Ahead of Print
Negative Pressure Wound Therapy An update for clinicians and outpatient care givers
Luc Téot, Jan Apelqvist, Ami Fagerdahl, Christian Willy
For referencing Apelqvist J, Fagerdahl A, Teót L, Willy C. Negative Pressure Wound Therapy: An Update for Clinicians and Outpatient Care Givers. J Wound Management, 2024;25(2 Sup1): S1-S56
DOI 10.35279/jowm2024.25.02.sup01
Abbreviations
|
BMI |
Body Mass Index |
|
CI |
Confidence Interval |
|
ciNPT |
closed incision Negative Pressure Therapy |
|
DFU |
Diabetic Foot Ulcer |
|
EBP |
Evidence-based practice |
|
ERAS |
Enhanced Recovery After Surgery |
|
EUR |
Euro |
|
EWMA |
European Wound Management Association |
|
EXS |
Exudate |
|
GP |
General Practitioner |
|
HaH |
Hospitalisation at Home (adapted from French “Hospitalisation á Domicile” (HaD)) |
|
Hb |
Haemoglobin |
|
HBA1C |
Glycated haemoglobin |
|
ICG-FA |
Indocyanine green fluorescence angiography |
|
iNPWT |
incisional NPWT |
|
LoS |
Length of stay |
|
mmHG |
Millimetre of mercury |
|
MMPs |
Matrix Metalloproteinases |
|
NHS |
National Health Service (UK) |
|
NPWT |
Negative Pressure Wound Therapy |
|
NPWTi-d |
Negative Pressure Wound Therapy with instillation and dwell-time |
|
PU |
Pressure Ulcer |
|
PVP |
Polyvinylpyrrolidone |
|
QoL |
Quality of Life |
|
RCT |
Randomised controlled trials |
|
ReOP |
Re-Operation |
|
RR |
Relative risk |
|
RTM |
Remote Therapy Monitoring |
|
sNPWT |
single-use NPWT |
|
sO2 |
Oxygen saturation |
|
SSI |
Surgical Site Infection |
|
SSO |
Surgical Site Occurrence |
|
WHO |
World Health Organisation |
1. Introduction
In 2015 EWMA established an interdisciplinary working group with the objective of preparing a guidance document about Negative Pressure Wound Therapy (NPWT) including its use in clinical practice, as well as its implications for organisation of care, documentation, communication, patient safety and patient perspective, and health economic aspects. This document was published in 2017 with the title Negative Pressure Wound Therapy: Overview, Challenges and Perspectives. 1
By late 2020, talks began about updating this document with the aim of creating a shorter, more easily accessible document, to focus on recent developments and utilisation of the NPWT technology, as well as utilisation of the NPWT technology outside of the hospital setting. The timing coincided with the COVID19 pandemic which provided a new focus on treatment of patients at home or outside of hospital settings, but also impacted negatively on the writing by channelling focus and attention towards urgent tasks in the healthcare system. Work on the document was resumed in 2022, leading to a key session presentation at the EWMA-CICA 2022 conference.2 While continuing work on preparing the current document, the author group has also hosted a key session with a full room at the EWMA 2023 conference.3
1.1 Objectives and focus topics of this document
Objectives of this document are to focus on the following topics:
- Present technological advances and emerging ways of utilising the NPWT technology
- Provide hands-on clinical guidance based on the newest available evidence about the use of NPWT, including solutions, such as reticulated open cell foam dressings with through-holes, closed incision Negative Pressure Therapy (ciNPT) and disposable NPWT.
- Address the use of NPWT in primary care in a home care or out-of-hospital setting, to the extent possible through the use of telemedicine services, with the aim of increasing the standardisation outside of hospitals, and the accessibility of NPWT for home care treated patients.
Development of the NPWT technology and clinical practice since the publication in 2017 of the EWMA Negative Pressure Wound Therapy: Overview, Challenges and Perspectives document has been strongest regarding procedures involving ciNPT, as well as negative pressure therapy with instillation and dwell-time (NPWTi-d). Thus, these topics will figure prominently in this document.
Further, when NPWT was first recognised as an established treatment method, patients were in general treated in an in-patient, hospital setting. However, in accordance with the ongoing, general transition towards primary care-based treatments, this has also been the case for NPWT.
To shed more light on the use of NPWT in primary care across Europe, a survey was conducted by inviting the appointed representatives of the 53 European national wound management associations, which currently make up the group of EWMA Cooperating Organisations, to respond to a questionnaire. The analysis of the data gathered clarified that NPWT was regularly used in primary care to some extent, with only one country representative stating that the treatment was not used in primary care at all. The most common description (by 63 per cent of the respondents) of NPWT organisation in primary care was that the treatment is initiated by the hospital prescribing it, and that the hospital is also responsible for providing the treatment. The survey showed that NPWT in primary care is used, organised, monitored, and financed differently across Europe. The general perception was that NPWT in primary care is limited but could and should be expanded. 4
Regarding the combination of NPWT and telemedicine services, the need for and value of these solutions was highlighted during the COVID19 pandemic. There are lessons learned and experiences to draw on in particular from France that was ahead of other countries in developing a rapid legislative strategy to keep access to the patients. The authors expect that this mode of utilisation of NPWT services will increase in effective wound management in the future. Some reflections about this are included in Chapter 7: Future Perspectives and Trends.
Organisational aspects of treatment by use of NPWT are not covered in this document. Health economy regarding ciNPT is briefly covered in chapter 5.9 and Appendix 3 provides an overview of publications covering costs of ciNPT and cost-savings.
2. Methodology and Terminology
2.1 Literature search methodology
The methodology of this document comprises a general literature review supplemented with individual searches on specific topics along with the addition of the authors’ clinical expertise. Specific literature searches were made regarding the study design, endpoints, and outcomes in comparative/randomised controlled trials (RCTs) of NPWT. Each of the chapters 4−6 include information about search terms used for that particular chapter.
Most research regarding wound healing and NPWT has been related to acute wounds and to a lesser extent chronic/problematic/non-healing wounds.5–9
The opinions stated in this document have been reached by a consensus of the authors involved, based on evidence-based literature, published research articles and clinical experience. This paper is not purely evidence-based or an evaluation of existing products, as this would compromise the primary objective.
The literature review shows that there is a growing body of high-quality evidence for the benefits of NPWT in its different forms (conventional NPWT, NPWTi-d, ciNPT). However, as not every statement in this document can be based on a high-level evidence base, we had to rely on existing information and experience. Health care today is encouraged to be built on evidence-based practice (EBP). EBP refers to the use of best available knowledge, not only from scientific results but also, as importantly, from clinicians’ experience and knowledge together with the patients’ wants and needs.10 In surgical management of difficult cases, the homogenisation imposed by RCT can be difficult to obtain. This is why EBP is a valuable substitute when based on expert opinion.
Since the authors are residents of European countries and EWMA is a European association, the document will particularly take European patients and healthcare perspectives into consideration. The document will focus on the human (clinical) perspective; however, animal-related studies will be mentioned when applicable.
2.2 Document elaboration methodology
Each author was responsible for drafting one chapter in the document, after which the full group of authors provided feedback. This process was repeated several times; thus, the author group has together edited the final document with all authors discussing and agreeing on controversies, statements and discussions.
To get an overview of the current organisation of NPWT in primary care throughout Europe an electronic survey was sent to 53 national wound care associations across 35 countries, which currently make up the group of Cooperating Organisations of EWMA. In total, representatives from 32 Cooperation Organisations in 22 countries distributed throughout Europe responded to the survey. The data was processed and analysed, whereupon a short report was written, submitted to, and published in the Journal of Wound Management. 4
The final draft of the full document has been going through a peer-review and validation process by members of the EWMA Council and other external persons.
2.3 Terminology
The term NPWT refers to a controlled negative pressure (sub-atmospheric) system that is applied topically onto the wound. Stated simply, the method consists of application of negative pressure of usually 75−125 millimetre of mercury (mmHg), in single indications as an exception up to 200 mmHg, to a porous foam specifically designed with pores the size of 400 microns that has been placed inside the wound. Immediate sealing of the wound with an airtight adhesive drape prevents subsequent entry of air from the environment, hence the term ‘vacuum sealing’, which has been used for years with NPWT. The suction is propagated from the vacuum source to the wound bed, leading to a negative pressure in the foam, mechanical force changes on the surface of the wound and removal of exudate.
Two important modifications of NPWT are discussed:
- NPWTi-d: NPWT with a repeated computer-controlled retrograde instillation (i) mostly of saline, as well as antiseptic or antibiotic substance into the sealed wound, followed by a dwell-time (d). This technology was introduced from US, where it was first applied.
- ciNPT, that refers to any type of NPWT with foam or multilayer dressing over closed incisions.
In 20171 it was expected that use of NPWT in outpatient service would increase significantly. However, the earlier mentioned survey documents that this has not been the case. This development is investigated because if we want to use NPWT effectively in primary care, use in outpatient services must increase.
3. Principles of NPWT
NPWT can be regarded as an established wound care method, as it has been in use since the mid-1990s. The dissemination of the method and technology has increased significantly during the last 20 years and it is now available in a large number of countries, assumed to include approximately 80 per cent of the global population.
From today’s perspective, the two modifications NPWTi-d and ciNPT of the conventional NPWT are of particular importance. In the following chapters the principles of conventional NPWT, as well as the modifications ciNPT, introduced in 2006,11 and NPWTi-d, introduced in 2011, will be presented.
3.1 Functional principle of NPWT
The principle of NPWT involves extending the usually narrowly defined suction effect of drainage across the entire area of the wound cavity or surface using an open-pore filler that has been fitted to the contours of the wound. NPWT aids the optimisation of wound healing through the application of sub-atmospheric pressure to help reduce inflammatory exudate and promote granulation tissue. To prevent air from being sucked in from the external environment, the wound and the filler that rests inside or upon the wound are hermetically sealed with an airtight adhesive polyurethane drape that is permeable to water vapour, transparent, and bacteria-proof. A connection pad is then applied over a small hole that has been made in the drape and connected to a vacuum source by means of a tube. The following effects on wound healing and the affected tissue, resulting from applied suction that acts evenly on the entire wound surface, are considered the primary clinically significant benefits of NPWT.12–20
- Reduction of the wound area due to negative pressure acting on the foam, derived from either polyurethane (black) or polyvinyl alcohol (white), pulls together the edges of the wound (wound retraction).
- Stimulation of granulation tissue formation in an optimally moist wound environment. In several situations even over bradytrophic tissue, such as tendons and bone, NPWT was able to stimulate granulation tissue formation.
- Continuation of effective mechanical wound cleansing (removal of small tissue debris by suction) and continuous removal of wound exudate (and, consequently, fewer dressing changes) within a closed system.
- Pressure-related reduction of interstitial oedema with consecutive improvement of microcirculation, stimulation of blood flow and oxygenation.
- Hygienic wound closure; bacteria-proof wound dressing for sealing the wound so no external bacteria can enter the wound and the patient’s own wound bacteria are not spread (reduction of the risk of cross-infections and development of resistance within the health care setting).
- Odourless and clean dressing technique; constant seeping through the dressing onto the patient’s clothing and bedding can be avoided, reducing demands on the nursing staff.
- Reduction in the number of required dressing changes (only necessary every two to three days), which reduces nursing time requirements, particularly in patients with exudating wounds.
- Easy and early patient mobilisation.
3.2 Functional principle of NPWTi-d: Negative Pressure Wound Therapy with instillation and dwell-time
Instillation therapy is a variation of conventional NPWT that has been used since 1996 for the complementary treatment of acute and chronic wound infections after initial surgery.21
This modification involves the retrograde instillation of saline. It is a process recommended by international consensus after 201522,23 and an RCT comparing saline versus saline plus antiseptics.24,25 Antiseptics, such as pyrrolidinone homopolymer compound with iodine or octenidine dihydrochloride, were proposed in case of a patient infection or the presence of a biofilm before 2015. 26,27 Antibiotic substance into the sealed wound via a dedicated tube system has been proposed in extremely selected indications and should not be used as a routine practice.
NPWTi-d permits constantly controlled instillation without burdening the patient or nursing staff. Using today’s computer-controlled programmable therapy units it is possible to automatically control the instillation therapy (the amount of fluid and time for which the substance is allowed to take effect, e.g. 20 min dwell time), duration of the suction period (e.g. 4−6 hours) and then, after a set time during which the instillation is left to take effect with no suction applied, removing the solution by suction and continuation of standard NPWT. NPWTi-d has been successfully used for the treatment of acute wound infections after surgical wound debridement28,29 and on mature biofilm30 but there is growing evidence of benefit for non-infected wounds, as it boosts granulation tissue formation.31 Further, with recently developed foams, an NPWTi-d device can be used as a debridement tool.32–37
An overview of NPWTi-d devices/systems confirmed to be available on the European market in August 2023 can be found in Appendix 1.
3.3 Functional principle of ciNPT: closed incision Negative Pressure Therapy
In this modification of NPWT, negative pressure therapy is applied immediately postoperatively over closed incisions in a variety of surgical specialties and clinical situations, primarily with the aim of preventing the incidence of surgical site infections (SSI), as well as other surgical wound complications, like seroma and hematoma. Since the introduction of this technique in 2006, numerous studies have reported improved outcomes with its use. Similarly, ciNPT appears to have a beneficial effect on wound healing by reducing the lateral tension of the incision line, reducing oedema, thus improving oxygenation of the wound area, and effectively hygienically closing the fresh surgical wound for several days “undisturbed” (e.g., until suture removal).
An overview of ciNPT devices /systems confirmed to be available on the European market in June 2023 can be found in Appendix 1.
4. NPWTi-d
4.1 What is the advantage of NPWTi-d compared to standard NPWT?
The technical interest lies in the ability to bring a liquid into the wound that can modify the healing process of the wound by boosting granulation tissue formation. This liquid can be different and can contain active products, but the recommendation of the international consensus is to use saline as primary intention.22,23 Several studies have been done comparing two groups of patients on NPWTi-d, one with saline alone, the other with saline plus polyhexanide. 24,25 They found no statistically significant difference. Several authors use sodium hypochlorite, polyvinylpyrrolidone (PVP), other antiseptics, and exceptionally, antibiotics, not adapted to administration over an open wound and not supported by published studies.
The chronological analysis of the literature shows a first description of NPWT with instillation by Wolvos in 2006, after the very preliminary work of Fleischmann in 1986 21, which used the principle of instillation with rudimentary means, and technical refinements brought by Rycers. 26 Instillation, different from irrigation which reproduces a shower effect, can be defined as pouring, promoting the proliferation of the collagen tissue. Comparative studies propose a figure of 35 percent increase in granulation tissue formation when using NPWTi-d compared to standard NPWT. 30
In 2013 Fluieraru et al described the clinical use of NPWT instillation with saline, without antiseptics, reporting cases where standard NPWT had failed to bud the wound, while switching to instillation had filled the detachment with a budding tissue.38 This study was followed by a larger study by Brinkert et al39 reporting 131 cases of treatment of complex wounds by saline instillation alone, some of which exposed osteosynthesis material. The superiority of NPWT over standard NPWT, therefore seemed to be confirmed by clinical trials of low statistical value but involving large numbers of patients from different centres.
Kim et al 24 showed in a randomised study the absence of difference between the group with antiseptics and without antiseptics, and a new consensus was developed, confirmed by a second randomised study by the same authors, recommending the use of saline alone in the primary intention, the use of antiseptics being reserved for situations of proven biofilm and significant risk of local infection. Multiple clinical indications followed in different surgical disciplines.40–48
In 2017 Téot et al49 described a new foam shape, with holes 1cm in diameter, regularly distributed, this foam being covered by a second layer of non-perforated foam. This was followed by an extended study by Kim et al.50 The system works on two levels, the first being that of holed foam scraping the surface of the wound to detach the devitalized tissues and guide them through the holes to the top of these macro-columns of budding tissue. The second layer of foam scrapes the surface of the macro-columns and removes accumulated devitalized tissue. This dual effect of rasping removes fibrinous, necrotic tissue from the wound surface within days (Figure 1). This new step therefore makes it possible to offer NPWT with instillation no longer as a solution for draining fluids and stimulating the budding tissue but as a tool covering all the steps from debridement to obtaining a uniform bud filling the entire wound regardless of its depth. It is then possible either to cover the wound surgically with a skin graft, an artificial dermis, or a flap, or to let it evolve towards epidermisation spontaneously.
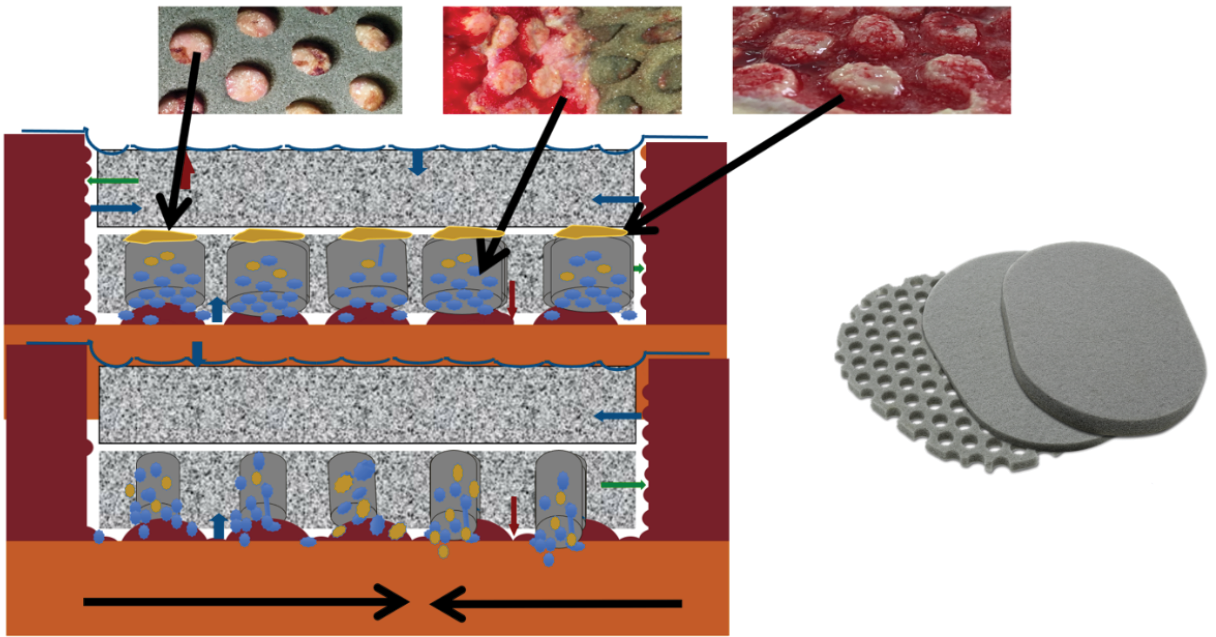
Figure 1. The first foam layer scratches the wound surface by movements initiated by the alternative pressure of the saline coming in and going out, undesired tissues fill the holes, creating macro columns, covered with slough. The second layer is affected by the movements of the first layer and passively scratches the summit of the columns debriding the rest of the undesired tissues. The third foam layer is just to adapt the height of the filling to the height of the edges. Illustration by Luc Téot.
Several publications since 2019 show the interest of this new cleansing approach in complex, deep wounds on patients where surgery is not an option.34–37,51,52
In these situations, the effect of NPWT with instillation is demonstrated by analysis of the literature based on descriptions of clinical cases. The ability to apply to the edges of the wound a combination over time of alternating washing-soaking phases acting as drainage associated with solubilization of local matrix metalloproteinases (MMPs), followed by a negative pressure phase, this repeated several times a day, creates a stimulating local cell support for faster and more effective healing. Even if NPWT is not considered a treatment to achieve complete healing, the reduction in the time to obtain budding tissue filling the wound is major. This is the reason most surgical teams and/or wound and healing experts use this technique before surgically covering. It is still necessary to allow time to fill the wound and not to consider NPWT as an exclusive washing tool, an error still frequently committed by many surgeons, who persist in believing that a flap will obtain filling and coverage. If the filling is already obtained by NPWTi-d, coverage is simplified.
Search terms applied for searching in PubMed, Embase and response in terms of number of references are as follows: “NPWT Instillation”: 312, “NPWTi dwell”: 109, “NPWTi debridement”: 132. The number of systematic reviews identified was 12. A recent Cochrane review about NPWT did not mention instill or NPWTi-d.53
Figure 2 illustrates a healing process with NPWTi-d applied.
