Volume 23 Number 1
Intact fish skin grafts result in faster time to healing and lower costs to patients with venous leg ulcers and diabetic foot ulcers compared to the standard of care: An outcome-based pricing model
Thomas Zehnder, Marlise Blatti
Keywords venous leg ulcers, diabetic foot ulcers, economic outcomes, fish skin
DOI 10.35279/jowm2022.23.01.05
Abstract
Introduction Between 10 and 34% of healthcare expenditures are wasted. Given the expansion of the European advanced wound care market to $3.51 billion by 2025, healthcare systems will face problems unless they adopt models that help curb waste and prevent myopic spending. This manuscript introduces an outcome-based pricing model.
Methods For one year, a Swiss hospital prospectively examined and treated all venous and diabetic wounds meeting inclusion criteria with either standard of care or fish-skin graft. A model for expected time to wound closure was used to evaluate the conditions and assess how well surrogate endpoints predict wound outcomes.
Results Data show that surrogate endpoints are predictive enough to facilitate clinical decision-making regarding the use of intact fish skin grafts much earlier than the grafts used in usual models. While the intact fish skin graft products are initially more expensive than standard of care, they allow decision-makers to take hospital length of stay and other factors into account. Further, the difference in cost was quite substantial in favour of fish skin grafts over the long term. This model allows payors to mitigate risks when evaluating novel products, as healthcare systems do not pay for worse outcomes in new products. Even with this added cost, innovators benefit by reaping more data to use when refining products.
Conclusions Balancing innovation, care and cost with the system stresses to come will likely require a new means of understanding all three. This model provides a potential solution to one thorny juncture of these forces.
INTRODUCTION
The move towards outcome-based pricing agreements
An OECD report on healthcare spending1 reported that, on average, between 10 and 34% of healthcare expenditures are wasted on unnecessary procedures or drugs, inappropriate patient care, administrative costs or even corruption. These inefficient expenditures, coupled with the drastically rising costs of healthcare and treatment, are prompting many healthcare systems to explore different reimbursement models, such as outcome or value-based healthcare.
At present, there is no single definition of value-based healthcare, or what ‘value’ means in a health context; here, ‘value’ is defined as patient outcomes relative to the money spent to achieve that outcome, that is, the cost-effectiveness of treatments or procedures. Health technology assessment bodies in European countries tend to make decisions on the reimbursement of new health technologies at the national level based on their cost-effectiveness; this value is often only evidenced by late-stage clinical trial data, early pricing agreements or limited post-market data.
Despite the above, a novel approach to cost-effectiveness is emerging where healthcare bodies agree to pay only for the benefit that is shown in individual patients; this is called ‘outcome-based pricing’. As the prices of novel drugs and technology continue to rise, these agreements are becoming critical for maintaining the balance of introducing new therapies to patients while ensuring there is an appropriate cost benefit in state-funded healthcare systems.
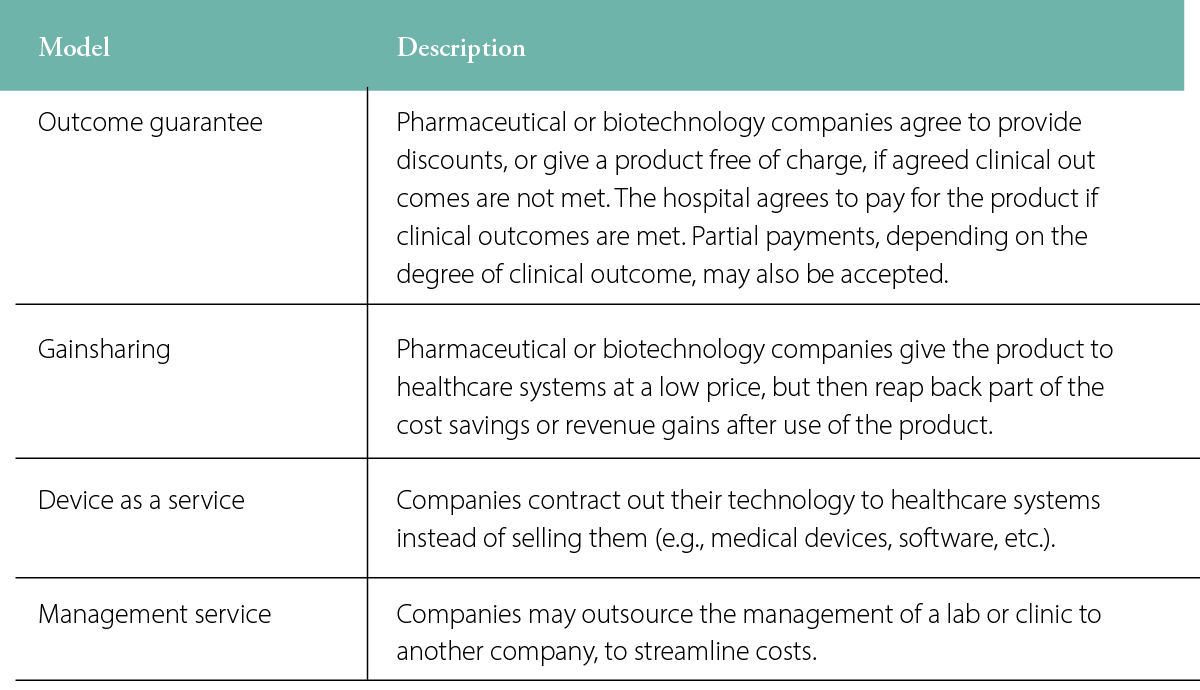
Several other unique payment models may be deployed, depending on the product being offered, such as an outcome guarantee model, a gain sharing model or varieties of service models (Table 1). The outcome guarantee model is most applicable to pharmaceuticals and health-benefiting medical devices (i.e., not diagnostics or equipment).
Table 1: Examples of pricing models available to healthcare systems
Chronic wound healing and outcome-based pricing
Chronic wounds are an example of the types of indications on which healthcare systems could improve using outcome-based pricing. Because care is longer-term, these wounds represent a great burden to healthcare systems. It is of interest to payors to have wounds treated and healed as soon as possible; however, public healthcare systems, such as those found throughout Europe, often fall into the trap of focusing on the initial cost of treatment versus standard of care (SOC). Biological and skin substitute products are inherently more expensive than basic SOC regimens, and this often puts hospitals off using them for hard-to-heal wounds, so that these treatments are typically employed only in more extreme cases.
