Volume 30 Number 2
In vitro effects of the alpha-blocker prazosin on urothelial cancers and bladder function
Mr Liam O’callaghan, Professor Russ Chess-Williams, Dr Katie Powell,
Associate Professor Catherine McDermott
Licensed under CC BY 4.0
DOI 10.33235/anzcj.30.2.35
Objective: Bladder cancer, one of the ten most common cancers diagnosed worldwide, presents as a common, complex, and costly disease. Current treatments, including intravesical chemotherapy and immunotherapy, are associated with frequent recurrences and local side effects that significantly affect quality of life.1 These side effects, including urinary frequency, urgency, dysuria and hematuria, contribute to or intensify urinary incontinence, posing significant challenges in patient care.2 Consequently, there is an important need for novel therapeutic approaches that offer effective cancer treatment with reduced side effects. In this context, α1-ADR antagonists, known for their cytotoxic effects on prostate cancers, emerge as promising candidates. This study aims to investigate the cytotoxicity of the α1-ADR antagonist prazosin (Minipress) in bladder cancer cell lines, considering their potential as a bladder cancer treatment with minimal side effects. Additionally, the research explores the impact of luminal prazosin treatment on bladder function using a porcine model, to provide insights relevant to the management of urinary incontinence in bladder cancer patients.
Methods: Human malignant urothelial cells lines (invasive T24 cells and non-invasive RT4 cells) were incubated with prazosin (1-300 µM) or vehicle control (DMSO) for either a 30-minute or 2-hour period (mimicking the duration of intravesical treatment). Cell viability was then assessed 24, 48, and 72 hours after prazosin incubation using the resazurin reduction assay. To examine the effects of prazosin on bladder function, the luminal surface of female pig bladders were treated with prazosin (300 µM) or vehicle control in modified Ussing chambers for 2 hours, followed by organ bath studies to assess functional responses to agonists carbachol, isoprenaline, adenosine triphosphate (ATP), electrical field stimulation (EFS) and high potassium Krebs.
Results: Incubation of T24 and RT4 cells with prazosin (300µM) resulted in a statistically significant concentration-dependent decrease in cell viability at 72 hours (Figure 1A). In bladder functional experiments, pre-treatment with 300 µM prazosin did not significantly affect nerve evoked contractile bladder responses (Figure 1B). Acetylcholine (ACh) remained the dominant neurotransmitter in control and treated tissues. Responses to purinergic stimulation, β-adrenoceptor relaxation to isoprenaline and general bladder contractility to high potassium were similarly unchanged. However, the maximal response of intact bladder tissues to carbachol was significantly enhanced after prazosin pre-treatment (P<0.001) (Figure 1C), while responses of denuded detrusor and urothelium/lamina propria to muscarinic stimulation were unchanged.
Conclusions: The study highlights prazosin’s concentration-dependent cytotoxicity against both invasive T24 and non-invasive RT4 bladder cancer cells, highlighting this drug as a promising candidate for targeted bladder cancer therapy. Significantly, prazosin demonstrates minimal impact on normal bladder function in the porcine model, suggesting a lower risk of exacerbating urinary incontinence; a major concern with current intravesical treatments. Future research should investigate prazosin’s long-term effects and potential integration into existing treatment regimes, offering a holistic approach to bladder cancer treatment that priorities both tumor suppression and quality of life.