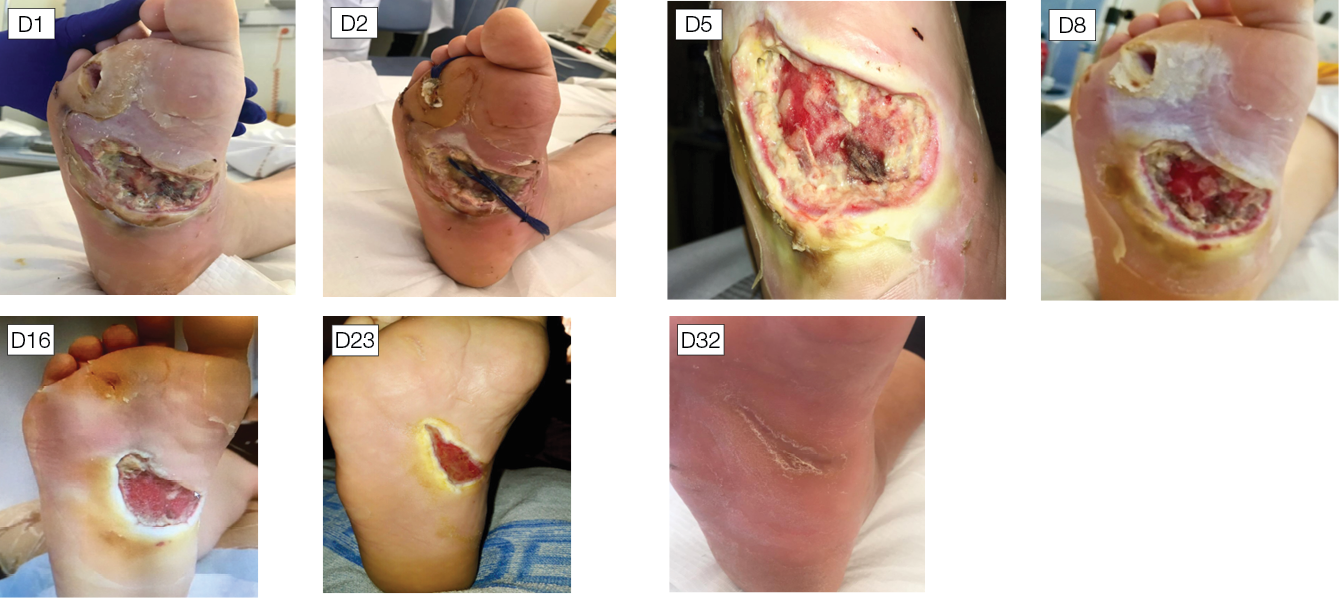
Figure 2. Healing process with NPWTi-d applied
Infected penetrating wound of the foot of a patient with diabetes, without bone involvement. The application of NPWTi-d with perforated foam for 8 days, followed by an application of NPWTi-d with foam for 15 days, combined with the discharge, ensures the promotion of granulation tissue, dries the infected channel, allows the healing of both ends of the path without surgery. D1: At admission the foot shows two communicating wounds. D2: At day 2 the drainage realised in the ward using polyethylene filiform drainage. NPWTi-d using through holes is applied on the proximal wound, adapted foam is applied over the distal wound. Same machine, -125mmHg, 10cc instillation fluid saline every two hours (dwell time 10 minutes). D5: During the second surgical debridement the NPWTi-d true holes foam effect is visible as some macro-columns begin to appear on the surface of the wound. D8: Macro-columns are more obvious, and the distal wound is beginning to close. D16: Changing the foam to adapted foam, wounds are retracting. D23: Stop NPWT. D32: Spontaneous closure is obtained. Credits: Luc Téot, 2018.
4.2 What are the clinical indications for NPWTi-d?
Complex wounds are candidates for the use of NPWTi-d. 54 A wound is recognised as being complex when it is assessed as such by the healthcare professional who takes care of it. The absence of signs of healing and the complex nature of the wounds are most often multifactorial and can be explained by:
- Patient-related factors (lack of evaluation of comorbidities or factors contributing to the wound, difficulties related to patient behaviour and cooperation)
- Factors related to the wound (surface, volume, involvement of noble tissues, erroneous diagnosis of the aetiology of the wound, absence of diagnosis of infectious or ischemic complications of the wound)
- Factors related to the skills and knowledge of healthcare professionals (lack of standardised or appropriate care protocols)
- Factors related to environmental or social difficulties in terms of resources available for the treatment of the wound.
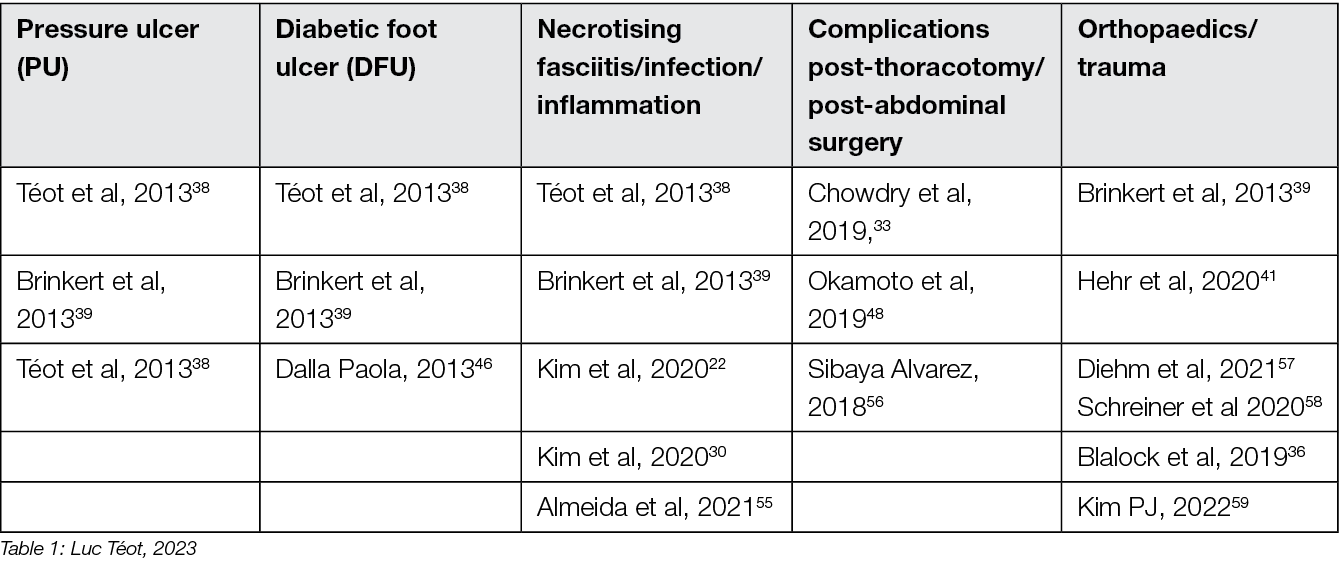
The examples of complex wounds most often described concern Wagner’s Grade 3 and 4 diabetic foot wounds, Stage 3 and 4 pressure ulcers, leg ulcers exposing a bone or tendon structure or large post-traumatic or post-debridement losses in case of devastating infection. Deep detachment wounds resistant to standard NPWT treatment are also considered complex (Table 1). In recent years, studies have shown that NPWT can be used also in inflammatory wounds, once the inflammation process has been reduced by immunosuppressants. 55
Table 1: Principal indications of NPWTi-d in the literature
4.3 What are the obstacles to the use of NPWTi-d?
Several authors highlight the positive impact that NPWTi-d can bring in the management of complex wounds, but the addition of instillation to NPWT adds a level of complexity in the care process, which can in practice lead to difficulties. 43
The perceptions of caregivers have a significant impact on the care and treatment provided to patients. Levels of care can vary widely depending on the clinician’s attitude, knowledge, and skills, and make a substantial difference in outcomes. The introduction into daily care of modern technologies, which do not consider all the innovative parameters, can prove to be problematic.
Clear and accurate information must also be provided to patients about these high-tech treatments so that they can be enlightened about their benefits. Pressure ulcers are in general considered a type of wound that not all clinicians want to deal with. Ageism can also play a role, and if the patient is elderly and in poor health, the misconception that a pressure ulcer is just a manifestation of the end of life remains unfortunately tenacious. However, understanding of the pathophysiology of pressure ulcers has evolved over the past decade and clinicians now recognise that pressure ulcers do not only affect the elderly. Spinal-cord injured patients are also candidates. PU can occur at any age, from new-borns to the elderly.
A patient-centred approach by an expert multidisciplinary team is essential. Responsibility must be shared to best meet the needs of patients.
Cost considerations can also be a barrier to introducing modern technologies, especially if NPWT is to be applied for a long time. The difficulty lies in the dispersion of billings to ensure that the benefit of a reduction in the duration of complete healing does not come from a single action billed to a single actor. The introduction of a modern technology such as NPWTi-d must be accompanied by clear instructions for use, which must be understood and applied by healthcare providers. Currently, it is recommended to use NPWTi-d with perforated foam for 6 days and NPWTi-d without perforated foam for 15 days. Beyond that, the standard NPWT or a portable type NPWT60 can be used if necessary. It is quite possible that the NPWTi-d is used in hospital settings, then relayed to a local follow-up and rehabilitation unit. Furthermore, many of these advanced treatments can only be delivered at home if the primary caregiver has the knowledge and skills to do so, the notion of ‘complex dressing’ is purely a reimbursement quote and does not imply that the care provider is truly capable of mastering the applied technique. Practical training in NPWTi-d is therefore necessary and for instance in France this is offered to all relevant clinicians by the national wound healing association on an annual basis.
Resistance to change remains a non-negligible factor in the refusal to change dressings. Training and communication remain necessary, with a clear message that the NPWTi-d technique improves results, and that it is therefore essential to take ownership of it.
Lack of knowledge of healthcare professionals, as well as accessibility to the technique, are other practical issue that must be resolved by all practitioners and their providers of the technology. There is still too much resistance to change on the principle that the price of the technology is not offset by a recognised benefit. These delays in management are extremely detrimental and it is obvious that the NPWTi-d technique is underused to the detriment of the healing of complex wounds with the consequence of a delay in the management of tissue salvage and a rate of high amputation.61
Summing-up, the development, and introduction of this technology illustrates the challenges of introducing a resource-demanding technology in health care systems.
5. Closed incision Negative Pressure Therapy (ciNPT)
5.1 Introduction
The World Health Organization (WHO) estimate that 230 million major surgeries − operative procedures involving significant risks to the patient − are performed globally each year. In industrialised countries, Surgical Site Infections (SSI) occur in general surgery in about 5% of patients and in high-risk surgical procedures reaching over 50% lengthening the average length of stay of 12.6 days. SSIs burden patients, their families, the healthcare system, and society with loss of productivity, prolonged hospital stays, increased health-care provider visits, and increased financial costs. With a mortality of SSIs of up to 20% in, for example, deep sternal wound infections after median sternotomy for cardiac surgery62, this occurrence can be a rare but devastating complication. Current standards of care for preventing SSIs include the implementation of defined procedures and standardising processes using preoperative prophylactic systemic antibiotics, preoperative soap or antiseptic showesr/baths, aseptic incision site surgical preparation and sterile and meticulous surgical technique and pre- and perioperatively adopted use of appropriate hair removal, antibiotic prophylaxis, avoidance of hypothermia and perioperative glycaemic control.
Also several research groups are trying to reduce the high SSI rate via new wound treatment devices (such as cold-plasma), new suture techniques, antibiotic-coated sutures, silver-impregnated dressings, antiseptic wound irrigation, and iodine-impregnated skin drapes. Additionally, some authors have tried to reduce the SSI-rate through the implementation of comprehensive, multidisciplinary wound management teams, as well as implementation of the Enhanced Recovery After Surgery (ERAS) concept, involving pain treatment, early mobilisation and social help. Yet, the continued high SSI rates in surgery patients demonstrates the need for further preventative methods.
Traditionally, surgeons have closed surgical incisions with primary intention using sutures, staples, tissue adhesives, paper tape or a combination of these methods. However, NPWT has become a viable wound care option since its introduction almost three decades ago. Commercial negative pressure dressings are increasingly used in various clinical settings and for many types of acute and chronic open wounds. Since the early 2000s, surgeons have recognised that foam-based negative pressure dressings applied over closed incisions can also be useful in preventing incision complications. Thus, the use of NPWT on closed incisions (ciNPT) must be considered as a component of a modern bundle approach to reduce postoperative wound infection.
Today, there is a rapidly emerging literature on the effect of NPWT on closed incisions. Initiated and confirmed first with a randomised controlled trial in orthopaedic trauma surgery,11 studies in abdominal, plastic, and vascular surgery with high rates of complications have been reported recently.
5.2 What does ciNPT mean?
The term ‘closed incision negative pressure therapy’ (ciNPT) refers to any type of NPWT with foam or multilayer negative pressure dressing over closed incisions. ciNPT is the commonly used abbreviation. The abbreviation ‘iNPWT’, used for incisional NPWT, and used in the most recent meta-analysis 63 does not seem very suitable to the authors, as it appears too similar to the NPWTi-d used for the instillation technique. Another abbreviation ‘sNPWT’ for single use NPWT seems confusing, as single use elements are generally always used in NPWT, and this property is not limited to use in surgical incisions.
5.3 What is the aim of ciNPT?
The aim of ciNPT is to reduce the surgical site infection rate and the formation of superficial seromas and haematomas.
5.4 What is the underlying mechanism of action of ciNPT?
CiNPT appears to manage the surgical incision by reducing incision line tension, decreasing oedema with a resulting better oxygenation of the wound tissue (respectively decreasing interstitial fluid and increasing blood flow), increasing lymph clearance, and providing a hygienic airtight seal, beneficial in preventing incision complications.
Details
There exist differences between the mechanism of action of ciNPT on closed incisions and NPWT on open wounds. Conventional NPWT on open wounds causes mechanical stress on the wound edges (pressure and gravitational forces), which alters tissue perfusion and has an anti-oedematous effect, leading to angiogenesis and the formation of granulation tissue. There are several articles that deal specifically with the mechanisms of action of NPWT over closed incisions 64–74. The evidence supports the hypothesis that reduction of lateral tension and haematoma or seroma, coupled with an acceleration of the elimination of tissue oedema, are the main mechanisms of action of incisional NPWT.
Lateral tension
ciNPT on closed wounds seems to reinforce healing by reducing the lateral tension in suture lines. The wound then gapes less, and the risk of scarring may decrease. Reductions in lateral tension have been demonstrated during NPWT with computer modelling and in vitro measurements. 73 There is also evidence from animal studies that the breaking strength of wounds is increased through the application of continuous NPWT to closed incisions,75,76. The reduced tension also appears to have a favourable effect on wound scarring and cosmesis.77 It was also observed that ciNPT-treated wounds had significantly lower upregulated genes associated with inflammation, hypoxia, delayed re-epithelialisation, impaired wound healing, and scar formation. Thus, it could be explained that these wounds showed significantly improved mechanical properties (strain energy density, peak strain) and a narrower cutaneous scar,69 as well as a tendency to promote collagen synthesis in closed wounds with dead space, indicating increased tensile strength and enhanced healing72 (Figure 3).
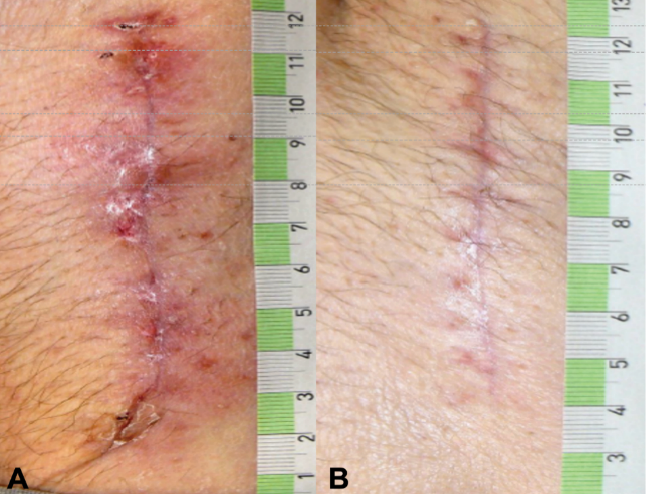
Figure 3. Postoperative wound healing situation 42 days after bilateral prothesis (male patient, age 58, p AOD II b both sides, aortobifemoral prosthesis right/left (Y-Prosthesis). A: traditional dressing. B: ciNPT use for 5 days (Engelhardt M, Rashad NA, Willy C, et al 71). Picture by Michael Engelhardt.
Tissue perfusion:
There are few reports on ciNPT on the effect of perfusion adjacent to closed incisions. An experimental study shows that while conventional NPWT affects perfusion in defect wounds, there is little effect on perfusion in incisional wounds. Using laser Doppler flow measurement, it could be shown that perfusion increased significantly in the patients who underwent ciNPT, while it decreased in the control subjects. Thus, ciNPT could even compensate for the decreased perfusion after mammary artery harvesting.64 In the context of skin flap reconstructions, a significantly higher perfusion of the flap was demonstrated by using the indocyanine green fluorescence angiography (ICG-FA) technique.65 Similar results were also demonstrated by another research group using white-light infrared spectroscopy. The researcher observed a significantly higher increase in oxygen saturation at the wound edge after resection of a soft tissue tumour (with a diameter of more than 10cm on the extremities or trunk) in the study group compared to the control group.66 Using combined laser Doppler spectrophotometry and an ICG-FA system to determine oxygen saturation, haemoglobin content and perfusion patterns, the use of ciNPT was also shown to renew significantly more favourable healing conditions in post-bariatric wounds. These studies have shown that ciNPT has a positive effect on oxygen saturation and tissue perfusion, both of which are associated with the wound healing process.71
Oedema
An experimental study in pigs indicated an effect by NPWT over closed incisions in oedema. The results from studying how radiolabel microspheres are cleared to lymph nodes beneath incisions treated with NPWT suggested increased lymphatic drainage.68
Haematoma and seroma:
Collections of blood and serum in sub-incisional tissues create dead spaces that may predispose patients to wound healing disorders and SSIs. NPWT over closed incisions has been shown to result in reductions in haematoma volume.68,72 This has also been demonstrated clinically for seroma in small RCTs.78–80
5.5 What evidence is there today for the benefit of ciNPT?
There is a rapidly emerging literature on the preventive effect of ciNPT in SSI. There are currently approximately 250 peer-reviewed articles that have been published about ciNPT to manage closed incisions. From these there exist 69 studies comparing ciNPT with standard postoperative wound management in randomised controlled trials (RCTs). To conclude from the experience to date: ciNPT is used in many different surgical disciplines and, overall, most of these studies reported that ciNPT use was associated with decreases in wound complications, wound dehiscence, hematoma/seroma formation and reduction in SSI.
Details
Since 2004, numerous published articles in all surgical disciplines have reported on the experience and results of treating incisions with ciNPT. Thus, to date, there are approx. 250 peer-reviewed articles published on the topic of ciNPT for the treatment of closed incisions. These papers were published between 2004 and 31 January 2024. The keywords included: ‘ciNPT’, ‘active incisional management’, ‘incisional vacuum therapy’, ‘incisional negative pressure wound therapy’, ‘incisional NPWT’, iNPWT, ‘incisional wound vacuum assisted closure’, ‘closed incisional negative pressure therapy’, ‘closed incision management’, ‘active incision management’, sNPWT, ‘preventive negative pressure therapy’ and ‘prophylactic negative pressure therapy’.
All the peer-reviewed articles include a wide variety of studies. In addition to animal studies, economic analyses and some preclinical studies, reviews, meta-analyses, consensus papers and case reports as well as case series have been published. Most of the studies analyse the results of the comparison of ciNPT with standard wound closure methods. A limited number (n=69) of robust, prospective, randomised, comparative, controlled studies on ciNPT use over closed surgical (all surgical disciplines) incisions that might most benefit from this therapy exist. Full, detailed information is available in Appendix 2.
Most publications come from the US, Germany, and Italy. ciNPT has been used in many surgical disciplines, such as trauma and orthopaedic surgery, plastic and reconstructive surgery, general surgery, colorectal surgery, hernia repair surgery, post-bariatric surgery, thoracic and cardiovascular surgery, vascular surgery, gynaecology and obstetrics, as well as urology. The available studies summarise the results and experiences with the treatment of a total of over 25,000 patients (RCTs include 7190 patients, see Table 2).
