Volume 29 Number 3
Pessary management for pelvic organ prolapse: a review of clinical practice and Australian medical device regulations
Zanna Franks, Hannah G Krause
Licensed under CC BY 4.0
Keywords management, prolapse, pessary, device, regulation
For referencing Franks Z & Krause HG. Pessary management for pelvic organ prolapse: a review of clinical practice and Australian medical device regulations. Australian and New Zealand Continence Journal 2023; 29(3):67-73.
DOI
https://doi.org/10.33235/anzcj.29.3.67-73
Submitted 25 March 2023
Accepted 8 August 2023
Abstract
Pelvic organ prolapse affects up to 50% of women throughout life. Management can be conservative or surgical. Pessaries have become an integral component to management of pelvic organ prolapse, providing symptom improvement and enhancing quality of life. After careful clinical assessment, women choosing pessary management are offered either self-care or clinician-based pessary care. Pessary care can be offered by a range of clinicians, including doctors, nurses and physiotherapists. In this article we review the literature on the historical use of pessaries and how they have changed to be the device manufactured today. We then outline the Australian regulation of vaginal pessaries as per the Therapeutic Goods Administration (TGA). We discuss device classification in relation to clinical practice and guidelines.
The TGA classifies pessary devices as either Class 1: device which is intended for transient use, Class 2A: to be used continuously for at least 60 minutes but not more than 30 days, and Class 2B: continuous use for more than 30 days. The majority of pessaries available in Australia are classified as 2A devices.
The TGA classification of pessaries and commonly accepted standards of care in many Australian centres are not always synergistic. In Australia, varied models of care are offered for pessary management. Recent literature has identified a need for clinician guidelines and training for pessary care. The TGA device classification should be considered in Australian training and guidelines. Information on TGA device classification needs to be discussed with each patient that is offered pessary management. If 2A pessaries are used in conjunction with clinical led care, it is unlikely that the device will be removed every 30 days. Therefore, 2A devices may be used off-label and the patient should be informed of this deviation from the regulation.
Introduction
Pelvic organ prolapse (POP) affects up to 50% of women1,3–5. POP has been defined as the descent of at least one of the vaginal walls to or beyond the hymen with maximal Valsalva, plus the presence of bothersome characteristics such as vaginal bulge or functional compromise. Women with POP describe feelings of vaginal dragging, protrusion into or outside of the vagina, bladder or bowel symptoms, and sexual dysfunction6.
Management of POP is largely based on symptomatology and bother. It includes both non-surgical and surgical options. Non-surgical management includes pelvic floor muscle training (PFMT) and pessaries to provide support6. This paper will discuss pessaries for the management of symptomatic prolapse. We review the evolution in engineering of the current day pessary. We explore the Therapeutic Goods Administration (TGA) regulation of pessary use and consider this in relation to current standards of clinical practice and training. In particular, we apply this to the Australian pessary practitioner6. We seek to provide clarity surrounding the TGA regulations on pessaries to all health professionals fitting and managing pessaries.
The History of Pessary Use
Pessary management for POP has had a variable course throughout history7. The word pessary stems from Greek and Latin literature, originating from the Greek word pessós and Latin word pessarium meaning an oval stone used in a checkers-like game8,9.
Pessary devices have been used since 400BC; there are reports detailing Hippocrates inserting pomegranates into the vagina to reduce prolapse9,8. Further historical literature on POP management from AD1050 describes a “ball pessary” constructed from strips of linen to fill the vagina9. German literature from 1559 describes using a sponge tightly rolled and bound with string, dipped in wax, and covered with oil or butter as a pessary9. The late 16th century saw developments to pessaries as they became oval-shaped and crafted from hammered brass and waxed cork8,9.
The vulcanisation of rubber in the 1860s and then polystyrene plastics in the 1950s saw modernisation of pessary design9. Currently, pessaries are manufactured using silicone, polyvinyl-chloride, polythene, acetyl-copolymer or latex materials4,6.
The development and implementation of silicone devices has provided advantageous properties. Silicon is non-absorbent of secretions or odours, it has a long half-life, withstands sterilisation and repeated cleaning processes, and is inert and hypoallergenic10,11.
