Volume 24 Number 2
Pyoderma gangrenosum and peripheral arterial disease: a case series and literature review
Adriana L Caixinha, Karsten Fogh, Rikke Bech
Keywords Pyoderma gangrenosum, peripheral arterial disease, prognosis, risk factor
For referencing Caixinha A, Fogh K & Bech R. Pyoderma gangrenosum and peripheral arterial disease: a case series and literature review. Journal of Wound Management 2023;24(2):14-18.
DOI
https://doi.org/10.35279/jowm2023.24.02.05
Submitted 1 February 2023
Abstract
Background Pyoderma gangrenosum (PG) is a neutrophilic dermatosis associated with systemic inflammatory diseases. Peripheral arterial disease (PAD) is a manifestation of atherosclerosis, which is a chronic inflammatory disease.
Hypothesis Is the presence of PAD a risk factor for the prognosis of PG?
Methods We performed a retrospective medical chart review of seven patients with an overlap of PAD and PG and compared treatment strategies and outcomes.
Results Six out of seven patients had a poor outcome, requiring amputation, even though adequate treatment was initiated.
Conclusions We propose that PAD, whether by reducing the healing potential or by partially contributing to the pathophysiology of the wounds, is a risk factor for the prognosis of PG. Furthermore, the fact that three of the patients developed PAD within a very short period of time after the diagnosis of PG suggests that PG could itself be a risk factor for the development of PAD. However, more clinical data is required to adequately assess this possible relation.
Implications for clinical practice Raise awareness to the possible co-existency of both diagnoses and the importance of early screening.
Key messages
- In this case series we describe the comorbidities, treatment strategies and outcomes of seven patients that were diagnosed with both pyoderma gangrenosum (PG) and peripheral arterial disease (PAD).
- Our goal is to raise awareness to the possible co-existence of these diagnoses in the presence of treatment refractory wounds.
- Our data suggests that the presence of PAD is a risk factor for the prognosis of PG. Furthermore, our data suggests that PG could itself be a risk factor for the development of PAD. However, more clinical data is required to adequately assess this possible relation.
Introduction
Pyoderma gangrenosum (PG) is a rare neutrophilic dermatosis characterised by a single or multiple, rapidly expanding and painful ulcerations, typically located on the lower extremities. Its pathophysiology is not yet well understood, but PG is thought to be an autoinflammatory disorder driven by T cells1. Different variants have been described, such as bullous, pustular, peristomal, vegetative and postoperative PG, as well as the ulcerative subtype which is the most common2.
The diagnosis of PG is challenging due to the fact that there are no specific laboratory tests or histopathological findings, and that the clinical presentation is variable. As a result, multiple diagnostic criteria have been proposed, initially in 2004 by Su et al3, followed by Maverakis et al4 in 2018 with the Delphi process and lastly by Jockenhöfer et al who proposed the PARACELSUS score system in 20195.
PG has been associated to different systemic diseases, especially inflammatory bowel disease, rheumatologic and haematologic disorders. Furthermore, a relatively high prevalence of cardiovascular disorders has been described among PG patients, of which peripheral arterial disease (PAD) is of particular interest6–8.
PAD is a common manifestation of atherosclerosis, with different clinical presentations. The major risk factors for PAD are smoking, hypertension, hyperlipidaemia, diabetes mellitus (DM), obesity and family history of vascular disease. It has also been associated to different inflammatory diseases, as inflammation is involved in the pathogenesis of atherosclerosis9,10.
According to the American College of Cardiology/American Heart Association (ACC/AHA)11 there are four main clinical presentations of PAD – asymptomatic, intermittent claudication, chronic limb ischaemia and acute limb ischaemia. PAD is often generalised, but the involvement of the lower extremities is common11.
The diagnosis of PAD involves the measurement of the ankle-brachial index (ABI), the toe-ankle index (TBI) and/or vascular imaging techniques such as duplex ultrasound, digital subtraction angiography, computed tomography angiography and magnetic resonance angiography12. An ABI of under 0.9 is diagnostic for PAD. TBI values lower than 0.7 are considered abnormally reduced, indicating presence of PAD.
