Volume 39 Number 4
Management of complex head wounds involving the temporalis and sternocleidomastoid muscles: a case series
Angela Yi Jia Liew and Yee Yee Chang
Keywords Head and neck, wound breakdown, chronic wound, chemo-radiation therapy, split skin graft, wound bed preparation
For referencing Liew AYJ & Chang YY. Management of complex head wounds involving the temporalis and sternocleidomastoid muscles: a case series. WCET® Journal 2019;39(4):41-44
DOI https://doi.org/10.33235/wcet.39.4.41-44
Abstract
The use of a comprehensive wound assessment tool and appropriate selection of dressing products can aid clinicians in the management of complex wounds. This paper describes the care of two patients presenting with head wounds. The first case is a patient who suffered from a right temporalis flap failure and exposed periosteum after wide excision. The second case explores the management of a sternocleidomastoid abscess that developed during radiotherapy for head and neck cancer. The paper outlines how the choice of healing by secondary intention can prevent patients from undergoing additional surgeries where outcomes may not be favourable.
Introduction
Medical advancements in the management of head and neck cancers allow patients the flexibility of choice in their treatment. However, factors such as active immunotherapy treatments or previous surgical resection can affect the healing outcome or result in an unhealthy wound bed1. In addition, patients may not be suitable candidates for surgery or may prefer conservative management.
This case series will explore two cases which utilised conventional dressing products to assist with wound closure. The first case discusses a patient with a failed right temporal split skin graft with exposed cranium after wide excision for treatment of basal cell carcinoma. The second case focused on a patient who developed a neck abscess at the sternocleidomastoid region during chemotherapy and had undergone incision and drainage. Both cases were reviewed using the triangle of wound assessment which provided the clinicians with a guide for holistic evaluation2.
Case one
Mr A was a 77-year-old Chinese gentleman who presented at the head and neck specialist outpatient clinic in an acute tertiary hospital with a right temporal lesion on 29 April 2016. The lesion had been present since birth; however he had noticed an increase in size to 1x3cm after an accidental injury during a haircut two years ago. The lesion had no contact bleeding, no discharge and was non-tender. Head and neck examination revealed no palpable cervical lymph nodes. Mr A underwent an excision biopsy of the right temporal lump in May 2016. Histology showed apocrine adenocarcinoma and basal cell carcinoma arising from a nevus sebaceous cyst. A positron emission tomography (PET) scan showed no significant Fludeoxyglucose (FDG) uptake in other areas, but Mr A required a wider excision for adequate clearance of the tumour.
On 23 May 2016, Mr A underwent a wide excision in which a right temporalis flap was used to cover the exposed periosteum, followed by a split skin graft to cover the wound bed. The split skin graft was anchored down with a polyurethane foam dressing, and the patient was discharged 2 days later. Initial wound inspection of the right temporalis flap 10 days later showed that the split skin graft had taken well. However, wound inspection a week later showed slough at the edges of the split skin graft, and that the periosteum was exposed. The patient’s wound was managed with Aquacel® Ag (United Kingdom), a hydrofiber with silver, and he was offered another surgery for flap coverage as the wound remained stagnant despite every other day change of dressing. His primary surgeon referred him to the wound care team as Mr A refused surgery and requested a conservative approach instead.
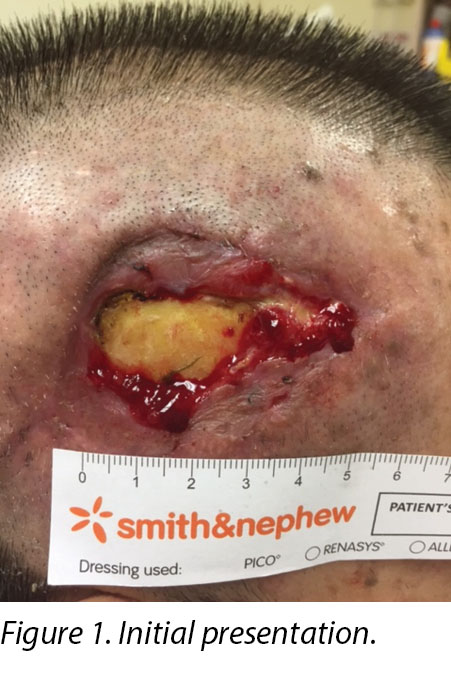
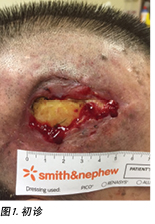
On inspection, the right temporalis wound measured 3x5cm overall with a depth of 1cm (Figure 1). The wound bed contained the exposed periosteum measuring 2x3cm with sloughy wound edges and areas of grafted skin which had taken. No signs of local infection were detected. Shaving of hair around the periwound region was done to maintain a good margin for the application of dressings. Wound clinicians did conservative sharp debridement of devitalised tissue at the wound edges. The wound was cleaned with normal saline and primarily dressed with Heliaid®, a collagen sheet dressing. The patient was taught to change the external gauze if stained for exudate control.
