Volume 24 Number 2
Effects of local antibiotics in calcium-sulphate granules for the treatment of diabetic forefoot osteomyelitis: a propensity-matched observational study
Benedetta Ragghianti, Alberto Piaggesi, Edoardo Mannucci, Matteo Monami
Keywords economic evaluation, foot ulcer, diabetes mellitus, osteomyelitis
For referencing Ragghianti B et al. Effects of local antibiotics in calcium-sulphate granules for the treatment of diabetic forefoot osteomyelitis: a propensity-matched observational study. Journal of Wound Management 2023;24(2):19-25.
DOI
https://doi.org/10.35279/jowm2023.24.02.06
Submitted 20 March 2023
Abstract
Background To assess the effects of a local antibiotic delivery system on the incidence of post-surgical infective complications after surgical procedures in patients with diabetic foot osteomyelitis (DFO).
Methods A retrospective study was carried out on patients with forefoot DFO associated with soft tissue infection undergoing minor amputations using local antibiotics in calcium-sulphate granules. Patients were matched with a historical series using propensity-score matching. The principal endpoint was a composite of relapse/recurrence/new onset of DFO, infection/dehiscence at the surgical site, re-intervention for abscesses drainage, and major amputation. Direct costs were analysed as a secondary endpoint.
Results Composite endpoint occurred in 19% and 36.4% (p=0.17) of cases and controls, respectively. Only three patients in the control group had recurrent DFO. After adjusting for ulcer duration, the risk of infective complications and major amputation was significantly lower (Hazard Ratio [HR] 0.20 [0.04;0.95], p=0.047) and the 90-day healing rate was significantly higher (HR 4.44 [1.03;19.07], p=0.045) in cases than in controls. The median direct healthcare costs for cases and controls during the 90-day follow-up were €2,050 [1,829;3,946] and €1,731 [1,028;14,817] per patient, respectively (p=0.072). Median costs for antibiotics were lower for cases than controls (p<0.001).
Conclusions The use of calcium-sulphate granules as an add-on therapy to surgical treatment of DFOs reduces post-surgical infections and complications, without increasing direct costs.
Introduction
Diabetic foot ulcers (DFUs) are a major complication of diabetes mellitus (DM) and are associated with a high risk of major amputations and mortality1–3. In patients with DM, the incidence of lower extremity amputations (LEA) ranges from 70 to 700 per 100,000 person-years4 and 5-year survival after an amputation appears to be similar to that of patients with malignancies (around 60–70%)5. Moreover, DFUs have a detrimental effect on quality of life and disability6. Last but not least, DFUs have a relevant economic impact, with estimated mean yearly costs ranging from US$650 million to over US$1 billion7–13.
Diabetic foot osteomyelitis (DFO) further increases the risk of major amputations and mortality in patients with DFU, with relevant consumption of economic resources14. Moreover, DFO often requires prolonged antibiotic therapy and extensive surgical debridement with a relevant risk of perioperative complications, possibly delaying healing15,16. Patients with DFO are often affected by multiple comorbid conditions such as peripheral artery disease, which can limit the efficacy of systemic antibiotic therapy for insufficient tissue penetration17, as well as renal or liver insufficiency which may contra-indicate a prolonged antibiotic therapy. Moreover, antibiotic-resistant bacteria can prevent the use of many antibiotics18,19.
Recently, several devices capable of releasing antibiotics at local level have been developed to overcome some of these criticisms, thus reducing the risk of post-surgical infections, possibly accelerating healing processes; however, clinical reports are still scarce and related to limited experiences20–22. The assessment of the economic impact of this approach is also lacking.
The aims of the present cohort study are the assessment of the incidence of post-surgical infective complications, healing rates and time-to-healing, and direct healthcare costs in patients with forefoot DFO undergoing surgical procedures and treated with local antibiotics in calcium-sulphate granules, as compared with a propensity-matched historical control sample.
Patients, materials and methods
Patients
The present analysis was retrospectively performed on a consecutive series of patients with diabetes and forefoot DFO who underwent minor amputation (i.e. toe amputation, metatarsal-phalangeal osteoarthrotomy and/or metatarsal osteotomy) using local antibiotics in calcium-sulphate granules (Stimulan®, Biocomposites Ltd, UK) at the Diabetic Foot Unit of Careggi Hospital, Florence, Italy, between 1 June 2021 and 1 June 2022. Patients were included if they fulfilled the following criteria:
- Diagnosis of DM.
- Texas 3B or 3D23 forefoot DFO and clinical infection of deep tissue.
