Volume 24 Number 2
Lower leg ulcer diagnosis and principles of treatment
Kirsi Isoherranen, Elena Conde, Leanne Atkin, Mark Collier, Annette Høgh, John D. Ivory, Klaus Kirketerp-Møller, Sylvie Meaume, Hayley Ryan, Ewa K. Stuermer, George-Sorin Tiplica, Sebastian Probst
For referencing Isoherranen K, Montero EC, Atkin L, Collier M, Høgh A, Ivory JD, Kirketerp-Møller K, Meaume S, Ryan H, Stuermer EK, Tiplica GS, Probst S. Lower Leg Ulcer Diagnosis & Principles of Treatment. Including Recommendations for Comprehensive Assessment and Referral Pathways. J Wound Management, 2023;24(2 Sup1):s1-76
DOI https://doi.org/10.35279/jowm2023.24.02.sup01
Abreviations
1. Introduction
Author: Kirsi Isoherranen
Chronic wounds are a huge burden on the individual’s quality of life (QoL) and healthcare system (1–3). Right and early diagnostics of chronic wounds is essential for successful wound management (4, 5); however, there is a dearth of literature describing the outcomes of wound healing related to the timing and accuracy of diagnostics.
Chronic lower limb wounds can be divided into six main categories: venous, arterial, mixed venous and arterial, diabetic foot, pressure and atypical ulcers (6, 7). However, more and more wounds are multiaetiological in the clinical setting (8, 9), and one diagnosis does not exclude another. In the clinical setting, it is not unusual to see a patient suffering from arterial and venous insufficiency, and also a wound with an atypical cause (see Figure 1). There are also other reasons for leg oedema than venous insufficiency (see Chapter 5). In our opinion, the definition of a chronic wound is somehow constrained, as some acute wounds can be “chronic or hard to heal” from the beginning. For instance, an acute wound might develop into a chronic wound within some weeks if the patient has arterial or venous insufficiency or other reasons for leg oedema, and this is not considered in the treatment plan from the beginning.
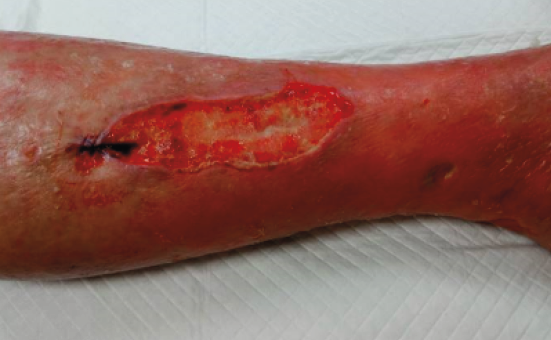
Figure 1. 78-year-old woman, with peripheral arterial disease (PAD), venous insufficiency and Martorell ulcer in the left leg. Image courtesy of Kirsi Isoherranen, permission granted for inclusion
In many European countries, individuals with a chronic wound are first seen in primary care by a nurse or by a general practitioner (GP). This poses a huge demand on the respective professionals; they should have enough education and tools to assess the patient and perform the necessary referrals. For instance, if the patient is not recognised to have arterial insufficiency, the referral for revascularisation is delayed and may lead to amputation (10). Additionally, compression therapy should be introduced from the very beginning in every lower leg ulcer, if there are no contraindications. It is widely recognised that GPs might not have sufficient knowledge of leg ulcer assessment and treatment (11). Likewise, nurses and allied health professionals treating lower leg ulcers should be aware of the differential diagnostics and referral pathways so that the individual does not have to self-manage too long, and adequate referrals are done.
Justification for this document
In response to the lack of uniform diagnostic guidelines in the field of lower leg ulcers, the European Wound Management Association (EWMA) established this working group to gather the best available evidence on the diagnostics of a lower leg ulcer. Focus is on the diagnostics of infection, arterial and venous insufficiency, leg oedema and atypical causes. The one-pager published together with the document aims to be a practical tool to aid for the diagnosis of a lower leg ulcer (see Chapter 13). Earlier documents have focused on venous leg ulcers (VLUs), but there are no documents covering VLU and other aetiologies behind a lower leg ulcer. Treatment options are focused on the most important, i.e., revascularisation, endovenous treatment, compression therapy and local treatment. Debridement, advanced treatments and treatment of wound infection are covered in other EWMA documents and are beyond the scope of this document (12–14). Diabetic foot ulcers and pressure ulcers are also excluded, as uniform guidelines exist in these areas (15, 16). However, the differential diagnostics for an individual with a diabetic foot ulcer and pressure ulcer are included in the one-pager explanatory document.
For the main document a comprehensive systematic search (Appendix 1: Methodology) with the focus on systematic reviews on diagnostics was performed. Relevant articles were included in the chapters related to those topics, and for every chapter, a separate literature search was undertaken and the PRISMA guidelines were used to report the results (Appendix 1). We found a lack of systematic reviews focusing on diagnostics and therefore in some chapters, such as Acute and atypical wounds (Chapter 2), Checklists (Chapter 7), The patient perspective (Chapter 10), other studies were included as well. With the chapters Compression therapy (Chapter 8) and Local Treatment (Chapter 9) only systematic reviews were included. Regarding the grading of evidence, the modified Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was used (Table 1).
Table 1. Summary of grading of recommendations and the quality of evidence
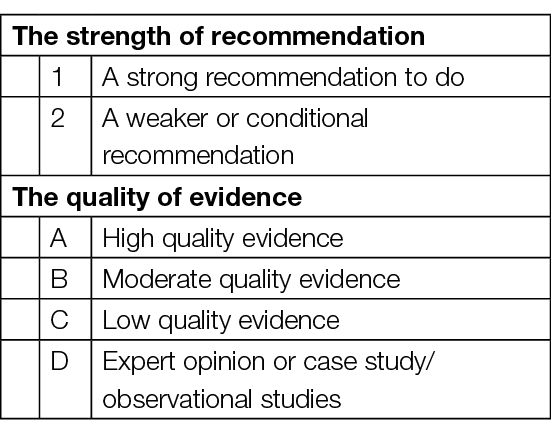
Table 2. Summary of recommendations
The strength of the recommendation is graded as 1 or 2
A Grade 1 recommendation is a strong recommendation to do (or not do) something, where benefits clearly outweigh risks (or vice versa) for most, if not all, patients. Most clinicians and patients would want to follow a strong recommendation unless there is a clear rationale for an alternative approach. A strong recommendation usually starts with the standard wording: “We recommend …” or “It is recommended …”.
A Grade 2 recommendation is a weaker or conditional recommendation, where the risks and benefits are more closely balanced or are more uncertain. Alternative approaches or strategies may be reasonable depending on the individual patient’s circumstances, preferences and values. A weak or conditional recommendation usually starts with the standard wording: “We suggest …” or “It is suggested …”.
The strength of a recommendation is determined not only by the quality of evidence for defined outcomes but also the balance between desirable and undesirable effects of a treatment or intervention, differences in values and preferences and, where appropriate, resource use. Each recommendation concerns a defined target population and is actionable.
The quality of evidence is graded from A to D and is defined as follows:
Grade A evidence means high-quality evidence that comes from consistent results from well-performed randomised controlled trials (RCTs), or overwhelming evidence from another source (such as well-executed observational studies with consistent strong effects and exclusion of all potential sources of bias). Grade A implies confidence that the true effect lies close to the estimate of the effect.
Grade B evidence means moderate-quality evidence from randomised trials that suffers from serious flaws in conduct, inconsistency, indirectness, imprecise estimates, reporting bias, or some combination of these limitations, or from other study designs with specific strengths such as observational studies with consistent effects and exclusion of the majority of the potential sources of bias.
Grade C evidence is low-quality evidence from controlled trials with several serious limitations, or observational studies with limited evidence on effects and exclusion of most potential sources of bias.
Grade D evidence is based only on case studies, expert judgement or observational studies with inconsistent effects and a potential for substantial bias, such that there can be little confidence in the effect estimate.
The aim of the document is to:
– Present the most important diagnostic procedures of lower leg ulcers and the evidence underpinning these diagnostic criteria.
– Present the evidence for compression and local therapy in lower leg ulcers.
– Provide an insight into the patient and health economy perspectives in lower leg ulcers.
– Provide tools to prevent diagnostic and treatment delay in lower leg ulcers.
2. Ageing and acute wounds
Authors: Elena Conde Montero, Kirsi Isoherranen, Sylvie Meaume
Leg trauma in the elderly, simply because of the changes in the skin and vessels associated with age itself, can become complicated, even after mild trauma. If there are other comorbidities, such as chronic venous insufficiency (CVI) or peripheral arterial disease (PAD), the risk of wound stagnation is higher. In the acute and primary care setting, it is important to make vascular assessment and assessment of leg oedema in a patient with a traumatic lower leg ulcer, skin tear or dissecting haematoma (26). Indeed, the ageing of the population means that many injuries, despite an acute traumatic trigger, have a multifactorial explanation (9, 27, 28). Over-cleansing and over-frequency of dressing changes or misplaced bandages can also complicate wounds and damage fragile perilesional skin (29). Importantly, hygiene and emollient interventions for maintaining skin integrity in older people in hospital and residential care settings may have a positive impact on preventing these lesions, by protecting the fragile skin barrier. (30).
Table 3. Risk factors for stagnation of acute wounds
Dermatoporosis
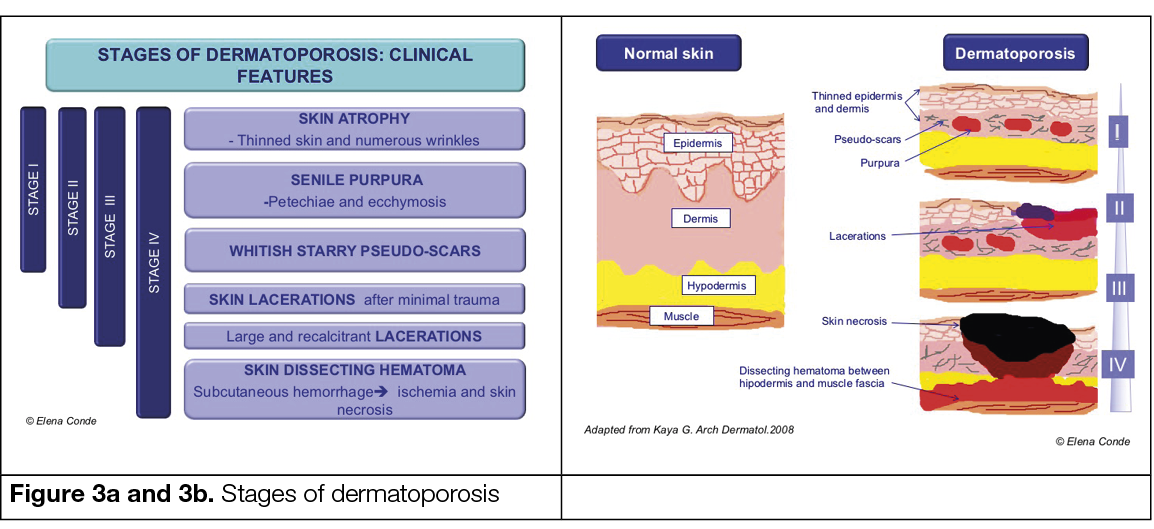
The term dermatoporosis includes the different manifestations and complications of skin fragility inherent to the physiological ageing process. Considering increased life expectancy, it has become a common problem. Sun damage, prolonged treatment with topical or systemic corticosteroids, anticoagulant treatment, malnutrition and dehydration are exacerbating factors. (28, 31)
The initial stage of dermatoporosis is characterised by thinning of the dermis and epidermis (skin atrophy), senile purpura (vascular rupture due to wall fragility) and stellate pseudo scars (loss and degeneration of collagen and elastic fibres) resulting in the increased risk of wounds after trauma (Figure 2) (28).
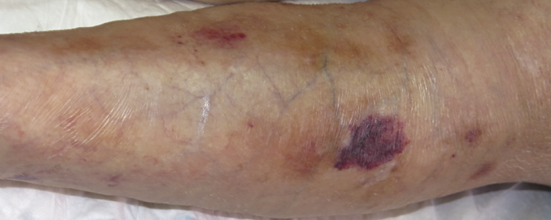
Figure 2. Skin atrophy and senile purpura. Image courtesy of Elena Conde Montero, permission granted for inclusion
Traumatic wounds caused by mechanical forces, including adhesive removal, called skin tears, which do not extend beyond the subcutaneous layer, may occur in this context. (26, 32).
Skin tears are classified as type 1 (no skin loss), type 2 (partial flap loss) or type 3 (total flap loss exposing the entire wound bed) (26).
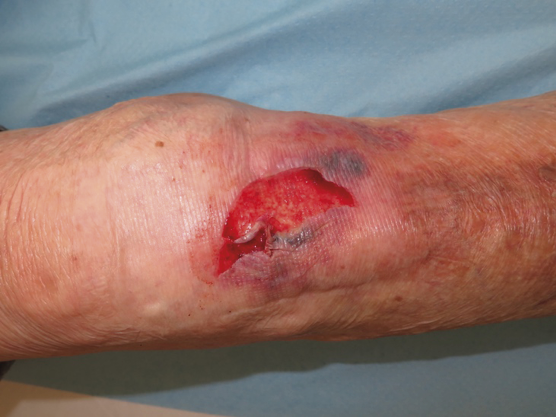
Figure 4. Type 2 skin tear. Image courtesy of Elena Conde Montero, permission granted for inclusion
Deep dissecting haematoma represents the highest degree of expression of dermatoporosis (Figure 5). Early diagnosis and proper management are essential to avoid potential complications and prolonged hospitalisation. Its occurrence is secondary to minimal trauma. This occurs because the vessels of the subcutaneous cellular tissue have a fragile wall and the skin, due to its atrophy, does not maintain its protective role. The onset of this condition can be misdiagnosed with cellulitis, as it manifests with erythema, oedema and local heat. If the haematoma is not evacuated in this first phase, skin ischaemia occurs and an extensive eschar appears. (33).

Figure 5. Deep dissecting haematoma. Image courtesy of Elena Conde Montero, permission granted for inclusion
Arteriolosclerosis
Arteriolosclerosis includes a spectrum of histological features such as thickening and loss of elasticity, calcifications and decreased caliber of the arterioles, which has a direct impact on possible impaired cutaneous microcirculation. Even if it is associated to ageing itself, cardiovascular risk factors such as hypertension or diabetes contribute to its occurrence. (34-35)
In patients with arteriolosclerosis, the normal initial vasoconstriction after trauma may imply a compromise of tissue irrigation prolonged in time, since baseline cutaneous perfusion is already reduced with the consequent ischaemia. Subsequent vasodilatation with release of inflammatory mediators increases necrosis and starts a vicious cycle of necrosis-inflammation with the development of recalcitrant wounds.
Initially, wounds secondary to arteriolosclerosis usually have an insignificant clinical aspect, but progressively acquire a purplish or blackish colour and, in a few days, become extensive, deep and very painful wounds. It is an under-diagnosed type of wound and is more prevalent than is commonly thought. Diagnosis of these wounds is clinical, as histological findings are not pathognomonic and can be found in any skin biopsy of the leg of an elderly person, even if there is no wound (34).
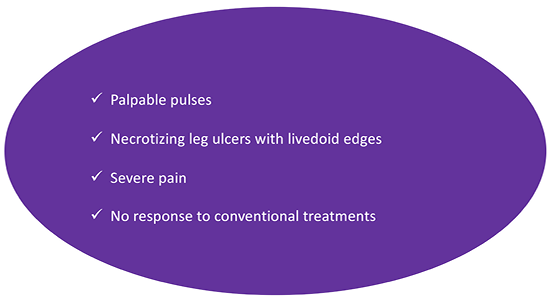
Figure 6. Clinical signs of acute wounds in the context of arteriolosclerosis
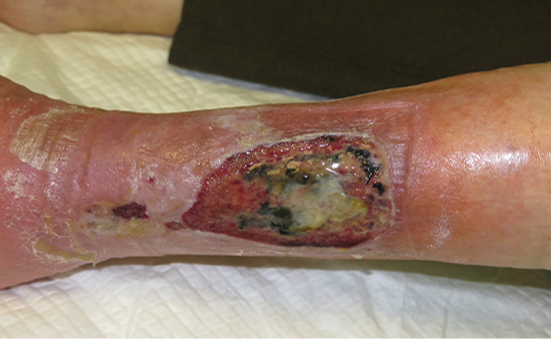
Figure 7. Clinical aspect of an arteriolosclerotic ulcer. Image courtesy of Elena Conde Montero, permission granted for inclusion
A biopsy will only be required if we want to rule out another type of wound, such as pyoderma gangrenosum (PG) (35).
Oedema can exacerbate the deterioration of an arteriolosclerotic ulcer, as it increases necrosis by decreasing perfusion.
Chapter 5 discusses the histological (and even clinical) findings of this type of wound which are also found in the so-called Martorell ulcer or calciphylaxis – all can be considered wounds within the same clinical-histological spectrum; therefore, the management will be similar in all of them (35).
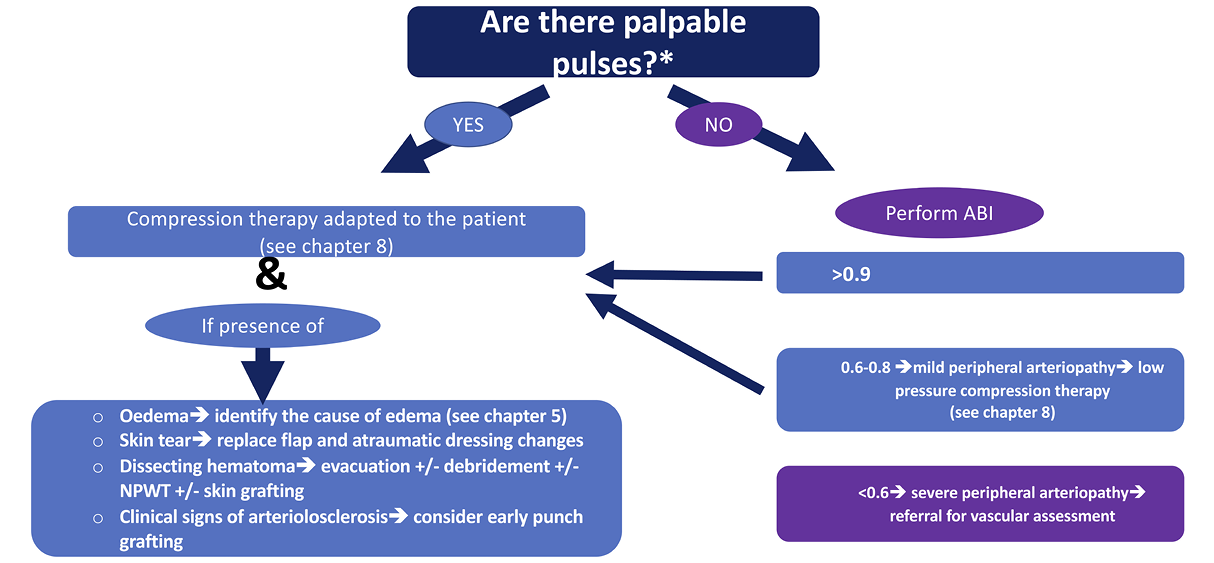
* The reliability of pulse palpation depends on the experience of the healthcare professional. If the practitioner is not confident in pulse palpation, an ABI should be performed
NPWT= Negative pressure wound therapy
Figure 8. Treatment algorithm in traumatic leg ulcers
Summary
– Predisposing factors for acute ulcers after trauma include skin fragility and arteriolosclerosis associated with age and oedema from different causes, especially decompensated heart failure or CVI.
– Oedema control, i.e. compression therapy, is fundamental in avoiding the chronification of any acute leg wound.
3. Infection
Authors: Ewa K. Stuermer, Klaus Kirketerp-Møller
All wounds should be primarily screened for possible inflammation or infection. This should be undertaken by visual diagnosis. Studies show that approximately 35% of all chronic wounds lead to recurrent (systemic) infections. Moreover, in up to 40% of chronic wounds, wound infection is the trigger of a prolonged or chronic healing process (36). Every wound, regardless of whether it is acute or chronic, is contaminated within a few hours with microorganisms of the skin microbiome and environmental bacteria which may also contain pathogenic species. This situation is referred to as “wound contamination”. External wound cleansing, which is carried out at this time, reduces microbial colonisation. At the same time, the body’s own immune system and the microbioma counteract local spreading. If these processes do not take place or if the local, autogenic immune response is not efficient enough, the local colonisation of the wound with microorganisms increases continuously. It is referred to as ‘colonised’, and is accompanied by a more or less severe local reaction (Figure 9) (37). The leading bacterial species on the leg ulcer are Staphylococcus aureus, including its methicillin-resistant variant (MRSA), Pseudomonas aeruginosa, and enterobacteria (38, 39). The successive progression to local infection of the wound is a continuum as illustrated in Figure 9. The transition from ‘colonised’ to ‘infected’ is difficult to determine clinically. Evidence suggests that VLUs are polymicrobial with an average of four to five species making the choice of narrow spectrum antimicrobial treatment difficult (38, 40, 41). Finally, every wound is colonised by bacteria, but the evidence is scarce that eradication of them would lead to faster wound healing.
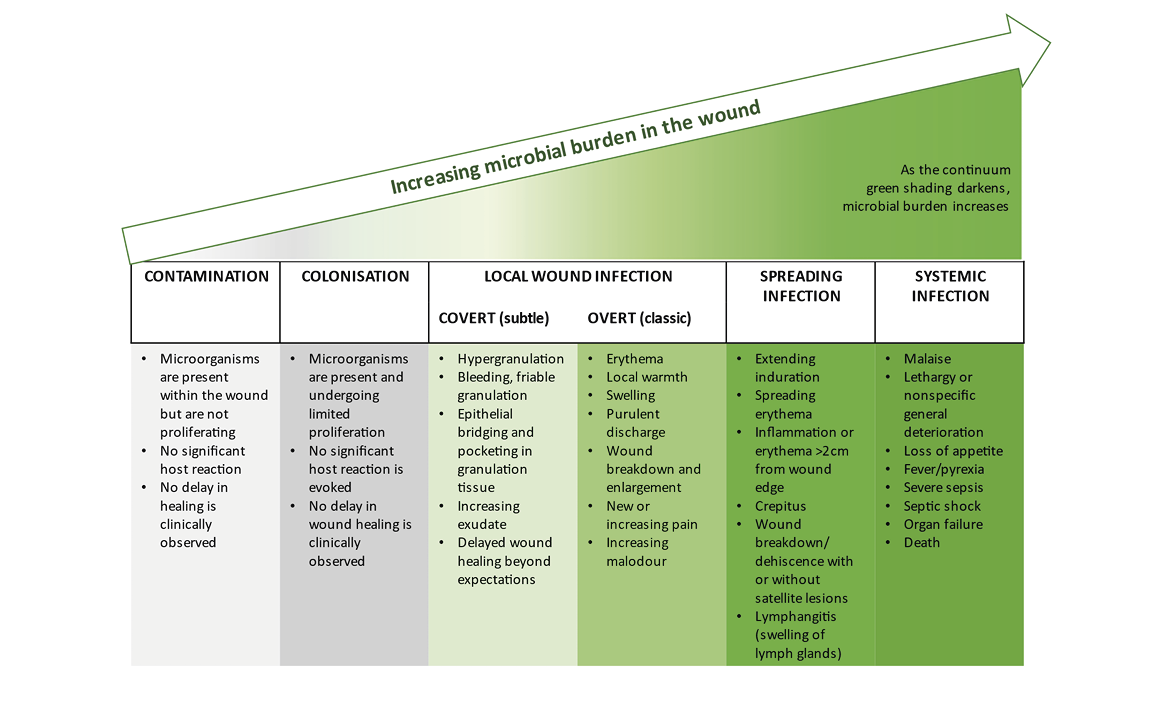
Figure 9. Schematic illustration of the infection continuum and biofilm formation over the course of time.
Reproduced with permission from International Wound Infection Institute (IWII), Wound Infection in Clinical Practice consensus document. Wounds International. 2022. (37)
Most patients diagnosed with a wound infection show the typical clinical signs of acute infection and inflammation (redness, swelling, heat and loss of function), report pain and may present with purulence. Systemic signs such as fever, chills and general fatigue may also be accompanying symptoms. In the wound swab, the (bacterial) triggers of the infection in many cases are detectable by conventional culture, and the clinical markers in the peripheral blood (for example, C-reactive protein (CRP), neutrophil count, leukocyte count) are elevated. Any colonisation of microorganisms will affect the healing process, but is usually confined to the local area and often covert. Many publications exist that address the topic of ‘wound infection diagnostics’; however, there is a lack of a sound evidence base for interventions. The following descriptions and recommendations summarise the most relevant information.
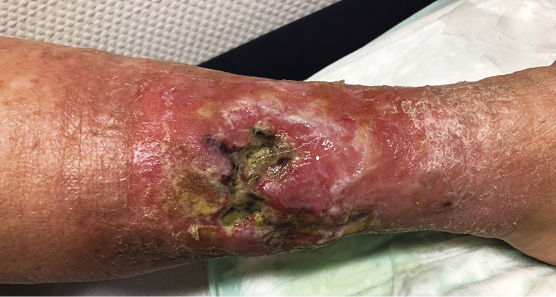
Figure 10. Local wound infection of a leg ulcer related to PAD. Swelling, erythema, surrounding dermatitis with strong exudation. Local antimicrobial therapy is indicated. Decision regarding antibiotic therapy depends on systemic signs of infection. Image courtesy of Ewa Stürmer, permission granted for inclusion
Classifications and scoring systems
Irrespective of the underlying pathophysiology of the wound, for example PAD, CVI, diabetes or immunological diseases, all wounds are initiated by a loss of skin integrity, which allows pathogenic microorganisms to invade and be destructive to the epidermis and subcutis [definition in: Swanson et al., 2016 (42)]. Commonly local (antimicrobials) or systemic support (antibiotics) depending on the level of infection (43) are required as the individual’s immune system may not be sufficient to combat the invading micro-organisms. Identifying this pivotal stage clinically is one of the greatest challenges in the therapy of infected wounds.
To aid decision-making, scoring systems have been developed to classify postoperative wound infections to better assess whether a wound is at risk of infection or whether a wound infection has already manifested. In particular, two scores are frequently applied for this purpose: the W.A.R. Score (Wounds-at-risk (44)) and the TILI Score (Therapeutic Index for Local Infections) (45).The modified classification of the American Centers for Disease Control and Prevention (CDC) is primarily used to classify postoperative wound infection (46).
The W.A.R. Score (44) is used for early detection of infection risk based on characteristics and risk factors of the wound. The score was originally developed for use in chronic wounds, although it is also applicable to acute and postoperative wounds. The rationale of the score has two purposes, firstly to identify wounds at risk of infection at an early stage and thus indirectly to provide antimicrobial therapy, and secondly to reduce unnecessary and prolonged use of local antimicrobial therapies. Thus, the W.A.R. Score not only serves as a recognition system, but also as a decision-making tool. However, a risk assessment is not the same as diagnosing infection. Treatment with antibiotics or antiseptics must always be accompanied by close re-evaluation of the outcome of therapy. The therapeutic regimen should always be adapted to the clinical findings.
To simplify the indication of local antiseptic wound therapy (especially for practitioners who are not specialised in wound treatment), the TILI Score was developed (45). This score distinguishes between indirect (e.g., typical signs of inflammation) and direct criteria (e.g., pus, evidence of pathogenic microorganisms) of local infection and helps in the decision to use antiseptic local therapy. Particularly due to the graduated interpretation, a limited local reaction based on individual signs of inflammation is not overestimated. This can improve the indication and reduce the non-indicated use of local antimicrobials.
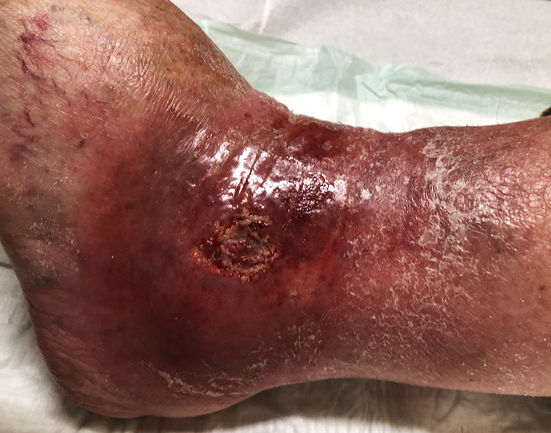
Figure 11. Deep wound infection located above the ankle. Marked swelling (glossy skin), deep redness. Local antimicrobial therapy required. Pus and debris may be drained. Antibiotics are required, but subject to systemic signs of infection (fever, severe pain, leukocytosis). Image courtesy of Ewa Stürmer, permission granted for inclusion.
Wound biofilm
The definition of wound biofilm is: “Biofilm is a structured community of microbes with genetic diversity and variable gene expression (phenotype) that creates behaviors and defenses that result in the production of unique (chronic) infections with significant tolerance to antibiotics and antimicrobials while being protected from host immunity” (37).
Based on a meta-analysis (47), approximately 78% of all chronic wounds are colonised with pathogenic microorganisms in the form of biofilms. Recent studies have revealed that even acute infections contain bacteria in biofilm mode of growth (48, 49), so it would be a reasonable assumption that all chronic colonisation, including VLU, host bacteria in biofilm. Although it has been stated that bacterial biofilm plays a significant role in delayed healing (50), we can assume that even VLUs with no signs of infection host bacterial biofilm. Biofilm mode of growth is a survival strategy of bacteria. The protection from the host immune response, like from neutrophils and macrophages, may be the physical protection from the so-called extrapolymeric substance (EPS) which mainly consists of polysaccharides and proteins (51). More likely the protection is based on the excretion of virulence factors regulated by Quorum Sensing as part of the biofilm phenotype bacteria (52). The biofilm matrix acts as a biochemical barrier against antimicrobial agents (53, 54). Thus, many of its components can directly chemically interact with and neutralise them, resulting in loss of efficacy of antimicrobial agents (55). Signaling molecules enable the interaction of different microorganisms to communicate with each other (56) or lower the metabolic activity of specific bacteria resulting in a high resilience (57) and ‘tolerance’ of biofilm to antimicrobial agents. The term antimicrobial ‘tolerance’ and not ‘resistance’ is used in this context because the antimicrobial agents have not lost their efficacy against (planktonic) microorganisms and thus cannot act due to the dormant status of the biofilm phenotype of the bacteria.