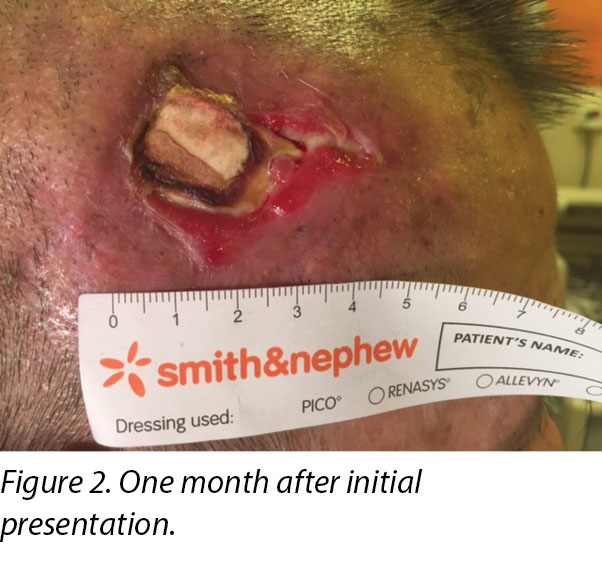
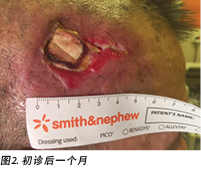
On presentation 1 month later, the patient’s wound showed friable, hyper granulating tissue at the inferior aspect of the wound bed (Figure 2). Shave excision using a blade was done to manage the hyper granulating tissue weekly, and the collagen dressing was not changed if it had not degraded as it served as a scaffold for granulating tissue to migrate and cover the exposed periosteum3.
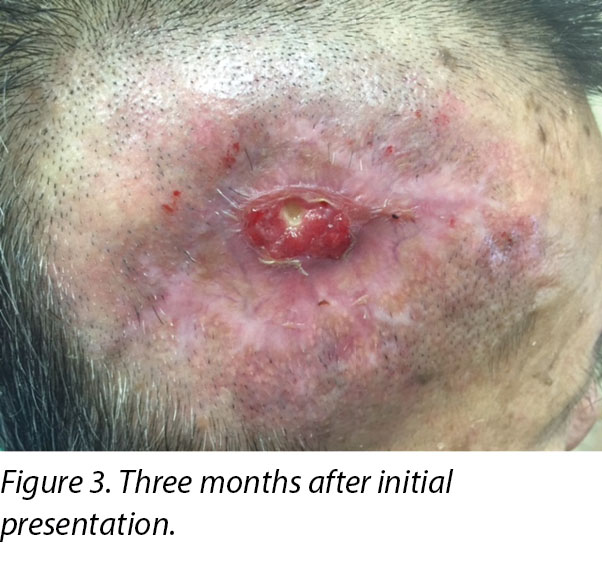
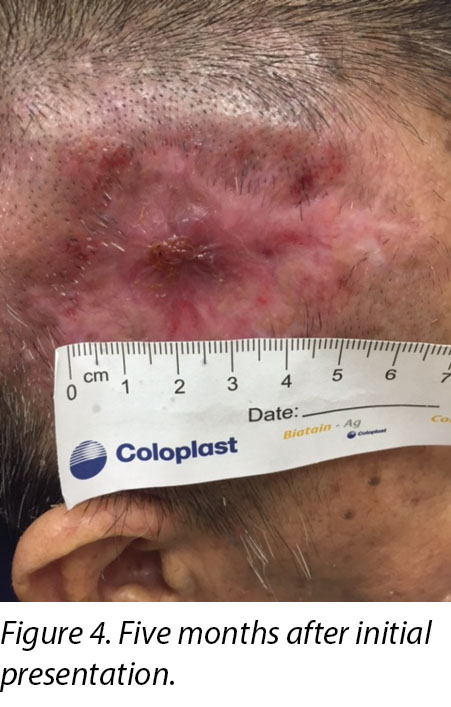
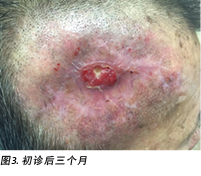
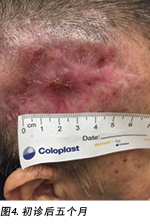
A review by the surgeon 3 months later showed the edges of the wound contracting, covering the periosteum (Figure 3). Hypertrophic granulation was still present at wound edges and required periodic shave excision. Collagen dressing was used until full epithelialisation occurred 5 months after the initial consultation with the wound care team (Figure 4).