Wound care treatment in Switzerland
The Swiss Society for Dermatology and Venereology (SGDV) and the Swiss Association for Wound Care (SafW) have developed guidelines that define the use of skin replacement products. The regulation of Switzerland’s Federal Department of Home Affairs on benefits in compulsory healthcare insurance specifies the use of skin replacement products as a compulsory service and refers to the SafW and SGDV guidelines. These guidelines are based on the scientifically founded knowledge that wounds that show a reduction in area of less than 40–50% following four weeks of adequate local therapy (SOC) should be treated with a skin replacement product. The reasoning is that, if skin replacement products cause a significant reduction in time to wound closure over SOC, a significant cost reduction can be achieved.
In line with the idea of outcome-based pricing, it was agreed that treatment with an intact fish skin graft* should accelerate wound healing by a minimum of 25% over expected healing time with SOC. If this criterion was met, then the payment was received. If the intact fish skin graft failed to accelerate wound healing over SOC, then the product was provided without payment.
This is a pilot feasibility study designed to evaluate a novel performance-based pricing model with a focus on the treatment of venous leg ulcers (VLUs) and diabetic foot ulcers (DFUs) using SOC treatment or intact fish skin graft at Spital Thun, Switzerland.
METHODS
VLU model
Background
The model’s purpose is to predict, with reasonable accuracy, which wounds are unlikely to heal within a specified time frame, based on surrogate wound healing markers. The observation period necessary to reach the endpoint of wound healing can be shortened to just four weeks using the surrogate markers, instead of the typical >12-week endpoint. The ability to predict which wounds are unlikely to heal based on SOC treatment alone allows for early intervention with definitive therapy, accelerating wound healing, reducing the risk of being hospitalised, improving quality of life and lowering the overall costs of wound treatment costs.
Predicting healing of VLUs using surrogate endpoints
Gelfand et al. looked at the probability of wound healing at 12 or 24 weeks, based on a surrogate endpoint of reduction in wound area at Week 4, in 11,472 patients with VLUs. The time points of 12 and 24 weeks were chosen as they are consistent with most VLU clinical trials. The surrogate endpoint of 43.76% wound reduction observed at Week 4 correctly predicted complete wound healing at 24 weeks in 66–81% of cases.2
Predicting wound healing trajectories
The surrogate endpoint identified by Gelfand et al. had to be compared against a standardised VLU wound healing trajectory, both in wounds that were predicted to heal by the endpoint and those that were not, in order for this model to be meaningful.
Steed et al. reported the wound healing trajectories of 232 patients with VLUs.3 The plotted mean values show wound healing trajectories split by patients who had fully healed by Week 20 and those who had not.
The wound healing trajectories presented by Steed et al. were tabulated for patients likely to heal at Week 20 and those unlikely to heal at Week 20.3 The time was expressed as a relative value to wound healing so it could be plotted and extrapolated. Quadratic equations were fitted to each curve to give the best R2 value possible. Fitted equations were limited to second-degree polynomials so that only two roots could be calculated, one within the 0–100% time frame and another beyond that, which could be ignored.
Assumption 1: For the model to be functional, it was assumed that non-healing patients would eventually heal under their current treatment and follow the trajectory plotted above. The assumption is unlikely to be true for all patients in the real world.
The quadratic equation was solved by giving two solutions: x1 and x2, where x1 represents the relative time passed based on the measured reduction in wound area between Week 0 and Week 4, where the patient receives only SOC.
Assumption 2: For the purpose of the model, a healing area >98% was considered fully healed to prevent plateauing of the curve, so as not to extend the estimated healing time unrealistically.
Using the equation, the estimated time to fully healed can be calculated using the following equation:
Relative time at >98% healed (based on assumption 2) x 4 Solution x1
This process was repeated for patients with <43.76% wound area reduction at Week 4, which were therefore predicted to not heal by Week 24 using the second quadratic equation.
Simplifying the model
For simplicity and adherence to SAfW and SGDV guidelines, the surrogate endpoint was rounded to 50% for this model. Therefore, patients who have a wound area reduction of <50% at Week 4 are unlikely to heal and should be eligible for treatment with intact fish skin graft. The endpoint of Week 24 described by Gelfand et al. was also changed to Week 20 to fit with the wound trajectories described by Steed et al.2,3
Treatment with a fish skin graft
The patients demonstrating a wound area reduction of less than 50% are unlikely to heal through SOC treatment and thus eligible to be allocated to the fish skin graft group. The patients demonstrating a wound area reduction of more than 50% are likely to heal, continuing with SOC.
Assumption 3: Patients treated with intact fish skin graft will have a minimum of 25% improvement over SOC, in terms of the time to fully healed, to be considered successful.
As patients are treated with an intact fish skin graft at Week 4, following four weeks of SOC treatment, an improvement of >25% from Weeks 4 to 8 and from Week 4 to time-to-fully-healed was calculated and displayed as the final output.
DFU model
The process described above was repeated for DFUs based on data from Margolis et al., who looked at the probability of wound healing at 12 or 20 weeks, based on surrogate endpoints in 28,624 patients with DFUs.4 The maximised dichotomous surrogate endpoint of wound reduction of 61% at Week 4 was found to correctly predict complete wound healing at 20 weeks in ~69% of cases.4 Based on these data, wound healing trajectories of 160 patients with DFUs were plotted for patients likely to heal at Week 20 and those unlikely to heal at Week 20.3 The data were then extrapolated and modelled to provide an estimate of healing at Week 8 and time-to-fully-healed, based on the reduction in wound area at Week 4 and the probability of healing at Week 20.
For simplicity, the surrogate endpoint was rounded to 50% for this model. Therefore, patients who have a wound area reduction of <50% at Week 4 are unlikely to heal by Week 20 and should be eligible for treatment with an intact fish skin graft. The intact fish skin graft product is expected to have a minimum of 25% improvement in healing time over SOC.