Table 2. The distribution of pooled patient numbers across the surgical disciplines of all randomised controlled ciNPT studies
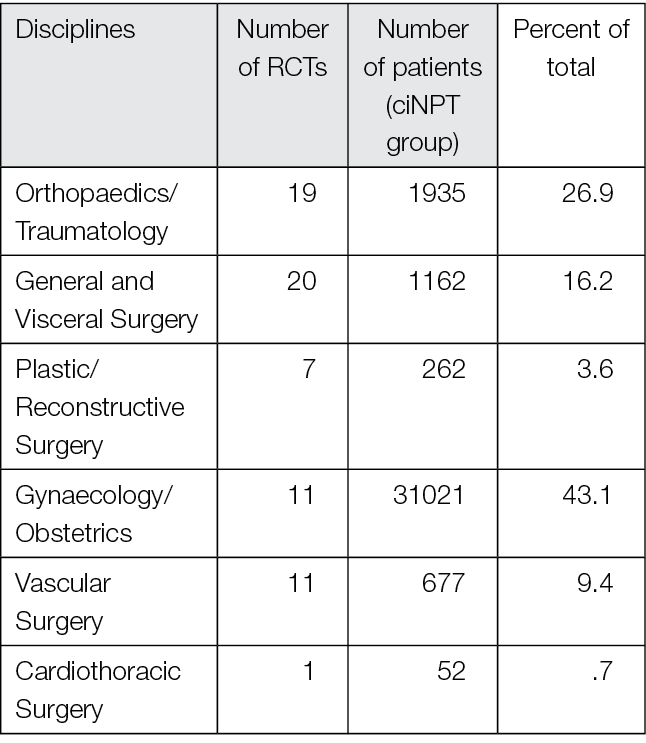
Since 2006, several RCT’s (n=69) and meta-analyses (n=36) have described the effect of NPWT on closed incisions in all surgical fields (see Table 2 and Appendix 2). These studies encompass various wound types and surgical interventions, including high-risk open fracture types (tibial plateau, tibia, pilon, calcaneus), total joint replacement procedures, lower extremity amputations and elective, open colorectal resection. Enrolled patients often had comorbidities, including obesity (BMI ≥30 kg/m2), diabetes mellitus, peripheral vascular disease, or chronic obstructive pulmonary disease.
Overall, the RCTs show a significant benefit of ciNPT for patients in the surgical disciplines of orthopaedics and trauma surgery, vascular surgery, and plastic and reconstructive surgery. Since many studies are under-powered, an even more significant positive effect of ciNPT must be assumed against the background of all studies, including cohorts and case-control studies. However, not only the underpowering of the studies but also the inclusion of partially low-risk patients in collectives that already show low infection rates and in which even a ‘comprehensive’ bundle approach to reduce SSI rates and wound healing disorders can no longer trigger a major effect mean that the benefit of ciNPT cannot be derived with sufficient validity.
The overall positive effect of ciNPT on wound healing rates is also seen in the WHO Global guidelines for the prevention of surgical site infection.81
The 2022 Cochrane Systematic Review/meta-analysis investigating the evidence for the effectiveness of NPWT on postoperative wounds healing by primary closure also underlines the importance of ciNPT for modern wound treatment. The primary outcomes were measures of SSI, mortality, and wound dehiscence.53 The analysed studies evaluated ciNPT in a wide range of surgeries, including orthopaedic, obstetric, vascular, and general procedures. All studies compared ciNPT with standard dressings. The results presented were:
Moderate-certainty evidence; probably results in fewer SSIs (8.7% of participants) than treatment with standard dressings (11.75%); risk ration (RR) = 0.73 (95% CI: 0.63 to 0.85; I(2) = 29%).
Moderate-certainty evidence; probably little or no difference in wound dehiscence between people treated with ciNPT (6.62%) and those treated with standard dressing (6.97%), but there is uncertainty around this as the confidence intervals include risks of benefits and harm; RR = 0.97 (95% CI: 0.82 to 1.16; I(2) = 4%).
Low-certainty evidence for the outcomes of reoperation and seroma; reduced risk of reoperation favouring the standard dressing arm, but there is uncertainty around this as the confidence intervals include risk of benefits and harm. RR = 1.13 (95% C: 0.91 to 1.41; I(2) = 2%; 18 trials with 6272 participants). There may be a reduced risk of seroma for people treated with ciNPT, but there is uncertainty about this as described above. RR = 0.82 (95% CI: 0.65 to 1.05; I(2) = 0%; 15 trials with 5436 participants.)
Low-certainty evidence; there may be a reduced risk of death after surgery for people treated with ciNPT (0.84%) compared with standard dressings (1.17%), but there is uncertainty around this as described above. RR = 0.78 (95 percent CI: 0.47 to 1.30; I (2) = 0 percent).
The August 2023 meta-analysis and trial sequential analysis supports the Cochrane-data while identifying eight previously published general meta-analyses investigating ciNPT and compared their results to present meta-analysis. For the updated systematic review, 57 RCTs with 13,744 patients were included in the quantitative analysis for SSI, yielding a RR of 0.67 (95% CI: 0.59 to 0.76, I (2) = 21%) for ciNPT compared with standard dressing. Certainty of evidence was high. Compared with previous meta-analyses, the RR stabilised, and the confidence interval narrowed. In the trial sequential analysis (TSA), the cumulative Z-curve crossed the trial sequential monitoring boundary for benefit, confirming the robustness of the summary effect estimate from the meta-analysis. In this up-to-date meta-analysis, Grading of Recommendations Assessment, Development and Evaluation( GRADE) assessment shows high-certainty evidence that ciNPT is effective in reducing SSI, and uncertainty is less than in previous meta-analyses. TSA indicated that further trials are unlikely to change the effect estimate for the outcome SSI; therefore, if future research is to be conducted on ciNPT, it is crucial to consider what the findings will contribute to the existing robust evidence.
5.6 What are the indications for ciNPT?
ciNPT appears to have the potential to reduce surgical incision complications in high-risk surgical procedures or under high-risk surgical conditions.
Details
While the experiences up to today with ciNPT devices were obviously encouraging, all studies failed to provide strong evidence of the efficacy of the device to reduce surgical site infections and wound complications. Nevertheless, due to the emerging benefits of this form of therapy, it seems necessary to define indications for the use of ciNPT already today. It must be considered that a larger number of ongoing studies can certainly change today’s decisions about the use of NPWT, the surgical indications and the setting.
In high-risk surgical procedures, ciNPT appears to have the potential to reduce surgical incision complications. High-risk incisions included those with specific characteristics (e.g., incisions that were re-opened or under high tension) as well as those associated with specific surgical procedures (e.g., high-energy extremity fractures).
Using these considerations, the most appropriate use of ciNPT seems to be for the following operations:
- Medial sternotomy
- Post-bariatric surgery (e.g., abdominoplasty and brachioplasty)82
- Major complex abdominal wall repair
- Vascular (bypass) surgery using the femoral cutdown approach
- High energy trauma fractures, lower limb (calcaneus, pilon)
- Periprosthetic fracture surgery
- Caesarean sections
- Breast surgery
The use of ciNPT should be seriously considered in these surgeries especially if additional patient-specific risk factors that contribute to reduced soft tissue perfusion or oxygenation are likely to negatively influence the postoperative wound healing process, such as:
- BMI ≥30 kg/m2
- Smoking
- Diabetes mellitus (HbA1c > 8%/64 mmol/mol)
- Poor nutrition status
- Previous wound infection
- ASA score ≥ 3
This combination of surgery-immanent and patient-immanent risk factors is of particular importance for the indication for the use of ciNPT when the general conditions of the surgery are also unfavourable and associated with the likelihood of an increased introduction of patient-intrinsic or environmental pathogens:
- Emergency surgery
- Revision surgery
- Duration of operation >75 percent percentile (most of the data on a possible benefit of ciNPT with longer operating times are available for arthroplasty. Here, the 75 percent percentiles are 90 min and 120 min (hip and knee arthroplasty, respectively).
Based on the results of all published experiences, it is possible to present the potential evidence-based benefits of use of ciNPT and to define individual patient groups and specific surgical procedures. Thus, against the background of the above-mentioned reflections, consensus recommendations were made for the most appropriate use of ciNPT (i.e., in patients with one or more comorbidities or in patients with a surgical incision at high risk for developing SSIs) (Figure 4).83
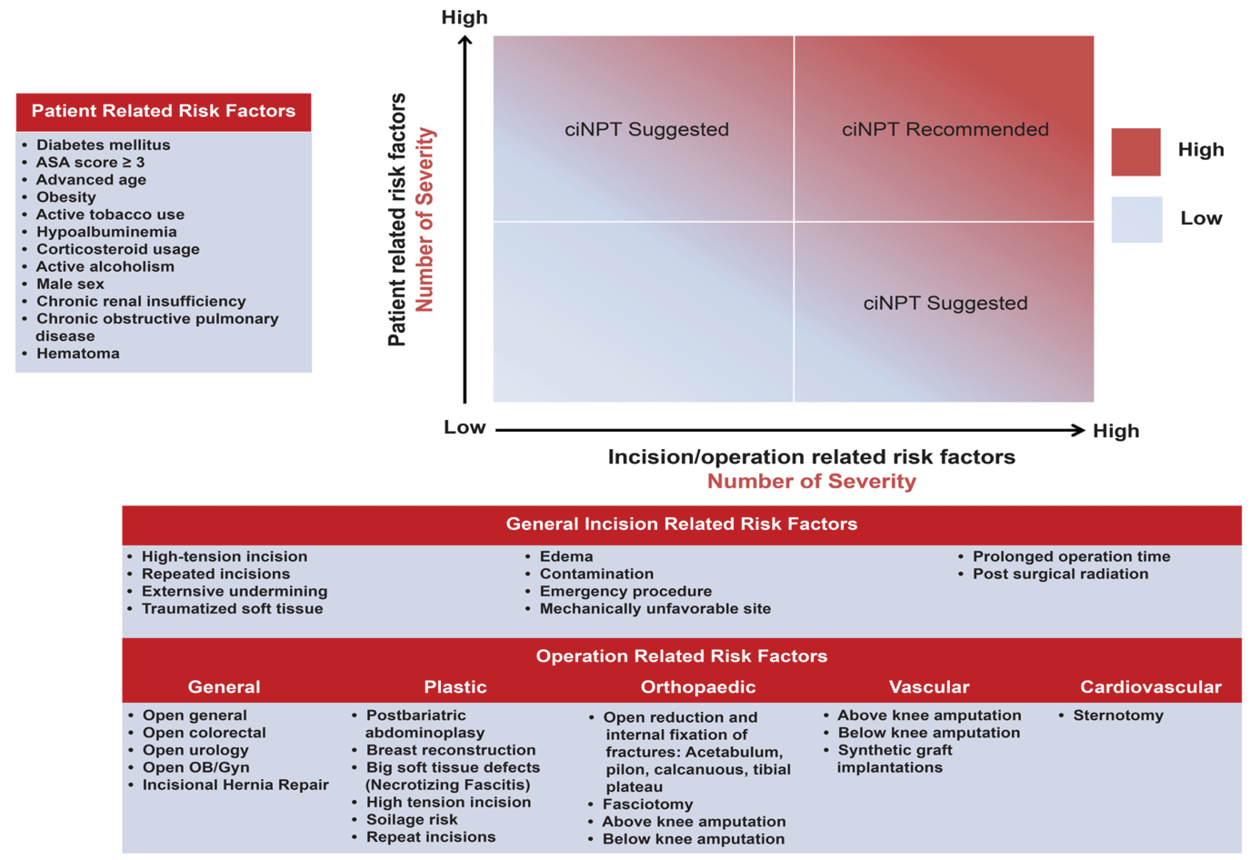
Figure 4. ciNPT risk factors assessment. Blue indicates low risk for SSI while red indicates high risk for SSI. ciNPT use is recommended in patients with an increased number of patient risk factors and incision risk factors. Willy C et al83.
However, a clear identification of the high-risk area leading to a ciNPT indication, in which closed incision management should be performed in any case, cannot be determined based on Figure 4. Nor will the risk calculation tools, some of which consider a large number of risk factors, be suitable for a targeted indication. These tools are generally not implemented in everyday hospital practice and cannot (yet) be automatically used preoperatively in clinical digital information systems. Against the background of the proven benefit of ciNPT for individual surgical disciplines, however, it appears to be purposeful to create an individualised decision algorithm for the typical patient population in one’s own field of work, considering the previous findings in the literature. This decision path should lead to the targeted reinforcement of preventive measures with the use of ciNPT, in addition to the bundled approach that is always chosen anyway.
5.7 What devices are available for ciNPT?
There are several commercially available devices on the market. For an overview, see Appendix 1.
Details
The technology of ciNPT has been developed to involve the application over surgical incisions. Special wound dressings have been designed to be applied over closed incisions. These are made of a material that has high-skin compatibility, such as a silicone adhesive. Wound fillers, such as foam or gauze should not be applied directly on intact skin. The ciNPT systems described in the literature in 2024 are represented by:
- A polyurethane foam placed over the length of the incision, secured with a protective occlusive tape, and attached to a commercially-available suction source (NPWT device) set at between -75 mmHg and -125 mmHg, in a continuous suction. Using this system, the surgeon can decide how long the ciNPT system should remain on the incision, should it stay in place until the removal of the sutures or for a shorter time.
- An integrated, one-piece dressing comprised of a polyurethane film with acrylic adhesive that provides adhesion of the dressing to the skin surrounding the incision and a polyurethane shell that encapsulates the foam bolster and interface layer, providing a closed system. The dressing is connected to a conventional large vacuum source, which is also used in the clinic for traditional NPWT, or to a smaller portable, battery-powered, usually disposable suction source with a small-volume canister. These smaller, easier-to-handle devices are particularly suitable for inpatients preparing to be discharged from hospital and for the treatment of wounds in outpatient settings.
- A portable, disposable, canister-less device with a dressing consisting of a silicone contact layer to minimise pain on removal, a pressure transfer lock (or airlock proprietary to one supplier). All ciNPT dressings feature a pressure transfer layer, thus this is a generic feature. It allows even distribution of negative pressure across the dressing, an absorbent layer that draws exudates away from the wound, and a top sheet with high water vapour permeability. The dressing is connected to a portable disposable system that provides a continuous negative pressure of -80 mmHg for 7 to14 days.
5.8 Any side effects of using ciNPT to be considered?
Apart from very few reports describing avoidable skin blistering, no side effects have been reported.
Details
Adverse effects with ciNPT use were only noted in a few studies.53,83–89 The most recent systematic review and other articles address one issue that is considered a disadvantage of ciNPT over standard wound care. This is blistering under the dressing. This has also been described in other studies. 84,87–89 One RCT study describes that due to the development of skin blisters at the interface between skin and dressing, 63 percent of patients in the ciNPT group discontinued the study prematurely.87 This adverse effect was most likely due to improper dressing configuration (such as a lack of a non-adherent film dressing, or drape used to protect the skin from the foam dressing and too much tension when using the dressing). It is noteworthy that in this study, ciNPT was used for only 48 hours instead of the recommended 7 days. So, it must be stressed, however, that this blister formation should rather be seen as an application error or because of the use of unsuitable or not sufficiently technically mature products.
5.9 Is the use of ciNPT cost-effective?
As mentioned in the introduction, this document does not include any deeper analysis/investigation of cost perspectives. However, published literature provides evidence suggesting that ciNPT in high-risk patients in certain indications could be cost-beneficial. A detailed overview of cost of ciNPT and cost-savings is included in Appendix 3.
Details
Treatment costs are an important issue in patient care. To date, several studies analysed costs of ciNPT use and compared wound complication rates, quality of life and cost savings of ciNPT to routine incision care.80,88–101 The authors in these studies provide evidence suggesting that ciNPT in high-risk patients in certain indications could be cost-beneficial. While these results are encouraging, large cohort studies examining cost savings in various surgical fields are needed.
6. Use of NPWT in primary care
6.1 Introduction
Through recent years there has been a shift from inpatient hospital care out to primary care in a home care setting for many patients with different diseases and conditions.102
Primary care had been defined by the Institute of Medicine (IOM) as: “the provision of integrated, accessible health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community.”103
Primary care in this definition includes all health care given to patients outside the hospital or in collaboration with the hospital but performed in the patient´s home or community.
When NPWT was first recognised as an established treatment method the patients were treated mainly in an inpatient setting in hospitals. However, with the ongoing transition to more primary care-based treatments in general, this has also been the case for NPWT. In primary care you can now use the standard or the canister-free dressing-based NPWT. The later can be a safer option since it is still an excellent dressing without the negative pressure, if the pressurisation malfunctions. This chapter, however, deals with foam or gauze based “traditional” NPWT with the canister. The patients thus have the same need of education and support no matter which treatment method is used.
Search terms applied in literature searches for this chapter in the data bases PubMed and CINAHL with different combinations include: “clinical competence”, “community care”, “community health services”, “education”, “home care”, “monitoring negative pressure wound therapy”, “patient compliance”, “patient discharge”, “patient education”, “patient satisfaction”, “primary health care”, “professional-patient relations”, “remote consultation”, “remote sensing technology”, “self-care”, “quality of life”, “wound healing” and “wounds and injuries”.
6.2 How is NPWT in primary care organised in European countries?
The 2017 EWMA NPWT-document discussed hopes that in the future treatment with NPWT in community settings would increase due to ongoing changes in the organisation of care. This development has not unfolded as expected.
EWMA surveyed 53 national wound care associations, across the 33 European countries linked to EWMA via Cooperating Organisations. These organisations are the closest collaborating partners of EWMA. The aim of the survey was to examine the use of NPWT in primary care and describe the organisation of the treatment. Responses were received from 32 associations across 22 countries.
The findings showed that 97% of the countries used NPWT in primary care, however in 60% of the countries using NPWT in primary care, it was hospital staff who were both responsible for the care and performed the treatment. In almost all countries participating in the survey the treatment was manually monitored though visits to hospitals for dressing changes. The only country that stated that they used electronic monitoring was France. In comparison, in Denmark the treating primary care health personnel had potential to use telemedicine to communicate with the hospital for patient safety.4
6.3 How effective and safe is treatment with NPWT in primary care?
NPWT in primary care has been shown in research to be safe, effective and cost effective.
Treatment with NPWT in outpatient settings has been shown to be more cost effective and associated with lower costs than treatment in the hospital. For example, in a study from the Czech Republic treating diabetic foot ulcers the cost for treatment with NPWT outside the hospital compared with inpatient treatment was significantly lower, EUR 600 versus EUR 1300 (p=0.001).104 A report from the NHS in the United Kingdom also showed lower costs when using NPWT in outpatient settings, along with other benefits including the possibility of earlier discharge from hospital.105
In a study comparing children treated with NPWT involved 1621 inpatients and 1563 outpatients. It showed that treatment in an outpatient setting was associated with both lower costs and lower complication rates.106 However, the higher complication rate among inpatient children may be partly explained by selection bias since these children had a worse health conditions than the outpatients, which may lead to being more prone to complications.
In an interview study from Sweden with patients being treated postoperatively with NPWT at home the participants described several benefits of the transition from inpatient to primary care. For example the relief of being at home in their normal environment. This meant sleeping in their own bed, not being disturbed by others and having the freedom of getting on with everyday life. However, the results from the study showed one key component for the patient to have a positive experience of being treated at home and that was the feeling of being well prepared at discharge.
6.4 Which criteria must be achieved for successful treatment with NPWT in primary care?
Self-care management is a key criterion for a successful treatment with NPWT in primary care. This means that the patients have the strength, confident and resources to manage the treatment at home. There is also a need for clear routines at discharge from the hospital into primary care.
To create sufficient self-care management for patients to handle treatment at home some aspects have been described in the literature as important. The feeling of being prepared has been described as essential for self-care management. Patient education is another a key component for preparation to be treated with NPWT at home.107
Another aspect to self-care management is support, not only from health care personnel but, more importantly, from next-of-kin, often relatives. These people need to be included in the patients’ care and can support and help with handling the devise and troubleshooting when alarms ring, which may reduce anxiety and worries for the patient.107–109
Additional important criterion for successful treatment with NPWT in primary care are clear routines and policies at discharge from the hospital into primary care. This is an organisational issue and needs to be addressed in collaboration between hospitals and primary care facilities who will continue management of the patients’ NPWT.108
6.5 Can advanced NPWT, such as NPWTi-d, be used in primary care?
Advanced NPWT, such as the NPWTi-d, has traditionally only been used in a hospital setting. However, in France a unique framework has been developedto allow the application of advanced treatments in home care settings, The framework is called of Hospitalisation at Home (HaH).
The NPWTi-d at home in HaH is unique and is only available in France. This service aims to decrease the cost of traditional hospitalisation by providing a medico-paramedical coverage of at home hospitalisation. The serives provides a medical visit twice a week, a paramedical service every day, plus emergency call availability 24/7 offering technical and logistical support, while guaranteeing the quality and safety of patient care. Qualities deemed essential by health authorities for the management of complex and/or chronic wounds and, of course, for patients who require NPWTi-d.
