Volume 24 Number 1
The impact of pressure injury on quality of life in adults: protocol for a systematic review
Shiwen Liu, Helen Rawson, Victoria Team
Keywords quality of life, systematic review, pressure injury, pressure ulcer
For referencing Liu S, Rawson H & Team V. The impact of pressure injury on quality of life in adults: protocol for a systematic review. Journal of Wound Management 2023;24(1):4-9.
DOI
https://doi.org/10.35279/jowm2023.24.01.03
Submitted 22 September 2022
Abstract
Background Pressure injuries are a common healthcare problem and are associated with impaired quality of life.
Objective To investigate the impact of pressure injuries on the quality of life of adults aged 18 years and older.
Design Systematic review.
Eligibility criteria Both quantitative and qualitative studies that report on quality of life in adults with a pressure injury from acute care, rehabilitation, long-term care and com- munity settings will be included.
Methods Studies conducted between January 2009 and July 2022 and found in the Ovid Medline, Ovid Embase, CINAHL EBSCO, Scopus and Central Register of Controlled Trials databases will be identified. Two independent review authors will perform the study selection and data extraction. Methodological qual- ity will be appraised using the Critical Appraisal Skills Programme checklists. A thematic synthesis will be used to analyse qualitative evidence. A meta-analysis will be conducted for quantitative data if no signifi- cant heterogeneity is detected. Where statistical pooling is not possible, findings will be reported narratively. The Grading of Recommendations As- sessment, Development and Evaluation (GRADE) and the GRADE-Confidence in the Evidence from Reviews of Qualitative research (GRADE-CERQual) tools will be used to determine confidence levels of findings from quantitative and qualitative studies.
Introduction
Description of the condition
A pressure injury (PI), also referred to as pressure ulcer, pressure sore, bed sore or decubitus ulcer, is defined as ‘localised damage to the skin and/or underlying tissue, as a result of pressure or pressure in combination with shear1 and is a common healthcare problem2,3. In adults, PIs most commonly occur at the bony prominences of the hips and sacrum4. Other locations, such as the ischial tuberosity, greater trochanter, heel and lateral malleolus, are also common sites for PIs4. The National Pressure Injury Advisory Panel (NPIAP) categorises PIs into four stages: non-blanchable erythema, partial-thickness skin loss with exposed dermis, full-thickness skin loss, full-thickness skin and tissue loss. For a PI that is obscured by slough or eschar, the depth of wound is unknown, so it is classified as an unstageable PI: obscured full-thickness skin and tissue loss. A deep tissue PI is staged as persistent with a non-blanchable deep red, maroon or purple discoloration1. The latest review5 and recent studies6,7 have reported that the risk factors for PIs in adult patients include physiological factors (advanced age, body mass index (BMI) < 18.5, malnutrition, incontinence, impaired mobility, poor perfusion, dehydration and comorbid conditions such as diabetes), extrinsic factors that affect skin integrity (friction/shear, interface pressure and turning and repositioning) and mental status/ neurological disorders (traumatic brain injury, dementia or other cognitive disorders). The European Pressure Ulcer Advisory Panel (EPUAP) and the NPIAP collaboratively published the first edition of the Prevention and Treatment of Pressure Ulcers/ Injures: Clinical Practice Guideline-The International Guideline (The International Guideline)1 in 2009. It was later updated in 2014 and 20191,8 to ensure supporting resources are in place for healthcare professionals, patients and informal caregivers to manage and prevent PIs1. PIs remain a significant problem in hospitals and long-term care facilities, with the prevalence ranging from 13% to 15%4. PIs are often associated with a long healing period, severe pain, exudate, odour, sleep and mood disturbances and infection9,10. A PI can also lead to a reduced quality of life (QoL), impaired mobility, high cost for patients and healthcare systems and increased morbidity and mortality4,9,10.
