Volume 25 Number 1
Antimicrobial effects of an acidified nitrite foam on drip flow reactor biofilm
C Michael Miller, Garth James, David Bell, Greg Schultz
Keywords biofilm, wound healing, Infection, nitric oxide, acidified nitrite foam
For referencing Miller C M et al. Antimicrobial effects of an acidified nitrite foam on drip flow reactor biofilm. Journal of Wound Management 2024;25(1):16-21.
DOI
10.35279/jowm2024.25.01.05
Submitted 31 October 2023
Accepted 8 January 2024
Abstract
Background Nitric oxide (NO) plays critical roles in wound healing, including stimulating vasodilation, angiogenesis and broad antimicrobial activity.
Aim To measure the effect of an acidified nitrite foam (ANF) on biofilms created by six different microbes.
Methods A novel method of generating, delivering and topically applying NO gas at the point of care was developed using ANF in a mixed bubble foam and was tested in vitro against six common microbial wound pathogens.
Results A single 5-minute topical exposure of the NO bubble gas formulation generated a 5.8-log10 reduction of mature biofilm of Pseudomonas aeruginosa, a 5.1-log10 reduction of Staphylococcus aureus biofilm, a 4.0-log10 reduction of Staphylococcus epidermidis biofilm, a 3.2-log10 reduction of Proteus mirabilis biofilm, a 2.7-log10 reduction of Acinetobacter baumannii biofilm, and a 1.5-log10 reduction of Candida albicans biofilm.
Conclusion The efficacy of a 5-minute treatment of ANF used on biofilms of P. aeruginosa, A. baumannii, S. aureus, C. albicans, P. mirabilis and S. epidermidis was confirmed. The treatment resulted in a significant reduction in colony-forming units per square centimetre (CFU/cm2) comparable to or surpassing other methods of NO gas application, suggesting NO containing foam’s utility as a point of care solution for chronic wounds with elevated bioburden and biofilms where levels of endogenously produced NO may be insufficient for wound healing completion.
Key messages
- Nitric oxide (NO) plays a critical role in wound healing, including stimulating vasodilation, angiogenesis and broad antimicrobial activity.
- Deployment of NO as a topical treatment has traditionally been challenging due to NO’s short half-life.
- The micro foam-based means of NO generation, transport and exogenous application takes full advantage of NO’s potential to engage and disrupt biofilms, destroy bacterial pathogens, and serve as a real-time exogenous NO supplementation agent for chronic wounds.
- Acidified nitrite foam (ANF) significantly reduced the colony-forming units per square centimetre (CFU/cm2) of each microbe tested after 5 minutes of treatment.
- Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis and Proteus mirabilis seem particularly susceptible to NO antimicrobial actions.
Reflective Questions
- Can these in vitro results translate to improved wound healing in humans?
- Could ANF be the antimicrobial solution for managing biofilms?
- How beneficial would a 5-minute treatment of topical NO be for patients suffering with painful and costly chronic wounds?
Introduction
Chronic wounds are complex and inflammatory in nature. High wound bioburden and the development of polymicrobial biofilms result in pathologically prolonged inflammation which plays a significant role in the disruption of the normal healing cascade and wound chronicity1,2. However, these may be overcome by bactericidal treatments, such as that of nitric oxide (NO), which is produced and used by the body to combat biofilm-forming bacteria colonies.
Biofilms are bacteria living in heterogeneous, multicellular communities encapsulated in self-produced extracellular polymeric substances (EPS)1,3. The biofilm’s protective polymer matrix provides an optimal environment for bacteria to evade host immune response and antimicrobial action. Once established, these biofilm colonies surround themselves by free swimming planktonic cells dispersed to colonise new habitats4,5. When compared to their planktonic counterparts, biofilm colonies generally exhibit greatly increased resistance and up to 10,000 times higher tolerance to immune defences, biocides and antibiotics, thus leading to chronic infections3,4. For this reason, effectual modes of action for eradication of biofilms must include a potentiated broad spectrum antimicrobial mechanism6–8.