The medical and care competence of a HaH establishment is in principle generalist, and not reserved for wound treatment. In fact, the HaH caries out more than 25%of its interventions in palliative care and more than 20% in complex dressings. It also has the skills to take care of heavy nursing needs, nutrition, respiratory assistance, intravenous treatments and more. Specialised activities have also been developed in certain territories, for example in obstetrics, cancer treatment and neurological rehabilitation.
The principle aim of HaH is home care, while providing the patient with medical and/or technical procedures comparable to those performed in the hospital. This structure makes it possible to reduce the hospital stay time, and in some cases even to avoid further hospitalisation.99
6.6 How can NPWT be effectively and safely monitored during primary care?
The use of a remote monitoring service system with both an alarm function on the device, and a follow-up system using phone calls can enhance the effectiveness and safety of the treatment in a home care setting.
One key factor for successful outcome is adherence to the treatment. This can be a challenge, particularly when patients are receiving the treatment in a home care setting. Adherence can be defined as “persistence in the practice and maintenance of desired health behaviours and is the result of active participation and agreement.”110 One way to control adherence during treatment with NPWT at home can be via monitoring the treatment. Because of the benefits of monitoring the treatment, the RTM (Remote Therapy Monitoring) service was developed (Figure 5).
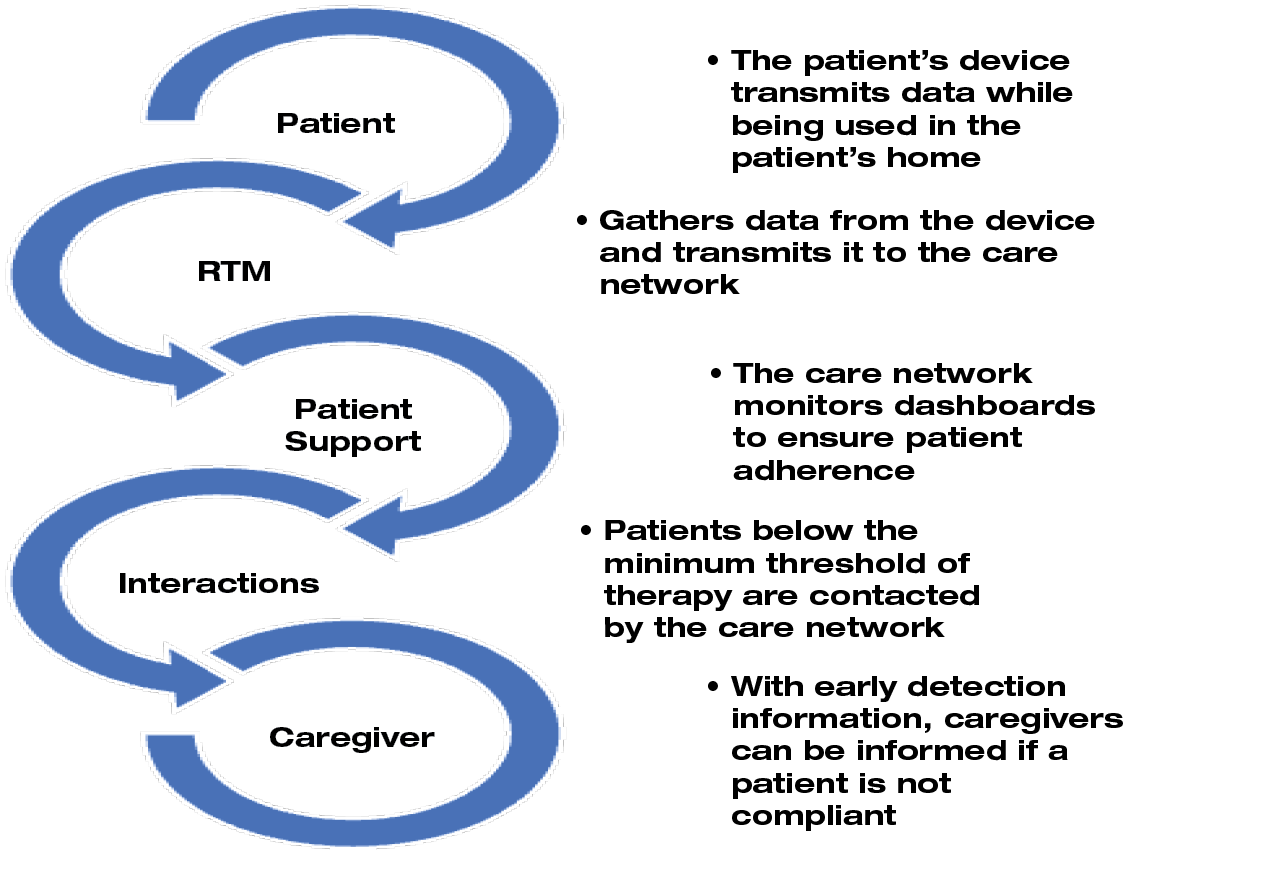
Figure 5. Remote Therapy Monitoring (RTM) service. Adapted from Lumpkins A, Stanton T.111
The RTM service is designed for use with NPWT devices in home care settings. The system allows data from NPWT devices to be remotely transferred to the responsible health care providers and alarms ring when the NPWT applications malfunction. The system alerts health care providers who can make a phone call when NPWT use is declining.112 A large retrospective study of 510 patients using the RTM service with monitoring and phone calls showed a 74% increase in adherence after intervention.113 In addition to indications of improved adherence to NPWT with the RTM service system, it also was shown in a small pilot study to statistically significantly reduce the length of time in therapy and decrease the cost.
6.7 Which are the key components of health education for patients treated with NPWT in primary care?
The patients need education, not only information, to feel confident and involved in their self-care management of their treatment at home.
Studies have shown that there is a need for health education for patients when being treated with NPWT in home settings. Edcuation improves patients’ capacity for self-care management, which boosts confidence and feelings of security.107,114,115
Health education should be person-centred and can be divided into three timeframes:
A. Education before initiating NPWT
B. Education for the first day of treatment at the hospital before discharge, and
C. Education for being treated with NPWT at home.
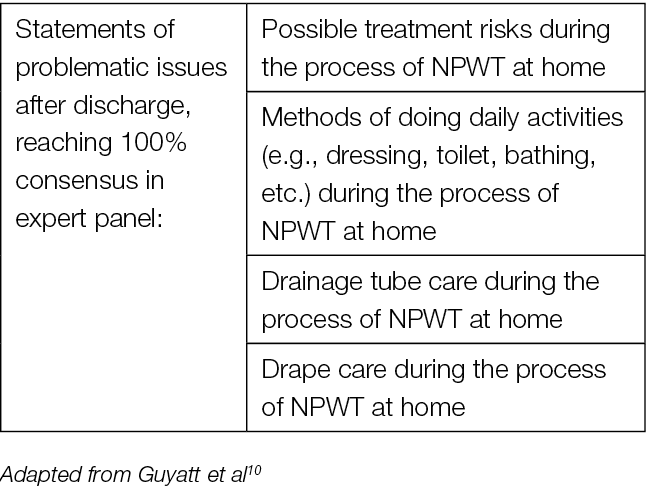
The most critical timeframe described in research is from the time of discharge to the first few days at home. It is while being at home that many of the issues and problems with the treatment arise (Table 3). Therefore, additional follow-up education may be needed throughout the entire treatment period for the patient to be able to address these upcoming issues.116
The main objective for health education in all of the timeframes is to involve the patient in his/her treatment and to achieve sufficient and competent self-management.107
Table 3. Problematic issues after discharge
6.8 What are the educational demands for health care personnel treating patients with NPWT in primary care settings?
The health care personnel who perform NPWT need formal and recurrent education. If there is a lack of competent staff to manage the treatment in primary care, delaying patient discharge from the hospital should be considered.
There is a need for formal training for the health care personnel both before initially starting NPWT and on an ongoing basis. Nurses need to be and to feel competent when handling the devices and to have confidence in their ability to troubleshoot the machines when they malfunction. Cray117 suggests in an interview study with nurses that educational study days for staff should be arranged, with both theoretical teaching and practical handling of the treatment. Online educational programs have also been suggested, however the lack of practical training in those programs has been a major problem leading to a sort of ‘trial and error’ procedure by the health care personnel. Moffatt et al108 showed that this may be seen by the patient as lack of competence of the health care personnel that can cause anxiety and a sense of being unsafe while being treated at home. Also, Bolas and Holloway118 described patients’ experiences with health care personnel lacking competence in performing NPWT. They raised an important point questioning whether health care personnel could achieve adequate skills in management of NPWT when seldom being exposed by the treatment. There is a need for clarifying the criteria, on a local level, to be used to decide who is considered competent enough to perform and be responsible for the treatment. This could be supported by a certification system, including basic education, for this type of treatment.
Ousey and Milne115 point out that there often is a lack of competent nurses to manage NPWT when patients are discharged from hospital. It is therefore important to make sure that there is sufficient staffing for this in primary care, otherwise the patients should continue the treatment in hospital.
7. Future perspectives and trends
7.1 Telemedicine and NPWT
Telemedicine was developed during the COVID19 period. In some countries (France, Italy, others) the development of telemedicine aimed at covering the lack of experts in wound healing observed in some areas. ‘Domoplaies’ was proposed by the French Wound Healing Occitanie Network.119,120 It offers different services like orientation, expertise, support, coordination of chronic wounds and continuing education.
Orientation is provided thanks to a centralised call center covering the whole French Occitanie territory (6,000,000 persons, over 20,000 km2). Expertise is assumed by 60 experts spread over the whole territory and covering the different disciplines, including vascular, diabetic, geriatrics and rehabilitation. Support by specialised nurses working in cooperation with the primary care team, consisting of GPs and local nurse, allows for following the patient pathway, mostly at home, along the whole healing trajectory. Monthly meetings between the experts provide a forum for continuing education and discussion of complex cases.
This model allows treatment of 300 patients per month and it is on the way to be generalised to the national level.The financial coverage being provided as a lumpsum for each patient. Thanks to this model NPWT follow up at home increased, especially for complex cases needing NPWTi-d. It provided maximum security for the primary care team and for the patient, limiting the cost of ambulance use and hospitalisations. The local nurse is guided via live stream by the expert during the dressing change, on a secure line and with some cost coverage of the expenses due to the extra work for the local team.
Author(s)
Luc Téot, MD PhD,
Assistant Professor of Plastic Surgery, Honorary Head of Plastic Surgery Department, Montpellier University Hospital, Montpellier, France
Jan Apelqvist, MD, PhD,
Associate Professor, Department of Endocrinology, Skåne University Hospital, Malmö, Sweden
Ami Fagerdahl, RN, PhD,
Associate professor, Department of Clinical Research and Education, Södersjukhuset, Karolinska Institutet, Stockholm, Sweden
Christian Willy, MD, PhD,
Professor of Surgery, Colonel, Trauma & Orthopedic Surgery, Septic & Re-constructive Surgery, Bundeswehr (Military) Academic Hospital Berlin, Germany
Corresponding author:
Luc Téot, MD PhD
l-teot@chu-montpellier.fr
Editorial support:
Eskild Bang Heinemeier, EWMA Secretariat
Jan Nikolai Kristensen, EWMA Secretariat
References
- Apelqvist J, Willy C, Fagerdahl AM, Fraccalvieri M, Malmsjö M, Piaggesi A, et al. EWMA Document: Negative pressure wound therapy: Overview, challenges and perspectives. J Wound Care. 2017 Mar 1;26(Sup3): p1–154.
- Focus session: NPWT: Focus perspectives of EWMA guidance document under preparation. In Paris, France, Room 352A; 2022.
- Key session: Negative pressure wound therapy. In Milan, Italy, Room Space 1; 2023.
- Fagerdahl A. Organisation of NPWT in primary care in Europe – a descriptive survey. J Wound Manag [Internet]. 2023 Jul;24(2). doi: https://doi.org/10.35279/jowm2023.24.02.03
- Dumville JC, Owens GL, Crosbie EJ, Peinemann F, Liu Z. Negative pressure wound therapy for treating surgical wounds healing by secondary intention. Cochrane Database Syst Rev. 2015 Jun 4;(6):CD011278.
- Dumville JC, Webster J, Evans D, Land L. Negative pressure wound therapy for treating pressure ulcers. Cochrane Database Syst Rev. 2015 May 20;(5):CD011334.
- Gottrup F, Apelqvist J, Price P, European Wound Management Association Patient Outcome Group. Outcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care. 2010 Jun;19(6):237–268.
- Rossi P, Camilloni L, Todini A, Fortino A, Bernardo LD, Frigerio L, et al. Health technology assessment of the negative pressure wound therapy for the treatment of acute and chronic wounds: efficacy, safety, cost effectiveness, organizational and ethical impact. Ital J Public Health. Vol 9, 2012(2):46–66.
- Game FL, Attinger C, Hartemann A, Hinchliffe RJ, Löndahl M, Price PE, et al. IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2016 Jan;32(S1):75–83.
- Guyatt GH, Haynes RB, Jaeschke RZ, Cook DJ, Green L, Naylor CD, et al. Users’ guides to the medical literature: XXV. Evidence-based medicine: principles for applying the users’ guides to patient care. JAMA. 2000 Sep 13;284(10):1290.
- Stannard JP, Robinson JT, Anderson ER, McGwin G, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high-energy trauma. J Trauma Inj Infect Crit Care. 2006 Jun;60(6):1301–1306.
- Fleischmann W, Becker U, Bischoff M, Hoekstra H. Vacuum sealing: indication, technique, and results. Eur J Orthop Surg Traumatol. 1995 Dec;5(1):37–40.
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997 Jun;38(6):563–76; discussion 577.
- Banwell PE, Téot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care. 2003 Jan;12(1):22–28.
- Armstrong DG, Lavery LA, Abu-Rumman P, Espensen EH, Vazquez JR, Nixon BP, et al. Outcomes of subatmospheric pressure dressing therapy on wounds of the diabetic foot. Ostomy Wound Manage. 2002 Apr;48(4):64–68.
- Deva AK, Buckland GH, Fisher E, Liew SC, Merten S, McGlynn M, et al. Topical negative pressure in wound management. Med J Aust. 2000 Aug;173(3):128–131.
- Avery C, Pereira J, Moody A, Whitworth I. Clinical experience with the negative pressure wound dressing. Br J Oral Maxillofac Surg. 2000 Aug;38(4):343–345.
- Banwell PE. Topical negative pressure therapy in wound care. J Wound Care. 1999 Feb;8(2):79–84.
- Banwell P, Holten I, Martin DL. Negative pressure therapy: clinical applications and experience with 200 cases. Wound Repair Regen. 1998(6):460.
- Fleischmann W, Lang E, Russ M. Infektbehandlung durch Vakuum-versiegelung. Unfallchirurg. 1997 Apr 17;100(4):301–304.
- Fleischmann W, Russ M, Westhauser A, Stampehl M. Vacuum-sealing-technique used as drug release system for topical treatment of wound infections. Unfallchirurg. 1998 Aug;101(8):649–654.
- Kim PJ, Attinger CE, Constantine T, Crist BD, Faust E, Hirche CR, et al. Negative pressure wound therapy with instillation: International consensus guidelines update. Int Wound J. 2020 Feb;17(1):174–186.
- Gupta S, Gabriel A, Lantis J, Téot L. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int Wound J. 2016 Apr;13(2):159–174.
- Kim PJ, Silverman R, Attinger CE, Griffin L. Comparison of negative pressure wound therapy with and without instillation of saline in the management of infected wounds. Cureus [Internet]. 2020 Jul 7 [cited 2024 Feb 13]; doi: 10.7759/cureus.9047
- Meshkin DH, Fan KL, Charipova K, Hill C, Evans KK, Steinberg JS, et al. Long-term outcome assessment between antiseptic and normal saline for negative pressure wound therapy with instillation. Adv Wound Care. 2021 Oct 1;10(10):535–543.
- Rycerz AM, Allen D, Lessing MC. Science supporting negative pressure wound therapy with instillation. Int Wound J. 2013 Dec;10(s1):20–24.
- Kim PJ, Attinger CE, Crist BD, Gabriel A, Galiano RD, Gupta S, et al. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds Compend Clin Res Pract. 2015 Dec;27(12):S2–19.
- Kim PJ, Attinger CE, Steinberg JS, Evans KK, Lehner B, Willy C, et al. Negative-pressure wound therapy with instillation: international consensus guidelines. Plast Reconstr Surg. 2013 Dec;132(6):1569–1579.
- Giri P, Krishnaraj B, Chandra Sistla S, Sistla S, Basu D, Shankar G, et al. Does negative pressure wound therapy with saline instillation improve wound healing compared to conventional negative pressure wound therapy? A randomized controlled trial in patients with extremity ulcers. Ann Med Surg. 2021 Jan;61:73–80.
- Kim PJ, Lavery LA, Galiano RD, Salgado CJ, Orgill DP, Kovach SJ, et al. The impact of negative-pressure wound therapy with instillation on wounds requiring operative debridement: Pilot randomised, controlled trial. Int Wound J. 2020 Oct;17(5):1194–1208.
- Tahir S, Malone M, Hu H, Deva A, Vickery K. The effect of negative pressure wound therapy with and without instillation on mature biofilms in vitro. Materials. 2018 May 16;11(5):811.
- Fletcher J, Mosahebi A, Younis I, Geraghty J, Khalil H, Maries M, et al. Negative pressure wound therapy with instillation for Category 3 and 4 pressure ulcers: Findings of an advisory board meeting. Wounds UK. 2019;Vol 15(3).
- Chowdhry SA, Wilhelmi BJ. Comparing negative pressure wound therapy with instillation and conventional dressings for sternal wound reconstructions. Plast Reconstr Surg − Glob Open. 2019 Jan;7(1):e2087.
- Obst MA, Harrigan J, Wodash A, Bjurstrom S. Early-stage management of complex wounds using negative pressure wound therapy with instillation and a dressing with through holes. Wounds Compend Clin Res Pract. 2019 May;31(5):E33–36.
- McElroy EF. Use of negative pressure wound therapy with instillation and a reticulated open cell foam dressing with through holes in the acute care setting. Int Wound J. 2019 Jun;16(3):781–787.
- Blalock L. Use of Negative pressure wound therapy with instillation and a novel reticulated open-cell foam dressing with through holes at a Level 2 trauma center. Wounds Compend Clin Res Pract. 2019 Feb;31(2):55–58.
- Teot L, Ohura N. Challenges and management in wound care. Plast Reconstr Surg. 2021 Jan;147(1S-1):9S-15S.
- Fluieraru S, Bkara F, Naud M, Herlin C, Faure C, Trial C, et al. Sterile-water negative pressure instillation therapy for complex wounds and NPWT failures. J Wound Care. 2013 Jun;22(6):293–299.
- Brinkert D, Ali M, Naud M, Maire N, Trial C, Téot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J. 2013 Dec;10(s1):56–60.
- Burusapat C, Sringkarawat S. Efficacy of negative-pressure wound therapy with tetrachlorodecaoxygen-anion complex instillation compared with standard negative-pressure wound therapy for accelerated wound healing: a prospective, randomized, controlled trial. Plast Reconstr Surg. 2021 Aug;148(2):339–52.
- Hehr JD, Hodson TS, West JM, Schulz SA, Poteet SJ, Chandawarkar RY, et al. Instillation negative pressure wound therapy: An effective approach for hardware salvage. Int Wound J. 2020 Apr;17(2):387–393.
- Horch R, Braumann C, Dissemond J, Lehner B, Hirche C, Woeste G, et al. Einsatz der Vakuuminstillationstherapie für die Wundbehandlung – Ergebnis einer Expertenkonsensuskonferenz. Zentralblatt Für Chir - Z Für Allg Visz Thorax- Gefäßchirurgie. 2018 Dec;143(06):609–616.
- McKanna M, Geraci J, Hall K, Hauan B, Howell M, Huey T, et al. Clinician Panel recommendations for use of negative pressure wound therapy with instillation. Ostomy Wound Manage. 2016 Apr;62(4):S1–14.
- Coccolini F, Gubbiotti F, Ceresoli M, Tartaglia D, Fugazzola P, Ansaloni L, et al. Open abdomen and fluid instillation in the septic abdomen: Results from the IROA Study. World J Surg. 2020 Dec;44(12):4032–4040.
- Garcia-Ruano A, Deleyto E, Garcia-Fernandez S. VAC-instillation therapy in abdominal mesh exposure: a novel indication. J Surg Res. 2016 Dec;206(2):292–297.
