Volume 39 Number 3
Application of irrigation and negative pressure wound therapy (INPWT) to treat diabetic foot gangrene: a case report
Ai-hua Chen, Huiling Liu, Chunmei Zhang and Ping Zou
Keywords negative pressure wound therapy, Diabetic foot gangrene, irrigation, moist dressing
For referencing Chen A et al. Application of irrigation and negative pressure wound therapy (INPWT) to treat diabetic foot gangrene: a case report. WCET® Journal 2019; 39(3):20–25
DOI https://doi.org/10.33235/wcet.39.3.20-25
Abstract
This case study summarises the treatment of a patient with diabetic foot gangrene. By undergoing irrigation and negative pressure wound therapy (INPWT) with moist dressing, foot amputation was avoided. The treatment process included: the comprehensive assessment of systemic and local condition; choosing suitable debridement such as sharp surgical debridement; preventing the spread of infection; applying INPWT to reduce endotoxin absorption; and active treatments of primary disease such as controlling blood sugar and blood pressure, and improving microcirculation and nutrition. After 2 months of vigorous INPWT, the patient’s wound bed improved. After the application of a moist dressing, the wound closed and healed successfully at 3 months.
Introduction
The global prevalence of diabetes mellitus is 8.5%, with roughly 90% of cases being type 2 diabetes1,2. Over the last several decades, the prevalence of diabetes has drastically increased worldwide – with rates quadrupling since 19802. This can be attributed to a quickly aging population, general population growth, and increased incidences of obesity. It is estimated that there will be nearly 550 million people with diabetes globally by 2030, making diabetes a key public health issue and a global concern. China currently houses the most diabetics in the world. According to the latest epidemiological survey conducted by the National Health Committee in 2018, 11.6% of the Chinese population has diabetes, totalling 114 million people. Furthermore, China has a low treatment rate (32.2%) and control rate (49.2%), resulting in many incidences of complications, including the risk of heart disease, stroke, blindness, kidney failure and amputation. These complications have serious consequences on the quality of life of patients.
One of the most prevalent comorbidities of diabetes is foot ulcers. The World Health Organization (WHO) classifies a diabetic foot disease as a foot infection, ulcer and/or deep tissue damage related to local nerve abnormality and lower limb distal peripheral vascular pathological changes3. The incidence of foot ulcers in individuals with diabetes is 6.3%, with a lifetime incidence of between 19–34% in this population4,5. A main concern with foot ulcers in people with diabetes is the prolonged period the wound takes to heal. The likelihood of the wounds healing decreases in diabetics due to a number of factors, including circulatory dysfunction, hyperglycaemia, neuropathy, hypoxia and impaired neuropeptide signalling, as well as comorbidities such as end-stage kidney disease, congestive heart failure and peripheral artery disease4,6. Many of these factors contributed to the initial development of the diabetic foot ulcers. Additional factors that advance ulcer development include a high body mass index, foot deformity, being of the male gender, prolonged infliction of diabetes, advanced age, and poor foot self-care habits7.
For individuals with diabetic foot ulcers, the infection rate is over 50%8. Infection is a process comprised of invading microorganisms, the body’s inflammatory response to the invasion, and tissue degradation and destruction9. Factors that contribute to an infection in an individual with diabetic foot ulcers include wounds that last longer than 30 days, deep wounds, wounds that were caused traumatically, and wounds that are comorbid with peripheral arterial disease8,9.
Additionally, if diabetic foot ulcers become infected they may progress to gangrene10. Diabetic foot gangrene is the final stage of diabetic foot disease, which is often comorbid with nerve and vascular lesions of other organs2. Infections can include osteomyelitis, cellulitis, abscesses, fasciitis or septic arthritis11. These infections can be caused by both fungi and bacteria such as Staphylococcus, Proteobacteria, Pseudomonas aeruginosa, Streptococcus and coliform bacteria10.
Due to infection and slow healing times, diabetic foot ulcers may eventually lead to the need for amputation; individuals with diabetic foot ulcers are 10–20 times more likely to need amputation compared with their non-diabetic counterparts2,7. Amputation can lead to additional complications – the 5-year survival rate post-amputation is 30% for individuals with diabetes4. One study found that amputation rates were around 46–78% for moderate to severe infections respectively8.
Infection is generally managed through antibiotic therapy, surgical intervention and wound debridement8,11. Other adjunct therapies used with these methods include negative pressure wound therapy (NPWT) and hyperbaric oxygen therapy. Comprehensive treatment and local management of diabetic foot gangrene is therefore important to reduce the amputation rate of patients with diabetic foot disease, to maintain foot function and mobility, and to improve the quality of life for individuals afflicted with diabetic foot disease12.
NPWT is a form of wound management wherein negative pressure is exhibited across the entire surface area of the wound and is maintained by a dressing that is sealed and connected via a tube to an external vacuum. It acts to bring the edges of the wound together in healing – this promotes granulation tissue formation, reduces oedema, thereby increasing microcirculation, and removes small debris and exudate via suction13. This therapy can be combined with other approaches, such as instillation, which delivers a controlled amount of a cleansing solution – such as antiseptics or antibiotics – in order to treat diabetic foot infections13,14.
In May 2016, a case of Wagner IV diabetic foot gangrene was admitted to our hospital. This paper will explore the clinical history of the patient, patient assessment and treatment. After 3 months of treatment, the patient’s wound was healed successfully, and foot amputation was avoided.
Case report
Clinical history and assessment
The patient was an 88-year-old male who was admitted to the hospital on 22 May 2016. He had experienced polydipsia and polyuria for more than 3 years. Upon physical examination, the patient had a temperature of 37.8oC, pulse of 84 beats/min, and blood pressure of 160/90 mmHg. The patient appeared alert and oriented. The patient had a fasting blood glucose of 11 mmol/L, haemoglobin of 110 g/L, albumin level of 38.1 g/L, and a white blood cell count of 11x109/L.