Quality of life refers to ‘the functional effect of an illness and its consequent therapy upon a patient, as perceived by the patient’11. QoL can be affected by an individual’s physical, social and psychological states12 and is generally recognised as a valid indicator for unmet needs. It has also been used for monitoring the efficacy of health services, evaluating the effectiveness of interventions, and analysing costutility as a supplementary objective clinical measure13,14. Measuring QoL can provide a deep understanding of the impact of disease on individuals15. There are two primary instruments developed for QoL measurement; these include either generic or disease-specific instruments. Generic instruments, such as the 36-Item Short Form Survey (SF-36), can be used to assess the outcome of health status across multiple diseases, interventions or populations. In contrast, disease-specific instruments, such as the Pressure Ulcer Quality of Life Scale (PU-QOL), are used for QoL measurements related to specific conditions or diseases15,16. The International Guideline reports on the complex relationship between QoL and its contributory factors, such as comorbidities, coping ability and knowledge, all of which interact with one another1.
Why it is important to undertake this review
We conducted a preliminary search of MEDLINE and the Cochrane Database of Systematic Reviews. A systematic review17 published in 2009 and one literature review18 published in 2014 on this topic were identified. The previously published systematic review identified the impact of PI on QoL in older adults17; however, it was published before the first edition of the International Guideline1. The previously published literature review18 demonstrated the impact of PI on QoL, but it was not a systematic review, and the cited studies were published in or before 2010. In this study, we address this gap and systematically review the evidence on the impact of PI on QoL of adults aged 18 years and older. We include studies on the QoL of people with PI published after the first edition of the International Guideline published in 2009 and the evidence-based recommendations implemented into practice.
Objective
In this protocol for a systematic review, we present a transparent process, listing every step of the review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) statement19. The objective of the planned systematic review was to investigate the impact of PI on the QoL of adults aged 18 years and older.
Research question: How do pressure injuries impact the quality of life of adults aged 18 years and older?
Methods
In this protocol, we present a transparent process of the planned methods for our systematic review guided by the PRISMA 2020 statement20. This protocol is registered with the PROSPERO (CRD42022350983) and has been deposited in Open Science Framework (OSF) repository21.
Eligibility criteria
Types of studies
This review will include both quantitative and qualitative research studies on the impact of PI on the QoL of adults aged 18 years and older.
Quantitative studies, including:
- Randomised controlled trials
- Quasi-randomised trial
- Non-randomised studies
- Cross-sectional studies
- Cohort studies
- Before and after evaluation studies
- Case-control studies
Qualitative studies, including:
- Studies that use qualitative methods to collect data, such as group or individual interviews, focus group discussions and observations
- Studies that use qualitative methods of data analysis, such as thematic analysis, grounded theory, qualitative content analysis and phenomenological analysis
Studies not focused on QoL or the impact of QoL will be excluded, as will studies that merely report the measurement properties of QoL instruments. Further, any studies that report the impact of a specific product or drug on the QoL will be disregarded. Conference abstracts, all kinds of reviews, letters to the editor, abstract-only publications, editorials and comments will also be excluded from this review, as will animal studies.
Types of participants
Adults aged 18 years and older with a PI from acute care, rehabilitation, long-term care and community settings will be included in this systematic review. Participants younger than 18 years old, or those with ulcers/injuries other than PI will be excluded.
Outcome measures
The outcome in this review will be an assessment of the QoL of adults with PI. Quantitative studies usually measure QoL with generic and disease-specific instruments, and the QoL is reported as the score or scores on different subscales of the QoL instruments, such as subscale scores ranging from 0–100 in the SF-36. In qualitative studies, the outcome measures will be participants’ descriptions of how the PI affects their QoL, including physical symptoms, social and psychological functioning, financial status and other aspects of life and wellbeing.
Report characteristics
This systematic review will consider studies published between January 2009, the year when the first edition of the International Guidelines was published, and July 2022. Only studies published in English will be included, but no geographical limitations will be imposed.