Bacteria, even in biofilm, are not visible to the naked eye. A wound swab may also be negative due to the lack of phenotype-related ability to grow on media of clinical microbiological routine, due to a non-random distribution in the wound which leads to an inadequate absorption during swab collection, and thus to an insufficient dissolution in the analysis process (58, 59). This can be circumvented by microbiological analysis of curettage tissue. Yet, routine microbiological analysis does not detect all bacterial and fungal species present in the biofilm. Here, DNA analysis using polymerase chain reaction (PCR) technique currently provides the most accurate information about the microbial diversity in multispecies biofilm. To what extent this information is relevant, clinically or therapeutically, remains a matter for further research.
The wound biofilm locally induces a permanent, more or less strong immune reaction or inflammation. It can be the trigger of a wound infection but can also persist for weeks and months without causing it. Biofilm or even critical colonisation of the wound (>104KFE/mm2) may be visualised to the human eye using UV-near light with a special device (60). Light of wavelength 450nm allows areas of high bacterial metabolic activity to be detected by fluorescence of deposited bacterial metabolic products. These products include porphyrins (red fluorescence of e.g. Staphylococcus Spp. and Enterobacteriaceae) or by the cyan-blue fluorescence of pyoverdine secreted by Pseudomonas Spp. (60, 50). A larger study already showed clinical support by this fluorescence technology (61).
Swabbing – indication and technique
Because chronic wounds are always colonised with bacteria and the switch to local infection is also blurred, the timing and type of wound swab collection for microbiological analysis is still under discussion (62-65). The only consensus is that to determine any existing colonisation with multi resistant germs, area-wide swabs should be taken both from wounds and from patients themselves (nose, throat, wound, inguinal if necessary) in Z-technique and under rotation of the swab. A positive swab alone is never an indication for antimicrobial treatment. On the other hand, it is always indicated to identify the pathogen if an antimicrobial treatment is initiated.
Four main techniques are described for the diagnosis of lower leg ulcers: deep tissue biopsy, Levine technique (66), the Z-technique, and the so-called Essen Rotary (67). Tissue biopsy can be performed with a 2-mm-diameter punch near the wound edge in a full-thickness fashion to obtain viable tissue. The Levine technique addresses a 1cm2 area of viable tissue in the wound where the swab is rotated with a small amount of pressure to collect exudate and bacteria. In the Z-technique, the entire wound is swabbed once using the swab in a zigzag pattern. With the Essen Rotary, swabbing begins at the centre of the wound and the swab is moved centrifugally from there to the edge of the wound in a circular motion.
Many studies compare the efficacy of bacterial and fungal detection by swab collection (various techniques) and deep wound biopsy (65, 68). The overall conclusion of these analyses is that wound biopsy is not superior to wound swabbing, and in particular to Levine swabbing, which is the most widely studied technique. This may be to the non-heterogenous distribution of bacteria in the wound bed (69). However, it should be concluded that standard clinical microbiological lab analysis detects only 17% of all bacteria identified by PCR technique in a wound swab (70).
Pitfalls in the diagnosis of wound infections
Patients’ possible comorbidities should be considered in a comprehensive holistic wound assessment – for example, malnutrition (71), immunologic suppression, and in particular the metabolic syndrome associated with diabetes mellitus – as they can mask bacterial infection (63). The blood levels of leucocytes and/or CRP or even the swelling and redness of the wound surrounding may be low, even if wound infection is present. In addition, some bacteria, such as P. aeruginosa, have developed strategies not to be recognised by the human immune system and thus not to be targeted (72). For these so-called covert infections, experts have defined secondary signs of wound infection (73). These are discolouration of granulation tissue, friable granulation tissue, delayed healing or wound breakdown, serous exudate with concurrent inflammation, pocketing at the base and malodour. In addition, the effect of bacteria residing in the wound tissue may range from none to seriously hampering any part of the healing process and even cause necrosis. It is also important to recognise that inflammatory conditions like PG can be very difficult to distinguish from bacterial infections in chronic wounds (see Chapter 6). An attempt for approaching the diagnosis of PG have been published (74, 75). Moreover, infectious conditions like the sandfly disease or Leishmaniasis, a parasite mediated disease, may resemble chronic wound infections.
Treatment of leg ulcer infections
For the treatment of the different degrees of wound infections in leg ulcers, reference can be made to the International Wound Infection Institute Consensus document (37) and EWMA positions document Antimicrobials and non-healing wounds. Evidence, controversies and suggestions published in 2022 (13).
Practical aspects of leg ulcer infection
- Consider debridement (removal of dead tissue) as part of the reduction of the bacterial load.
- Include the wound edge and perilesional skin in wound cleansing and care.
- Do not treat with antibiotics without systemic clinical signs of infection.
- If treatment with antibiotics is needed, obtain microbiological specimens first to guide the antibiotic treatment.
- Review and reconsider chosen therapy at least weekly.
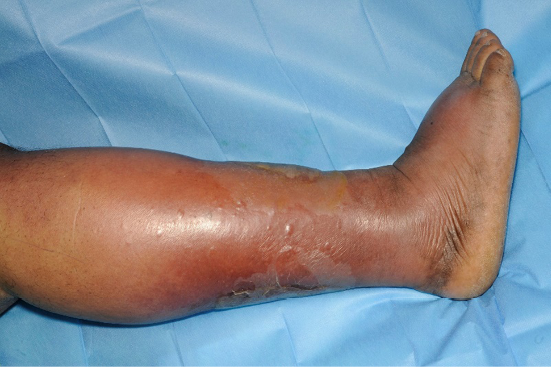
Figure 12. Cellulitis in dark skin. Image courtesy of Samantha Holloway, permission granted for inclusion
4. Vascular assessment and treatment
Author: Annette Høgh
Oxygen and nutrients are essential for wound healing and tissue cells to survive. The arterial system (high-pressure system) carries oxygen and nutrients to the body tissue via the arteries whereas the deoxygenated blood returns to the heart via the vein system (low-pressure). The exchange of gases and transfer of nutrients between blood and tissues takes place in the capillaries. Lymph capillaries weave between tissue cells and the blood capillaries. The lymph system drains the excess fluid (leaking between the blood vessels and the tissues) and empties it back into the bloodstream via the lymph vessels hereby managing the fluid levels in the body. Dysfunction or imbalance in these systems may lead to a changed condition in the skin leading to ischaemia and/or oedema causing chronic ulceration (76). Assessment of the function of arterial and venous systems may help to identify the underlying causes to formation of chronic wounds and categorise the clinical severity of the vascular disease and expected response to treatment.
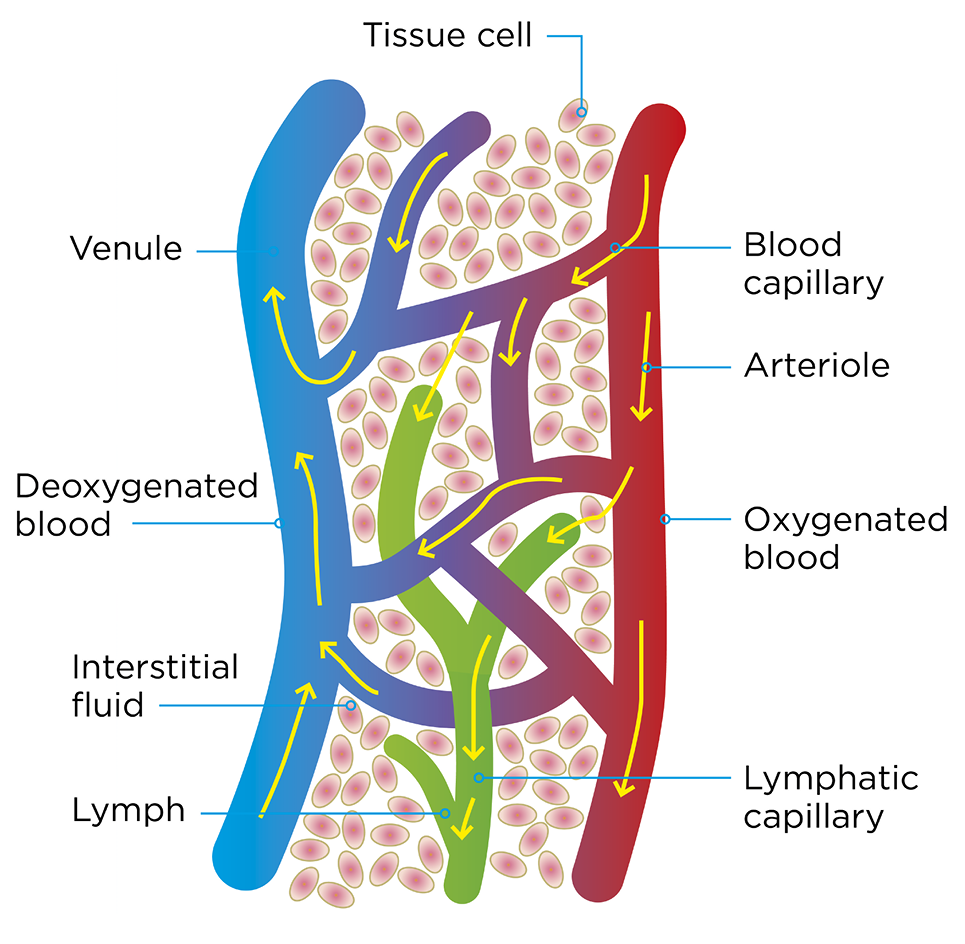
Figure 13. The arterial and venous system
Arterial assessment and treatment
Pathophysiology
Over 90% of arterial disease in the lower limbs derive from narrowing of the peripheral arteries obstructing the deliverance of oxygen to the peripheral tissue cells. The underlying disease is atherosclerosis that affects all vascular territories. Atherosclerosis of the coronary arteries may present as angina, ischaemic heart disease or coronary events such as myocardial infarction. Atherosclerosis of carotid arteries can result in transient ischaemic attack or stroke. Severe atherosclerosis of the lower limbs can result in inadequate tissue perfusion, leading to cell death and the formation of ulceration, necrosis or gangrene.
The decreased oxygen inflow and the abolition of metabolic waste products combined with extended diffusion pathway entail decreased resistibility to tissue load and infection leading to reduced wound healing potential. However, the underlying pathogenic factors are frequently multifactorial (76).
PAD describes the clinical manifestations of atherosclerosis affecting the circulation in the legs. PAD is graduated over a spectrum, from asymptomatic, to exercise induced pain (intermittent claudication, (IC) and in the most severe form can result in chronic limb threatening ischaemic (CLTI) wounds. The severity of PAD is classified according to signs and symptoms that reflect the degree of circulatory compromise. The final stage of PAD in the lower limbs is rest pain and/or ischaemic wound, typically placed on the tiptoe or on bony protrusions on the foot, representing the tip of the iceberg among patients with PAD (Figure 14).
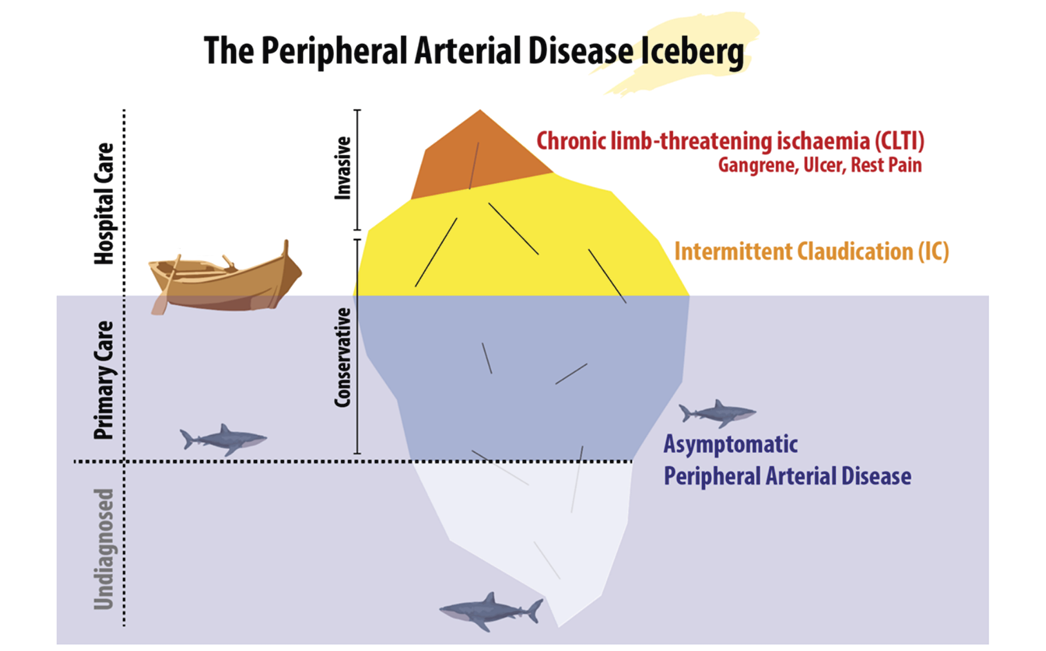
Figure 14. The PAD Iceberg. From Sogaard M et al., A thought-provoking statement regarding the treatment of patients with peripheral arterial disease. Vasa. 2023;52(2):(77).
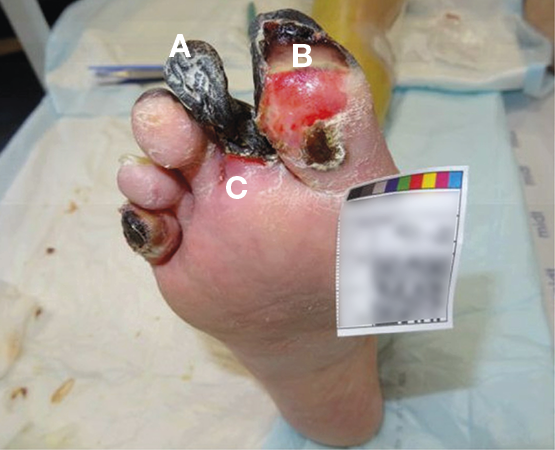
Figure 15. Example of dry gangrene (A), wet gangrene (B) and marked zone between vital and avital tissue (C). Image courtesy of Annette Hoegh, permission granted for inclusion
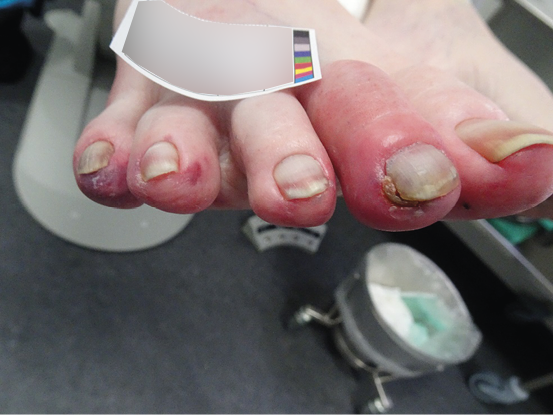
Figure 16. Early phase peripheral ischaemia. Image courtesy of Annette Hoegh, permission granted for inclusion
Dry gangrene is a focal necrosis where the affected skin becomes chilly, dry and changes colour to dark purple, eventually turning into a black dry up mummified area (see Figure 15 as an example). A marked zone between the vital and avital tissue appears. The process causes disabling pain combined with decreased mobility and disturbed sleep; if pain is absent or pain sensitivity is reduced, peripheral neuropathy should be considered. If an infection occurs, the dry gangrene will turn wet, leading to the potential of a lower limb- or life-threatening septic condition. Surgical wound revision in areas with compromised oxygen inflow is pointless as necrosis quickly regenerates.
Diagnosing / assessment
The diagnosis ‘ischaemic wound’ is based on clinical characteristics combined with an objective and physical examination of the patient. Describe the location, the extent, and the appearance of the wound (see Table 4). Palpation of pedal pulses and, if absent, measurement of the ankle-brachial index (ABI) are first-line screening tests for assessing the presence and severity of PAD. Acknowledgement of leg/foot or wound related pain is a crucial point in the diagnostic process (78). However, patients may have severe peripheral atherosclerosis diseases without symptoms, which can be related to their incapacity to walk enough to reveal symptoms (e.g. heart failure or chronic obstructive pulmonary disease which reduce the patient’s walking ability do to shortness of breath) and/or reduced pain sensitivity (e.g. due to diabetic neuropathy). Critical PAD can also be clinically masked in one leg when the other one has a more disabling disease. Often the patients have coexisting peripheral oedema as a consequence of pain with decreased functional capacity and/or by sleeping with the affected limb over the edge of the bed in an attempt to improve the blood flow to the foot.
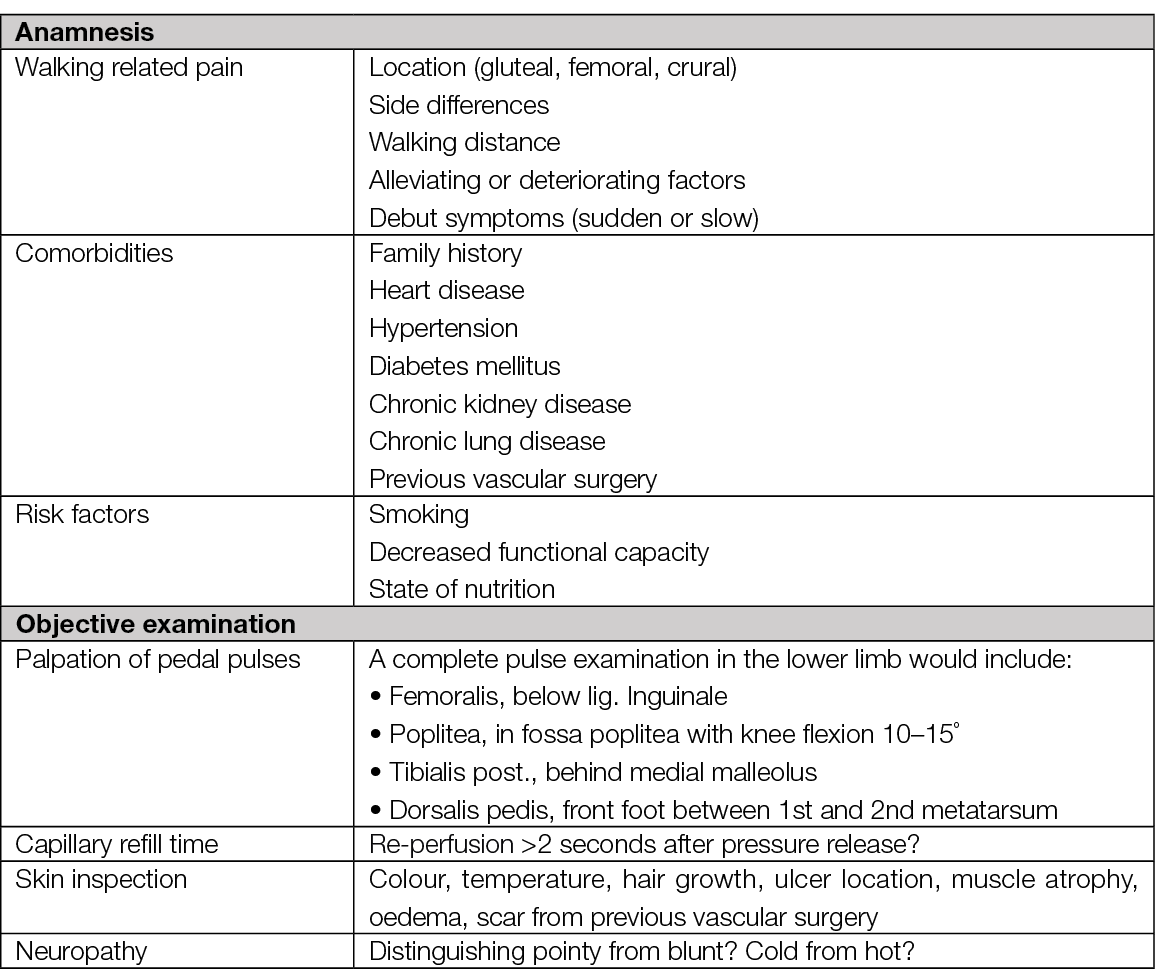
Table 4. Anamnesis and objective examination of patients with chronic ischaemia in lower extremity
Pulse palpation and capillary refill time
As a part of the clinical examination, systematic assessment of the pedal pulses (the posterior tibial and the dorsal pedal artery) must be performed. Absent pedal pulses and / or capillary refill time >2 seconds are associated with an abnormal ABI and, thus, these patients must be referred to ABI measurement. Vascular assessment is crucial to prevent amputations due to PAD (20).
Pitfalls: Pulse palpation and capillary refill time
- Oedema and obesity (the pulse become weakened because of the increased distance between the artery and the surface of the skin)
- Skin temperature <27˚C (at low temperatures the perfusion to the extremities is impaired)
- 5–10% have an alternative anatomic position of the pedal arteries
- Your own pulse can be interpreted as the patients
- Small muscle movements in the foot can imitate a pulse
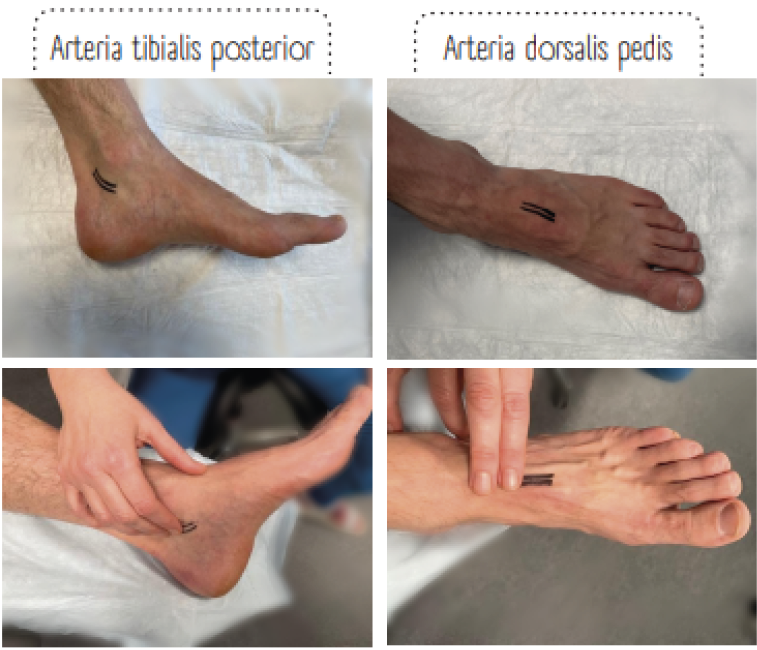
Figure 17. Where to perform pedal pulses. Image courtesy of Annette Hoegh, permission granted for inclusion
The ankle-brachial index (ABI)
The ABI index compares the systolic blood pressure (SBP) on the arms and the legs to give a ratio that expresses the severity of PAD. The ABI is considered to be an accurate and reliable method which current guidelines recommend, with cutoff ABI ≤0.9, with a 75% sensitivity and 86% specificity for the PAD diagnosis (82, 83). The sensitivity of ABI is poorer in patients with diabetes or end-stage chronic kidney disease because of medial calcification, where ABI is high (>1.40) because of incompressible arteries (84). Alternative tests such as toe pressure, toe-brachial index (TBI) or measurement of the oxygen level in the skin or wound edge can be useful. When clinically suspected, a normal ABI (>0.9) does not definitely rule out the diagnosis of ischaemic wounds or PAD; thus, a referral to vascular department is needed where further assessment or arterial imaging/intervention can be performed. Also, the guidelines threshold value for performing monitored compression therapy in mixed ulcers (0.5 vs 0.6) differs depending on the publications (23, 85).
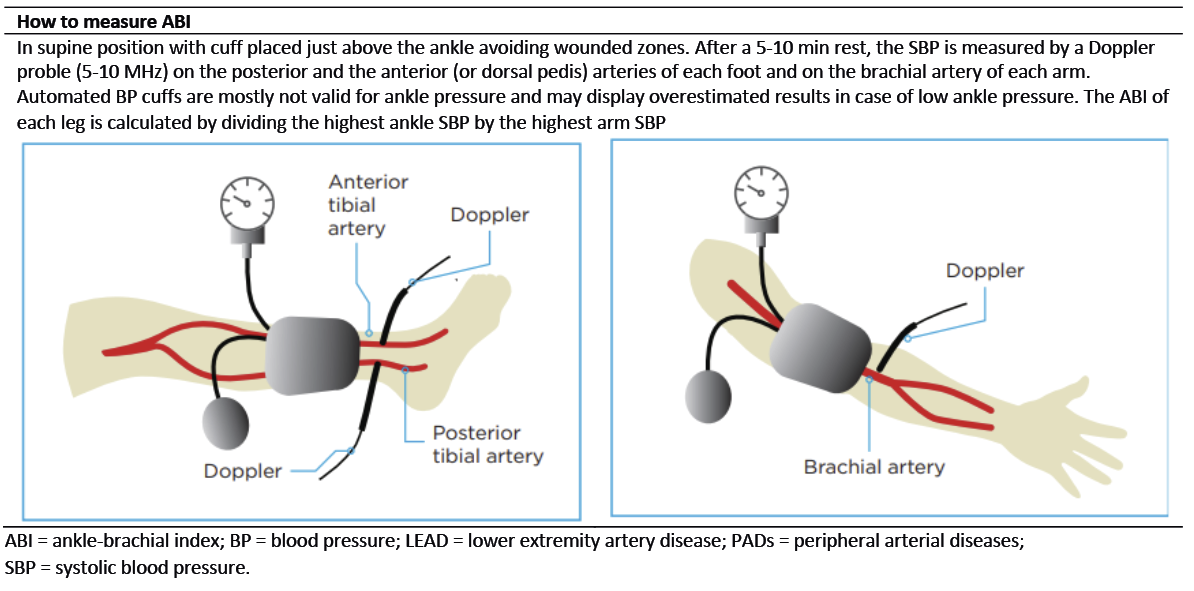
Figure 18. The Ankle-brachial index. Modified with permission from Aboyans V. et al., Editor’s Choice - 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(3):313. (82)
The gold standard method for conducting ABI measurement is a handheld Doppler, which requires specialist training and can lead to poor diagnostic accuracy and delays in treatment if used incorrectly (86). A variety of automated ABI / TBI systems have been introduced through the last decade to optimise the usability by being less time consuming and costly with a limited request for training. A systematic review (based on 11 published studies) evaluated the diagnostic accuracy of four portable ABI / TBI systems used in a community-based PAD screening program. They found a moderate level of sensitivity (20–95%) and high level of specificity (77–99%) (87). The article emphasises that diagnostic accuracy must be balanced with usability when choosing a system.
Interpretation of ABI
ABI should not be used in isolation but as an additive to a combination of the patient anamnesis and objective examination (Table 4). According to current guidelines, ABI indexes between 0.9 and 1.4 are normal. The wound healing potential diminishes parallel with decrease in ABI and/or TBI. Patients with wounds and an ABI <0.6 or TBI <50mmHg should be referred to a vascular specialist. This is mandatory for limb salvage. TBI measurements <30mmHg are not compatible with wound healing without revascularisation. Furthermore, ABI is an independent risk factor (ABI <0.9 or ABI >1.4) for cardiovascular morbidity and mortality, as it reflects the extent of the underlying universal atherosclerosis (90). Patients with PAD and wounds have a 3-fold increased risk of myocardial infarction, stroke or vascular death as compared to patients with IC. Regarding the limb risk, at 5 years 27% of patients with PAD and an ischaemic wound have major amputations (88). Patients in this ABI interval (<0.9 or ABI >1.4) will benefit from most cardiovascular preventive strategies, especially strict control of risk factors.
ABI measurement does not focus on microcirculation. On the other hand, several diagnostic techniques have been introduced to enable tissue perfusion measurements in the lower limbs; e.g. transcutaneous oxygen pressure or skin perfusion pressure by laser Doppler flowmetry or silastic strain gauge plethysmography (89). However, there is no clear consensus on using estimations of the microcirculation in the skin / wound edges as a predictive marker for wound healing in the existing international literature.
Pitfalls of ABI
- Skin temperature <27˚C (at low temperatures the perfusion to the extremities is impaired)
- 5–10% have an alternative anatomic position of the pedal arteries
- The wound is in the same place as where the cuff is placed
- Rest time less than 5–10 minutes (the blood pressure may be misleading high without rest prior to assessment)
- The patient is asymptomatic and ABI measurement has not been performed
- The patient has untreated hypertension (high blood pressure) which may lead to misinterpreting of the ABI
- Diabetes or other causes for medial calcification
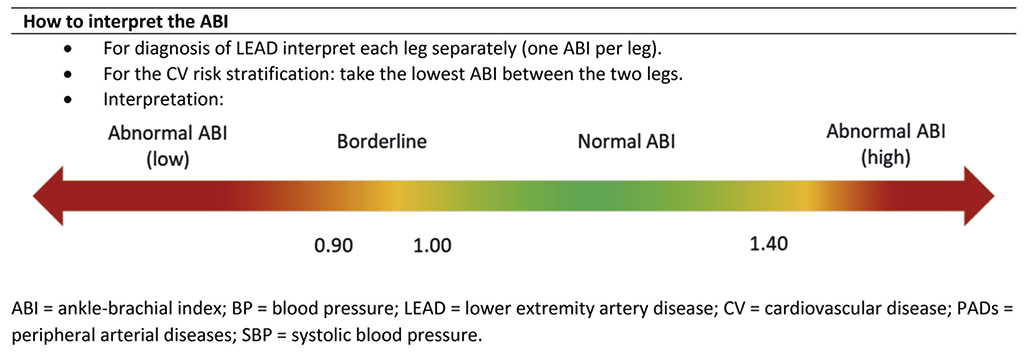
Figure 19. How to interpret the Ankle-brachial index. Modified with permission from Aboyans V. et al., Editor’s Choice - 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(3):313. (82)
Revascularisation
One of the main reasons to perform endovascular or open revascularisation procedures in patients with ischaemic wounds is to facilitate healing by improving tissue perfusion and skin oxygenation of the lower leg and foot. Imaging is a cornerstone in the planning of the revascularisation and it includes a variety of angiographies – digital subtraction, computed tomography (CT), magnetic resonance imaging (MRI) or duplex ultrasound scanning (DUS). Imaging is used to map the extent of the atherosclerosis and afterwards used in the planning of interventional angiology or vascular surgery reconstruction of the blood flow to the affected area.