- TcPO2 (transcutaneous oximetry) measured on the dorsum of the affected foot before surgical procedure ≥30mmHg.
- Follow-up of at least 90 days.
Cases were compared with an historical cohort of patients undergoing the same procedures with the exception of the antibiotic-loaded calcium-sulphate granules inception.
A propensity score using variables that might have affected treatment assignment or outcomes was developed, following the protocol used by Kosiborod and colleagues24. Candidate variables used in the development of the propensity score were: age, sex, duration of diabetes, and site of DFO. Matching was performed by randomly selecting (the first patient in the historical dataset) at least one patient with the same propensity score (with a ratio of at least 1:1) ± 1SD.
The study protocol was approved by the local ethical committee (Protocol number: 22331_OSS), and informed consent was obtained from all patients before inclusion in the analysis.
Baseline data collection
Demographic and clinical data were collected from clinical records, including a medical history with detailed information on the duration of diabetes, complications and concomitant medical conditions, current pharmacological treatment, cardiovascular risk factors, self-reported smoking habits, and any other relevant medical conditions. At the first visit, following an established standard procedure of the Clinic, all patients underwent a physical examination, during which their weight, height and blood pressure were recorded. Results of laboratory determinations (HbA1c, creatinine, LDL cholesterol calculated with Friedewald formula25) performed within 3 months prior to the first visit were recorded.
Pain at the first visit was assessed using a visual analogue scale (VAS) ranging from 0–100mm. As per local standard of care, transcutaneous pressure of oxygen (TcpO2; Radiometer Medical ApS; Brønshøj, Denmark) at the basis of the first toe and/or ankle-brachial index (ABI) were measured, and arterial duplex-scanning of lower limb arteries was performed. Diagnosis of diabetic neuropathy was performed by measuring both vibratory perception threshold (VPT)26 with a biothesiometer (METEDA, San Benedetto del Tronto, Italy) and a 10g monofilament; diagnosis of neuropathy was performed in case of non-sensitivity of 10g monofilament on at least one of the application points and/or VPT ≥25 volts at the hallux or malleolus. DFU were classified according to the University of Texas score23, which ranges from 0=no lesions to 3=lesions deep to bone and joints, and graded from A=no infection and no ischaemia to D=infection and ischaemia. When more than one lesion was present, only the largest ulcer was taken into account (index lesion). Diagnosis of DFO was made using the probe-to-bone test using a sterile metal probe (considered positive when the probe met the hard surface of the bone at the base of the wound) in conjunction with a plain X-ray of the foot, which was performed in all patients and considered positive for DFO when it showed bone destruction, cortical reabsorption, periosteal reaction, or a sequestrum.
In all patients, a tissue biopsy with a scalpel or punch biopsy instrument, following Levine technique27, was taken for bacteriological analysis and culture.
Renal failure was defined as a reported previous diagnosis of renal failure, or as serum creatinine >1.5mg/dl. Ischaemic heart disease (IHD) and cerebrovascular disease were diagnosed when patients reported previous myocardial infarction/angina or stroke/transient ischaemic attack. Comorbidity was assessed through the calculation of Charlson’s comorbidity score (CCS)28.
DFU treatment
All patients underwent minor amputations following the standard of care of our unit; amputations were performed using loco-regional anaesthesia in an ambulatory setting.
Necrotic tissues, pus and infected soft tissues were removed until exposing healthy bleeding tissue. If DFO was located in the diaphysis, devitalised bones at the base of DFU were exposed and excised to the level of healthy cancellous and cortical bone. If possible, the bases of the metatarsal and phalangeal bones were preserved for healthy tendons attaching. When infection was located in interphalangeal or metatarsophalangeal joints, both the joints and partial distal and proximal bones were excised. Fibrous tissues, fascia and tendons nearby were also completely removed. Following bone resection, irrigations with a solution of iodopovidone were performed. Antibiotic-impregnated calcium-sulphate was then prepared. Vancomycin and/or tobramycin, on the basis of the results of the antibiogram, was mixed into the synthetic calcium-sulphate (Stimulan®, Biocomposites Ltd, UK) with the manufacturer-recommended ratio: 0.5g vancomycin with 5ml calcium-sulphate or 120mg tobramycin with 5ml calcium-sulphate. Then they were dissolved in a sterile saline solution and injected into the void space created by bone resection.