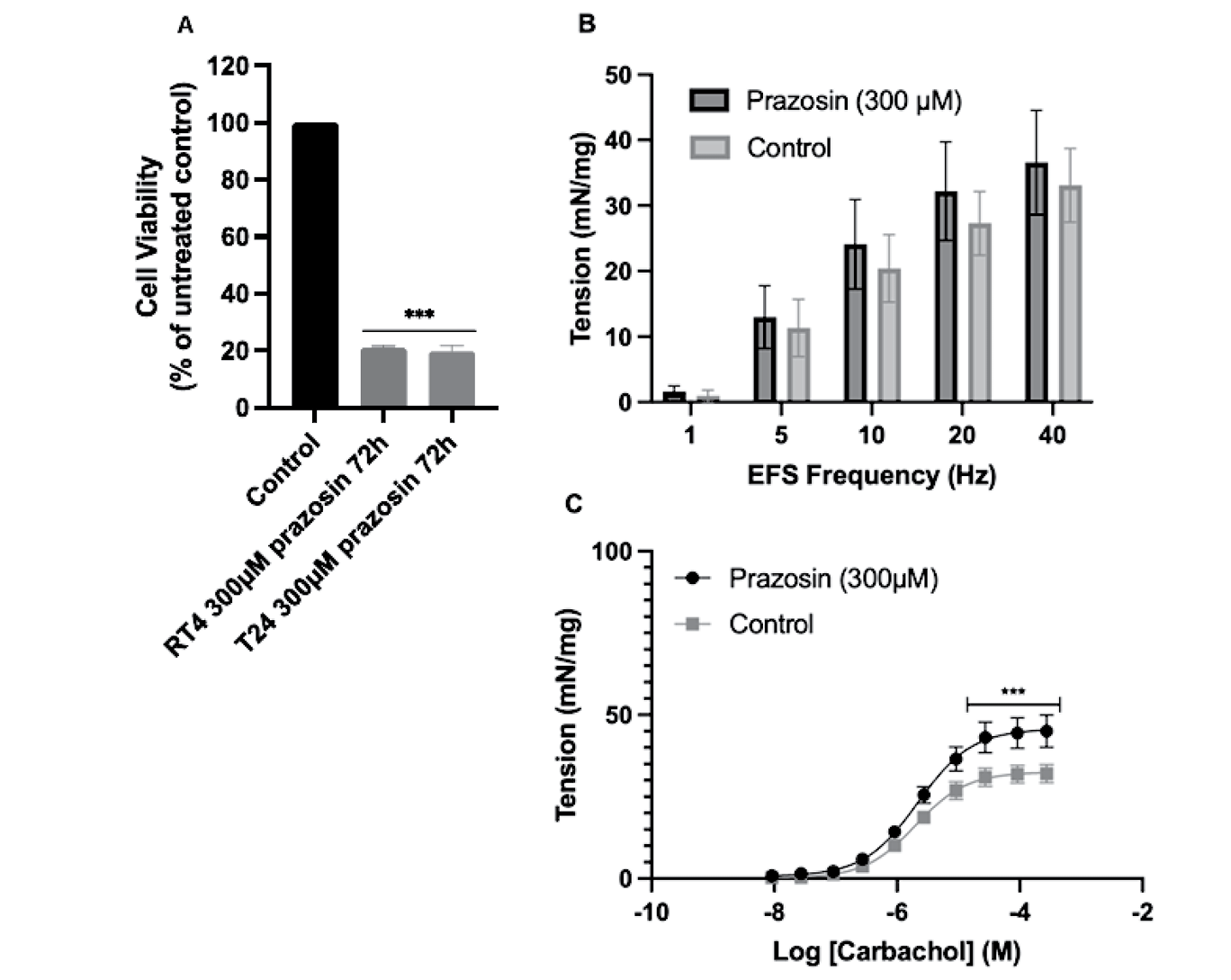
Figure 1. (A) Effects of a 2-hour incubation with prazosin on the viability of T24 and RT4 bladder cancer cells 72 hours after treatment (***P<0.001 compasred to control). Responses of (B) porcine detrusor to electricsal field stimulation (EFS) and (C) responses of intact porcine tissues to carbachol, after pretreatment with prazosin or control (***P<0.001). Data are displayed as mean ± SEM (n=6).
Author(s)
Mr Liam O’callaghan1, Professor Russ Chess-Williams1, Dr Katie Powell1, Associate Professor Catherine McDermott1
1Bond University, Gold Coast, Australia
References
- Shariat SF, Chade DC, Karakiewicz PI, Scherr DS, Dalbagni G. Update on intravesical agents for non-muscle-invasive bladder cancer. Immunotherapy. 2010;2(3):381-392. doi:10.2217/imt.10.1.
- Koch GE, Smelser WW, Chang SS. Side Effects of Intravesical BCG and Chemotherapy for Bladder Cancer: What They Are and How to Manage Them. Urology. 2021;149:11-20. doi: 10.1016/j.urology.2020.10.039.