Case Study 2
Mr B, a 71-year-old Chinese gentleman who had radiotherapy for squamous cell carcinoma (SCC) of the right tonsil presented to the emergency department with an abscess over the right sternocleidomastoid region. A computerised tomography (CT) scan of the neck showed the development of a multi-loculated abscess along the entire length of the right sternocleidomastoid muscle with overlying cellulitis. He underwent emergency incision and drainage of the abscess. As Mr B was undergoing active radiotherapy treatment and was therefore not a surgical candidate for removal of the SCC, he was referred to the wound care team for follow-up management of his surgical site.
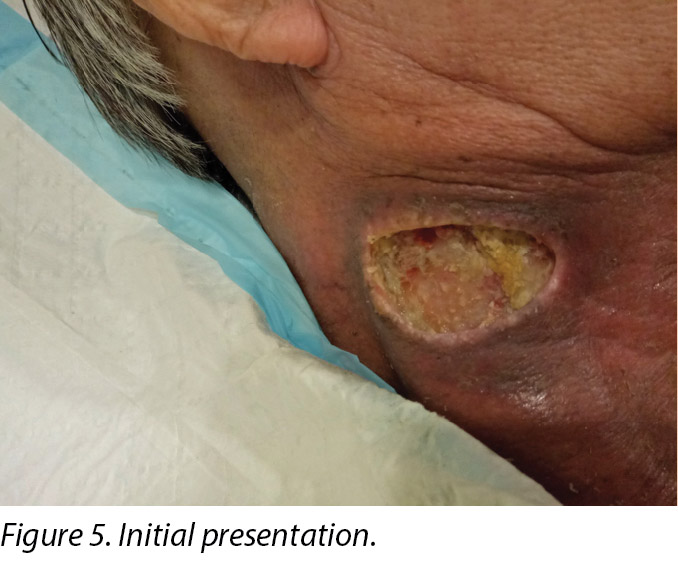
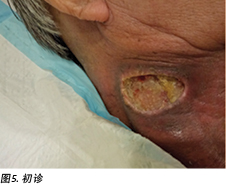
An initial assessment was done on 15 August 2016 in the wound specialist clinic in an acute tertiary hospital. The right sternocleidomastoid wound was a sloughy wound base that measured 3x4.5cm with a depth of 1cm and undermining from 12–2 o’clock to a length of 1cm (Figure 5). Periwound erythema, acute radiotherapy hyperpigmentation, and tenderness with a minimal amount of exudate was present. In addition, as the wound bed was near major neck vessels, sharp debridement was not considered an advisable option. As the patient needed to complete his prescribed radiotherapy treatment, plans were made for the initial management of his wound with a topical antimicrobial. Hence the patient was managed with daily povidone-iodine dressing as it was cost-effective and reduced the bioburden on the wound bed4.