Study criteria
The feasibility study to evaluate the proposed payment model was performed from January through December 2020. A signed informed consent document was collected from each patient, and patients were treated in accordance with the requirements of the SAfW and SGDV guidelines. For 12 months, all venous and diabetic wounds that met inclusion criteria were prospectively examined and treated. After the initial four-week treatment according to SOC, wounds become dependent on the wound area reduction in the first four weeks and were treated further either with the continuation of SOC or with intact fish skin graft. A model that shows the expected time for wound closure and the expected wound area reduction, calculated as the period from Weeks 5–8 depending on the wound area reduction for the period from Weeks 1–4, served as basis for further documentation and outcome-based payment.
For wounds treated with SOC, the modelled expected value for the time to wound closure and the effective date of wound closure were documented. For wounds treated with intact fish skin graft, the product costs were paid by Spital Thun only if the effective wound area reduction between Weeks 5 and 8 was greater than 25% of the modelled value based on SOC treatment.
Patient recruitment
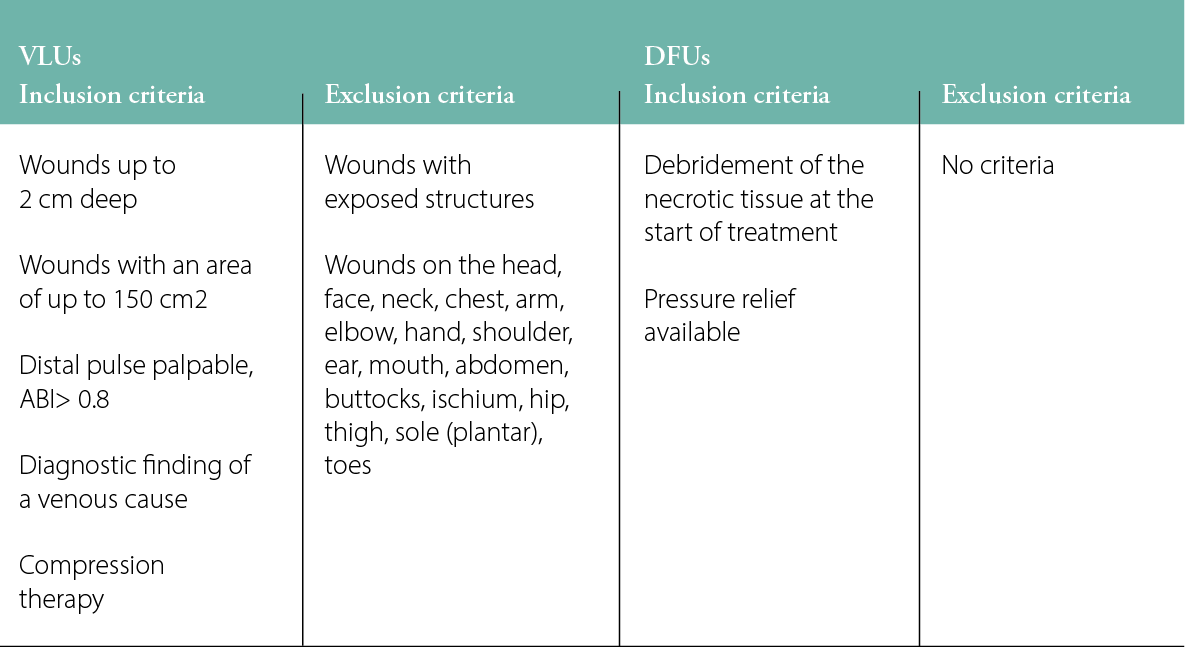
Regardless of whether the patient was treated on an outpatient or inpatient basis, the following first steps were carried out: adequate therapy for the cause of the wound was implemented and an assessment was made of the inclusion and exclusion criteria based on the model publications (Table 2).2–5
Table 2: Inclusion and exclusion criteria for VLUs and DFUs in the model
As soon as the adequate treatment of the wound cause had been initiated/completed, a four-week start period with adequate local therapy (‘SOC phase’) was initiated. At the beginning of the SOC phase, the wound was photographed and the wound area was measured.
During the four-week SOC phase, the wound care was provided according to international standards, with a particular focus on wound debridement, infection treatment and general wound treatment.
At the end of the four-week SOC phase, the wound was photographed and the wound area was measured. The values of the wound areas at the beginning and end of the SOC phase were entered in the model.
The following two decisions were possible:
- Wound area reduction Weeks 1–4 ≤50%: switch treatment to fish skin graft
- Wound area reduction Weeks 1–4 >50%: continue treatment with SOC
For wounds that are treated with SOC, the following criteria were documented:
- Wound area reduction between Weeks 1 and 4
- Expected value for the time until wound closure according to the model at the end of the SOC phase
- Type of further processing, if there is a deviation from SOC
- Time to full wound closure
For wounds that are treated with fish skin graft, the following must be documented:
- Expected value for the time until wound closure, according to the model at the end of the SOC phase
- Expected value for wound area reduction with SOC for Weeks 5–8 (‘SOC value’)
- Minimum value for expected wound area reduction with fish skin graft (the ‘threshold value’)
The threshold value is calculated as follows: Threshold = SOC value * 1.25
According to the literature, DFU wounds with a wound area reduction in Weeks 1–4 of <10% have a very low probability of healing at all. In these cases, the threshold is generally 5%.
Fish skin grafts were used according to the manufacturer’s instructions and the experience of the service providers. The type of debridement before use of the fish skin graft was documented, along with a photograph with the fish skin graft visible on the wound; the date, number and size of the product used; and patient compliance.
Paid treatment
At the end of Week 8, the wound was photographed and the wound area calculated as centimetres squared. If the effective wound area reduction during Weeks 4–8 was greater than the threshold value, then the agreed sum was due to be paid.
The following points were documented during further treatment from Week 9:
- Type of debridement before using intact fish skin graft
- Image with intact fish skin graft visible on the wound
- Date, number and size of the products used
- Patient compliance (compression, pressure relief, etc.)
- Date of wound closure
The outcome of further treatment with intact fish skin graft from Week 9 had no effect on the charged product costs.