When stenotic or occlusive arterial disease is found, reconstruction should be considered. The treatment options include endovascular angioplasty +/– stenting or surgical bypass operations. The choice of the surgical approach is based on a number of factors including the severity and location of the arterial disease, and the general health of patient.
Bypass surgery is used to send oxygenated blood around an occluded area, most common from the groin to the popliteal artery or to one of the three main arteries of the lower leg. Optimally, a large vein from the patient’s leg is used as bypass material, but it is also possible to use a fabric graft if the patient’s own vein can’t be used. Thrombendarterectomy is also a common operation where the artery is opened longitudinally and the atherosclerotic plaque, including the intima layer of the artery, is removed in total. In more complex lesions, a hybrid procedure combining open surgery and endovascular procedure, is used for the treatment of multilevel arteriosclerosis.
In ischaemic wounds, once the blood flow is re-established, the focus can be re-centred on standard wound treatment, especially compression therapy.
Venous assessment and treatment
VLUs are the most common type of leg ulcer with a significant socioeconomic burden due to slow healing and frequent recurrence. A VLU arise from venous insufficiencies in the lower extremities due to valvar incompetence, reflux, venous obstruction, or a combination of these with consequent distal ambulatory hypertension. All patients with a VLU should be considered for venous intervention in addition to compression therapy to improve ulcer healing and prevent recurrence (21).
Anatomy / pathophysiology
The venous system on the lower extremities includes a superficial system (the great and small saphenous veins) localised in the subcutis above the muscle fascia and a deep system place in the muscles below the muscle fascia. There are >40 perforators between these two systems. The venous system is endowed with valves. In cooperation with the muscles on the calf and foot an effective venous pump system is created ensuring a unidirectional flow back towards the heart. An efficient venous pump system decreases the pressure in the lower extremity veins from 70–100mmHg in supine position to 20–30mmHg by walking.
If the venous pump system is dysfunctional, CVI arises and consequent distal hypertension. The risk of VLU grows as the distal hypertension progresses. Furthermore, perforators with reflux may contribute to chronicity of VLU.
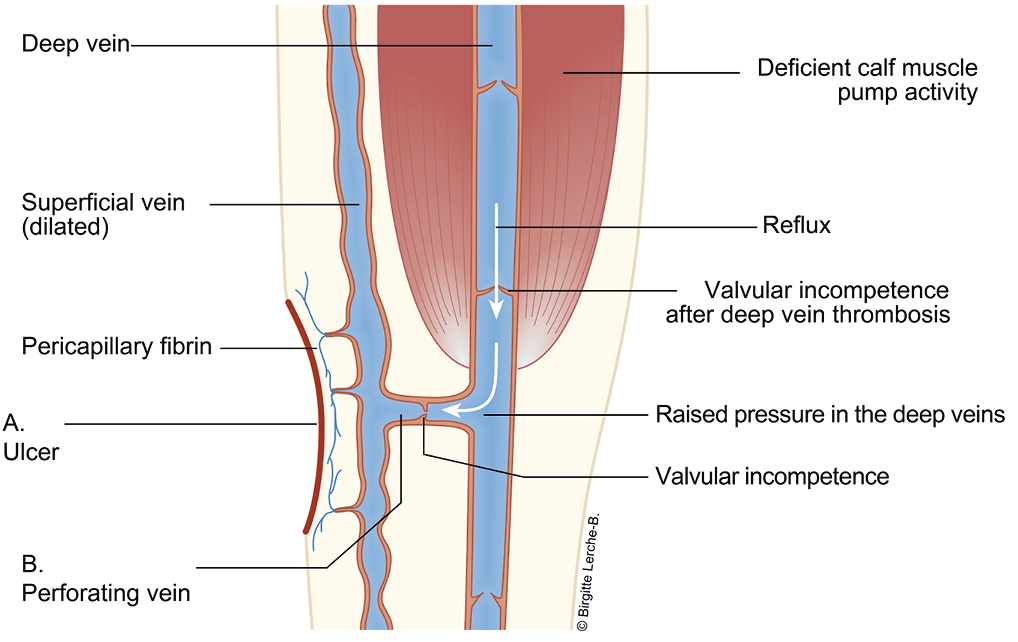
A: Distal hypertension in the veins which decrease the microcirculation in the skin
B. Dysfunctional perforator vein; lack of ability to decrease the venous pressure during muscle activity
Figure 20. The venous system on the lower leg. Modified with permission from Høgh A. [Wounds related to vascular changes - Venous Wounds]. In: Gottrup F, Karlsmark T, Kirketerp- Moller K, editors. [Wounds - Aetiology, diagnosis and treatment]. Copenhagen: Forfatterne og Mungsgaard; 2021. p. 28. (90)
Other causes of venous insufficiency in the lower extremities include extrinsic vein compression (e.g. intra-abdominal masses, including pregnancy), previous deep vein thrombosis (DVT) resulting in occlusion (post-thrombotic syndrome), raised general venous pressure followed by right heart failure, impaired calf muscle pump (e.g. immobilisation), or obesity.
The pathophysiology behind the development of CVI is twofold. Firstly, inflammatory response may lead to vascular remodeling with degenerative loss of elastin and collagen, with the consequent destruction of the intraluminal valves combined with increased wall thickness due to fibrosis. This transformation leads to changes in vasomotor tone and reflux (i.e. reversed blood flow) creating impaired venous emptying and chronic venous hypertension. Secondly, changes occur in the microcirculation by dysfunctional post-capillary venules affecting the surrounding tissues and skin. Inflammatory mediators combined with cell migration generate oedema and hypoxia followed by loss of normal integrity of the skin with VLU formation.
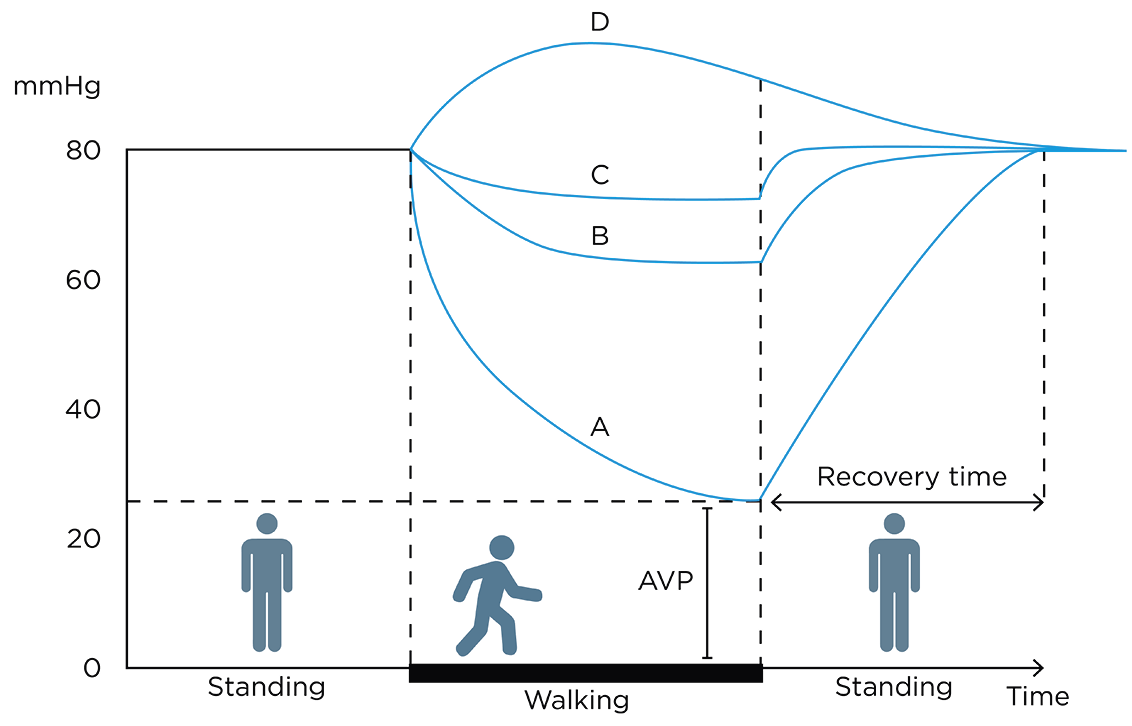
AVP: ambulatory venous pressure
RT: recovery time
Figure 21. Schematic illustration of the ambulatory venous pressure in mmHg in (A) healthy subjects, (B) patients with superficial and perforator dysfunction, (C) patients with additional deep venous dysfunction, and (D) patients with deep venous outflow obstruction.
Compression of the lower extremities veins and soft tissue leads to improved haemodynamics and thereby reduces the effects on the skin of venous hypertension and oedema. Non-adherence to compression may be the principal cause of slow healing or recurrence of VLU. Individual variation in the vulnerability to venous hypertension exist and presence of reflux is not an indication of treatment alone
Clinical examination
The diagnosis of VLU is based on clinical characteristics combined with a subjective and objective examination of the patient. To identify venous insufficiency an ultrasound examination is needed to assess the return flow of the blood from the lower leg back to the heart.
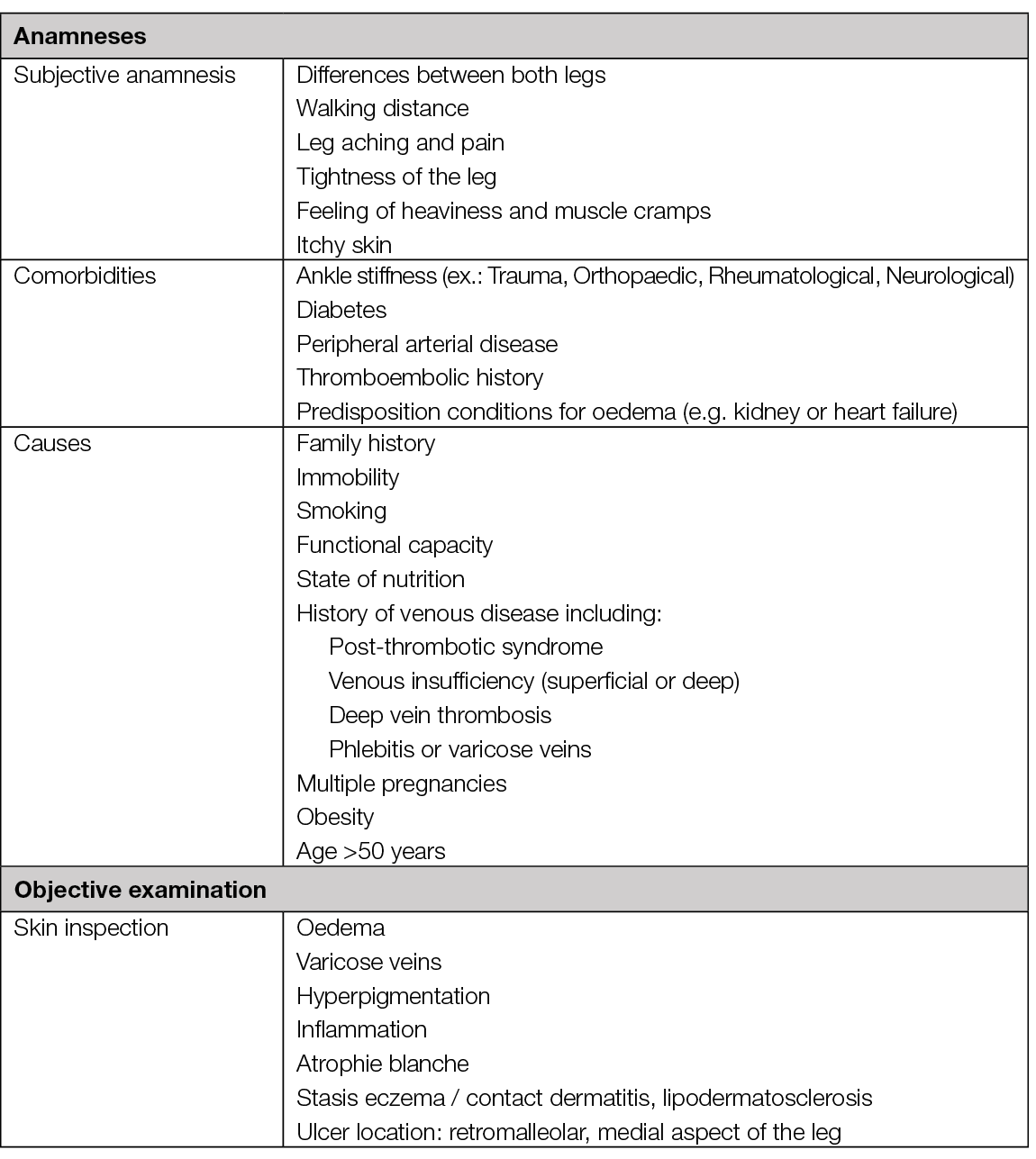
Table 5. Anamnesis and objective examination of patients with VLU
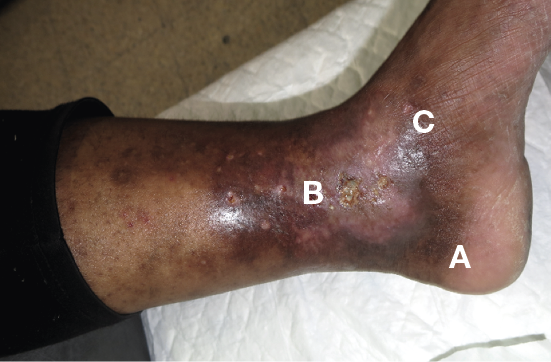
Figure 22. Cutaneous signs of CVI – hyperpigmentation (A), lipodermatosclerosis (B), atrophie blanche (C). Caution: CVI is very prevalent. Wounds of atypical causes can present on a leg with these signs. Image courtesy of Elena Conde Montero, permission granted for inclusion
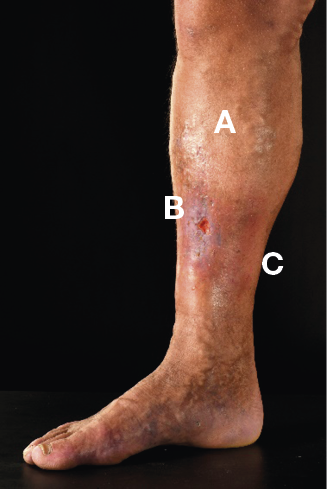
Figure 23. VLU with varicose veins (A), lipodermatosclerosis (B) and hyperpigmentation (C) © Mid Yorks NHS Trust used with permission
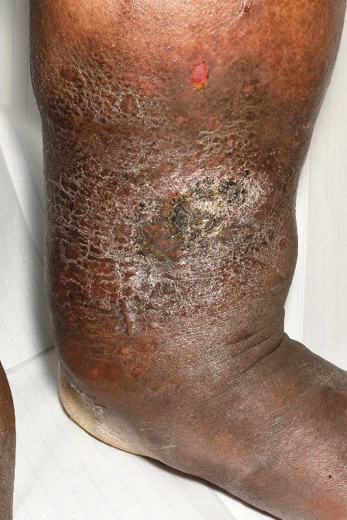
Figure 24. Stasis eczema and lipodermatosclerosis in dark skin © Mid Yorks NHS Trust used with permission
Ultrasound
Duplex ultrasound (DUS) (probe 5–17MHZ) is the primary diagnostic test for patients with suspected or clinically evident chronic venous disease assessing the morphology and the haemodynamics of the flow in lower limb veins. DUS is both non-invasive, fast and reproducible technique (23). DUS is used to assess direction and velocity of the passing venous blood flow to identify occlusions and/or reflux (i.e. reversed blood flow). The examination is done with the patient in standing position with the knee of the investigated leg slightly bent. By manual compression of the thigh, calf or foot, a ‘blood column’ is moving towards the venous valves to test for signs of insufficient veins. Venous insufficiency is defined as a reversed flow duration >1 second for the femoral vein and popliteal vein as well as >0.5 seconds for the superficial veins and perforators (23).
In addition to diagnostic procedures, DUS is used as a tool in planning customised treatment by graphical representation of the findings. For patients with suspected supra-inguinal venous obstruction, in addition to full leg DUS, ultrasound of the abdominal and pelvic veins should be considered as part of the initial assessment (23).
Classification system
The Clinical Etiological Anatomical and Pathophysiological (CEAP) classification system facilitates a worldwide universally understandable description of venous disease and works as an instrument to standardise the diagnosis and allow better communication of chronic venous disorder diagnosis between healthcare professionals. The name CEAP classification stands for Clinical (C), Etiological (E), Anatomical (A), and Pathophysiological (P) (91). Additionally, CEAP contains 18 named venous segments to locate the venous pathology. In clinical practice, we normally use only the clinical subclassification (C) which gives a lot of information about the severity and prognosis of chronic venous disease. We speak of CVI from stage C3 onwards. Finding these clinical signs on the perilesional skin of a leg with a wound must make us suspect a venous ulcer.
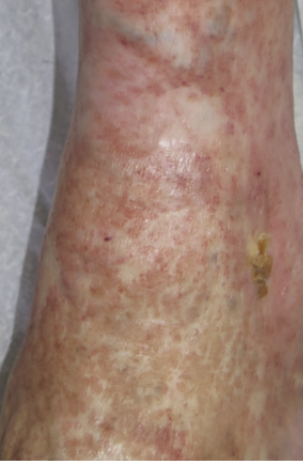
Figure 25. Atrophie blanche. Image courtesy of Elena Conde Montero, permission granted for inclusion
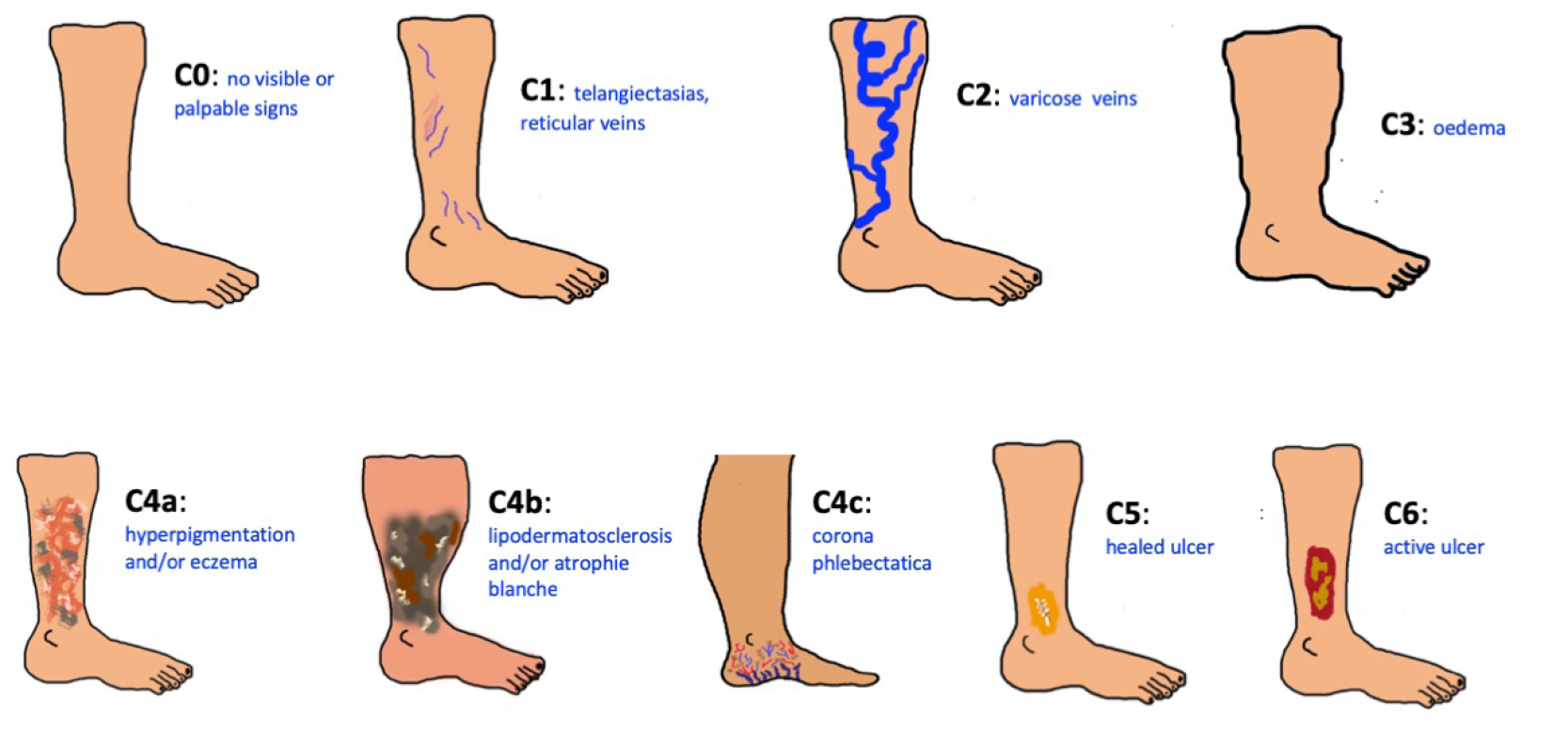
Figure 26. The problem of chronic venous disease. CEAP classification. Images courtesy of Elena Conde Montero, permission granted for inclusion
Venous intervention
The target for treating venous insufficiency is to relief symptoms, accelerate wound healing and prevent VLU occurrence and/or recurrence. In addition to venous intervention, compression is a key treatment and obligation in all oedema and venous insufficiency treatment (see Chapter 8). Furthermore, the treatment of the surrounding skin and the VLU itself combined with pain control is of great significance (see Chapter 7).
The European Society for Vascular Surgery (ESVS) guidelines from 2022 recommend endovenous intervention to patients with chronic venous disease of the lower extremities to prevent VLU, accelerate ulcer healing and/or reducing the risk of ulcer recurrence (23-25). The recommendations include patients with active or healed VLU based on superficial venous incompetence presenting with varicose veins (92), CEAP clinical class C2, oedema because of chronic venous disease (CEAP clinical class C3), skin changes because of chronic venous disease (CEAP clinical class C4–C6) as well as patients with combined superficial and deep venous insufficiency (95, 96).
Minimally invasive endovenous techniques have grown over time to be the ‘gold standard’ based on the technique efficiency, safety, low price and patient friendliness, compared to classical surgery (ligation and or stripping of incompetence saphenous veins) (23). Endovenous techniques are all DUS-guided to increase efficacy and safety, and include: endovenous laser or radiofrequency, ultrasound guided foam sclerotherapy (USGFS) or catheter directed injection of cyanoacrylate glue. Treatment of perforants and sub ulcer venous plexus should be considered as part of the treatment strategy (class 2A) (23). According to the ESVS guidelines, endovenous thermal ablation should be the first line treatment (23). There is no guarantee that recommendation of lifelong compression use may be expendable after surgical intervention.
VLUs with coexisting PAD (mixed ulcers)
15–20% of patients with VLU have coexisting PAD (93, 94) (defined as an ABI <0.9 – see arterial assessment (23)). Identifying the aetiology behind the ulcer formation is essential and basic assessment of arterial status (ABI) should be performed to quantify the degree of a potential arterial component. With decreasing ABI, the risk of iatrogenic skin damage due to compression therapy, increases. However, compression therapy is still one of the keystones in treatment of chronic ulcers, independently of the underlying aetiology. An ABI >0.8 may be considered as normal and allows commencement of full compression therapy (97). With ABI 0.6–0.8 modified compression under close clinical supervision is mandatory. Compression therapy should be continuously re-assessed and adapted to the patient’s needs. Exceptional attention is needed among patients suffering from peripheral neuropathy to avoid skin damage along with the use of compression therapy.
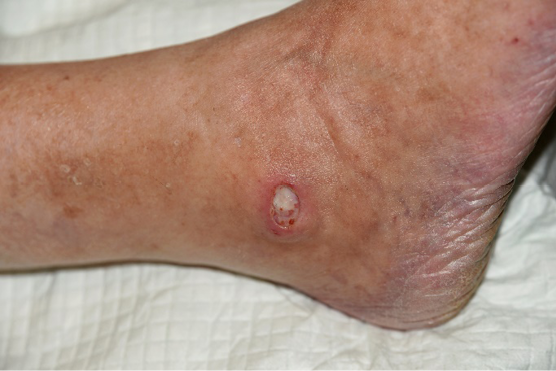
Figure 27. A VLU with coexisting PAD in the right ankle region. Image courtesy of Kirsi Isoherranen, permission granted for inclusion
5. Leg oedema
Authors: Mark Collier, George-Sorin Tiplica
Definition
Oedema is the clinical manifestation of an accumulation of fluid within the interstitial spaces of the body. In most cases it develops when the balance between the flow of fluid out of capillaries and the return of fluid to the vascular space via capillary reabsorption and lymphatic flow is disrupted. In rare cases it is produced by an excess of soft tissue (98). Oedema can be localised or develop up-to generalised oedema (99). One form of localised oedema is peripheral oedema which is most frequently gravity related.
Aetiology
Leg oedema is produced by a multitude of aetiologic factors (Table 6) (98, 100). Local or systemic conditions can induce the occurrence of leg oedema by acting alone or in combination. Identifying the complex aetiology of the leg oedema is often challenging for the medical staff. The systemic disorders that include leg oedema in their clinical tableau are not the subject of this document. The local conditions producing leg oedema most frequently involve the leg veins (venous insufficiency/oedema) or the lymphatic circulation (lymphoedema). A combination of aetiologic factors can develop, producing complex modifications (phlebo-lymphoedema). Phlebolymphoedema results from a secondary accumulation of lymph fluid due to CVI. The circulatory and lymphatic systems maintain a delicate balance in the body (see also Chapter 4). If the venous system is damaged, it will typically affect the lymphatic vessel system (101). Due to this complex aetiology, the patient suffering with leg oedema is usually managed in a multidisciplinary setting involving (but not limited to): a dermatologist, vascular surgeon, cardiologist, endocrinologist, nephrologist, infectious disease doctor, internal medicine doctor, rheumatologist, orthopaedist as clinically indicated.
Epidemiology
Venous oedema is reported in 8% of the general population (102). However, this estimate will vary between countries. For example, a UK study reported a total crude prevalence of 3.93/1000 population (95% CI 3.69–4.19), with a prevalence of 2.48 for men and 5.37 for women (103). Lymphoedema has been reported to affect an estimated 35 million patients in the United States of America (USA), over 200,000 people in the United Kingdom (UK) and 250 million people worldwide. Accurate data reporting both current prevalence and incidence rates of oedema and lymphoedema is sparse however, as both conditions are often associated with a variety of co-morbidities (Table 6). It is acknowledged that primary lymphoedema is rare and is thought to affect around one in every 6,000 people whereas secondary lymphoedema is much more common. For example, in the UK, secondary lymphoedema affects five in 10 people with vulval cancer. About three in every 10 people with penile cancer will get lower limb lymphoedema (104, 105). Lymphoedema is often present in the obese patient (body mass index (BMI) >30), a group with alarmingly increasing numbers. In one UK based lymphoedema service it was reported an increase in the percentage of obese patients being referred, from 60% to 68% over a 10-year period. It has been reported that often patients had been managed in the community without success for long periods – months and years. It has therefore been argued that a community-based approach that combines the wound care expertise of community nursing teams and the advice and guidance of lymphoedema specialists on appropriate management techniques, including compression therapy, would promote optimal patient management practices. However, it has also been acknowledged that any management plan must still include addressing weight issues if any long-term success in reducing chronic/obesity induced lymphoedema is to be achieved (106).
Table 6. Aetiology of leg oedema (98, 100). Several aetiologies may overlap in a patient to explain acute oedema, chronic oedema or acute exacerbation.