Advancements in pessary materials used has allowed for variation in structure to pessary devices. The materials used to manufacture a pessary, although important to improve function, are not likely to affect adverse events. Device shape is more likely to be associated with complications compared to materials used in manufacturing. Evidence shows that complications such as erosions are more common in Gellhorn or donut pessaries rather than ring pessaries12. It is important for a practitioner to understand the materials pessaries are made from; however, this is only one aspect that should be considered in practice.
Pessary Use in Clinical Practice
Pessary devices for POP have a beneficial therapeutic impact on quality of life, sexual function and body image. Pessary use promotes a significant reduction in POP symptoms, with low complication rates1,4.
Clinical practice is affected by clinical efficacy, risk–benefit profile, patient-reported outcomes and cost. NICE guidelines (2019) report that up to 98% of clinicians managing POP offer pessaries for management13–15. They are commonly offered first-line; a multiple disciplinary survey of UK practice published in 2020 found that 75% of clinicians managing pessaries will use them as first-line16.
High patient acceptability and symptomatic improvement of POP is reported with pessary use6–7,17. An observational study found 76% of women newly fitted with a pessary will continue use for at least four weeks. In the same study, 86% of the women continuing with pessary management maintained use for over five years6,18. Further to this, medium-term satisfaction rates are high (70–92%), reducing POP-related bother, and improving quality of life and positivity of body image7,12,19.
Management for prolapse can be either through surgery or pessary use20. Evidence supports that pessary management can provide comparable treatment outcomes to surgery in reported symptoms and quality of life1. A prospective study comparing pessary management with surgery in women with symptomatic POP reports equivocal outcomes in urinary and bowel symptoms, sexual function and quality of life improvements21. A small (n=160) prospective cohort study from the United States20 compared pessary and surgical management for POP. It showed comparable outcomes between the treatment arms for goal attainment and improvements in physical, social and emotional functioning; only slightly better outcomes were found in surgery20.
Similar findings are again reported in a recent (2019) observational study comparing pessary and surgery for advanced POP in women with a uterus. It showed similar outcomes in success of pessary and recurrence of prolapse symptoms post-surgery22. A prospective study using validated questionnaires compared vaginal pessaries and surgery for POP using the International Consultation on Incontinence Questionnaire-Vaginal Symptoms (ICIQ-VS) and Urinary Incontinence (ICIQ-UI) Short Form18. Although limited by only one-year follow-up, women with symptomatic POP did report improvement in vaginal, bowel, urinary and quality of life scores in both groups18.
Pessary management for POP has well documented clinical efficacy and should be considered for patients presenting with symptomatic POP. In addition to improving symptoms, vaginal pessaries are often selected as a cost-effective treatment6,23,24. Cost efficacy of pessary management for POP has been supported through cost analysis studies25.
Complications of pessaries
A 2020 Cochrane review on pessaries for POP includes an analysis of the number of women reporting adverse events from pessary use6. The most commonly reported adverse event is vaginal discharge, bleeding and erosions12. Increased urinary incontinence, irritation and discomfort during intercourse, vaginal odour and increase in bacterial vaginosis are also reported6,9.
Common adverse effects are usually straightforward to treat. Vaginal ulcers or erosions can be managed with vaginal oestrogen and removing the pessary for a period of time to allow the ulcer to heal6,9. Additional concerns such as spontaneous expulsion, difficulty with defecation and de novo stress incontinence can also require amendments to the size or shape of pessary used6,9.
If pessaries are left in situ for prolonged periods serious complications can arise9,26,27. Poorly fitted devices have resulted in reports of the cervix, uterus or bowel herniating and strangulating through, an impacted or embedded pessary, cervical incarceration and infection28,29.
Fistulas are rare and result in significant complications for patients. The association between pessary use and fistula formation was described in 1868 by Thomas Addis Emmet30. Although infrequent, fistulas have been well documented throughout the literature with multiple case reports26,31–33.
When should a pessary review be performed to minimise complications
There is paucity in data to support optimal pessary review to minimise adverse events. A 2020 prospective cohort review assessed efficacy of routine follow-up for pessary cleaning using a measure of visual analogue scale on pain, discharge and irritation one week before and after cleaning at three and nine months. They found there was no difference in outcome pre- or post-cleaning and reported no serious adverse events related to pessary use34.