RESULTS
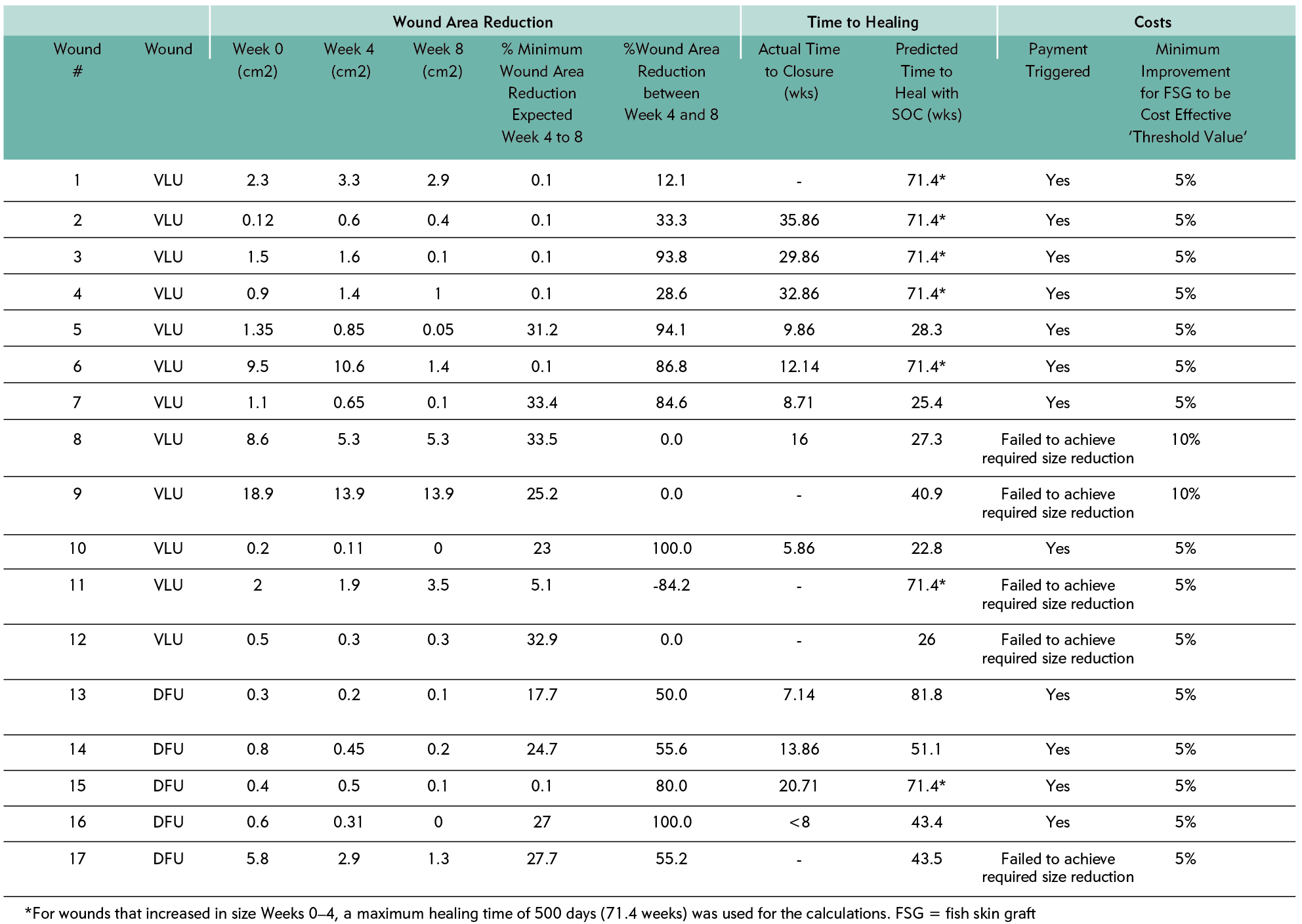
A total of 51 wound cases (25 DFU cases and 26 VLU cases) were enrolled in this study as of 31 December 2020. Nine cases (7 DFUs and 2 VLUs) dropped out between Weeks 0 and 4 on SOC, prior to allocation to the treatment group. The reasons for dropout varied: five wounds had healed before Week 4; one patient had an amputation; two were hospitalised; and one patient died, unrelated to the management of the wound. At the allocation event, there were 42 wounds (18 DFUs and 24 VLUs). Based on the model design, for wounds with an area reduction <50%, 21 cases were put into the fish skin graft group, and 21 cases were put into the SOC group (Figure 2). The original wounds ranged from 0.12 cm2 to 36.2 cm2 in size. The median and mean wound sizes were 1.4 cm2 and 3.51 cm2, respectively. Between Weeks 5 and 8, five patients dropped out: one case from the SOC group and four cases from the fish skin graft group; none of the dropouts were related to either treatment of the wound. In the fish skin graft group, one patient tested positive for COVID-19 infection, two had unrelated infections of a wound not receiving intact fish skin and one patient did not attend the final in-person follow-up appointment due to concerns of contracting COVID-19.
