Volume 29 Number 1
A multidisciplinary approach to incontinence-associated dermatitis in a rural setting: a case study
Catherine Leahy, Suud Nahdi, Michelle Lai, Fiona Coyer, Michelle Barakat-Johnson
Members of the IMBED Project Steering Committee
Licensed under CC BY 4.0
Keywords Incontinence-associated dermatitis, incontinence, case study, plan of care
For referencing Leahy C et al. A multidisciplinary approach to incontinence-associated dermatitis in a rural setting: a case study. Australian and New Zealand Continence Journal 2023; 29(1):10-15.
DOI
https://doi.org/10.33235/anzcj.29.1.10-15
Submitted 19 June 2022
Accepted 23 January 2023
Abstract
Incontinence-associated dermatitis (IAD) is a common complication often unrecognised and mistreated in individuals with incontinence. It is the erosion of the skin from prolonged exposure to urine and/or faeces from incontinence and is often mistaken for a pressure injury. People experience considerable discomfort such as pain, burning and itching in the affected areas (buttocks, perineum and gluteal clefts). The prevention and management of IAD involves assessing and managing incontinence and implementing a skin care regimen to protect and maintain the integrity of the person’s skin. We report a case study of a 71-year-old female to illustrate the benefits of an interdisciplinary plan of care for IAD to prevent further complications. The woman at the centre of our case study had multiple co-morbidities and severe urinary stress incontinence prior to her admission. A multidisciplinary team – continence nurse practitioner (NP), wound clinical nurse consultant (CNC), occupational therapist (OT) – were engaged to devise a management plan to manage her incontinence, treat the IAD and improve skin integrity. The purpose of this case study is to present their treatment journey through a regional health service, the issues encountered, and the prevention and management plans implemented in order to highlight best practices and the benefits of an interdisciplinary plan of care.
Introduction
Incontinence-associated dermatitis (IAD) is skin erosion/damage that occurs when exposed to urine, faeces or both for prolonged periods1. Symptoms of IAD may include significant pain, discomfort and itching in the affected areas such as the buttocks, perineum and gluteal clefts2,3 which may result in reduced quality of life, including loss of independence, depression and sleep disturbance4–7.
The prevalence of IAD is reported to be 8%8 among residents of residential aged care facilities and, in the acute setting, prevalence is reported to be 19%8, 29%9 and even as high as 45.7% among adult patients who have urinary or faecal incontinence in acute care facilities3. In accordance with the best practice guidelines, prevention and management of IAD should consist of appropriate assessment and management of incontinence, and the implementation of a structured skin care regimen1.
The aim of this case study is to present the journey of a woman with incontinence and IAD through a regional health service, the issues encountered, and the prevention and management plans implemented in order to highlight best practices in the prevention and management of IAD. This regional health service offers a nurse-led virtual consultancy service to support rural and remote clinicians within the local health district, including for wound prevention and management and continence management. Clinical reviews and assessments are conducted via telehealth.
Ethical approval for this case study was provided by the Royal Prince Alfred Hospital Human Research Ethics Committee (2019/ETH08742). Written informed consent was provided for the case details to be reported. Written consent was provided for the organisation’s clinical archive of images to be published.
Case presentation
Margaret (pseudonym), a 71-year-old female living in a small remote town in New South Wales, Australia, was admitted to a regional hospital and diagnosed with intertriginous dermatitis and bacterial cellulitis on her abdominal pannus. Margaret had experienced heavy urinary incontinence for over 30 years for which she had sought treatment for in the past. On admission to hospital, Margaret was enrolled as a participant in a multi-site funded implementation science study that focused on implementing the best practice guidelines on the prevention and management of IAD across NSW10.
Medical history
Margaret’s medical history and comorbidities included urinary stress incontinence, high body mass index (BMI) of 42, poorly controlled insulin dependent diabetes mellitus, bilateral knee osteoarthritis, hypertension, gastroesophageal reflux disorder, previous pulmonary embolism and gout. Table 1 lists the multiple medications Margaret was taking to manage these conditions. Margaret was ambulant and independent using her four-wheel walker, but her activities of daily living (ADLs) were restricted due to her body habitus and bilateral knee osteoarthritis.
