Volume 25 Number 1
Cross sectional survey of patients with pressure ulcers in primary care settings in Northern Portugal
Paulo Ramos, Cátia Borges, Isabel Azevedo, Assunção Magalhães, Pedro Almeida, António Soares,
Filipa Fontes
Keywords prevalence, pressure ulcer, demographics, epidemiology, primary care
For referencing Ramos P, Borges C, Azavedo I, Magalhães A, Almeda P, Soares A et al. Cross sectional survey of patients with pressure ulcers in primary care settings in Northern Portugal. Journal of Wound Management. 2024;25(1):36-42.
DOI
10.35279/jowm2024.25.01.08
Submitted 29 October 2023
Accepted 23 February 2024
Abstract
Background Pressure injuries, commonly referred to as pressure ulcers (PUs) represent a localised damage to the skin and the underlying tissue due to the persistent application of pressure or pressure combined with shear. The aging demographic in developed countries and the rising incidence of chronic diseases calls for a concerted effort to enhance PU management. For that we need epidemiological studies to understand the magnitude of the problem, and they are scarce in the primary care settings.
Aim The study aims to characterise the patients’ suffering from PUs in our healthcare facilities, categorise the wounds based on their location, type, source of origin and duration of treatment. The information gathered will serve as a valuable reference point in devising strategies to enhance care and optimise cost saving.
Methods This observational, non-randomised, cross-sectional study enrolled 54 primary care patients with PUs in the area covered by ACeS Póvoa de Varzim/Vila do Conde (ACeS PVVC), primary healthcare facility in Northern Portugal, conducted during the month of January of 2019.
Results/ findings The study enrolled 54 patients, with a gender distribution of 35.2% males and 64.8% females. The mean age of participants was 82.63±9.92 years. Almost all 96.3% received their treatment at home, and 1.9% in the ambulatory (outpatient) setting. The mean number of pressure ulcers per patient was 1.47±0.73. A total of 29.6% presented with Stage II pressure ulcers, 29.6% with Stage III and 38.9% with Stage IV or unstageable.
Conclusions/ implications for clinical practice The findings of this study serve as a catalyst for further discussion about the effective management of pressure ulcers at the organisational level. The development of effective strategies for assessment, prevention, and intervention for homecare patients requires a comprehensive understanding of the occurrence of pressure ulcers within the community.
Key messages
This work describes the characteristics of the patients with pressure ulcers in a primary care setting in Portugal that are receiving home care by their family healthcare teams.
The study aims to characterise the patients’ suffering from PUs in our healthcare facilities, categorises the wounds based on their location, type, source of origin and duration of treatment.
The study enrolled 54 patients, with a gender distribution of 35.2% males and 64.8% females. Over one third of the population presented Stage IV PUs or unstageable.
Introduction
Pressure injuries, commonly referred, in the European context, as pressure ulcers (PUs), represent a localised damage to the skin and the underlying tissue due to the persistent application of pressure or pressure combined with shear. This phenomenon, although typically observed over bony prominences, can also be caused by the use of medical devices or other objects.1 PUs are a ubiquitous healthcare challenge that put a significant burden on patients.1 They are characterised by pain, costly treatment and healing difficulties, yet they have a high degree of preventability.2
Several factors are associated with the development of PUs: immobility, low perfusion and altered skin/PU status, moisture, older age, hematological problems, poor health and nutritional status, institutionalisation, incontinence, longer hospitalisation, presence of co-morbidities, among others.3 The assessment of these risk factors is crucial in devising measures to optimise patient outcomes.4 We took into account that these risk factors are not only related to PU development, but are also associated with other dependence-related injuries. The lack of appropriate treatment and care can worsen the severity of PUs, increasing the risk of infection and mortality.5
The aging demographic in developed countries and the rising incidence of chronic diseases calls for a concerted effort to enhance PU management. This requires access to evidence-based best practices, a comprehensive understanding of PU prevalence and characterisation, and an awareness of patient-specific issues. Despite the fact that over four decades have elapsed since the first study on community prevalence of PUs was published in 1977, there have been limited studies conducted in primary care settings that address this issue.6,7 The reported rates of PU prevalence are variable, depending on a multitude of factors, such as study methodologies, individual differences, organisational variables, and sample sizes.3 In 2015 Alves estimated that the prevalence of PUs was 0.7 PU cases per 1000 inhabitants; that they represent 67.2% of all chronic wounds; and that 61.8% of the patients with PUs are in the primary care setting.8 Lopes et al reported a prevalence of 5.76% in hospitalised patients, 4.03% in nursing home residents and 0.02% in the community population.3 The emphasis on providing adequate healthcare in the community has increased worldwide, with studies finding that countries with strong primary care systems have better outcomes and lower healthcare costs. 7
PUs are frequently associated with other disorders and are often seen as an inevitable complication of underlying diseases, resulting in the undervaluation of their impact on the healthcare system. This silent problem often leads to inadequate planning and poor implementation of prevention, treatment and management strategies.9
