Volume 28 Number 2
Busting for Botox®: an analysis of rebooking methods and delay to reinjection of intravesical Botulinum toxin A
Josh Kealey, Henry H. Yao, Janice Cheng, Helen E. O’Connell and Johan Gani
Keywords urinary bladder, overactive bladder, botulinum, urodynamic
For referencing Kealey J et al. Busting for Botox®: an analysis of rebooking methods and delay to reinjection of intravesical Botulinum toxin A. Australian and New Zealand Continence Journal 2022; 28(2):23-28
DOI
https://doi.org/10.33235/anzcj.28.2.23-28
Submitted 19 October 2021
Accepted 14 March 2022
Abstract
The objective of this study was to determine if the method of rebooking intravesical Botulinum neurotoxin A injections for treatment of detrusor overactivity causes treatment delays. The records of patients diagnosed with urodynamically proven detrusor overactivity treated with Botox® between March 2005 and October 2018 were included in a retrospective multicentre case series. Rebooking method was categorised into: (i) patient-initiated rebooking methods, (ii) doctor-initiated rebooking methods and (iii) automatic rebooking. Primary outcome was the proportion of patients with delay in reinjection >1 month after cessation of effect. A total of 336 patients were included in this study and results showed that 180/336 underwent a second and 122/180 a third cycle of Botulinum neurotoxin A. Patient- reported efficacy ranged from 73–84%, urinary tract infection rate was 8–11% per cycle and de novo urinary retention rate was 8.2–16.1% per cycle. The method of rebooking was patient-initiated in 45% (n=68) of cases and doctor-initiated in 55% (n=83) for the second injection. The rate of delay to retreatment was not clinically significant between the two groups at 33% and 37%, respectively. For those who progressed to a third cycle, the method of rebooking was automated in 11% (n=12) of patients and doctor- or patient-initiated rebooking in 89% (n=97). Automatic rebooking method resulted in a significantly lower rate of delay to injection (8% vs 44%, p=0.026). Significant delays occur in the reinjection of intravesical Botulinum neurotoxin A for detrusor overactivity. These delays can be reduced by utilising an automatic rebooking method once dose and duration of effect are established.
Introduction
Intravesical Botulinum neurotoxin A (BoNT-A) is a well-established therapy for detrusor overactivity1. It has been increasingly employed to treat patients with idiopathic detrusor overactivity with success2,3. Meta-analyses comparing anticholinergic medication, mirabegron and intravesical BoNT-A have shown that BoNT-A is more likely to improve overactive bladder symptoms and continence4.
Botulinum neurotoxin A is formed from the Clostridium botulinum bacteria. It causes flaccid muscle paralysis by inhibiting calcium-mediated release of acetyl-choline vesicles at the pre-synaptic neuromuscular junction5. Neuromuscular blockade is achieved by extracellular glycoprotein binding on cholinergic nerve terminals and blockade of intracellular acetyl-choline secretion. A large prospective multicentre study of 430 patients by Nitti and colleagues demonstrated the sustained efficacy of BoNT-A over several years without increased safety concerns6. Patients in that study displayed fewer urge episodes and micturitions per day compared to placebo and the researchers report a median therapeutic duration of 7.6 months per injection. Sustained benefit with repeat injections has also been demonstrated without any loss of therapeutic effect7,8. Unfortunately, patients may have prolonged wait times to reinjection after symptoms return, as was indicated by Veeratterapillay and colleagues9. This delay between return of symptoms and reinjection of intravesical BoNT-A likely has a significant impact on the quality of life of patients as detrusor overactivity returns.
Following the first injection, the duration of effect is unknown. Patients are usually followed up at 1–2 weeks with a voiding flow rate and post-void residual, and then again at 3 months to determine the efficacy of BoNT-A. Depending on these findings, the patient and clinician will decide whether to proceed with subsequent doses. At this stage, the patient either returns for regular reviews and is booked for the subsequent injections when effect wanes or calls to inform of cessation of effect. In the Australian public health system it is difficult for patients to inform clinicians when effect wanes as patients rarely have a sole treating clinician. Following the second dose of BoNT-A, the duration of efficacy is known and patients can be rebooked for the next dose at set intervals. This is a standard practice in many institutions around the world.