NO is an endogenous gasotransmitter that plays a vital role in wound healing. The reported and previously demonstrated bactericidal capability of NO gas supports myriad reasons for its clinical application. Common exogenous applications of NO rely on some form of acidified nitrite following the pathway shown in the equations below9,10.
|
2 NO2 +2H+ → N2 O3+H2 O |
|
2 N2 O3 → NO2 +NO |
Emerging evidence suggests that NO can induce biofilm dispersal, increase bacteria susceptibility to antibiotic treatment, and induce cell damage or cell death via the formation of reactive oxygen or reactive nitrogen species3. The antimicrobial activity of NO is due to both nitrosative and oxidative mechanisms which eventually result in the production of dinitrogen trioxide (N2O3) and peroxynitrite (ONOO–)11,12. Dinitrogen trioxide induces DNA deamination, while peroxynitrite causes membrane lipid peroxidation13,14.
The full advantage of NO treatment can be accessed on site by applying the acidified nitrite as a foam (ANF)15,16. In this context, a foam is ideally comprised of micro bubbles of gas surrounded by thin films of liquid and surfactant. Presenting an ANF requires mixing of one foam derived from a liquid solution of acid, surfactant and water, and a similar foam derived from a solution of nitrite salt, surfactant and water, dispensed by a hand pump that creates a foam when depressed.
These bubbles are comprised of a thin layer of water sandwiched between layers of surfactant molecules. Because the bubble walls are comprised primarily of water and surfactant, the acid and nitrite foams easily coalesce when mixed, exposing the reactants to each other. Importantly, the NO product that is produced is engulfed inside the bubble wall, which forms a cluster of ultra thin filmed bubbles containing NO. These bubbles create an ‘airtight net’ that prevents the entrapped NO from escaping to the ambient air during formation and promotes transport of NO to the tissue site on which the NO bubbles are applied, as illustrated in Figure 1.
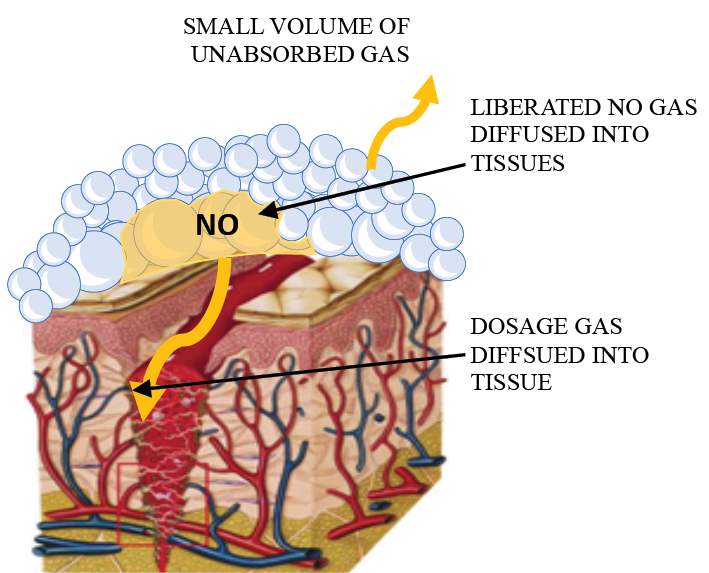
Figure 1. Graphic showing ANF delivery of NO gas
Methods
To address the challenges reported herein, a micro foam-based means of NO generation, transport and exogenous application as reported in two US Patents was used17,18. A series of experiments were designed to test whether the effectiveness of ANF is equal to or greater than that reported by other modes of action at disrupting specific biofilms and killing their respective microbial pathogens.
The testing was conducted by the Medical Biofilms Laboratory (MBL) of the Center for Biofilm Engineering (CBE) at Montana State University. Biofilms were created using an in vitro model system, Drip Flow Biofilm Reactor® (DFR 110-6, Biosurface Technologies Corp., Bozeman, MT). The DFR (Figure 2) is designed to model a low shear environment and has been approved by ASTM as a standard method for growing Pseudomonas aeruginosa biofilms (method E-2647-20). Testing was performed using the modified procedure described below because this procedure was found to be more relevant to the treatment of wound biofilms.