Further treatments
According to the Global Vascular Guidelines29, patients with critical limb ischaemia underwent percutaneous or surgical revascularisation prior to minor amputation. Local medications were performed following the International Working Group on Diabetic Foot (IWGDF) guidelines30. Empiric systemic antibiotic therapy was prescribed for up to 4 weeks and eventually modified on the basis of antibiogram30 in all patients. In all cases antibiotic therapy was stopped when clinical signs and symptoms of infections were resolved. Antibiotic therapy was continued after surgical procedure only in case of persistence of signs and/or symptoms of infection.
Therapeutic shoes (with pressure-relieving insoles) were used in all patients with plantar DFU, for pressure relief, as per the standard procedure of the Clinic.
Follow-up data
During the 90-day follow-up, re-infections, new surgical procedures for DFO recurrence, the total number of visits, duration of systemic antibiotic therapy, laboratory and instrumental exams, and hospital admissions were monitored.
Plain X-ray was repeated in all patients at 90 days to evaluate the eradication of DFO.
For patients with missing information on 1 October 2022, an attempt to retrieve information (including vital status) was made via telephone.
Endpoints
The primary endpoint of the study (within 90 days) was a composite of:
- Recurrence of DFO.
- New onset of DFO in other sites.
- New onset of tissue infection/dehiscence at the surgical site.
- Re-intervention for abscesses drainage or recurrence of DFO
- Major amputation.
New DFO or DFO recurrence was diagnosed if radiological signs of DFO were detected in the surgical site or in adjacent sites within 90 days. Dehiscence was defined as any infective process (PEDIS >131) in the surgical site within 7 days from surgical procedures.
Infective complications were defined as any infective process (PEDIS >1) in the surgical site that occurred between 7 days after surgical procedure and the end of the study.
Re-intervention was defined as the need for a new surgical intervention at the site of previous DFO in case of abscesses drainage or relapse of DFO.
Major amputation was defined as an amputation proximal to the ankle joint.
The choice of individual components of the primary endpoint were made in order to explore the putative protective effects of local antibiotics in calcium-sulphate granules on post-surgical infective complications.
Secondary endpoints were:
- Healing rate.
- Time to healing.
- Total number of visits.
- Major amputation rate.
- Re-intervention rate.
