Volume 23 Number 1
VenUS 6 – A randomised controlled trial of compression therapies for the treatment of venous leg ulcers: Study design and update
Sabeen Zahra, Catherine Arundel, Katherine Jones, Tom Davill, Gareth Roberts, Jo Dumville
Keywords randomised controlled trial, wound healing, venous leg ulcer, Compression therapies, time to healing
DOI 10.35279/jowm2022.23.01.02
Abstract
Background Venous leg ulcers (VLU) are common wounds, mainly in the gaiter region of the leg. Compression therapy is an effective treatment for reducing the time to healing of VLU. The four-layer bandage and two-layer hosiery protocols are supported by good evidence for clinical and cost effectiveness. There is more limited evidence for other treatments, such as the two-layer bandage and compression wraps. Robust evidence is required to compare the clinical and cost effectiveness of these and to investigate which is the best compression treatment for reducing time to healing of VLU whilst offering value for money.
Aim VenUS 6 aims to investigate the clinical and cost effectiveness of evidence-based compression, two-layer bandage and compression wraps for time to healing of VLU.
Method This multicentre, pragmatic, three-arm parallel group study aims to recruit 675 eligible participants aged ≥18 years with at least one VLU, and who can tolerate full compression and give written consent. Participants are allocated 1:1:1 to receive evidence-based compression, two-layer bandage or compression wrap. Participants are followed up with weekly assessments until the participant’s reference leg is ulcer-free and no further nursing assessments are required to treat the leg. Reference ulcer healing is confirmed by a healthcare professional/treating nurse. Participant-reported outcomes are collected at baseline and at 1, 3, 6 and 12 months. The primary outcome is time to healing of the reference ulcer. Secondary outcomes include clinical events and participant-reported ulcer related pain, quality of life, treatment adherence and resource use.
INTRODUCTION
Venous leg ulcers (VLU) are common, recurring open wounds on the lower leg resulting from venous insufficiency. Wound care is mainly delivered in community-based clinics, patient homes or outpatient settings. Delivering effective treatments remains important to maximise ulcer-free days and improve the health-related quality of life of people with VLU. Evidence suggests full (high) compression treatment, aiming to deliver around 40mmHg compression at the ankle, is effective for reducing ulcer healing time and is cost effective.1 More recently, the Early Venous Reflux Ablation (EVRA) trial has reported the early provision of endovenous ablation surgery to further reduce time to healing and to be cost effective.2,3 Due to the long waiting times for surgery referrals, however, such treatment may be delayed, resulting in people receiving compression therapies in the interim. Furthermore, some people with VLU might not be eligible for this surgery and so would require compression therapy as their primary treatment. Understanding the most clinically and cost-effective compression treatment for VLU remains an important aim in wound care.
The main full compression treatments currently in use in the UK’s National Health Service (NHS) are: 1) two-layer hosiery or the four-layer bandage (termed here as ‘evidence-based compression’, or EBC), 2) the two-layer bandage and 3) compression wraps. The effectiveness of EBC therapies was assessed previously with the four-layer bandage and shown to be more clinically and cost effective than the short stretch bandage.4 A subsequent trial with 457 participants showed little difference in healing times between the four-layer bandage and two-layer hosiery.5,6 Two-layer hosiery was also found to be a cost effective treatment compared with the four-layer bandage, although this treatment is not suitable for all patients.
There is more limited evidence for other compression therapies. Three trials involving a total of 299 participants have compared two-layer with four-layer bandages, with uncertainty around the relative effects of the two treatment options.7–9 For compression wraps, only one small trial has been reported (24 participants), with a relatively short follow-up period of 12 weeks. Time to healing or complete wound healing was not reported.10 Whilst the relative effectiveness evidence is limited, two-layer bandages and, increasingly, compression wraps are widely used as treatments for VLU. The generation of further robust evidence through a sufficiently powered randomised controlled trial to evaluate which is the best full compression treatment for reducing time to healing for VLU can inform future decision making in this area.
Given the varying evidence for different compression systems, the VenUS 6 study has been designed to undertake the following comparisons for time to healing of VLU and address the uncertainties identified above in a way that is most relevant to decision makers. The key objectives are, therefore:
- To compare compression wraps with EBC in terms of the time to healing of VLU;
- To determine whether two-layer bandages are non-inferior to EBC for time to healing of VLU;
and
- To determine which is the most cost effective, full compression treatment for VLU.