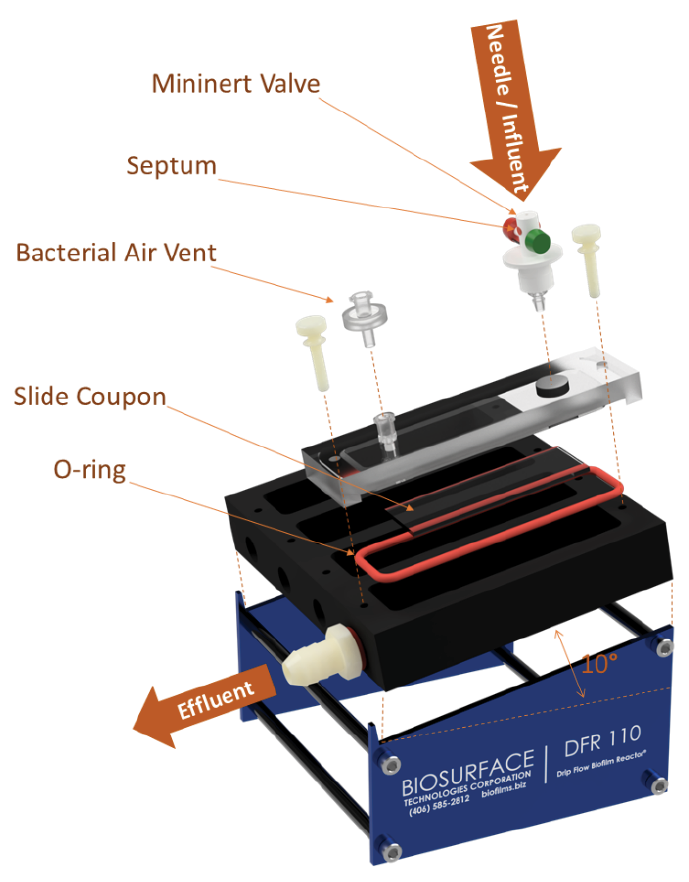
Figure 2. Schematic of the DFR
Although numerous in vitro wound models have been proposed, none have been clinically validated to predict effectiveness of anti-biofilm treatments. The DFR method for biofilm growth used in this study was based on an ASTM standard method (E-2647-20) that was vetted through the ASTM process, including the evaluation of repeatability and reproducibility based on results from 10 independent laboratories. For this study, changes to the standard method were made to better represent the wound microenvironment, including a collagen-coated surface. The DFR certainly captures one of the most important characteristics of biofilms, tolerance to antibiotics19 and other antimicrobial agents20. The DFR has also recently been used to test other wound care products21.
Pathogens
Testing was performed on biofilms of P. aeruginosa MBL Strain SWR 215 (a clinical chronic wound isolate obtained from a wound biopsy by the Southwest Regional Wound Center in Lubbock, TX and preserved by the MBL), Acinetobacter baumannii (ATCC BAA-1797), Staphylococcus aureus (ATCC BAA-1556), Candida albicans (ATCC 10231), Staphylococcus epidermidis (ATCC 35984), and Proteus mirabilis (ATCC 7002). These strains are maintained as frozen stock cultures at –80°C in the MBL.
Treatments
NOxy Health Products supplied the NO producing foam. The foam was obtained as a mixture of two components (a solution A and a solution B) contained in separate foam pumps. Solution A comprised a solution of citric acid. Solution B comprised a solution of sodium nitrite. Both contained a portion of surfactant. The steps to apply an exogenous NO foam treatment are listed below.
Dispense an amount of Solution A foam into an appropriate container by depressing the Solution A pump head consistently and briskly.
Dispense an amount of Solution B foam into the same container by depressing the Solution B pump head consistently and briskly.
Using a plastic paddle or mixing tool, stir the two foams vigorously for 5 seconds to mix.
Apply the mixed NO foam by pouring the foam out of the container onto the site or using a spatula/spoon for a more measured/precise placement of the foam.
Biofilm growth
Six-channel DFRs, equipped with hydroxyapatite- and collagen-coated (HAC) glass coupons, were operated at 33°C (approximate wound temperature) under aerobic conditions. Hydroxyapatite-coated glass slides, prepared by Clarkson Chromatography, were collagen-coated using a coating matrix kit (Life Technologies Corporation) following the manufacturer’s instructions.
Approximately 20 minutes prior to inoculation, sterile media (1%-strength brain-heart infusion broth with 0.5% adult bovine serum) was dripped into each channel and allowed to collect over the coupons. A conditioning layer on the surface of the coupons was observed to form.