- Severe adverse events (SAE).
- Direct costs.
Healing was defined as complete epithelialisation of the wound with the absence of drainage, confirmed at two follow-up visits (the first one was taken as referral to calculate time to healing).
SAE were defined as any event or adverse reaction which corresponds to one or more of the following criteria: a) fatal outcome; b) life–threatening; c) requires hospitalisation or determines a prolongation of it.
Economic assessment
The economic assessment was performed taking the local health system perspective into account. Thus, only direct healthcare costs were considered, and costs associated with healthcare resource use for the duration of follow-up were extracted from clinical records. In detail, direct costs included specialists’ visits, diagnostic procedures, hospital admissions (related to diabetic foot), major and minor amputations, antibiotic therapy, grafts, and off-loading orthesis (Tables 1&2).
Table 1. Microbiological findings in two groups
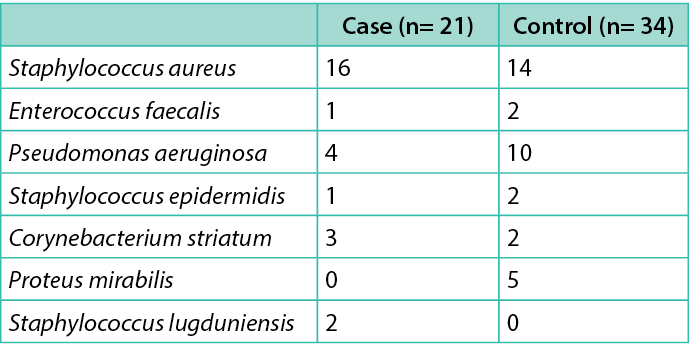
Table 2. Costs (€) for hospital admission for foot-related conditions
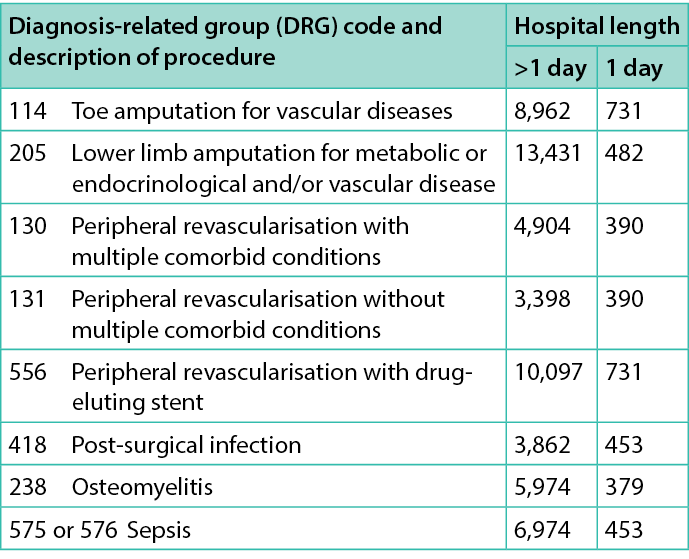
Costs for hospitalisations were estimated on the basis of established regional tariffs (https://www.salute.gov.it/portale/temi/p2_6.jsp?id=3662&area=programmazione SanitariaLea&menu=vuoto), i.e. tariffs established for the diagnosis-related group (DRG) associated with each episode for hospital admissions (either day-hospital or full-length stay) and recorded in clinical records; similarly for costs related to specialistic visits and outpatient procedures performed (e.g. RX, MRI, laboratory exams, etc.). The cost of antibiotic therapy was estimated considering ex-factory prices (https://www.salute.gov.it/portale/temi/p2_6.jsp?id=3662&area=programmazione SanitariaLea&menu=vuoto),while current market prices were used to value costs for orthopaedic shoes/orthesis. The health economic analysis performed tried to estimate costs born to the healthcare system, mainly using tariffs related to different healthcare services over 1 year. All costs were referred to 2020.
Statistical analysis
Statistical analysis was performed on SPSS 25.0 (SAS Institute, Cary, Ill). Data were expressed as mean ± standard deviation, or as median (25–75th percentile), depending on their distribution. Costs were reported considering both mean ± standard deviation and median (25–75th percentile). Comparisons between groups were performed using Student’s t-test for independent samples or Mann–Whitney U test when appropriate. Chi-square and Fisher exact test were used for between-group comparisons of categorical variables when appropriate. Kaplan–Meier method was used to derive the probability of healing over time. Conditional logistic regression was used for multivariate analysis, in order to adjust for duration of ulcer; no other adjustments were made, as cases and controls had been matched for the other potential confounders.