The right side of dorsalis pedis artery pulse was weak and the front of his right foot appeared black–purple in colour. The patient’s first and second toe were missing on the right foot, with toes three, four and five appearing purple–black with necrosis. Tissue was only partially connected with the plantar skin. Yellowish tissue covered 100% of the wound bed, with large amounts of grey, purulent, malodourous exudate at the wound site indicating severe infection. The edge of wound was irregular with oedema, high skin temperature and appeared purple–black. The patient’s skin temperature on his right lower limb was higher than that of the left lower limb. The wound was classified as a Wagner Grade 4. On a numeric rating scale, pain was scored by the patient as eight out of 10 on a scale where 0 is no pain and 10 the worse pain.
The patient’s admission diagnosis was as follows: type II diabetes mellitus and diabetic foot gangrene and infection; coronary atherosclerotic heart disease with unstable angina, with a chronic non-ST segment elevations myocardial infarction event 2 years previously, arrhythmia, and third stage of paroxysmal atrial fibrillation; hypertension level 3 (high risk); cerebral infarction sequela; and artery plaque formation in double lower limbs. The doctor recommended amputation of the foot, but the patient refused.
In June 2016 the patient was referred to an enterostomal (ET) nurse for foot treatment and wound care. The patient had right femoral artery stenosis (90%) and popliteal artery and posterior tibial artery stenosis (61%, 80%). A right lower extremity artery stent was implanted, which was in place for 5 months, and resulted in positive postoperative arterial blood flow. After a comprehensive assessment, the ET nurse found no contraindications in the patient to NPWT. This is the first case of using irrigation and NPWT (INPWT) in this large hospital to treat diabetic foot ulcer. Factors affecting wound healing in the patient were being of an older age, and having a variety of comorbidities such as diabetes, cardiovascular disease, poor nutrition, long-term chronic disease as well as poor mental health.
Systemic treatment
As outlined above, the treatment process included preventing the spread of infection, as well as active treatments of primary disease such as controlling blood sugar and blood pressure, and improving microcirculation and nutrition.
The goal for wound healing rate is 0.2 cm per week, but when the wound bacterial count is at or above 106/mm2, healing speed is expected to decrease to 0.055 cm/week. The more serious a bacterial infection, the slower wound healing is to be expected3. It is therefore vital to have systemic infection control practices in place when performing local wound management. The patient was prescribed a peracillin sodium intravenous infusion at a rate of 2.5 g once per 8 hours. Wound cultures showed growth of Staphylococcus aureus and Pseudomonas aeruginosa, suggesting the need for ofloxacin; therefore, a levofloxacin sodium chloride injection of 0.4 g was given through intravenous infusion once a day for 1 week. The patient’s temperature was normal, and his condition was stable.
To control blood glucose, phosphate sieglidine tablets at a dosage of 100 mg were given orally once a day. The patient was also prescribed acarbosaccharides (baicana) 50 mg orally three times a day, which helped control his fasting blood glucose at 7 mmol/l and postprandial blood glucose around 10 mmol/l.
To reduce blood pressure, the patient was given amberyl mertolol (betatolak) 47.5 mg orally once a day, mononitrate isosorbate sustained-release capsule (isole) 50 mg orally once a day, and valsartan lodipine (combination of amlodipine 5 mg and valsartan 80) one tablet orally once every 12 hours, which kept blood pressure at 130/80 mmHg.
The patient was given pancreatopeptidase (yi-open) 240 units orally three times daily to increase local blood circulation and promote wound healing. The patient had anaemia, low protein, and other nutritional deficiencies which affected wound healing. To counterbalance this, the patient was given a high protein and high vitamin diet which was distributed via small meals under the precondition of controlling blood sugar.
Wound management process
To manage the wound, the inactivated tissue was removed to prevent the spread of infection. Necrotic tissues and microorganisms were also removed to prevent the absorption of toxins. To promote granulation growth, the patient’s dead bone tissue was also excised, and the sinus cavity was closed. Eschar was removed to repair the wound edge and promote epithelium creep. The edges of the wound were trimmed to protect granulation and epithelial tissue. Skin moisturisers were used around the wound to promote blood circulation.
At initial wound management (28 June 2016), an iodine solution was used to disinfect the wound, and a sodium chloride solution was used to rinse the wound. Tissue cultures were taken from the wound to assist in guiding antimicrobial therapy. Surgical debridement was used to remove senescent cells and decrease bacterial burden. INPWT was then applied to the wound. The irrigation liquid that was used was 0.01% iodide normal saline (NaCl 500ml+5% iodide 10 ml), with a speed of 20 drops per min. The negative pressure was kept between 80–125 mmHg (Figure 1).
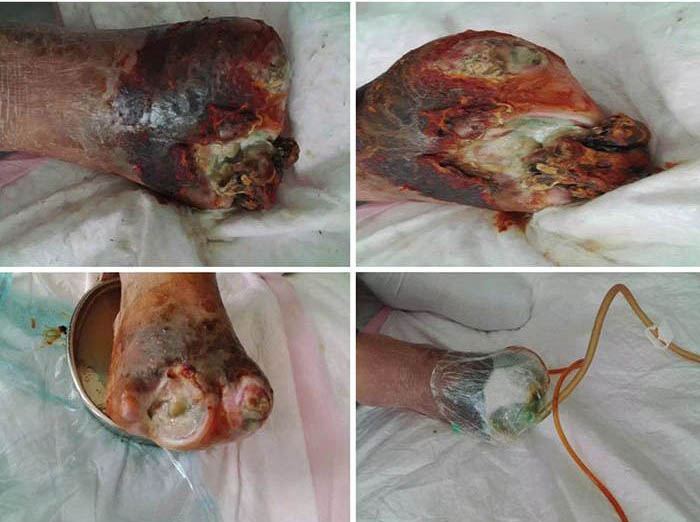
Figure 1. Initial stage of wound management (28 June 2016).
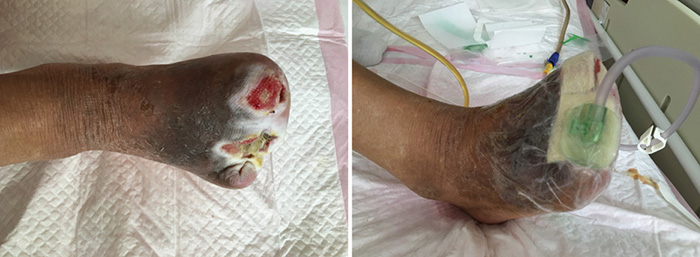
At the second stage of wound management (5 July), the wound bed was 50% erythematous and 50% yellowish (Figure 2). The wound continued to have a foul odour, with oedema around the wound, dark pigmentation, and edge impregnation. Treatment included continued debridement, and other methods as stated before. INPWT was also continued.