This multi-institutional, multi-surgeon retrospective observational cohort study aims to determine whether differing rebooking methods have an impact on the delay of subsequent BoNT-A injections.
Material and Methods
Medical records were reviewed in this retrospective multi-centre and multi-surgeon case series involving three urological centres performing high volumes of intravesical BoNT-A (one multi-site public health system and two private practices) in an operating theatre under anaesthesia. Each participating site contributed their entire intravesical BoNT-A database to the study. The records of patients who underwent first treatment of intravesical BoNT-A (Botox ®) between March 2005 and October 2018 were included. Ethics approval was obtained from Western Health, Melbourne, VIC, Australia HREC (HREC/20/WH/54761) to conduct this study. Patient information was deidentified and consent for inclusion in this retrospective analysis was not specifically sought.
All patients who underwent intravesical Botulinum toxin for urodynamically proven detrusor overactivity in the study period were included. Patients were excluded from analysis if efficacy, duration of effect or rebooking method were unclear. Decision regarding rebooking method was collaboratively decided upon by clinicians and patients after efficacy had been established. Patients were given the option for doctor- vs patient-initiated rebooking. Whilst the specific factors that determined patient rebooking method were not specifically documented, decision making around this took into account individual patient's health literacy, ease of attending appointments and patient preference. More complex patients who required more frequent reviews would likely have undergone doctor-initiated rebooking at these reviews.
Clinicopathological data were retrospectively collected from medical records and included aetiology for overactive bladder, date of intravesical BoNT-A injection, dose used, urinary tract infection (UTI) rates, urinary retention rates, efficacy rates, duration of efficacy, and method of rebooking for further treatment. Efficacy of BoNT-A was determined from a combination of postoperative questionnaires Incontinence Quality of Life questionnaire (I-QOL), self-reported urinary incontinence questionnaires including the International Consultation on Incontinence Questionnaire–Urinary Incontinence (ICIQ-UI) and routine practice follow-up within 6 weeks of injection. Duration of effect was determined from regular questionnaire use, patient-initiated contact and practice nurse follow-up. Cases were categorised into three groups – no efficacy (no improvement in symptoms), partial efficacy (improved but incomplete resolution of symptoms) or full efficacy (achieved complete resolution of symptoms). Patients whose efficacy could not be assessed were excluded from analysis.
Following the initial treatment with BoNT-A, patients were offered two methods of rebooking for subsequent cycles. The first method is patient-initiated rebooking when efficacy has subsided. The second method is doctor-initiated rebooking and requires interval-based assessment of the ongoing efficacy of BoNT-A at timed interval telephone or in-person appointments – these were completed at least once at the 6–9-month mark post-injection.
Following the second cycle, the duration of efficacy is known, and the rebooking method was categorised into an automated rebooking based on cycle 1 duration of efficacy versus patient- or doctor-initiated rebooking method as above depending on patient preference. An elective operating booking form with a date was completed and a hospital booking was made for the approximate duration of effect from cycle 1. Patients who were rebooked automatically were reviewed pre-operatively on the day of procedure to ensure that further BoNT was indicated and they had the ability to contact the health provider and delay their treatment if required.
The primary outcome of this study was the presence of delay in receiving subsequent doses of intravesical BoNT-A treatment. For the purposes of this study we have defined delay in treatment as greater than 1 month of symptoms returning before receiving subsequent doses. This timeframe was chosen to allow for peri-operative scheduling to occur prior to treatment.
Statistical analyses were performed using SPSS® 25 (SPSS Inc, Chicago, USA). Chi-squared and Fisher’s exact test were used compare rates and proportions.