Information sources
The electronic databases searched in this review will include Ovid Medline, Ovid Embase, CINAHL EBSCO, Scopus and the Central Register of Controlled Trials. Grey literature will be searched through Open Grey, Greylit.org, clinicaltrials.gov and Google Scholar for works’ first ten pages. The reference lists of the included studies will be screened for potentially eligible studies. Studies’ authors will be contacted in cases of missing data, or for additional data for clarification, if necessary. If no data are found, the study will be excluded.
Search strategy
The Patient/population, Intervention, Comparison and Outcomes (PICO) model was used to build the initial search terms. An expert research librarian was also consulted to support the development of the search strategy. An initial search of Ovid Medline (Figure 1) was conducted in July 2022, focused on keywords and subject headings contained in the relevant papers to develop the search strategy of this systematic review. All keywords and index terms of the search strategy were modified for the different databases by using phrase searching, wildcards, Boolean operators and quotations. The keywords in the search strategy were ‘pressure injury’, ‘pressure wound’, ‘pressure ulcer’, ‘pressure sore’, ‘bed sore’, ‘decubitus ulcer’ and ‘quality of life’.
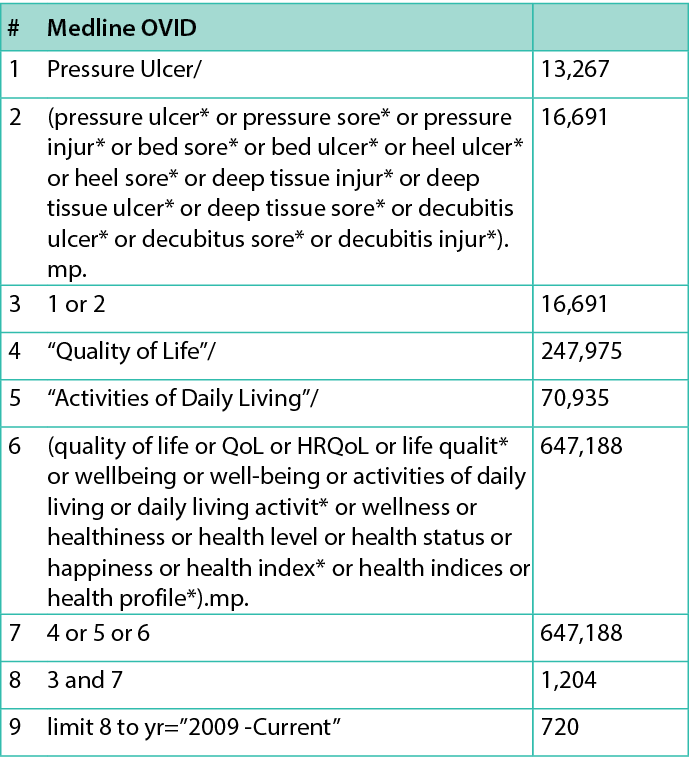
Figure 1: Search strategy
Study records
Data management
All studies will be imported into Endnote 20 to remove duplicate citations and then imported to Covidence for the management of the review process.
Selection process
Two review authors will independently screen all titles and abstracts based on the eligibility criteria. The full text of selected studies will be retrieved and imported in Covidence. The full-text studies will be independently screened by two review authors against the eligibility criteria, and the reason for any exclusion will be noted in Covidence. Any discrepancies of inclusion or exclusion will be resolved by discussion or consulting with a third author. The results of the selection process and reasons for exclusion will be presented in the PRISMA flow diagram20 (Figure 2).
Figure 2. PRISMA flow diagram
Data collection process
Data from included studies will be extracted independently by two review authors. A data extraction template developed by the author team will be used for data collection. The details of extraction templates are shown in Figures 3 and 4. Two review authors will extract data from the first three studies to ensure the consistency of data collection. Discrepancies that arise between the two review authors will be resolved by discussion or consulting with a third review author.