Clinicians often lack specialised training in the diagnosis and treatment of wounds, as wound care is not a defined specialisation, with various specialists and professionals such as dermatologists, podiatrists, endocrinologists, nurses, vascular surgeons and dietitians, among others, potentially involved in care to varying extents in different healthcare systems. This fragmented responsibility, from a policy perspective, has resulted in a lack of prioritisation of resources and capabilities for wound care, leading to inconsistent treatments, prolonged healing times, and an uncoordinated approach to prevention.10 Additionally, there is a lack of standardised education and formal training programs in place, as well as a lack of implementation of PU prevention policies. For example, in the United States, only 21% of Medicare-certified home care agencies use a validated scale for risk assessment, while 72% of the facilities rely on clinical nursing judgment. Only 28.1% of the facilities have protocols in place for preventive interventions. In the Netherlands, a PUk protocol is available in 78% of home care settings, but these protocols are often of low quality or not updated according to the latest guidelines, and healthcare workers frequently do not receive any formal training on their implementation. Furthermore, process-oriented studies, about the quality of care for nursing home residents, revealed that measures for pressure ulcer prevention were rarely consistent with evidence-based guidelines.11
The Agency for Healthcare Research and Quality (AHRQ) estimates that PUs care costs more than $11 billion annually in the United States, and it is predicted that the global market for PUs care products will reach $4.5 billion by 2024.12
In Portugal, despite international research on PU prevalence in the population and characterisation of wounds starting in the 1970s and 1980s, respectively, PUs were, until recently, not recognised as a healthcare problem, with no management or prevention policies implemented. It was only in 2015 that the National Plan for Patient Health Safety 2015-2020 defined as a strategic goal to achieve 95% rate of implementation of practices to evaluate, prevent, and treat PUs, with the aim of reducing their prevalence by 50% compared to 2014. However, to date, little has been done to achieve these goals. Currently, there is no national funding available to provide coordinated programs for the prevention and management of PUs, nor to implement educational programs for healthcare professionals. There is also a lack of reinforcement of healthcare teams with specialised professionals and limited nationally funded grants for research programs to establish new interventions, practices, and procedures in care.
Although several countries include the implementation of good PU management practices among their patient-safety strategies, there are no standardised epidemiological indicators of chronic wound management.13 It is important to note that, besides healthcare direct costs, the impact of PUs on patient quality of life must also be considered. PU pain can be debilitating, reducing individuals’ ability to participate in physical and social activities, assume comfortable positions, move, walk, and undergo rehabilitation. Although patients often report pain, this does not always prompt action, and many healthcare professionals dismiss such reports. Local pain or discomfort at a potential PU site may be a precursor of pressure damage.14
The present study aims to characterise patient suffering from PUs in our healthcare facilities, categorise the wounds based on their location, type, source of origin and duration of treatment. The information gathered will serve as a valuable reference point in devising strategies to enhance care and optimise cost saving.
Materials and Methods
This observational, non-randomised, cross-sectional study enrolled 54 primary care patients with PUs within the area covered by ACeS Póvoa de Varzim/Vila do Conde (ACeS PVVC), primary healthcare facility in Northern Portugal, conducted during January of 2019.
The community settings in Portugal are organised by geographical areas, and for a better understanding of the sample selection we will briefly explain this organisation. ACeS (Portuguese initials for Health Care Centers Group), is a middle management organisation that aggregates several family health centers, community care independent units, shared assistance resources and public health units. The family health teams are constituted by a family doctor (GP), family nurse and administrative services, if the patient needs a nutrition, psychology or social worker assessment they are referred to the shared assistance resources unit. The community care independent units’ teams are specialised for rehabilitation of patients at home, assistance during pregnancy and in post childbirth and work in the implementation of health prevention programs in schools. Patients can be referred to these units by the family teams or by the hospital teams. In ACeS PVVC all patients have a family health team and that is why we used these teams to identify all patients with PU in home care in our geographical area.
We used a convenience sample, encompassing all patients with an active diagnosis of PU at the time that the study was conducted and their informal caregivers. The healthcare facility’s organisation, population selection, and inclusion/exclusion criteria have previously been described.15
As independent variables, we evaluated the patient PU status (stage, location, area, tissue classification, exudate), as well as relevant clinical and demographic data from their medical and nursing records. The nursing team used the validated Braden Scale16 to assess the risk of PU development, and the Barthel Scale17 for dependency risk. These assessments are mandatory by the national healthcare regulatory board, in all dependent patients, with or without PUs. The burden experienced by the caregivers was evaluated by the Zarit Burden Interview, Short Form Version (ZBI), that has been widely used in different clinical settings, and is validated for the Portuguese population.18,19
A survey was created by the researchers, taking into account European Pressure Ulcer Advisory Panel (EPUAP) recommendations for data collection, to characterise the PUs. All tests used were previously validated for the Portuguese population and are available for public use.