Results
Patient demographics
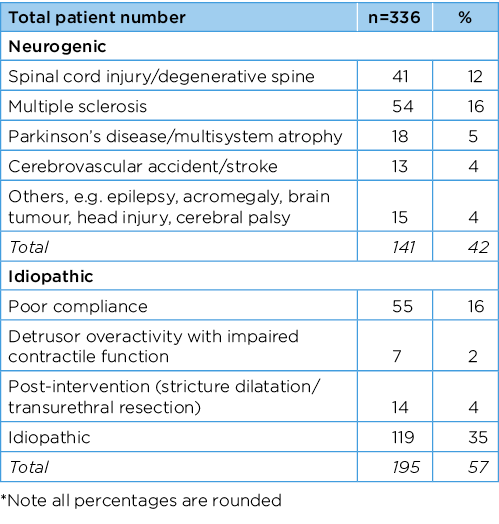
A total of 336 patients who underwent initial intravesical BoNT injection were included in this study. The median age was 63 years old (IQR 47–72). Of the 336 patients, 235 (70%) were female and 99 (30%) male. Table 1 shows the aetiology of detrusor overactivity identified for all patients included in the study.
Table 1. No. patients who received BoNT with idiopathic and neurogenic aetiologies
Efficacy and complication rates
Of patients who underwent initial intravesical BoNT-A injection, partial or complete efficacy was reported in 285/336 (84%) patients. A majority of patients 250/336 (74%) received 100 units of BoNT-A, 41/336 (12%) received 200 units and 36/336 (11%) received 300 units. Of the 336 patients, UTI was documented in 36/336 (11%) of patients. De novo urinary retention was documented in 54/336 (16%) of patients, with another 54/336 (16%) of patients dependent on permanent catheterisation or intermittent self-catheterisation prior to treatment. Prior to treatment all patients were counselled regarding the risk of urinary retention post-treatment.
Of the patients who progressed to a second cycle of BoNT-A, 150/180 (83%) of patients reported partial or complete efficacy. Compared to the first cycle, only 111/180 (62%) of patients received 100 units of BoNT-A, 32/180 (18%) received 200 units and 32/180 (18%) received 300 units. UTI was documented in 15/180 (18%) patients following the second dose. De novo urinary retention was documented in 18/180 (10%) patients, with another 43/180 (24%) dependent on permanent catheterisation or intermittent self-catheterisation pre-treatment.
Of patients who progressed to a third cycle, 89/122 (73%) reported partial or complete efficacy. Doses of BoNT-A for the third cycle were more varied, with 56/122 (46%) patients receiving 100 units, 28/122 (23%) receiving 200 units, 35/122 (29%) receiving 300 units and 1/122 (1%) receiving 400 units. De novo urinary retention was reported in 10/122 (8%) patients with 39/122 (32%) dependent on permanent catheterisation or intermittent self-catheterisation pre-treatment.
Rates of delay to booking
Of patients rebooked for a second injection, there was no clear documentation of return of symptoms for 28/180 (16%) cases and these were excluded from analysis. The method of rebooking was not known in 4/180 (2%) cases and these were also excluded from analysis. Of the remaining cases, the method of rebooking was for patient-initiated rebooking in 68/151 (45%) cases and doctor-initiated rebooking in 83/151 (54.9%) cases. Given that the duration of intravesical BoNT-A is not known after the first cycle, automatic rebooking was not utilised. There was no statistical difference in the rate of delay to subsequent doses regardless of whether the method of rebooking was patient-initiated (32%) versus doctor-initiated (37%). Figure 1 indicates the initial rebooking method that was utilised and the rates of delay to reinjection.
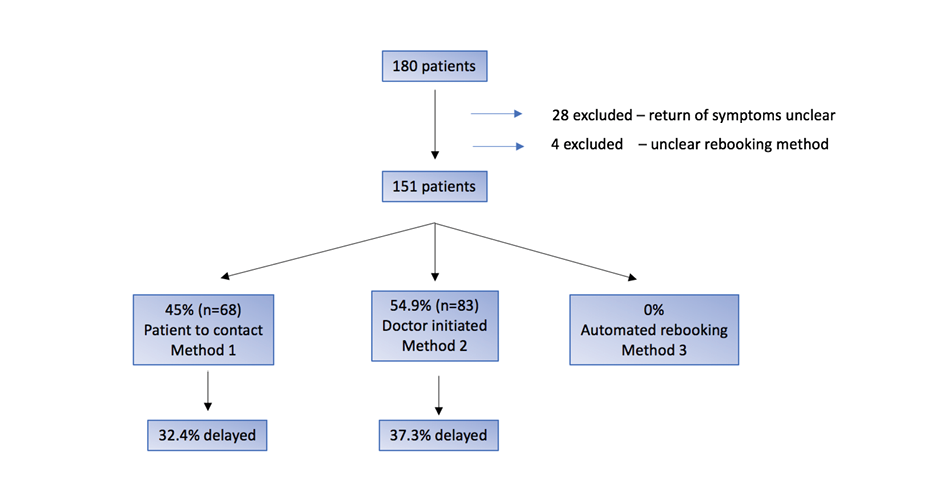
Figure 1. BoNT-A cycle 2 rebooking method and delay to reinjection
Note: This figure compares the rate of delay for automated rebooking methods and other rebooking methods after recurrence of symptoms after cycle 1 of intravesical BoNT-A.