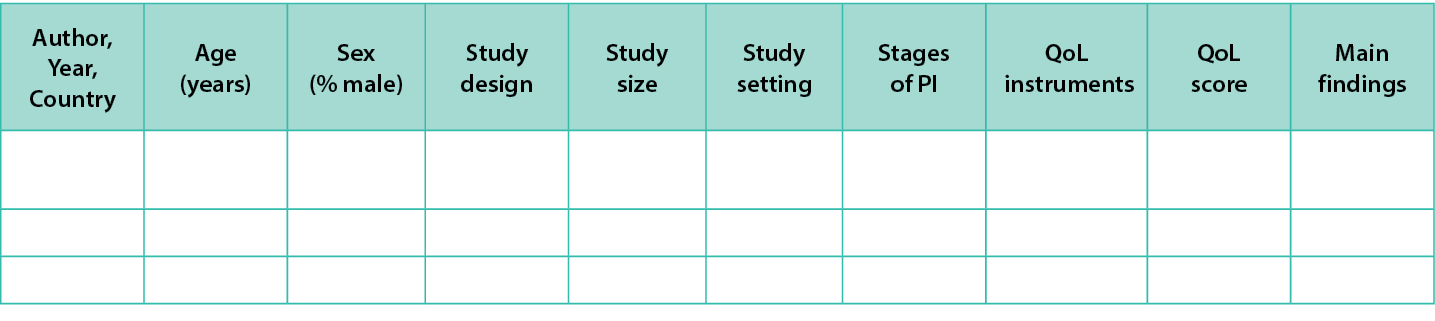
Figure 3. Data extraction instrument-Quantitative studies

Figure 4: Data extraction instrument - Qualitative studies
Data items
For quantitative studies, the following data will be extracted from the included studies:
- Author, publication year, country
- Age of participants
- Sex of participants
- Study design type
- Study size
- Study setting
- Aim of the study
- Main findings
- Stages of PI
- QoL score
- QoL assessment instrument: Generic or disease-specific instruments
For qualitative studies, the review authors will extract the following data:
- Author, publication year, country
- Age of participants
- Sex of participants
- Study design
- Study size
- Study setting
- Stages of PI (if reported)
- Aim of the study
- Participants’ quotes related to the QoL
- Authors’ descriptions or analyses of the participants’ quotes
Risk of bias in individual studies
The methodological quality of eligible studies will be critically appraised by two independent review authors using the Critical Appraisal Skills Programme (CASP) checklists for evaluating different study designs, such as randomised controlled trials, cohort studies and qualitative studies22. The CASP is the most commonly used appraisal tool used in healthcare-related qualitative evidence synthesis23. Discrepancies between the two review authors will be resolved by discussion or consulting with a third review author, if necessary. The results of studies’ methodological quality will be reported narratively. Studies that do not meet the quality threshold will not be excluded; however, quality will be considered when developing themes.
Data synthesis
The qualitative and quantitative results will be reported separately. To evaluate the impact of PI on QoL, quantitative data will, where possible, be pooled for statistical meta-analysis. The heterogeneity will be examined using an inconsistency indexI2. If no significant heterogeneity is detected (I2< 75%), a meta-analysis will be performed using a random effects model24. Effect sizes of risk ratios (dichotomous data) or the mean difference (continuous data) will be calculated for analysis. Where statistical pooling is not possible, the findings of effectiveness will be reported narratively.
This systematic review will use a thematic synthesis as described by Thomas and Harden25. This approach is recommended in the Cochrane Handbook for Systematic Reviews of Qualitative Evidence to investigate participants’ perspectives and experiences25,26. NVivo software will be used for thematic synthesis.
The first stage in the process of thematic synthesis is coding25. Two review authors will select a study for independent, line-by-line coding for themes. They will then discuss with each other to agree on themes.
The first review author will continue the line-by-line coding for the remaining studies. When a new theme is generated during the coding process, the second review author will be consulted. The second review author will review one third of the studies, to check the accuracy of the coding. Any discrepancies will be resolved by discussion or consultation with a third review author.
The second stage in the thematic synthesis is the identification of descriptive themes25. Two review authors will work together to discuss the similarities and differences of coded themes and then group related themes to form the descriptive themes.