Data on age, gender, primary diagnosis, co-morbidities, technical aids, pain level, treatment frequency, and anatomical location, number and staging of PUs were documented. If a patient had multiple PUs, the total number was recorded, but only the most serious wound was characterised in detail. The amount of time spent in direct patient contact, specifically for dressing changes, was recorded but did not include travel time to and from the facility. Other wound-related activities, such as risk assessment and wound condition monitoring, were not taken into account. The origin of PUs acquisition was recorded if known, i.e. hospital stay, community care, or unknown. Exudate amount was classified using categories of none, scant, moderate, and abundant, based on the consensus document of the World Union Wound Healing Society.20 All data was collected by the family nurse who performs the home care. Patients’ demographics and clinical backgrounds were collected by consulting the clinical records. The characterisation of PUs was done by direct observation. To avoid misclassification and to take into account the differential diagnosis between PUs and other dependence-related lesions all family nurses were trained by the research team nurse wound specialist, and a leaflet was provided to help in the characterisation of the PUs and the how to perform the correct differential diagnosis. The family nurses could consult the research team to clarify any queries.
The family nurses collected written informed consent from PU patients, when possible, if not the main caregiver signed the consent. The study was approved by the Local Ethics Committee and it was conducted in accordance with the Declaration of Helsinki, as revised in 1983.
Statistical analysis was performed using SPSS version 25.0 for Windows 10. The Kolmogorov-Smirnov test was used to assess normality and continuous variables were calculated as mean and standard deviation. Categorical variables, which did not have a normal distribution, were calculated as frequencies and percentages.
Results
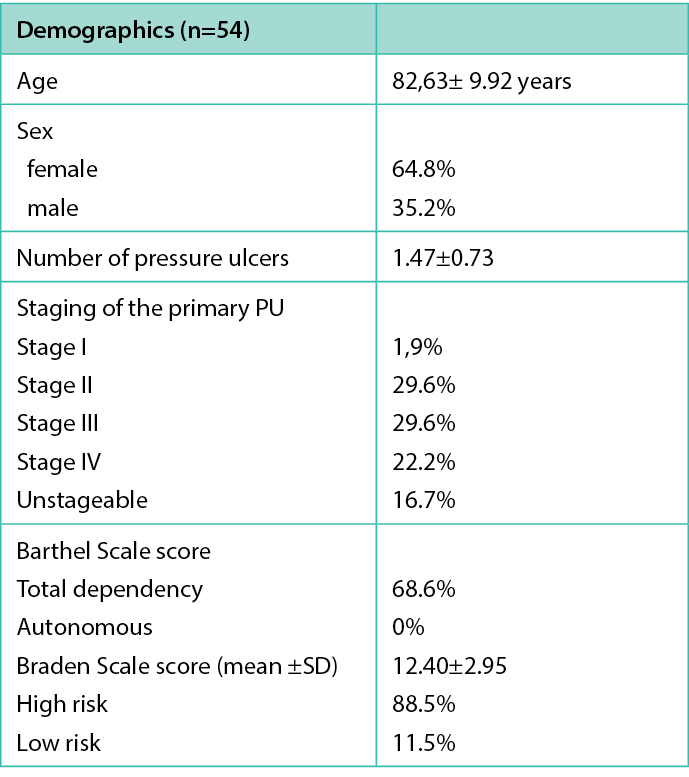
The study enrolled 54 patients, with a gender distribution of 35.2% males and 64.8% females. The mean age of participants was 82.63±9.92 years. Almost all 96.3% received their treatment at home, and 1.9% in the ambulatory setting. Almost all treatments (96.3%), were administered at the patients’ homes (96.3%), with only one performed in the primary care facility. Only one case did not have available information. The mean number of PUs per patient was 1.47±0.73. A total of 1.9% of patients presented Stage I, 29.6% Stage II, 29.6% Stage III and 38.9% Stage IV or unstageable PUs. An assessment using the Braden Scale indicated that 88.5% of the patients were at high risk of developing a PU. The Barthel Scale revealed that 68.6% of the patients were dependent in their daily activities (Table 1).
Table1. Patient demographics
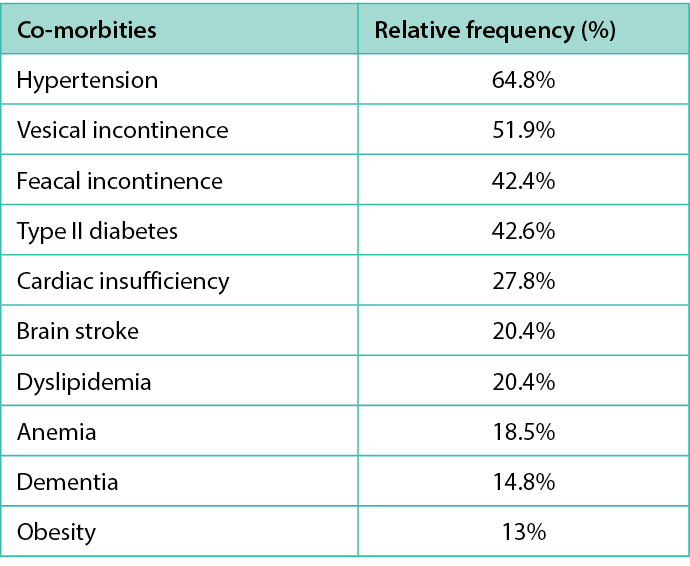
The present study identified that 57.5% of our population was bedriden or confined to wheelchairs, with 18.5% having a prior history of PUs. The most prevalent co-morbidities among the study participants were hypertension (64.8%), urinary incontinence (57.4%), fecal incontinence (42.4%) and Type II diabetes (42.6%) (Table 2). A total of 85.2% had three or more co-morbidities. The data indicated that a mere 59.7% of the study population used a specialised mattress designed for the prevention of PUs, with 40.7% relying on alternating pressure systems and 19% using viscoelastic foam. Interestingly, 42.6% of the patients employed heel protectors, which could be linked to the elevated incidence of PUs observed in the calcaneal region (33.3%).