Of the patients who proceeded to a third cycle, 109/122 (89%) had documented symptom return and a known rebooking method. Of these cases, 12/109 (11%) were enrolled in an automated rebooking program. The remaining 97/109 (89%) patients underwent patient- or doctor-initiated rebooking after detection of return of symptoms. The rate of delay to subsequent dose of intravesical BoNT-A was significantly lower for patients who were automatically rebooked compared with patient- or doctor-initiated rebooking at 1/12 (8%) vs 43/109 (44%), p=0.026, Fisher’s exact test. Figure 2 compares patient- or doctor-initiated rebooking to automatic rebooking and the associated rates of delay. There was no significant difference between doctor- and patient-initiated rebooking in cycle 2. The median delay in for those who experienced a significant delay was 19 weeks. There was no significant difference in aetiology of detrusor overactivity in patients who utilised an automatic booking system.
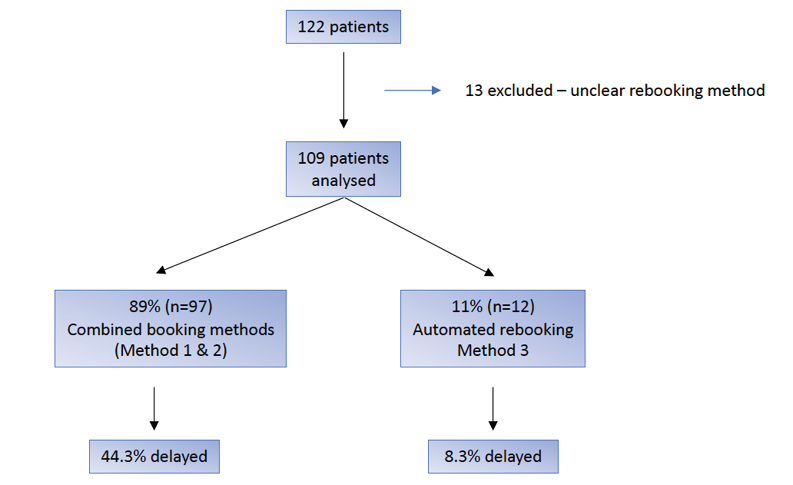
Figure 2. BoNT-A cycle 3 rebooking method and delay to reinjection
Note: This figure compares the rate of delay for automated rebooking methods and other rebooking methods after recurrence of symptoms after cycle 2 of intravesical BoNT-A
Discussion
This case series has shown that automatic rebooking methods for intravesical BoNT-A show a significant reduction in treatment delays for those with detrusor overactivity. This reduction in treatment delay likely corresponds to a reduction in often debilitating symptoms that accompany detrusor overactivity and therefore an improvement in quality of life. Many urology practices across the globe already adopt this strategy for booking patients for subsequent doses of BoNT-A. The results of this study should prompt practices not using this strategy to consider incorporating the practice of automatic rebooking for patients after the second dose of BoNT-A.
The efficacy rate of 84.8% reported in this study is consistent with the 86% reported by previous studies of intravesical BoNT-A9 . Previous studies report BoNT-A has a median duration of effect of 33 weeks; however, time to return of symptoms can be highly variable between patients6. This makes automatic rebooking for every patient at the outset challenging as many will be over- or under-treated based on their individual response duration. Despite multiple studies identifying duration of effect between 7–12 months, inter-injection times are often far greater than this and likely leave patients with the return of often debilitating symptoms prior to retreatment10,11. Veeratterapillay and colleagues reported a mean interval between first and second injections of 17.6 months, approximately twice the expected duration of effect9,10.