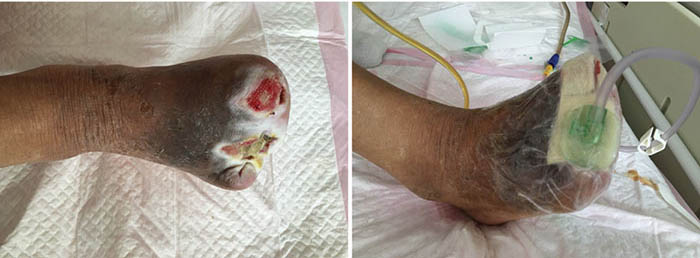
Figure 2. Second stage of wound management (5 July 2016).
At third stage management (8 July), after the removal of the dead bone, sinus cavities 3.5 cm and 2.5 cm in depth were found in the first and third digits respectively (Figure 3). The total size of wound was 3x7.5 cm. To heal the wounds, conservative debridement was applied, with washing of the sinus cavities and an implanted sponge to ensure that washing fluid reached the bottom of the sinus cavity without leaving a dead cavity. Continued INPWT was used in the treatment.
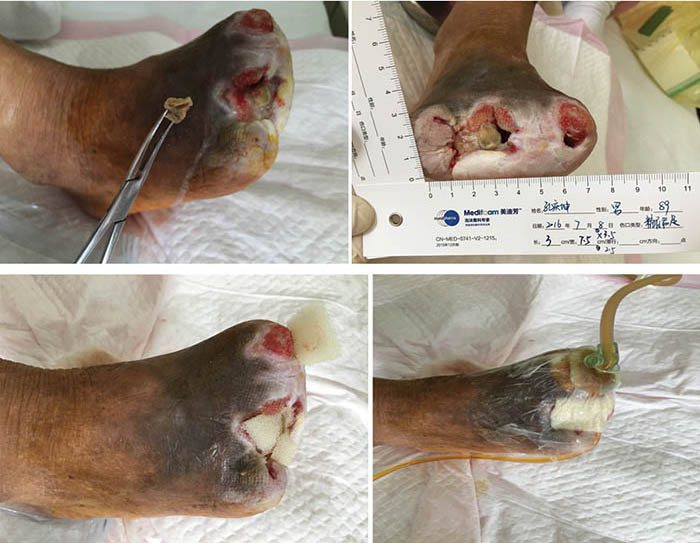
Figure 3. Third stage of wound management (8 July 2016).
After roughly 6 weeks of treatment, at ninth stage of wound management (12 August), the sinus cavity in the first toe closed (Figure 4). The wound bed was fully erythematous. The wound was managed with continued conservative debridement using NPWT; however, irrigation was discontinued.
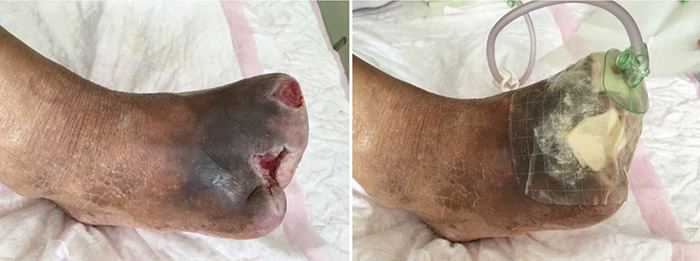
Figure 4. Ninth stage of wound management (12 August 2016).
At the 12th stage of wound management (23 August), the wound bed continued to be fully erythematous (Figure 5). The edges of the wound were macerated, and the wound no longer smelled foul. Conservative debridement to remove necrotic tissue was applied. NPWT was stopped and treatment was switched to moist dressings. A hydrocolloid film was applied to the inner later of the dressing to promote epithelisation, while a silicon foam dressing was applied to the outer layer to promote the absorption of exudate.
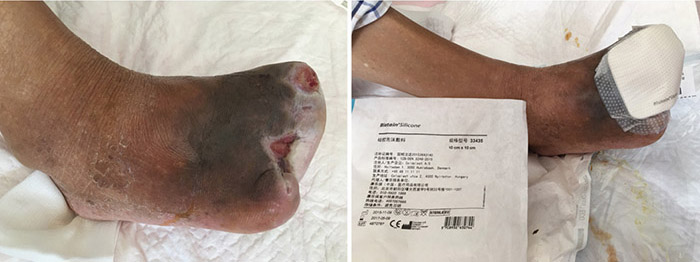
Figure 5. Twelfth stage of wound management (23 August 2016).
At the 15th stage of wound management at 3 months (27 September), toes one and three were totally healed through wound care management (Figure 6). Figure 7 shows a comparison of the initial presentation and the wound after 3 months of treatment. Figure 8 shows the fully healed wound in May 2019, some 3 years after initial presentation.
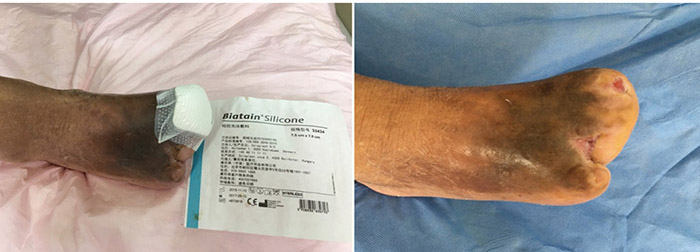
Figure 6. Fifteenth stage of wound management (27 September 2016).