Table 2. Co-morbities of the patients.
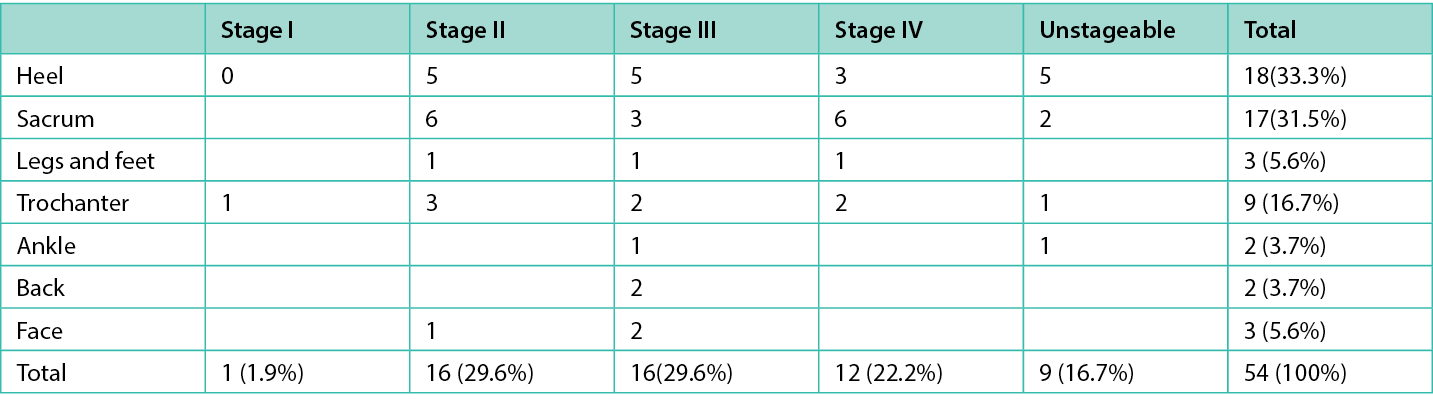
It was observed that the majority of the PUs occurred at the patients’ place of residence (75.9%), while 16.7% originated in the hospital, and in 7.4% of the cases the origin was unknown. The lower extremities were the most frequent location for PUs, accounting for 59.3% of the total. The most common anatomical sites for PUs in our study were the heels (33.3%), sacrum (31.5%), and trochanter (16.7%) as it is described in table 3.
Table 3. Stage and location of the primary PU
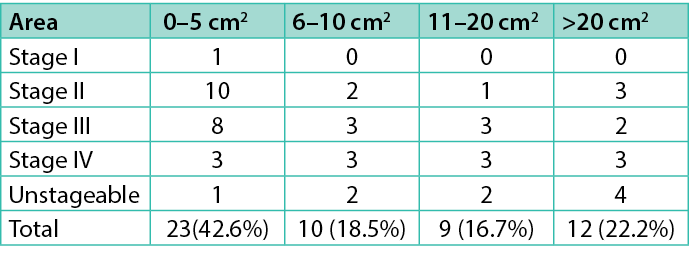
The stage and size of the primary PU is described in Table 4. Most of the primary PU evaluated under this study (61.1%) had between 0 and 10 cm2 of ulcer area, but 22.2% had more than 20 cm2.
Table 4. Stage and size of the primary PU
Most of the treaments were performed twice a week (52.8%), and 35.9% were provided three or more times a week, and 11.3% once a week. Our findings revealed that the prevalence of deep pressure ulcer infection was 5%. The PU infection diagnosis was done taking into account only the clinical signs and symptoms. Among the signs of infection identified, exudative wounds were most prevalent at 22.2%, followed by the presence of devitalised tissue at 20.4%, stalled wound healing at 7.4%, pain at 9.3%, red and friable granulation tissue at 5.6%, and malodour at 3%. To affirm that a PU was infected it was necessary to have 3 or more of the signs previously mentioned(Table 5).21
Table 5. PU stage with the tissue type and exudate level
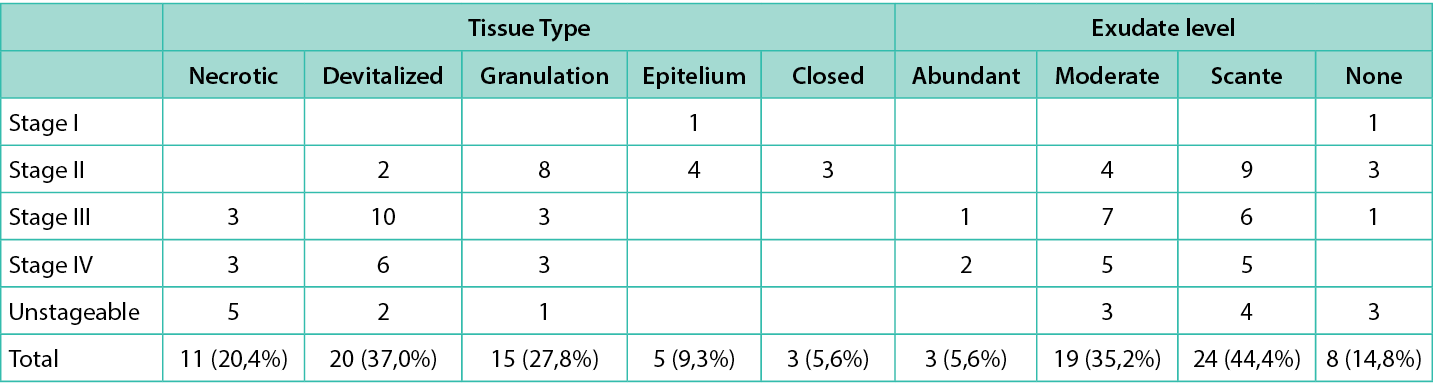
Regarding the surrounding skin, our results indicated that 37% of the patients displayed hydrated skin without notable changes, whereas 63% exhibited some degree of alteration. Specifically, 22.2% showed signs of maceration, 16.7% had dry skin, 14.8% demonstrated redness, and 9.3% presented scaly skin.