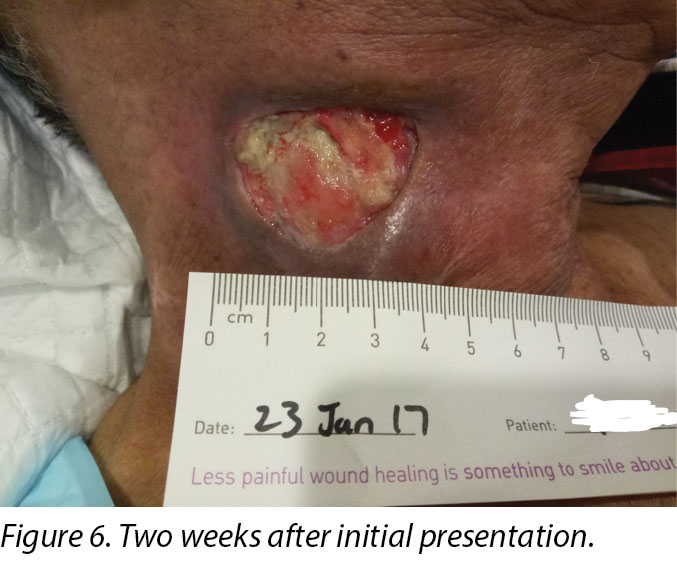
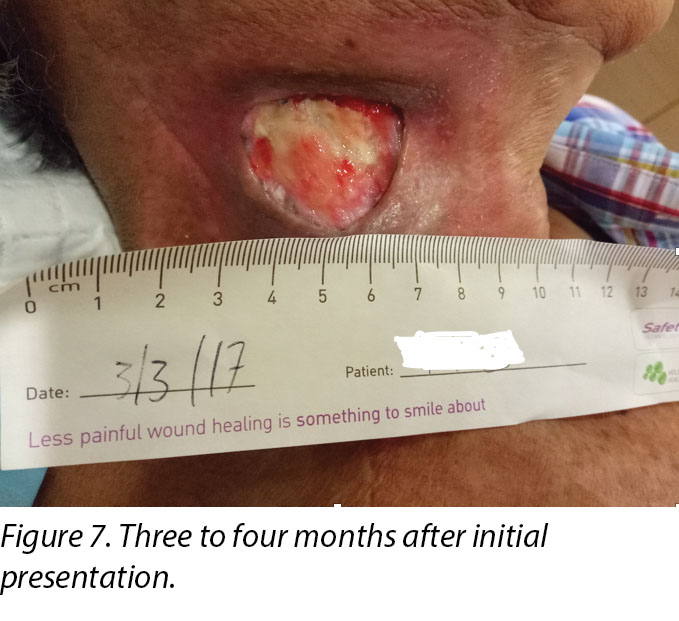
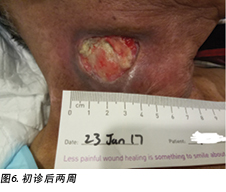
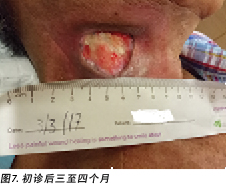
Upon completion of his oncology treatment 2 weeks later, reassessment of his wound was done (Figure 6). Wound measurements were 3x4.5cm with a 1cm depth. Minimal areas of granulation were noted, and the wound bed was mostly covered by adherent slough. Cleansing of the wound was done with normal saline. Mr B was initially started on Iodosorb® (New Zealand) cadexomer iodine powder then switched to cadexomer iodine ointment with Duoderm® CGF® (Denmark) hydrocolloid wafer as a secondary dressing to promote a moist wound healing environment4. As the wound progression was slow, after 3–4 months the treatment modality was switched to Askina® Calgitrol (United Kingdom), a silver gel, but this was stopped after 1 month as more fibrotic tissue was deposited on the wound bed (Figure 7). Mr B was then placed back on cadexomer iodine ointment with a hydrocolloid wafer. He was seen twice a week at the wound care clinic for careful debridement of loose devitalised tissue given the anatomy of major vessels around the wound bed. As the periwound skin was dry, Mr B was advised on the application of moisturiser daily.