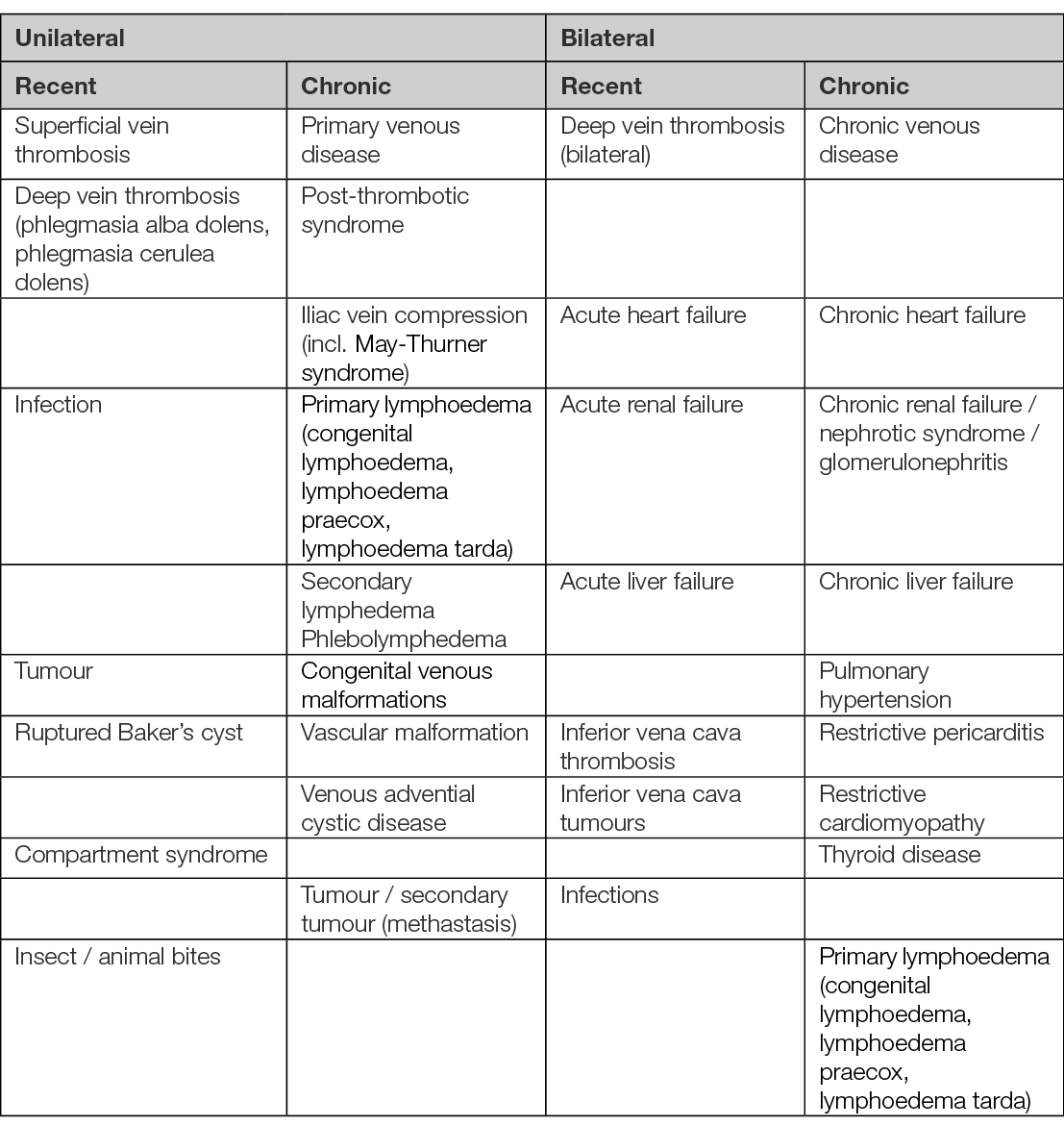
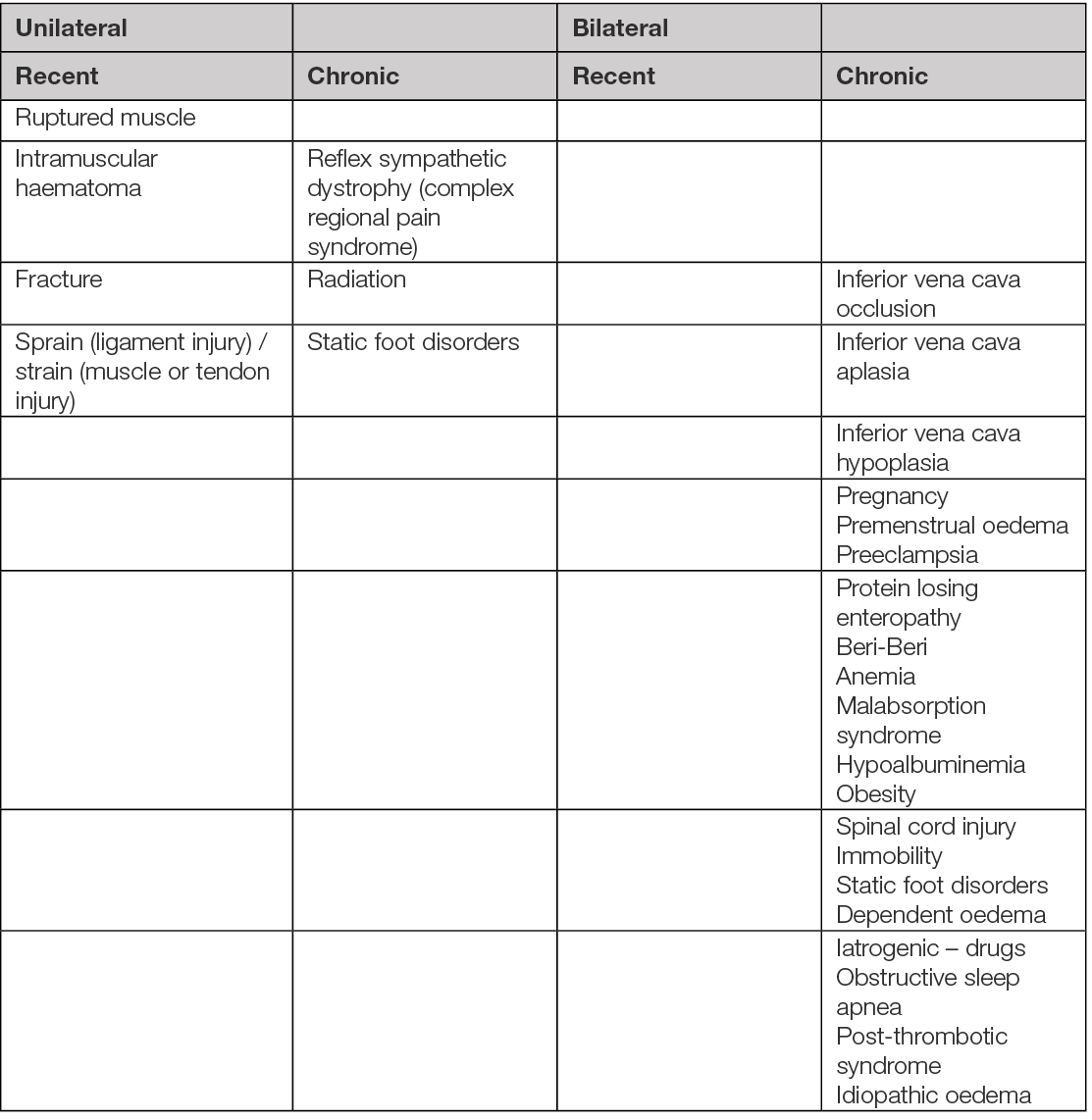
Classification
Leg oedema can be classified as recent (includes leg oedema with acute onset <3 days) and this usually reduced to some extent with elevation, or chronic (when the duration is of 3 months or more) (107). Following, the clinical distribution of the leg oedema can be unilateral (asymmetrical) or bilateral (symmetrical). Depending on the tissue depression induced by digital pressure, leg oedema can be pitting (e.g. renal disease, heart failure) or non-pitting (e.g. lymphatic disease, thyroid disease).
Venous oedema represents the stage C3 from the CEAP classification (see Chapter 4).
Lymphoedema is classified as (108):
- Stage 0: subclinical oedema. No volume change observed
- Stage 1: the amount of extracellular or subcutaneous water can be measured to quantify lymphoedema by bioimpedance spectroscopy (BIS)
- Stage 2: swelling noted of the affected anatomical region
- Stage 3: the accumulation of adipose tissue and fibrotic changes are noted (the affected area feels hard to touch).
Assessing the patient with leg oedema
History and clinical examination
The assessment and diagnosis of leg oedema should be undertaken utilising a holistic framework that should incorporate patient history (including medication and social history) and a thorough general physical examination (height, weight and BMI), vital signs (blood pressure, pulse, breath), the patient’s gait and joint mobility, venous and arterial assessment, clinical neurological examination, the presence of chronic oedema and the condition of the patient’s skin.
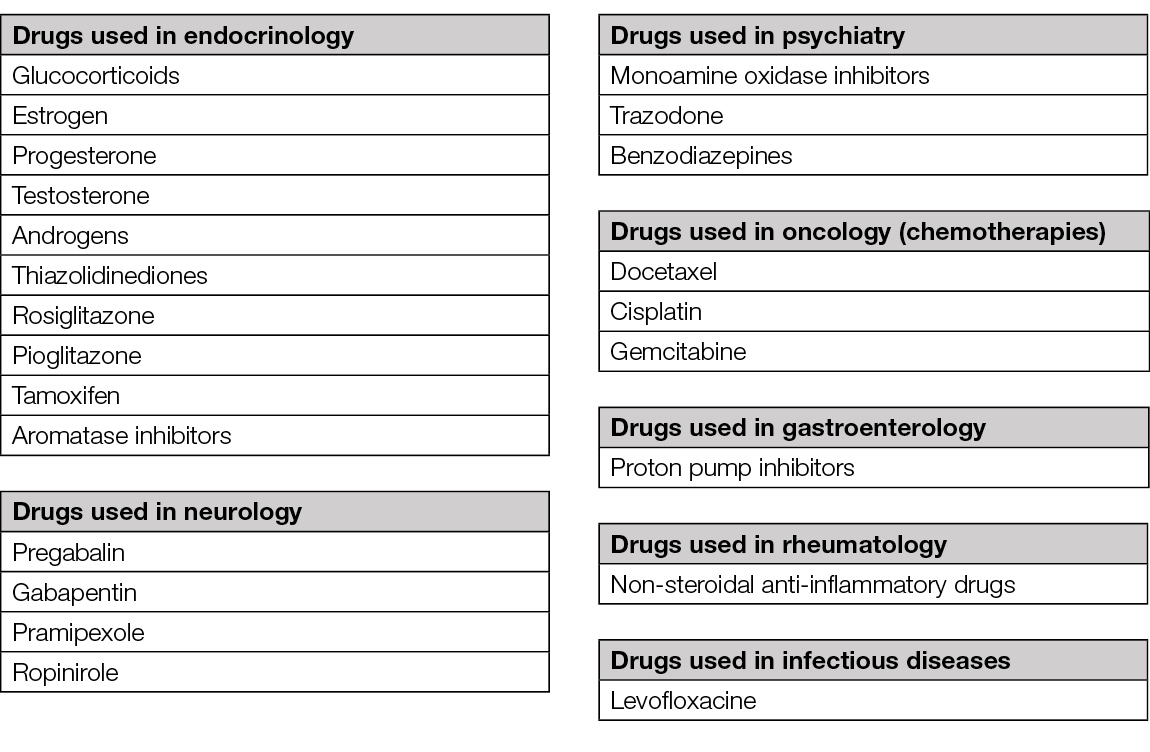
History will identify the duration of the disease with particular attention to the acute manifestations (<72h) generated by trauma, DVT, infection or trauma. Medical history will focus on the existing (internal) conditions and on their (systemic) treatment. There are many drugs that can induce the apparition of leg oedema (Table 7) (98, 100, 109) – in more than half of these drug-related cases, leg oedema is related to the intake of calcium channel blockers (e.g., amlodipine) (110). Surgical history is also important as pelvic surgery can induce leg oedema. For differential diagnosis, it is important to note the evolution of leg oedema during the day (e.g., in venous diseases of the lower limb leg oedema worsens in gravitational positions and improves when the limb is elevated). Symptomatology is crucial: pain can be intense in arterial diseases, heaviness or fatigue of the legs can occur in venous disease.
Table 7. Drugs inducing leg oedema (98, 100, 109).
The venous oedema (CEAP classification C3) (Figure 26, Chapter 4) starts as lower leg oedema, at the end of the day and disappears when the patient starts daily activities. Lipoedema is frequent in women and evolves with symmetrical fatty deposit accumulations from the lateral parts to the medial aspects of the skin but there will be no fatty deposit accumulations below the ankle (111).
Clinical examination involves both observation and palpation of the limbs. Measuring the circumference of the calves and ankles is also useful for monitoring treatment outcomes. A simple clinical test referred to as Stemmer’s Sign can help support the differential diagnosis of oedema/lymphoedema (112). To perform the Stemmer’s Test, pinch a fold of skin at the base of the second toe or finger. If the skin can be lifted, the result is likely negative. If the skin can’t be pinched, the result is likely positive. A positive result is a likely predictor for primary and secondary lymphoedema of the lower limb. A negative result does not rule out lymphoedema and will typically be noted in patients with a normal BMI and/or who have stage 1 disease.
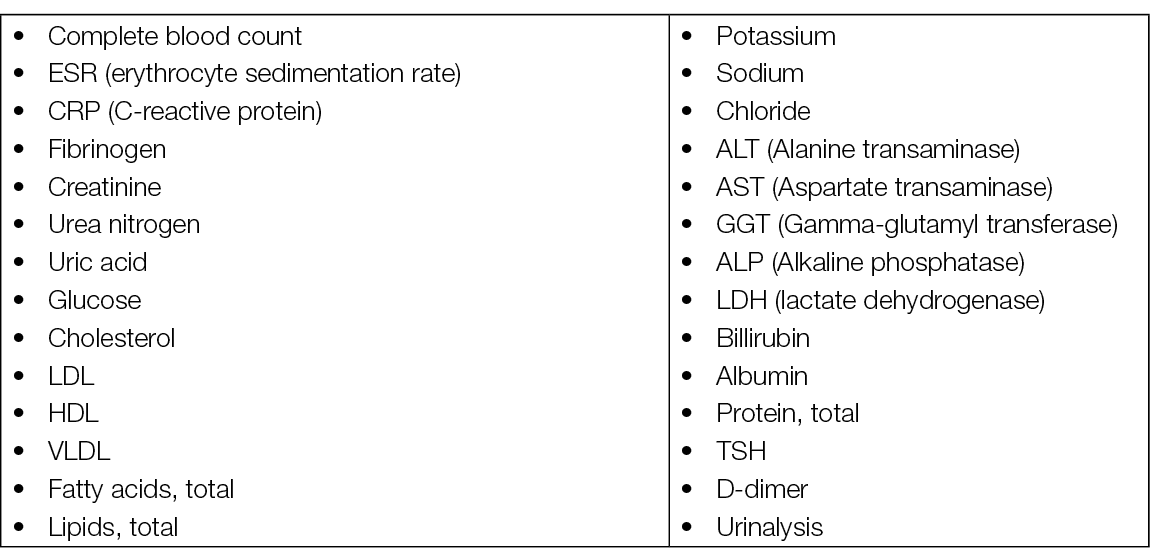
Basic laboratory screening (blood)
Laboratory tests needed for the aetiologic diagnostic of leg oedema must cover a large range of possible systemic diseases (Table 8) (98, 113). The D-dimer level is essential when acute DVT is suspected.
Table 8. Leg oedema – laboratory tests
Specific laboratory / imaging investigations
DUS is the most used imaging investigation in leg oedema to support the aetiologic diagnosis and the treatment recommendations. It is a non-invasive examination that provides information on the venous anatomy, valve function, and other non-vascular anatomic structures of the lower leg. In the pelvic region DUS can offer information on the anatomic structures, vascular anatomy and function, possible tumoral masses and lymphatic nodules (114).
Lymphoedema measuring instruments with evidence for good reliability are the BIS, water volumetry, tape measurement and perometry. It has been reported that BIS can detect alterations in extracellular fluid in stage 1 lymphoedema and the other measurement instruments can detect alterations in volume starting from stage 2 (115). There exists clear evidence for good reliability of BIS in the lower limb (Level of evidence: 2C) and high reliability and excellent validity of water volumetry, tape measurement and perometry in measuring lymphoedema in the upper limbs (Level of evidence: 1B). Based on the authors’ analysis, the use of BIS, volumetry, tape measurement and perometry can be recommended in clinical practice. Measurement of midline and lower limb lymphoedema warrants more research.
Magnetic resonance lymphology (MRL) can also be used for the evaluation of peripheral lymphoedema (116). MRL identifies superficial lymphatic vessels with a diameter of >0.5mm with high sensitivity and specificity and accurately shows abnormal lymphatics and lymphatic drainage patterns. MRL is based on a gadolinium-based contrast enhanced T1-weighted gradient-recalled echo sequence combined with T2-weighted MRI, with acquisition 30 minutes after injection of contrast material.
Other imaging investigations can be used, mainly for the diagnosis of leg oedema induced by conditions affecting the pelvis or the abdomen. Contrast-enhanced (CT) or (MRI) can provide accurate information on tumours, lymph nodes, venous anatomy (including obstructions of the ilio-caval segment).
Tools for leg oedema evaluation (patients and medical staff)
Visual Analogue Scale is used for subjective characteristics (pain, heaviness, itching etc) and represents a continuous (analogue) scale, most frequently from 0=minimal to 10=maximum.
Numeric Rating Scale is also used for subjective characteristics (pain, heaviness, itching, etc.) and represents a discrete scale from 0=minimal to 10=maximum.
Leg oedema: summary of principal management techniques and treatments
There are several techniques that have been reported as being effective for the treatment of lower limb oedema, both conservative and invasive (surgical).
Conservative techniques include the use of compression; however, applied and maintained (e.g. bandages/stockings/wraps), used alone or combined with exercise and/or elevation, or with the use of intermittent pneumatic compression (IPC) devices from the authors’ experience will be of benefit to many patients. Compression products with specific properties, reflecting different materials, design and fabrication processes, can be further grouped by elasticity, stretching and the number of layers incorporated into the finished product (117). The effect of different types of compression modalities on chronic oedema has been compared (118), leading to the general conclusion that some compression is better than no compression for the reduction of patient oedema and associated pain. Recently, a review article has been published comparing leg elevation with compression therapy (119). To summarise, the author reported that best practice is to firstly manage any underlying health issues and to reduce swelling using compression (after appropriate assessment) and leg elevation (see Chapters 4 and 8). Whilst it is generally accepted that compression remains the gold standard of management to aid the reduction in swelling and oedema, it is also acknowledged that it may not be suitable for all individuals. When individuals have difficulty elevating their lower limb above heart level (e.g. when supine) due to another existing co-morbidity, there are various appliances that may assist with the patient’s leg elevation needs, ranging from simple straps to mechanical leg raising devices.
IPC has been used for over 40 years in the management of lymphoedema. Although the method of application has evolved during that time (e.g., the development of IPC systems applying compression via multi-chamber sleeves), there has been little consensus regarding optimal treatment parameters or dosage (duration of treatment time). The authors of a systematic review (120) reported low evidence of moderate quality, indicating significant outcomes were achieved with dosage times between 45 and 60 minutes.
It has been reported that aquatic therapy has several proposed benefits for people with lymphoedema; however, prior to the publication date of one paper (121), no systematic review of the evidence for aquatic therapy in lymphoedema management had been conducted. Yeung and Semciw reported that there was a moderate level of evidence of no significant short-term differences in lymphoedema status (as measured by lymphoedema relative volume) between patients who completed aqua lymphatic therapy compared to land-based standard care.
Surgical approaches can be divided into reductive (e.g., liposuction, skin/subcutaneous excision) or physiological (e.g., lymphovenous anastomosis [LVA], vascularised lymph node transfer [VLNT]). Two systematic reviews of the literature analyse the evidence for the effectiveness of surgical interventions for the management of lymphoedema (122, 123). Both acknowledged that there is no cure for lymphoedema, and that conservative management remains the first line approach to manage patients with the condition. Carl et al. (2017) reported that the results of their study indicate that surgical management of lymphedema is effective for all clinical stages (122). They argue that LVA is the most appropriate choice due to its low complication profile and validated success. Although LVA requires at least partially functioning lymph connectors, VLNT can be performed with obstructed lymph channels. Ideally, this determination should be made at a preoperative visit via lymphoscintigraphy. Although VLNT improves lymphatic function in late-stage disease, there are considerable risks. When reported, the rate of complications for VLNT was 30.1%, suggesting that this invasive procedure should be reserved for only severe forms of lymphoedema.
A further systematic review and meta-analysis, published in 2021, reported the results of a consensus conference, the focus of which was the surgical treatment of lymphoedema (123). Although the authors acknowledged that only two RCTs were identified during their extensive literature search, the consensus delegates agreed various consensus recommendations using the GRADE criteria. See summary of consensus statements in Table 9.
Table 9. Summary of consensus recommendations / strength of evidence (GRADE) – modified after Chang et al. (2021) (123)
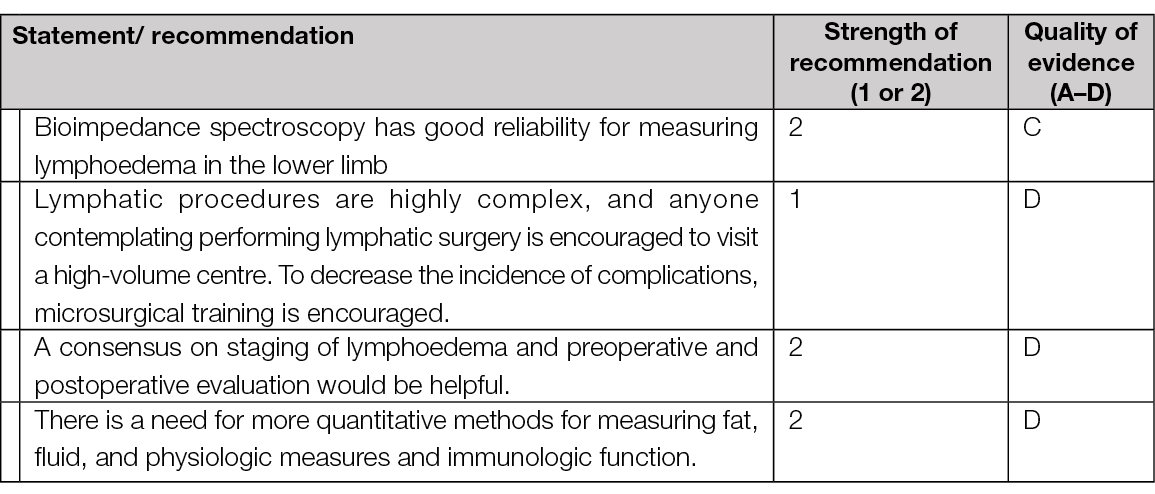
Figure 28. Lymphoedema and papillamotosis in dark skin. Image courtesy of Samantha Holloway, permission granted for inclusion
Guidelines
A literature search to highlight recent systematic reviews of guidelines for lymphoedema identified two papers published in 2020. O’Donnell et al. (124) identified just four clinical practice guidelines (CPGs) – The lymphoedema framework best practice for the management of lymphoedema; A practice guideline for the management of lymphoedema (Japanese Lymphoedema Study
Group); Clinical Resource Efficiency Support Team Guidelines for the diagnosis, assessment and management of lymphoedema and Guidelines of the American Venous Forum. It was concluded that the four CPGs all lacked contemporary literature and were of low study quality. Therefore, the authors argue, it is imperative for vascular societies to develop contemporary high quality evidence-based CPGs for lymphoedema, as they have for other vascular diseases.
Tan et al. (125) also reviewed full text CPGs reporting on evidence-based recommendations in lymphoedema diagnosis or management (in English) and further concluded that no lymphoedema CPG was considered adequate for use in clinical practice. All current lymphoedema CPGs have areas for improvement with elements of methodological quality lacking, particularly with respect to rigour of development. It was also argued that CPGs present a uniform treatment protocol for patients and, consequently, both the effectiveness and the quality of care should be improved if a CPG is adopted for clinical practice. Furthermore, a consequence-driven protocol is usually associated with an overall reduction in the cost of care (124).
6. Atypical wounds
Authors: Elena Conde Montero and Kirsi Isoherranen
Table 10. Types and characteristics of atypical wounds
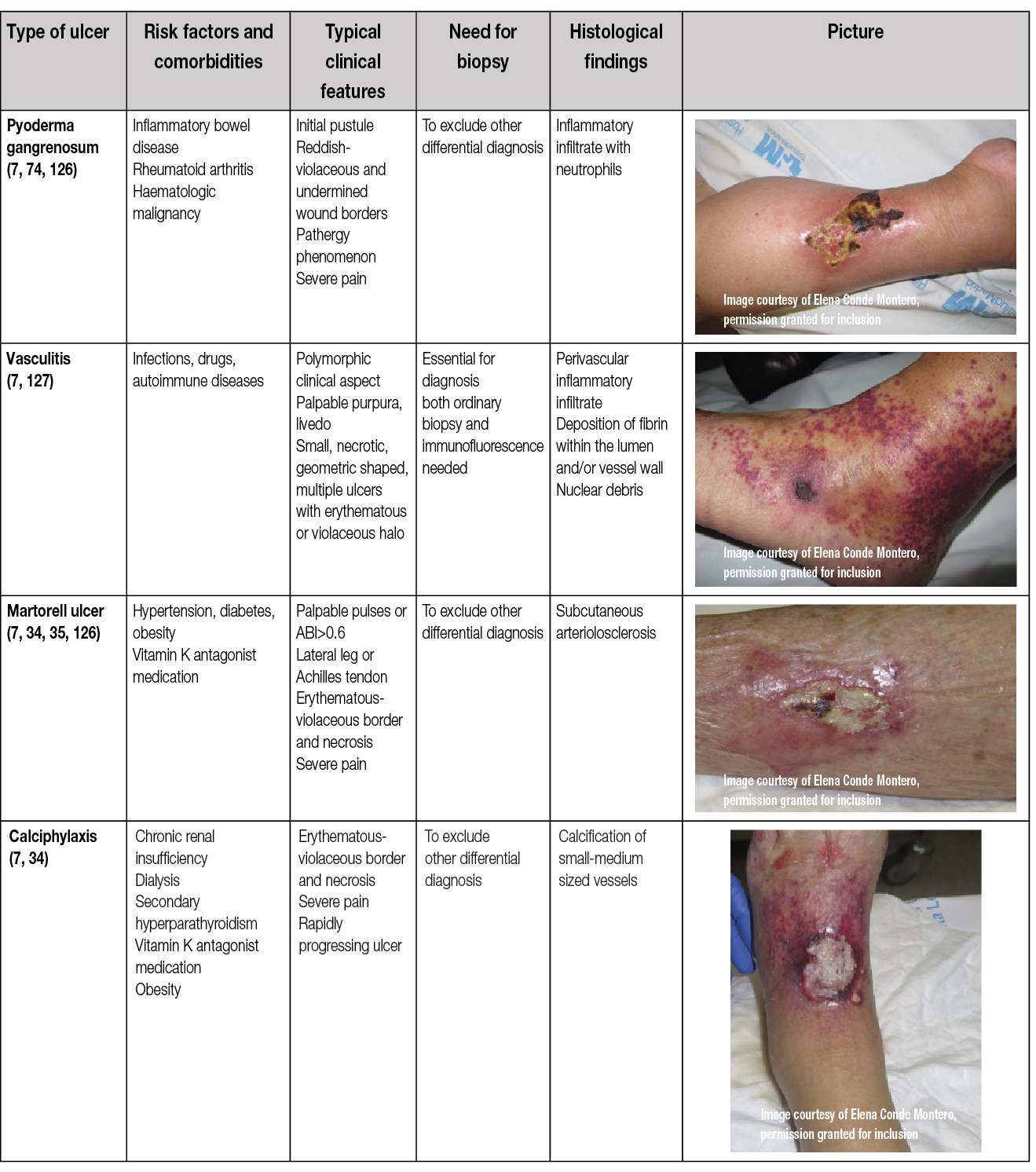
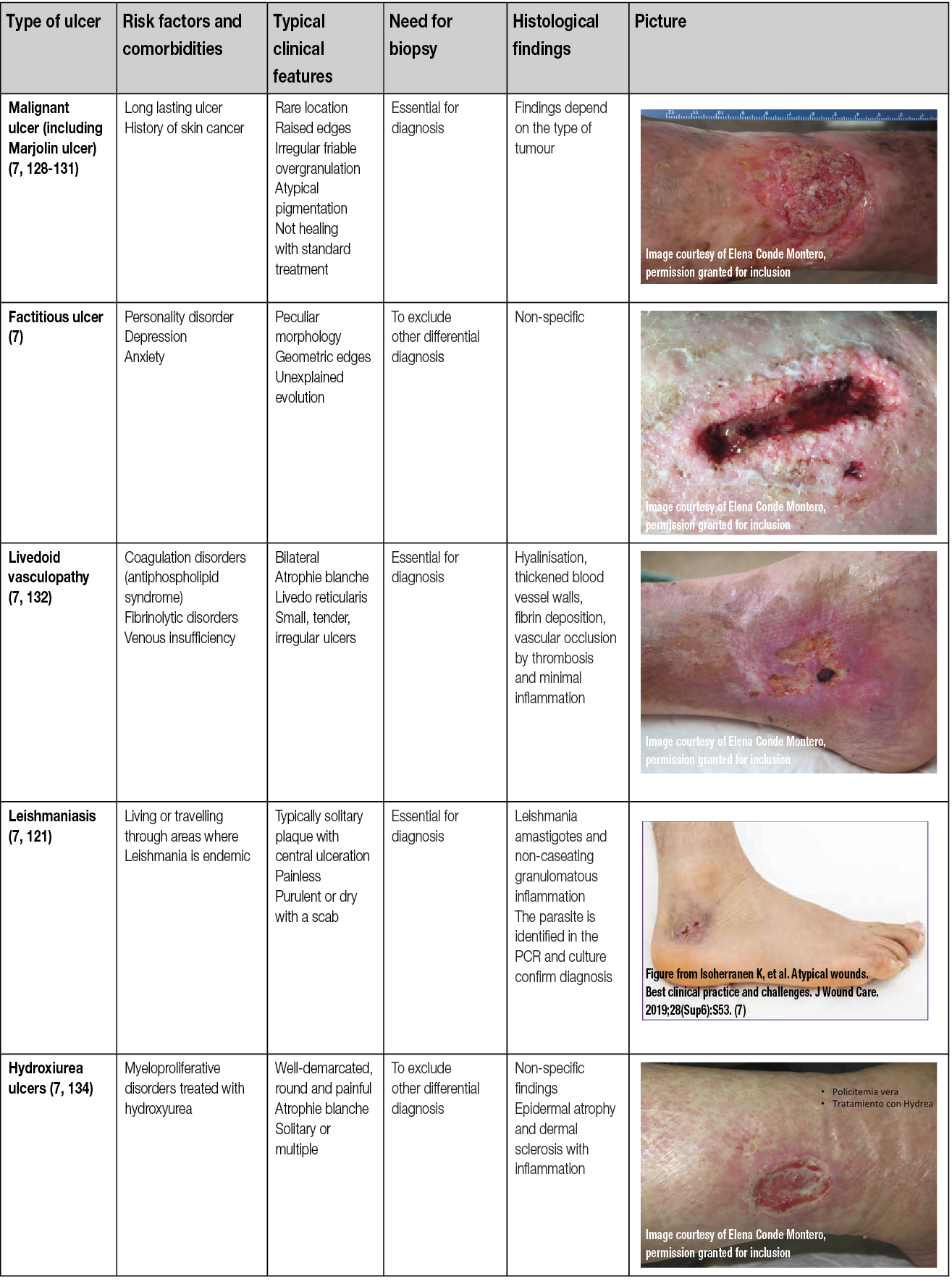
Even if venous, arterial and mixed ulcers are the most common leg ulcers, other less frequent causes may produce wounds in the lower limbs and may be considered ‘atypical wounds’. Consequently, if the wound does not fit within the clinical presentation of the typical leg ulcers, an atypical cause should be suspected (7, 135). However, in addition to an atypical wound on the leg, patients may also have concurrent arterial and/or venous insufficiency. Therefore, vascular evaluation is needed for every patient (7, 135).