- Dalla Paola L. Diabetic foot wounds: the value of negative pressure wound therapy with instillation. Int Wound J. 2013 Dec;10(s1):25–31.
- Fernández L, Ellman C, Jackson P. Use of negative pressure wound therapy with instillation in the management of complex wounds in critically ill patients. Wounds Compend Clin Res Pract. 2019 Jan;31(1):E1–4.
- Okamoto K, Matsumoto K, Iga N, Komatsu S. Negative pressure wound therapy with instillation without open-window thoracostomy for empyema. Respirol Case Rep. 2019 Jul;7(5):e00417.
- Téot L, Boissiere F, Fluieraru S. Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int Wound J. 2017 Oct;14(5):842–848.
- Kim PJ, Applewhite A, Dardano AN, Fernandez L, Hall K, McElroy E, et al. Use of a novel foam dressing with negative pressure wound therapy and instillation: recommendations and clinical experience. Wounds Compend Clin Res Pract. 2018 Mar;30(3 suppl):S1–17.
- De Pellegrin L, Feltri P, Filardo G, Candrian C, Harder Y, Galetti K, et al. Effects of negative pressure wound therapy with instillation and dwell time (NPWTi -d) versus NPWT or standard of care in orthoplastic surgery: A systematic review and meta-analysis. Int Wound J. 2023 Aug;20(6):2402–2413. doi: 10.1111/iwj.14072
- Scarpa C, Grigatti M, Rizzato S, Crema A, Vindigni V, Bassetto F. Novel foam dressing with through holes and negative pressure wound therapy with instillation and dwell time: A retrospective cohort study. Wounds. 2024;36(3):67–72.
- Norman G, Shi C, Goh EL, Murphy EM, Reid A, Chiverton L, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Wounds Group, editor. Cochrane Database Syst Rev [Internet]. 2022 Apr 26 [cited 2024 Feb 14];2022(4). Available from: http://doi.wiley.com/10.1002/14651858.CD009261.pub7
- Bassetto F, De Antoni E, Rizzato S, Scarpa C. Management of acute and chronic wounds using negative pressure wound therapy with instillation and dwell time: a retrospective. Wounds Compend Clin Res Pract [Internet]. 2021 Aug 14: doi: 10.25270/wnds/081421.01
- Almeida IR, Coltro PS, Gonçalves HOC, Westin AT, Almeida JB, Lima RVKS, et al. The role of negative pressure wound therapy (NPWT) on the treatment of pyoderma gangrenosum: A systematic review and personal experience. Wound Repair Regen. 2021 May;29(3):486–494.
- Alvarez PS, Betancourt AS, Fernández LG. Negative pressure wound therapy with instillation in the septic open abdomen utilizing a modified negative pressure therapy system. Ann Med Surg. 2018 Dec;36:246–51.
- Diehm YF, Fischer S, Wirth GA, Haug V, Orgill DP, Momeni A, et al. Management of acute and traumatic wounds with negative-pressure wound therapy with instillation and dwell time. Plast Reconstr Surg. 2021 Jan;147(1S-1):43S-53S.
- Schreiner W, Ludolph I, Dudek W, Horch RE, Sirbu H. Negative pressure wound therapy combined with instillation for sternoclavicular joint infection. Ann Thorac Surg. 2020 Nov;110(5):1722–5.
- Kim P. Negative Pressure Wound Therapy With Instillation: An adjunctive therapy for infection management in orthopaedic trauma. J Orthop Trauma. 2022 Sep;36(4):S12–16.
- Fong KD, Hu D, Eichstadt S, Gupta DM, Pinto M, Gurtner GC, et al. The SNaP System: Biomechanical and animal model testing of a novel ultraportable negative-pressure wound therapy system. Plast Reconstr Surg. 2010 May;125(5):1362–1371.
- Rys P, Borys S, Hohendorff J, Zapala A, Witek P, Monica M, et al. NPWT in diabetic foot wounds—a systematic review and meta-analysis of observational studies. Endocrine. 2020 Apr;68(1):44–55.
- Zukowska A, Zukowski M. Surgical Site Infection in Cardiac Surgery. J Clin Med. 2022 Nov 26;11(23):6991.
- Groenen H, Jalalzadeh H, Buis DR, Dreissen YEM, Goosen JHM, Griekspoor M, et al. Incisional negative pressure wound therapy for the prevention of surgical site infection: an up-to-date meta-analysis and trial sequential analysis. eClinicalMedicine. 2023 Aug;62:102105.
- Atkins BZ, Tetterton JK, Petersen RP, Hurley K, Wolfe WG. Laser doppler flowmetry assessment of peristernal perfusion after cardiac surgery: beneficial effect of negative pressure therapy. Int Wound J. 2011 Feb;8(1):56–62.
- Chien YC, Lin YH, Chen CC, Lin HC. Compromised flap salvage with closed incision negative pressure therapy under real-time indocyanine green fluorescence assessment. Ann Plast Surg. 2021 Feb;86(2S):S96–101.
- Dadras M, Ufton D, Sogorski A, Wallner C, Wagner JM, Lehnhardt M, et al. Closed-incision negative-pressure wound therapy after resection of soft-tissue tumors reduces wound complications: results of a randomized trial. Plast Reconstr Surg. 2022 May;149(5):972e–980e.
- Gui D, Casaroli A, Borrello A, Magalini S. Closed incision negative pressure therapy (ciNPT): does depression affect underlying tissues? Eur Rev Med Pharmacol Sci. 2021 Sep;25(17):5458–5462.
- Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): Hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen. 2011 Sep;19(5):588–596.
- Kilpadi DV, Lessing C, Derrick K. Healed porcine incisions previously treated with a surgical incision management system: mechanical, histomorphometric, and gene expression properties. Aesthetic Plast Surg. 2014 Aug;38(4):767–778.
- Kilpadi DV, Olivie M. Evaluation of closed incision negative pressure therapy systems on the closure of incisional space model. J Wound Care. 2019 Dec 2;28(12):850–860.
- Renno I, Boos AM, Horch RE, Ludolph I. Changes of perfusion patterns of surgical wounds under application of closed incision negative pressure wound therapy in postbariatric patients1. Clin Hemorheol Microcirc. 2019 Aug 5;72(2):139–150.
- Suh H, Lee AY, Park EJ, Hong JP. Negative pressure wound therapy on closed surgical wounds with dead space: animal study using a swine model. Ann Plast Surg. 2016 Jun;76(6):717–22.
- Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (cim): biomechanics. Surg Innov. 2012 Mar;19(1):67–75.
- Timmermans FW, Mokken SE, Smit J, Bouman M, van de Grift TC, Mullender MG, et al. The impact of incisional negative pressure wound therapy on scar quality and patient-reported outcomes: A within patient-controlled, randomised trial. Wound Repair Regen. 2022 Mar;30(2):210–221.
- Meeker J, Weinhold P, Dahners L. Negative pressure therapy on primarily closed wounds improves wound healing parameters at 3 days in a porcine model. J Orthop Trauma. 2011 Dec;25(12):756–761.
- Glaser DA, Farnsworth CL, Varley ES, Nunn TA, Sayad-Shah M, Breisch EA, et al. Negative pressure therapy for closed spine incisions: a pilot study. Wounds Compend Clin Res Pract. 2012 Nov;24(11):308–316.
- Gabriel A, Singh D, Silverman RP, Collinsworth A, Bongards C, Griffin L. Closed Incision Negative Pressure Therapy Versus Standard of Care Over Closed Plastic Surgery Incisions in the Reduction of Surgical Site Complications: A Systematic Review and Meta-Analysis of Comparative Studies. Eplasty. 2023;23:e22.
- Pauser J, Nordmeyer M, Biber R, Jantsch J, Kopschina C, Bail HJ, et al. Incisional negative pressure wound therapy after hemiarthroplasty for femoral neck fractures – reduction of wound complications. Int Wound J. 2016 Oct;13(5):663–667.
- Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz-Drost S, Schlechtweg P, et al. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop. 2012 Apr;36(4):719–722.
- Nordmeyer M, Pauser J, Biber R, Jantsch J, Lehrl S, Kopschina C, et al. Negative pressure wound therapy for seroma prevention and surgical incision treatment in spinal fracture care. Int Wound J. 2016 Dec;13(6):1176–1179.
- World Health Organization. Global guidelines for the prevention of surgical site infection [Internet]. 2nd ed. Geneva: World Health Organization; 2018 [cited 2024 Apr 8]. 184 p. Available from: https://iris.who.int/handle/10665/277399
- Facchin F, Pagani A, Marchica P, Pandis L, Scarpa C, Brambullo T, et al. The role of portable incisional negative pressure wound therapy (piNPWT) in reducing local complications of post-bariatric brachioplasty: a case-control study. Aesthetic Plast Surg. 2021 Aug;45(4):1653–1659.
- Willy C, Agarwal A, Andersen CA, Santis GD, Gabriel A, Grauhan O, et al. Closed incision negative pressure therapy: international multidisciplinary consensus recommendations. Int Wound J. 2017 Apr;14(2):385–398.
- Ailaney N, Johns WL, Golladay GJ, Strong B, Kalore NV. Closed incision negative pressure wound therapy for elective hip and knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2021 Jul;36(7):2402–2411.
- Gillespie BM, Webster J, Ellwood D, Thalib L, Whitty JA, Mahomed K, et al. Closed incision negative pressure wound therapy versus standard dressings in obese women undergoing caesarean section: multicentre parallel group randomised controlled trial. BMJ. 2021 May 5;n893.
- Gomez TW, Gomez JW, Gopal R. Clinical applications and benefits of using closed-incision negative pressure therapy for incision and surrounding soft tissue management: a novel approach for comorbid wounds. Cureus [Internet]. 2020 Jul 30 [cited 2024 Feb 14]; doi: 10.7759/cureus.9469
- Howell RD, Hadley S, Strauss E, Pelham FR. Blister formation with negative pressure dressings after total knee arthroplasty. Curr Orthop Pract. 2011 Mar;22(2):176–179.
- Norman G, Goh EL, Dumville JC, Shi C, Liu Z, Chiverton L, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Wounds Group, editor. Cochrane Database Syst Rev [Internet]. 2020 May 1 [cited 2024 Feb 14]; Available from: https://doi.wiley.com/10.1002/14651858.CD009261.pub5
- Webster J, Scuffham P, Sherriff KL, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. In: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley & Sons, Ltd; 2012 [cited 2024 Feb 14]. p. CD009261.pub2. Available from: https://doi.wiley.com/10.1002/14651858.CD009261.pub2
- Karl T, Woeste S. Prevention of inguinal wound healing disorders in vascular surgery. Results of using an epidermal negative pressure system (Prevena (TM)). Gefasschirurgie. 2013;18(2):120–125.
- Dohmen PM, Markou T, Ingemansson R, Rotering H, Hartman JM, Van Valen R, et al. Can post-sternotomy mediastinitis be prevented by a closed incision management system? GMS Hyg Infect Control. 2014;9(3):Doc19. doi: 10.3205/dgkh000239
- Lewis LS, Convery PA, Bolac CS, Valea FA, Lowery WJ, Havrilesky LJ. Cost of care using prophylactic negative pressure wound vacuum on closed laparotomy incisions. Gynecol Oncol. 2014 Mar;132(3):684–689.
- Gillespie BM, Rickard CM, Thalib L, Kang E, Finigan T, Homer A, et al. Use of negative-pressure wound dressings to prevent surgical site complications after primary hip arthroplasty: a pilot RCT. Surg Innov. 2015 Oct;22(5):488–495.
- Tuffaha HW, Gillespie BM, Chaboyer W, Gordon LG, Scuffham PA. Cost-utility analysis of negative pressure wound therapy in high-risk cesarean section wounds. J Surg Res. 2015 May;195(2):612–622.
- Chopra K, Gowda AU, Morrow C, Holton L, Singh DP. The economic impact of closed-incision negative-pressure therapy in high-risk abdominal incisions: a cost-utility analysis. Plast Reconstr Surg. 2016 Apr;137(4):1284–1289.
- Manoharan V, Grant AL, Harris AC, Hazratwala K, Wilkinson MPR, McEwen PJC. Closed incision negative pressure wound therapy vs conventional dry dressings after primary knee arthroplasty: a randomized controlled study. J Arthroplasty. 2016 Nov;31(11):2487–2494.
- Kwon J, Staley C, McCullough M, Goss S, Arosemena M, Abai B, et al. A randomized clinical trial evaluating negative pressure therapy to decrease vascular groin incision complications. J Vasc Surg. 2018 Dec;68(6):1744–1752.
- Gabriel A, Maxwell GP. Economic analysis based on the use of closed-incision negative-pressure therapy after postoperative breast reconstruction. Plast Reconstr Surg. 2019 Jan;143(1S):36S-40S.
- Hyldig N, Joergensen J, Wu C, Bille C, Vinter C, Sorensen J, et al. Cost-effectiveness of incisional negative pressure wound therapy compared with standard care after caesarean section in obese women: a trial-based economic evaluation. BJOG Int J Obstet Gynaecol. 2019 Apr;126(5):619–627.
- Licari L, Campanella S, Carolla C, Viola S, Salamone G. Closed Incision Negative Pressure Therapy Achieves Better Outcome Than Standard Wound Care: Clinical Outcome and Cost-Effectiveness Analysis in Open Ventral Hernia Repair With Synthetic Mesh Positioning. Cureus [Internet]. 2020 May 26 [cited 2024 Feb 14]; doi: 10.7759/cureus.8283
- Bloom JA, Tian T, Homsy C, Singhal D, Salehi P, Chatterjee A. A cost-utility analysis of the use of closed-incision negative pressure system in vascular surgery groin incisions. Am Surg. 2023 Jun;89(6):2237–2246.
- Probst S, Seppänen S, Gerber V, Hopkins A, Rimdeika R, Gethin G. EWMA Document: home care-wound care: overview, challenges and perspectives. J Wound Care. 2014 May 1;23(Sup5a):S1–41.
- Primary Care: America’s Health in a New Era [Internet]. Washington, D.C.: National Academies Press; 1996 [cited 2024 Feb 14]. Available from: http://www.nap.edu/catalog/5152
- Stryja J, Staffa R, Říha D, Stryjová K, Nicielniková K. Cost-effectiveness of negative pressure wound therapy in outpatient setting. Rozhl V Chir Mesicnik Ceskoslovenske Chir Spolecnosti. 2015 Aug;94(8):322–328.
- Dowsett C, Davis L, Henderson V, Searle R. The economic benefits of negative pressure wound therapy in community-based wound care in the NHS. Int Wound J. 2012 Oct;9(5):544–552.
- Santosa KB, Keane AM, Keller M, Olsen MA, Sears ED, Snyder-Warwick AK. Inpatient versus outpatient management of negative pressure wound therapy in pediatric patients. J Surg Res. 2020 Oct;254:197–205.
- Monsen C, Acosta S, Kumlien C. Patients experiences of negative pressure wound therapy at home for the treatment of deep perivascular groin infection after vascular surgery. J Clin Nurs. 2017 May;26(9–10):1405–1413.
- Moffatt CJ, Mapplebeck L, Murray S, Morgan PA. The experience of patients with complex wounds and the use of NPWT in a home-care setting. J Wound Care. 2011 Nov;20(11):512–527.
- Ottosen B, Pedersen BD. Patients’ experiences of NPWT in an outpatient setting in Denmark. J Wound Care. 2013 Apr;22(4):197–206.
- Cohen SM. Concept analysis of adherence in the context of cardiovascular risk reduction. Nurs Forum (Auckl). 2009 Jan;44(1):25–36.
- Lumpkins A, Stanton T. Benefits of a patient-centered remote therapy monitoring program focusing on increased adherence to wound therapy. Wounds Compend Clin Res Pract. 2019 Aug;31(8):E49–53.
- Griffin LP, Sifuentes MM. Remote monitoring saves costs in outpatient negative pressure wound therapy. Am J Manag Care. 2022 Feb 1;28(2):53–58.
- Griffin L, Leyva Casillas LM. A patient-centered remote therapy monitoring program focusing on increased adherence to wound therapy: a large cohort study. Wounds Compend Clin Res Pract. 2018 Aug;30(8):E81–83.
- Janssen AH, Wegdam JA, de Vries Reilingh TS, Eskes AM, Vermeulen H. Negative pressure wound therapy for patients with hard-to-heal wounds: a systematic review. J Wound Care. 2020 Apr 2;29(4):206–212.
- Ousey K, Milne J. Focus on negative pressure: exploring the barriers to adoption. Br J Community Nurs. 2010 Mar;15(3):121–124.
- Huang Y, Mao B, Hu J, Xu B, Ni P, Hou L, et al. Consensus on the health education of home-based negative pressure wound therapy for patients with chronic wounds: a modified Delphi study. Burns Trauma. 2021. doi: 10.1093/burnst/tkab046.
- Cray A. Negative pressure wound therapy and nurse education. Br J Nurs. 2017 Aug 10;26(15):S6–18.
- Bolas N, Holloway S. Negative pressure wound therapy: a study on patient perspectives. Br J Community Nurs. 2012 Mar;17(Sup3):S30–35.
- Sood A, Granick MS, Trial C, Lano J, Palmier S, Ribal E, et al. The role of telemedicine in wound care: A review and analysis of a database of 5,795 patients from a mobile wound-healing center in Languedoc-Roussillon, France. Plast Reconstr Surg. 2016 Sep;138(3S):248S-256S.
- Téot L, Geri C, Lano J, Cabrol M, Linet C, Mercier G. Complex wound healing outcomes for outpatients receiving care via telemedicine, home health, or wound clinic: A randomized controlled trial. Int J Low Extrem Wounds. 2020 Jun;19(2):197–204.