In this study we describe seven patients with the combination of PG and PAD.
Case Report/Case Presentation
Patients meeting inclusion criteria were identified by specialists in dermatology at the dermatology department of Aarhus University Hospital in Aarhus, Denmark.
Patients were included if they presented ulcerations that clinically and, when available, histologically were consistent with PG, and if the patients concomitantly had a diagnosis of PAD confirmed by TBI and/or CT-angiography. We identified an ulcer as PG based on the clinical presentation of a rapidly expanding painful ulcer with undermined violet edges.
We identified seven patients within the period of 1 January 2012 and 1 January 2022 and performed a retrospective medical chart review and literature review. Baseline characteristics of the seven patients that were included are summarised in Table 1.
Table 1. Summary of clinical characteristics in the current series of patients with PG and PAD
The mean age of PG onset was 69 years (range 60–82) and the mean age of PAD onset was 71 years (range 55–84). Four patients were men. All ulcerations were located in the lower extremities. All the patients, apart from Cases 2, 5 and 7, were overweight, with a mean BMI of 32.3. Three patients were active smokers, one was a previous smoker and three had never smoked. Other comorbidities included arterial hypertension (all patients), type 2 DM (two patients), type 1 DM (one patient), ischaemic heart disease (three patients), atrial fibrillation (two patients), and venous insufficiency (one patient).
All of the biopsies from our patients showed non-specific findings with evidence of inflammatory infiltrates. Among these, neutrophilic infiltrates were observed in biopsies from four patients, which is typically associated with histologic findings in PG. Unfortunately, there are no specific histologic features that support a diagnosis of PG. However, the absence of characteristic histologic findings for other types of ulcers is helpful in excluding other differential diagnoses, such as Martorell’s ulcer.
Martorell’s ulcer is a skin ulcer that is commonly associated with poorly controlled hypertension. Given that all patients in our case series had a diagnosis of arterial hypertension, we considered Martorell’s ulcer as a relevant differential diagnosis to exclude. This type of ulcer usually presents on the posterior side of the lower extremities and has a distinctive appearance, with a central black eschar surrounded by a yellow or red halo. However, none of our patients showed these typical signs of Martorell’s ulcer. Furthermore, the histological findings from biopsies of our patients did not support a diagnosis of Martorell’s ulcer. Specifically, there was no evidence of hypertrophy in the dermis, subcutaneous tissue or vessel walls, nor was there any narrowing of the vessel lumen or hyper- or para-keratosis of the epidermis, which are typical findings of Martorell’s ulcer.
With the exception of one patient (Case 1), all of the patients were diagnosed with PG before being diagnosed with PAD, with a mean elapsed time of 2.57 years between diagnoses. Cases 2, 3 and 7 had, shortly after being diagnosed with PG, normal TBI values, developing severely reduced TBI within a mean period of time of 55.3 months.
Table 2 summarises the treatment strategies and the outcomes of the seven patients. All patients but one (Case 1) were treated with oral prednisolone. Moreover, all patients were treated with other immunomodulatory agents such as azathioprine, methotrexate, mycophenolate mofetile, ciclosporine, intravenous immunoglobulins and dapsone. Five patients were treated with infliximab. Case 5 was diagnosed with chronic lymphocytic leukaemia and was treated with rituximab in the haematologic department.
Table 2. Summary of treatment strategies and outcomes in the current series of patients with PG and PAD
Patients were commenced on both conservative and, when indicated, invasive treatment strategies due to their PAD. Out of the seven patients, three underwent bilateral femur amputation (Cases 3 (Figure 1), 5 and 7), three unilateral femur amputation (Cases 1 (Figure 2), 2 and 6) while only one patient experienced resolution of the ulcerations (Case 4 (Figure 3)).