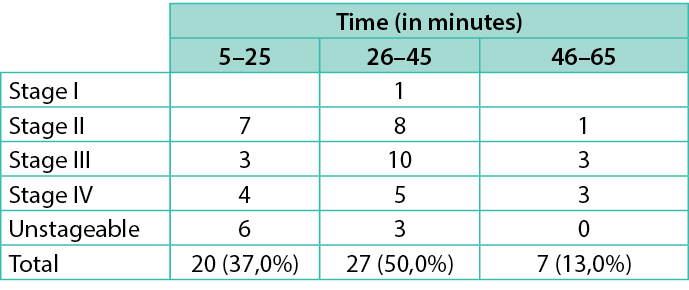
As for the time required for the treatment, the majority of family nurses reported requiring between 16 to 44 minutes (52%). A smaller proportion (22%) required less than 15 minutes and 26% required more than 45 minutes (Table 6). It is worth noting that these estimates did not include the time necessary for the family nurse to travel to the patients’ residences.
Table 6. Stage of PU crossed with the time needed for treatment
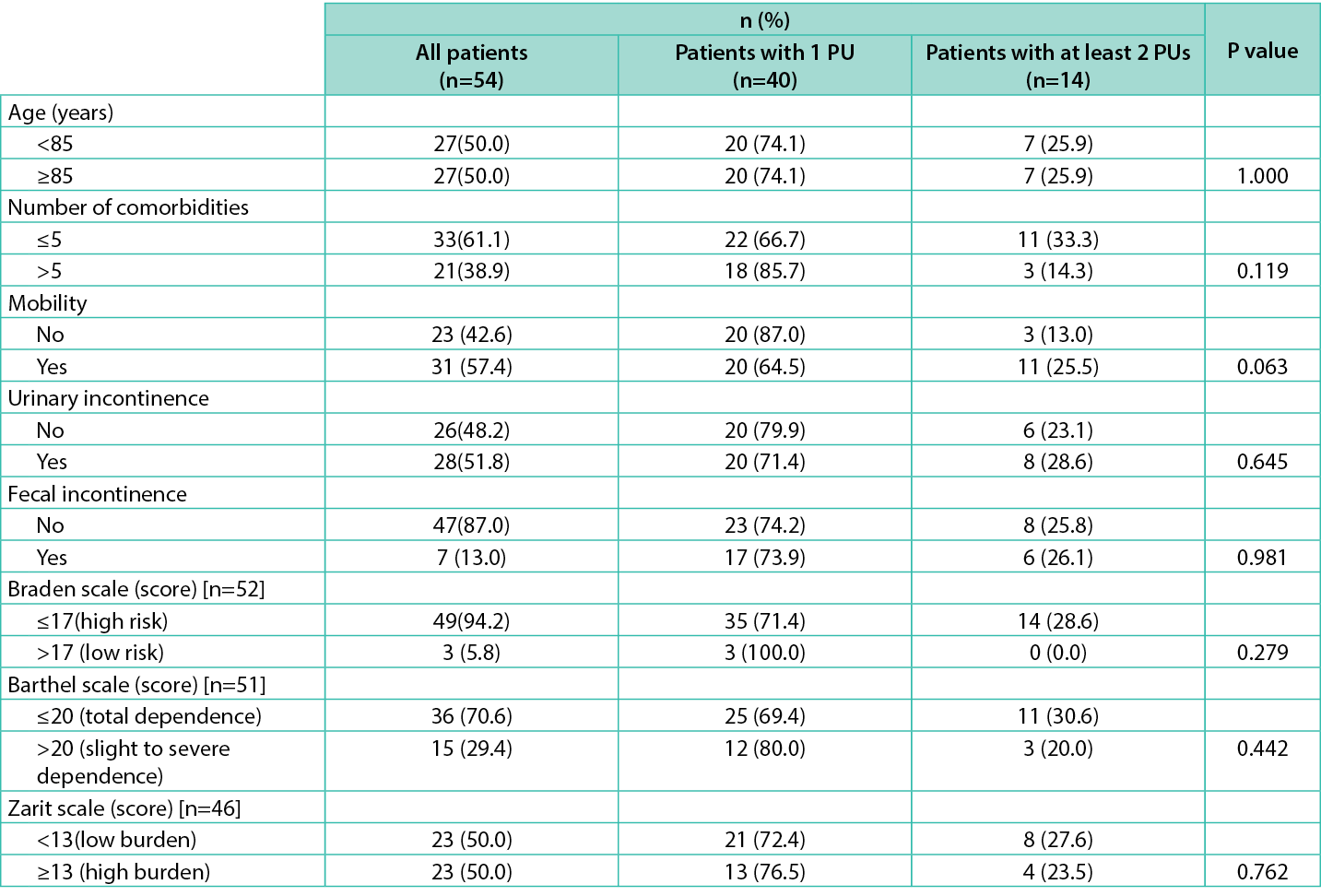
We tried to understand if there were any correlations between the number of PUs and the age, number of co-morbidities, mobility, urinary and feacal incontinence, Braden and Barthel score and level of burden reported by the informal caregiver. There were no correlations found (Table 7), maybe due to the small sample size.