A prospective observational study looked at ring pessaries. Patients were reviewed at four weeks then six-monthly until 24 months. In this time pessaries were not removed, rinsed or replaced. They found 91.8% of women continued pessary use at 24 months. Adverse events occurred in 27% of cases. Adverse events were grouped as extrusion of pessary, bleeding, excoriation, pain and increase in vaginal discharge requiring pessary removal35. However, there remains a lack of clarity on the optimal timing of pessary review by a clinician to reduce complications. A nine-year longitudinal study in the USA suggests there is a 3% risk of developing a vesicovaginal or rectovaginal fistula and a 5% risk of developing a mechanical genitourinary device complication on follow-up of pessary insertion36. When considering optimal timing for pessary review, clinical indications, evidence to support practice and regulation should be considered.
Who regulates pessaries
In Australia, vaginal pessaries for management of prolapse are classified as a medical device and are regulated through the TGA37. The TGA has a set of principles including safety requirements, infection and microbial contamination protocols, construction and environmental properties37. The current process for TGA classification relies on the submission of evidence on safety of use by Australian device sponsors to the TGA for review37. This assessment process is known as a conformity assessment, and it is how a sponsor shows the safety, quality and performance of their medical devices37.
The regulatory framework comprises pre-market and post-market requirements. Compliance with Australian safety and performance requirements must be met for all medical devices supplied to Australia37.
What is a medical device?
A medical device (as per the Therapeutic Goods Act 1989) is any instrument, apparatus, appliance, software, implant, reagent, material, or other to be used for human beings for the purpose of one or more of the following38:
(i) Diagnosis, prevention, monitoring, prediction, prognosis, treatment, or alleviation of disease.
(ii) Diagnosis, monitoring, treatment, alleviation of or compensation for an injury or disability.
(iii) Investigation, replacement, or modification of the anatomy or of a physiological or pathological process or state.
(iv) Control or support of conception.
(v) In vitro examination of a specimen derived from the human body for a specific medical purpose.
It does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means. Medical devices are classified by the TGA based on risk (Table 1).
Table 1. Classification of medical devices37–38
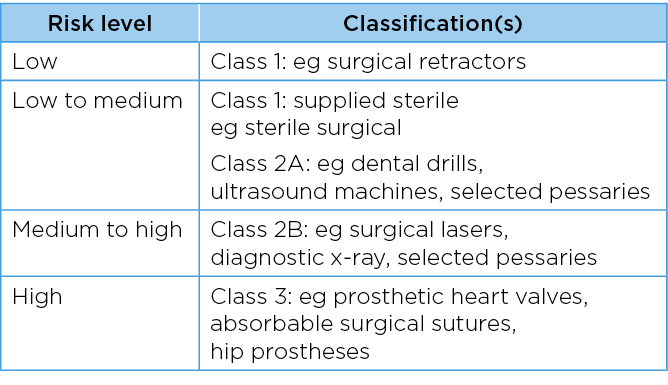
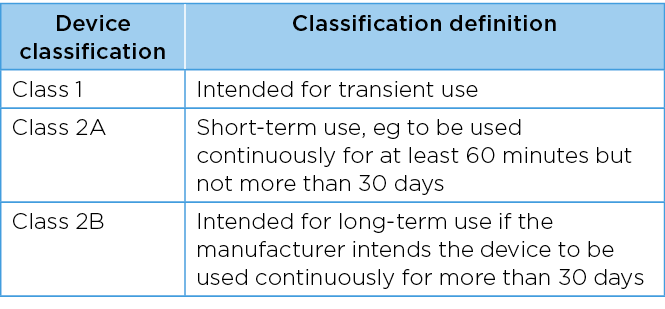
The Therapeutic Goods Regulation 2002, schedule 2 classification, details the regulation for invasive medical devices. This applies to an invasive medical device that is intended by the manufacturer to be used to penetrate a body orifice of a patient2. Invasive medical devices (not intended to be connected to an active medical device) are classified as Class 1: device is intended for transient use, Class 2A: short-term use ie, to be used continuously for at least 60 minutes but not more than 30 days, and Class 2B: device is intended for long-term use of more than 30 days (Table 2)2.
