Volume 24 Number 3
Compression therapy in a patient with Klippel-Trénaunay syndrome
Hanna Hyvönen, Päivi Salminen, Milla Kallio, Heli Lagus, Kirsi Isoherranen
Keywords lymphoedema, compression therapy, compression garment, Klippel-Trénaunay syndrome, swelling
For referencing Hyvönen H et al. Compression therapy in a patient with Klippel-Trénaunay syndrome. Journal of Wound Management 2023;24(3):77-80.
DOI
10.35279/jowm2023.24.03.02
Submitted 31 May 2023
Accepted 19 September 2023
Abstract
Background Klippel-Trénaunay syndrome (KTS) is a rare congenital vascular malformation syndrome defined as capillary and venous malformations, and hypertrophy of the limb with or without lymphatic malformation. Currently there is no cure for KTS but the majority of patients are advised to wear compression garments on the affected limb to control swelling and discomfort.
Aim To describe used compression therapy methods and their results in a paediatric patient with KTS.
Methods The patient records were assessed for the used treatment methods for KTS. Our patient was treated with multimodal treatments to alleviate symptoms, and compression therapy was used specially to control lymphoedema and venous insufficiency.
Results A 13-year-old boy with KTS had lymphoedema and venous insufficiency of the right lower extremity since birth. Compression therapy was started at the age of 2 months with a custom-made, single-leg compression pantyhose with gradual compression from distal to proximal regions. Furthermore, a manual lymphatic drainage and intermittent pneumatic compression were added on the regimen respectively. Other treatment methods included sclerotherapy, surgery and medication. None of treatment methods was sufficient alone to reduce limb oedema but the patient reported improved performance when using compression garments and a positive impact after enhanced compression therapy.
Conclusion This report shows the importance of continuous and individualised compression therapy in the treatment of lymphoedema associated to KTS.
Key messages
- Compression therapy is essential in the treatment of Klippel-Trénaunay syndrome (KTS).
- To describe the compression therapy utilised in a paediatric patient with KTS.
- Individualised compression therapy is beneficial for alleviating the swelling and lymphoedema associated with KTS.
Introduction
Klippel-Trénaunay syndrome (KTS) is a rare congenital vascular malformation syndrome defined by the capillary and venous malformations with soft tissue and/or bone hypertrophy of the affected extremity, with or without lymphatic malformation1,2. This condition was first described in 1900 by two French physicians, Maurice Klippel and Paul Trénaunay3. KTS has been linked to somatic mutations in the phosphatidylinositol-4-5-bisphosphate 3 kinase, catalytic subunit (PIK3CA) gene, and it belongs to the PIK3CA-related overgrowth spectrum (PROS) which includes several overgrowth syndromes with overlapping clinical manifestations4. Herein, we report a case of a paediatric KTS patient with lymphoedema and venous insufficiency of the right lower extremity receiving multimodal treatments.
Methods
The patient records were assessed for the treatment options utilised for KTS. Our patient was treated with multimodal treatments to alleviate his symptoms, and compression therapy was used in an attempt to control the patient’s lymphoedema and venous insufficiency. The patient and his caregiver provided their written informed consent for the publication of this case report.
Case report
A male newborn was delivered by an elective caesarean section at a gestational age of 39 weeks with a birth weight of 3950g and a length of 51.5cm. The foetal ultrasound in the second trimester, as well as an MRI in the third trimester, showed cystic fluid collections and an oedema in the right lower extremity, leading to a suspicion of KTS.
At birth, a hypertrophy of the right lower extremity was confirmed. The right leg was longer and thicker than the left leg, and red-to-purple marks, known as a port-wine stain (capillary malformation), were noted in the skin of the right foot reaching the thigh and gluteal area (Figure 1). Varicose veins were found around the right knee. A post-birth MRI of the whole body revealed venous and lymphatic malformations of the right lower extremity, extending to the right side of the pelvis and underneath the right kidney. Based on the clinical and radiological evaluation, a diagnosis of KTS was set.