Table 7. Sociodemographic and clinical characteristics of the patients according to the number of PUs.
Discussion
In our study, 54 patients with PUs were analysed. The findings indicated that 88.5% of the patients were at an elevated risk for developing additional PUs, as assessed by the Braden Scale. The predominant stages of PUs among the sample population were Stages II and III, accounting for 59.2% of the total. The average number of PUs per individual was 1.43±0.73, which is concordant with previous research conducted by Passadouro et al in 2016, who reported an average of 1.9 PUs per individual.22 Lopes et al reported that in their PU population of Cova da Beira, 38.3% had Stage III and 21.7% Stage IV, but the study included nursing homes, which were excluded from our study.3
The majority of the population in this study were “the older elderly” (80 years or older). As individuals age, they tend to experience a reduction in subcutaneous fat, a decrease in dermal thickness, and impaired sensory perception, making them more susceptible to the development of PUs. Furthermore, older patients are more likely to have comorbidities, which serve as additional risk factors for PU formation. In this study, a remarkable 85.3% of the participants had three or more co-morbidities, including conditions that can impact tissue perfusion, such as Type 2 diabetes (42.6%), cardiac insufficiency (27.8%), anemia (18.5%), and obesity (13%). It is important that these comorbid conditions are properly managed in order to improve wound healing and reduce the risk of new PUs.2 Our study found that 57.5% of the population was bedridden or confined to a wheelchair, which exacerbates the risk of developing PUs. The immobility associated with these conditions leads to prolonged exposure to external mechanical forces, thereby increasing the likelihood of developing PUs. As expected, we observed a high level of dependency (68.6%), which may also negatively impact the outcome of the wound treatment.2
The most commonly observed site of the primary PU was the calcaneus (33.3%) and the sacrum (31.3%). These results align with those reported by Lopes et al. This phenomenon can likely be attributed to several factors, including the high percentage of bedridden patients (68.6%), inadequate use of specialised mattresses designed for PU prevention (40.3%), and the limited use of heel protectors (42.6%).3,4
In terms of PU classification, we verified some inconsistencies in the Stage II classification, because in two cases the data collector classified as a Stage II PU, but in the type of tissue marked the presence of devitalised tissue. According to EPUAP/ NPIAP/ PPPIA a Stage II PU must have a clean wound bed without any necrotic or devitalised tissue.1 Being aware of this fact, the local wound commission created a tool to help family nurses to perform PU classification.
Most of the primary PUs evaluated in this study (61.1%) had between 0–10cm2 of ulcer area, but 22.2% had more than 20cm2. This is very concerning because of the potencial of severe complications; in fact, large PUs are more prone to infection and impaired healing status. Berlowitz et al found that PU size was important; both Stage II (OR=5.2) and Stage III ulcers (OR=1.5) were more likely to heal compared with Stage IV ulcers.23,24
Since this task is very time-consuming, with direct impact in the work organisation of the clinic and with a huge impact in the heathcare budget, we collected data on the frequency of treatment needed. Most of the treatments were performed twice a week (52.8%), and 35.9% were provided three or more times a week, and 11.3% once a week. We did not collect any data about the dressings used on local wound treatment.
Pain is a significant factor that contributes to the quality of life and its ramifications on treatment protocols should not be overlooked. In many cases, there may be an underestimation of PU-related pain during the day and during treatment due to the majority of patients’ inability to effectively communicate their discomfort. Our study found that 9.3% of the sample population reported experiencing pain. However, it is important to note that this study did not consider the impact of analgesia management on the reported prevalence of pain. The frequency of PU-related pain reported appeared to be remarkably low. This underappreciation of pain associated with PUs may stem from a widespread lack of understanding among healthcare professionals regarding the role of pain in the development and treatment of these wounds. Previous studies have reported much higher estimates of PU pain, with percentages ranging from 37% to 66% in the two largest studies that included over 100 patients. According to the research conducted by McGinnis et al, 75.6% of patients reported experiencing pain related to PU care.14 The low percentage reported in our study could be due to the inability of many patients to report pain, given that 68.6% of our sample was highly dependent.