Prompt diagnosis of these atypical ulcers is essential to establish an adequate treatment, which will be the key to avoid morbidity and mortality (7, 135).
As shown in Table 10, there are comorbidities and clinical features that can guide us in the suspicion of one of these less frequent causes and make an early referral to a dermatologist.
Skin biopsy may be essential for diagnosis, as in the case of vasculitis or tumour ulcers, and in other cases it may support diagnosis and exclude other differential diagnoses, as in PG or Martorell’s ulcer. Moreover, a biopsy is mandatory to exclude malignancy if a wound that has been initially classified as venous or mixed ulcer does not present signs of healing during 4–12 weeks despite an adequate treatment.
In case of suspecting infectious ulcers, mostly in immunosuppressed patients or travellers from tropical areas, a wide biopsy for tissue cultures including atypical bacteria, mycobacteria and fungi should be taken.
When suspecting Martorell and calciphylaxis ulcers, a wide and deep biopsy should be performed from the edges of the wound.
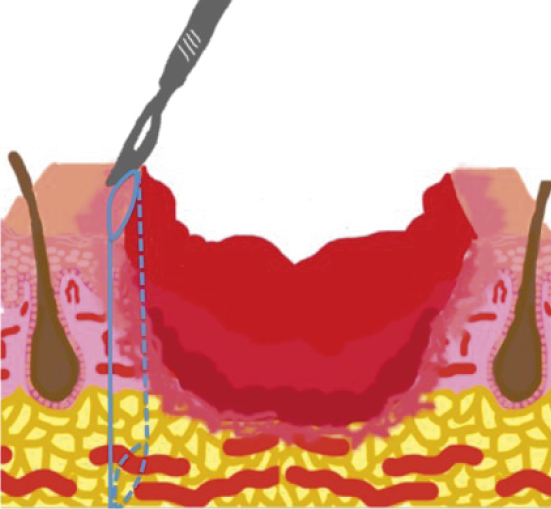
Figure 29. Skin biopsy of the edge of the wound. Figure from Isoherranen K, et al. Atypical wounds. Best clinical practice and challenges. J Wound Care. 2019;28(Sup6):S55. (7)
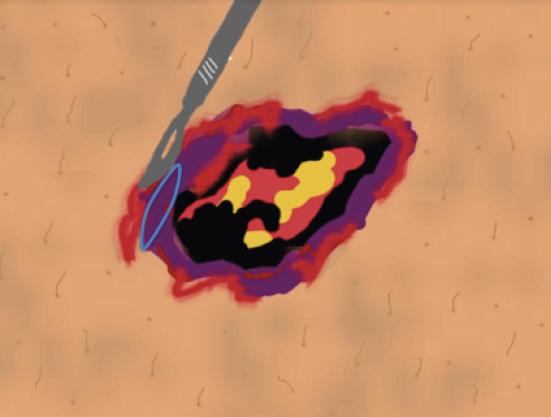
Figure 30. Necrotic tissue should not be included in the biopsy. Figure from Isoherranen K, et al. Atypical wounds. Best clinical practice and challenges. J Wound Care. 2019;28(Sup6):S55. (7)
Punch biopsies can be used if malignancy is suspected and should be repeated if the result is negative but suspicion is high (136).
In the case of vasculitis, in addition to the biopsy specimen, a specimen for direct immunofluorescence (DIF) should be taken. For this, an acute lesion (<24 hours old) should be chosen. Additionally, if vasculitis or vasculopathy are considered, testing should include anti-nuclear antibodies, anti-neutrophilic cytoplasmic antibodies, rheumatoid factor, anticyclic citrullinated peptide, serum protein electrophoresis, hepatitis profile, cryoglobulins, lupus anticoagulant, antiphospholipid and anticardiolipin antibodies, protein C, protein S, factor V Leiden and antithrombin III levels, cryofibrinogens and coagulation profile (137).
The treatment of atypical wounds will be directed at their cause. Once the aetiology has been confirmed, local treatment to promote healing is similar for all wounds, regardless of their cause (7). It is important to highlight that, regardless of the cause (trauma, surgery, vasculitis, PG...), if no contraindication exists, compression therapy, adapted to each patient, seems to be a good anti-gravity and anti-inflammatory treatment for any leg wound, always associated with anti-oedema measures (during rest, legs should be elevated as much as possible).
The document Atypical wounds: Best clinical practices and challenges develops the different types of atypical wounds in more detail (7).
Summary
– In the case of ulcers of rare location, with irregular, raised or hyperpigmented borders, friable overgranulation and absence of response to compression therapy, an atypical cause must be suspected.
– If an atypical ulcer is suspected, skin biopsy for histological, and even microbiological study in infectious aetiologies, will help to guide the diagnosis.
– Regardless of the cause of the wound, compression therapy adjuvant to the aetiological treatment can reduce inflammation and promote healing.
7. Checklist for assessing a patient with a leg ulcer
Author: Kirsi Isoherranen
Many instances, such as airlines, army and maritime transportation have implemented safety checklists to prevent human error (138). Presently, multifactorial wounds represent a major wound category (9), and the patients take more and more medications and have multiple comorbidities, such as diabetes, obesity, depression, hypertension and vascular disease (139, 140). This makes the assessment of a patient with lower leg ulcer challenging, with various items to be considered, and also poses a high risk for neglecting some items important for wound healing. Therefore, checklists can act as a practical, rapid method for a thorough, safe and efficient assessment. In order to be useful and implemented, checklists should be straightforward to use and not too complicated (141).
There are few published checklists regarding the assessment of patients with lower leg ulcers. Most of them focus on specific items (140, 142, 143) and three papers describe a more thorough view (144-146). The most recent article was written by a panel of authors who highlighted that a checklist is not an algorithm nor a guideline, but a simple tool and a guide to be useful for the majority of clinicians (144). This checklist was constructed in a specific checklist form asking simple questions to achieve its goal to develop the assessment process.
On the contrary, several wound assessment tools have been published and a recent systematic review concluded that most of the available assessment tools have indeterminate or inadequate ratings and are based on low to very low quality of evidence (147).
Our author group consisted of a multidisciplinary team of different medical specialities and nurse experts. The aim of our checklist is to highlight the holistic assessment of a patient with a leg ulcer (Table 11). It does not focus in wound assessment per se, but includes also the assessment of periwound skin which often is neglected (148, 149). The checklist does not necessarily assist in making a diagnosis but helps the clinician in the systematic assessment and appropriate management plan. Additionally, based on our document, we have developed an algorithm that also contains recommendations for referral (see the one-pager).
Table 11. Checklist for comprehensive assessment of a patient with a lower leg ulcer (150)
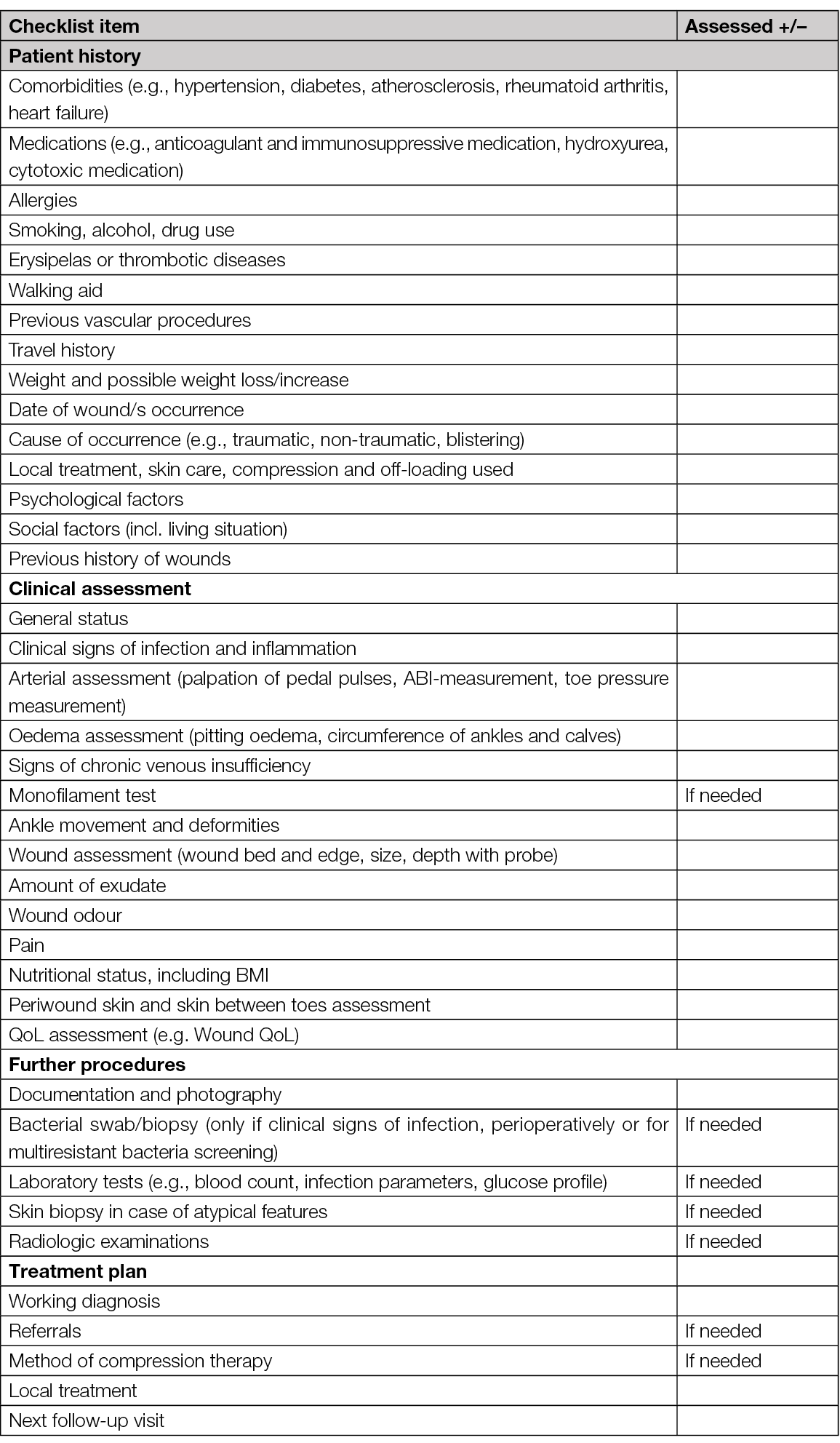
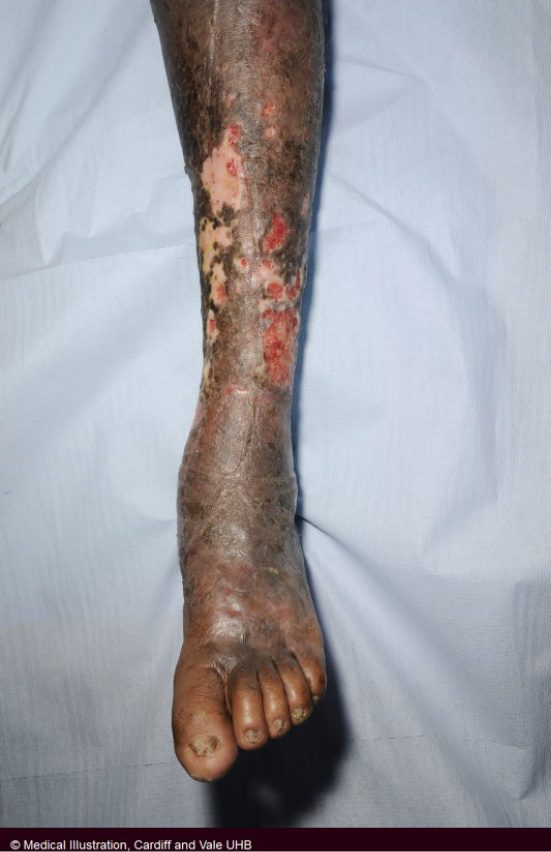
Figure 31. VLU in dark skin. Image courtesy of Samantha Holloway, permission granted for inclusion
8. Compression therapy
Authors: Sebastian Probst, Hayley Ryan
After a database search for systematic reviews about the use of compression therapy for lower leg ulcers, 352 articles were retrieved, but only 15 met our inclusion criteria and were kept for detailed analysis. Most included systematic reviews report data from RCTs, followed by real-world studies or single-arm observational cohort studies. Some of the included studies focused on comparison of different types of compression systems comparing either with no compression or other compression types. The most common primary outcome was wound healing, and QoL and pain as a secondary outcome. Other systematic reviews focused on VLU recurrence, the association of compression with surgery, or physical activity. One included systematic review demonstrated the effect of modified compression therapy (MCT) with or without revascularisation on mixed ulcer healing. We decided to use the RAL classification in this chapter (Table 12).
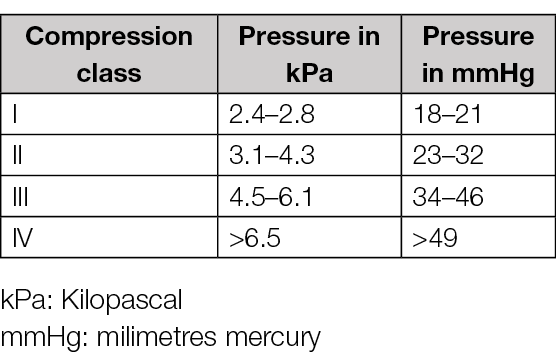
Table 12. Compression classes using the RAL classification
Compression compared to no compression
Two systematic reviews compared the effectiveness of several types of compression using complete healing time and complete VLU healing with no compression (151, 152). Shi and colleagues analysed 14 RCTs comparing some compression types – short-stretch bandage (SSB), 4-layer bandage (4LB) compression and Unna’s boot – with no compression (151). As primary outcomes they investigated time-to-complete wound healing and complete healing. Pooling a hazard ratio from five studies, a shorter time to complete healing (2.17, 95% confidence interval (CI) 1.52 to 3.10; n=84) for patients wearing compression was found. The same systematic review reports in eight studies a better complete healing risk ratio (RR) (1.77, 95% CI 1.41 to 2.2; n=1120) as secondary outcomes pain and patients’ QoL were investigated. A lower pooled mean difference (MD) for pain (–1.39, 95% CI −1.79 to −0.98; n=495) in patients wearing compression was shown in three studies. Two studies (n=426) showed an improvement in patients wearing compression for the disease-specific QoL in a follow-up from 12 weeks to 12 months using the total score of the Charing Cross Venous Ulcer Questionnaire (lower scores=better QoL) (MD=–6.87, 95% CI –13.10 to –0.64). Different types of compression were reported by O’Meara et al., including 48 RCTs including 4321 patients (153). One of the included smaller RCTs compared Unna’s boot (compression) with simple bandages (no compression), resulting in complete healing at 12 months for patients wearing the Unna’s boot (RR=2.30, 95% CI 1.29 to 4.10; P=0.005; n=36). Another RCT showed that SSB had a significant effect on complete healing compared to usual care (percentage of healed patients, 71% vs 25%; n=53). This systematic review also reported data from three trials assessing 4LB. One of them showed significantly more patients with complete healing at 3 months (RR=4.0, 95% CI 1.35 to 11.82; P=0.01; n=36).
Faster healing time was also reported in two other trials. One larger study used median weeks to healing (20 vs 43; P=0.03; n=233) and the other showed a p-value (P=0.006). Another systematic review focusing on QoL (153), demonstrated with eight studies that any VLU treatment (compression therapy, 4LB, 3-layer bandaging (3LB), SSB, advanced wound dressing, and superficial venous surgery) led to an improvement of QoL. Moreover, patients that had superficial venous surgery in addition to compression reported a significantly better QoL than patients with compression only.
Comparison between different compression systems
Comparing the efficacy of different compression bandage types, O’Meara and colleagues reported results from one study which showed that 4LB had better healing at 24 weeks than SSB (RR=0.74, 95% CI 0.59 to 0.92; n=245) and five others demonstrated faster healing for 4LB than for SSB (152). The median days to healing were estimated at 90 for 4LB and 99 days for SSB (hazard ratio=1.31, 95% CI 1.09 to 1.58). Three trials compared three-component systems containing an elastic component with the same system without elastic part. Two studies showed that elastic bandages healed significantly more ulcers at 3 to 4 months (RR=1.83, 95% CI 1.26 to 2.67), but another study observed no difference at 6 months (P=0.67). Four studies (n=317) showed that high-compression stockings are associated with better VLU healing outcomes than SSB at 2 to 4 months (RR=1.62, 95% CI 1.26 to 2.10). Similarly, another review pooled eight studies together and demonstrated that ulcer healing was better in the stockings group than SSB (pooled RR=1.33, 95% CI, 1.02–1.74) (156). This same review also demonstrated that there was a significantly lower median time to ulcer healing for 3LB (2.8 months) than for 4LB (3.7 months; P=0.04). Mauck and colleagues found 15 trials comparing long-stretch bandages (LSBs) and SSBs, but pooling the studies together (pooled RR=0.98; 95% CI 0.91–1.06), they saw no significant difference in healing outcomes (154).
Another review compared the efficacy of a 2-layer bandaging (2LB) compression system with other compression bandages, presenting five studies (155). At six months, a study demonstrated better ulcer healing for 2LBA compared to another 2LBB (odds ratio (OR)=1.57; 95% CI, 1.10–2.24; P=0.03) and to a 4LB (4LBA) (OR=1.93, 95% CI, 1.26–2.97, P=0.05). Real-world studies showed that 2LBA resulted in a significantly shorter healed time at 6 months (MD, months=–0.40, 95% CI, –0.74 to –0.06; P=0.02 (2LBs); MD=–0.50, 95% CI, –0.86 to –0.14; P=0.007 (for 4LBs). However, no significant difference between the groups was found. This result was maybe due to the presence of only newly diagnosed VLU in the real-world studies, but the presence of both old and new VLU in the study population, thus hard-to-heal VLU could have also been present. As a secondary outcome, 2LBA was also linked to a better health-related quality of life (HRQoL) (MD, months=0.02, 95% CI, 0.02 to 0.02; P<0.0001 (2LBs); MD, months=0.02, 95% CI, 0.01 to 0.03; P=0.003 (4LBs). Another review found a tendency for faster healing with 4LB compared with 2LB. However, the QoL would seem to be improved rather by 2LB than 4LB (156). Finally, Welsh investigated the impact of bandage systems containing both elastic and inelastic components, called mixed component systems (157). She found no significant difference in ulcer healing rates compared to alternative compression systems like 4LBs (P=0.3).
Effects of compression on VLU recurrence
VLU recurrence was investigated in two reviews (158, 159). One trial showed a significantly lower recurrence rate at 12 months in people that wore compression stockings (RR=0.43, 95% CI, 0.27–0.69; P=0.001). One study, investigating the lipodermatosclerosis area, observed a significantly lower area in the stocking group at 12 months (−33.1, 95% CI, −61.9–15.07) compared to without stocking (+11.9, 95% CI, −24.6 to 122.2; P=0.04). A trial also showed that treatment adherence reduced the risk of recurrence 6 times. Nelson et al. presented four RCTs, and one trial demonstrated significant lower recurrence rates with compression than without at 6 months (RR=0.46, 95% CI 0.27 to 0.76) (158). One trial presented a better efficacy of high compression stockings than medium ones in recurrence at 3 years (RR=0.57, 95% CI 0.39 to 0.81). Nevertheless, medium pressure stockings (class II) showed better compliance than the high ones (class III).
Similarly, Dahm and colleagues reported results from studies investigating recurrence (160). One study showed a reduction in leg ulcer recurrence at 12 months for class II stockings (medium pressure) compared to class I (mild pressure) (RR 0.52; 95% CI 0.30 to 0.88). Another study compared class II and class III (medium to high pressure) stockings, but saw no significant difference on recurrence rates after 6 months (RR 0.64; 95% CI 0.20 to 2.03). Finally, a trial showed lower recurrence with class III stockings compared with no compression at 6 months (RR 0.46; 95% CI 0.27 to 0.76) and 12 (RR 0.43; 95% CI 0.27 to 0.69). Mauck and colleagues found one study showing that fewer ulcers recurred when wearing stockings (24/167) compared to 4LB (41/176) (HR 0.56; 95% CI 0.33–0.94; P=0.03) (154). The authors conclude that these results may be related to better compliance with lower compression.
Compression combined with physical activity
We found two reviews assessing the combination of physical activity with compression (161, 163). Jull and colleagues pooled a risk difference for any type of exercise from five RCTs (n=190) and found that there were 14 cases healed per 100 patients at 12 weeks (RD=14%, 95% CI, 1–27%; P=0.04) (164). Among these five trials, two studies assessed the effectiveness of progressive resistance exercise (different sets of heel raises), but did not find a significant result (RD=−6%; 95% CI, −32% to 21%; P=0.67, n=53). Two trials assessed prescribed physical activity with resistance exercise. One trial was conducted in a sport facility and the other had 30 minutes of walking three times per week as a physical activity. The exercises in facilities were calf raises, partial squats and 30 minutes of aerobic exercises three times per week and showed significantly more healed patients at 12 weeks. The pooled risk difference for both studies was significant even if the one not conducted in a facility did not present significant results alone (RD=27%; 95% CI, 9% to 45%; P=0.004). A trial also demonstrated that ankle exercises provided a significant median ulcer area change of 1.67cm2. Concerning the review of Smith et al., they found six RCTs looking at the effect of exercise on VLU healing (161). Three studies together showed no significant results in doing a progressive resistance exercise program in addition to compression (RR=1.14, 95% CI 0.71 to 1.84; n=116 participants) and the same trials also showed no difference in QoL (MD=3 points better on 100 points scale with exercise, 95% CI −1.89 to 7.89; n=59 participants). They found one study showing that exercise may increase risk of adverse events (OR 1.32, 95% CI 0.95 to 1.85, one RCT; n=40).
Compression combined with endovenous interventions
One of the reviews looked at the combination of surgery with compression (163). De Carvalho and colleagues found four RCTs regarding this topic (163). After 24 weeks, two studies showed no significant difference of surgery in addition to compression (P=0.85; n=500). However, 428 patients who had their ulcers treated within the 24 weeks showed significant lower recurrence rates at 12 months when they had surgery in addition to compression (12% vs 28%; P<0.0001). These rates were still significant after 4 years (31% vs 56%; P<0.001). Another study (n=103) observed significant longer ulcer-free periods during the follow-up of the surgery group (62% vs 33% in the conservative group; P=0.02). This follow-up consisted of 36 months divided in intervals of 3 months.
Another review presented a RCT that showed a healing rate of 85.6% for early endovenous ablation of superficial venous reflux combined with compression and 76.3% (p=0.001) in the compression group with a deferred surgery (n=450) (164). The median ulcer-free time was 306 days for the early ablation group and 278 days for the deferred one (p=0.002). In addition, more patients healed their ulcers with the early intervention (hazard ratio for healing, 1.38; 95% CI, 1.13 to 1.68; p=0.001).
Another study investigating USGFS showed that patients who had USGFS in addition to compression healed at a faster rate than those only treated with compression (9.7% vs 4.2%; p=0.001). A trial investigating recurrence rate showed that during a 4-year period, patients that had surgery and compression (31%) had less chance to have recurrence than those with compression only (56%; p<0.001). This change in recurrence rate was demonstrated through other studies shown by Elstone, where either surgery or USGFS showed better recurrence rate or longer ulcer-free time (164). Tollow and colleagues showed that patients who had had superficial venous surgery in addition to compression reported a significantly better QoL than patients with compression only (153).
Compression for mixed ulcers
We found one review investigating treatment for the mixed ulcers and arterial ulcers (MAVLU), i.e. VLUs with coexisting PAD, and presented the effect of the MCT with or without revascularisation depending on patients ABI (85). Lim and colleagues showed that MCT with a pressure between 20 and 30mmHg can promote healing in mixed ulcers when moderate arterial insufficiency is present (0.5 ≤ ABI ≤0.8), but if ABI is <0.5 (arterial ulcers), MCT can only be considered when acceptable ABI is achieved (87). They showed a study comparing healing rates of VLU and MAVLU with MCT. This trial presented similar healing rates between patients with MAVLU (60%; n=24) and VLU (65%; n=20). Lim and colleagues found several studies presenting similar healing rates between VLU and MAVLU when they are treated with MCT (87). Another study showed that 33 limbs with moderate peripheral arterial occlusive disease (PAOD) (ABI of 0.5–0.85) were treated with MCT at a compression of 30mmHg compared to 13 limbs with severe PAOD (ABI<0.5) that were treated with arterial revascularisation. After 36 weeks, it resulted in a healing of 64% (n=21) for patients treated with MCT who presented moderate arterial disease, and 23% (n=3) of patients with severe PAD healed.
Intermittent pneumatic compression (IPC)
IPC was the topic of one identified systematic review (165); nine RCT were included in this review assessing the impact of IPC on the healing of patients with VLU. In one trial (80 people), more ulcers healed with IPC than with dressings alone (62% vs 28%; p=0.002). Five trials compared IPC plus compression with compression alone. Two of these (97 people) found increased ulcer healing with IPC plus compression than with compression alone. The remaining three trials (122 people) found no evidence of a benefit for IPC plus compression compared with compression alone. Two trials (86 people) found no difference between IPC (without additional compression) and compression bandages alone. One trial (104 people) compared different ways of delivering IPC and found that rapid IPC healed more ulcers than slow IPC (86% vs 61%). The authors’ conclusions were that IPC may increase healing when compared with no compression, but it is unclear whether it can be used instead of compression bandages. There is some limited evidence that IPC may improve healing when added to compression bandages.
Summary
The search for systematic reviews for the use of compression for lower leg wounds retrieved 352 articles, but only 15 were kept for detailed analysis. The included reviews mainly reported data from RCTs and focused on the effectiveness of different compression types for VLUs compared to no compression or other compression types. Most reviews demonstrated that compression therapy improves complete healing time and complete healing of VLUs compared to no compression. Additionally, any VLU treatments (including compression therapy) lead to an improvement of QoL, and patients who had superficial venous surgery in addition to compression reported significantly better QoL than patients with compression only. Furthermore, some studies showed that high-compression stockings and 3LB were associated with better VLU healing outcomes than SSBs.
The available evidence from systematic reviews supports the use of compression therapy for the treatment of VLUs, with high-compression stockings and 4LB showing better outcomes than SSBs. However, more studies are needed to determine the optimal compression type, duration, and frequency of use for leg wounds. In addition, most studies use complete healing as their primary outcome. A clear definition of healing was lacking. Future research should also focus on understanding the mechanisms behind compression therapy and its cost-effectiveness, as well as exploring patient perspectives and strategies to improve adherence to treatment regimens. Overall, compression therapy should be used, if there are no contraindications, from the first visit for the management of lower leg ulcers.
In evidence other than systematic reviews and clinical practice it has been shown that along with the outlined compression bandages and stockings, also adjustable compression wrap devices using hook and look fasteners can be used. They also present an opportunity for improving treatment outcomes, supporting patient independence and self-management in the use of compression therapy. However, further research is necessary to optimize their effectiveness and ensure their widespread adoption in clinical practice.