Each channel of the reactor was then inoculated with 1mL of an overnight culture of the test organism containing approximately 8x1010 CFU per mL for the bacteria and approximately 7x1010 CFU per mL for the yeast. The reactor was then set at a 10° angle and sterile media was dripped through the reactor at a rate of 10mL/hr per channel for 72 hours to mimic the relatively slow fluid flow of wound exudate.
Biofilm treatment and sampling
For treatment, flow to the DFR was halted and the mixed NO foam or individual solution A and B foams were applied as directed to a coupon. Enough of the foam was applied to completely cover the entire DFR coupon (approximately 20mL of foam). Before applying the foams, the untreated control coupon was removed from the DFR and analysed by plate count, as described below. Following the contact time, the coupon(s) were removed from the DFR and rinsed with phosphate-buffered saline (PBS) to remove residual foam and unattached bacteria.
Viable plate counts
The number of viable bacteria on each of the coupons was determined by viable plate count. After rinsing, the coupons were placed in 10mL of 2X-strength Dey-Engley (D/E) neutralisation broth. Biofilm on the coupons were scraped and rinsed with the D/E, then sonicated (30 seconds in a 50mL conical centrifuge tube at 60kHz in an ultrasonic cleaner Model CSU3HE, Tuttnauer, Hauppauge, NY), vortexed (2 minutes), and sonicated (30 seconds) to further remove and disaggregate the biofilms. The biofilm suspensions were then serially diluted with PBS and plated on Tryptic Soy Agar (TSA). The plates were then incubated at 37°C for 24–48 hours, following which the number of CFU were counted. Based on the dilution and surface area of the slide, the number of CFU per unit area was calculated and logarithmically (base 10) transformed. Log differences between the treatment and untreated control biofilms were calculated for each experiment set. Each CFU/cm2 measurement was reported and is available upon qualified request.
Results
For each test organism, the viable plate counts in CFU/cm2 for an untreated control (no treatment), a Solution A foam treatment, a Solution B foam treatment, and the NO foam mixture treatments were measured. The NO foam treatments were applied to three coupons in the DFR. These six measurements corresponded to the six plates in the DFR. All foam treatments lasted 5 minutes.
To determine if there were differences in the CFU/cm2 between the no-treatment or control treatments versus the other treatments, an ANOVA analysis was conducted using the Tukey Honest Statistic Difference (HSD) test on JMP 16.1 (SAS Institute Inc.).
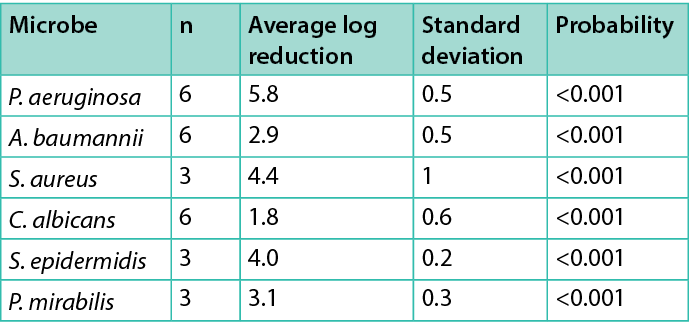
Figure 3 shows the control and each treatment CFU/cm2 for each organism tested. Table 1 summarises the average log reduction between the control and the 5-minute NO foam treatment. Since a 5-minute treatment of NO foam was significant for all organisms tested, Table 1 also lists the probability that the measured difference is not significant for each microbe tested.
ANF significantly reduced the CFU/cm2 of each microbe tested after 5 minutes of treatment. The P. aeruginosa, S. aureus, S. epidermidis and P. mirabilis seem particularly susceptible to NO antimicrobial actions. The A. baumannii biofilm was less susceptible (2.9 average log reduction), and the C. albicans biofilm was the least vulnerable (1.8 average log reduction), which is consistent with the large volume of results of acidified nitrite gels/creams and NO in general.