Results
The study enrolled 21 patients, compared with 34 matched controls, with a ratio 1:1.6. No significant differences were observed between cases and controls, except for duration of ulcer (Table 3). Microbiological findings in cases and controls are summarised in Table 1. All patients were followed for 90 days (or until they died or underwent major amputation) after the surgical procedure. No patient was lost to follow-up. Two patients in the control group, and none among cases, died (after major amputation) during follow-up. Four more patients in the control group, and none among cases, underwent a hospitalisation during follow-up (three for revascularisation and one for sepsis). The mean duration of follow-up was 75±21 days.
Table 3. Main anthropometric and demographic characteristics of the enrolled cohort and of observed ulcers
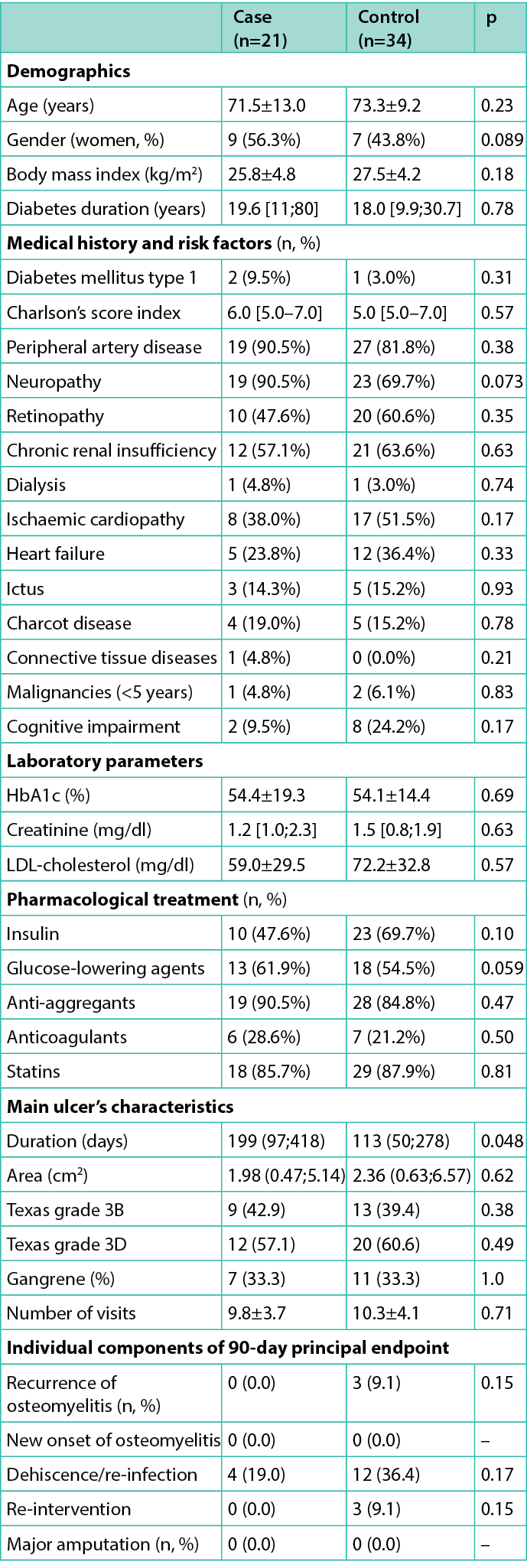
The 90-day composite endpoint was achieved by four cases (19.0%) and 15 controls (45.5%) as reported in Figure 1 (p=0.057). Information on individual components of the primary endpoint are reported in Table 3: dehiscence (within 7 days from surgical procedure) and re-infection (after 7 days) occurred in 19.0% and 36.4% (p=0.17) of cases and controls, respectively. Only three patients (9.1%) in the control group had a recurrence of DFO (p=0.15) and underwent a new surgical operation; no episode of new onset DFO was observed. No major amputations occurred within 90 days.
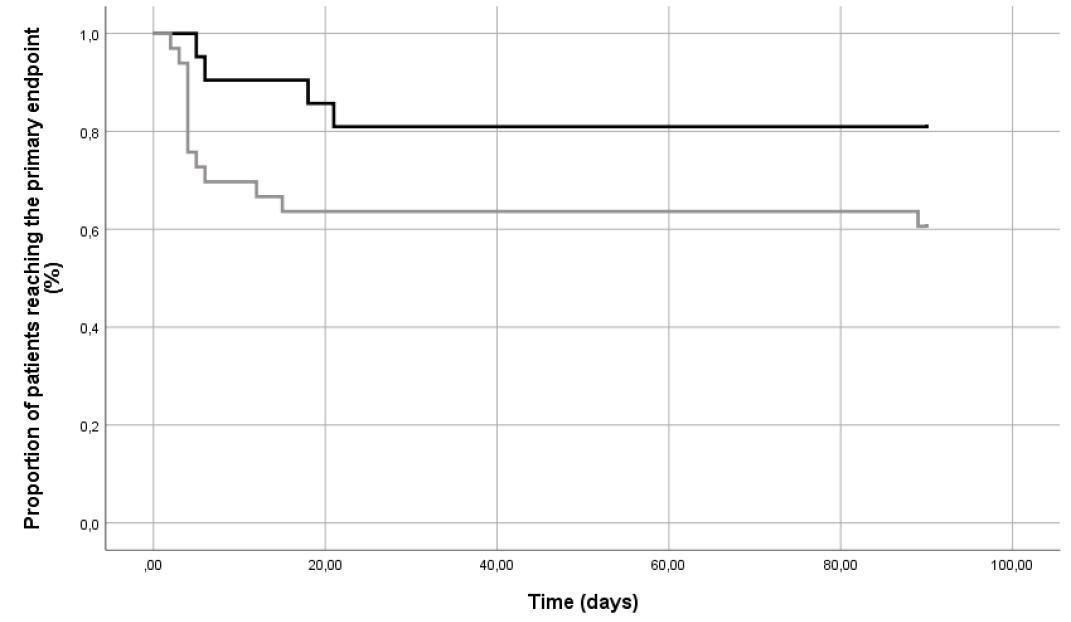
Figure 1. Proportion of patients (%) reaching the primary endpoint during the follow-up. Black line: cases; grey line: controls
Healing rate within 90 days for cases and controls was 61.9% and 45.5% (p=0.18), with time-to-healing of 78 (45;90) and 90 (73;90) days (p=0.26), respectively.
At conditional regression analysis, after adjusting for ulcer duration, the risk of post-surgical re-infection (recurrence of DFO, new onset of DFO, new onset of tissue infection/dehiscence and re-intervention) and major amputations (primary endpoint) was significantly lower in cases than in controls (HR 0.20 [0.04;0.95], p=0.047); in addition, the 90-day healing rate was significantly higher in cases than in controls [HR 4.44 (1.03;19.07), p=0.045].