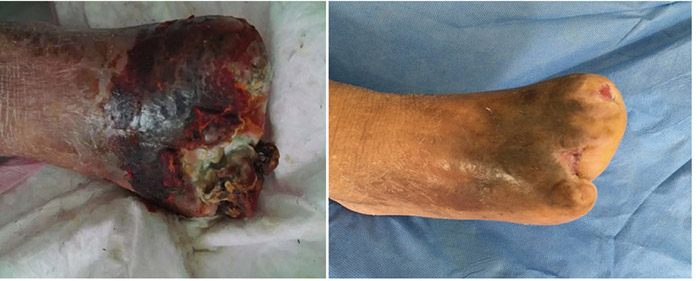
Before treatment Three months later
Figure 7. Comparison of treatment from initial presentation to after 3 months of treatment.
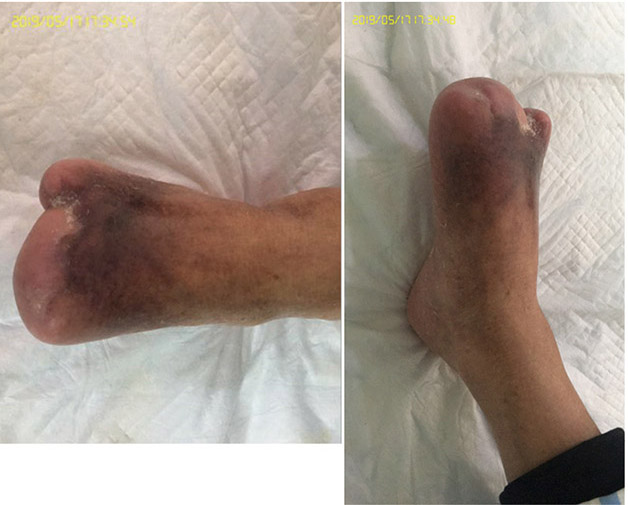
Figure 8. Fully healed wound, 3 years after initial presentation (May 2019).
Prevention
Diabetic foot is prone to relapse after healing or may reoccur in the other foot. Therefore, prevention is of vital importance. The patient needs to control their blood sugar levels and blood pressure, refrain from smoking, and participate in a consistent exercise regime. Moreover, the patient needs to diligently preform self-checkups on the problematic foot daily, practise proper foot care, wear comfortable diabetic shoes, practise regular foot hygiene, and visit the hospital for annual checkups. If any issues should arise, the patient must contact a diabetic podiatrist for examination and early treatment.
Discussion
This case report indicated that INPWT can be used to treat diabetic foot ulcers when combined with other therapies to promote wound healing. The total treatment process lasted for 3 months and cost 27,500 RMB. The success in wound treatment prevented foot amputation which could negatively impact patient health and quality of life. In addition, life-threatening infection and sepsis were prevented. The patient and their family were very satisfied with the effectiveness of the treatment.
Managing a wound in a diabetic foot is a clinical challenge – it requires multiple care strategies which are interdependent in order to reach the ultimate goal of wound healing. The literature suggests that the treatment process of a diabetic foot ulcer is lengthy, expensive and has a low curation rate12. However, this case study adds to the growing body of knowledge about utilising INPWT and adjunct therapies to heal advanced diabetic foot ulcers.
In the case of our patient, INPWT, managing patient comorbidities, and good nutrition played key roles in successfully healing his wound. The INPWT used a water bath therapy combined with closed aspiration. The main principle of INPWT is to bathe the infected wound in the cleansing solution to dissolve debris such as liquefied necrotic tissue or bacteria, inflammatory mediators, and purulent secretions. Debris can then be removed from the wound bed through a suction device. When an abundance of irrigation solution is utilised, the concentration of harmful cellular debris and metabolic by-products is effectively lowered, thus efficiently eliminating harmful substances on the surface of the wound.
Fully draining the wound secretions also reduced the wound burden and promoted local blood circulation, speeding up growth of granulation tissue15. Draining is an important process as bacterial by-products – such as endotoxins and metalloproteases from cellular metabolism during the colonisation process – can interfere with wound healing at various stages. This interference can occur through increased metabolic demands, negative microcirculatory changes, and can signal an inflammatory response. INPWT acts to produce mechanical stress on cells, removes interstitial fluid, and stimulates new cell proliferation. Meanwhile, the irrigated solution enhances these affects or introduces antimicrobial properties16.
The successful healing of our patient’s wound matches other research findings regarding INPWT. Gabriel et al.16 found that instillation of an irrigation solution in combination with NPWT enhanced wound healing through autolytic processes, prevention of glycocalyx establishment, mechanical debridement, and increased the viscosity of wound exudate which allowed for easier removal through the INPWT system. Additionally, Zelen and colleagues14 found that INPWT was effective at closing both small and large wounds resulting from diabetic neuropathy by promoting epithelialisation and granulation at the wound.
Our patient’s case was of greater complexity as he had many comorbidities alongside a severe wound infection. In addition to managing the patient’s local wound, emphasis was therefore placed on systemic treatment such glycaemic control, blood pressure reduction, improving microcirculation and ensuring better nutritional status. These strategies are in alignment with the Practical guidelines on the management and prevention of the diabetic foot 201117 which notes the importance of proper nutrition and maintaining blood sugar levels at less than 8 mmol/L.
Another study highlighted the importance of good nutrition for preventing amputation in individuals with diabetic foot ulcers. Their findings illuminated how nutritional status has a significant impact on limb preservation. The proposed mechanism through which poor nutritional status impacts wound healing is multi-faceted. Nutrients are lost in wound exudate, and comorbidities can impact nutrient uptake which are major concerns due to the metabolic requirements for the products of wound healing such as collagen formation and fibroblast proliferation18. Thus, if nutrition is inadequate, wound healing can be delayed or severe progressions in the ulcer can develop and lead to the need for amputation. Through comprehensive treatment, the patient’s wound healed successfully, and amputation was avoided. A plethora of research has demonstrated that amputation will drastically decrease quality of life, therefore avoiding amputation was of vital importance for the patient19.
Conflict of Interest
The authors declare no conflicts of interest. The patient consented to the usage of the photographs in this article.
Funding
The authors received no funding for this study.