Environment
She lived alone in social housing, in a single storey home where she had access to a toilet in the home. Due to the severity of the incontinence, the floors and her mattress were soaked with urine. The only person she regularly encountered was her professional carer who assisted her with ADLs such as personal care, including hygiene and shopping. At the time of her admission to hospital, Margaret was in the process of finding alternative accommodation.
Incontinence profile
Margaret’s urinary stress incontinence began when she was aged in her twenties, post-childbirth. She raised her incontinence issues with a number of her general practitioners (GPs) over the years but said her concerns were dismissed, and she did not pursue this further due to embarrassment and a belief that it was normal and that she could not seek help beyond her GP. Margaret described it as if the “flood gates” would open each time she stood up and then leakage occurred when she sat down, coughed or sneezed. The heavy urinary incontinence led to urine soaking her mattress and carpet in the house. She had tried many continence aids such as pull up padded pants, wrap around pads and pad liners. Her BMI and body habitus made it challenging to find a correct fitting continence aid. She therefore relied on liners. As a result of this, she was fearful of leaving the house and was socially isolated, she did not go out to shop or get a haircut and did not attend family gatherings due to fear of others smelling her body odour from her urinary incontinence. She did not have any formal diagnosis of depression.
When asked about incontinence management at home, Margaret and her carer said the liners used in the pads were ineffective as urine still trickled down her legs. Margaret accessed the continence assistance payment scheme (CAPS), an Australian government financial support scheme for patients with severe incontinence.
Upon further review by the continence nurse practitioner (NP), Margaret was found to be constipated. She reported episodes of faecal incontinence, once or twice per month, in the past. A mid-stream urine (MSU) collection and culture were analysed and revealed a Candida albicans urinary tract infection, which is one of the most common causes of nosocomial fungal urinary tract infections11.
Skin integrity profile
Margaret's skin was assessed on admission and revealed:
- Moisture-associated skin damage (MASD) under her breasts, abdominal pannus and underarms.
- A stage two pressure injury (PI12) on her sacrum.
- A stage one PI on each buttock.
A stage two PI involves partial-thickness skin loss into but no deeper than the dermis; stage one PIs are characterised by non-blanchable erythema and the skin is intact13.
Bacterial cellulitis, which was confirmed via culture, was found in the skin fold of her abdominal pannus with satellite erythema. In the area her skin was hot to touch, oedema was present, and micro lesions were noted with weeping serous fluid. Margaret complained of pain, tenderness and an itch in the area.
Margaret was referred to the wound clinical nurse consultant (CNC) for virtual review while she was in hospital. This service is a nurse-led consultancy service that provides wound prevention and management outreach and support to rural and remote health facilities within the local health district.
The wound CNC determined that Margaret did not have PIs but instead had IAD on each buttock and the sacral region. The IAD on the buttocks extended down her thighs to her knees and was diagnosed as a category 1A (redness without infection)14. On the sacrococcygeal region, IAD was diagnosed as a category 2A (broken skin and without signs of infection)14 with linear lesions noted. Figures 1 and 2 show examples of the two IAD categories from the clinic archives. Margaret was confirmed to have intertriginous dermatitis underneath her breasts, underarms and abdominal pannus. An example of intertriginous dermatitis under the abdominal pannus from the clinic archives is presented in Figure 3.