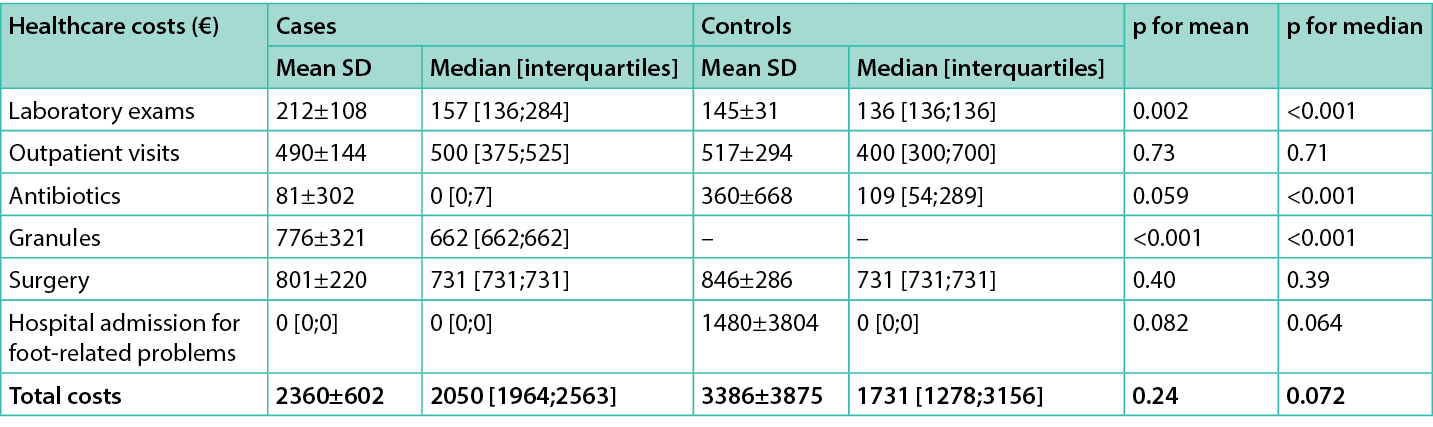
Direct healthcare costs for hospital admission for foot-related conditions and for ulcer management are listed in Tables 2&4. The mean and median direct healthcare costs per-patient for cases during the 90-day follow-up were €2,360±602 and €2,050 [1,964;2,563], respectively. The same figure for controls was €3,386±3,875 and €1,731 [1,278;3,156]. No statistically significant differences were observed for overall costs (all p>0.05).
Costs (median) during the 90-day follow-up for antibiotics were significantly lower in cases, whereas costs for exams were significantly lower in the control group. A non-statistical trend toward reduction of costs for hospital admission was also observed in favour of cases (Table 5).
Table 4. Costs (€) associated with procedures and laboratory examinations

Table 5. Average costs (€) per cases and controls over the 90 day follow-up
Discussion
We show here for the first time a comparison between patients with DFO treated with antibiotic-impregnated calcium-sulphate granules, and control patients, matched for propensity score. The principal endpoint (a composite of infection and amputation) was reduced by 80%, and healing showed a more than four-fold increase in comparison with controls, after adjusting for the duration of DFUs.
These results are of interest considering the high risk of major amputation in patients with DFO. In fact, DFO treatment is particularly challenging for several reasons. The penetration of antibiotics at bone level is difficult, usually achieving insufficient therapeutic concentrations, mainly because of concomitant peripheral vascular disease. Moreover, the presence of many comorbidities, such as renal insufficiency, limits the long-term use of many antibacterial compounds.
Several different local delivery systems have been explored as adjunctive therapies in order to overcome these problems, such as antibiotic-impregnated collagen sponges which have been demonstrated in randomised clinical trials to significantly improve the DFO prognosis32–34. An alternative approach is represented by antibiotic-impregnated calcium-sulphate granules, despite the scarce evidence mainly derived from retrospective uncontrolled studies35,36. The putative advantages of calcium-sulfate granules in comparison with other local delivery systems (e.g., carriers with protein or synthetic polymers, grafts37 etc.) include biodegradability, predictable elution characteristic osteoconductivity, and ability to fill void space after bone resection38. All these favourable characteristics could have positive effects on healing and amputation rates, as suggested by retrospective studies. However, available studies are affected by a number of limitations, such as heterogeneity of DFO (for site, concomitant deep-tissue infection, etc.), small sample sizes, and heterogeneity of surgical techniques used in combination with antibiotic-impregnated calcium-sulphate granules. There is only one other case control study on this device, reporting a reduction of DFO recurrence, without increasing the healing rate22. In our study, we observed a similar reduction of post-surgical infective complications, but with a higher proportion of patients healed at 90 days. Since outcomes are largely affected by the characteristics of enrolled samples, results obtained with this treatment should be compared with those of a control group.