Table 2. Classification of vaginal pessary devices2
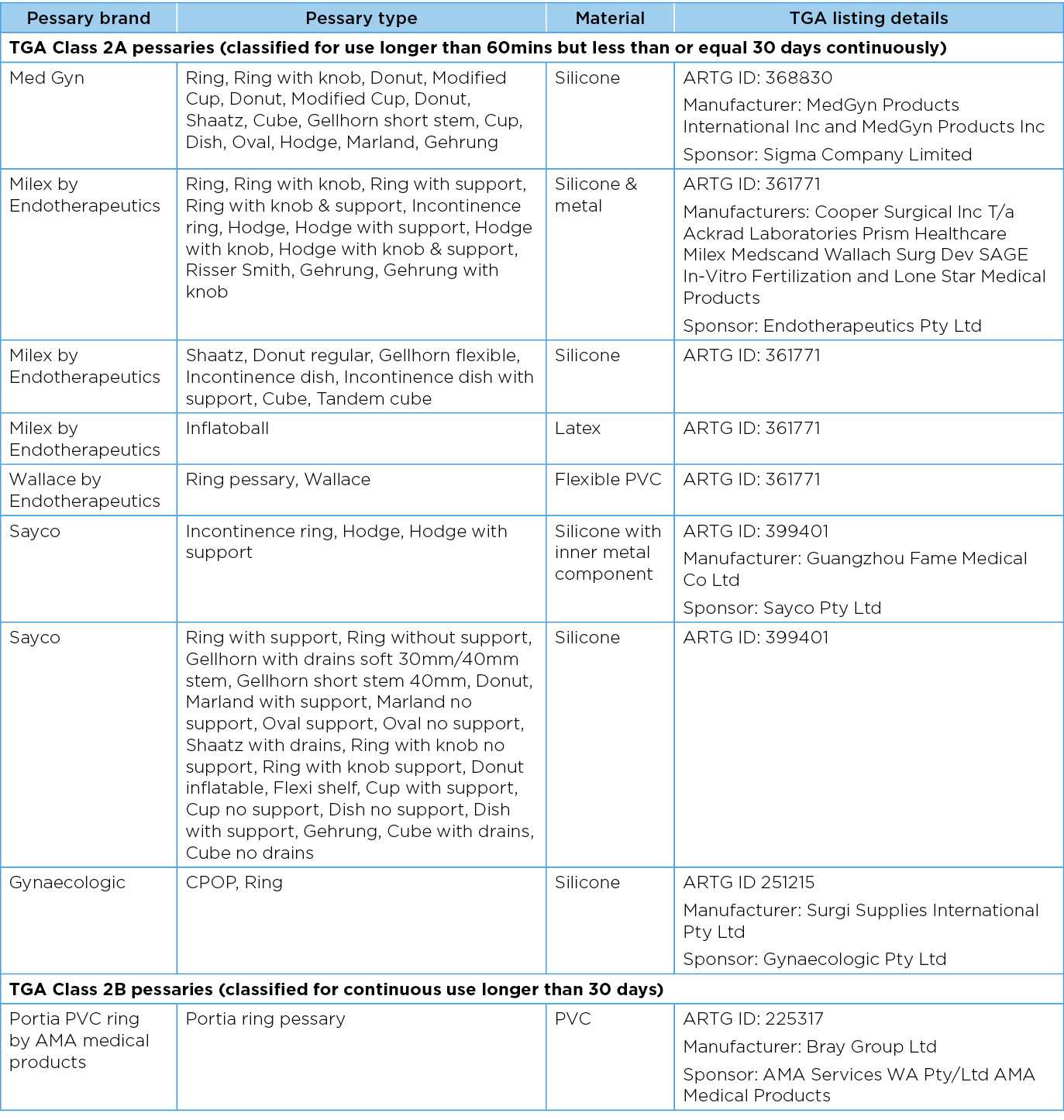
A vaginal pessary device is classified as an adaptable medical device. On review of the TGA classification of pessary devices (Class 2A/2B), the TGA classification may not always be in alignment with standard clinical practice in many Australian centres. Table 3 details pessaries commonly used in Australia and registered with the TGA39–43. Most of the available pessaries used in Australia are categorised by the TGA as 2A devices. This means the device is approved to use for longer than 60 minutes but not more than 30 days continuously. 2B devices are approved for use longer than 30 days39,41.