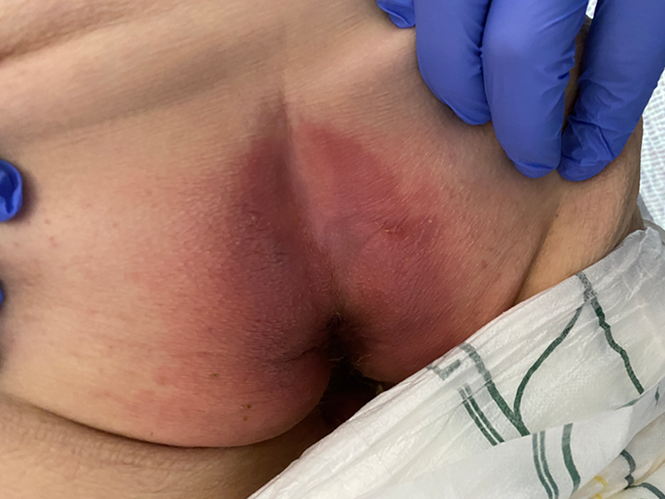
Figure 1. Example of IAD category 1A on bilateral buttocks
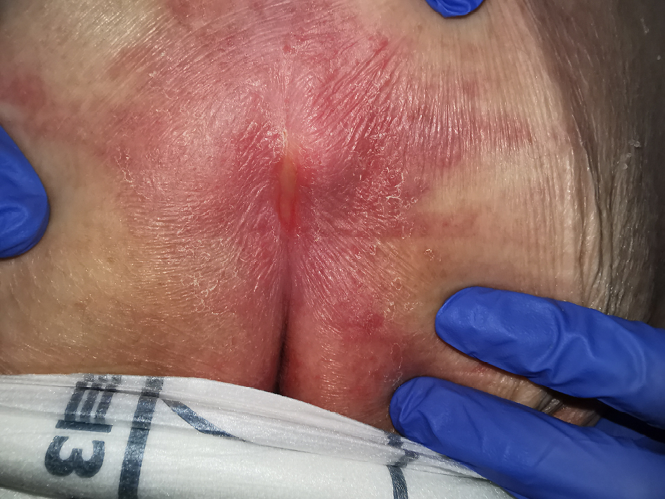
Figure 2. Example of IAD category 2A on sacrum
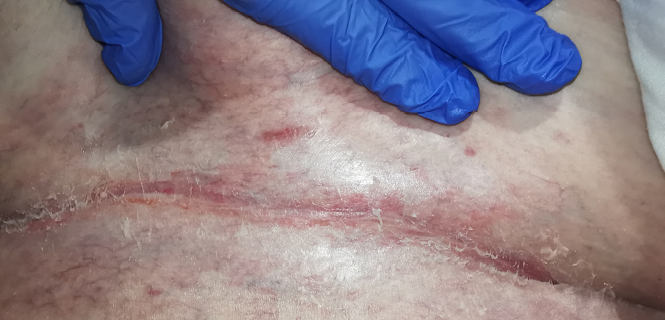
Figure 3. Example of intertriginous dermatitis under abdominal pannus
Management
Physical and functional assessment was undertaken by a multidisciplinary team comprising of a wound CNC and continence NP, occupational therapist (OT) and Margaret’s GP. The wound CNC, continence NP and OT provided consultation via telehealth. The multidisciplinary team developed a care plan to treat infection, manage incontinence and address mobility and self-care issues.
Incontinence management
The continence NP found that the extra large wrap around incontinence pads supplied did not fit and layers were used on the chair and bed to 'catch' the overflowing urine. The CAPS support package level was deemed insufficient to cover all her incontinence product needs and further financial support was sourced from other agencies. The extra funding allowed her to acquire bariatric wrap around pads that fitted correctly to provide social continence and improve her skin condition, and which had a positive effect on her quality of life, including feeling comfortable when leaving the house.
There was a structured toileting regimen implemented as an intervention to manage her urinary incontinence. This involved going to the toilet every 2 hours to empty her bladder. To address the issue of constipation and episodes of faecal incontinence, the continence NP and the wound CNC recommended implementing an individualised bowel toileting regimen, twice daily. The C. albicans urinary tract infection was managed with oral antifungal medication.
An OT review via telehealth occurred 6 days after admission to assess her mobility, including gait and use of mobility equipment and aids. The focus of that review was on her mobilising to the toilet and pelvic muscle assessment and exercises to address the stress urinary incontinence. The OT also recommended using a pressure relieving cushion and air mattress for PI prevention during her inpatient stay.
Skin integrity management
The wound CNC’s treatment plan involved:
- A daily nursing assessment involving visual and tactile inspection of her skin.