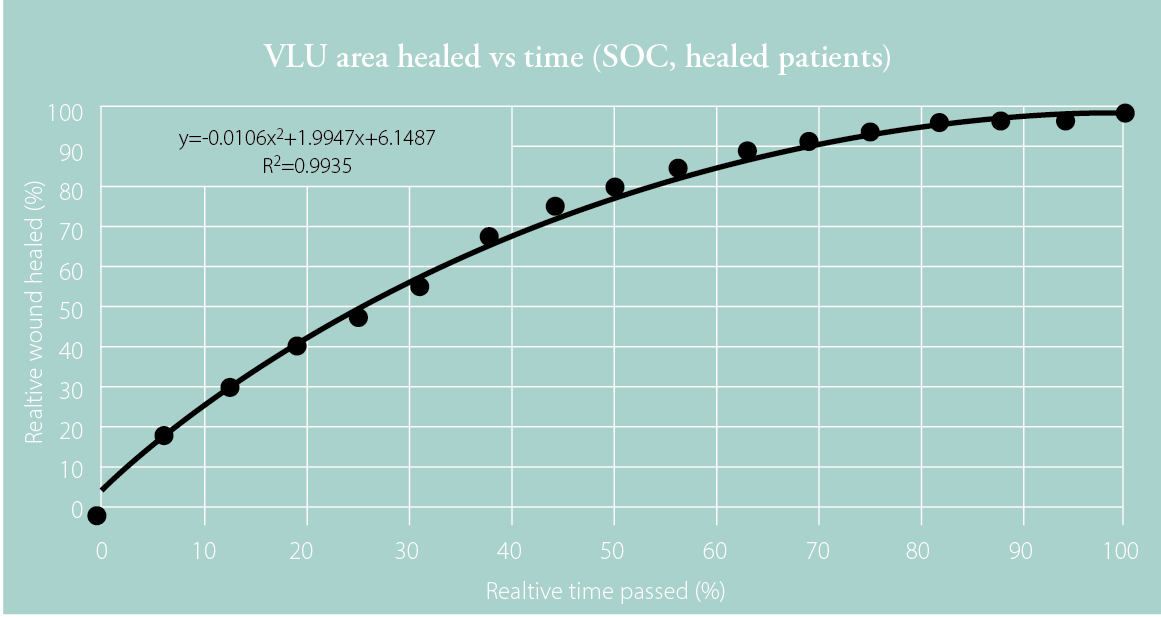
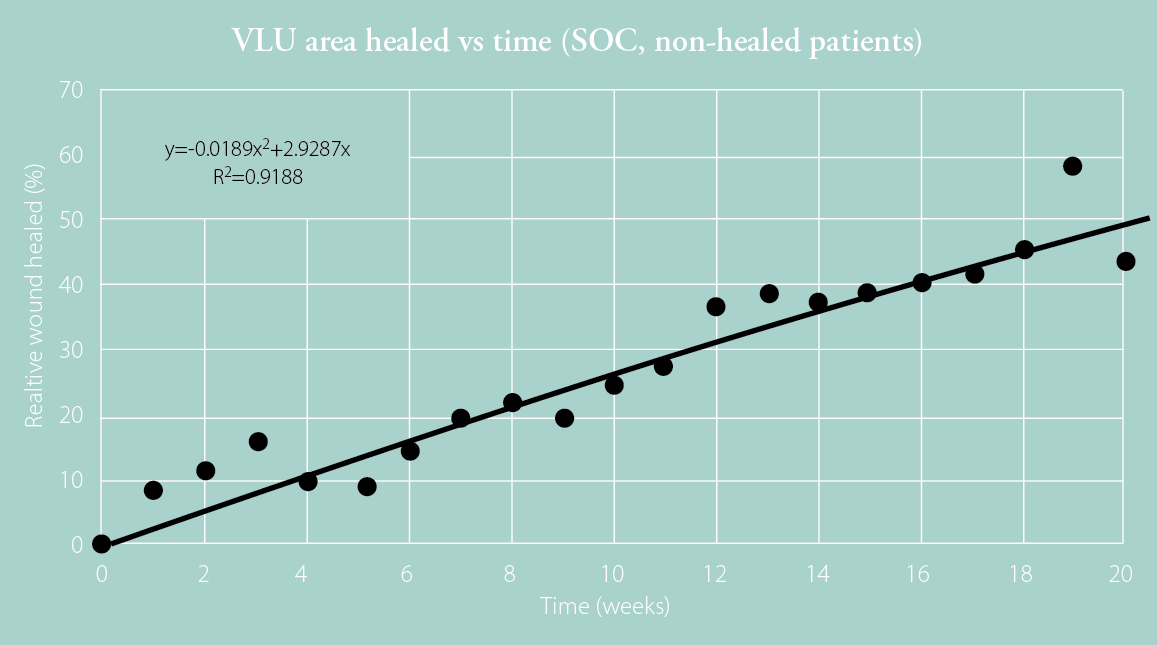
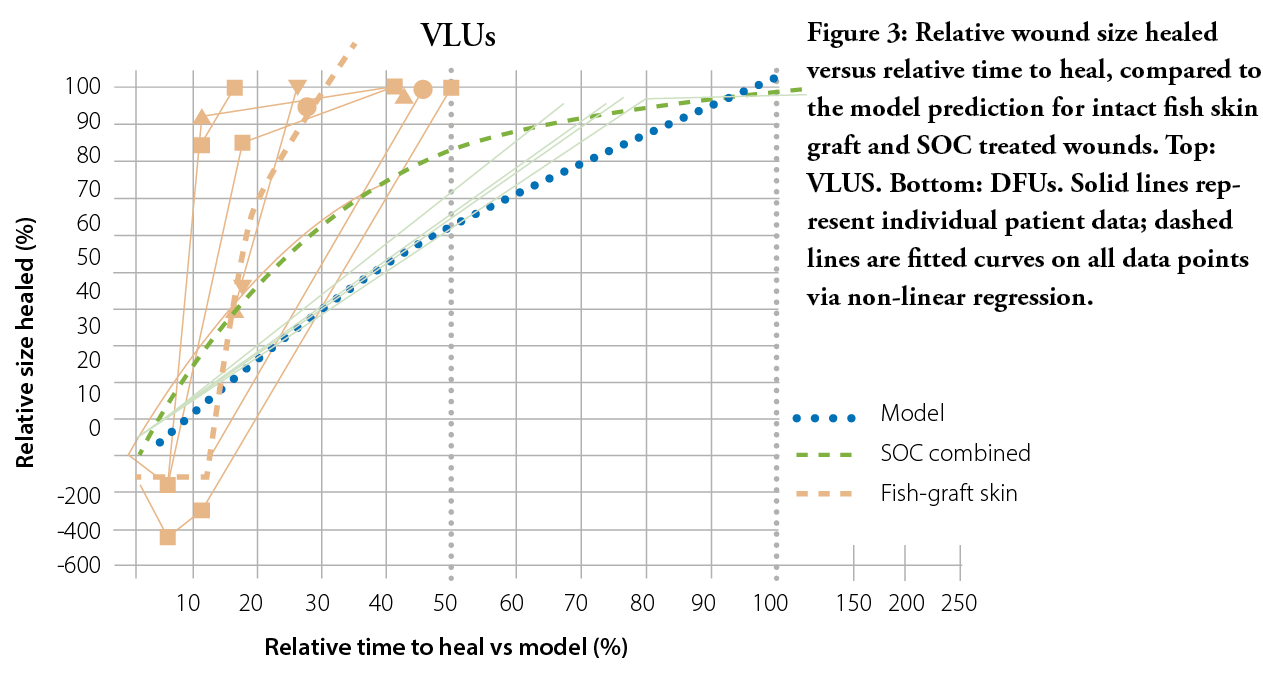
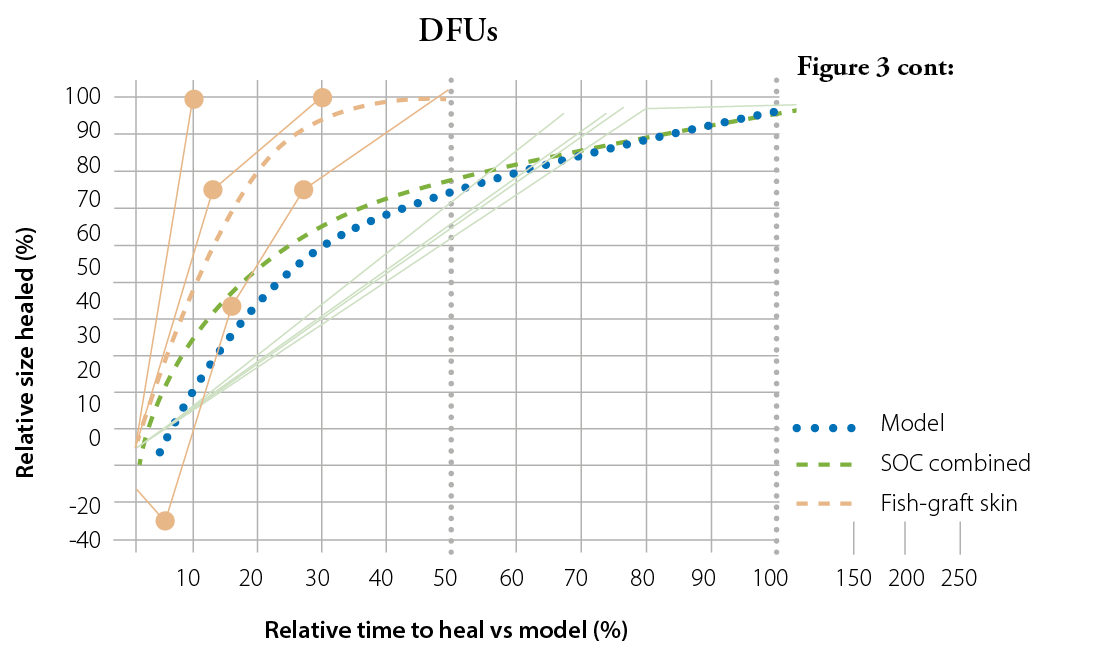
Figure 1: Plotted data for healed patients (top) and non-healed patients (bottom) at 20 weeks with fitted quadratic curves and corresponding equations based on the wound healing trajectories presented by Steed et al.
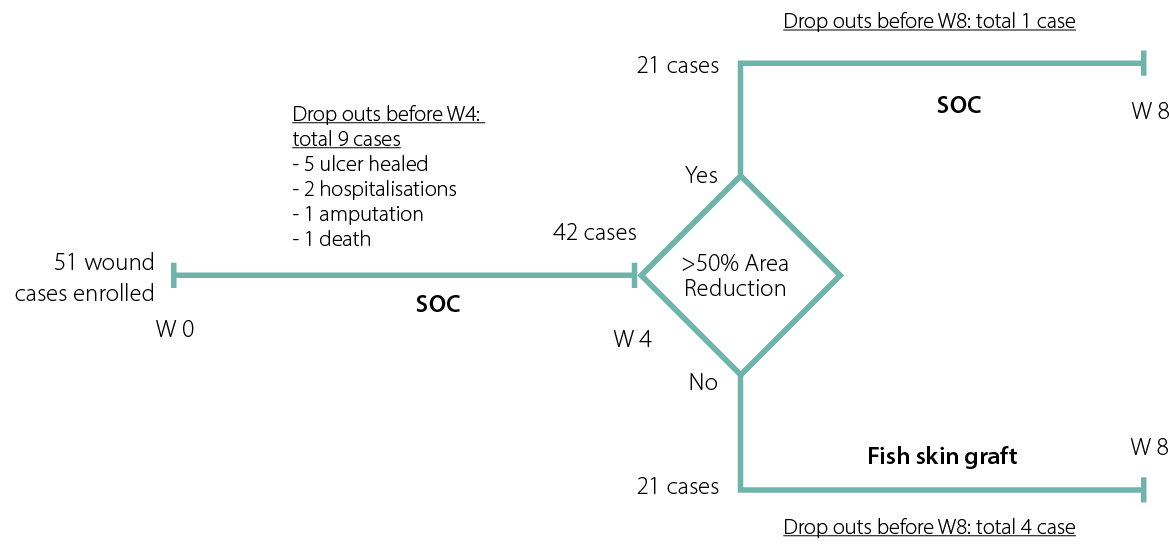
Figure 2: Patient recruitment and allocation in the model pilot study
Ultimately, 17 case wounds received treatment with an intact fish skin graft, as the wounds achieved less than 50% wound area reduction from Week 0 to Week 4, so a response in the SOC phase was deemed unlikely to heal by the Week 20 and 24 endpoints. The remaining 20 cases, which had a wound area reduction of more than 50%, continued the treatment with the SOC they had been receiving in Weeks 0–4.
Of these 17, five wounds failed to achieve the size reduction in Weeks 5 to 8; consequently, the graft was given free of charge. All remaining intact fish skin graft-treated wounds (N=12) beat the model predictions, so the hospital paid for the product used. Most wounds healed >50% sooner than was predicted, and as early as <10% of the time predicted by the model. Seven wounds increased in the area over the first four weeks on SOC; of those wounds only did not achieve the projected wound area reduction. Consequently, an accurate healing time could not be predicted for these wounds. For data analysis purposes, a maximum endpoint of 500 days was selected as a surrogate – an optimistic timeline for wounds that increased in area as much as +400%. Even these wounds, which were likely to remain chronic without intervention, managed to beat the optimistic healing time of 500 days.
In the fish skin graft group, there was a significant (p=0.027) increase in the wound area reduction between Weeks 5 and 8 between the minimum expected [model prediction] mean of 16.6% (standard deviation {SD}: 14.2), compared to the actual at 46.5% (SD: 49.9), based on the paired two-tailed t-test. For the 12 wounds that reached definitive closure, time to healing was significantly reduced (p<0.001), with 16.7 mean weeks to healing, compared to the predicted time to heal with SOC at a mean of 53.1 weeks.