灌注联合负压伤口治疗(INPWT)在糖尿病足坏疽治疗中的应用:一项病例报告
Ai-hua Chen, Huiling Liu, Chunmei Zhang and Ping Zou
DOI: https://doi.org/10.33235/wcet.39.3.20-25
摘要
本病例研究总结了一例糖尿病足坏疽患者的治疗过程。通过进行灌注联合负压伤口治疗(INPWT)并使用湿性敷料,避免了足部截肢。治疗过程包括:全面评估全身和局部情况;选择适当的清创术,如锐器手术清创;防止感染扩散;采用INPWT减少内毒素吸收;积极治疗原发疾病如控制血糖和血压,改善微循环和营养状态。进行2个月的积极INPWT治疗后,患者伤口床改善。敷上湿性敷料后,伤口闭合,并在3个月后成功愈合。
前言
糖尿病的全球患病率为8.5%,约90%的病例为II型糖尿病1,2。过去几十年间,全球范围内的糖尿病患病率急剧增长,增长率自1980年以来翻了两番2。造成这种情况的可能原因包括人口快速老龄化、人口的总体增长和肥胖发病率上升。据估计,到2030年,全球将有近5.5亿人患有糖尿病,糖尿病将成为重要的公共健康问题和全球关注焦点。目前,中国的糖尿病患者人数居世界首位。根据国家卫生健康委员会在2018年进行的最新流行病学调查,11.6%的中国人群患有糖尿病,总计1.14亿人。另外,中国的治疗率(32.2%)和控制率(49.2%)均较低,导致并发症发病率较高,包括心脏疾病、中风、失明、肾功能衰竭和截肢的风险。这些并发症对患者生活质量产生了严重后果。
糖尿病患病率最高的合并症之一是足溃疡。世界卫生组织(WHO)将糖尿病足病分为与局部神经异常和下肢远端外周血管病变相关的足部感染、溃疡和/或深部组织损伤3。糖尿病患者足溃疡的发病率为6.3%,该人群的终生发病率为19–34% 4,5。糖尿病患者足溃疡的主要问题在于伤口愈合时间延长。由于以下几个因素,糖尿病会减小伤口愈合的概率,包括:循环功能障碍、高血糖、神经病变、组织缺氧和神经肽信号传导受损,以及终末期肾病、充血性心力衰竭和外周动脉疾病等合并症4,6。这些因素中的许多因素都会造成糖尿病足溃疡的初期发展。其他促成溃疡发展的因素包括体质指数较高、足畸形、男性、长期糖尿病、高龄和足部自我护理习惯不良7。
糖尿病足溃疡患者中,感染率超过50% 8。感染过程包括微生物侵入、身体对侵入的炎症反应、组织降解和破坏9。造成糖尿病足溃疡患者发生感染的因素包括伤口至少30日未愈合、深部伤口、创伤性伤口以及伤口合并外周动脉疾病8,9。
另外,如果糖尿病足溃疡受到感染,则可能进展为坏疽10。糖尿病足坏疽是糖尿病足病的最终阶段,通常合并有其他器官的神经和血管病变2。感染可能包括骨髓炎、蜂窝织炎、脓肿、筋膜炎或化脓性关节炎11。这些感染可能由真菌和细菌导致,如葡萄球菌、变形菌、绿脓杆菌、链球菌和大肠菌群10。
由于感染和愈合时间缓慢,糖尿病足溃疡可能最终导致需要截肢;糖尿病足溃疡患者需要截肢的概率比非糖尿病对照者高10-20倍2,7。截肢可能导致其他并发症:糖尿病患者截肢后的5年存活率仅为30% 4。一项研究发现,中度到重度感染的截肢率为46–78%左右8。
一般通过抗生素治疗、手术干预和伤口清创术控制感染8,11。与这些方法联合使用的其他辅助治疗包括负压伤口治疗(NPWT)和高压氧疗。因此,糖尿病足坏疽的综合治疗和局部管理对于降低糖尿病足病患者的截肢率、维持足部功能和活动、改善糖尿病足病患者的生活质量非常重要12。
NPWT是一种伤口管理形式,涉及在整个伤口表面施加负压,并通过密闭且经由管路连接至外部负压系统的敷料维持。这种方法能在愈合过程中将伤口边缘聚拢,这会促进肉芽组织形成、减少水肿,从而增加微循环,并通过负压吸引清除碎屑和渗出液13。这种疗法可以与其他方法联用,如滴注法,其输送受控剂量的清洁液(如杀菌剂或抗生素),以治疗糖尿病足感染13,14。
在2016年5月,我院收治了一例Wagner IV级糖尿病足坏疽患者。本文将探索该患者的临床病史、患者评估和治疗。该患者治疗3个月后,伤口成功愈合,避免了足部截肢。
病例报告
临床病史和评估
患者为88岁男性,于2016年5月22日入院。他有烦渴和多尿症三年余。体检时,患者体温37.8°C,脉搏84次/分,血压160/90 mmHg。患者警觉、可定向。患者空腹血糖为11 mmol/L,血红蛋白水平110 g/L,白蛋白水平38.1 g/L,白细胞计数11×109/L。
右侧足背动脉脉搏微弱,右足前部呈黑紫色。患者右足第1和第2趾缺失,剩下的第3、4、5趾呈紫黑色并伴有坏死。仅部分组织与足底皮肤相连。伤口床100%由黄色组织覆盖,伤口部位存在大量灰色脓性恶臭渗出液,表明存在重度感染。伤口边缘不规则,伴有水肿,皮肤温度高,呈紫黑色。患者右下肢皮肤温度高于左下肢。伤口归类为Wagner 4级。按10分为最痛、0分为无痛的数值评定量表,将患者疼痛评分为10分量表中的8分。
患者入院诊断如下:II型糖尿病和糖尿病足坏疽伴感染;冠状动脉粥样硬化性心脏病伴不稳定型心绞痛,2年前发作慢性非ST段抬高型心肌梗死事件,心律不齐和阵发性房颤第三阶段;高血压3级(高危);脑梗死后遗症;以及双下肢动脉斑块形成。医生建议足部截肢,但患者拒绝。