- Twice daily cleansing and protecting of the areas using barrier cream cloths to protect and restore her skin (the barrier cream cloths used were impregnated with 3% dimethicone to provide all-in-one skin cleansing, moisturising, deodorising treatment and barrier protection).
- Application of thin, absorbent sheets designed to absorb skinfold moisture, minimise friction, and keep moisture away from the skin; these were placed between the abdominal pannus skin folds and were changed daily or when required.
- Additionally, the intertriginous dermatitis under Margaret’s breasts, underarms and pannus were managed with the application of hydrocortisone 1% and clotrimazole 1% cream three times daily.
- During her in-patient stay, Margaret was initially administered intravenous antibiotics (Ceftriaxone), and then, after 4 days, transitioned to oral antibiotics (Cephalexin) to manage the bacterial cellulitis. The misdiagnosed PIs were managed with the application of hydrocolloid dressings on the sacrum and both buttocks. A hydrocolloid dressing is a dressing that provides a moist and insulated healing environment.
This plan of care is in line with the best practice guidelines for the management of IAD1,10, and included recommending super absorbent polymer (SAP) pads be placed underneath Margaret as needed but not daily. For further prevention and management of Margaret’s intertriginous dermatitis and IAD, barrier cream cloths were used during personal care in the pelvic region and skin folds and continued post-discharge to prevent IAD recurring.
Discharge planning and ongoing care
Margaret was in hospital for 5 days by the time her care plan was in place. The care plan was in place for 10 days until she was discharged. Overall, Margaret's length of stay in hospital was 15 days.
The multidisciplinary team commenced discharge planning at the beginning of Margaret’s admission. Nursing staff referred Margaret to the local community nursing service and made other referrals to link her into non-government organisation carer support. The continence NP liaised with Margaret and her carer to ensure that appropriate continence funding and supply and product selection for aids and skin hygiene were in place. The wound CNC provided consumer and carer skin integrity education to ensure continuity of care upon discharge. The OT conducted a virtual home assessment and worked with social services to arrange alternative, stable, independent and long-term accommodation. Margaret’s GP provided ongoing support and care upon discharge to arrange specialist urology and plastic surgeon reviews into the future.
Upon discharge, Margaret’s intertriginous dermatitis and IAD were resolved, and she did not develop any PIs. The IAD resolved within 3 days following the initiation of her management plan. Margaret described significant improvement of her stress urinary incontinence, skin condition and quality of life and was successful in finding a new housing option to move into.
A few months after the resolution of Margaret’s abdominal cellulitis, she was referred to a urology service in a larger regional centre and plastic surgeon in Sydney for further assessment of the cause of her urinary incontinence. Margaret required plastic surgeon consultation to address her weight and volume of excess skin prior to undergoing corrective uterine prolapse surgery. Subsequently, she was diagnosed with a urinary prolapse and urinary retention. The recommendation was for Margaret to lose weight and undergo an abdominoplasty and apronectomy before correction of the prolapse.
Discussion
Incontinence and IAD management can be problematic for clinicians and patients alike; Margaret’s case is an exemplar of this. Many people are severely affected by incontinence for years and, because of stigma, embarrassment and fear of odour associated with the condition, can suffer social isolation and lack of access to healthcare15–17. In Margaret’s case, psychosocial factors such as lack of independence and unstable housing impacted on the management of her incontinence and hospital discharge planning. These psychosocial factors (not specifically addressed in our case study) must be addressed by a multidisciplinary team that includes a social worker and other community services to assess social conditions that can affect the management and prevention of IAD and then develop an appropriate management plan.
Margaret’s case highlights the benefit of multidisciplinary expert clinical assessment and management of incontinence to prevent the severe complications of IAD and PI. The initial misdiagnosis of the IAD as a PI highlights a need for further clinician education on the differences between the two conditions as misdiagnosis is common4,18–20 and can lead to incorrect or delayed management, prolonging pain and discomfort3,18. A recent study reported major gaps in current practice, including clinician knowledge of IAD21, its identification, prevention and management.