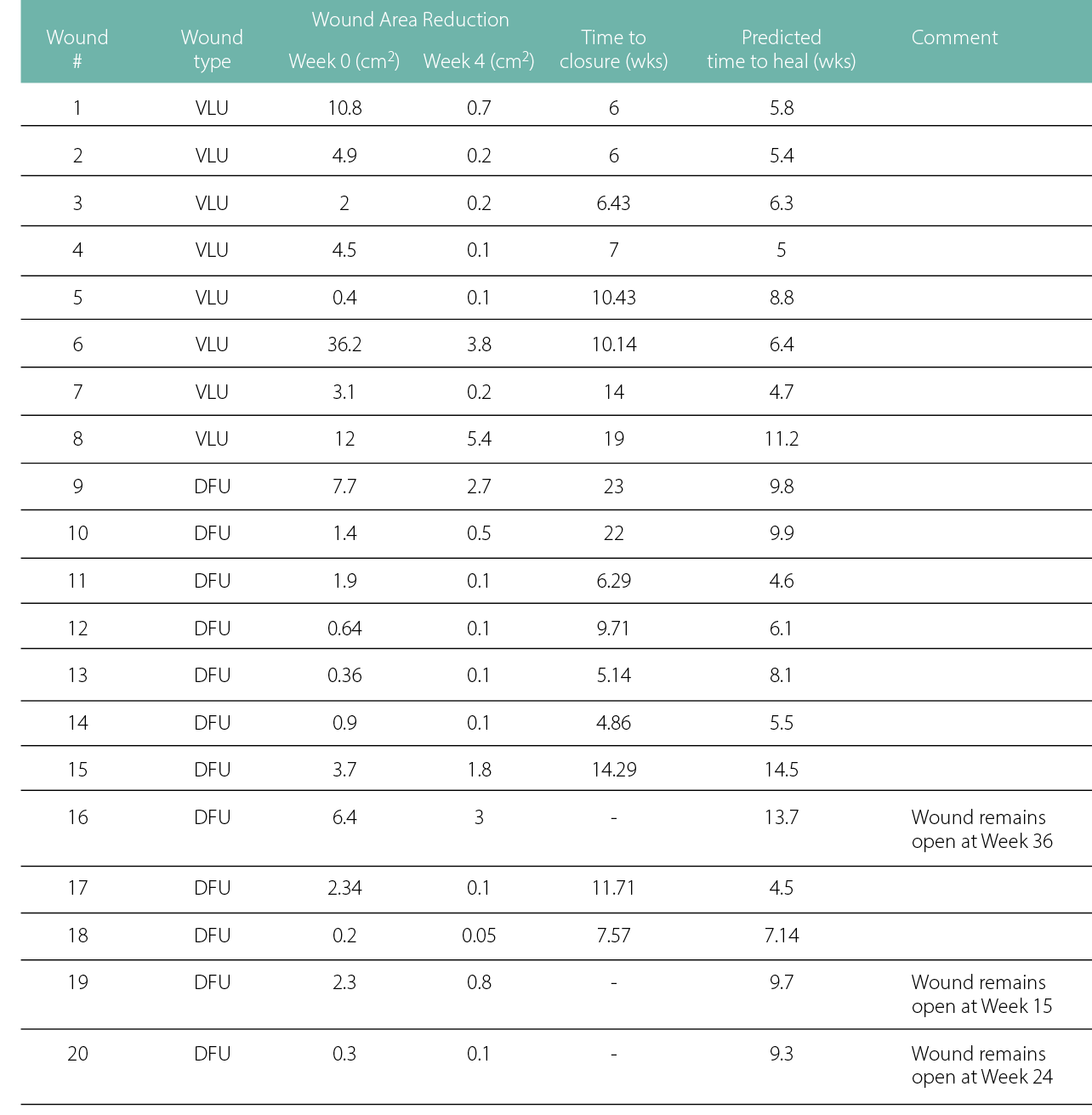
In the SOC group, the results of the paired test indicated that there is a significant (p = .007) difference between the actual time to heal mean of 10.8 weeks (SD: 5.8), compared to the predicted healing mean time of 7.3 weeks (SD: 2.8).
Consequently, payment was made for 12 wounds treated with the intact fish skin graft, and the product was given free of charge in five wounds.
Cost savings
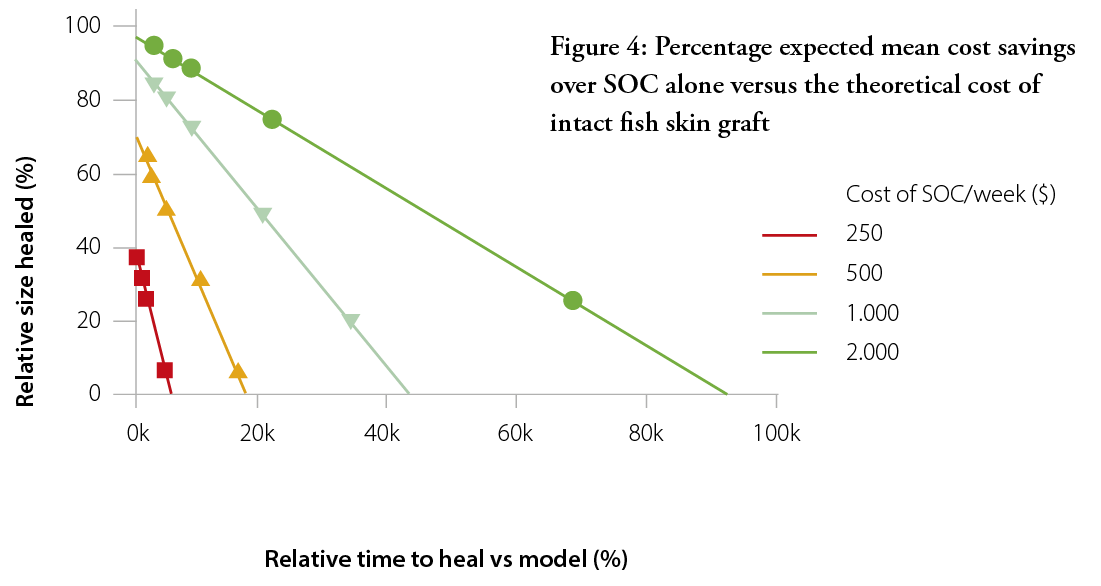
The purpose of this outcome-based pricing approach was to demonstrate that the use of intact fish skin graft results in faster healing wounds and, consequently, cost savings. Figure 3 shows the average expected cost saving in intact fish skin graft-treated patients versus if they had been treated with SOC alone, based on the modelled healing time. The cost of treatment was based on a weekly cost for SOC treatment at different values ranging from $100 per week (no cost savings) to $2000 per week. The cost of SOC treatment was kept the same for intact fish skin graft-treated patients, plus the cost of the intact fish skin graft on top.