An understanding of the TGA classification of devices is necessary to ensure patients are being counselled appropriately when a pessary is being offered. For example, a silicon ring (classified 2A) is selected as the most appropriate management option for the individual. It is often fitted with the longer-term intent to keep the device in situ for more than 30 days (Table 3). As such, the patient must be counselled that this is common practice but as per the TGA classification this would be considered an off-label use of their pessary2,29.
Table 3. Commonly used TGA approved pessaries39–43
Standards in training of pessary practitioners
In contrast to pessary regulation set by the TGA, standards of patient care and responsibility of clinical practice lies with the clinician. This is assisted by relevant professional codes of conduct, guidelines and policies29,44-48. A range of healthcare practitioners provide pessary care, including doctors, nurses and physiotherapists16,45–46. An Australian cross-sectional study has identified varied training experiences in pessary management across healthcare practitioners23. Practitioners report that current practice is largely based on information provided by manufacturers23.
Recent international literature has highlighted a lack of structured clinical training and guidelines for practitioners providing pessary management for POP44,47. Practitioner training in pessary care is needed to ensure appropriate patient selection, correct pessary fitting, and availability of long-term follow-up to minimise the potential for adverse events10,2,44. There is a lack of evidence to correlate complication rates with experience or skill of a practitioner. Yet, to ensure a high level of care is provided to women selecting pessary management and to protect pessary practitioners from liability and litigation, clear guidelines on best practice and clinician training are necessary44.
Internationally, progress has been made to guide pessary practice29. The UK pessary guidelines have created training standards for pessary practitioners29,39. A 2022 South Australian Delphi study details the development of a multinational, multidisciplinary competency framework developed for physiotherapy training in pessary management45.
To further advance pessary care and training, an understanding of pessary regulation with the TGA would be helpful to those considering pessary training. This will help to inform the way pessary practitioners may adequately counsel women to make an informed choice on pessary management.
Guidelines for clinical practice
The UK clinical guidelines for use of vaginal pessaries for POP is a best practice document. It provides an expert opinion on the timeline for review and pessary changes29. The document states that good practice for pessary follow-up is 4–6 weeks after initial fitting then six-monthly or longer if the pessary is self-managed successfully29. This expert opinion document is widely accepted in practice. As discussed previously, there is paucity of evidence to guide optimal timing of pessary review, with other studies reporting on outcomes from review times ranging from six weeks to 24 months34–36.
It is widely accepted in practice that there are limited risks related to pessary use. Yet regulation is lacking to support contemporary practice and timing of pessary review. Whilst risk to the patient remains minimal, the clinician needs to have clear documentation that risks have been discussed and understood, and that the patient accepts to use the pessary off -label if follow-up time will vary from TGA regulations. When delivering care, the TGA classification of pessary devices should be considered with the patient. The practitioner should document patient education and consent, the pessary type and size, pessary replacement, exchange or placement of a new device44.
Discussion
When a patient presents with a symptomatic POP, a detailed assessment and patient-centred discussion must take place prior to arriving at a decision on management. A thorough history should be taken, followed by physical examination. Examination includes assessment and clear documentation of the degree of prolapse of the anterior, posterior and central compartments of the pelvic floor using the POP-Q (Pelvic Organ Prolapse Quantification) system13. A careful pelvic exam should contain a bimanual examination to determine if coexisting pelvic pathology is present. Assessment of the pelvic floor muscles and vagina for atrophy or epithelial ulceration should also be performed and cervical screening test collected if indicated13. Sphincter tone and presence of rectal prolapse in those with bowel symptoms can be evaluated13. A validated pelvic floor symptom questionnaire may be considered to aid assessment and decision making. If obstructed defaecation, faecal incontinence or urinary symptoms are identified on assessment, then further investigations should be considered13,48.