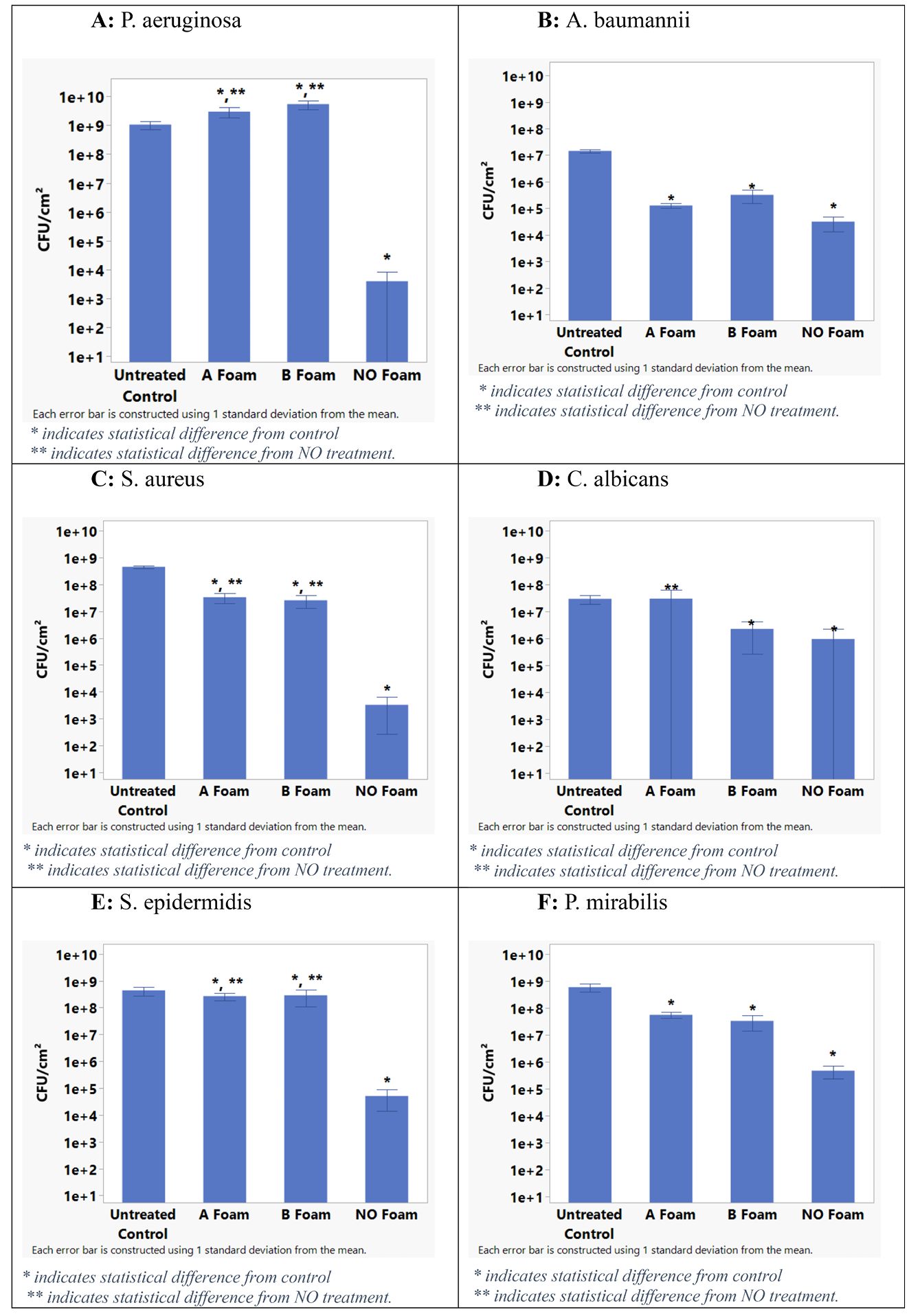
Figure 3. Log CFU/cm2 bar chart of pathogen tests
Table 1. Microbes tested, the number of tests, the average log reduction of CFU/cm2, one standard deviation of the average, and the probability that the reduction is not significant
Discussion
NO is emerging as a promising, novel approach to regulating inflammation, eradicating bacterial infections, and treating antibiotic resistant bacteria and biofilm infections3,8,11,12,22. NO offers broad-spectrum potential across a representative range of pathogens. Although it has been reported that care must be taken in its clinical use, NO’s ability to exogenously disrupt biofilms and destroy planktonic bacteria is finding its way into the literature with increasing frequency3,7,11,12,23,24.