Prevention of IAD requires the correct assessment and management of incontinence; this involves identifying and addressing any reversible causes of incontinence such as urinary tract infection, constipation or medications1. To manage incontinence, measures such as individualised toileting plans or use of continence containment pads, which in Margaret’s case was in place, could be implemented to reduce or avoid episodes of incontinence1. Toileting plans can establish when and how frequently patients should be toileted and can be tailored to a patient’s abilities and needs such as their mobility and nutritional status22. Margaret’s access to the toilet was further enhanced by engaging with mobility exercises under the guidance of the OT.
Other steps which contributed to maintaining Margaret’s skin integrity included minimising the bed protection layers and using an SAP bed pad, when necessary, as well as the use of correct sized continence aids which prevented urine leakage. These measures improved the microclimate of the skin23. Skin surface microclimate includes temperature and moisture. Exposure to moisture, such as urine and faeces, can lead to MASD due to inflammation of the epidermis and dermis4. Exposure to urine and faeces results in hyperhydration of the skin and increased skin pH, lowering tissue tolerance2. Faeces contain enzymes, intestinal flora and moisture which are harmful to the skin2. The regular use of barrier cream cloths improved healing of Margaret’s existing IAD compared with the zinc oxide cream and dressings that were previously applied to Margaret’s skin.
Conclusion
This case study reports an interdisciplinary best practice plan of care for the prevention and management of IAD. It demonstrates how coordinated efforts can improve outcomes by tackling multiple factors affecting the treatment of incontinence, mobility and skin integrity. This case study highlights the benefit of using best practice evidence in the assessment, prevention and management of IAD and the steps to be integrated into clinical practice to improve outcomes for patients with IAD.
Acknowledgements
We acknowledge Ivanka Komusanac, Executive Director of Nursing and Midwifery Services, Sydney Local Health District, executive sponsor of the IMBED study. We also acknowledge all members of the IMBED Project Steering Committee, IMBED Clinical Expert Group and IMBED Post-Research Implementation Advisory Committee.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Catherine Leahy
Quality, Clinical Safety and Nursing, Western New South Wales Local Health District, NSW, Australia
Suud Nahdi
Sydney Medical Program, Faculty of Medicine and Health, The University of Sydney, NSW, Australia
Michelle Lai
School of Nursing and Midwifery, Faculty of Medicine and Health, The University of Sydney, NSW, Australia
Skin Integrity, Nursing and Midwifery Executive, Sydney Local Health District, NSW, Australia
Fiona Coyer
School of Nursing, Midwifery and Social Work, The University of Queensland, QLD, Australia
School of Nursing, Faculty of Health, Queensland University Technology, QLD, Australia
Intensive Care Services, Royal Brisbane and Women's Hospital, QLD, Australia
Michelle Barakat-Johnson*
School of Nursing and Midwifery, Faculty of Medicine and Health, The University of Sydney, NSW, Australia
Skin Integrity, Nursing and Midwifery Executive, Sydney Local Health District, NSW, Australia
School of Human and Health Sciences, University of Huddersfield, United Kingdom
Patient Safety and Quality Unit, Level 7, King George V Building, Royal Prince Alfred Hospital, Missenden Road, Camperdown, NSW 2050, Australia
Email michelle.barakatjohnson@health.nsw.gov.au
Members of the IMBED Project Steering Committee
*Corresponding author
References
- Beeckman D, Campbell J, Campbell K, et al. Incontinence-associated dermatitis: moving prevention forward. Wounds Int 2015. Available from: https://eprints.soton.ac.uk/379190/
- Gray M, Black JM, Baharestani MM, et al. Moisture-associated skin damage: overview and pathophysiology. J Wound Ostomy Continence Nurs 2011;38(3):233–241.
- Gray M, Giuliano KK. Incontinence-associated dermatitis, characteristics and relationship to pressure injury: a multisite epidemiologic analysis. J Wound Ostomy Continence Nurs 2018;45(1):63–67.
- Beeckman D, Van Lancker A, Van Hecke A, et al. A systematic review and meta-analysis of incontinence-associated dermatitis, incontinence, and moisture as risk factors for pressure ulcer development. Res Nurs Health 2014;37(3):204–218.