2016年6月,患者转诊到一名肠造口(ET)护士处进行足部治疗和伤口护理。患者右股动脉狭窄(90%),腘动脉和胫后动脉狭窄(61%、80%)。右下肢动脉支架已植入5个月,因此术后有正向的动脉血流。经综合评估后,ET护士没有发现患者存在NPWT的禁忌症。这是该大型医院第一例应用灌注联合NPWT (INPWT)治疗糖尿病足溃疡的病例。影响患者伤口愈合的因素包括高龄及伴有多种合并症,如糖尿病、心血管疾病、营养不良、长期慢性疾病和精神健康不良。
全身治疗
如上所述,治疗过程包括防止感染扩散和积极治疗原发疾病,如控制血糖和血压,改善微循环和营养状态。
伤口愈合率的目标是每周0.2 cm,但是当伤口细菌计数等于或超过106/mm2时,预期愈合速度将降低到0.055 cm/周。细菌感染越严重,预期伤口愈合速度越低3。因此,进行局部伤口管理时,必须实施全身感染控制措施。给予患者哌拉西林钠静脉注射,每8小时一次,每次2.5 g。伤口细菌培养显示金黄色葡萄球菌和绿脓杆菌生长,说明需要氧氟沙星;因此,静脉注射0.4 g左氧氟沙星氯化钠注射液,每日一次,持续1周。患者体温正常,病情稳定。
为控制血糖,给予口服磷酸西格列汀片,剂量为100 mg,每日一次。还给予患者口服阿卡波糖(baicana),剂量为50 mg,一日3次,帮助将其空腹血糖控制在7 mmol/l,餐后血糖控制在10 mmol/l左右。
为降低血压,给予患者口服47.5 mg琥珀酸美托洛尔(倍他乐克),每日一次,口服50 mg单硝酸异山梨酯缓释胶囊(isole),每日一次,以及口服一片缬沙坦氨氯地平(5 mg氨氯地平和80 mg缬沙坦复合药),每12小时一次,将血压控制在130/80 mmHg。
患者每日3次口服240单位的抑肽酶(yi-open),以增强局部血液循环,促进伤口愈合。患者有贫血、低蛋白及其他营养缺乏,这影响了伤口愈合。为此,在控制血糖的前提条件下,通过副餐给予患者高蛋白和高维生素饮食。
伤口管理过程
为了管理伤口,清除无活性组织,防止感染扩散。同时去除坏死组织和微生物,防止内毒素吸收。为促进肉芽组织生长,同时切除患者坏死骨组织,封闭窦腔。去除焦痂,修复伤口边缘,促进上皮蔓延。修整伤口边缘,以保护肉芽和上皮组织。在伤口周围使用皮肤保湿剂,促进血液循环。
在伤口初期管理阶段(2016年6月28日),使用碘液为伤口消毒,使用氯化钠溶液冲洗伤口。取伤口组织培养,以指导抗生素疗法。通过手术清创去除衰老细胞,减少细菌负担。然后在伤口上进行INPWT。使用的灌注液为0.01%含碘生理盐水(500 ml NaCl+10 ml 5%碘),灌注速度为每分钟20滴。负压维持在 80–125 mmHg (图1)。
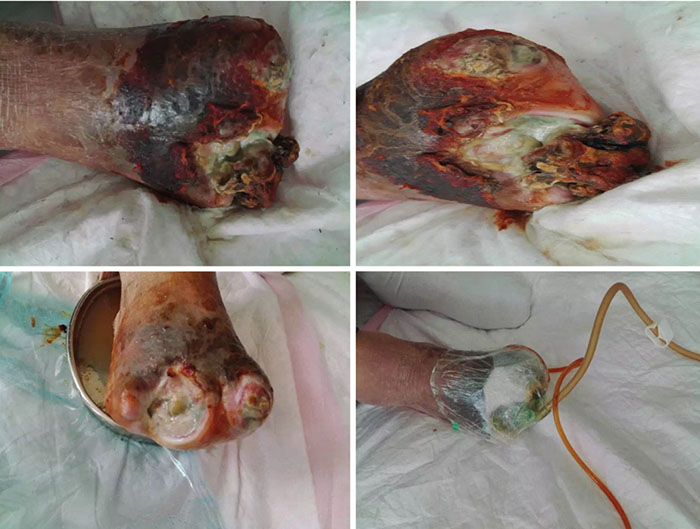
图1. 伤口管理的初期阶段(2016年6月28日)。
在伤口管理的第2阶段(7月5日),伤口床50%呈红肿,50%泛黄(图2)。伤口继续散发恶臭,伤口周围水肿,呈深色色素沉着,边缘浸软。治疗包括继续清创和其他上述方法。还继续进行INPWT。
F图2. 伤口管理的第2阶段(2016年7月5日)。
在伤口管理的第3阶段(7月8日),去除坏死骨组织后,在第1和第3趾分别发现3.5 cm和2.5 cm深的窦腔(图3)。伤口总大小为3×7.5 cm。为了治愈伤口,进行保守性清创,并冲洗窦腔及植入的海棉以确保冲洗液达到窦腔底部,不留死腔。治疗时持续进行INPWT。
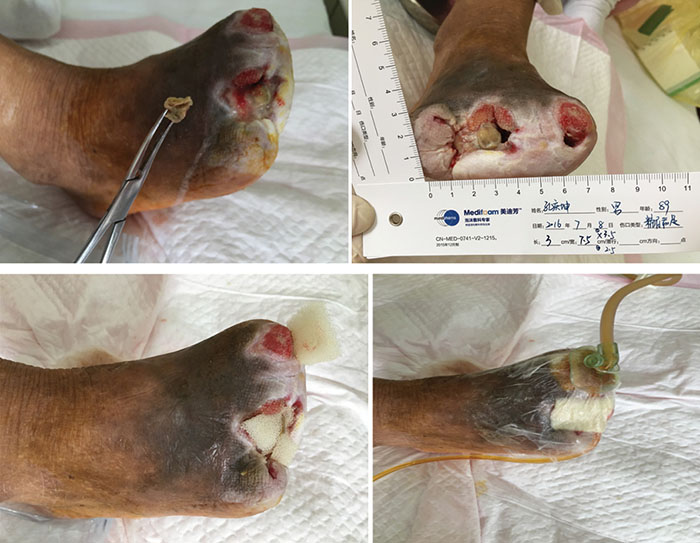
图3. 伤口管理的第3阶段(2016年7月8日)。
约治疗6周后,在伤口管理的第9阶段(2016年8月12日),第1趾窦腔闭合(图4)。伤口床完全红肿。采用持续的保守性清创并使用NPWT进行伤口管理;但是停止灌注。
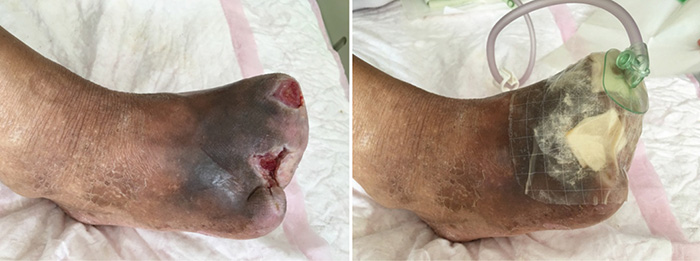
图4. 伤口管理的第9阶段(2016年8月12日)。
在伤口管理的第12阶段(2016年8月23日),伤口床继续呈完全红肿(图5)。伤口边缘浸软,伤口不再恶臭。采用保守性清创来去除坏死组织。停止进行NPWT,治疗转为湿性敷料。在敷料内层铺一层水胶体膜,促进上皮化,同时在外层铺上硅胶泡沫敷料,促进渗出液吸收。
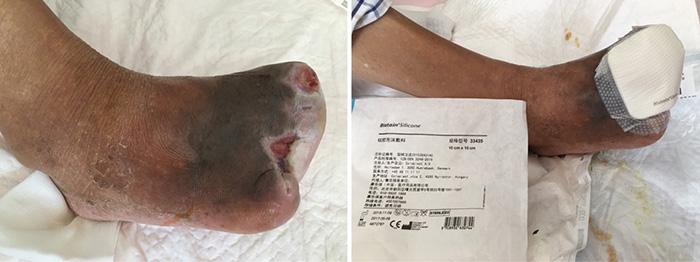
图5. 伤口管理的第12阶段(2016年8月23日)。
在伤口管理的第3个月时,即第15阶段(2016年9月27日),第1、3趾经伤口护理管理后完全愈合(图6)。图7显示了初诊和治疗3个月后的伤口比较。图8显示了初诊约3年后,即2019年5月,伤口完全愈合。
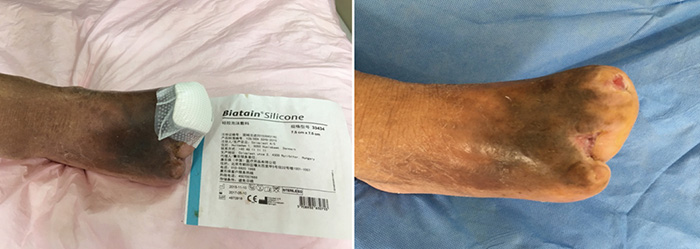
图6. 伤口管理的第15阶段(2016年9月27日)。
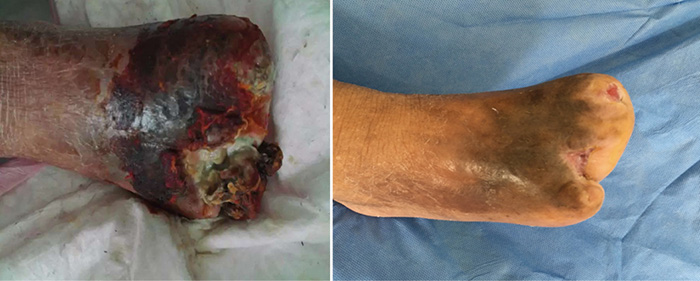
图7. 治疗时的初诊和治疗3个月后的比较。
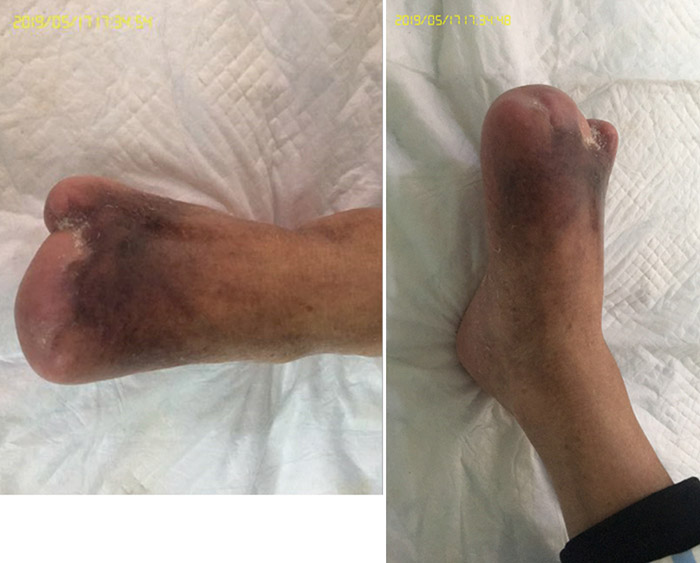
图8. 初诊3年后,伤口完全愈合(2019年5月)。
预防
糖尿病足治愈后容易复发,并且可能在另一只足上复发。因此,预防非常重要。患者需要控制血糖和血压,戒烟,持续进行锻炼。另外,患者需要坚持每天对患足进行自我检查,进行适当的足部护理,穿上舒适的糖尿病鞋,定期整理足部卫生,每年去医院复查。如果出现任何问题,患者必须联系糖尿病足病医生进行检查和及早治疗。
讨论