METHODS
Trial design
VenUS 6 is a multicentre, three–arm, parallel group, pragmatic randomised controlled trial evaluating the clinical and cost effectiveness of EBC, two-layer bandages and compression wraps. The trial has a 32-month recruitment period, including an initial 6-month internal pilot. Follow up will be variable, with participants followed for minimum of 4 months and a maximum of 12 months, following randomisation. This is a pragmatic trial and so, following randomisation and application of the allocated treatment, each participant will receive treatment as per routine clinical practice. The trial is registered with ISRCTN (reference: 67321719) and approved by the Research Ethics Committee (reference: 20/WS/0121), Health Research Authority and Health and Care Research Wales (HCRW). It is funded by the National Institute Health and Research Health Technology Assessment programme (NIHR128625) and sponsored by the Manchester University NHS Foundation Trust.
Study setting
The study will enlist up to 35 sites, including secondary care NHS trusts, community NHS trusts and primary care centres.
Participants: We will recruit adult (≥18yrs) participants with at least one VLU. Patients will be eligible for inclusion if they have least one VLU wholly or partially within the gaiter region of the leg; have an ankle brachial pressure index of ≥0.8 taken within last 3 months, or any locally approved alternate assessment to rule out peripheral arterial disease; and if they are able to tolerate full compression. Participants will be excluded if they: have a VLU that has non-venous aetiology and is confined to the foot only, are an active participant in another study evaluating treatments for VLU, have already been a VenUS 6 participant or if they have a planned treatment to remove/close superficial veins within the next 28 days. Figure 1 shows the VenUS 6 study design.
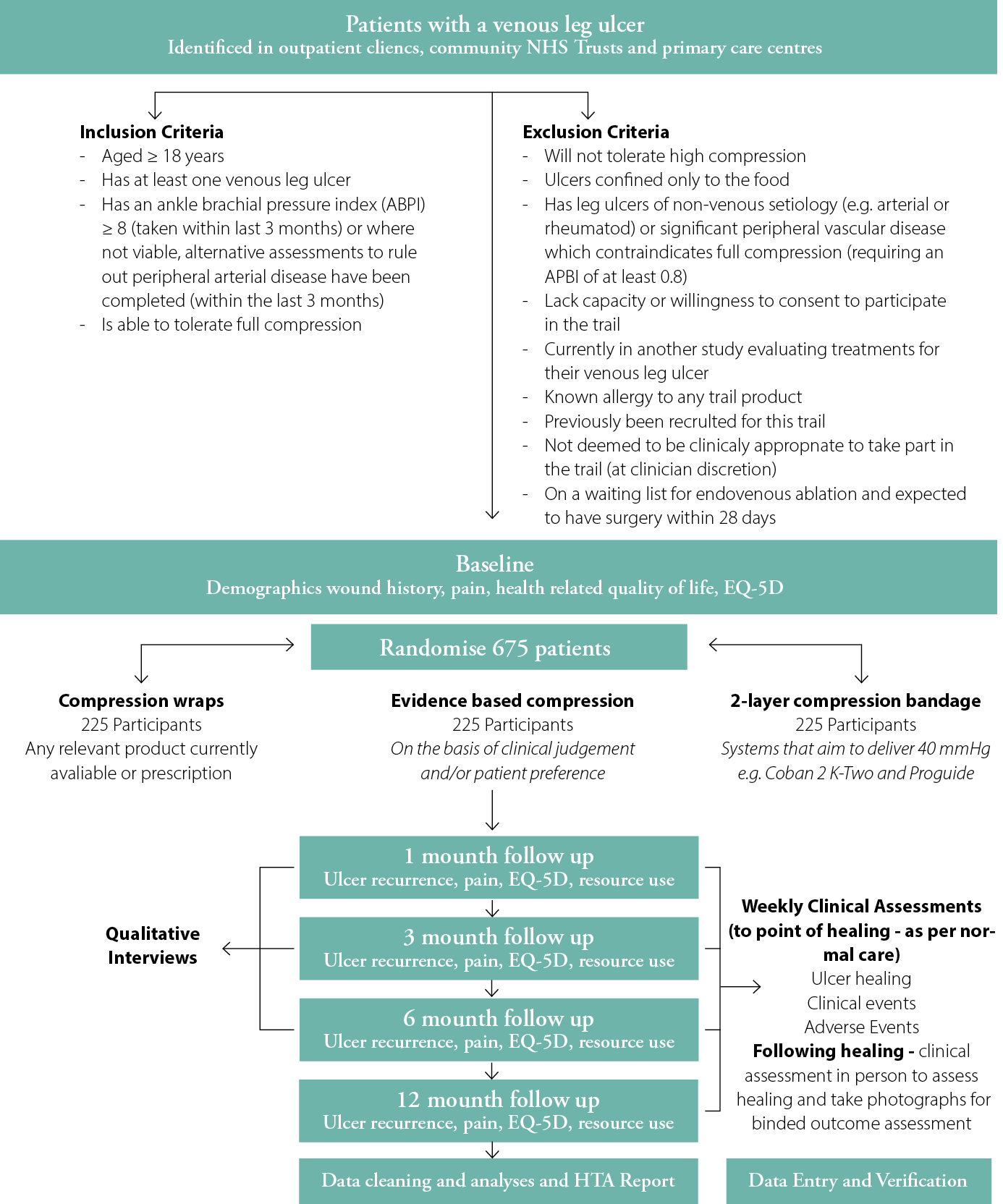
Figure 1: VenUS 6 design/participant pathway throughout the study
Randomisation and allocation
In all, 675 eligible participants will be equally randomised and allocated to one of the three compression therapies. A secure, centralised, online randomisation service hosted by the University of York Trials Unit will be used to randomise eligible participants. Randomisation will be stratified by ulcer duration (≤6 months or >6 months) and ulcer area (≤5cm2 or >5cm2) using permuted blocks.
Interventions
A total of 225 participants will be received into each of the following three intervention arms:
1. Evidence-based compression:
This is the choice arm for applying a four-layer bandage or two-layer compression hosiery system based on clinical discretion or participant preference. Any recognised brand of four-layer bandage can be used if it aims to deliver ≥40mmHg compression at the ankle. Compression hosiery consists of two layers of compression aiming to deliver graduated, sustained compression of ≥40mmHg at the ankle. The first layer is an understocking or liner, over which a second layer/overstocking is applied.
2. Two-layer bandage:
This contains an initial bandage layer covered with a top cohesive layer bandage to apply ≥40mmHg compression at the ankle. Any recognised two-layer system listed under the British National Formulary as a ‘multilayer compression bandaging/two-layer system’ may be used.11
3. Compression wrap:
Compression wraps are defined as using hook and loop systems (such as Velcro) to secure the compression sleeve around the foot and leg. Wraps may be used in VenUS 6 if they are designed to be worn on the lower leg and foot, aim to deliver ≥40mmHg compression at the ankle and are CE marked and available on FP-10. These may be used with a compressive or non-compressive liner.
Outcomes:
The primary outcome of the trial is time to healing of the reference ulcer, defined as complete epithelial cover in the absence of a scab (eschar) with no dressing required. We will undertake a blinded outcome assessment by assessing images of VLU taken at baseline and once per week for four weeks following healing. Secondary outcome measures are ulcer recurrence, adverse events, health-related quality of life, resource use, wound-related pain, treatment adherence and ease of use. These will be collected through study-specific case report forms. Data on treatment use; clinical events, including the healing of the VLU; and adverse events will be collected during nurse assessments until the participant’s leg is ulcer-free, and via monthly telephone contact following healing until the end of the participant’s follow up period. During the trial, participants will also be asked to complete postal questionnaires sent at months 1, 3, 6 and 12, following randomisation, to collect information regarding quality of life, pain, treatment adherence and resource use in relation to VLU. We will integrate VenUS 6 into a wider economic model to explore the relative cost effectiveness of alternative compression treatments.
VenUS 6 progress update:
Sites update:
To date, 18 sites have been opened and are actively recruiting participants to VenUS 6. This includes: 12 secondary care trusts, 4 community trusts and 2 GP practices. In addition, there are 12 sites currently in the process of being set up for the study. Full support is provided to sites during the set-up process and to help in delivering VenUS 6 study activities locally. There is also a per-participant fee of £469 available to sites to support the study’s activities.