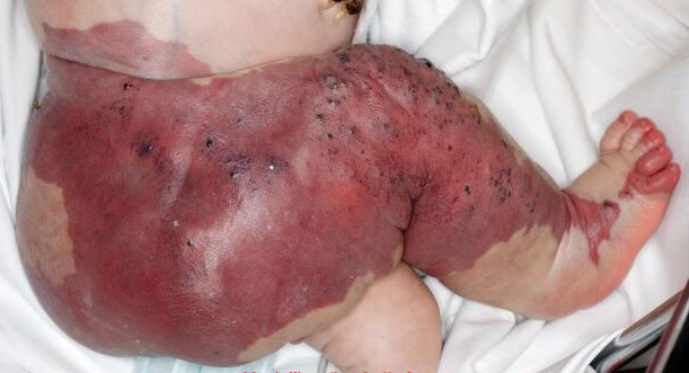
Figure 1. Cutaneous findings after birth referring to KTS
At the first follow-up visit after being discharged from the hospital at the age of 1.5 months, a referral for a custom-made, single-leg compression pantyhose with gradual compression from the distal to proximal regions was made. Furthermore, a manual lymphatic drainage was initiated once a week. At the age of 10 months, the compression magnitude of the pantyhose was increased – the area of the foot and lower leg transitioned from pressure class 2 to pressure class 3, and in other parts (thigh and gluteal area), from 1 to 2. Moreover, an anti-slip sole was designed on the bottom of the sock. Due to malformation, the patient had a deformed right foot, and the custom-made shoes did not fit well.
At the age of 2 years and 8 months, a debulkectomy of the lymphatic malformation and the cutaneous and soft tissue from the right leg and ankle was conducted to reduce the size of the limb. The treatment result was not permanent due to the tendency of the malformation to grow back. Later, a non-valvular marginal vein of the right lower extremity was occluded with an interventional radiological procedure. At the age of 3 years, the patient started to suffer from repeated erysipelas infections leading to antibiotic prophylaxis, and low-molecular-weight heparin was initiated as a prophylactic thromboembolic dose. To control the growing circumference of the right lower extremity and leakage from the cutaneous microcysts, the lymphatic malformation was treated with 11 sclerotherapy sessions by the age of 8 years. The manual lymphatic drainage was continued weekly to control lymphoedema, and a thigh-high open-toe compression stocking was added on the top of the one-leg compression pantyhose to intensify the compression pressure after the manual drainage. The port-wine stains and leaking veins in the right knee were treated with a pulsed dye laser, and special attention was given to management of sensitive skin to prevent infections.
At the age of 9 years, the patient reported increased pain and functional impairment due to the size and heaviness of the right lower extremity. A length discrepancy in favour of the right lower extremity had reached 3cm compared to the left leg, leading to epiphysiodesis. The disproportionately large size of the right leg limited the patient’s participation in sports activities. The girth of the right thigh was 42cm (vs the left thigh with a girth of 28cm), the calf had a girth of 47.5cm (vs the left calf with 24cm), and the ankle was 32cm (vs the left ankle with 16.5cm). There was no difference in the girths of upper extremities, and no signs of scoliosis was noted. Due to the increasing lymphoedema and functional impairment in the right lower extremity, the patient began taking Sirolimus, a mTOR inhibitor. 2 months after initiating Sirolimus, at the clinical follow-up visit, the right thigh had reduced in size, the colour of the port-wine stain on the affected skin had lightened, and there had not been skin bleeds. An MRI was repeated 18 months after the initiation of Sirolimus, but it did not show a clear difference in the volume of the malformation.
To gain more detailed knowledge of KTS, and to consider other potential treatment options, a genetical analysis of the affected tissue was conducted. Based on the genetical findings, a mosaic mutation of PIK3CA c.1633G>A, p.(Glu545Lys) was found. At the age of 10 years and 11 months, Alpelisib, a PIK3CA inhibitor, was initiated instead of continuing Sirolimus. 1 year after initiating the Alpelisib medication, in addition to the positive effects from the previously administered Sirolimus medication, the MRI showed a decrease in the soft tissue hypertrophy of the right lower extremity. At the age of 12 years, the patient received an intermittent pneumatic compression device to be used at home for approximately 1 hour per day. Furthermore, he continued wearing the one-leg compression pantyhose and the upper layer stocking. These daily routine treatments eased the swelling, and the clinical evaluation showed reduction in the size of the right lower extremity. At the follow-up visit (Figure 2), the patient and the guardian assessed the right leg being concretely smaller and lightweight than its initial appearance at the start of enhanced compression therapy.