Appendix 1
A) ciNPT devices / systems on the market
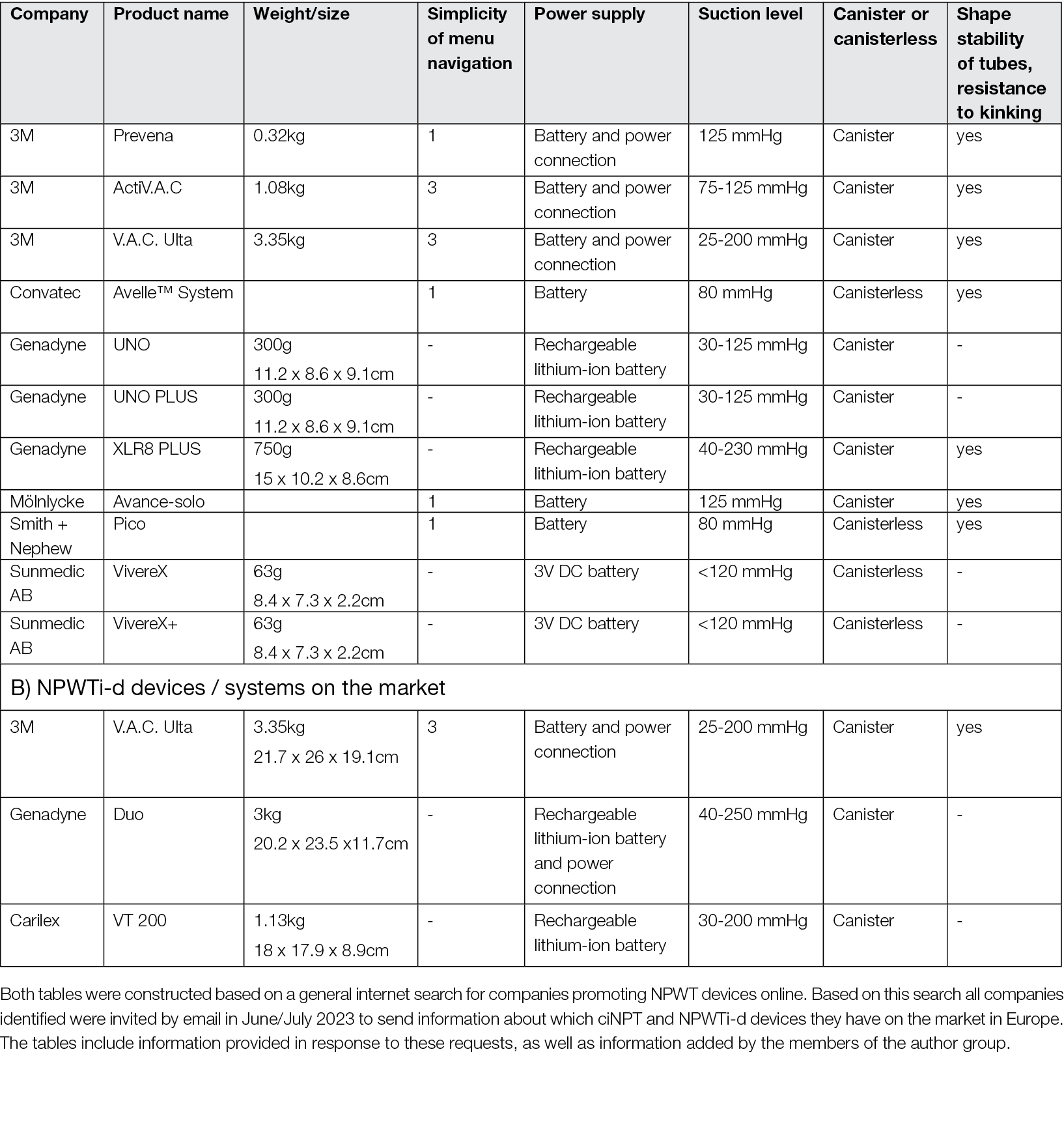
Appendix 2: RCTs 2004-2023 analysing benefits of ciNPT: Complete list, sorted by medical discipline
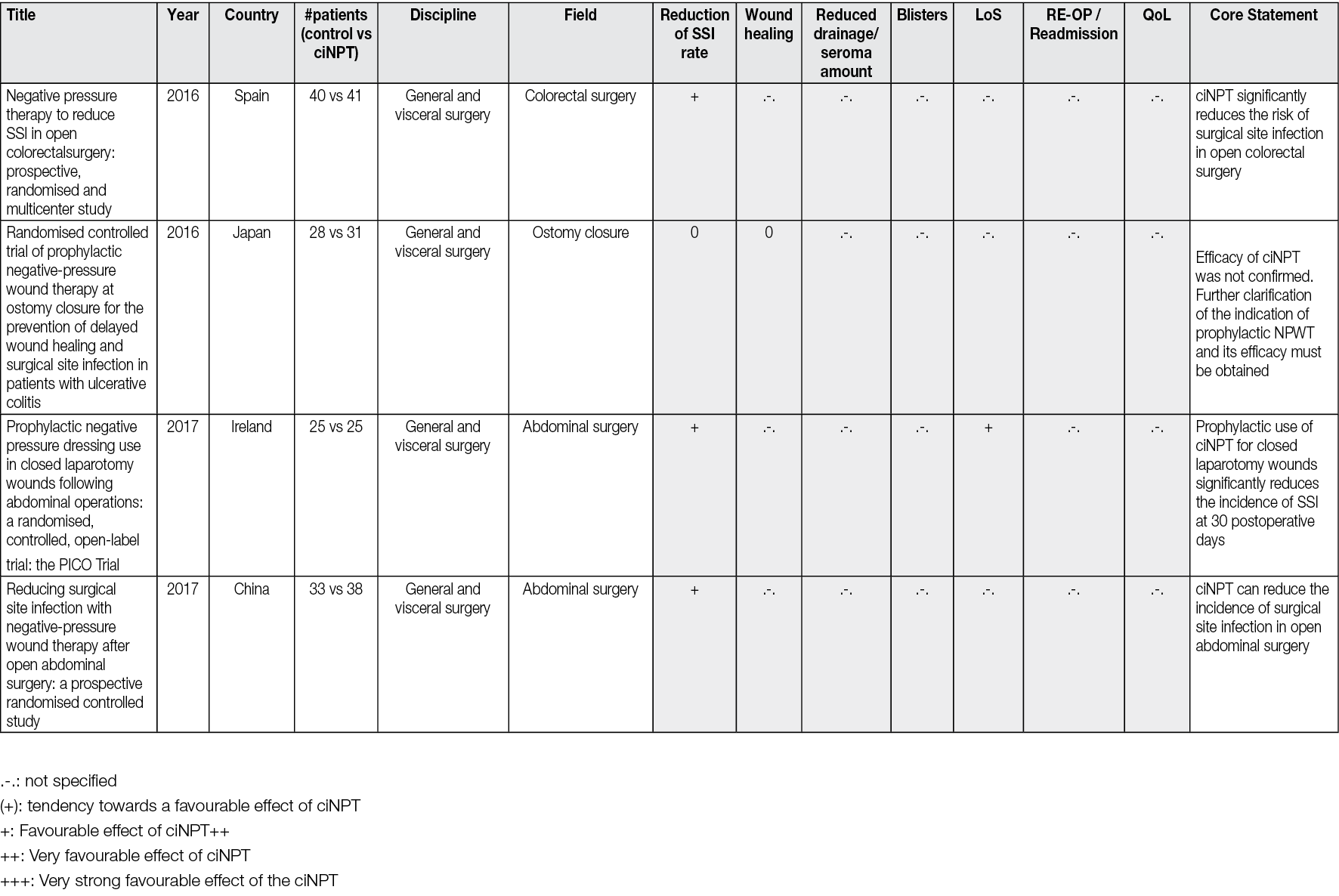
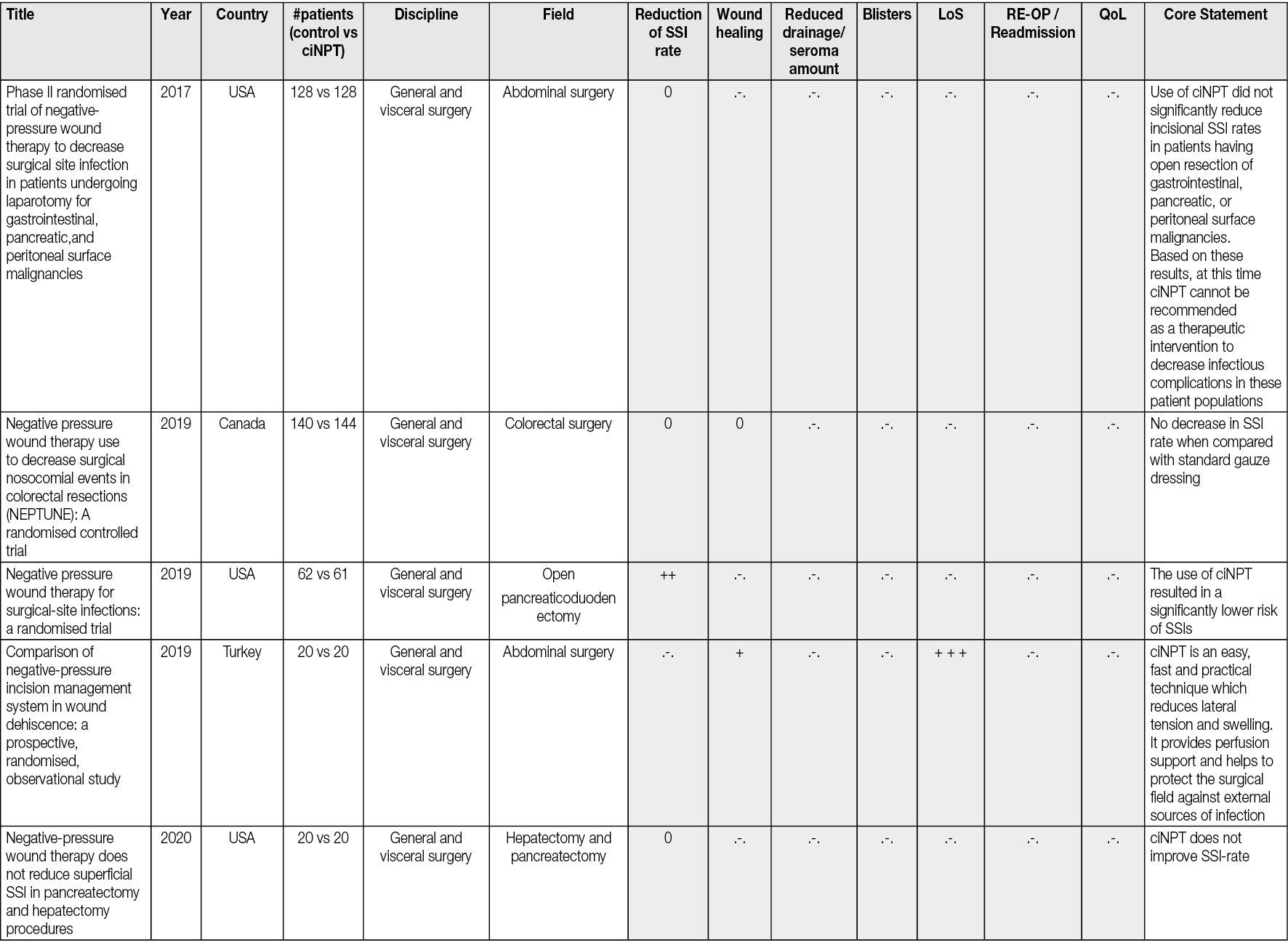
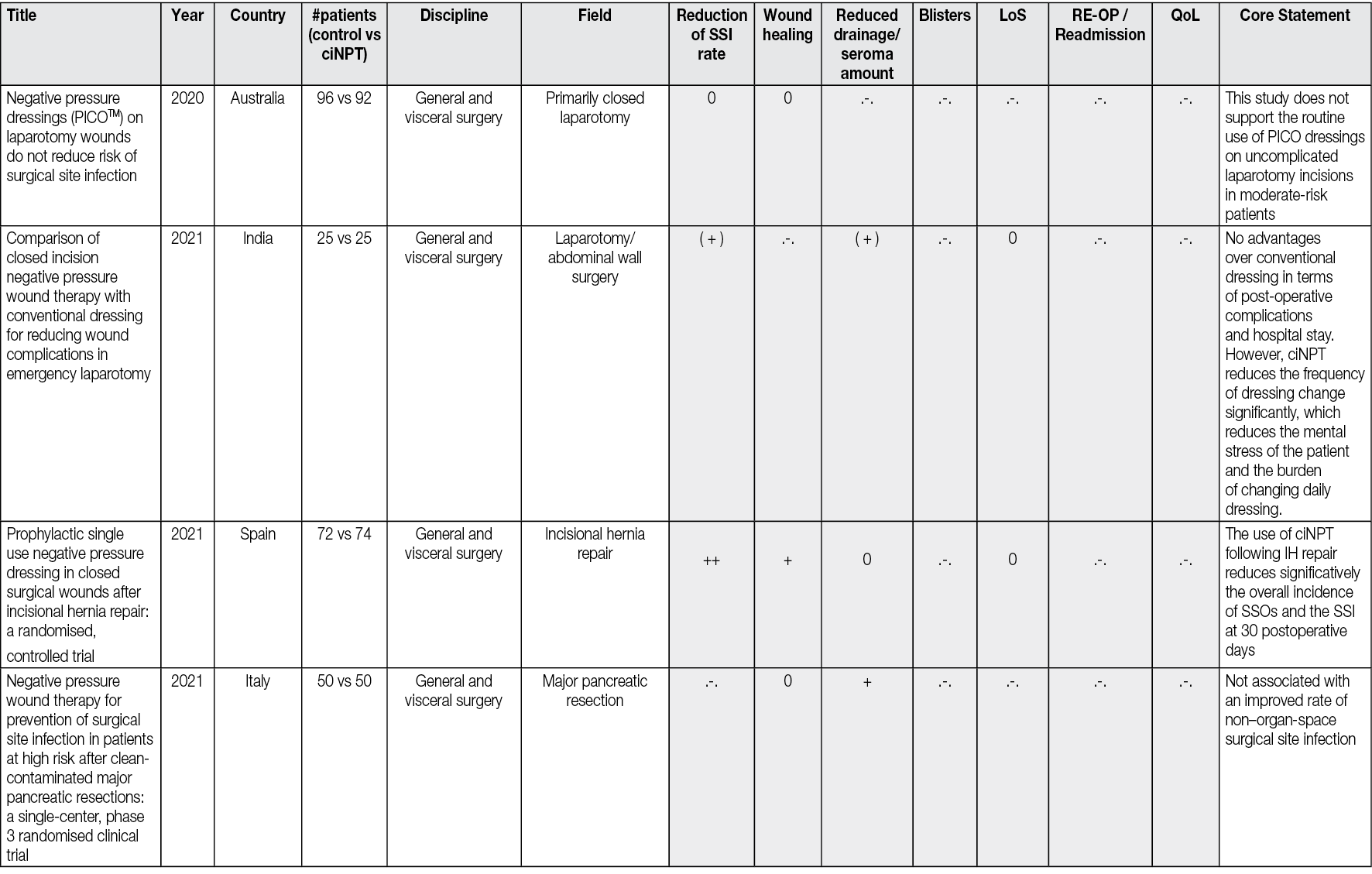