Case 4 received treatment with high doses prednisolone up to 35mg daily for 22 months, mycophenolate mofetile up to 1,500mg daily for 18 months, ciclosporine up to 300mg daily for 2 months, methotrexate up to 15mg weekly for 24 months and infliximab for a period of 40 weeks (1.5 years after diagnosis). There was no indication for invasive treatment of the PAD, but the patient received statins and anticoagulant. The treatment strategies for this patient did not substantially differ from the strategies applied to the other patients.
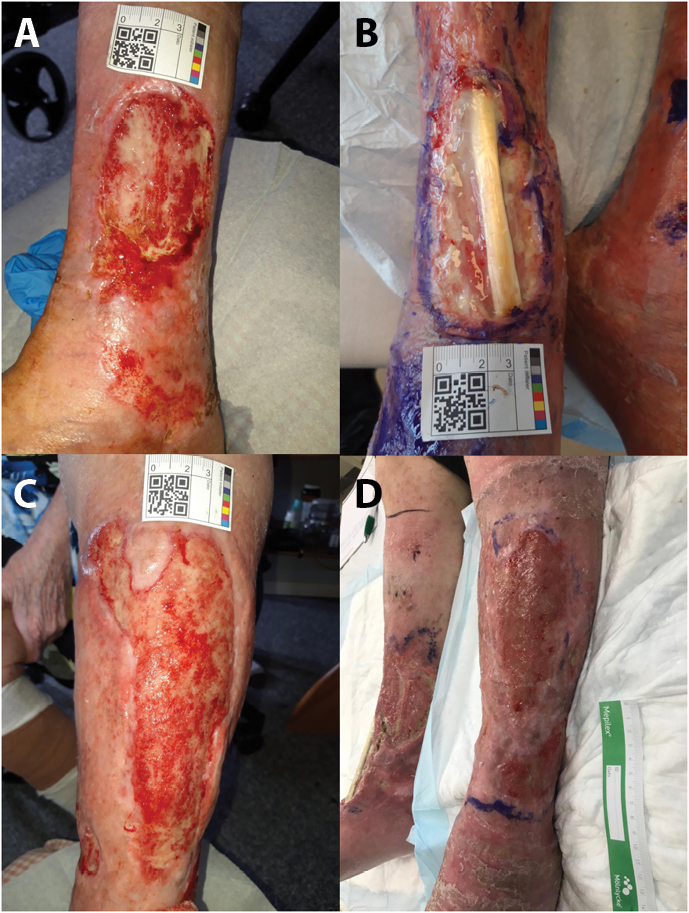
Figure 1. Case 3
A: 6 September 2021 showing a PG ulcer on the medial side of the right crus
B: 8 November 2021 showing severe progression after 2 months
C: 6 September 2021 showing a PG ulcer on the lateral side of the left crus
D: 8 November 2021 showing severe progression after 2 months
The patient had a bilateral amputation on 15 November 2021
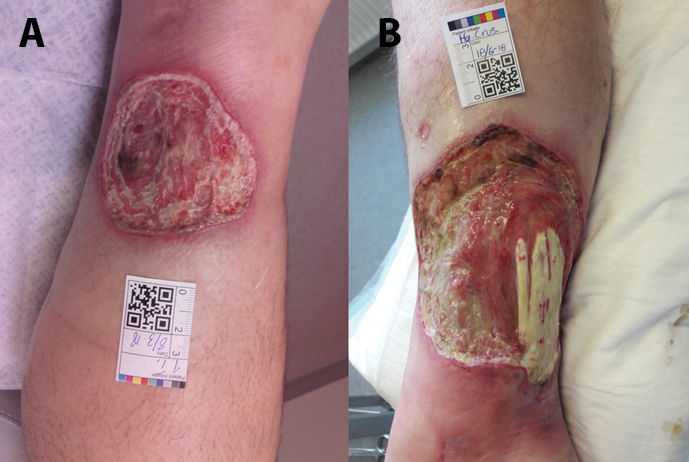
Figure 2. Case 1
A: 8 March 2018 showing a PG ulcer on the medial side of the right crus
B: 18 June 2018 showing severe progression
The patient had a unilateral right femur amputation on 24th July 2018
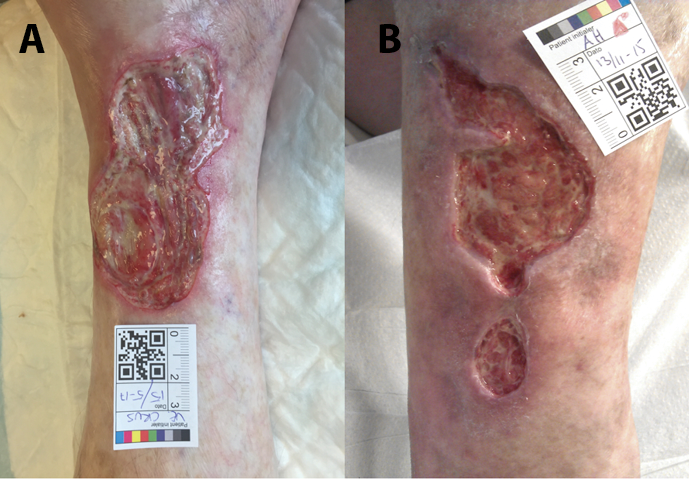
Figure 3. Case 4
A: 15 May 2017 showing an ulcer on the medial side of the left crus
B: 11 November 2015 of another ulcer on the medial side of the right crus
The patient’s ulcers healed completely
Discussion/Conclusion
The management of PG is challenging and poorly described in the literature. The association of PG and systemic diseases is extensively described. However, reports of simultaneous PAD and PG are only found in few epidemiologic reports13,14.
The presence of PAD restrains the amount of oxygenated blood that reaches the extremities, leading to the formation of ischaemic ulcers in severe cases. If wounds of other aetiology are present, the reduced oxygenation of the peripheral tissues will inhibit or prolong the healing potential of these wounds.
Wound healing is a complex process divided in three main phases: inflammation, proliferation and remodelling. Multiple local factors, such as ischaemia and infection, can influence wound healing. Healing, especially in the proliferative phase, requires large quantities of energy which demands a rich blood supply to provide glucose and oxygen15.