When discussing treatment, conservative management options include observation, lifestyle intervention, PFMT, topical oestrogen and pessary13,45. Some patients may choose to have a pessary if they have not yet completed their family, they have a high risk of recurrence, surgical timing doesn’t suit their lifestyle, they want to avoid surgical risks, or they are not fit for surgery1. When conservative management is selected, this does not preclude the individual from reconstructive or obliterative surgery in the future37,48.
Current options for women choosing pessary management for prolapse are either self-care or clinician-based pessary care. Clinician care involves regular review, usually 3–6 monthly, where the pessary is removed and vaginal tissues examined prior to replacing the pessary device1. If choosing the self-care option, women are taught to remove, clean and change their pessary regularly1. Silicone pessaries are soft and flexible; thus, self-care options are more feasible for this type of device. This enables a TGA class 2A (i.e., ≥60 minutes but ≤30 days) device to be used as per TGA instructions.
When clinician-led care is the chosen model, it is not always feasible for a healthcare practitioner to perform a pessary check every 30 days or less as per the TGA regulations for class 2A devices (eg silicon rings). Class 2B pessaries can be utilised within the current regulatory guidance as they can be left in situ more than 30 days continuously. If 2A devices are being left in situ for more than 30 days continuously they are being used off-label. Evidence and guidelines suggest minimal and acceptable patient risk from having a pessary left in situ for more than 30 days continuously. However, patients need to be informed that this is off-label use and the clinician should clearly document this.
Conclusion
The vaginal pessary is an effective management strategy for symptomatic POP. Prior to prolapse management, a patient should always undergo a thorough assessment and discussion on individual risk factors and all available treatment options. When a pessary device is the chosen management strategy, then patient ability to self care or preference of clinician care should guide choice of pessary offered. The TGA device classification needs to be considered and discussed with the patient. If 2A pessaries are used in conjunction with clinician-led care, it is unlikely that a review will be performed every 30 days. Therefore, 2A devices may be used off-label and the patient should be informed of this deviation from the regulation.
It is likely that future TGA device classifications of commonly used pessaries will change as device suppliers seek amendments to their certifications to align with standard clinical care. For reclassification to be considered by the TGA, the Australian sponsor must submit relevant data to the TGA to increase the length of use. The current TGA regulatory perspective is that the classification of a device reflects the maximum period of use that the manufacturer and sponsor have intended37.
Currently a large proportion of available pessaries in Australia remain 2A. It is imperative that the clinician providing treatment to the individual has appropriate training in pessary management, adheres to guidelines of clinical practice, and is familiar with the TGA regulations to provide safe and evidenced-based care.
Author(s)
Zanna Franks*
Gold Coast University Hospital, QLD, Australia
Email zanna.franks@gmail.com
Hannah G Krause
University of Queensland, Queen Elizabeth II Jubilee Hospital, Gold Coast University Hospital, Greenslopes Private Hospital, QLD, Australia
*Corresponding author
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
References
- Dwyer L, Dowding D, Kearney R. What is known from the existing literature about self-management of pessaries for pelvic organ prolapse? A scoping review protocol. BMJ Open 2022;12(7).
- Commonwealth Consolidated Regulations. Therapeutic Goods Regulations 2002 – Schedule 2 2022; 2023. Available from: http://classic.austlii.edu.au/au/legis/cth/consol_reg/tgdr2002400/sch2.html
- Your Pelvic Floor, International Urogynaecological Association. Vaginal pessary for pelvic organ prolapse. 2023. Available from: https://www.yourpelvicfloor.org/conditions/vaginal-pessary-for-pelvic-organ-prolapse/.
- Zeiger BB, Carramão S, Del Roy CA, Da Silva TT, Hwang S, Auge A. Vaginal pessary in advanced pelvic organ prolapse: impact on quality of life. Int Urogynecol J 2022;22:2013–20.
- Collins S, O’Shea M, Dykes N, Ramm O, Edenfield A, Shek K, Van Delft K, Beestrum M, Kenton K. International Urogynecological Consultation: clinical definition of pelvic organ prolapse. International Urogynecol J 2021;32:2011–9.