The significance of multidisciplinary involvement in the management of patients with PUs is emphasised by current guidelines. Nonetheless, our data reveals a lack of engagement with allied healthcare professionals, such as nutritionists and social workers, with only 23.9% of patients being referred. This shortfall in involvement is a matter of concern and merits further examination.
Studies in Portugal of PUs characterisation and prevalence are scarce, especially those performed in the primary care settings, hampering result comparisons. The fact that our study was conducted in a primary care community setting within a context of a universal healthcare system, encompassing all the healthcare centers in the region of the ACeS Póvoa de Varzim/Vila do Conde, which serve most of the patients receiving some type PU care, is one of the strengths of this study. In addition, data regarding PUs was collected with direct observation of the patients’ skin by their family nurses.
Study Limitations
This study is limited by its nature as a single-center, cross-sectional investigation, with a modest number of participants, hence we did not perform inferential statistics tests. Additionally, as a prevalence survey, its reliability is primarily suited for the measurement of long-standing diseases rather than those that are rapidly evolving, as is the case in populations with high turnover. Consequently, our findings primarily capture lower-grade, short-lived PUs, alongside a small proportion of patients with prolonged, chronic wounds, which often represent a significant economic burden to local health systems.25 Moreover, our study population comprised patients in the community under the care of primary care centers in the region, thereby excluding those in nursing homes who are typically treated for PUs in-house and only referred to community nursing services for complex wounds, which may have resulted in an underestimation of the actual prevalence. The nursing staff involved in this study received no formal training in pressure ulcer prevention and treatment from the healthcare institution, it was done only by the research team. Any additional education must be sought through personal initiative, and at their own expense, leading to potential variations in clinical judgment. Even with adequate training, several factors may have contributed to an underestimation of the prevalence of PUs, including difficulties in differentiating Stage I ulcers (non-blanchable erythema) from blanchable erythema, and difficulty in doing a differential diagnosis between PUs and other skin lesions such as incontinence-associated dermatitis, vascular ulcers, and skin tears. According to recent guidelines from the EPUAP/NPIAP/PPPIA, it is crucial to differentiate between PUs and other types of wounds and to attain clinical agreement among healthcare professionals responsible for classifying PUs.1 Besides these issues, we verified in our data same incongruences between the classification of the PUs and type of tissue present, for example in terms of PUs classification we verifed some inconsistencies in the Stage II classification, because in two cases the data collector classified as a Stage II PU but in the type of tissue marked the presence of devitalised tissue. According to EPUAP/ NPIAP/ PPPIA a Stage II PU must have a clean wound bed without any necrotic or devitalised tissue.1 Being aware of this fact, the local wound commisson created a tool to help all the family nurses in our institution to perform a more accurated PU classification.
We verified that in 22.3% of the patients the duration of the treatment was equal or less than 15 minutes, which might mean that the travel time between the heath care center and the patient home was not included. The related activities like preparing the materials, documentation and registering the procedures of care were also not taken into account in this estimation. Otherwise, in 26% of our sample the treatment time equals or surpasses 45 minutes, which represents a very time demanding task for our community care nurses.
Regarding exudate classification, the categories were based on empirical findings, hence the results are highly dependent on the observer training and experience.
Since we did not collect the data regarding all the materials used on local wound management, we could not perform an economic cost analysis.
Conclusions
In this study we conclude that most patients that require home PU treatment presented Stage II and IV PUs, and that the majority (88.5%) presented risk of developing new PUs. There was a lack of devices for preventing PUs in an important percentage (40.3%) our population. There is no correlation between the number of PUs and age, comorbidities, mobility, incontinence, Braden and Barthel scores and caregiver burden.
While causality cannot be established through the data collected in this study, the findings serve as a catalyst for further discussion on the effective management of PUs at the organisational level and can be a starting point to develop more robust research involving several primary care facilities.
To address the specific needs of this patient population, investment in the standardisation of care procedures, patient and caregiver education, the development of a specialised wound care workforce, and the integration of quality technology are imperative. Inadequate management of PUs leads to an increase in healthcare costs and reduces the quality of life for both patients and caregivers.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Paulo Ramos1,2, Cátia Borges1,2, Isabel Azevedo1,2, Assunção Magalhães1, Pedro Almeida1,
António Soares1, Filipa Fontes3,4,5,6
1ARS Norte, ACES Póvoa de Varzim/ Vila do Conde, Wound Care Commission, Portugal
2Portuguese Wound Management Association, Portugal
3Oncology Nursing Research Unit, IPO Porto Research Center (CI-IPOP), Portuguese Oncology Institute of Porto (IPO Porto), Portugal
4EPIUnit - Instituto de Saúde Pública, Universidade do Porto, Portugal
5Laboratório para a Investigação Integrativa e Translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal
6Departamento de Ciências da Saúde Pública e Forenses e Educação Médica, Faculdade de Medicina da Universidade do Porto, 4200-450 Porto, Portugal
*Corresponding author email pramos@arsnorte.min-saude.pt
References
- European Pressure Ulcer Advisory Panel NPIAP and PPPIA. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance.; 2019.