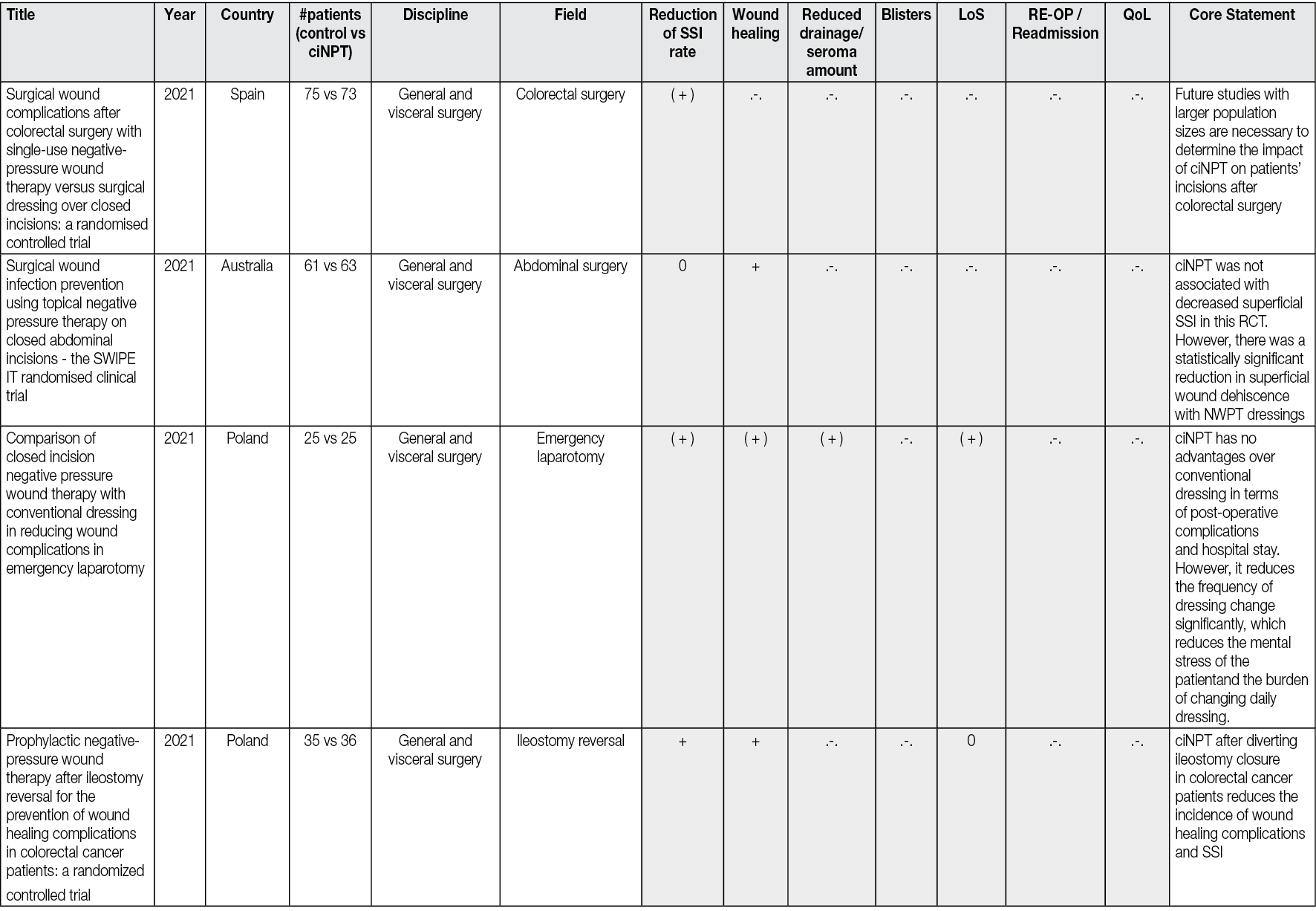
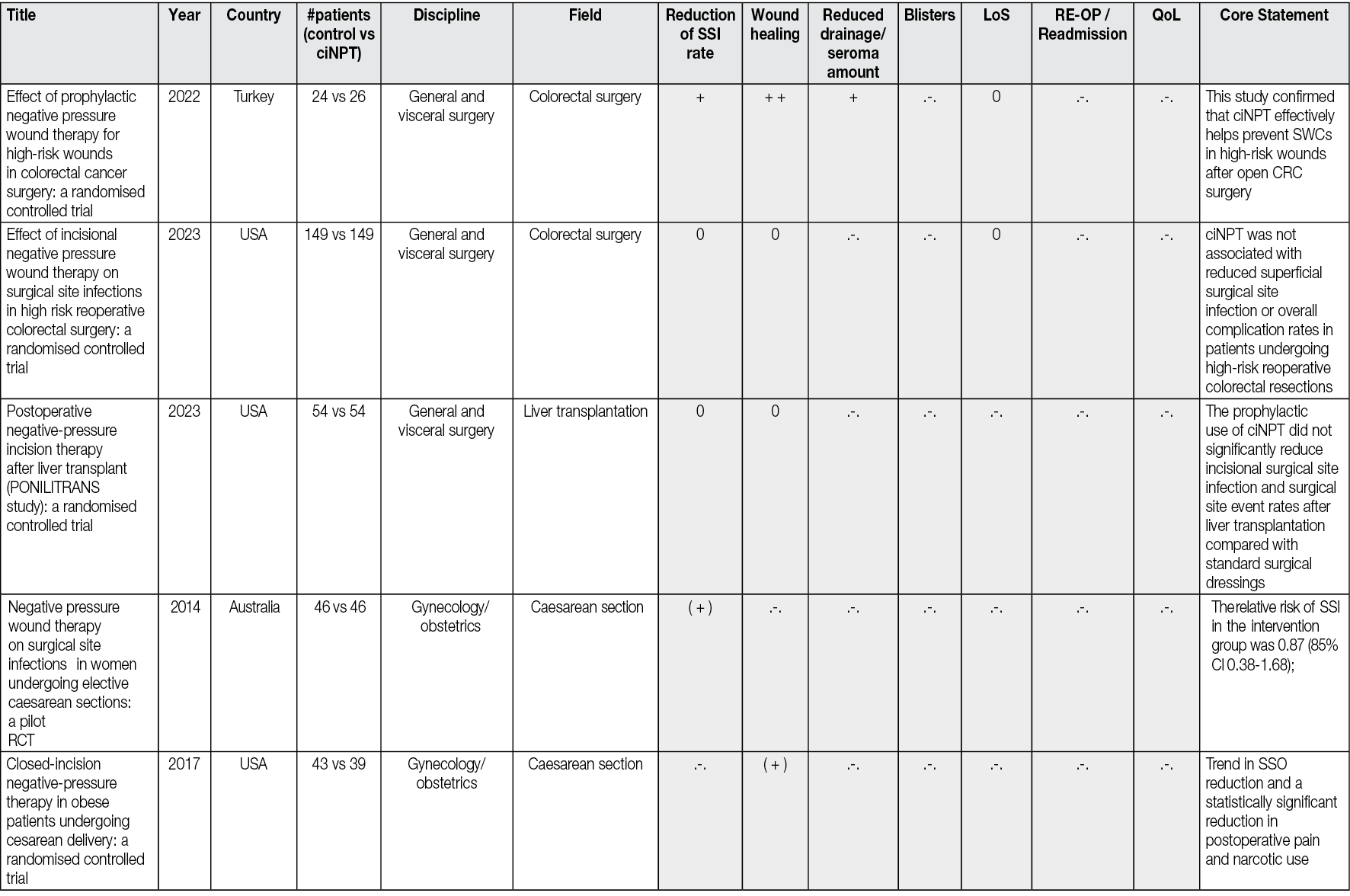
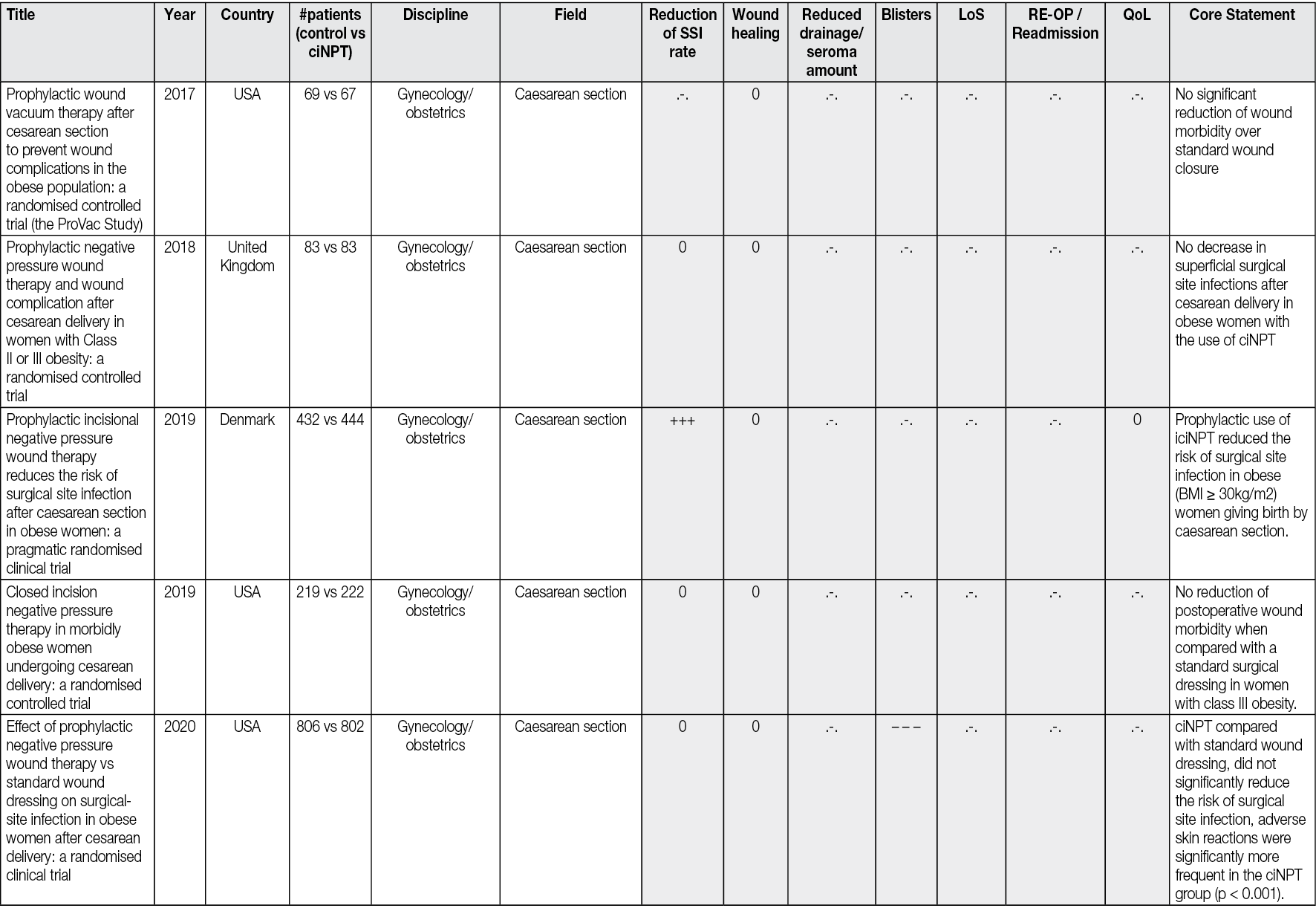
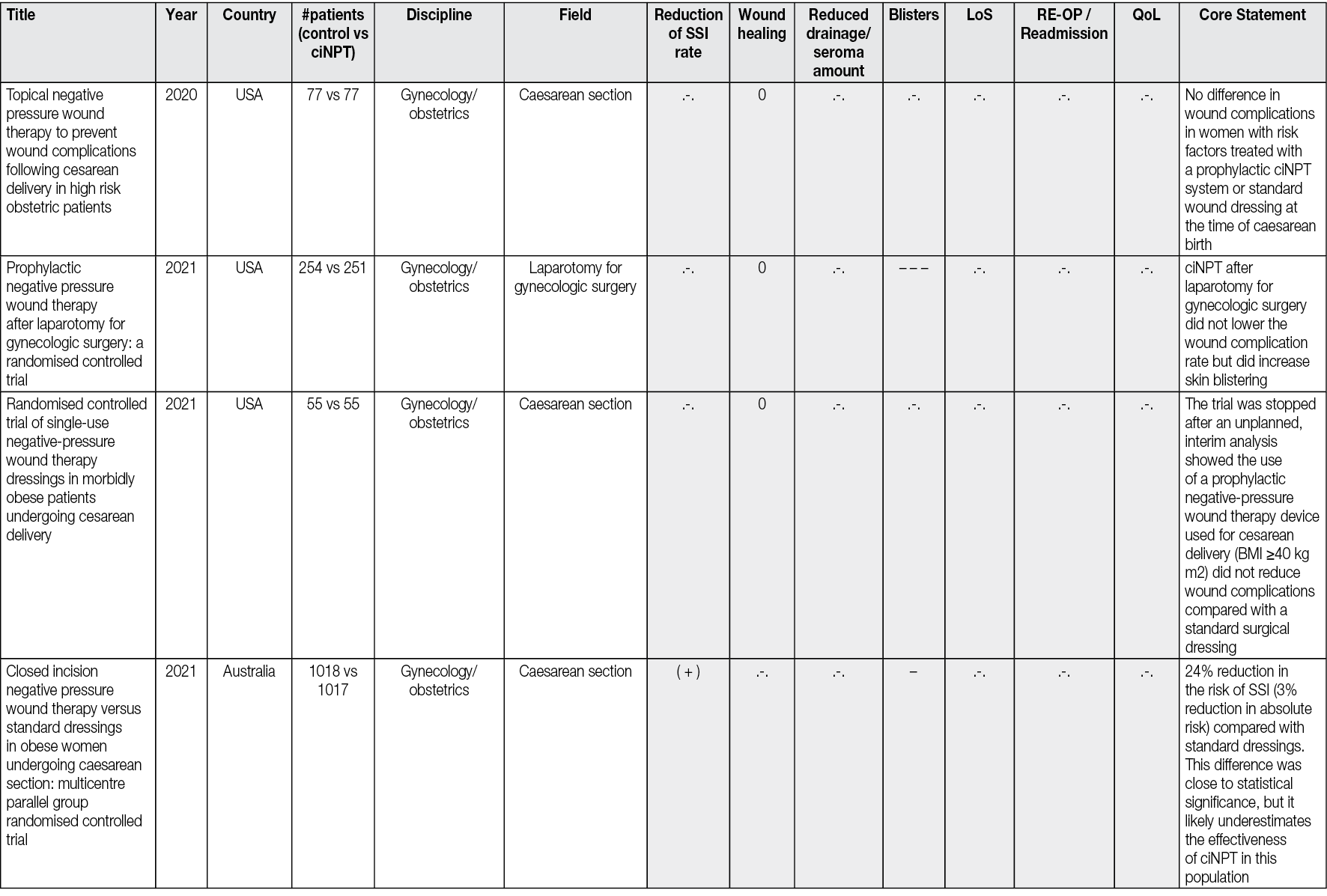
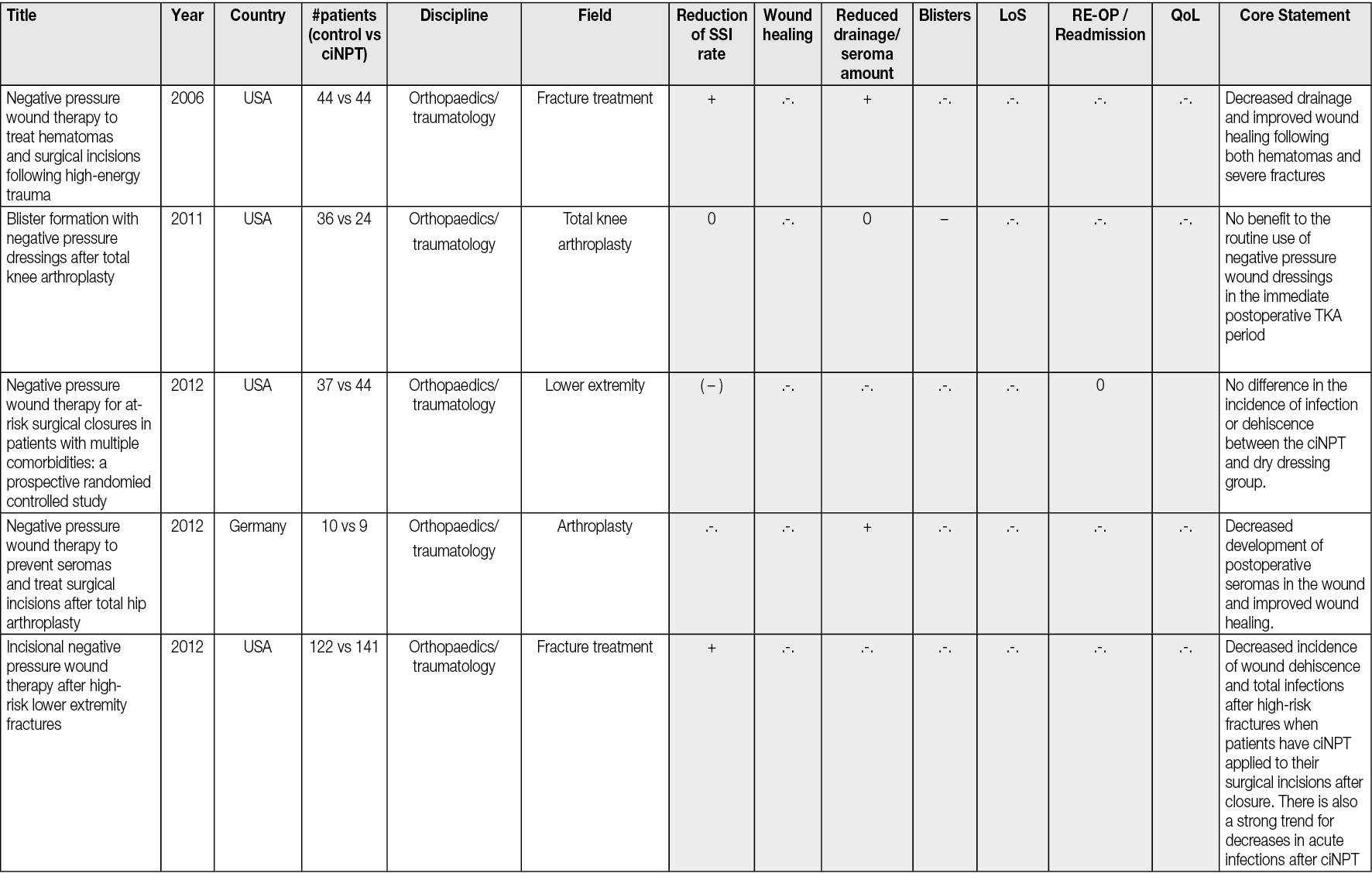
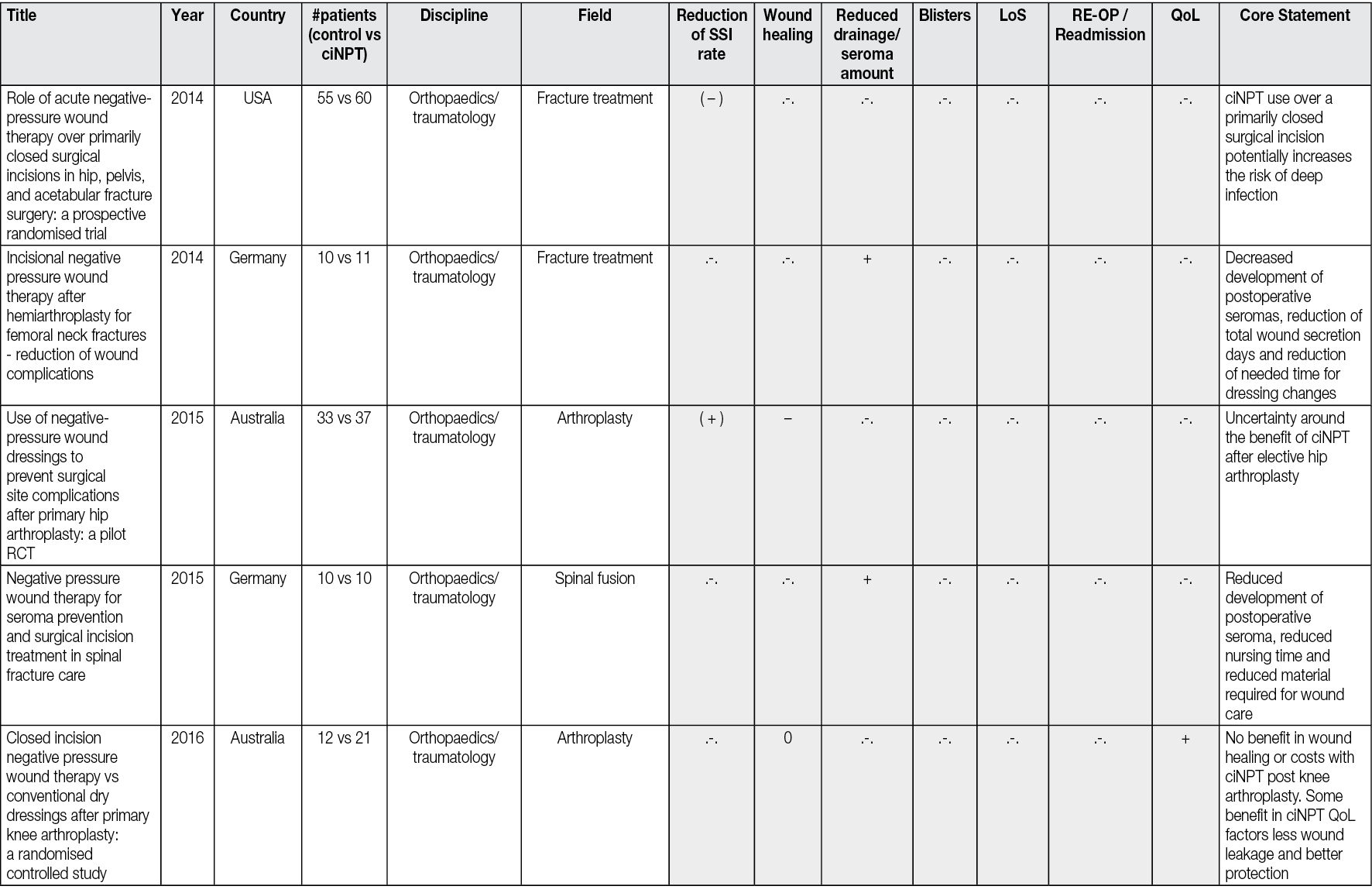
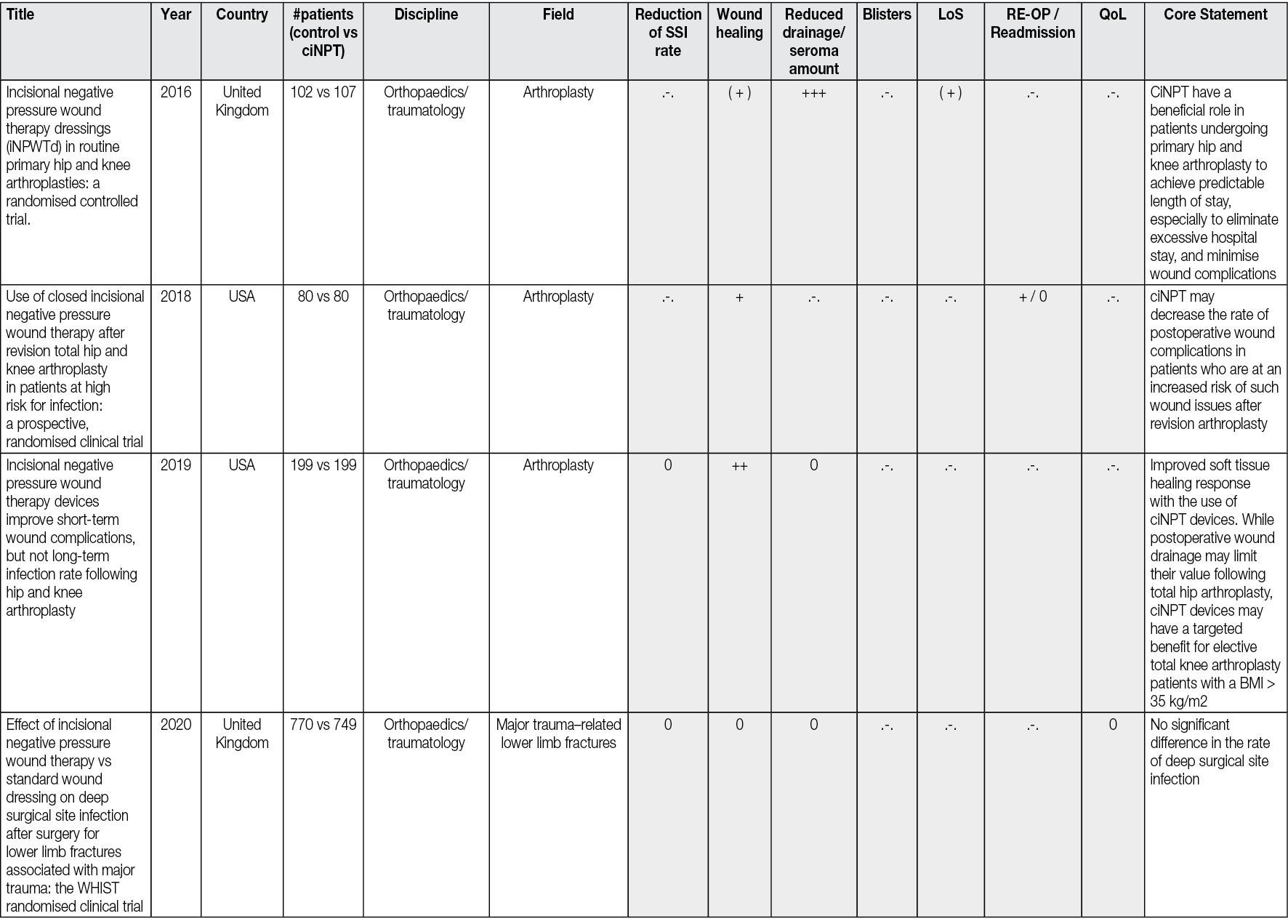