- Bugge C, Adams E, Gopinath D, Stewart F, Dembinsky M, Sobiesuo P, Kearney R. Pessaries (mechanical devices) for managing pelvic organ prolapse in women (review). Cochrane Database System Rev 2020(11).
- Lamers B, Broekman B, Milani A. Pessary treatment for pelvic organ prolapse and health-related quality of life: a review. Int Urogynecol J 2011;22(6):637–44.
- Oliver R, Thakar R, Sultan A. The history and usage of the vaginal pessary: a review. Eur J Obstet Gynecol Reprod Biol 2011;156(2):125–30.
- Lewicky-Gaupp C. Contemporary use of the pessary. The Alliance for Global Women’s Medicine; 2010. doi:10.3843/GLOWM.10025
- Continence Foundation Australia and University of South Australia. Guidelines for the use of support pessaries in the management of pelvic organ prolapse; 2021 [cited 2023]. Available from: https://www.continence.org.au/resource/pessary-guidelines?v=7099.
- Shah S, Sultan A, Thakar R. The history and evolution of pessaries for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2006;17(2):170–5.
- Kakkar A, Reuveni-Salzman A, Bentaleb J, Belzile E, Merovitz L, Larouche M. Adverse events associated with pessary use over one year among women attending a pessary care clinic. Urogynecol J 2023.
- National Institute for Health and Care Excellence (NICE). Urinary incontinence and pelvic organ prolapse in women: management. NICE; 2019. Available from: nice.org.uk/guidance/ng123.
- Bugge C, Hagen S, Thakar R. Vaginal pessaries for pelvic organ prolapse and urinary incontinence: a multiprofessional survey of practice. Int Urogynecol J 2013;24(6):1017–24.
- Pott-Grinstein E, Newcomer J. Gynaecologists’ patterns of prescribing pessaries. J Reprod Med 2001;46(3):205–5.
- Brown C, Pradhan, A, Pandeva I. Current trends in pessary management of vaginal prolapse: a multidisciplinary survey of UK practice. Int Urogynecol J 2021;32:1015–22.
- Panman C, Wiegersma M, Kollen B, Berger M, Lisman-van Leeuwen Y, Vermeulen, K, Dekker J. Effectiveness and cost-effectiveness of pessary treatment compared with pelvic floor muscle training in older women with pelvic organ prolapse: 2-year follow-up of a randomized controlled trial in primary care. Menopause 2016;23:1307–18.
- Lone F, Thakar R, Sultan A, Karamalis G. A 5 year prospective study of vaginal pessary use for pelvic organ prolapse. Int J Gynecol Obstet 2011;114(1):59–9.
- Patel M, Mellen C, O’Sullivan D, Lasala C. Impact of pessary use on prolapse symptoms, quality of life, and body image. Am J Obstet Gynecol 2010;202(5):499.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol 2016;215(5):659.e1-.e7.
- Abdool Z, Thakar R, Sultan A, Oliver R. Prospective evaluation of outcome of vaginal pessaries versus surgery in women with symptomatic pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2010;22(3):273–8.
- Miceli A, Dueñas-Díez J-L. Effectiveness of ring pessaries versus vaginal hysterectomy for advanced pelvic organ prolapse. cohort study. Int Urogynecol J 2019;30(12):2161–9.
- McEvoy K, Griffin R, Harris M, Moger H, Wright O, Nurkic I, Thompson J, Das R, Neumann P. Pessary management practices for pelvic organ prolapse among Australian health care practitioners: a cross-sectional study. Int Urogynecol J 2023.
- Vardona D, Martin-Lasnel M, Aubert A, Raffaèle F, Pizzoferrato A. Vaginal pessary for pelvic organ prolapse: a survey among French gynecological surgeons. J Gynecol Obstet Human Reprod 2021;50(101833).
- Hullfish K, Trowbridge E, Stukenborg G. Treatment strategies for pelvic organ prolapse: a cost-effectiveness analysis. Int Urogynecol J 2011;22:507–15.
- Torbey M. Large rectovaginal fistula due to a cube pessary despite routine follow-up; but what is ‘routine’? J Obstet Gynaecol Res 2014;40(11).