Invasive treatment of PAD was indicated in four out of the seven cases. The diagnosis of PAD in Cases 4, 5 and 6 was made at a stage that was too advanced to allow the performance of these procedures. In the presence of PG, it is, therefore, important to keep in mind the possible concomitant presence of PAD and examine the patient by palpation of peripheral pulses and measure TBI in early stages.
In the present case series, six out of seven of our patients had a poor outcome, ultimately requiring either bilateral or unilateral amputation, even though aggressive therapies were initiated in early stages. These findings suggest that the presence of PAD is a risk factor for poor prognosis of PG.
Furthermore, in three of our cases (Cases 2, 3 and 7), normal or close-to-normal TBI values were initially obtained, while subsequent TBI measurements, obtained between 21 and 79 months later, showed severely decreased values, representing severe PAD. Inflammation is central in the pathophysiology of both PAD and PG, and the rapid development of PAD in almost half of our patients could suggest that the presence of PG could be a trigger or a risk factor for the development of PAD. This assumption suggests that inflammation is a major component in the progress of both PAD and PG.
The development of PAD can also be explained by other factors, given that other known risk factors, such as arterial hypertension and sedentarism, were also present. Likewise, the fact that these patients had a close follow-up with multiple consultations and regular examinations, can partly explain the findings of a rapid evolving PAD.
PG is a rare entity, and its management is challenging. The concomitant presence of PAD seems to aggravate the prognosis, resulting in amputation in a significant proportion of our patients. Early screening and diagnosis of PAD in the clinical setting of PG is important for the prognosis.
The rapid development of severe PAD in patients with initial normal TBI could suggest that presence of PG could trigger the development of PAD. More studies are required to optimise the management of this complex subgroup of PG patients and to investigate a possible causality effect between PAD and PG.