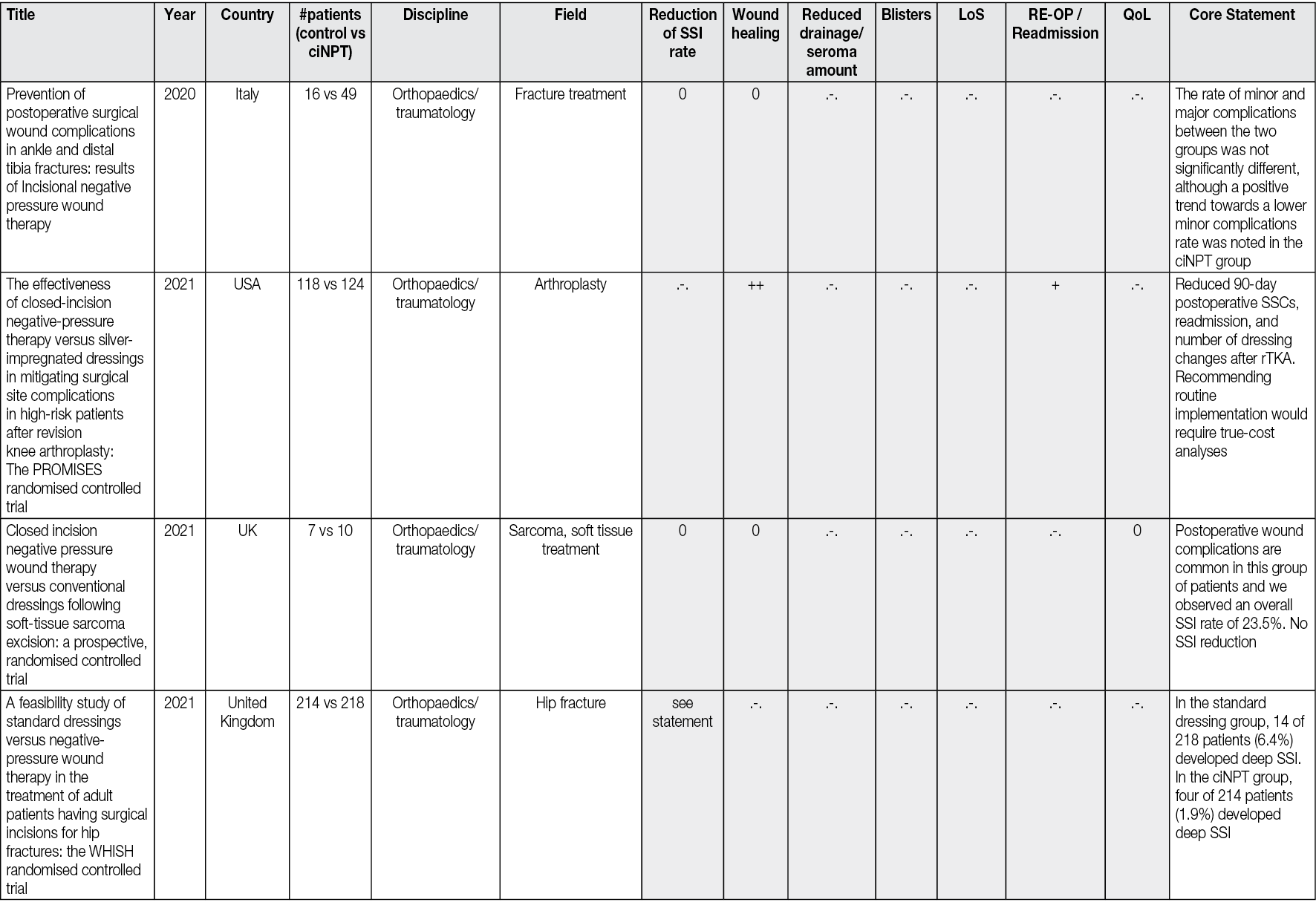
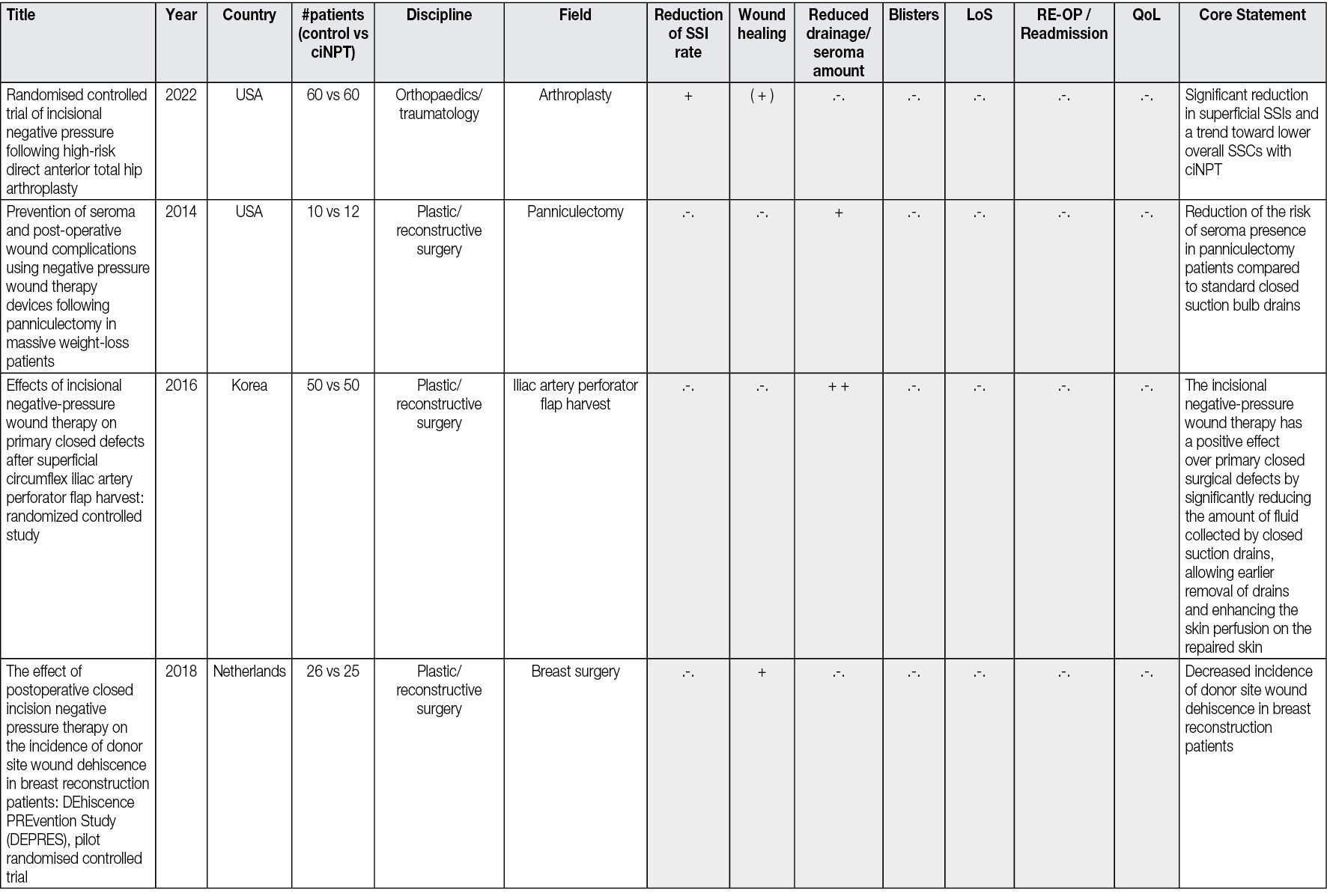
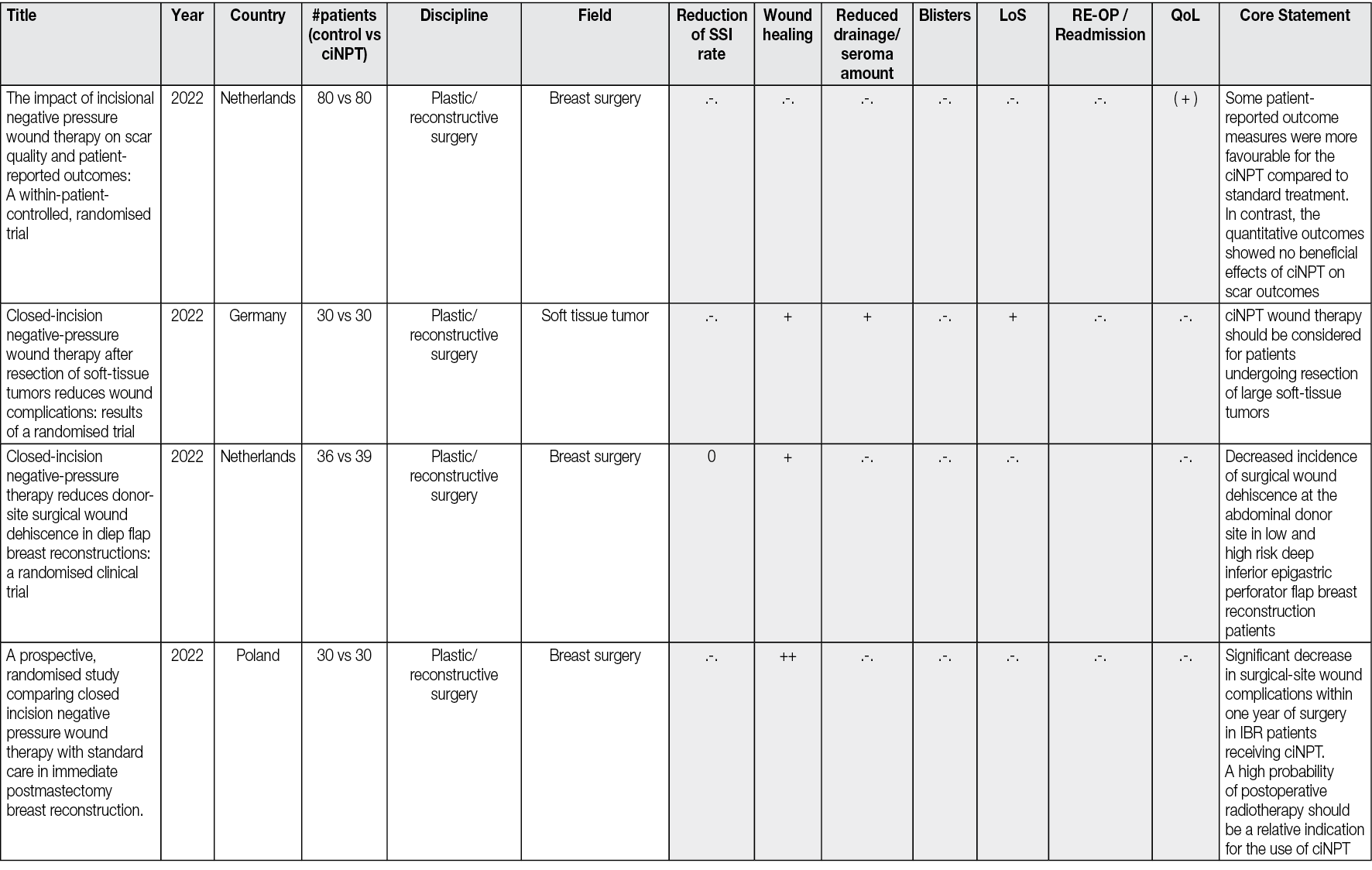
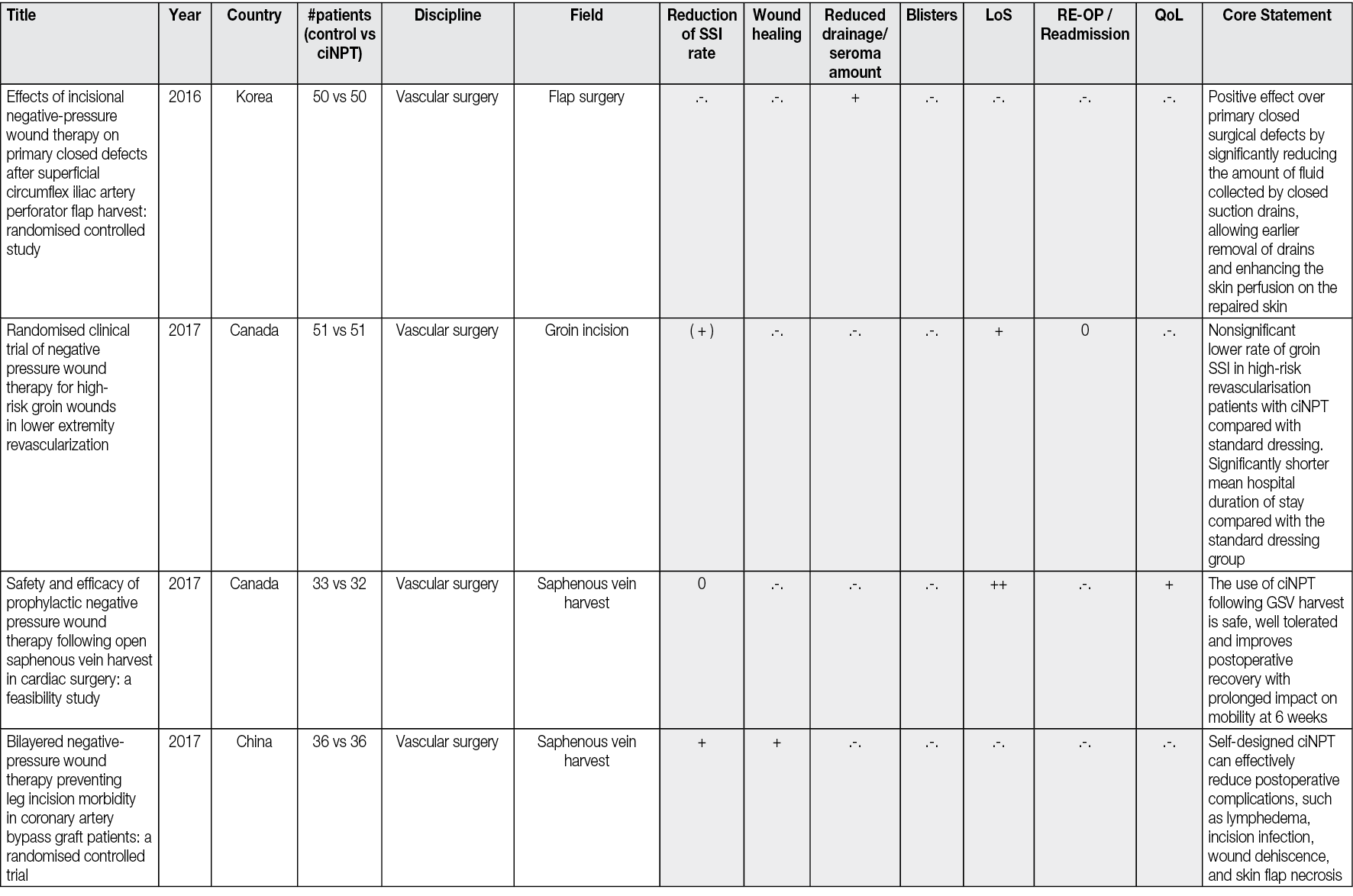
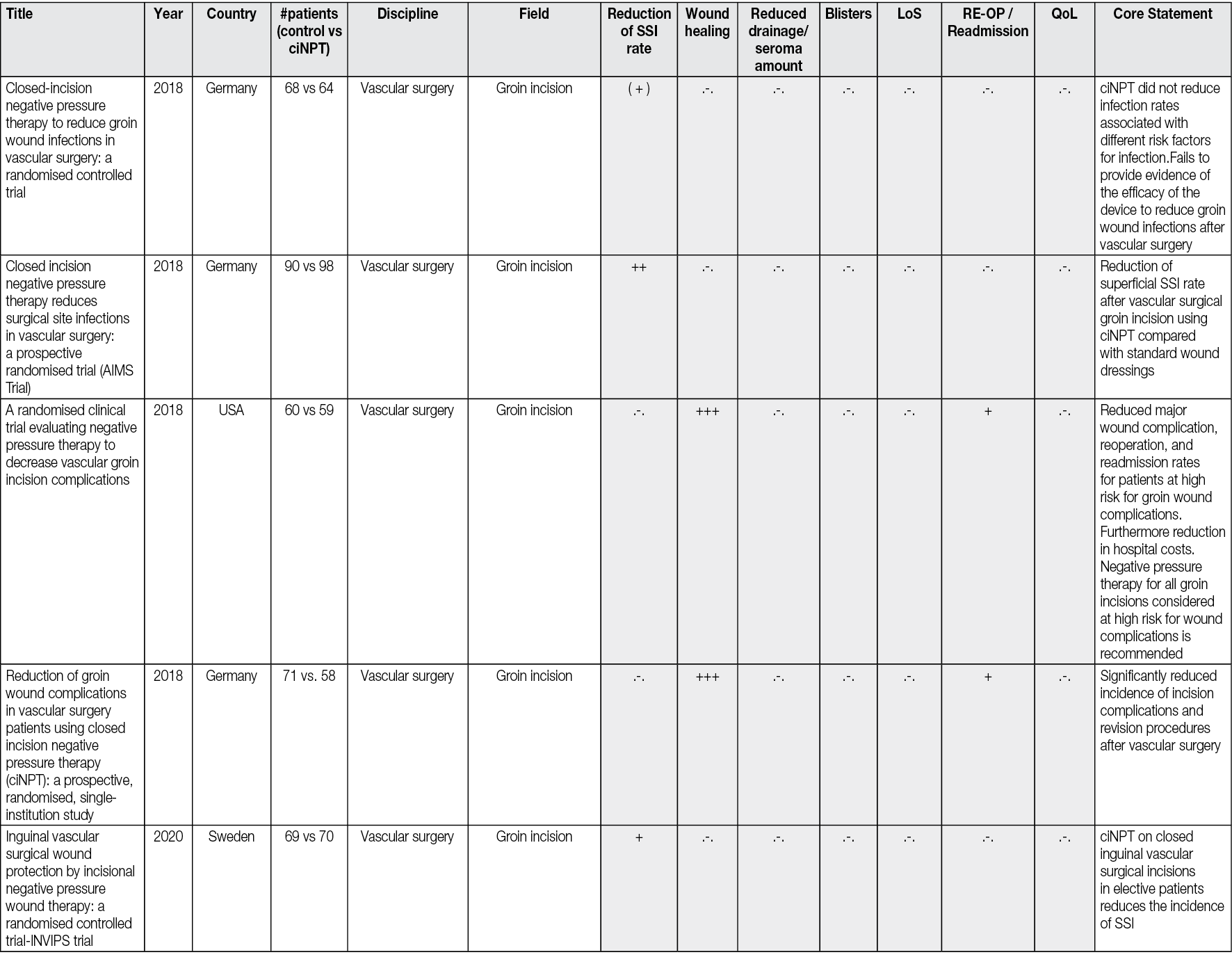
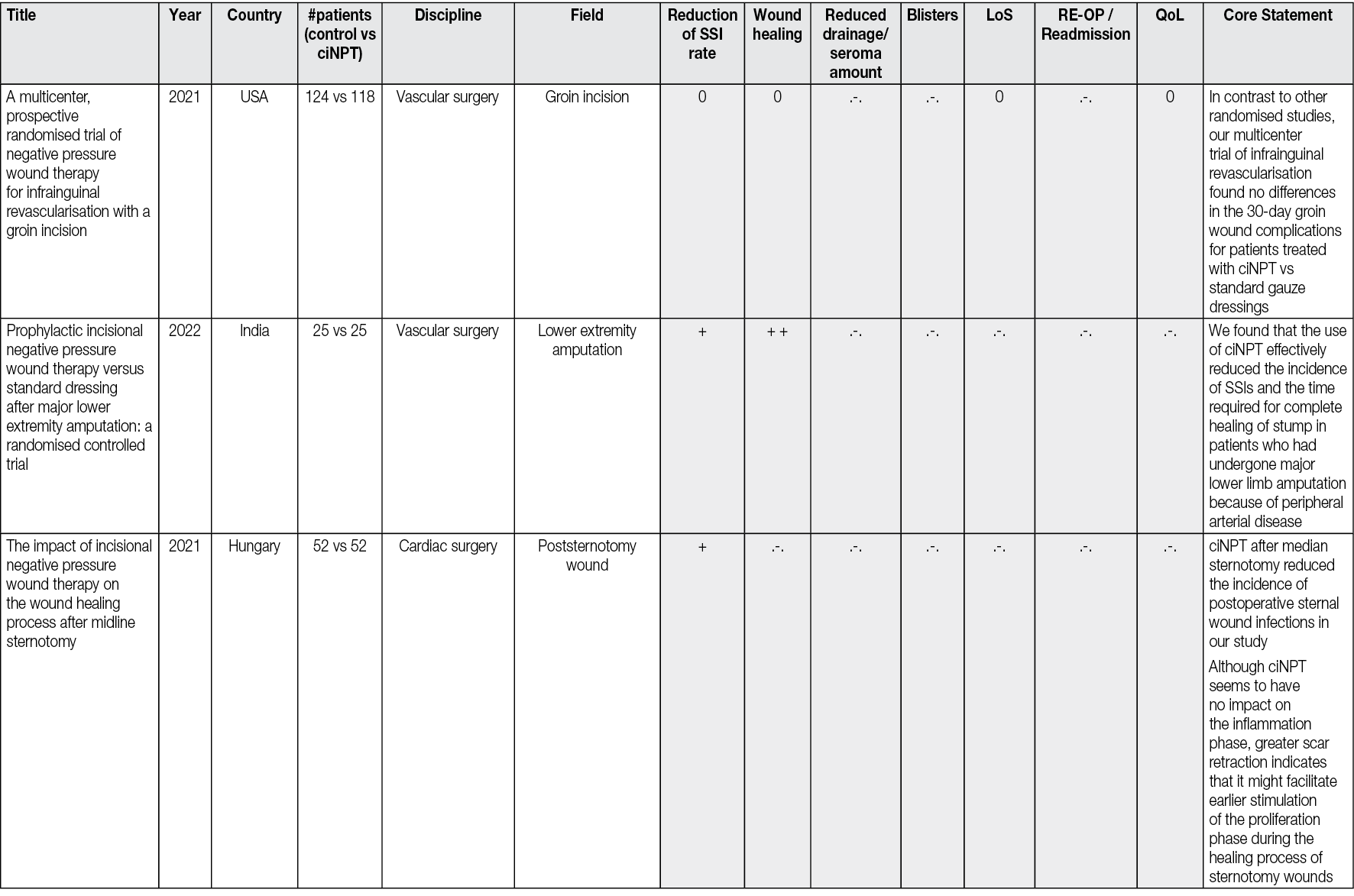
Appendix 3: Costs of ciNPT and cost-savings