In our study, we decided to use propensity score matching, a technique capable of minimising the distortion determined by prescription bias in observational studies24. With this approach, the cohort of patients receiving treatment is compared with a cohort of control patients selected within the same reference population, matched for a score that summarises the chance of receiving the investigated treatment. Although this technique can improve the reliability of results, in comparison with traditional adjusted analyses of cohort or case control studies, the possibility of residual confounders cannot be completely ruled out. It is therefore possible that the difference in outcome between cases and controls is partly determined by differences in characteristics of the two samples which were not accounted for in the definition of propensity score.
Moreover, a multivariate analysis was performed in order to adjust for the duration of DFUs, which was significantly different between cases and controls. It is well known that the duration of DFUs is associated with a greater severity of DFO, possibly affecting the results obtained. After adjusting for DFU duration, the risk of major adverse lower limb events was significantly lower in patients allocated to the treatment group.
The use of new devices and techniques is often limited by high costs. However, the cost of the device is only a small part of direct costs for the clinical management of DFOs, which also include antibiotics, hospital admission, specialists’ visits, laboratory examination, etc. For this reason, we performed an analysis of direct costs recorded in cases and controls, suggesting that the use of antibiotic-impregnated calcium-sulphate granules is affordable and cost-effective. In fact, the cost of the device seems to be balanced by savings for antibiotic therapy and hospital admission.
Despite the limitations of the present retrospective single-centre study, we believe that the use of antibiotic-impregnated calcium-sulphate granules in DFO treatment could be safe and cost-effective; the actual efficacy and safety of antibiotic-impregnated calcium-sulphate granules in DFO should be confirmed by randomised controlled trials.
Author contribution
BR and MM designed the study and contributed to data collection, interpretation and writing. AP revised the manuscript and contributed to interpretation of data. EM contributed to writing and supervised the data quality control. MM is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
The authors declared no potential conflict of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and or publication of this article.
Ethical Approval
This study was submitted to local Ethical Committee (Area Vasta Centro, Firenze) and received formal approval (Protocol number: 22331_OSS).
Author(s)
Benedetta Ragghianti1, Alberto Piaggesi2, Edoardo Mannucci1, Matteo Monami*1
1Diabetology, Careggi Hospital and University of Florence, Florence, Italy
2Diabetic Foot Section, Department of Medicine, University Hospital of Pisa, Italy
*Corresponding author email matteo.monami@unifi.it
References
- Lazzarini PA, Pacella RE, Armstrong DG, van Netten JJ. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabet Med 2018 May 23. doi:10.1111/dme.13680. Epub ahead of print.
- Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: from ulceration to death, a systematic review. Int Wound J 2016;13(5):892–903.
- Brownrigg JR, Davey J, Holt PJ, et al. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia 2012;55(11):2906–12.
- Narres M, Kvitkina T, Claessen H, et al. Incidence of lower extremity amputations in the diabetic compared with the non-diabetic population: a systematic review. PLoS One 2017;12(8):e0182081.
- National Institute for Health and Care Excellence. Surveillance report 2017 – peripheral arterial disease: diagnosis and management. NICE guideline CG147. London: National Institute for Health and Care Excellence; 2012.
- Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev 2001;17(4):246–9.
- Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018;21(1):27–32.
- Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabet Med 2014;31(12):1498–504.
- Kähm K, Laxy M, Schneider U, Rogowski WH, Lhachimi SK, Holle R. Health care costs associated with incident complications in patients with type 2 diabetes in Germany. Diabetes Care 2018;41(5):971–978.
- Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 2010;100(5):335–41.
- Girod I, Valensi P, Laforêt C, Moreau-Defarges T, Guillon P, Baron F. An economic evaluation of the cost of diabetic foot ulcers: results of a retrospective study on 239 patients. Diabetes Metab 2003;29(3):269–77.
- Tchero H, Kangambega P, Lin L, et al. Cost of diabetic foot in France, Spain, Italy, Germany and United Kingdom: a systematic review. Ann Endocrinol (Paris) 2018;79(2):67–74.
- Habacher W, Rakovac I, Görzer E, et al. A model to analyse costs and benefit of intensified diabetic foot care in Austria. J Eval Clin Pract 2007;13(6):906–12.