However, there is a scarcity of literature regarding the important issue of delay to reinjection. Baron and colleagues investigated patient factors associated with non-repetition of treatment and found that, despite an acceptable therapeutic effect, patient may not return for treatment due to time constraints, treatment discomfort and minimising outpatient appointment8. Whilst there is no definitive evidence that identifies the barriers to BoNT-A reinjection, some researchers have concluded barriers may be similar to those around not accessing other incontinence treatments and rebooking methods12,13.
The results in this case series demonstrate that both patient- and doctor-initiated rebooking results in significant delays. The median was 19 weeks, indicating patients are likely to suffer for some time when symptoms return before re-treatment. There are several hypothetical reasons for each method leading to significant delays. Patient-initiated booking relies on the patient’s ability to effectively contact the health service. Possible impediments to this include cognitive impairment and difficulty negotiating clinic or hospital systems to reach their treating clinician14. Additionally, some patients may be reluctant to contact their treating clinician, believing this bothersome to the clinician or placing pressure on the health system15. Patients may also misconstrue the nature of the treatment, not comprehending that re-treatment is the norm. Doctor-initiated rebooking relies on timely follow-up appointments to book reinjections. In the public healthcare system, rebooking follows a treat-in-turn policy and is prioritised against conditions likely to be associated with emergencies. In Victoria, Australia, time from booking to operation in non-urgent operations was 17–212 days in 201916.
The requirement for face-to-face review also allows for several barriers to impede swift review and rebooking including access to hospital grounds, mobility and geographical distance that may preclude patients from attending17. With increasing use of Telehealth, in the setting of the current COVID‑19 pandemic, an increase in phone and video consultations may help alleviate these issues. As there is no demonstrated difference in the rates of delay to BoNT-A between patient- and doctor-initiated rebooking, clinicians should choose the method of rebooking subsequent injection of BoNT-A based on the patient factors discussed previously.
Once the duration of efficacy is established, rebooking can be automated. This study demonstrates that an automatic booking system is associated with less delay to reinjection compared with a patient- or doctor-initiated rebooking. This reduction in delay to treatment translates to shorter periods of recurrent overactive bladder symptoms prior to retreatment. This foreseeably equates to an improved quality of life, as these patients often have severe and refractory overactive bladder symptoms. An automatic rebooking system may also reduce the requirement for outpatient appointments, leading to a decreased burden on patients and the healthcare system.
However, care needs to be taken in pre-operative assessment to ensure no significant health changes have occurred between scheduled treatments that would preclude or change treatment. This pre-operative assessment is an opportunity to determine dosage and timing adjustments. If an operative delay was required then a new set date could be organised at the time of delay.
Limitations to this study include the retrospective nature and the small proportion of patients with incomplete clinical records which can impact the outcomes assessed in this study. Secondly, the number of cases enrolled in the automated rebooking program was small, which may indicate established referral patterns or clinician hesitancy to utilise this method. The drop-out rate between cycles was also high, despite efficacy above 80%, indicating multiple other factors impacting ongoing treatment. Finally, the non-randomised nature of this study may be subject to selection bias. Patient- and disease-related factors including health literacy, age, severity and geographical location may all have affected clinician choice of rebooking method. A prospective comparative study to confirm the findings of this study is warranted and may also assess other methods of minimising delay including dedicated BoNT or functional urology clinic utilisation.
Conclusion
The findings of this case series suggest that an automatic rebooking of patients for subsequent treatment of intravesical BoNT-A results in a significant reduction in the proportion of patients experiencing delay to BoNT-A treatment. We propose that, to reduce treatment delays, clinicians consider the introduction of an automated booking program that alerts when a patient is due to be rebooked for their next treatment of BoNT-A based on their previous duration of efficacy.