- Summers J, Ford M. The forgotten pessary: a medical oddity. Am J Obstet Gynecol 1991;111(307).
- Poma P. Management of incarcerated vaginal pessaries. J Am Geriatr Soc 1981;29(325).
- United Kingdom Continence Society, Pelvic Obstetric and Gynaecological Physiotherapy. UK clinical guideline for best practice in the use of vaginal pessaries for pelvic organ prolapse; 2021.
- Wall L. Thomas Addis Emmet, the vesicovaginal fistula, and the origins of reconstructive gynecologic surgery. Int Urogynecol J Pelvic Floor Dysfunct 2002;13(3):145–55.
- Knight DG Kernohan N. The “forgotten” vaginal ring pessary – an unusual cause of rupture of the urinary bladder. Br Med J 1986;292(992).
- McElin T, Paalman R. Pessary complications in the management of uterine prolapse. Am J Obstet Gynecol 1959;78:643.
- Mohamed WI, Emmanuel D, Maqbool S, Asad A, Jawad A, Ihtesham, A. Rectovaginal fistula due to an erosive pessary: a case report. Int J Med Stud 2022;9(4):304–6.
- Thys S, Hakvoort R, Asseler J, Milani A, Vollebregt AS, Roovers J. Effect of pessary cleaning and optimal time interval for follow-up: a prospective cohort study. Int Urogynecol J 2020(8):1567–74.
- Miceli A, Fernández-Sánchez M, Polo-Padillo J, Dueñas-Díez J-L. Is it safe and effective to maintain the vaginal pessary without removing it for 2 consecutive years? Int Urogynecol J 2020;31(12):2521–8.
- Alperin M, Khan A, Dubina E, Tarnay C, Ning W, Pashos C, Anger J. Patterns of pessary care and outcomes for Medicare beneficiaries with pelvic organ prolapse. Female Pelvic Med Reconstr Surg 2013;19(3):142–7.
- Australian Government Department of Health and Aged Care: Therapeutic Goods Administration. Medical devices regulation basics; 2021 [cited 2023]. Available from: https://www.tga.gov.au/medical-devices-regulation-basics.
- Australian Government Department of Health and Aged Care: Therapeutic Goods Administration. Medical devices overview; April 2022 [cited 2023]. Available from: https://www.tga.gov.au/products/medical-devices/medical-devices-overview.
- Australian Government Department of Health and Aged Care: Therapeutic Goods Administration. Australian register of therapeutic goods; 2023 [cited March 2023]. Available from https://www.tga.gov.au/resources/artg?keywords=pessary]
- Medgyn Products Inc. Pessaries; 2016 [cited August 2023]. Available from: https://www.medgyn.com/product-category/pessaries/2016
- AMA Medical Products. Portia Flexible PVC pessary rings; 2023 [cited August 2023]. Available from: https://amamedicalproducts.com.au/search?q=pessary&options%5Bprefix%5D=last.
- Endotherapeutics Pty Ltd. Milex and Wallace pessaries; 2023 [cited August 2023]. [Available from: https://www.endotherapeutics.com.au/product/pessaries/.
- ProFem by Sayco. ProFem; 2023 [cited August 2023]. Available from: https://www.profem.com.au.
- Dwyer L Stewart E, Rajai A. A service evaluation to determine where and who delivers pessary care in the UK. Int Urogynecol J 2021;32(4):1001–1006
- Neumann P, Radi N, Gerdis T, Tonkin C, Wright C, Chalmers KJ, Nurkic I. Development of a multinational, multidisciplinary competency framework for physiotherapy training in pessary management: an E-Delphi study Int Urogynecol J 2022(2):253–65.
- Neumann P, Scammell A, Burnett A, Thompson J, Briffa NK. Training of Australian health care providers in pessary management for women with pelvic organ prolapse: outcomes of a novel program. ANZCJ 2015;21(1):6–12.
- Dwyer L, Kearney R, Lavender T. A review of pessary for prolapse practitioner training. Br J Nurs 2019;28(9):S18–24.
- Royal College of Obstetricians & Gynaecologists, British Society of Urogynaecology. Post-hysterectomy vaginal vault prolapse. Green-top Guideline No 46; 2015.