The third stage in the thematic synthesis is analytical themes. Comparing descriptive themes that are closely related to the original findings means analytical themes tend to ‘go beyond’ the findings to explore understanding or hypotheses25. This review aims to develop analytical themes about the impact of different implications of PI on the QoL. If discrepancies arise between the two review authors, a third review author will be consulted.
Confidence in cumulative evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methods will be used to assess the quality of quantitative evidence. Evidence will be graded as high, moderate, low or very low27. The results of the GRADE assessments will be presented as tables. The GRADE-Confidence in the Evidence from Reviews of Qualitative research (GRADE-CERQual)28 will be used to assess the confidence in the synthesis findings. The confidence will be judged as high, moderate, low or very low. These results will also be shown in tables.
Discussion
Pressure injuries are an ongoing challenge for patients, caregivers and healthcare professionals. This protocol was developed following the PRISMA-P statement. Three independent review authors with experience in systematic review methodology will perform the screenings and data extraction to avoid bias in selecting studies. In this review, studies written in languages other than English will be excluded, due to the review team’s language limitations. This is a limitation of the systematic review. Findings of this systematic review will be of interest to health planners in designing promotion programmes for improving the QoL of people with PIs. The findings will also enable healthcare professionals, patients and caregivers to gain a greater understanding of how PIs impact QoL. Finally, the findings of this review will play an important role in improving the prevention of PIs.
Acknowledgments
We thank Mario Sos, an expert librarian from the Faculty of Medicine, Nursing, Health Sciences at Monash University, for his assistance with the search strategy development.
Key messages
- Pressure injuries are associated with impaired quality of life.
- The study’s aim is to investigate the impact of pressure injuries on the quality of life of adults aged 18 years and older.
- A systematic review, including both quantitative and qualitative studies, will be conducted.
Author(s)
Shiwen Liu* BN (Hons) RN1, Helen Rawson PhD, RN1, Victoria Team DrPH, MD1
1Nursing and Midwifery, Monash University, Melbourne, Australia
*Corresponding author email shiwen.liu@monash.edu
References
- European Pressure Ulcer Advisory Panel, National Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: clinical practice guideline. The International Guideline: EPUAP/NPIAP/PPPIA; 2019.
- Jackson DE, Durrant LA, Hutchinson M, Ballard CA, Neville S, Usher K. Living with multiple losses: Insights from patients living with pressure injury. Collegian. 2018; 25(4):409-14. doi:10.1016/j. colegn.2017.10.008
- Karadağ A, Çakar V. Evidence-based prevention and management of pressure injuries in home care: A scoping review. Adv Skin Wound Care. 2022; 35(3):172-9. doi:10.1097/01. ASW.0000815484.50141.5d
- Mervis JS, Phillips TJ. Pressure ulcers: Pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019; 81(4):881-90. doi:10.1016/j. jaad.2018.12.069
- Chung M-L, Widdel M, Kirchhoff J, Sellin J, Jelali M, Geiser F, et al. Risk factors for pressure injuries in adult patients: A narrative synthesis. Int J Environ Res Public Health. 2022; 19(2):761-778. doi: 10.3390/ ijerph19020761
- Brimelow RE, Wollin JA. The impact of care practices and health demographics on the prevalence of skin tears and pressure injuries in aged care. J Clin Nurs. 2018; 27(7-8):1519-28. doi:10.1111/jocn.14287
- Osis SL, Diccini S. Incidence and risk factors associated with pressure injury in patients with traumatic brain injury. Int J Nurs Pract. 2020; 26(3):e12821. doi:10.1111/ijn.12821
- Kottner J, Cuddigan J, Carville K, Balzer K, Berlowitz D, Law S, et al. Prevention and treatment of pressure ulcers/injuries: The protocol for the second update of the International Clinical Practice Guideline 2019. J Tissue Viability. 2019; 28(2):51-8. doi:10.1016/j. jtv.2019.01.001