Author(s)
Josh Kealey
Urology Registrar, Master of Surgery Candidate,
Eastern Health Clinical School, Monash University, Melbourne, VIC, Australia
Henry H. Yao
Urologist, Department of Urology, Western Health, University of Melbourne, Melbourne, VIC, Australia
Janice Cheng
Urologist, Department of Urology, Western Health, University of Melbourne, Melbourne, VIC, Australia
Helen E. O’Connell
Urologist, Department of Urology, Western Health, University of Melbourne, Melbourne, VIC, Australia
Johan Gani
Urologist, Department of Urology, Western Health, University of Melbourne, Melbourne, VIC, Australia
*Corresponding author
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
References
- Dykstra DD, Sidi AA, Scott AB, et al. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139(5):919–22.
- Duthie JB, Vincent M, Herbison GP, et al. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database of Systematic Reviews, 2011 Issue 12. Art. No.: CD005493.
- Herschorn S, Gajewski J, Ethans K, et al. Efficacy of botulinum toxin A injection for neurogenic detrusor overactivity and urinary incontinence: a randomized, double-blind trial. J Urol 2011;185(6):2229–2235.
- Drake MJ, Nitti VW, Ginsberg DA, et al. Comparative assessment of the efficacy of onabotulinumtoxinA and oral therapies (anticholinergics and mirabegron) for overactive bladder: a systematic review and network meta-analysis. BJU Int 2017;120(5):611–622.
- Dressler D, Saberi FA. Botulinum toxin: mechanisms of action. Eur Neurol 2005; 53(1): 3–9.
- Nitti VW, Ginsberg D, Sievert KD, et al. Durable efficacy and safety of long-term onabotulinumtoxinA treatment in patients with overactive bladder syndrome: final results of a 3.5-year study. J Urol 2016;196(3):791–800.
- Owen RK, Abrams KR, Mayne C, et al. Comparison of the effectiveness of repeated injections of onabotulinum toxin A for refractory idiopathic detrusor overactivity: analysis of an open label extension of a randomized trial (the RELAX study). Neurourol Urodynam 2017;36(4):1201–1207.
- Baron M, Payronet B, Aublé A, et al. Long-term discontinuation of botulinum toxin A intradetrusor injections for neurogenic detrusor overactivity: a multicenter study. J Urol 2019;201(4):769–776.
- Veeratterapillay R, Harding C, Teo L, et al. Discontinuation rates and inter-injection interval for repeated intravesical botulinum toxin type A injections for detrusor overactivity. Int J Urol 2014;21(2):175–8.
- Dowson C, Khan MS, Dasgupta P, et al. Repeat botulinum toxin-A injections for treatment of adult detrusor overactivity. Nat Rev Urol 2010;7(12):661–7.
- Eldred-Evans D, Sahai A. Medium- to long-term outcomes of botulinum toxin A for idiopathic overactive bladder. Ther Adv Urol 2017;9(1):3–10.
- Borello-France D, Burgio KL, Goode PS, et al. Adherence to behavioral interventions for stress incontinence: rates, barriers, and predictors. Phys Ther 2013;93(6):757–773.
- Horrocks S, Somerset M, Stoddart H, et al. What prevents older people from seeking treatment for urinary incontinence? A qualitative exploration of barriers to the use of community continence services. Fam Pract 2004;21(6):689–696.
- van Gaans D, Dent E. Issues of accessibility to health services by older Australians: a review. Public Health Rev 2018;39:20–20.
- Norton JM, Dodson JL, Newman DK, et al. Nonbiologic factors that impact management in women with urinary incontinence: review of the literature and findings from a National Institute of Diabetes and Digestive and Kidney Diseases workshop. Int Urogyn J 2017;28(9):1295–1307.
- Australian Institute of Health and Wellbeing. AIHW national elective surgery waiting times data collection. 2020 [cited 2020 Jul 19]. Available from: https://www.aihw.gov.au/reports-data/myhospitals/intersection/access/eswt.
- Fradgley EA, Paul CL, Bryant J. A systematic review of barriers to optimal outpatient specialist services for individuals with prevalent chronic diseases: what are the unique and common barriers experienced by patients in high income countries? Int J Equity Health 2015; 14: Art. No. 52.