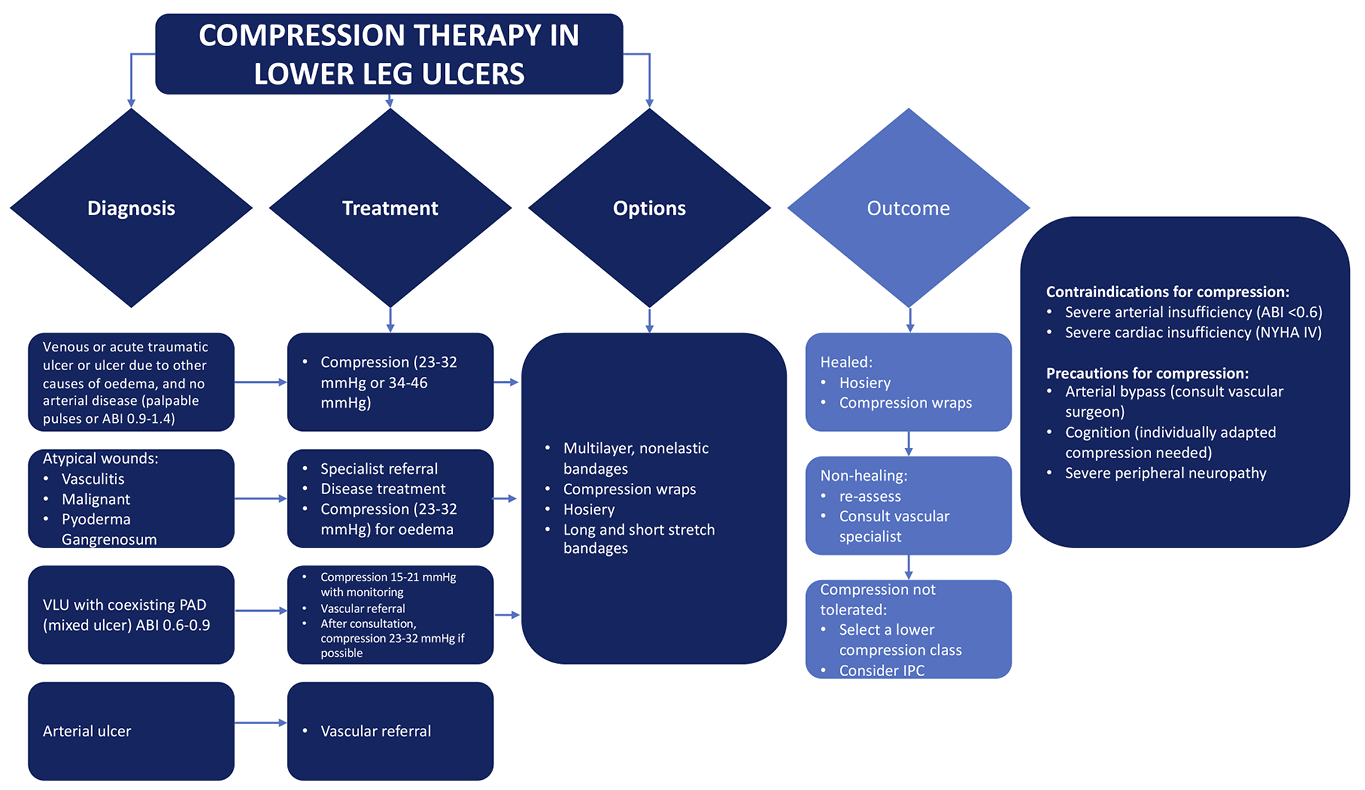
Figure 32. Compression therapy in lower leg ulcers
9. Local treatment of lower leg ulcers
Authors: Sebastian Probst, Leanne Atkin
This chapter discusses the local treatment of lower leg ulcers. Local treatment involves a series of procedures including dressing selection and monitoring. There is big variety in methods of local treatment of lower leg ulcers available. This is due not only to the many different aetiologies of such lower leg ulcers but also to the widely varying preferences of physicians and nurses, and to the lack of strong evidence and relevant guidelines regarding the most appropriate form of local wound treatment. For healable wounds, local wound treatment interventions can range anywhere along the spectrum from maintaining a moist environment to sharp debridement (166). On the other hand, treatment of maintenance and non-healable wounds is focused on selective debridement or manage infection, exudate or odour (166). In the following section, a synthesis of systematic reviews discussing the local treatment of lower leg ulcers is outlined. Excluded was compression therapy (see Chapter 8).
Wound cleansing and debridement in lower leg ulcers
Only two systematic reviews were identified addressing the topics of wound cleansing and debridement of lower leg ulcers. McLain and colleagues assess in their work the effects of wound cleansing, wound cleansing solutions and techniques for treating VLUs (167). The results show that it is uncertain whether aqueous oxygen peroxide makes any difference to change in ulcer size after 8 weeks (MD –1.38cm2, 95% CI –4.35 to 1.59 cm2) or 12 months (RR 1.88, 95% CI 1.10 to 3.20) when compared with sterile water. No difference in wound size reduction was also observed comparing propyl betaine and polyhexanide. The same results are shown when comparing octanedione dihydrochloride/phenoxyethanol with Ringer’s solution (RR 0.96, 95% CI 0.53 to 1.72). The authors concluded that there is insufficient evidence to demonstrate whether the above-mentioned solutions make any difference in the treatment of VLUs.
A similar conclusion was drawn by Gethin, Cowman and Kolbach (168). They were determining the effects of different debriding methods on the rate of debridement and wound healing in VLUs which included 10 RCTs with 715 participants. The review shows that 80% of the ulcers treated with dextranomer beads and 14% treated with Edinburgh University Solution of Lime (EUSOL) achieved complete debridement (RR 5.71, 95% CI 2.84 to 11.52). Using hydrogel (76%) was also effective compared to the use of paraffin gauze (45%) (RR 0.67, 95% CI 0.45 to 0.99). One study (n=48) reported that by 12 weeks, 15/18 (84%) ulcers treated with biocellulose wound dressing had achieved a 75% to 100% clean, granulating wound bed versus 4/15 (26%) treated with non-adherent petrolatum emulsion-impregnated gauze. A reduction in wound size was assessed comparing cadexomer iodine with paraffin gauze (MD 24.9cm², 95% CI 7.27 to 42.53, P value 0.006) and hydrocolloid compared to paraffin gauze (MD 23.8cm², 95% CI 5.48 to 42.12, P value 0.01).
Wound dressings in lower leg ulcers
Wound dressings support the healing process while promoting an optimal environment. To select the optimal wound dressing a holistic approach is needed. Several systematic reviews were identified focused on different wound dressings. One systematic review included five RCTs including 295 participants aiming to compare alginate dressing in combination with compression compared with other dressings (169). One RCT included 20 participants comparing different alginate dressings, three RCTs included 215 participants comparing alginate and hydrocolloid dressings, and one RCT with 60 participants compared alginate and plain non-adherent dressings. All studies show that there is no statistical significance between the alginate group with the comparison (169). A meta-analysis was feasible for one comparison (alginate and hydrocolloid dressings), with data from two RCTs (n=84) pooled for complete healing at 6 weeks (RR 0.42 (95% CI 0.14 to 1.21) (170). The authors conclude that alginate dressings are more or less effective in the healing of VLUs than hydrocolloid or plain non-adherent dressings, and there is no evidence to indicate a difference between different proprietary alginate dressings.
The effects of topical hydrogel wound dressings on the healing of VLU were studied by Ribeiro and colleagues (171). The systematic review included four RCTs (272 participants) comparing hydrogels to a wide variety of wound dressings including gauze and saline, alginate dressing, manuka honey and hydrocolloid. The authors conclude that there is inconclusive evidence to determine the effectiveness of hydrogel dressings compared with gauze and saline, alginate dressing, manuka honey or hydrocolloid on VLU healing. One of the limitations is that different dressing categories were compared with hydrogel. Similar conclusions were made when reviewing the use and effects of topical honey dressing on VLU healing (172); they were not able to determine any significant effect of use due to the small number of papers, all of which were of low-quality evidence. The evaluation of the impact of foam dressings on VLU healing was also unable to find evidence of effect, reporting that there was no difference in healing outcome when hydrocellular foam dressings and polyurethane foam dressings were used compared to a variety of wound dressings (paraffin gauze, knitted viscose, hydrocapillary dressings or protease modulating matrix (PMM) dressings). Pooled data across five RCTs (418 participants) showed no statistically significant difference between foam dressings and hydrocolloid dressings in the proportion of ulcers healed at 12 to 16 weeks (RR 1.00, 95% CI 0.81 to 1.22).
The use of antimicrobials in lower leg ulcerations was studied in four systematic reviews. Norman and colleagues (170) included 78 RCTs with 7014 participants assessing the effects of dressings and topical agents for healing of VLUs in any care setting. They show that the included evidence was of low certainty. The authors argue that this low certainty was continued when the results were considered by ranking the treatments in terms of the probability that they were the most effective for lower leg ulcer healing, with many treatments having similar, low, probabilities of being the best treatment. The two most highly ranked treatments (with a 50% probability) were sucralfate and silver dressings. It has to be taken in consideration that the sucralfate study was small. If sucralfate and silver dressings were compared with other dressings, there was some evidence that silver dressings may increase the probability of VLU healing, compared with non-adherent dressings (RR 2.43, 95% CI 1.58 to 3.74). Otherwise, the literature is unclear whether the intervention (antimicrobial dressing) increased the probability of healing. A systematic review assessing the evidence supporting the use of dialkylcarbamoyl chloride (DACC)-coated dressings in the clinical environment was performed (173). The authors show data from a pilot-RCT comparing the effects of DACC-coated dressings and silver impregnated dressings in chronically infected or heavily colonised leg ulcers of vascular origin and a cohort study including 19 patients (20 wounds) with chronically infected vascular ulcers. Both studies demonstrate a statistically significant reduction (p<0.01) of bacterial load and a positive outcome in relation to wound size reduction.
Another systematic review assessing the effects of the use of systemic antibiotics, topical antibiotics and antibiotics on VLU healing reported by 45 RCTs using 53 comparisons (n=4486) show that more participants were healed when they were prescribed levamisole (systemic antibiotics) compared with placebo: RR 1.31 (95% CI 1.06 to 1.62) (174). No between-group differences were detected in terms of complete healing for other comparisons (other antibiotics). However, the same systematic review highlighted that more participants were healed when given cadexomer iodine compared with standard care. The pooled estimate from four RCTs for complete healing at 4 to 12 weeks was RR 2.17 (95% CI 1.30 to 3.60). No between-group differences in complete healing were detected when cadexomer iodine was compared with hydrocolloid dressing, paraffin gauze dressing, dextranomer, and silver-impregnated dressings. The same systematic review demonstrates there were no between-group differences in complete healing detected comparing the use of povidone-iodine compared with hydrocolloid, moist or foam dressings or growth factors. Additionally, it was shown that the use of peroxide-based preparations when compared with usual care for surrogate healing outcomes (change in ulcer area). Using honey-based products compared with usual care showed no difference in time to healing or complete healing. Moreover, there were no between-group differences in complete healing observed when using 1% silver sulphadiazine ointment compared with standard care/placebo or tripeptide copper complex or different brands of silver-impregnated dressings or when silver-impregnated dressings were compared with non-antimicrobial dressings. O’Meara et al. showed further that more participants healed at 4 weeks when treated with an enzymatic cleanser (a non-antibiotic preparation) compared with a chloramphenicol-containing ointment (additional active ingredients also included in the ointment): RR 0.13 (95% CI 0.02 to 0.99) (174). The use of an antiseptic ointment (ethacridine lactate) was responsive (defined as >20% reduction in area) at 4 weeks when compared with placebo: RR 1.45 (95% CI 1.21 to 1.73). However, the authors concluded that there is no evidence available to support the routine use of systemic antibiotics in promoting healing of VLUs. A systematic review by Broderick and colleagues (175) determined whether topical agents and wound dressings affect healing in arterial ulcers. It compared healing rates and patient-centred outcomes between wound dressings and topical agents. The results show that there is an accelerated wound healing in the 2% ketanserin ointment in polyethylene glycol group. Another topical agent studied was the application of blood-derived concentrated growth factor compared with polyurethane film or foam. Two studies were found and in both studies the sample size was small, the reported results were inadequate, and the methodological quality was rated low. However, the results show that 66.6% of patients with a diabetic arterial ulcer (6/9) receiving blood-derived concentrated growth factor showed more than a 50% decrease in ulcer size compared to 6.7% (2/30) of patients with non-healing ulcers treated with standard dressing.
Topical agents or wound dressings to manage wound-associated pain
The use of topical agents or wound dressings for the management of patients’ pain from VLU was explored in a systematic review by Briggs et al. (176). The review found six studies (involving 343 participants) evaluating the use of local lidocaine cream (EMLA: Eutectic Mixture of local anaesthetic [Lidocaine/Prilocaine]) to help manage procedural pain (debridement). They found a statistical difference in pain score in favour of EMLA cream (MD –20.65, 95% CI –12.19 to – 29.11). Additionally, they reviewed two studies (470 participants) assessing the use of ibuprofen slow-release foam dressings for persistent VLU pain. Compared to local best practice, one paper reported significant benefits in total maximum pain relief scores, but the second paper reported no significant difference between the two groups. The conclusion of this systematic review was that EMLA cream provides effective pain relief during debridement and that there was some evidence to suggest that ibuprofen dressings may offer pain relief to people with painful VLUs, but further research was needed to assess the true impact.
Autologous platelet-rich plasma (PRP) in lower leg ulcerations
The use of autologous platelet-rich plasma (PRP) promoting wound healing was assessed by Martinez-Zapata et al. (177). The results reported on wounds of the lower leg (VLU) show that it is unclear if autologous PRP affects healing (RR 1.02, 95% CI 0.81 to 1.27).
Ultrasound therapy
The impact of local ultrasound therapy on VLU healing was reviewed by Cullum and Liu (178); their systematic review identified 11 research papers (10 of which were judged as being at an unclear or high risk of bias). There were nine trials which evaluated high-frequency ultrasound, seven provided data for ulcer healing, and two showed data on ulcer change size with two trials which evaluated low frequency ultrasound; both reported ulcer healing. They concluded that it was uncertain whether therapeutic ultrasound (either high or low frequency) improves the healing of VLUs due to low and very low-quality evidence.
Electromagnetic therapy (EMT)
One systematic review was identified investigating the impact of electromagnetic therapy (EMT) on the healing of VLUs (179). There were three RCTs identified, and all compared the use of EMT with sham-EMT. Two trials reported ulcer healing rates; one small trial (n=44) reported that significantly more ulcers healed in the EMT group than the sham-EMT group; however, this result was not robust due to the assumptions made about the outcomes of participants who were lost to follow-up. The second trial that reported numbers of ulcers healed found no significant difference in healing. The third trial was also small (n=31) and reported significantly greater reductions in ulcer size in the EMT group but did not report overall healing data. It remains unclear whether EMT influences the rate of healing of VLUs.
Protease modulating matrix (PMM) treatments
A systematic review by Westby et al. (180) aimed to determine the effect of PMM treatments on the healing of VLU; it included 12 studies (784 participants). Nine of the included studies compared PMM treatments with other treatments and reported healing as primary outcomes, seven of which recruited participants described as having ‘non-responsive’ or ‘hard-to-heal’ ulcers. Comparators were other types of wound dressings, and in all studies PMM was used as an adjunct to standard compression therapy. They reported that there was uncertainty whether PMM dressing regimens heal VLUs quicker than non-PMM dressing regimens (low-certainty evidence from one trial with 100 participants) (HR 1.21, 95% CI 0.74 to 1.97) and it was unclear whether PMM dressing regimens influence venous ulcer healing in comparison with to dressing regimens without PMM activity.
Summary
The synthesis of the systematic reviews (1A) shows that there is not enough evidence that one wound dressing would be superior to another neutral dressing when chosen according to wound bed and exudation. However, when consulting PROSPERO and clinical trials.gov there are promising ongoing publications, systematic reviews and clinical trials, to manage exudate in lower leg ulcerations (181, 182). Nevertheless, more methodologically robust clinical trials are needed to close this gap. The authors therefore conclude that the characteristics of the wound (for example, exudate, odour and/or pain) should be the deciding factor in choosing the most appropriate dressing for treating a lower leg ulceration (1A).
10. The patient perspective
Author: John D. Ivory
Sackett et al. defined evidence-based medicine (EBM) in 1996 as “the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients”, and wrote that in order to practise EBM, integration of systematically obtained, best available external clinical evidence and individual clinical expertise is necessary (183). Today, EBM incorporates patient values and expectations as a third and equally important foundational pillar and it manifests in the wound care community as part of the framework for the wound bed preparation paradigm (166, 184). The paradigm stresses the importance of patient involvement and asks that in addition to identifying and treating the cause of a wound, determining its ability to heal and providing local care, clinicians also address patient/family centred concerns which can include, among others, support structures, patient education and patient empowerment (166). A synthesis of qualitative evidence from 2017 concluded that treatment plans should include coping, wellbeing and symptom management through person-centred, holistic care (185). Indeed, Moore et al. stressed that to ensure optimal functioning of a universal model for team management of wounds, patient needs are a primary consideration and the patient should play a central role in all decision-making (186). We conducted a literature review to illustrate and summarise some of the areas where patient perspectives have been explored in wound care research during the last 10 years.
Lived experience
Living with chronic wounds is difficult, often to the point of devastation, and the adverse effects influence multiple areas of a patient’s life including finances, QoL, wellbeing, professional and social interaction (187-190).
Measurement of individual perception of disease by the WHOQOL BREF-IPQ short questionnaire about perception of disease in a Croatian sample of 100 persons aged 31–89 with chronic wounds/lower limb ulceration found that, in their opinion, their lives were significantly affected by their condition. It had multiple symptoms that affected them, and it could last for a relatively long time. They also thought that they controlled their condition relatively well and considered treatment to be helpful. These patients were very concerned about their disease and thought that it affected their emotional states moderately (191).
A Brazilian qualitative study incorporated a Symbolic Interactionism framework which reflects how human beings interact, interpret, define and act in their day-to-day lives according to the meaning they attribute to an experienced situation. The study methodology was based on Bardin’s content analysis. From interviews of five individuals, four elderly and one aged 45 years with VLU, themes of having limitations, of feeling pain and discomfort and feeling fear emerged. On a more positive note, study participants expressed having faith and were expectant of a cure. The phenomenon in its entirety was characterised as living in a difficult situation (192). The theme of hope and belief that healing would occur also emerged in 65 (81%) participants in a mixed-methods Portuguese study of 81 individuals with venous, arterial, mixed ulcers, lymphoedema-related, vasculopathic and ‘other’ aetiology ulcers (187). However, chronic leg ulcers had large effects on QoL in 14 (18%) participants. They were life changing events that affected professional, social and functional aspects of their lives with high frequency, and the changes resulted in feelings of sadness, depression, anger and loneliness in more than 30% (>24) of participants. Other feelings included those of shame and of being a burden to others. Pain and permanent presence of bandages were found to be the most distressing physical factors. Subsequently, work from Denmark using a phenomenological-hermeneutical approach that included semi-structured interviews and a focus group interview uncovered themes of “constant pain without the possibility of relief”, an “eternal battle to survive against the ulcer”, “living in a state between despair and hope” and the sense that “the ulcer controlled every-day life” in a cohort of six patients with arterial or mixed venous-arterial leg ulcers aged between 65–77 years. Patients used adjectives such as “terrible”, “horrible”, “painful” and “dreadful” to describe life and expressions such as “It’s been hell, like a living hell”. When combined, the themes, adjectives and expressions gave an overall finding of “A life with a leg ulcer is a living hell” to the study (193).
Recurrence
Given the sobering picture of life with a chronic wound as presented by patients themselves, one can intuit that ulcer recurrence and fear of recurrence must be a source of considerable concern.
In a sample of 145 Swiss VLU patients with ages ranging from 41–95 years, 19 (13.1%) had an ulcer recurrence after 3 months while 48 (33.1%) had a recurrence between 6–9 months (194, 195). Self-efficacy, social support and HRQoL were assessed in this study, but there was no association observed between those variables and ulcer recurrence. 17 (24.7%) of the study participants agreed to be interviewed. 13 (76.5%) of these reported that their recurrence was due to carrying out sudden, incautious moves in conjunction with a lapse in concentration which led to a fall or hitting the leg against an object. All 17 participants spoke about strategies to prevent accidental damage to the skin such as wearing compression stockings and skin hydration, and all wanted to avoid professional help with applying stockings to maintain autonomy. Three (17.6%) participants employed prevention strategies only when clinical signs such as oedema or dry skin presented. Two (11.8%) male participants preferred to take hot and cold showers and avoid stockings, thinking that they were only used by women. All participants worried about a recurring ulcer becoming chronic. Pain caused by wearing stockings was an issue and, in addition, prescribed stockings were thought to be visually unappealing and, when poorly fitting, capable of irritating the skin and causing ulcer recurrence.
Adherence to treatment
According to the World Health Organization (WHO), non-adherence to treatment estimates is as high as 50% among those living with chronic illnesses, and within the wound care community adherence can be particularly challenging (196).
In a Brazilian qualitative study based on Schutz’s social phenomenology using concepts of the social motivation theory as a guiding principle, 12 participants aged between 45–70 years with VLU were interviewed (197). Their perceptions of adherence to treatment were encapsulated in the following categories: “beliefs and personal and social activities hindering adherence to care” in which they found it difficult to maintain rest, appropriate nutrition and compression therapy and would adjust care according to their own beliefs and social activities, “being protagonists in the care relationship with venous ulcers” in which they felt dissatisfied with the way they were approached by clinicians in regard to having their needs met, and “motivation for adherence to care” in which they wanted improvement to their QoL.
Contemporaneously, semi-structured interviews were carried out with 31 participants aged between 39–89 years with VLU participating in an Australian RCT of aspirin as an adjunct to compression therapy (198). Analysis of the interviews guided by the theoretical domains framework revealed the following factors as influential to treatment adherence: understanding the management plan and its rationale (knowledge domain), able to wear compression (beliefs about capabilities domain), remembering self-care instructions (memory, attention and decision making domain), awareness of the consequences of failure to wear compression (beliefs about consequences domain), feeling overwhelmed because it’s not getting better (emotions domain), body image issues associated with compression (social influences domain), patience and persistence (behavioural regulation domain), discomfort associated with wearing compression in hot weather (environmental context and resources domain) and costs associated with compression (environmental context and resources domain).
In terms of recurrence and adherence to recurrence prevention measures, 71 VLU patients aged between 34–91 years responded to a survey carried out in seven outpatient clinics in New York and western Pennsylvania (197). Forty-one (73.2%) participants said they wore their compression stockings every day. Sixty (89.6%) participants reported that they received adequate discharge instruction on leg care, although approximately one in five would like to have received more comprehensive information. Fifty-eight (84.1%) received enough information to facilitate use and care of compression stockings. Thirty-three (47.1%) participants could recall verbal information and 34 (48.6%) could recall both verbal and written information. Thirty-four (60.7%) participants said that they could put on stockings without help and 38 (71.7%) could remove them without help. Fifteen participants (18%) who did not consistently adhere to compression said that stockings were too difficult to put on or remove, that they caused their legs to hurt, or that they could not reach their feet to put them on. In terms of elevation, 60 participants (86.9%) said that they elevated their legs for 3.6 hours per day on average. Those that didn’t, reported that elevation caused discomfort or that they couldn’t be bothered. Interestingly, one in four participants reported gaining 4.5– 9kg in weight. Study authors suggested that weight control should be investigated regarding prevention of ulcer recurrence.
Therapeutic benefit / relevant outcomes
There is little agreement on what constitutes therapeutic benefit, but consensus appears to favour patients assessing patient-relevant benefit themselves although patients’ needs have traditionally taken second place to the professional ‘user’ voice in terms of leg ulcer management (200, 202).
As part of an initiative to develop and validate a patient needs/patient benefit questionnaire for assessing patient-relevant benefit in wound therapy, 172 patients ranging in age from 18–91 years attending 98 centres in Germany were asked to rate 22 needs prior to and 22 benefits post treatment (200). The top ten needs in descending order of importance prior to treatment were to be healed from lesions, to be pain free, less dependent on doctor/clinic visits, able to lead normal lives, confident in the therapy, free of wound discharge, free of odour, unafraid of worsening of the condition, able to find a clear diagnosis and therapy, and to experience greater enjoyment of life. Post-treatment, the top ten benefits rated in descending order were having confidence in the therapy, finding a clear diagnosis and therapy, being free of wound discharge, free of odour, being unafraid of worsening of the condition, having fewer side effects, being able to experience of greater enjoyment of life, being pain free, needing less time for daily treatment and being healed from lesions. It should be noted that the sample consisted of an almost 1:1 ratio of acute to chronic wounds and included wound aetiologies beyond the scope of this document.
To understand patient experiences in the context of healthcare processes that impact care quality in chronic wound patients, a qualitative study recruited 60 participants from Denmark, the Netherlands, Canada and the USA and interviewed them in a one-on-one, semi-structured fashion (202). Interpretative description of transcriptions identified domains of care coordination, establishing/obtaining care, information delivery, patient-provider interaction, and treatment delivery as areas where quality of care could be measured and improved. Within these domains 21 sub-categories were identified (Table 13).
Table 13. Domains and sub-domains of healthcare processes influencing care quality (patient-reported) (202).
The sample for this study included multiple wound aetiologies of which VLU comprised 14 (23%) participants.
A systematic review from 2022 found that existing patient reported outcome measures (PROMs) for patients with CLTI do not cover all relevant domains for this population, are heterogeneous and are lacking in adequate validation and consistency (203). The review included data from 99 publications reporting on a PROM or a HRQoL metric in patients with CLTI. Table 14 illustrates the six most used PROM tools in this population.
Table 14. QoL instruments used in the CLTI population (205).
In terms of research, reasons for participation among persons with VLU include wanting to help others, to thank carers, to receive better care and to have a say on what works (204). A survey from the USA completed by 483 patients and caregivers across ten clinical sites found that patients most valued reduction of infection, improved strength of healing (tensile strength) and reduction of recurrence in a series of clinician-centred endpoints (205). When asked about activities of daily living/QoL endpoints, their priorities were improved ability to walk/do things for themselves, go outside of the home (isolation) and reduced pain. Endpoints that would make most difference to participants’ lives included faster healing time, reduction of infection and reduction of amputation, and improved ability to walk/do things for themselves. In this study 230 (53%) participants were diabetic and no other data relevant to wound aetiology was provided.
Delayed diagnosis
To facilitate evidence-based decision making with respect to leg ulcer management that will enhance patient safety, lead to faster healing or appropriate referral, timely and accurate diagnosis is essential (4). However, variability in approaches to assessment, diagnosis and management of VLU may result in, among other issues, delayed diagnosis (206).
Patient/family perspectives can be invaluable to the diagnostic process, especially with respect to identifying clinically relevant breakdowns and errors in the process that may not be immediately apparent to healthcare personnel or systems (207).
Searches of Medline and CINAHL between 2012 and end of year 2022 failed to capture any articles discussing the perspective of chronic wound patients in terms of delayed diagnosis.
A qualitative study carried out in the Northeast of England at the primary care level thematically analysed transcripts of interviews and focus groups from a sample of nurses, GPs and 17 patients with peripheral PAD (208). Patient participant views on diagnosing PAD fell under three themes. The first theme was of delays consulting with GPs. Delays tended to happen because they (participants) minimised symptoms as resulting from old age, or they prioritised symptoms of other existing comorbidities. The second theme was of delays in diagnosis where they received little explanation of what was causing their reported symptoms, or they did not receive any feedback/diagnosis after a test such as the ABI. The third theme was of not understanding PAD, where they struggled to understand the condition, its cause and associated risks. This problem persisted for some participants even after information had been provided by healthcare practitioners. Another qualitative study interviewed 77 adult inpatients with a median age decade of 60–69 years at six general medical-surgical units in a USA academic medical centre (209). Interview transcripts were analysed thematically using the Institute of Medicine definition of diagnostic error (“failure to establish an accurate or timely explanation for the patient’s health problem(s) or effectively communicate the explanation to the patient”) as a framework. The analysis characterised data under themes of underlying causes of diagnostic error, strategies to reduce future diagnostic error and impact of diagnostic error (Table 15).
Table 15. Patient perspectives of diagnostic error (209).
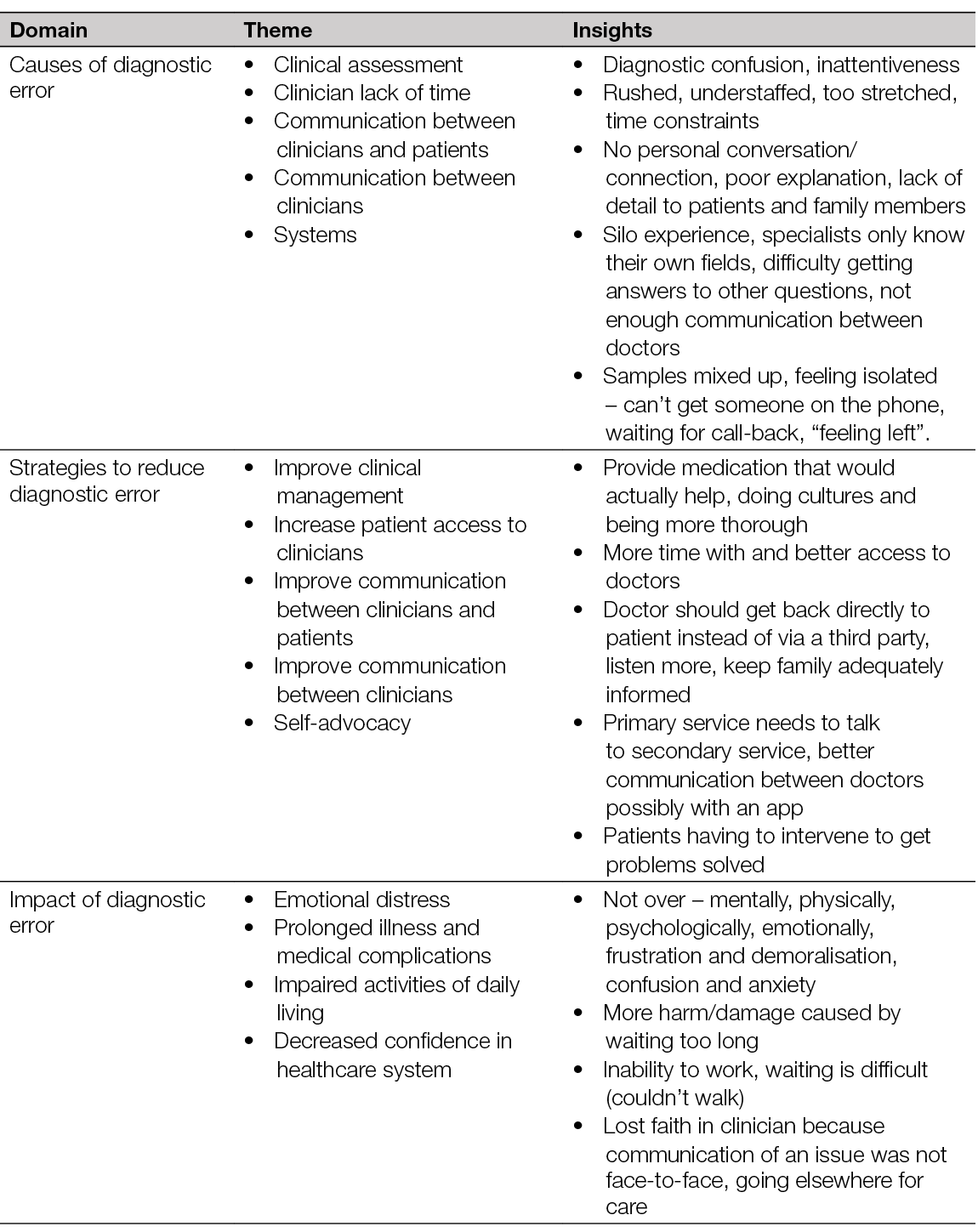
In terms of ambulatory care, a 14-person, multi-stakeholder group from the USA comprising patients, families, clinicians and experts in patient engagement and safety and diagnostic error convened to develop a framework to facilitate identification and classification of patient reported diagnostic process related breakdowns (PRDBs) (207). Patients in the group included persons with chronic illnesses or disabilities and parents of children with complex diagnoses. The group chose to work with process-related breakdowns rather than diagnostic errors and defined them as “a problem or delay reported by patients that could map to any part of the diagnostic process, as outlined in the National Academies of Medicine (NAM) conceptual model”. The framework was developed iteratively and incorporated a literature review for diagnostic error frameworks in the context of ambulatory care and patient/family reporting, and 2,165 patient-reported ambulatory errors taken from two surveys involving 25,425 USA respondents (Table 16). The group concluded that the PRDB framework fills a gap in existing diagnostic frameworks and can enhance organisational awareness of the diagnostic process. In addition, the framework can promote more meaningful engagement with patients and families in the diagnostic process.