As can be expected, the cost savings associated with the use of intact fish skin graft increases as the cost of SOC per week increases. At a total weekly cost of $2000, the intact fish skin graft can be expected to be cost-effective even at a ridiculously high price of >$90k. At a more reasonable $500 per week, the limit for cost-effectiveness lies at ~$16k.
DISCUSSION
The apparent limitation of this model is its inherent design – the wound time to heal was only predicted and varies greatly among individuals, depending on a multitude of factors ranging from underlying co-morbidities to patient compliance. Nonetheless, intact fish skin graft was indicated to treat wounds that were deemed unlikely to heal by Week 20 or 24, for VLUs and DFUs, respectively, and these wounds still healed in most cases. The treatment with the fish skin graft even managed to change the predicted trajectories and heal wounds that had increased in size up to +400% in the first four weeks.
In several wounds treated with SOC, the wound appeared to lag as it reached complete closure. A likely limitation is that most wounds entered in the study were relatively small, at a median of 1.38 cm2, thus measuring wounds of this size poses a risk of variability and may lead to inaccuracies in the reported results.
One aspect that is not taken into account in the outcome-based model is the patients’ quality of life. Yang et al. present real-world examples that show intact fish skin graft as a useful skin substitute for wound area reduction and the healing of ‘hard-to-heal’ lower extremity VLUs and DFUs.6 Even if intact fish skin grafts were to be priced above the cost-effectiveness threshold of $16,697 (total cost of treatment), patients would still likely benefit from the therapy, would have a better quality of life and would take up less of practitioners’ time. Thus, the willingness-to-pay could be expected to be greater than the cost-effectiveness threshold. Such calculations are beyond the scope of this model; however, Winters et al. recently published a paper showing the cost-effectiveness of intact fish skin graft versus SOC in treating chronic DFUs. They indicated that fish skin treatment could result in lower costs ($11,210 vs. $15,075 per wound), more healed wounds (83.2% vs. 63.4%), fewer amputations (4.6% vs. 6.9%) and a higher quality of life (0.676 vs. 0.605 quality-adjusted life-year ) than the SOC.7
Table 3: Individual data points for the fish skin graft patients
The global advanced wound care market is predicted to expand over the next five years. Specifically, in Europe, the current market, estimated at $2.8 billion in 2020, is expected to increase to $3.51 billion by 2025, with a compound annual growth rate of 4.63%, a tremendous increase in cost to healthcare systems when taken as a standalone number. However, outcome-based models such as the one described in this paper, and an increase in proven cost-effectiveness of these advanced therapies, could be used effectively to heal more wounds faster, thus increasing patients’ quality of life and decreasing overall costs of care.
If healthcare systems agree to try novel pricing models, the benefits are clear: greater adoption of novel products, driving innovation by biotechnology companies; lower overall costs for payors; lower burdens on healthcare workers; and higher quality of life for patients. One requirement for collecting these data, however, is the establishment of a secure registry so that clinics can easily access the model at a central location and the sponsor can continuously monitor the outcomes of treated patients and receive payments or donate products as necessary.
The work continues with hospitals and payors in Switzerland and other European countries to expand the roll-out of this outcome-based pricing model with the use of a central registry. Follow-up results from this feasibility study will be published in peer-reviewed journals as they become available.
Table 4: Individual data points for the patients continuing SOC
CONCLUSIONS
The results of this feasibility study showed that treatment with intact fish skin graft results in cost savings and faster healing wounds than would be predicted by SOC treatment. The treatment with intact fish skin graft resulted in a payment rate of 80%, indicating that any losses from providing the products free of charge are offset by the financial gains of the product sales. The model proposes a new pathway to overcome the financial and psychological hurdles of using skin substitutes in patients with chronic non-healing wounds where the SOC has been ineffective. There will be a need for larger-scale and more robust studies using this proposed outcome-based payment model.
Implications for clinical practice
- Economic considerations increasingly drive clinical decision-making.
- This novel pricing model allows for practitioners to investigate and use cutting-edge products and technologies more easily.
- The data collection involved allows for more informed clinical decisions.
Further research
A larger trial could demonstrate benefits on a systematic scale or illuminate any flaws that might arise if this model is adopted on a societal scale.
More research is warranted on making data collection and consultation as easy as possible for clinical practitioners.
KEY MESSAGES
With a massive increase in wound care expenditures on the horizon, healthcare payers require new methods to evaluate and reimburse treatments that save money over the long terms. This manuscript aims to provide an initial study of one such model. These results indicate that outcome-based pricing may spur better results, not only for patients and payers, but for medical innovators as well.
Author(s)
Thomas Zehnder, MD, Medical Department, Hospital of Thun, Switzerland
Marlise Blatti, Medical Department, Hospital of Thun, Switzerland
Correspondence: thomas.zehnder@spitalstsag.ch
Conflict of interests: None
References
- Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002; 119:1420-5.
- Steed DL, Hill DP, Woodske ME, Payne WG Robson MC. Wound-healing trajectories as outcome measures of venous stasis ulcer treatment. Int Wound J 2006; 3:40-7.
- Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003; 26:1696-700.
- Robson MC, Hill DP, Woodske ME, Steed DL. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg 2000; 135:773-7.
- Saeedi P et al. Diabetes research and clinical practice. 2019; 157:107843.
- Yang CK, et al. A prospective, postmarket, compassionate clinical evaluation of a novel acellular fish-skin graft which contains omega-3 fatty acids for the closure of hard-to-heal lower extremity chronic ulcers. Wounds 2016; 28(4):112-8.
- Winters C, et al. Cost-effectiveness of fish skin grafts versus standard of care on wound healing of chronic diabetic foot ulcers: A retrospective comparative cohort study. Wounds 2020; 32:283-90.