Statement of Ethics
Ethics approval is not required for this study in accordance with local guidelines. Written informed consent was obtained from the patients in Cases 1–5 and Case 7 for publication of the details of their medical case and any accompanying images. The next of kin of the deceased patient in Case 6 gave their written informed consent for the publication of the details of the patient’s medical case.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
None declared.
Author Contributions
Adriana Lourenco Caixinha read and analysed the medical charts data and wrote the manuscript, Rikke Bech and Karsten Fogh supervised and edited the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
The data in this study was obtained from private medical charts that are not publicly available. All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Author(s)
Adriana L Caixinha*1 MD, Karsten Fogh1 MD, DMSci, Rikke Bech1 MD, PhD
Department of Dermatology and Venereology, Aarhus University Hospital, Aarhus, Denmark
*Corresponding author email adrianacaixinha@gmail.com
References
- Maverakis E, Marzano AV, Le ST, Callen JP, Brüggen MC, Guenova E, et al. Pyoderma gangrenosum. Nat Rev Dis Primers 2020;6. doi:10.1038/S41572-020-0213-X.
- Powell FC, Su WPD, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol 1996;34:395–409. doi:10.1016/S0190-9622(96)90428-4.
- Daniel Su WP, Davis MDP, Weenig RH, Powell FC, Perry HO, Davis M. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004 Nov;43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x.
- Maverakis E, Ma C, Shinkai K, Fiorentino D, Callen JP, Wollina U, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi Consensus of International Experts. JAMA Dermatol 2018;154:461–6. doi:10.1001/JAMADERMATOL.2017.5980.
- Jockenhöfer F, Wollina U, Salva KA, Benson S, Dissemond J. The PARACELSUS score: a novel diagnostic tool for pyoderma gangrenosum. Br J Dermatol 2019;180:615–20. doi:10.1111/BJD.16401.
- Kridin K, Cohen AD, Amber KT. Underlying systemic diseases in pyoderma gangrenosum: a systematic review and meta-analysis. Am J Clin Dermatol 2018;19:479–87. doi:10.1007/s40257-018-0356-7.
- Jockenhöfer F, Gollnick H, Herberger K, Isbary G, Renner R, Stücker M, et al. Aetiology, comorbidities and cofactors of chronic leg ulcers: retrospective evaluation of 1 000 patients from 10 specialised dermatological wound care centers in Germany. Int Wound J 2016;13:821–8. doi:10.1111/iwj.12387.
- Schøsler L, Fogh K, Bech R. Pyoderma gangrenosum: a retrospective study of clinical characteristics, comorbidities, response to treatment and mortality related to prednisone dose. Acta Derm Venereol 2021;101. doi:10.2340/00015555-3776.
- Mascarenhas JV, Albayati MA, Shearman CP, Jude EB. Peripheral arterial disease. Endocrin Metab Clinic North Am 2014;43:149–66. doi:10.1016/j.ecl.2013.09.003.
- Campia U, Gerhard-Herman M, Piazza G, Goldhaber SZ. Peripheral artery disease: past, present, and future. Am J Med 2019;132:1133–41. doi:10.1016/J.AMJMED.2019.04.043.
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006;113:e463–654. doi:10.1161/CIRCULATIONAHA.106.174526.
- Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and the European Society for Vascular Surgery (ESVS). Eur Heart J 2018 Mar 1;39(9):763–816. doi:10.1093/eurheartj/ehx095.
- Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol 2011;165:1244–50. doi:10.1111/j.1365-2133.2011.10565.x.
- al Ghazal P, Körber A, Klode J, Dissemond J. Investigation of new co-factors in 49 patients with pyoderma gangrenosum. JDDG: J German Soc Dermatol 2012;10:251–6. doi:10.1111/j.1610-0387.2011.07734.x.
- Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg 2006 Jun;117(7 Suppl):1e-S-32e-S. doi:10.1097/01.prs.0000222562.60260.f9.