Table 16. Framework for PRDB in ambulatory care (207)
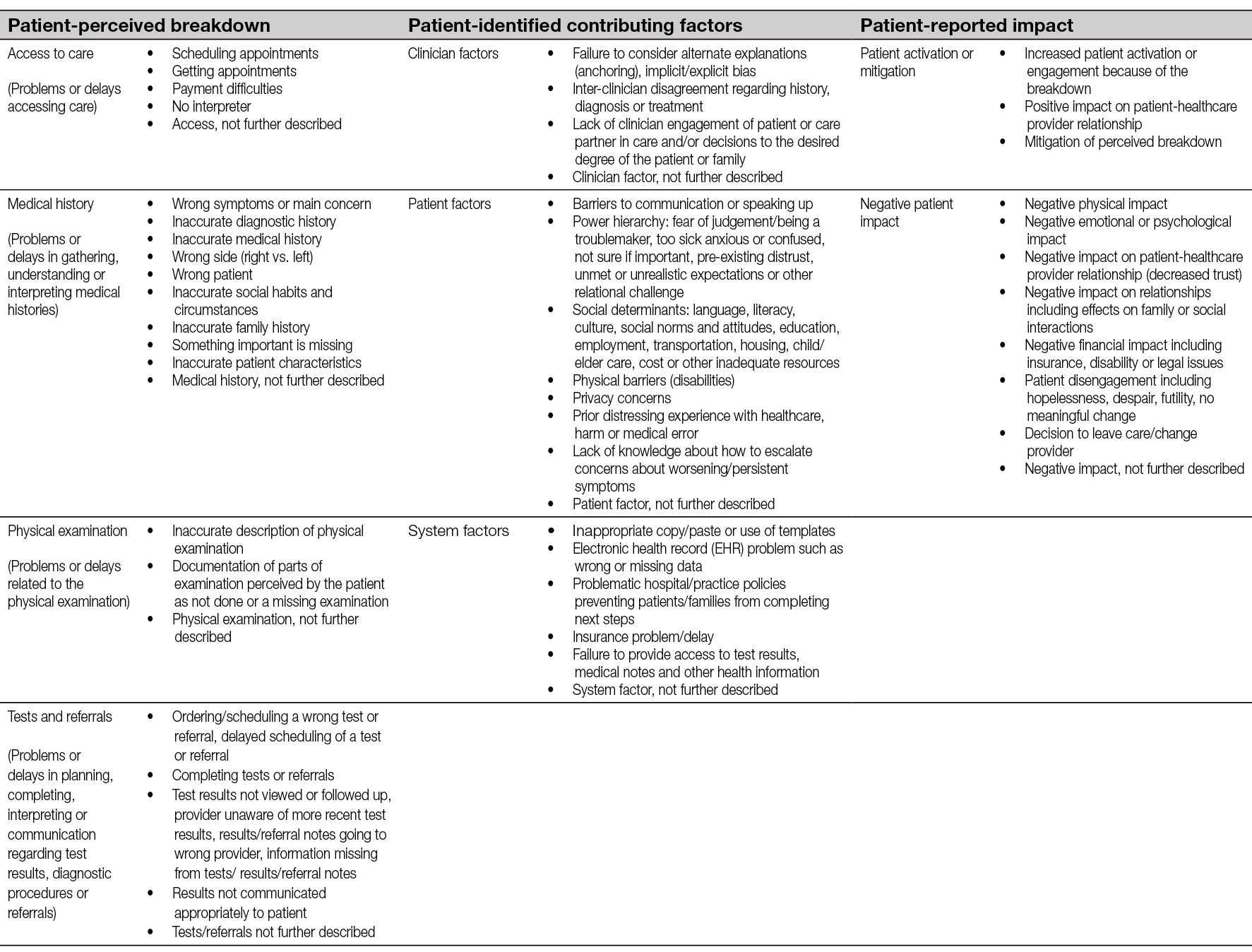
Summary
The lived experience of lower leg ulceration is difficult and often devastating, yet patients maintain hope and faith that they will receive a cure for their condition. Recurrence can happen suddenly and accidentally; patients fear recurrent ulcers becoming chronic and they tend to take measures to prevent this from happening. Motivations for adherence to treatment include wanting to improve QoL, understanding treatment plans and their rationale, and awareness of the consequences of non-adherence. Prior to initiating treatment, patients have given priority to, among others, outcomes of ulcer healing, freedom from pain and becoming more independent of doctors and clinic visits. With respect to research, they prioritised faster healing time, reduction of infection, reduction of amputation, and more mobility and independence as endpoints that would make most difference to their lives. We found no research that explored delayed diagnosis in the context of chronic wounds, but research in ambulatory care implies that patient and/or family perspectives can help to identify flaws in the diagnostic process that clinicians or systems may miss. Patients with PAD have highlighted themes of delay in consulting primary care providers or minimisation of symptoms, lack of feedback after a diagnostic test and not understanding the condition in their own diagnostic pathway. It could be that these findings are also applicable to the chronic wound population and that the research methodologies should be explored in this population.
Practising EBM requires that best available evidence, clinical expertise and experience, and patient values and expectations be combined. RCTs and systematic reviews are primarily designed to provide patients, clinicians and healthcare systems with evidence in terms of the safety and efficacy of an intervention. These RCTs frequently include measures of participant QoL, activities of daily living, pain or satisfaction, but one must interpret data for these measures in terms of the intervention under investigation and the rigorous RCT context. To truly understand patient values and expectations, we should listen to their perspectives, but on their terms and so it may be that a research approach prioritising the patient rather than an intervention could provide more appropriate information to support this aspect of the EBM paradigm. This short literature review included mostly qualitative research in an attempt to put the patient experience to the fore and gain insight into their perspectives on living with lower limb ulceration. More qualitative or mixed methods studies in wound care would provide a foundation for qualitative or mixed methods systematic reviews which would help us build a greater, more comprehensive understanding of the patient perspective.
11. Economic burden of lower leg ulcers
Authors: Kirsi Isoherranen, Leanne Atkin
The impact of chronic wounds to individual’s QoL and healthcare costs is well-known; but still often neglected and underappreciated (189). Additionally, there is a paucity of studies describing the effect of diagnostic delay in the total costs of wound care. We did not find any studies describing the effect of timing of diagnostics in total costs of wound care.
Most of the studies identified reported cost-of-illness of chronic wounds without separating leg ulcers from the data. We included only studies that reported original economic data from lower leg ulcers, and not, for example, diabetic foot or pressure ulcers (Table 17). We found one systematic review that used a cost-of-illness checklist to evaluate the quality of reports (210). In this review, economic evaluations reporting costs in a chronic ulcer population administered a specific intervention or clinical trial were excluded. In the end, 36 studies were included from 2267 articles. Mean costs were evaluated with different perspectives (i.e. healthcare public payer, patient out of pocket, societal and hospital) and with different follow-up durations. The mean one year cost from a healthcare public payer perspective for leg ulcers was US$11,000, compared to US$44,200 for diabetic foot ulcers and US$15,400 for pressure ulcers. The main limitations of this systematic review were the differences in study methods and the fact that almost half of the studies originated from the USA.
Table 17. Description of included studies describing the economic burden of lower leg ulcers
Costs at a national level
Other studies identified reported costs at a national level (211–214).
A recent cross-sectional survey from the UK reported the average 2-week per person cost of treating patients with VLU at £166.39 (95% CI £157.78– £175.00), and most of the costs resulted from community staff working time. The estimated annual costs were £102 million with a per person annual cost at £4787,70 (211). The main limitation of this study was that an assumption was made that the nine locales where the study was conducted are like the rest of the UK.
A cross-sectional study from the metropolitan area of Hamburg included 502 patients (212). Data was evaluated from three different perspectives: the impact of leg ulcers on HRQoL, the quality of leg ulcer care and the direct and indirect costs of leg ulcer. Most of the patients had a VLU (78.5%), other aetiologies were arterial (9.8%), post-traumatic (10.0%) vasculitis (1.6%) and unknown (9.0%). The mean total direct costs-of-illness were €8288 and ranged from €0.0 (no treatment) to €43,245. The limitation of the study was that the analysis was performed at only one time point with no longer follow-up.
An earlier study from Germany focused on VLUs (213). The study design was cross-sectional, including 23 specialised wound care centres and 218 patients. The mean total cost of the ulcer per year and patient was €9569 (€8658.10 direct and €911.20 indirect costs). The main limitations of the study were small sample size and the limitation to specialised wound centres.
One study evaluated the clinical and economic burden of wound care in the Tropics in the Singapore area, bringing thus important data from that area (214). The study was a population health analysis of inpatient and outpatient (both specialist and primary care) data from January 2013 to December 2017. Wounds were classified as neuro-ischaemic ulcers, VLUs, pressure injuries and surgical site infections. In patients with VLUs, the incidence of inpatient episodes was 1.5 wound episodes per 1000 patient episodes, and average gross charge per wound episode was US$7235. The estimated gross healthcare costs per patient for hospital care (inpatient and specialist outpatient) and primary care was US$16,761. Importantly, the average 1-year VLU recurrence was 52.5%, highlighting the importance of secondary prevention. Limitations of the study were the retrospective and registry-based design leading to associated selection and information bases. Also, the data may not be generalisable to other regions in the Tropics or Asia.
Summary
The data on cost-of-illness of lower leg ulcers is mainly limited to VLUs. Studies show that there is a substantial economic burden related to VLUs, but further research is needed to confirm these findings. There are many limitations regarding study methodologies and there is a clear need for robust prospective trials that provide empirical data both for the direct and non-direct costs of lower leg ulcers. In addition, there is a lack of studies describing the effect of right-timed and accurate diagnostics on the costs of treating leg ulcers.
12. Recommendations and future perspectives
Author: Kirsi Isoherranen
This document aims to highlight the complex and multifactorial nature of lower leg ulcers. In clinical practice, ‘simple’ VLUs become rarer and rarer, and the patients presenting such an ulcer present multiple comorbidities and aetiologies. A multidisciplinary assessment and treatment plan is needed otherwise these ulcers become chronic and are classified as ‘hard to heal ulcers’. It is therefore of the utmost importance that the patient is diagnosed promptly. In the worst case, a delay in diagnosis leads to an amputation or death of the patient. This poses a huge challenge to the first professionals who assess and treat the ulcer in primary care. Also in specialist care, multidisciplinarity is a key approach for successful wound management. It is not unusual that the patient has at the same time ischaemia and oedema due to heart failure and CVI.
Every patient with a lower leg ulcer should be assessed at the first appointment (also in the emergency setting) for infection, ischaemia, diabetes, causes of leg oedema, possible atypical causes or pressure injuries. Preferably a checklist should be used for consistency and to take into account various aspects affecting wound healing (see Chapter 7 and the one-pager). After this assessment, treatment (e.g. compression therapy) based on the specific causes should be started as early as possible and in case of ischaemia or atypical causes, prompt referral to specialist care is needed. In case of venous insufficiency, the patient needs also a venous assessment performed by a vascular surgeon, angiologist or a phlebologist in addition to compression therapy (see Chapter 4).
It is surprising how little focus has been attempted to the timing and accuracy of wound diagnostics as an outcome for wound healing. This document attempts to highlight the need for this. Even advanced treatments are predisposed to failure if the wound is not diagnosed correctly. This is important in many aspects: the patients’ and family’s QoL, health economy and sustainability.
The author group recommends the following for future research and health organisation projects:
- Future trials studying factors in determining healing outcomes of lower leg ulcers should incorporate as one factor the timing of diagnosis after occurrence of the wound. This is important also in respect to advocacy; enough resources for this should be allocated to primary care.
- There is a need for advanced and holistic techniques for the diagnosis of wound infection. Artificial intelligence-based technology could be one answer with the possibility of analysing patient factors (e.g. systemic symptoms, diabetes, differential diagnosis with PG) in addition to wound bed, periwound skin characteristics and wound bacteria.
- Patients with acute wounds should be assessed more holistically, for instance in emergency settings, to prevent them to become chronic or hard-to-heal wounds.
- At the organisational level, there is a need to restructure the flow of patients with a lower leg ulcer. This includes prompt comprehensive assessment aided by checklists.
- Regarding local treatment, there is a clear need for high-quality research, such as RCTs. It is important also in these studies that the diagnostical procedure is standardised and basic therapy, such as compression therapy, is performed.
- There is also a high need for more qualitative or mixed methods studies in wound care which would help build a greater, more comprehensive understanding of the patient perspective.
We hope that our document is implemented across Europe and all over the world and used in daily practice. By this, we strongly believe that it can make a true difference in the lives of persons with a lower leg ulcer. In addition, we want to acknowledge all the feedback and comments we received from professionals all over the world.
13.
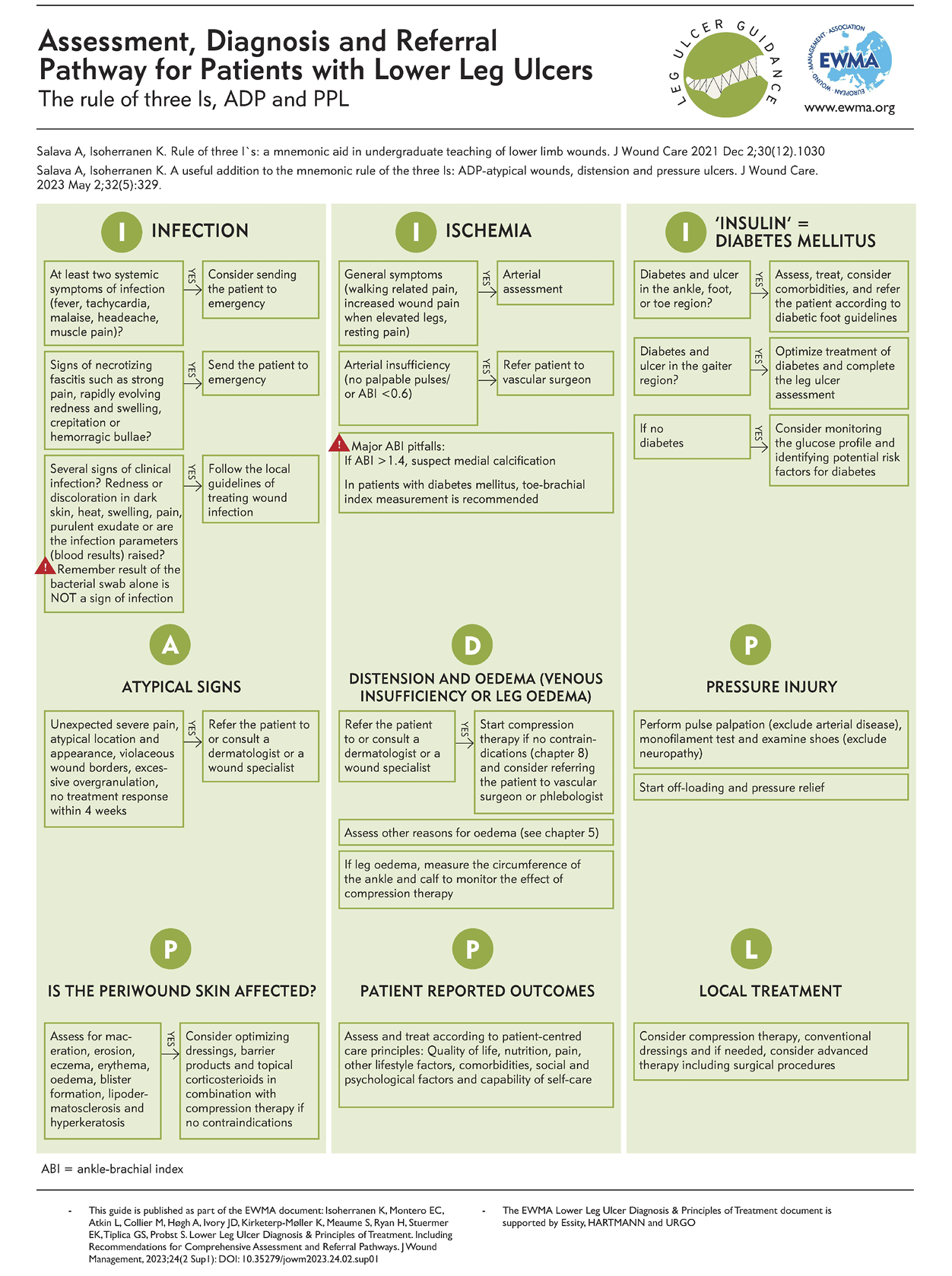
Author(s)
Kirsi Isoherranen (Editor), MD, PhD,
Helsinki University Hospital and Helsinki University, Dermatology Clinic and Helsinki Wound Healing Centre, Finland
Elena Conde Montero (Co-editor), MD, PhD,
Hospital Universitario Infanta Leonor y Virgen de la Torre, Dept. of Dermatology, Madrid, Spain
Leanne Atkin, PhD, Vascular Nurse Consultant, Mid Yorks NHS Trust & Research Fellow, University of Huddersfield, UK
Mark Collier Nurse Consultant and Associate Lecturer, Tissue Viability (UK) & Universities of Lincoln and Sunderland, UK
Annette Høgh MD, PhD Associate Professor and Senior Consultant, Department of Vascular surgery and Wound Care Center, HEM Viborg, & Department of Clinical Medicine Aarhus University Denmark
John D Ivory PhD Candidate, School of Nursing and Midwifery, University of Galway, Ireland
Klaus Kirketerp-Møller MD, PhD, Consultant, Department of Dermatology & Copenhagen Wound Healing Center, Bispebjerg University Hospital, Denmark
Sylvie Meaume MD, dermatologist and geriatrician, Rothschild Hospital, Assistance Publique Hôpitaux de Paris, University Hopital Sorbonne University, Paris
Hayley Ryan MBA, CNC, RN, AICGC, PGCertWM, Director at WoundRescue (Australia & New Zealand), Board Director & Chair at Wounds Australia, Wound Clinical Nurse Consultant ANZ, School of Nursing and Midwifery, College of Health, Medicine and Wellbeing, University of Newcastle PhD researcher
Ewa K Stuermer MD, Professor, Department of Vascular Medicine, University Heart Center, University Medical Center Hamburg-Eppendorf, Germany
George-Sorin Tiplica MD, PhD, Professor, Dermatology and Venereology, Dermatology II, Colentina Clinical Hospital, Carol Davila University of Medicine and Pharmacy Bucharest, Romania
Sebastian Probst DClinPrac, MNS, BNS, RN, Full Professor of Tissue Viability and Wound Care, Geneva School of Health Sciences, HES-SO University of Applied Sciences and Arts Western Switzerland, Geneva, Switzerland; Care Directorate, University Hospital Geneva, Switzerland; Faculty of Medicine Nursing and Health Sciences, Monash University, Melbourne, Australia; College of Medicine Nursing and Health Sciences, University of Galway, Galway, Ireland.
Corresponding author:
Kirsi Isoherranen
Email Kirsi.Isoherranen@hus.fi
Editorial support:
Matilde Bøgelund Hansen,
EWMA secretariat
© EWMA 2023 Copyright of published material and illustrations is the property of the European Wound Management Association. However, provided prior written consent for their reproduction, including parallel publishing (e.g. via repository), obtained from EWMA via the Editorial Board of the Journal, and proper acknowledgement, such permission will normally be readily granted. Requests to produce material should state where material is to be published, and, if it is abstracted, summarised, or abbreviated, then the proposed new text should be sent to Journal of Wound Management Editor for final approval. Although EWMA has taken great care to ensure accuracy, EWMA will not be liable for any errors of omission on inaccuracies in this publication. Published by the European Wound Management Association, Nordre Fasanvej 113, 2, 2000 Frederiksberg, Denmark Web: www.ewma.org. Email: ewma@ewma.org
The EWMA Lower Leg Ulcer Diagnosis & Principles of Treatment document is supported by Essity, HARTMANN and URGO
References
- Olsson M, Järbrink K, Divakar U, Bajpai R, Upton Z, Schmidtchen A, et al. The humanistic and economic burden of chronic wounds: A systematic review: The burden of chronic wounds. Wound Rep and Reg. 2019;27(1):114-25.
- Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020;10(12):e045253.
- Graves N, Phillips CJ, Harding K. A narrative review of the epidemiology and economics of chronic wounds. Br J Dermatol. 2022;187(2):141-8.
- Ahmajarvi K, Isoherranen K, Venermo M. Cohort study of diagnostic delay in the clinical pathway of patients with chronic wounds in the primary care setting. BMJ Open. 2022;12(11):e062673.
- Mooij MC, Huisman LC. Chronic leg ulcer: does a patient always get a correct diagnosis and adequate treatment? Phlebology. 2016;31(1 Suppl):68-73.
- Jockenhofer F, Gollnick H, Herberger K, Isbary G, Renner R, Stucker M, et al. Aetiology, comorbidities and cofactors of chronic leg ulcers: retrospective evaluation of 1 000 patients from 10 specialised dermatological wound care centers in Germany. Int Wound J. 2016;13(5):821-8.
- Isoherranen K, O’Brien JJ, Barker J, Dissemond J, Hafner J, Jemec GBE, et al. Atypical wounds. Best clinical practice and challenges. J Wound Care. 2019;28(Sup6):S1-S92.
- Martinengo L, Olsson M, Bajpai R, Soljak M, Upton Z, Schmidtchen A, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Annals of Epidemiology. 2019;29:8-15.
- Ahmajarvi KM, Isoherranen KM, Makela A, Venermo M. A change in the prevalence and the etiological factors of chronic wounds in Helsinki metropolitan area during 2008-2016. Int Wound J. 2019;16(2):522-6.
- Bolton L. Peripheral arterial disease: Scoping review of patient-centred outcomes. Int Wound J. 2019;16(6):1521-32.
- Friman A, Wiegleb Edstrom D, Ebbeskog B, Edelbring S. General practitioners’ knowledge of leg ulcer treatment in primary healthcare: an interview study. Prim Health Care Res Dev. 2020;21:e34.
- Strohal R, Dissemond J, Jordan O’Brien J, Piaggesi A, Rimdeika R, Young T, et al. EWMA Document: Debridement: An updated overview and clarification of the principle role of debridement. J Wound Care. 2013;22(Sup1):S1-S49.
- Probst S, Apelqvist J, Bjarnsholt T, Lipsky B, Ousey K, Peters E. Antimicrobials and Non-healing Wounds: An Update. Journal of Wound Management. 2022;23(3).
- Piaggesi A, Bassetto F, den Braber E, Dalla Paola L, Marques MA, Palla I, et al. New technologies for tissue replacement. Journal of Wound Management. 2023;24(1 Sup1). s1-130.
- European Pressure Ulcer Advisory Panel, National Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Emily Haesler (Ed.). EPUAP/NPIAP/PPPIA: 2019
- Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA, et al. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3266.
- Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336(7652):1049-51.
- British HIV Association (BHIVA). Guideline Development Manual. 2021: 1-37.
- Schunemann HJ, Brożek J, Guyatt GH, Oxman AD. Handbook for grading the quality of evidence and the strength of the recommendations using the GRADE approach. 2013.
- Aguirre A, Sharma K, Arora A, Humphries MD. Early ABI Testing May Decrease Risk of Amputation for Patients With Lower Extremity Ulcers. Annals of Vascular Surgery. 2022;79:65-71.
- Force USPST, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for Peripheral Artery Disease and Cardiovascular Disease Risk Assessment With the Ankle-Brachial Index: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(2):177-83.
- Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e686-e725.
- De Maeseneer MG, Kakkos SK, Aherne T, Baekgaard N, Black S, Blomgren L, et al. Editor’s Choice - European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur J Vasc Endovasc Surg. 2022;63(2):184-267.
- Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, et al. A Randomized Trial of Early Endovenous Ablation in Venous Ulceration. N Engl J Med. 2018;378(22):2105-14.
- Gohel MS, Mora MJ, Szigeti M, Epstein DM, Heatley F, Bradbury A, et al. Long-term Clinical and Cost-effectiveness of Early Endovenous Ablation in Venous Ulceration: A Randomized Clinical Trial. JAMA Surg. 2020;155(12):1113.
- LeBlanc Kea. Best practice recommendations for the prevention and management of skin tears in aged skin. Wounds International. 2018.
- Schofield M, Aziz M, Bliss MR, Bull RH. Medical pathology in patients with leg ulcers: a study carried out in a leg ulcer clinic in a day hospital for the elderly. J Tissue Viability. 2003;13(1):17-22.
- Michelerio A, Tomasini CF. The Alzheimer patient from the dermatologist’s point of view. Ital J Dermatol Venerol. 2021;156(4):422-7.
- Kaya G, Saurat JH. Dermatoporosis: a chronic cutaneous insufficiency/fragility syndrome. Clinicopathological features, mechanisms, prevention and potential treatments. Dermatology. 2007;215(4):284-94.
- Cowdell F, Jadotte YT, Ersser SJ, Danby S, Lawton S, Roberts A, et al. Hygiene and emollient interventions for maintaining skin integrity in older people in hospital and residential care settings. Cochrane Database Syst Rev. 2020;1:CD011377.
- Kaya A, Vuagnat H, Kaya G. Dermatoporosis: Clinical features, molecular mechanisms and novel therapeutic targets - A literature review. Journal of Wound Management Official journal of the European Wound Management Association. 2022(November 2022).
- Serra R, Ielapi N, Barbetta A, de Franciscis S. Skin tears and risk factors assessment: a systematic review on evidence-based medicine. Int Wound J. 2018;15(1):38-42.
- Kaya G, Jacobs F, Prins C, Viero D, Kaya A, Saurat JH. Deep dissecting hematoma: an emerging severe complication of dermatoporosis. Arch Dermatol. 2008;144(10):1303-8.
- Hafner J. Calciphylaxis and Martorell Hypertensive Ischemic Leg Ulcer: Same Pattern - One Pathophysiology. Dermatology. 2016;232(5):523-33.
- Monfort JB, Cury K, Moguelet P, Chasset F, Bachmeyer C, Frances C, et al. Cutaneous Arteriolosclerosis Is Not Specific to Ischemic Hypertensive Leg Ulcers. Dermatology. 2018;234(5-6):194-7.
- Yao Z, Niu J, Cheng B. Prevalence of Chronic Skin Wounds and Their Risk Factors in an Inpatient Hospital Setting in Northern China. Adv Skin Wound Care. 2020;33(9):1-10.
- Swanson T, Ousey K, Haesler E, Bjarnsholt T, Carville K, Idensohn P, et al. IWII Wound Infection in Clinical Practice consensus document: 2022 update. J Wound Care. 2022;31(Sup12):S10-S21.
- Gjodsbol K, Christensen JJ, Karlsmark T, Jorgensen B, Klein BM, Krogfelt KA. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J. 2006;3(3):225-31.
- Jockenhofer F, Gollnick H, Herberger K, Isbary G, Renner R, Stucker M, et al. Bacteriological pathogen spectrum of chronic leg ulcers: Results of a multicenter trial in dermatologic wound care centers differentiated by regions. J Dtsch Dermatol Ges. 2013;11(11):1057-63.
- Burmolle M, Thomsen TR, Fazli M, Dige I, Christensen L, Homoe P, et al. Biofilms in chronic infections - a matter of opportunity - monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol. 2010;59(3):324-36.
- Dowd SE, Sun Y, Secor PR, Rhoads DD, Wolcott BM, James GA, et al. Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 2008;8:43.
- Swanson T, Haesler E, Angel D, Sussman G. IWII Wound infection in clinical practice consensus document 2016 update. Wound Practice and Research. 2016;24:94-198.
- Ribet D, Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015;17(3):173-83.
- Dissemond J, Assadian O, Gerber V, Kingsley A, Kramer A, Leaper DJ, et al. Classification of wounds at risk and their antimicrobial treatment with polihexanide: a practice-oriented expert recommendation. Skin Pharmacol Physiol. 2011;24(5):245-55.
- Dissemond J, Gerber V, Lobmann R, Kramer A, Mastronicola D, Senneville E, et al. Therapeutic index for local infections score (TILI): a new diagnostic tool. J Wound Care. 2020;29(12):720-6.
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606-8.
- Malone M, Bjarnsholt T, McBain AJ, James GA, Stoodley P, Leaper D, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care. 2017;26(1):20-5.