- Monami M, Ragghianti B, Nreu B, et al. Major amputation in non-healing ulcers: outcomes and economic issues. data from a cohort of patients with diabetic foot ulcers. Int J Low Extrem Wounds; 2022 Apr 28. Epub ahead of print.
- Monami M, Longo R, Desideri CM, Masotti G, Marchionni N, Mannucci E. The diabetic person beyond a foot ulcer: healing, recurrence, and depressive symptoms. J Am Podiatr Med Assoc 2008;98(2):130–6.
- Smith-Strøm H, Iversen MM, Igland J, et al. Severity and duration of diabetic foot ulcer (DFU) before seeking care as predictors of healing time: a retrospective cohort study. PLoS One 2017;12(5).
- Mader JT, Shirtliff ME, Bergquist SC, Calhoun J. Antimicrobial treatment of chronic osteomyelitis. Clin Orthop Relat Res 1999;360:47–65.
- Hajdu S, Lassnigg A, Graninger W, Hirschl AM, Presterl E. Effects of vancomycin, daptomycin, fosfomycin, tigecycline, and ceftriaxone on Staphylococcus epidermidis biofilms. J Orthop Res 2009;27(10):1361–5.
- Asten SAV, Mithani M, Peters EJG, La Fontaine J, Kim PJ, Lavery LA. Complications during the treatment of diabetic foot osteomyelitis. Diabetes Res Clin Pract 2018;135:58–64.
- Drampalos E, Morrissey N, Jahangir N, Wee A, Pillai A. Adjuvant antibiotic loaded bio composite in the management of diabetic foot osteomyelitis: a multicentre study. Foot (Edinb) 2019;39:22–27.
- Morley R, Rothwell M, Stephenson J, McIlvenny L, Webb F, Barber A. Complex foot infection treated with surgical debridement and antibiotic loaded calcium sulfate: a retrospective cohort study of 137 cases. J Foot Ankle Surg 2022;61(2):239–247.
- Qin CH, Zhou CH, Song HJ, et al. Infected bone resection plus adjuvant antibiotic-impregnated calcium sulfate versus infected bone resection alone in the treatment of diabetic forefoot osteomyelitis. BMC Musculoskelet Disord 2019;20(1):246.
- Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. J Foot Ankle Surg 1996;35(6):528–31.
- Kosiborod M et al. Rates of myocardial infarction and stroke in patients initiating treatment with SGLT2-inhibitors versus other glucose-lowering agents in real-world clinical practice: results from the CVD-REAL study. Diabetes Obes Metab 2018;20(8):1983–1987.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499–502.
- Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med 1998;158(3):289–92.
- Lipsky BA et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36(S1):e3280.
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47(11):1245–51.
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;58(1S):S1–S109.
- Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA, on behalf of the International Working Group on the Diabetic Foot (IWGDF). IWGDF guidelines on the prevention and management of diabetic foot disease. IWGDF; 2019.
- Chuan F, Tang K, Jiang P, Zhou B, He X. Reliability and validity of the perfusion, extent, depth, infection and sensation (PEDIS) classification system and score in patients with diabetic foot ulcer. PLoS One 2015;10(4):e0124739.
- Lipsky BA et al. Topical application of a gentamicin-collagen sponge combined with systemic antibiotic therapy for the treatment of diabetic foot infections of moderate severity: a randomized, controlled, multicenter clinical trial. J Am Pod Assoc 2012;102:223–232.
- Varga M, et al. Application of gentamicin-collagen sponge shortened wound healing time after minor amputations in diabetic patients: a prospective, randomised trial. Arch Med Sci 2014;10:283–287.
- Uckay I et al. A randomized, controlled study to investigate the efficacy and safety of a topical gentamicin-collagen sponge in combination with systemic antibiotic therapy in diabetic patients with a moderate or severe foot ulcer infection. BMC Infect Dis 2018;18:361.
- Krause FG et al. Outcome of transmetatarsal amputations in diabetics using antibiotic beads. Foot Ankle Int 2009;0:486–493.
- Chatzipapas C et al. Local antibiotic delivery systems in the surgical treatment of diabetic foot osteomyelitis: again, no benefit? Int J Low Extrem Wounds 2020. J Low Extrem Wounds 2022;21(4):555-561.
- Lalidou F, Kolios, G, Drosos GI. Bone infections and bone graft substitutes for local antibiotic therapy. Surg Tech Int 2014;24:353–362.
- Gauland C. Managing lower-extremity osteomyelitis locally with surgical debridement and synthetic calcium sulfate antibiotic tablets. Adv Skin Wound Care 2011;24:515–23.