本病例报告表明,INPWT联合其他促进伤口愈合的疗法可以用于治疗糖尿病足溃疡。治疗全过程持续3个月,花费27,500元人民币。伤口治疗成功后,避免了对患者健康和生活质量产生负面影响的足部截肢。另外,也避免了危及生命的感染和败血症。患者及其家属对治疗效果非常满意。
糖尿病足的伤口管理是一个临床挑战,它需要相互依赖的多种护理策略,以实现伤口愈合的最终目标。文献指出,糖尿病足溃疡的治疗耗时长、花费昂贵,并且治愈率低12。但是,本病例研究为不断增长的知识体系贡献了新的一页,即利用INPWT和辅助疗法可治愈晚期糖尿病足溃疡。
本病例中,INPWT、管理患者合并症以及良好的营养在成功治愈伤口中发挥了重要作用。INPWT使用水浴疗法结合封闭吸引。INPWT的主要原则是用清洁液对感染的伤口进行水浴,以溶解液化坏死组织或细菌、炎症介质和脓性分泌物等碎屑。然后可通过吸引装置清除伤口床中的碎屑。经使用充分的灌注液后,有效降低了有害细胞碎屑和代谢副产品的浓度,从而有效清除伤口表面的有害物质。
此外,彻底引流伤口分泌物减少了伤口负担,促进局部血液循环,加快肉芽组织生长15。引流非常重要,因为细菌副产物(如在定植过程中的细胞代谢产生的内毒素和金属蛋白酶)可干扰多个阶段的伤口愈合。干扰方式包括增加代谢需求、不良的副循环变化等,并可能触发炎症发应。INPWT可以对细胞产生机械应力,清除间质液,刺激新细胞增殖。同时,灌注液增强了这些效果,或带来了抗菌特性16。
对本例患者伤口的成功治愈与其他关于INPWT的研究结果一致。Gabriel等人16发现,滴注灌注液联合NPWT可以通过自溶清创过程、防止糖萼形成、机械清创以及提高伤口渗出液粘性,更便于通过INPWT系统清除等方式增强伤口愈合。另外,Zelen及其同事14发现,INPWT可促进伤口的上皮化和肉芽组织生长,从而有效闭合糖尿病神经病变导致的大、小伤口。
本例患者为具有较高复杂度的病例,其除了重度伤口感染外,还有多种合并症。因此,除了管理患者的局部伤口,还重点进行了全身治疗,如控制血糖,降低血压,改善微循环及确保更佳的营养状态。这些策略符合《2011版糖尿病足管理和预防实践指南》17,其中指出了适当的营养状态和维持血糖低于8 mmol/L的重要性。
另一项研究也强调了良好的营养状态对于避免糖尿病足溃疡患者截肢的重要性。他们的发现说明了营养状态对于保全下肢的重要意义。关于不良营养状态影响伤口愈合这一点,已经提出了多种机制。营养物会流失在伤口渗出液中,合并症可能会影响营养吸收,而由于伤口愈合产物的代谢要求,如胶原形成和纤维细胞增殖等,这些是重点担忧的问题18。因此,如果缺乏营养,伤口愈合可能会延迟,或可能会发生溃疡的严重恶化,最终导致需要截肢。本例患者通过综合治疗,伤口成功愈合,避免了截肢。大量研究证明,截肢将大幅降低生活质量,因此避免截肢对于患者非常重要19。
利益冲突
作者声明没有利益冲突。患者同意将照片用于本文中。
资助
作者未因该项研究收到任何资助。
Author(s)
Ai-hua Chen
ET, RN, BScN
Director of Wenzhou Enterostomal Therapy Nursing Education Programs, Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, P. R. China
Huiling Liu
ET, RN
971 Hospital of the Chinese People’s Liberation Army Navy, Qingdao, Shandong Province, P. R. China
Chunmei Zhang
RN,PhD
Nursing Director of Second Affiliated Hospital and Yuying
Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, P. R. China
Ping Zou*
RN, PhD
Associate Professor, School of Nursing, Nipissing University, Toronto, ON, Canada
Email pingz@nipissingu.ca
* Corresponding author
References
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus: present and future perspectives. Nature Rev Endocrin 2012;8(4):228.
- World Health Organization. Global report on diabetes. WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland: World Health Organization; 2016.
- Xiao-bing F. The treatment of diabetic foot and management of its related chronic refractory wounds. People’s Military Medical Publishing Press; 2013.