Recruitment update:
Recruitment to VenUS 6 commenced on 3 February 2021 and is anticipated to be completed by 30 March 2023. As of 31 January 2022, 155 participants (23% of 675) had been recruited. For information related to VenUS 6 progress updates, please visit the VenUS 6 webpage at:
https://www.york.ac.uk/healthsciences/research/trials/research/trials/venus6/#tab-3
VenUS 6 is still open to new recruiting sites. For more information and to express interest in participating, please contact: venus6-trial-group@york.ac.uk
Declarations
Competing interests
All authors declare that they have no competing interests.
Acknowledgements
The research team would like to thank the study participants who have kindly agreed to take part in this study and our patient and public involvement representatives whose input has been indispensable in developing study documentation.
Funding
This project is funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (Project Reference: 128625).
The views expressed are those of the author(s) and
not necessarily those of the NIHR or the Department of Health and Social Care.
Availability of data and materials
Datasets and statistical code used in this study will be available from the corresponding author, on reasonable request, following completion of the trial.
Ethics approval and consent to participate
Ethical approval for this trial has been granted by the West of Scotland Research Ethics Committee 4 – reference 20/WS/0121 (approval date: 15.09.2020). Participants are required to provide informed consent prior to participation.
Consent for publication
Not applicable.
Key messages
- Full compression therapy options have been found to be the first-line treatment for venous leg ulcers (VLU) and are routinely used across the UK’s NHS. Despite this, identification of the most effective full compression therapy treatments remains important.
- VenUS 6 aims to evaluate the clinical and cost effectiveness of current compression therapies, evidence-based compression (four-layer bandage or two-layer compression hosiery), two-layer bandage and compression wraps, in terms of time to healing of venous leg ulcers.
- VenUS 6 is currently in progress and is looking for more sites to participate in recruiting participants to the study. For more information and to express an interest please, contact venus6-trial-group@york.ac.uk.
Author(s)
Sabeen Zahra PhD1, Catherine Arundel MSc1, Katherine Jones PhD1, Tom Davill MSc1, Gareth Roberts BSc1, Jo Dumville PhD2,
on behalf of the VenUS 6 trial team
1York Trials Unit, Department of Health Sciences, Faculty of Science, University of York, YO10 5DD
2Division of Nursing, Midwifery and Social Work, Jean McFarlane Building, University of Manchester, Oxford Road, Manchester, M13 9PL
Correspondence: Catherine.arundel@york.ac.uk
Conflict of interests: None
References
- O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012;11:CD000265.
- Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med 2018; 378(22):2105-4.
- Epstein DM, Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, et al. Cost-effectiveness analysis of a randomized clinical trial of early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration. Br J Surg 2019; 106(5):555-62.
- Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, Team V. VenUS I: A randomised controlled trial of two types of bandage for treating venous leg ulcers. Health Technol Assess 2004; 8(29):iii, 1-105.
- Ashby R GR, Ali S, Adderley U, Bland JB, Cullum N, Dumville J, et al. Compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): A randomised controlled trial. Lancet 2014; 383:871-9.
- Ashby R GR, Ali S, Saramago P, Chuang L-H, Adderley U, Bland JM, et al. Compression hosiery versus compression bandaging in the treatment of venous leg ulcers: A randomised controlled trial, mixed treatment comparison and decision analytic model. Health Technol Assess 2014; 18:1-293.
- Lazareth I, Moffatt C, Dissemond J, Lesne Padieu AS, Truchetet F, Beissert S, et al. Efficacy of two compression systems in the management of VLUs: Results of a European RCT. J Wound Care 2012; 21(11):553-4, 6, 8 passim.
- Moffatt CJ, Edwards L, Collier M, Treadwell T, Miller M, Shafer L, et al. A randomised controlled 8-week crossover clinical evaluation of the 3M Coban 2 Layer Compression System versus Profore to evaluate the product performance in patients with venous leg ulcers. Int Wound J 2008; 5(2):267-79.
- Szewczyk MT, Jawień A, Cierzniakowska K, Cwajda-Białasik J, Mościcka P. Comparison of the effectiveness of compression stockings and layer compression systems in venous ulceration treatment. Arch Med Sci 2010; 6(5):793-9.
- Blecken SR, Villavicencio JL, Kao TC. Comparison of elastic versus nonelastic compression in bilateral venous ulcers: A randomized trial. J Vasc Surg 2005; 42(6):1150-5.
- BNF [Internet]. Two layer systems. National Institute for Health and Care Excellence. Available from: https://bnf.nice.org.uk/wound-management/two-layer-systems.html.