- Australian Commission on Safety and Quality in Health Care [Internet]. Hospital-acquired complication - 1. Pressure injury fact sheet (long); 2018. Available from: https://www.safetyandquality.gov.au/sites/default/ files/2019-05/saq7730_hac_factsheet_pressureinjury_ longv2.pdf
- Team V, Bouguettaya A, Richards C, Turnour L, Jones A, Teede H, et al. Patient education materials on pressure injury prevention in hospitals and health services in Victoria, Australia: Availability and content analysis. Int Wound J. 2020; 17(2):370-9. doi:10.1111/iwj.13281
- Meaume S, Dompmartin A, Lok C, Lazareth I, Sigal M, Truchetet F, et al. Quality of life in patients with leg ulcers: Results from CHALLENGE, a double-blind randomised controlled trial. J Wound Care. 2017; 26(7):368-79. doi:10.12968/ jowc.2017.26.7.368
- Pequeno NPF, Cabral NLdA, Marchioni DM, Lima SCVC, Lyra CdO. Quality of life assessment instruments for adults: A systematic review of population-based studies. Health Qual Life Outcomes. 2020; 18(1):208-221. doi:10.1186/s12955-020-01347-7
- Phyo AZZ, Freak-Poli R, Craig H, Gasevic D, Stocks NP, Gonzalez-Chica DA, et al. Quality of life and mortality in the general population: A systematic review and meta-analysis. BMC Public Health. 2020; 20(1):1596-1616. doi:10.1186/s12889-020-09639-9
- Phyo AZZ, Ryan J, Gonzalez-Chica DA, Woods RL, Reid CM, Nelson MR, et al. Health-related quality of life and all-cause mortality among older healthy individuals in Australia and the United States: A prospective cohort study. Qual Life Res. 2021; 30(4):1037-48. doi:10.1007/s11136-020-02723-y
- Young T, Furtado K, Alves P. Health related quality of life (HRQOL) implications for people with pressure ulcers. In: Romanelli M, Clark M, Gefen A, Ciprandi G, editors. Science and practice of pressure ulcer management. London: Springer London; 2018. p.79-87.
- Rutherford C, Campbell R, Brown JM, Smith I, Costa DSJ, McGinnis E, et al. Comparison of generic and disease-specific measures in their ability to detect differences in pressure ulcer clinical groups. Wound Repair Regen. 2019; 27(4):396-405. doi:10.1111/ wrr.12716
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: A systematic review. J Am Geriatr Soc. 2009; 57(7):1175-83. doi:10.1111/j.1532-415.2009.02307.x
- Repiğ G, Ivanoviğ S. Pressure ulcers and their impact on quality of life. Acta Medica Medianae. 2014; 53(4):75-80. doi:10.5633/amm.2014.0412
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1-10. doi:10.1186/2046-4053-4-1
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71. doi:10.1136/ bmj.n71
- Liu S, Rawson H, Team V [Internet]. The impact of pressure injury on quality of life in adults: Protocol for a systematic review. 2022, August 4. Available from https://osf.io/5ckpx
- Critical Appraisal Skills Programme [Internet]. CASP check list. 2022. Available from: https://casp-uk.net/ casp-tools-checklists/
- Long HA, French DP, Brooks JM. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Research Methods in Medicine & Health Sciences. 2020; 1(1):31-42. doi:10.1177/2632084320947559
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions, version 6.1 (updated September 2020): Cochrane; 2020.
- Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008; 8(1):45-55. doi:10.1186/1471-2288-8-45
- Noyes J, Booth A, Cargo M, Flemming K, Harden A, Harris J, et al. Chapter 21: Qualitative evidence. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions, version 63 (updated February 2022): Cochrane; 2022;525-45.
- Schünemann H, Broğek, J., Guyatt, G., & Oxman, A. (editors). GRADE handbook for grading quality of evidence and strength of recommendations: The GRADE Working Group, 2013. Available from: http://guidelinedevelopment.org/handbook
- Lewin S, Booth A, Glenton C, Munthe-Kaas H, Rashidian A, Wainwright M, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings: Introduction to the series. Implement Sci. 2018; 13(1):2-12. doi: 10.1186/s13012-017-0688-3