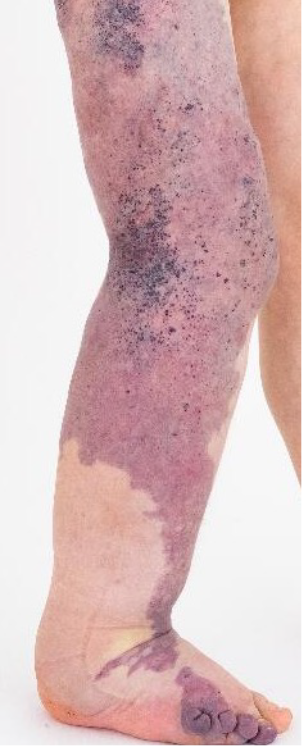
Figure 2. Achieved clinical outcome with multimodal treatment strategy
At the follow-up visit 10 months postoperatively, the patient reported being able to walk only 200 meters without the compression garments, whereas he could walk 2000 meters continuously with the combination of a single-leg compression pantyhose and an upper-layer compression stocking. The patient walked independently without a walking aid. The walking position was stooped forward, and the walk was slightly lame as the patient had to raise his right lower limb in the front through abduction. Bicycling went well, but running and jumping had been left out. When moving without the compression garments, he reported the difficulty of straining his body and even felt tachycardia. With the patient’s multidisciplinary team, the treatment and follow-up were continued in the clinic, and his occupational therapist and physiotherapist planned new exercises to build up the patient’s muscle strength and stretching ability.
Results
Manual lymphatic drainages, intermittent pneumatic compressions and custom-made compression garments played a role in our patient’s comprehensive treatment. Other treatment methods included sclerotherapy, surgery and medication. None of the treatment methods sufficiently reduced the patient’s limb oedema, but our case still highlights the importance of continuous individualised compression therapy in the treatment of lymphoedema combined with congenital vascular malformations.
Discussion
The clinical presentation and symptoms of KTS varies from patient to patient. Currently, there is no cure for KTS, but the mainstay is to reduce symptoms1. The management strategies for paediatric lymphoedema have been adapted from adults with lymphoedema – current principles include skin care, exercise, manual lymphatic drainages, the use of medication to control infections, and compression therapy5–7. Specific paediatric management strategies include teaching parents to manage participation, encourage normal physical activities, as well as provide psychological support and self-management5,8.
The majority of the KTS patients are advised to wear compression garments on the affected limb to control lymphoedema, venous insufficiency and discomfort1,2,9,10. Static compression decreases the fluid escaping from the venous system and improves the proximal lymph flow which promotes lymph drainage into lymphatic-venous anastomoses and slows adipose deposition. As a result, the volume of the extremity reduces11.
Even though lymphoedema is incurable, compression therapy has been consistently described in the management of paediatric lymphoedema7,8,11. Static compression applies continuous pressure to the affected extremity most effectively11. Brorson and Svensson indicated that controlled compression therapy with custom-fitted, gradually tightened layered stockings may decrease the limb volume by 47% over 1 year, and the continuous use of compression garments is necessary to maintain the reduced volume of the extremity12. However, very little research has been conducted on the use of compression garments for rapidly growing infants and young children10,13,14. For the garment(s) to fit correctly, the individual custom-made compression stockings should cover the affected area of the limb and extend to the toes or fingers, depending on the extremity14. In growing children, this means several new custom-fitted compression garments per year, as described in our patient’s case. Compression garments can also protect the affected area from incidental trauma and skin breaks, as well as prevent infections7.
Manual lymphatic drainages have long been a recommended treatment for adult lymphoedema15. Our patient received manual lymphatic drainages from in his early years of life without any notable adverse effects. Furthermore, intermittent pneumatic compressions were added to the treatment protocol with positive results. The review of Phillips and Gordon included five studies assessing pneumatic compression in the management of paediatric lymphoedema, all of which reported favourable outcomes without adverse effects13.
As a limitation, there is not a standardised measurement protocol for monitoring limb size and volume in paediatric lymphoedema patients. Due to the normal growth in infants and children, a different approach to monitoring paediatric lymphoedema patients is required, as adult lymphoedema patients do not experience the same type of paediatric growth5,16. Most studies have used an unaffected limb as a control, which is compared to the affected limb to evaluate management interventions. By doing so, the girth is presumably stable, which is not useful due to the natural changes in height, and the girth makes volume measurements incomparable5,13. In this case report, the lower limb circumferences were addressed only once to specify the size difference for the reader.