- Armstrong DG, Boulton AJ, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376(24):2367–75.
- Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Annals Med 2017;49(2):106–16.
- Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther 2014;31(8):817–36.
- Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes 2015;6(1):37.
- Hobizal KB, Wukich DK. Diabetic foot infections: current concept review. Diabetic Foot Ankle 2012;3(1):18409.
- Lipsky BA, Aragón‐Sánchez J, Diggle M, Embil J, Kono S, Lavery L, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes/Metab Res Rev;2016;32:45–74.
- Noor S, Zubair M, Ahmad J. Diabetic foot ulcer: a review on pathophysiology, classification and microbial etiology. Diabetes Metabo Synd: Clin Res Rev 2015;9(3):192–9.
- Barshes NR, Sigireddi M, Wrobel JS, Mahankali A, Robbins JM, Kougias P, et al. The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabetic Foot Ankle 2013;4(1):21847.
- Qiu X, Zhao W, Du X. Clinical analysis of 56 cases of diabetic foot gangrene treatment J North China Uni (natural science edition) 2011;18(4):506–7.
- Back DA, Scheuermann‐Poley C, Willy C. Recommendations on negative pressure wound therapy with instillation and antimicrobial solutions: when, where and how to use: what does the evidence show? Int Wound J 2013;10(s1):32–42.
- Zelen CM, Stover B, Nielson D, Cunningham M. A prospective study of negative pressure wound therapy with integrated irrigation for the treatment of diabetic foot ulcers. Eplasty 2011;11.
- Chou TH. The application of “TIME” principle in the preparation of wound beds. J Chin Nurs 2013;48(9):855–7.
- Gabriel A, Shores J, Heinrich C, Baqai W, Kalina S, Sogioka N, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 2008;5(3):399–413.
- Bakker K, Apelqvist J, Schaper NC, Board IWGotDFE. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes/Metab Res Rev 2012;28:225–31.
- Gau B-R, Chen H-Y, Hung S-Y, Yang H-M, Yeh J-T, Huang C-H, et al. The impact of nutritional status on treatment outcomes of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications 2016;30(1):138–42.
- Laiteerapong N, Karter AJ, Liu JY, Moffet HH, Sudore R, Schillinger D, et al. Correlates of quality-of-life in older adults with diabetes: The Diabetes & Aging Study. Diabetes Care 2011;DC_102424.