Recent developments in NO have proven it to be a novel approach to the treatment of antibiotic resistance and biofilm eradication3,4,25–29. However, the usefulness of NO on biofilms is dose-dependent and is therefore based on the effectiveness of the delivery method. NO from various donors and doses have been shown to inhibit biofilm formation as well as induce biofilm dispersal and potentiate antibiotic or antimicrobial treatment in Gram-negative bacteria, including P. aeruginosa, and the Gram-positive bacterium, S. epidermidis, as well as the fungal infection, C. albicans28–32. In vitro, ~170.2nmol NO/mg of NO-releasing chitosan film significantly decreased methicillin resistant S. aureus (MRSA) viability by more than 3 log in vitro, reduced biofilm biomass and improved wound healing by approximately seven times that of non-NO-releasing chitosan controls33. Conversely, S. aureus displayed increases in biofilm biomass when exposed to 0.9–2µM of NONOate, while biofilms were reduced at 125–1000µM of NONOate31,34. No studies were identified that demonstrated biofilm dispersal for A. baumannii and P. mirabilis, beyond the work presented in this manuscript.
Conversely, NO’s deployment as a topical mode of action remains extremely challenging. These difficulties often arise from various challenges in applying a gas to a wound. Mass transfer factors impact NO application at the point of care and include the user’s inability to control desired treatment vectors associated with the applied gas (e.g., how to force the direction of travel of the gas into and not away from affected tissues once applied). A related factor is the lack of a reasonable means of control over driving potentials such as pressure or concentration differentials requisite for diffusion into biofilm tissues. Additionally, the inevitable isolation or stranding of pico- to nanosised bubbles of the NO molecule within stratified viscous layers of common gelatinous carriers, employed in most exogenous treatment regimens, are also a great deterrent29,20. These factors reduce potential NO concentration when used as an exogenous supplement. NO’s reactivity also imposes limitations on its mode of application. NO’s short half-life, which measures only seconds when exposed to ambient conditions, further reduces the potential NO concentration gradient and impedes the delivery of NO to the wound.
The NO micro bubble foam formulation overcomes the serious diffusion barrier that is caused by a viscous mass of gel or cream topical formulations of NO. Gels and creams impede diffusion of NO out of those formulations to the wound bed. In contrast, the NO foam micro bubbles cover the entire wound surface, ensuring intimate contact with and sealing off the complicated surface topology of the wound from ambient air, while simultaneously releasing a predetermined dosage in direct proximity to the targeted bioburden. This mode of action purports to offer the advantages and none of the disadvantages previously encountered in exogenous supplementations.
Conclusions
The data reported above confirms that ANF is an effective antimicrobial agent against the tested biofilms. To date, P. aeruginosa, A. baumannii, S. aureus, C. albicans, P. mirabilis, and S. epidermidis biofilms were tested against a 5-minute treatment of ANF and all the microbes experienced a significant log reduction in CFU/cm2 at least equivalent to or greater than that of other means of NO gas application. These results demonstrate that a single, 5-minute treatment of topical NO gas foam was effective in significantly reducing CFUs of in vitro biofilms.
This study demonstrated that exogenous foam-based NO supplementation took full advantage of NO’s potential to engage and disrupt biofilms and destroy bacterial pathogens, and suggests that foam-based NO may be a point of care solution for rescue of chronic wounds stuck in the inflammation stage of wound healing due to high bioburden and biofilms. Exogenous foam-based NO supplementation may also serve as a resource in those instances where endogenous production of NO is sufficient to start but becomes insufficient to complete the wound healing sequence. Given the advantages of the ANF system and its effectiveness detailed herein, it is hoped that ANF will become a preferred treatment in the clinician’s toolbox to fight planktonic and biofilm infections.
Limitations
Biofilms were grown using a DFR model based on the ASTM-approved standard method E-2647-20 to test anti-biofilm effectiveness. Although modifications to ASTM E-2647-20 were made to better mimic the wound environment, no in vitro method has been developed that can completely capture the in vivo complexities of a wound. There is no in vitro wound model that has been validated to predict the effectiveness of antibiofilm agents for human chronic wounds.
In the method described herein, D/E neutralising broth is used for biofilm recovery because it contains a surfactant (Tween 80) which helps remove and disperse the microorganisms as well as inactivating a variety of antimicrobial agents. Inadequate neutralisation can be indicated in plate count results, with no counts at low dilutions but counts at medium to high dilutions. No evidence of this was seen, but the neutralising effect of the D/E broth was not validated for this specific formulation.
