organ prolapse at midlife, quality of life, and risk factors. Obstet Gynecol 2009;113(3):609–616.
- Toye F, Barker KL. A meta-ethnography to understand the experience of living with urinary incontinence: ‘Is it just part and parcel of life?’ BMC Urol 2020;20: Art. No 1. doi:10.1186/s12894-019-0555-4.
- Coolen ALWM, Troost S, Mol BWJ, et al. Primary treatment of pelvic organ prolapse: pessary use versus prolapse surgery. Int Urogynecol J 2018;29:99–107.
- Dumoulin C, Cacciari L, Hay-Smith E. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2018;10. Art. No. CD005654.
- Abhyankar P, Uny I, Semple K, et al. Women’s experiences of receiving care for pelvic organ prolapse: a qualitative study. BMC Women’s Hlth 2019;19: Art. No. 45. doi:10.1186/s12905-019-0741-2
- Peake S, Manderson L. The constraints of a normal life: the management of urinary incontinence by middle aged women. Women’s Hlth 2003;37(3):37–51.
- Health Quality Commission. A window on the quality of Aotearoa NZ’s Healthcare 2019; Wellington, NZ. Available from: https://www.hqsc.govt.nz/assets/Health-Quality-Evaluation/PR/Window_2019_web_final.pdf.
- Dawson P, Jaye C, Gauld R, et al. Barriers to equitable maternal health in Aotearoa New Zealand: an integrative review. Int J Equity Hlth 2019;18: Art.No. 168. doi:10.1186/s12939-019-1070-7.
- Jansen P, Bacal K. He Ritenga Whakaaro: Māori experiences of health services. Mauri Ora Associates, Auckland; 2008 Available from: https://www.moh.govt.nz/notebook/nbbooks.nsf/0/2A6CAF401ABBEFB9CC2575F400 0B6D0C/$file/He-Ritenga-Whakaaro.pdf
- Goodwin N, Smith J, Davies A, et al. A report to the Department of Health and the NHS Future Forum: integrated care for patients and populations: improving outcomes by working together. London: The King’s Fund and Nuffield Trust; 2012. Available from: https://www.pcrs-uk.org/sites/pcrs-uk.org/files/files/nuffield_trust_kings_fund.pdf
- Engel, G. The need for a new medical model: a challenge for biomedicine. Science 1977;196(4286):129–136.
- Ghetti C, Skoczylas LC, Oliphant SS, et al. The emotional burden of pelvic organ prolapse in women seeking treatment: a qualitative study. Female Pelvic Med Reconstr Surg 2015;21(6):332–38.
- Moseley GL, Butler DS. Fifteen years of explaining pain: the past, present, and future. J Pain 2015;16(9):807–813.
- Shahid S, Thomas S. Situation, Background, Assessment, Recommendation (SBAR) communication tool for handoff in health care – a narrative review. Safety Hlth 2018;4: Art. No. 7. doi:10.1186/s40886-018-0073-1.
- Bugge C, Dembinsky M, Kearney R, et al. Does self-management of vaginal pessaries improve care for women with pelvic organ prolapse? BMJ 2021;372: Art. No. 310. doi:10.1136/bmj.n310
- Bristol Urological Institute. International Consultation on Incontinence Questionnaire Female Lower Urinary Tract Symptoms; 2021. Available from: www.iciq.net.
- Avery K, Donovan J, Peters TJ, et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodynam 2004;23(4):322–330.
- Nyström E, Sjöström M, Stenlund H, et al. ICIQ symptom and quality of life instruments measure clinically relevant improvements in women with stress urinary incontinence. Neurourol Urodynam 2015;34(8):747–751.
- Price N, Jackson S R, Avery K, et al. Development and psychometric evaluation of the ICIQ Vaginal Symptoms Questionnaire: the ICIQ-VS. BJOG 2006;113(6):700–712.
- Davies N, Burdett T. Would integrating women’s professional care of pelvic organ prolapse improve the symptoms and quality of life: an integrative literature review. J Integr Care 2020;28(2):119–133.
- Mackie S, Darvill A. Factors enabling implementation of integrated health and social care: a systematic review. Br J Community Nurs 2016;21(2):82–87.
- Brown H, Barnes H, Lim A, et al. Better together: multidisciplinary approach improves adherence to pelvic floor physical therapy. Int Urogynecol J 2020;31:887–893.
- Chan M, Schulz J, Flood C, et al. A retrospective review of patients seen in a multidisciplinary pelvic floor clinic. J Obs Gynaecol Canada 2010;32(1):35–40.
- Strandberg-Larsen M, Krasnik A. Measurement of integrated healthcare delivery: a systematic review of methods and future research directions. Int J Integr Care 2009;9(E01). doi:10.5334/ijic.305