- Kolpen M, Kragh KN, Enciso JB, Faurholt-Jepsen D, Lindegaard B, Egelund GB, et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax. 2022;77(10):1015-22.
- Stuermer EK, Besser M, Debus ES, Smeets R, Dietrich M. Bacterial infiltration in biofilm-colonized wounds: Analyses in the hpBIOM ex vivo wound model and possible impact on swabbing and debridement. Int Wound J 2023 [accepted]
- James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37-44.
- Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, DeLeo FR, et al. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem. 2004;279(52):54881-6.
- Jensen PO, Bjarnsholt T, Phipps R, Rasmussen TB, Calum H, Christoffersen L, et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology (Reading). 2007;153(Pt 5):1329-38.
- Cowan T. Biofilms and their management: from concept to clinical reality. J Wound Care. 2011;20(5):220, 2-6.
- Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol. 2011;186(11):6585-96.
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623-33.
- Larsen T, Fiehn NE. Resistance of Streptococcus sanguis biofilms to antimicrobial agents. Apmis. 1996;104(4):280-4.
- Williams P, Winzer K, Chan WC, Camara M. Look who’s talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond B Biol Sci. 2007;362(1483):1119-34.
- Fazli M, Bjarnsholt T, Kirketerp-Moller K, Jorgensen B, Andersen AS, Krogfelt KA, et al. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol. 2009;47(12):4084-9.
- Frevert W, Wright TW, Farmer KW, Yang Q, Struk AM, Schultz G. Evaluation of Biofilms on Explanted Shoulder Prostheses Using Functional Biofilm Assay and Scanning Electron Microscopy. J Surg Orthop Adv. 2018;27(3):171-7.
- Rennie MY, Dunham D, Lindvere-Teene L, Raizman R, Hill R, Linden R. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:X(TM). Diagnostics (Basel). 2019;9(1).
- Le L, Baer M, Briggs P, Bullock N, Cole W, DiMarco D, et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Adv Wound Care (New Rochelle). 2021;10(3):123-36.
- Dow G, Browne A, Sibbald RG. Infection in chronic wounds: controversies in diagnosis and treatment. Ostomy Wound Manage. 1999;45(8):29-40.
- Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair Regen. 2006;14(5):548-57.
- Spear M. Best technique for obtaining wound cultures. Plast Surg Nurs. 2012;32(1):34-6.
- Rondas AA, Schols JM, Halfens RJ, Stobberingh EE. Swab versus biopsy for the diagnosis of chronic infected wounds. Adv Skin Wound Care. 2013;26(5):211-9.
- Levine NS, Lindberg RB, Mason AD, Jr., Pruitt BA, Jr. The quantitative swab culture and smear: A quick, simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma. 1976;16(2):89-94.
- Al Ghazal P, Korber A, Klode J, Schmid EN, Buer J, Dissemond J. Evaluation of the Essen Rotary as a new technique for bacterial swabs: results of a prospective controlled clinical investigation in 50 patients with chronic leg ulcers. Int Wound J. 2014;11(1):44-9.
- Copeland-Halperin LR, Kaminsky AJ, Bluefeld N, Miraliakbari R. Sample procurement for cultures of infected wounds: a systematic review. J Wound Care. 2016;25(4):S4-6, S8-10.
- Jakobsen TH, Xu Y, Bay L, Schonheyder HC, Jakobsen T, Bjarnsholt T, et al. Sampling challenges in diagnosis of chronic bacterial infections. J Med Microbiol. 2021;70(3).
- Rhoads DD, Cox SB, Rees EJ, Sun Y, Wolcott RD. Clinical identification of bacteria in human chronic wound infections: culturing vs. 16S ribosomal DNA sequencing. BMC Infect Dis. 2012;12:321.
- Ghaly P, Iliopoulos J, Ahmad M. The role of nutrition in wound healing: an overview. Br J Nurs. 2021;30(5):S38-S42.
- Bardoel BW, van der Ent S, Pel MJ, Tommassen J, Pieterse CM, van Kessel KP, et al. Pseudomonas evades immune recognition of flagellin in both mammals and plants. PLoS Pathog. 2011;7(8):e1002206.
- Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen. 2001;9(3):178-86.
- Jockenhofer F, Wollina U, Salva KA, Benson S, Dissemond J. The PARACELSUS score: a novel diagnostic tool for pyoderma gangrenosum. Br J Dermatol. 2019;180(3):615-20.
- Maverakis E, Ma C, Shinkai K, Fiorentino D, Callen JP, Wollina U, et al. Diagnostic Criteria of Ulcerative Pyoderma Gangrenosum. JAMA Dermatology. 2018;154(4).
- Oishi P, Hoffman JIE, Fuhrman BP, Fineman JR. Chapter 20 - Regional Circulation. In: Bradley PF, Zimmerman JJ, editors. Pediatric Critical Care: Pediatric Critical Care; 2011. p. 217-33.
- Sogaard M, Nordanstig J, Eldrup N, Behrendt CA. A thought-provoking statement regarding the treatment of patients with peripheral arterial disease. Vasa. 2023;52(2):77-80.
- NICE. Peripheral arterial disease: diagnosis and management. NICE Clinical Care Excellence. 2020;147.
- Cournot M, Boccalon H, Cambou JP, Guilloux J, Taraszkiewicz D, Hanaire-Broutin H, et al. Accuracy of the screening physical examination to identify subclinical atherosclerosis and peripheral arterial disease in asymptomatic subjects. J Vasc Surg. 2007;46(6):1215-21.
- Collins TC, Suarez-Almazor M, Peterson NJ. An absent pulse is not sensitive for the early detection of peripheral arterial disease. Fam Med. 2006;38(1):38-42.
- Khan NA, Rahim SA, Anand SS, Simel DL, Panju A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295(5):536-46.
- Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. Editor’s Choice - 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(3):305-68.
- Xu D, Zou L, Xing Y, Hou L, Wei Y, Zhang J, et al. Diagnostic value of ankle-brachial index in peripheral arterial disease: a meta-analysis. Can J Cardiol. 2013;29(4):492-8.
- Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890-909.
- Lim SLX, Chung RE, Holloway S, Harding KG. Modified compression therapy in mixed arterial-venous leg ulcers: An integrative review. Int Wound J. 2021;18(6):822-42.
- Casey S, Lanting S, Oldmeadow C, Chuter V. The reliability of the ankle brachial index: a systematic review. J Foot Ankle Res. 2019;12:39.
- Watson EL, Patel B, Katsogridakis E, Pepper CJ, Messeder SJ, Saratzis A, et al. Selecting Portable Ankle/Toe Brachial Pressure Index Systems for a Peripheral Arterial Disease Population Screening Programme: a Systematic Review, Clinical Evaluation Exercise, and Consensus Process. Eur J Vasc Endovasc Surg. 2022;64(6):693-702.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5-67.
- Franks PJ, Barker J, Collier M, Gethin G, Haesler E, Jawien A, et al. Management of Patients With Venous Leg Ulcers: Challenges and Current Best Practice. J Wound Care. 2016;25 Suppl 6:S1-S67.
- A H. [Wounds related to vascular changes - venous Wounds]. In: Gottrup F, Karlsmark T, Kirketerp-Moller K, editors. [Wounds - Aetiology, diagnosis and treatment]. Copenhagen: Forfatterne og Mungsgaard; 2021. p. 227-35.
- Lurie F, Passman M, Meisner M, Dalsing M, Masuda E, Welch H, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8(3):342-52.
- Michaels JA, Brazier JE, Campbell WB, MacIntyre JB, Palfreyman SJ, Ratcliffe J. Randomized clinical trial comparing surgery with conservative treatment for uncomplicated varicose veins. Br J Surg. 2006;93(2):175-81.
- Knipp BS, Blackburn SA, Bloom JR, Fellows E, Laforge W, Pfeifer JR, et al. Endovenous laser ablation: venous outcomes and thrombotic complications are independent of the presence of deep venous insufficiency. J Vasc Surg. 2008;48(6):1538-45.
- Marston WA, Brabham VW, Mendes R, Berndt D, Weiner M, Keagy B. The importance of deep venous reflux velocity as a determinant of outcome in patients with combined superficial and deep venous reflux treated with endovenous saphenous ablation. J Vasc Surg. 2008;48(2):400-5; discussion 5-6.
- Hedayati N, Carson JG, Chi YW, Link D. Management of mixed arterial venous lower extremity ulceration: A review. Vasc Med. 2015;20(5):479-86.
- Humphreys ML, Stewart AH, Gohel MS, Taylor M, Whyman MR, Poskitt KR. Management of mixed arterial and venous leg ulcers. Br J Surg. 2007;94(9):1104-7.
- Weller CD, Team V, Ivory JD, Crawford K, Gethin G. ABPI reporting and compression recommendations in global clinical practice guidelines on venous leg ulcer management: A scoping review. Int Wound J. 2019;16(2):406-19.
- Gasparis AP, Kim PS, Dean SM, Khilnani NM, Labropoulos N. Diagnostic approach to lower limb edema. Phlebology: The Journal of Venous Disease. 2020;35(9):650-5.
- Beelen LM, van Dishoeck A-M, Tsangaris E, Coriddi M, Dayan JH, Pusic AL, et al. Patient-Reported Outcome Measures in Lymphedema: A Systematic Review and COSMIN Analysis. Annals of Surgical Oncology. 2020;28(3):1656-68.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to Leg Edema of Unclear Etiology. The Journal of the American Board of Family Medicine. 2006;19(2):148-60.
- Farrow W. Phlebolymphedema-a common underdiagnosed and undertreated problem in the wound care clinic. J Am Col Certif Wound Spec. 2010;2(1):14-23.
- Salim S, Machin M, Patterson BO, Onida S, Davies AH. Global Epidemiology of Chronic Venous Disease. Annals of Surgery. 2021;274(6):971-6.
- Moffatt CJ, Keeley V, Franks PJ, Rich A, Pinnington LL. Chronic oedema: a prevalent health care problem for UK health services. Int Wound J. 2017;14(5):772-81.
- O’Donnell TF, Allison GM, Melikian R, Iafrati MD. A systematic review of the quality of clinical practice guidelines for lymphedema, as assessed using the Appraisal of Guidelines for Research and Evaluation II instrument. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2020;8(4):685-92.
- Lymphoedema: NHS; 2019 Available from: https://www.nhs.uk/conditions/lymphoedema/.
- Todd M. Chronic Oedema: Obesity-related lymphoedema. British Journal of Community Nursing. 2019;24(Sup10):S5-S.
- Moffatt C, Keeley V, Quéré I. The Concept of Chronic Edema—A Neglected Public Health Issue and an International Response: The LIMPRINT Study. Lymphatic Research and Biology. 2019;17(2):121-6.
- The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology. 2020;53(1):3-19.
- Adigun CG. Adverse Drug Reactions of the Lower Extremities. Clinics in Podiatric Medicine and Surgery. 2016;33(3):397-408.
- Evans NS, Ratchford EV. The swollen leg. Vasc Med. 2016;21(6):562-4.
- Bertsch T, Erbacher G, Elwell R. Lipoedema: a paradigm shift and consensus. J Wound Care. 2020;29(Sup11b):1-51.
- Stemmer R. [Stemmer’s sign--possibilities and limits of clinical diagnosis of lymphedema]. Wien Med Wochenschr. 1999;149(2-4):85-6.
- Trayes KP, Studdiford JS, Pickle S, Tully AS. Edema: diagnosis and management. Am Fam Physician. 2013;88(2):102-10.
- Garcia R, Labropoulos N. Duplex Ultrasound for the Diagnosis of Acute and Chronic Venous Diseases. Surgical Clinics of North America. 2018;98(2):201-18.
- Hidding JT, Viehoff PB, Beurskens CHG, van Laarhoven HWM, Nijhuis-van der Sanden MWG, van der Wees PJ. Measurement Properties of Instruments for Measuring of Lymphedema: Systematic Review. Physical Therapy. 2016;96(12):1965-81.
- Miseré RML, Wolfs JAGN, Lobbes MBI, van der Hulst RRWJ, Qiu SS. A systematic review of magnetic resonance lymphography for the evaluation of peripheral lymphedema. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2020;8(5):882-92.e2.
- Moffatt CJ, Keeley V, Franks PJ, Rich A, Pinnington LL. Chronic oedema: a prevalent health care problem for UK health services. International Wound Journal. 2017;14(5):772-81.
- Carvalho CA, Lopes Pinto R, Guerreiro Godoy MdF, Pereira de Godoy JM. Reduction of Pain and Edema of the Legs by Walking Wearing Elastic Stockings. International Journal of Vascular Medicine. 2015;2015:1-4.
- Chadwick SE. The use of leg elevation in the treatment of chronic peripheral oedema. British Journal of Community Nursing. 2022;27(Sup10):S28-S32.
- Phillips JJ, Gordon SJ. Intermittent Pneumatic Compression Dosage for Adults and Children with Lymphedema: A Systematic Review. Lymphatic Research and Biology. 2019;17(1):2-18.
- Yeung W, Semciw AI. Aquatic Therapy for People with Lymphedema: A Systematic Review and Meta-analysis. Lymphatic Research and Biology. 2018;16(1):9-19.
- Pedreira R, Cho B, Hassanein A, Clarke-Pearson E, Bello R, Walia G, et al. Systematic Review of the Surgical Treatment of Extremity Lymphedema. Journal of Reconstructive Microsurgery. 2017;33(06):412-25.
- Chang DW, Dayan J, Greene AK, MacDonald JK, Masia J, Mehrara B, et al. Surgical Treatment of Lymphedema: A Systematic Review and Meta-Analysis of Controlled Trials. Results of a Consensus Conference. Plastic & Reconstructive Surgery. 2021;147(4):975-93.
- O’Donnell TF, Jr., Allison GM, Iafrati MD. A systematic review of guidelines for lymphedema and the need for contemporary intersocietal guidelines for the management of lymphedema. J Vasc Surg Venous Lymphat Disord. 2020;8(4):676-84.
- Tan M, Salim S, Beshr M, Guni A, Onida S, Lane T, et al. A methodologic assessment of lymphedema clinical practice guidelines. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2020;8(6):1111-8.e3.
- Kolios AGA, Hafner J, Luder C, Guenova E, Kerl K, Kempf W, et al. Comparison of pyoderma gangrenosum and Martorell hypertensive ischaemic leg ulcer in a Swiss cohort. Br J Dermatol. 2018;178(2):e125-e6.
- Shanmugam VK, Angra D, Rahimi H, McNish S. Vasculitic and autoimmune wounds. J Vasc Surg Venous Lymphat Disord. 2017;5(2):280-92.
- Hayes S, Dodds SR. The identification and diagnosis of malignant leg ulcers. Nurs Times. 2003;99(31):50-2.
- Gil T, Pistunovich Y, Kulikovsky M, Elmalah I, Krausz Y, Mettanes I, et al. A prospective case-control study of non-healing wounds of the lower limbs - the value of biopsies for ulcerating carcinoma. J Eur Acad Dermatol Venereol. 2015;29(2):337-45.
- Misciali C, Dika E, Fanti PA, Vaccari S, Baraldi C, Sgubbi P, et al. Frequency of malignant neoplasms in 257 chronic leg ulcers. Dermatol Surg. 2013;39(6):849-54.
- Walsh R. Improving diagnosis of malignant leg ulcers in the community. Br J Nurs. 2002;11(9):604-13.
- Segui M, Llamas-Velasco M. A comprehensive review on pathogenesis, associations, clinical findings, and treatment of livedoid vasculopathy. Front Med (Lausanne). 2022;9:993515.
- Meireles CB, Maia LC, Soares GC, Teodoro IPP, Gadelha M, da Silva CGL, et al. Atypical presentations of cutaneous leishmaniasis: A systematic review. Acta Trop. 2017;172:240-54.
- Bulte CA, Hoegler KM, Kutlu O, Khachemoune A. Hydroxyurea: a reappraisal of its cutaneous side effects and their management. Int J Dermatol. 2021;60(7):810-7.
- Gottrup F, Karlsmark T. Leg ulcers: uncommon presentations. Clin Dermatol. 2005;23(6):601-11.
- Stansal A, Khayat K, Duchatelle V, Tella E, Gautier V, Sfeir D, et al. [When to ask for a skin biopsy in a patient with leg ulcer? Retrospective study of 143 consecutive biopsies]. J Med Vasc. 2018;43(1):4-9.
- Gonzalez CD, Florell SR, Bowen AR, Presson AP, Petersen MJ. Histopathologic vasculitis from the periulcer edge: A retrospective cohort study. J Am Acad Dermatol. 2019;81(6):1353-7.
- Snyder RJ WR, Hettrick H. Is there a place for checklists in the current wound care model? Podiatry Management.31(5):193-8.
- Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211-59.
- Hess CT. Arterial ulcer checklist. Adv Skin Wound Care. 2010;23(9):432.
- Kaari S, Vähätalo M, Kallio M, Lagus H, Isoherranen K. A digital wound management checklist to support clinical decision-making: A qualitative validation study. Journal of Wound Management Official journal of the European Wound Management Association. 2022(November 2022).
- Thomas Hess C. Checklist for laboratory tests to rule out atypical causes of leg ulcers. Adv Skin Wound Care. 2010;23(11):528.
- Thomas Hess C. Venous ulcer checklist. Adv Skin Wound Care. 2010;23(8):384.
- Snyder RJ, Jensen JL, Applewhite AJ, Couch KS, Joseph WS, Lantis Ii JC, et al. A Standardized Approach to Evaluating Lower Extremity Chronic Wounds Using a Checklist. Wounds : a compendium of clinical research and practice. 2019;31 5 Suppl:S29-S44.
- Hess CT. Checklist for differential diagnosis of lower-extremity ulcers. Adv Skin Wound Care. 2010;23(10):480.
- Hess CT. Lower-extremity wound checklist. Adv Skin Wound Care. 2011;24(3):144.
- Smet S, Probst S, Holloway S, Fourie A, Beele H, Beeckman D. The measurement properties of assessment tools for chronic wounds: A systematic review. Int J Nurs Stud. 2021;121:103998.
- Rippon MG, Rogers AA, Ousey K, Atkin L, Williams K. The importance of periwound skin in wound healing: an overview of the evidence. J Wound Care. 2022;31(8):648-59.
- Dini V, Janowska A, Oranges T, De Pascalis A, Iannone M, Romanelli M. Surrounding skin management in venous leg ulcers: A systematic review. J Tissue Viability. 2020;29(3):169-75.
- Finnish Medical Society Duodecim & Finnish Dermatological Society. Modified from Chronic lower leg ulcer. Current Care Guidelines. 2021. Available from www.kaypahoito.fi
- Shi C, Dumville JC, Cullum N, Connaughton E, Norman G. Compression bandages or stockings versus no compression for treating venous leg ulcers. Cochrane Database Syst Rev. 2021;7:CD013397.
- O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;11(11):CD000265.
- Tollow P, Ogden J, Whiteley MS. The comparative impact of conservative treatment versus superficial venous surgery, for the treatment of venous leg ulcers: A systematic review of the impact on patients’ quality of life. Phlebology. 2016;31(2):82-93.
- Mauck KF, Asi N, Elraiyah TA, Undavalli C, Nabhan M, Altayar O, et al. Comparative systematic review and meta-analysis of compression modalities for the promotion of venous ulcer healing and reducing ulcer recurrence. J Vasc Surg. 2014;60(2 Suppl):71S-90S e1-2.
- Goka EA, Poku E, Thokala P, Sutton A. Clinical and Economic Impact of a Two-layer Compression System for the Treatment of Venous Leg Ulcers: A Systematic Review. Wounds. 2020;32(1):11-21.
- Fulcher E, Gopee N. Effect of different compression bandaging techniques on the healing rate of venous leg ulcers: a literature review. Br J Community Nurs. 2020;25(Sup6):S20-S6.
- Welsh L. What is the existing evidence supporting the efficacy of compression bandage systems containing both elastic and inelastic components (mixed-component systems)? A systematic review. J Clin Nurs. 2017;26(9-10):1189-203.
- Nelson EA, Bell-Syer SE. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev. 2014(9):CD002303.
- Compression Stockings for the Prevention of Venous Leg Ulcer Recurrence: A Health Technology Assessment. Ont Health Technol Assess Ser. 2019;19(2):1-86.
- Dahm KT, Myrhaug HT, Stromme H, Fure B, Brurberg KG. Effects of preventive use of compression stockings for elderly with chronic venous insufficiency and swollen legs: a systematic review and meta-analysis. BMC Geriatr. 2019;19(1):76.
- Smith D, Lane R, McGinnes R, O’Brien J, Johnston R, Bugeja L, et al. What is the effect of exercise on wound healing in patients with venous leg ulcers? A systematic review. Int Wound J. 2018;15(3):441-53.
- Jull A, Slark J, Parsons J. Prescribed Exercise With Compression vs Compression Alone in Treating Patients With Venous Leg Ulcers: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018;154(11):1304-11.
- de Carvalho MR. Comparison of outcomes in patients with venous leg ulcers treated with compression therapy alone versus combination of surgery and compression therapy: a systematic review. J Wound Ostomy Continence Nurs. 2015;42(1):42-6; quiz E1-2.
- Elstone A. Does venous intervention combined with compression therapy improve outcomes for patients with venous ulceration? Wounds UK. 2020;16:6-123.
- Nelson EA, Hillman A, Thomas K. Intermittent pneumatic compression for treating venous leg ulcers. Cochrane Database Syst Rev. 2014(5):CD001899.
- Sibbald RG, Elliott JA, Persaud-Jaimangal R, Goodman L, Armstrong DG, Harley C, et al. Wound Bed Preparation 2021. Adv Skin Wound Care. 2021;34(4):183-95.
- McLain NE, Moore ZE, Avsar P. Wound cleansing for treating venous leg ulcers. Cochrane Database Syst Rev. 2021;3(3):CD011675.
- Gethin G, Cowman S, Kolbach DN. Debridement for venous leg ulcers. Cochrane Database Syst Rev. 2015;2015(9):CD008599.
- O’Meara S, Martyn-St James M, Adderley UJ. Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev. 2015(8):CD010182.
- Norman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst Rev. 2018;6(6):CD012583.
- Ribeiro CT, Dias FA, Fregonezi GA. Hydrogel dressings for venous leg ulcers. Cochrane Database Syst Rev. 2022;8(8):CD010738.
- Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev. 2015;2015(3):CD005083.
- Totty JP, Bua N, Smith GE, Harwood AE, Carradice D, Wallace T, et al. Dialkylcarbamoyl chloride (DACC)-coated dressings in the management and prevention of wound infection: a systematic review. J Wound Care. 2017;26(3):107-14.
- O’Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. 2014(1):CD003557.
- Broderick C, Pagnamenta F, Forster R. Dressings and topical agents for arterial leg ulcers. Cochrane Database Syst Rev. 2020;1(1):CD001836.
- Briggs M, Nelson EA, Martyn-St James M. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database Syst Rev. 2012;11(11):CD001177.
- Martinez-Zapata MJ, Marti-Carvajal AJ, Sola I, Exposito JA, Bolibar I, Rodriguez L, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;2016(5):CD006899.
- Cullum N, Liu Z. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev. 2017;5:CD001180.
- Aziz Z, Cullum N. Electromagnetic therapy for treating venous leg ulcers. Cochrane Database Syst Rev. 2015;2015(7):CD002933.
- Westby MJ, Norman G, Dumville JC, Stubbs N, Cullum N. Protease-modulating matrix treatments for healing venous leg ulcers. Cochrane Database of Systematic Reviews. 2016;2017(4).
- https://www.crd.york.ac.uk/prospero/
- https://clinicaltrials.gov/
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71-2.
- Szajewska H. Evidence-Based Medicine and Clinical Research: Both Are Needed, Neither Is Perfect. Annals of Nutrition and Metabolism. 2018;72(Suppl. 3):13-23.
- Fearns N, Heller-Murphy S, Kelly J, Harbour J. Placing the patient at the centre of chronic wound care: A qualitative evidence synthesis. Journal of Tissue Viability. 2017;26(4):254-9.
- Moore Z, Butcher G, Corbett LQ, McGuiness W, Snyder RJ, van Acker K. Exploring the concept of a team approach to wound care: Managing wounds as a team. J Wound Care. 2014;23(Sup5b):S1-S38.
- Cunha N, Campos S, Cabete J. Chronic leg ulcers disrupt patients’ lives: A study of leg ulcer-related life changes and quality of life. British Journal of Community Nursing. 2017;22(Sup9):S30-S7.
- Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6(1):15.
- Kapp S, Santamaria N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int Wound J. 2017;14(6):1108-19.
- Olsson M, Järbrink K, Divakar U, Bajpai R, Upton Z, Schmidtchen A, et al. The humanistic and economic burden of chronic wounds: A systematic review. Wound Rep and Reg. 2019;27(1):114-25.
- Brtan Romić R, Brtan A, Romić I, Cvitanović H, Duvančić T, Lugović-Mihić L. QUALITY OF LIFE AND PERCEPTION OF DISEASE IN PATIENTS WITH CHRONIC LEG ULCER. Acta Clin Croat. 2015;54(3):309-14.
- Castro SLS, Ferreira NMLA, Roque M, de Souza MBB. Living in a difficult situation: understanding the experience of persons with venous leg ulcers. Revista Estima. 2012;10(1):12-9.
- Lernevall LSD, Fogh K, Nielsen CB, Dam W, Dreyer PS. Lived experiences of life with a leg ulcer - a life in hell. EWMA Journal. 2017;17(1):15-21.
- Probst S, Séchaud L, Bobbink P, Skinner MB, Weller CD. The lived experience of recurrence prevention in patients with venous leg ulcers: An interpretative phenomenological study. Journal of Tissue Viability. 2020;29(3):176-9.
- Probst S, Bobbink P, Séchaud L, Buehrer Skinner M. Venous leg ulcer recurrences – The relationship to self-efficacy, social support and quality of life – A mixed method study. Journal of Advanced Nursing. 2020;77(1):367-75.
- Hurlow J, Hensley L,. Achieving Patient Adherence in the Wound Care Clinic. Today’s Wound Clinic. 2015.
- Silva MHD, Jesus MCP, Tavares RE, Caldeira EAC, Oliveira DM, Merighi MAB. Experience of adults and older people with adherence to venous ulcer care. Rev Gaucha Enferm. 2019;40:e20180024.
- Weller CD, Richards C, Turnour L, Team V. Patient Explanation of Adherence and Non-Adherence to Venous Leg Ulcer Treatment: A Qualitative Study. Frontiers in Pharmacology. 2021;12.
- Shannon MM, Hawk J, Navaroli L, Serena T. Factors affecting patient adherence to recommended measures for prevention of recurrent venous ulcers. J Wound Ostomy Continence Nurs. 2013;40(3):268-74.
- Augustin M, Blome C, Zschocke I, Schäfer I, Koenig S, Rustenbach SJ, et al. Benefit evaluation in the therapy of chronic wounds from the patients’ perspective-development and validation of a new method. Wound Rep and Reg. 2012;20(1):8-14.
- McNichol E. Involving patients with leg ulcers in developing innovations in treatment and management strategies. British Journal of Community Nursing. 2014;19(Sup9):S27-S32.
- Squitieri L, Tsangaris E, Klassen AF, van Haren E, Poulsen L, Longmire NM, et al. Patient-reported experience measures are essential to improving quality of care for chronic wounds: An international qualitative study. Int Wound J. 2020;17(4):1052-61.
- Goodney P, Shah S, Hu YD, Suckow B, Kinlay S, Armstrong DG, et al. A systematic review of patient-reported outcome measures patients with chronic limb-threatening ischemia. J Vasc Surg. 2022;75(5):1762-75.
- Weller CD, Richards C, Turnour L, Team V. Rationale for participation in venous leg ulcer clinical research: Patient interview study. Int Wound J. 2020;17(6):1624-33.
- Gould LJ, Liu J, Wan R, Carter MJ, Dotson M, Driver VR. Evidence supporting wound care end points relevant to clinical practice and patients’ lives. Part 3: The Patient Survey. Wound Rep and Reg. 2020;29(1):60-9.
- Weller CD, Evans S. Monitoring patterns and quality of care for people diagnosed with venous leg ulcers: the argument for a national venous leg ulcer registry. Wound Practice & Research, . 2014;22(2):68-72.
- Bell SK, Bourgeois F, DesRoches CM, Dong J, Harcourt K, Liu SK, et al. Filling a gap in safety metrics: development of a patient-centred framework to identify and categorise patient-reported breakdowns related to the diagnostic process in ambulatory care. BMJ Quality & Safety. 2022;31(7):526-40.
- Lecouturier J, Scott J, Rousseau N, Stansby G, Sims A, Allen J. Peripheral arterial disease diagnosis and management in primary care: a qualitative study. BJGP Open. 2019;3(3).
- Sacco AY, Self QR, Worswick EL, Couperus CJ, Kolli SS, Muñoz SA, et al. Patients’ Perspectives of Diagnostic Error: A Qualitative Study. Journal of Patient Safety. 2021;17(8):e1759-e64.
- Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M. Cost-of-illness studies in chronic ulcers: a systematic review. J Wound Care. 2017;26(sup4):S4-S14.
- Urwin S, Dumville JC, Sutton M, Cullum N. Health service costs of treating venous leg ulcers in the UK: evidence from a cross-sectional survey based in the north west of England. BMJ Open. 2022;12(1):e056790.
- Augustin M, Brocatti LK, Rustenbach SJ, Schäfer I, Herberger K. Cost-of-illness of leg ulcers in the community. Int Wound J. 2014;11(3):283-92.
- Purwins S, Herberger K, Debus ES, Rustenbach SJ, Pelzer P, Rabe E, et al. Cost-of-illness of chronic leg ulcers in Germany. Int Wound J. 2010;7(2):97-102.
- Lo ZJ, Lim X, Eng D, Car J, Hong Q, Yong E, et al. Clinical and economic burden of wound care in the tropics: a 5-year institutional population health review. Int Wound J. 2020;17(3):790-803.