Historically, some antimicrobial treatments have performed well in a laboratory setting but perform less well against biofilms in vivo. It is thought that the biofilm penetrates the substrate it is growing on, thereby yielding extra protection from an antimicrobial agent. In other cases, the in vivo environment may neutralise the antimicrobial agent, reducing its effectiveness against the biofilm pathogen.
Further animal model and/or human in vivo data is required to ascertain the effectiveness of topical NO gas therapies as potential benefits in chronic wound care. Testing has been completed to affirm the ANF’s full capabilities against an ex-plant porcine dermal model31. A First-In-Human study has been initiated in Israel to confirm the safety and efficacy of ANF in humans.
Conflict of Interest Statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mr Miller, Dr Bell and Prof Schultz report that financial support was provided by NOxy Health Products. Mr Miller, Dr Bell and Prof Schultz report a relationship with NOxy Health Products that includes consulting or advisory and equity or stocks. Mr Miller and Dr Bell have patents #US10052348 and #US10751364 issued to NOxy Health Products.
Consent in full
The authors herewith certify that they are responsible for the contents of the manuscript. The details of the specific findings detailed in this manuscript have not been published or distributed to any other entity. In consideration of the action of the Editors of the Journal of Wound Management (JWM), in reviewing and editing this submission, the authors hereby transfer, assign, or otherwise convey all copyright ownership to JWM if such work is published in the JWM.
Author(s)
C Michael Miller*1 MS, PE, Garth James2 PhD, David Bell1 PhD, Greg Schultz3 PhD
1NOxy Health Products, 115 Priebe Gulch Dr, Anaconda, MT 59711, USA
2CBE Medical Biofilms Laboratory, Montana State University, Bozeman, MT 59717, USA
3University of Florida, MD OBGYN Wound Research Center, Gainesville, FL 32610-0294, USA
*Corresponding author email miller@noxyhp.com
References
- Li P, Tong X, Wang T, Zhang W et al. Biofilms in wound healing: a bibliometric and visualised study. Int Wound J 2023;20(2):313–327. doi:10.1111/iwj.13878
- Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol 2020;10(9):200223. doi:10.1098/rsob.200223
- Poh WH, Rice SA. Recent developments in nitric oxide donors and delivery for antimicrobial and anti-biofilm applications. Molecule 2022;27(3):674. doi:10.3390/molecules27030674
- Barraud N, Kelso MJ, Rice SA, Kjelleberg S. Nitric oxide: a key mediator of biofilm dispersal with applications in infectious diseases. Curr Pharm Des 2015;21(1):31–42. doi:10.2174/1381612 820666140905112822
- Rather MA, Gupta K, Mandal M. Microbial biofilm: formation, architecture, antibiotic resistance, and control strategies. Braz J Microbiol 2021;52(4):1701–1718. doi:10.1007/s42770-021-00624-x
- Irving S. Managing chronic, nonhealing wounds stalled in the inflammatory phase: a case series using a novel matrix therapy, CACIPLIQ20. Br J Community Nurs 2019;24(Sup9):S33-S37. doi:10.12968/bjcn.2019.24.Sup9.S33
- Zhang Y, Young P, Traini D, Li M et al. Challenges and current advances in in vitro biofilm characterization. Biotechnol J 2023 Jul:e2300074. doi:10.1002/biot.202300074
- Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric oxide therapy for diabetic wound healing. Adv Healthc Mater 2019;8(12):e1801210. doi:10.1002/adhm.201801210
- Seitz W. Systems and methods for topical treatment with nitric oxide: United States Patent 7,048,951; 2006 May 23.
- Fine D. Method and apparatus for nitric oxide generation. United States Patent 7,040,313; 2006 May 9.
- Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 1998;25(4–5):434–456. doi:10.1016/ s0891-5849(98)00092-6
- Mourenza Á, Gil JA, Mateos LM, Letek M. Oxidative stress-generating antimicrobials, a novel strategy to overcome antibacterial resistance. Antioxidant 2020;9(5):361. doi:10.3390/antiox9050361
- Möller M, Botti H, Batthyany C, Rubbo H et al. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem 2005;280(10):8850–8854. doi:10.1074/jbc.M413699200
- Fang FC. Antimicrobial actions of reactive oxygen species. mBio 2011;2(5):e00141–11. doi:10.1128/mBio.00141-11
- Afzali H, Khaksari M, Norouzirad R, Jeddi S et al. Acidified nitrite improves wound healing in type 2 diabetic rats: role of oxidative stress and inflammation. Nitric Oxide 2020;103:20–28. doi:10.1016/j.niox.2020.07.001
- Afzali H, Khaksari M, Jeddi S, Kashfi K et al. Acidified nitrite accelerates wound healing in type 2 diabetic male rats: a histological and stereological evaluation. Molecule 2021;26(7):1872. doi:10.3390/ molecules26071872
- Ghaffari A, Neil DH, Ardakani A, Road J et al. A direct nitric oxide gas delivery system for bacterial and mammalian cell cultures. Nitric Oxide 2005 May;12(3):129–40. doi:10.1016/j.niox.2005.01.006
- Ghaffari A, Jalili R, Ghaffari M, Miller C et al. Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen 2007;15(3):368–377. doi:10.1111/j.1524- 475X.2007.00239.x
- Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, Watters C, Stewart PS, Dowd SE. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care 2010 Aug;19(8):320–8. doi:10.12968/jowc.2010.19.8.77709
- Buckingham-Meyer K, Goeres DM, Hamilton MA. Comparative evaluation of biofilm disinfectant efficacy tests. J Microbiolog Method 2007;70(2):236–244. doi:10.1016/j.mimet.2007.04.010
- Regulski M, Myntti MF, James GA. Anti-biofilm efficacy of commonly used wound care products in in vitro settings. Antibiotics (Basel) 2023 Mar 8;12(3):536. doi:10.3390/antibiotics12030536
- Torregrossa AC, Aranke M, Bryan NS. Nitric oxide and geriatrics: implications in diagnostics and treatment of the elderly. J Geriatr Cardiol 2011;8(4):230–242. doi:10.3724/SP.J.1263.2011.00230
- Nathan CF, Hibbs JB Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol 1991;3(1):65–70. doi:10.1016/0952-7915(91)90079-g
- Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol 1993;54(2):171–178.
- Nguyen HM, Ngoc Le TT, Nguyen AT, Thien Le HN et al. Biomedical materials for wound dressing: recent advances and applications. RSC Adv 2023;13(8):5509–5528. doi:10.1039/d2ra07673j
- Lundberg JO, Weitzberg E. Nitric oxide signaling in health and disease. Cell 2022;185(16):2853–2878. doi:10.1016/j. cell.2022.06.010
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 2008;7(2):156–167. doi:10.1038/nrd2466
- Barraud N, Storey MV, Moore ZP, Webb JS, Rice SA, Kjelleberg S. Nitric oxide-mediated dispersal in single- and multi-species biofilms of clinically and industrially relevant microorganisms. Microb Biotechnol 2009;2:370–378.
- Yang L, Teles F, Gong WD, Dua SA, Martin L, Schoenfisch MH. Antibacterial action of nitric oxide-releasing hyperbranched polymers against ex vivo dental biofilms. Dent Mater 2020;36:635–644.
- Deppisch C, Herrmann G, Graepler-Mainka U, Wirtz H, Heyder S, Engel C, Marschal M, Miller CC, Riethmüller J. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study. Infection 2016;44:513–520.
- Arora DP, Hossain S, Xu Y, Boon EM. Nitric oxide regulation of bacterial biofilms. Biochem 2015;54:3717–3728.
- Miller CM et al. Acidified nitrite foam anti-microbial action in an ex vivo porcine dermal model. J Wound Manage 2023;24(3):TBC. doi:10.35279/jowm2023.xxx
- Choi M, Hasan N, Cao J, Lee J, Hlaing SP, Yoo JW. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int J Biol Macromol 2020;142:680–692.
- Deja M, Busch T, Bachmann S, Riskowski K, Campean V, Wiedmann B, Schwabe M, Hell B, Pfeilschifter J, Falke KJ, et al. Reduced nitric oxice in sinuse epithelium of patients with radiologic maxillary sinusitis and sepsis. Am J Respir Crit Care Med 2003;168:281–286.