Conclusion
Lymphoedema associated with KTS is difficult to manage. Symptoms may be alleviated substantially with custom-fitted garments, manual lymphatic drainages and intermittent pneumatic compressions, improving the patient’s quality of life as KTS is a lifelong disease requiring frequent, continuous follow-up visits and multimodal treatments.
Further research
Research on the use of compression therapy for paediatric patients is needed to systemically determine the benefits and drawbacks.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Hanna Hyvönen*1 MD, Päivi Salminen2 MD, Milla Kallio3,4 MD, Heli Lagus4,5 MD, PhD, Kirsi Isoherranen1,4 MD, PhD
1Department of Dermatology and Allergology, Skin and Allergy Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
2Department of Pediatric Surgery, HUS Rare Diseases Center, VASCERN VASCA European Reference Centre, New Children’s Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
3Department of Vascular Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
4Helsinki Wound Healing Centre, Finland
5Department of Plastic Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
*Corresponding author email hanna.z.hyvonen@helsinki.fi
References
- John PR. Klippel-Trénaunay syndrome. Tech Vasc Interv Radiol 2019;22(4):100634. doi:10.1016/j.tvir.2019.100634. Epub 2019 Sep 23.
- Noel AA, Gloviczki P, Cherry KJ Jr, Rooke TW, Stanson AW, Driscoll DJ. Surgical treatment of venous malformations in Klippel-Trénaunay syndrome. J Vasc Surg 2000 Nov;32(5):840–7. doi:10.1067/mva.2000.110343.
- Klippel M, Trénaunay P. Memoires originaux: du Naevus variqueux osteohypertrophique. Arch Gen Med 1900;185:641–672.
- Canaud G, Hammill AM, Adams D, Vikkula M, Keppler-Noreuil KM. A review of mechanisms of disease across PIK3CA-related disorders with vascular manifestations. Orphanet J Rare Dis 2021 Jul 8;16(1):306. doi:10.1186/s13023-021-01929-8.
- Damstra RJ, Mortimer PS. Diagnosis and therapy in children with lymphoedema. Phlebol 2008;23(6):276–86. doi:10.1258/phleb.2008.008010.
- Todd M. Lymphoedema in children: an overview. Br J Nurs 2010 Apr 8–21;19(7):420, 422, 424–7. doi:10.12968/bjon.2010.19.7.47437.
- Schook CC, Mulliken JB, Fishman SJ, Grant FD, Zurakowski D, Greene AK. Primary lymphedema: clinical features and management in 138 pediatric patients. Plast Reconstr Surg 2011 Jun;127(6):2419–2431. doi:10.1097/PRS.0b013e318213a218.
- Smeltzer DM, Stickler GB, Schirger A. Primary lymphedema in children and adolescents: a follow-up study and review. Pediatr 1985 Aug;76(2):206–18.
- Asghar F, Aqeel R, Farooque U, Haq A, Taimur M. Presentation and management of Klippel-Trénaunay syndrome: a review of available data. Cureus 2020 May 8;12(5):e8023. doi:10.7759/cureus.8023.
- Sung HM, Chung HY, Lee SJ, Lee JM, Huh S, Lee JW, Choi KY, Yang JD, Cho BC. Clinical experience of the Klippel-Trénaunay syndrome. Arch Plast Surg 2015 Sep;42(5):552–8. doi:10.5999/aps.2015.42.5.552.
- Greene AK, Sudduth CL, Taghinia A. Lymphedema. Semin Pediatr Surg 2020 Oct;29(5):150972. doi:10.1016/j.sempedsurg.2020.150972.
- Brorson H, Svensson H. Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone. Plast Reconstr Surg 1998 Sep;102(4):1058–67; discussion 1068.
- Phillips JJ, Gordon SJ. Conservative management of lymphoedema in children: a systematic review. J Pediatr Rehabil Med 2014;7(4):361–72. doi:10.3233/PRM-140306.
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord 2017 Jul;5(4):587–595. doi:10.1016/j.jvsv.2016.10.084.
- Williams A. Manual lymphatic drainage: exploring the history and evidence base. Br J Community Nurs 2010 Apr;15(4):S18–24. doi:10.12968/bjcn.2010.15.Sup3.47365.
- Todd M, Welsh J, Moriarty D. The experience of parents of children with primary lymphoedema. Int J Palliat Nurs 2002 Sep;8(9):444–51. doi:10.12968/ijpn.2002.8.9.10689.