- Furtado KAX, Infante P, Sobral A, Gaspar P, Eliseu G, Lopes M. Prevalence of acute and chronic wounds – with emphasis on pressure ulcers – in integrated continuing care units in Alentejo, Portugal. Int Wound J. 2020;17(4). doi:10.1111/iwj.13364
- Lopes TS, Videira LMM dos S, Saraiva DMRF, Agostinho ES, Bandarra AJF. Multicentre study of pressure ulcer point prevalence in a Portuguese region. J Tissue Viability. 2020;29(1). doi:10.1016/j.jtv.2019.11.002
- Sumarno AS. Pressure ulcers: the core, care and cure approach. https://doi.org/1012968/bjcn201924Sup12S38. 2019;24:S38-S42. doi:10.12968/BJCN.2019.24.SUP12.S38
- Khor HM, Tan J, Saedon NI, Kamaruzzaman SB, Chin AV, Philip JHP et al. Determinants of mortality among older adults with pressure ulcers. Arch Gerontol Geriatr. 2014;59(3). doi:10.1016/j.archger.2014.07.011
- Barbenel JC, Jordan MM, Nicol SM, Clark MO. Incidence of pressure sores in the Greater Glasgow Health Board Area. Lancet. 1977;310(8037). doi:10.1016/S0140-6736(77)90676-6
- Stevenson R, Collinson M, Henderson V, Wilson L, Dealey C, McGinnis E, et al. The prevalence of pressure ulcers in community settings: An observational study. Int J Nurs Stud. 2013;50(11). doi:10.1016/j.ijnurstu.2013.04.001
- Alves PJP. Feridas: prevalência e custos. 2015.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6). doi:10.1111/j.1524-475X.2009.00543.x
- Järbrink K, Ni G, Sönnergren H, et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6(1). doi:10.1186/s13643-016-0400-8
- Paquay L, Wouters R, Defloor T, Buntinx F, Debaillie R, Geys L. Adherence to pressure ulcer prevention guidelines in home care: a survey of current practice. J Clin Nurs. 2008;17(5):627–636. doi:10.1111/J.1365-2702.2007.02109.X
- Sen CK. Human wounds and its burden: An updated compendium of estimates. Adv Wound Care. 2019;8(2). doi:10.1089/wound.2019.0946
- Díaz-Herrera MÁ, Martínez-Riera JR, Verdú-Soriano J, et al. Multicentre study of chronic wounds point prevalence in primary health care in the Southern Metropolitan Area of Barcelona. J Clin Med. 2021;10(4). doi:10.3390/jcm10040797
- McGinnis E, Briggs M, Collinson M, et al. Pressure ulcer related pain in community populations: a prevalence survey. BMC Nurs. 2014;13(1). doi:10.1186/1472-6955-13-16
- Ramos P, Borges C, Azevedo I, et al. Burden of informal caregivers of patients with pressure ulcers in a primary care setting. J Wound Care. 2022;31(10):864-871. doi:10.12968/JOWC.2022.31.10.864
- DGS. Escala de Braden: Versão Adulto e Pediátrica (Braden Q). Orientação da Direção Geral Saúde. 2011;017:1–10.
- Araújo F, Oliveira A, Pinto C, Ribeiro J. Validação do Índice de Barthel numa amostra de idosos não institucionalizados. Rev Port Saúde Pública. 2007;25(2):59–66.
- Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O’donnell M. The Zarit Burden Interview: A new short version and screening version. Gerontologist. 2001;41(5):652–657. doi:10.1093/geront/41.5.652
- Ferreira F, Pinto A, Adelaide Laranjeira1, et al. Escala de sobrecarga do cuidador de Zarit: características psicométricas na população portuguesa. Cad Saúde. 2010;3(2):13–19.
- World Union of Wound Healing Societies (WUWHS). Consensus Document. Wound exudate: effective assessment and management. Wounds Int. 2019.
- Woo, Kevin; Sibbald G. A Cross-sectional Validation Study of Using NERDS and STONEES to ASSESS Bacterial Burden. Ostomy wound Manag. 2009;(August):40–48.
- Passadouro R, Sousa A, Santos C, Costa H, Craveiro I. Características e Prevalência em Cuidados de Saúde Primários das Feridas Crónicas. J Port Soc Dermatology Venereol. 2016;74(1):45–51. doi:10.29021/spdv.74.1.514
- Berlowitz DR, Brandeis GH, Anderson J, Brand HK. Predictors of Pressure Ulcer Healing Among Long-Term Care Residents. J Am Geriatr Soc. 1997;45(1):30–34. doi:10.1111/J.1532-5415.1997.TB00974.X
- Oyibo SO, Jude EB, Tarawneh I, et al. The effects of ulcer size and site, patient’s age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet Med. 2001;18(2):133–138. doi:10.1046/J.1464-5491.2001.00422.X
- Hopkins A, Worboys F. Establishing community wound prevalence within an inner London borough: Exploring the complexities. J Tissue Viability. 2014;23(4):121–128. doi:10.1016/J.JTV.2014.10.002