- Minassian VA, Devore E, Hagan K, et al. Severity of urinary incontinence and effect on quality of life in women by incontinence type. Obstet Gynecol 2013;121(5):1083–1090.
- Bartlett L, Nowak M, Ho Y-H, et al. Impact of fecal incontinence on quality of life. World J Gastroenterol 2009;15(26):3276–3282.
- Beeckman D, et al. Proceedings of the Global IAD Expert Panel. Incontinence-associated dermatitis: moving prevention forward. London; 2015.
- Kayser SA, Phipps L, VanGilder CA, et al. Examining prevalence and risk factors of incontinence-associated dermatitis using the International Pressure Ulcer Prevalence Survey. J Wound Ostomy Continence Nurs 2019;46(4):285–290.
- Johansen E, Bakken LN, Duvaland E, et al. Incontinence-associated dermatitis (IAD): prevalence and associated factors in 4 hospitals in Southeast Norway. J Wound Ostomy Continence Nurs 2018;45(6):527–531.
- Barakat-Johnson M, Basjarahild S, Campbell J, et al. Implementing best available evidence into practice for incontinence-associated dermatitis in Australia: a multisite multimethod study protocol. J Tissue Viability 2020;30(1):67–77.
- Behzadi P, Behzadi E, Ranjbar R. Urinary tract infections and Candida albicans. Central Eur J Urol 2015;68(1):96–101.
- National Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: clinical practice guideline. Osborne Park, Western Australia: Cambridge Media; 2014.
- European Pressure Ulcer Advisory Panel (EPUAP). Prevention and treatment of pressure ulcers/injuries: clinical practice guidelines. The international guideline. 3rd ed 2019. Available from: https://www.internationalguideline.com/guideline
- Beeckman D, Van den Bussche K, Alves P, et al. The Ghent Global IAD Categorisation Tool (GLOBIAD). Skin Integrity Research Group. Ghent University; 2017 Available from: www.UCVVGent.be
- Pintos-Díaz MZ, Alonso-Blanco C, Parás-Bravo P, et al. Living with urinary incontinence: potential risks of women's health? A qualitative study on the perspectives of female patients seeking care for the first time in a specialized center. Int J Environ Res Public Health 2019;16(19):3781.
- Rada MP, Jones S, Betschart C, et al. An international collaboration for harmonising outcomes, research, and standards in urogynaecology and women’s health (i-chorus. org). A meta-synthesis of qualitative studies on stress urinary incontinence in women for the development of a core outcome set: a systematic review. Int J Gynaecol Obstet 2022;158(1):3–12.
- Toye F, Barker KL. A meta-ethnography to understand the experience of living with urinary incontinence: ‘is it just part and parcel of life?’ BMC Urol 2020;20(1):1–25.
- Barakat-Johnson M, Lai M, Barnett C, et al. Hospital-acquired pressure injuries: are they accurately reported? A prospective descriptive study in a large tertiary hospital in Australia. J Tissue Viability 2018;27(4):203–210.
- Barakat-Johnson M, Barnett C, Lai M, et al. Incontinence, incontinence-associated dermatitis, and pressure injuries in a health district in Australia: a mixed-methods study. J Wound Ostomy Continence Nurs 2018;45(4):349–355.
- Beeckman D. Incontinence-associated dermatitis (IAD) and pressure ulcers: an overview. In: Romanelli M, Clark M, Gefen A, Ciprandi G, eds. Science and practice of pressure ulcer management. London: Springer London; 2018:89–101.
- Barakat-Johnson M, Stephenson J, Basjarahil S, et al. Clinician knowledge of incontinence-associated dermatitis: a multisite survey of healthcare professionals in acute and subacute settings. J Wound Ostomy Continence Nurs 2022;49(2):159–167.
- O'Connell B, Ostaszkiewicz J, Sukkar K, et al. Continence tools for residential aged care: an education guide. Deakin University; 2009.
- Kottner J, Black J, Call E, et al. Microclimate: a critical review in the context of pressure ulcer prevention. Clin Biomech 2018;59:62–70.