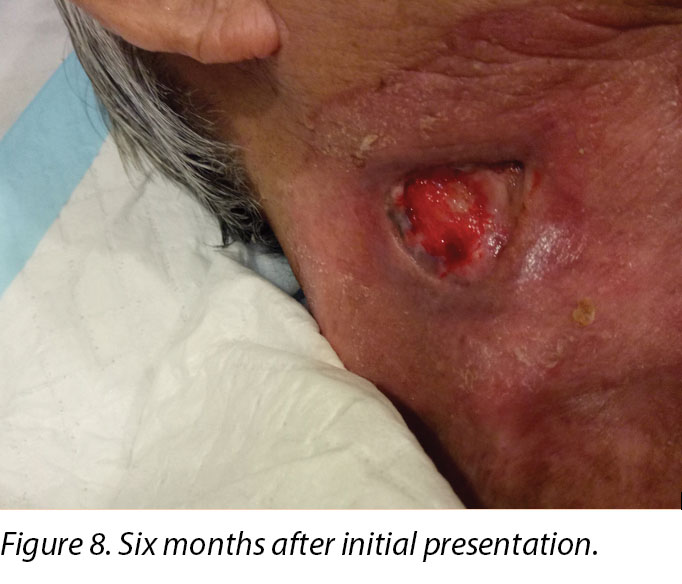
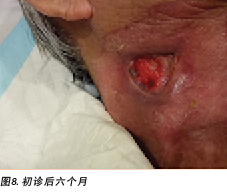
Six months after the initial treatment and review, the wound bed measured 3x4cm with a wound depth of 0.5cm (Figure 8). Mr B continued twice weekly dressings at the wound clinic for debridement of loose devitalised tissue with Iodosorb® cadexomer iodine ointment and Duoderm® CGF® hydrocolloid (Denmark) dressing. Once all fibrotic tissue had been debrided and the wound bed was superficial, the patient continued with hydrocolloid wafer primarily to facilitate granulation4. Full epithelialisation occurred 1 year after the initial presentation.
Discussion
Winter’s study5 on wound epithelialisation in porcine models showed that for wound healing to occur, a combination of three factors must be considered – reduction of bacterial burden on the wound bed, vascularisation, and wound hydration. In addition, a recent paper included debridement and oedema management as crucial factors to consider in wound healing6. Understanding these principles, combined with the usage of the triangle of wound assessment tool, aided wound clinicians in developing a patient-centric treatment plan which guided their choice of dressing products for these two cases7.
In the case of Mr A, his wound bed was the exposed periosteum which was devoid of blood supply8. The exudate level on this wound bed provided enough moisture for wound healing and was not excessive. Mr A’s wound did not present with any signs of local, spreading nor systemic infection. The wound edges consisted of hypertrophic tissue3. The temporal region, which was covered with hair, was the periwound area. The periwound skin condition was healthy, with no signs of eczema, maceration nor dehydration. The management plan was therefore to: promote granulation from the edges to close the wound; shear the hypertrophic tissue at the wound edge; and protect the wound bed from external sources of infection that can occur with non-adherence of the secondary dressing. The wound presentation narrowed down the dressing product selection to the ones that could promote angiogenesis and granulation such as collagen2.
The outer layer of the periosteum is cell poor; hence, epithelial advancement is challenging in such a wound bed due to the lack of blood supply and viable tissue for collagen synthesis9. Elastase, which activates matrix metalloproteinase (MMPs), is often present in such non-healing wounds3. It inhibits the extracellular matrix components of elastin and collagen by binding and depleting collagen levels within the wound bed. This resulted in the extracellular matrix degrading and a reduction in fibroblast levels crucial in the proliferative phase for wound healing3. Using a collagen dressing can disrupt the elastase levels, reducing MMP levels by binding to growth factors and further inactivating MMPs on the wound bed3. Also, using a collagen dressing can improve the deposition of new collagen within the wound bed, as fibroblast and macrophages anchor well to the three-dimensional collagen structure. This can propel the wound from the inflammatory to the proliferative phase, initiating angiogenesis3. Lastly, it stimulates cellular migration by providing a moist wound environment and fluid balance within the wound bed3.
Conservative sharp debridement was done for hypertrophic tissue at the wound edges periodically for easier cell migration laterally2. The application of a secondary dressing was challenging; hence shaving of the temporal region was necessary for better visualisation and to prevent foreign bodies such as hair from contaminating the wound bed10. Sebum from hair follicles also made adherence of secondary dressing difficult. Hence a layer of non-sting barrier spray was applied to the periwound area before the application of any secondary dressing.
As for Mr B, the wound presentation differed as he continued on radiotherapy treatment. Initial wound presentation was a wound bed which was dry; the initial treatment goal post-incision and drainage was to reduce the bioburden on the wound bed. Subsequent assessment of his wound post-radiation therapy was a fibrotic, dry and sloughy wound bed. The wound edge and periwound skin were also dry. The management plan was to: rehydrate the wound bed, wound edge and periwound skin to promote a moist wound healing environment; debride the non-viable tissue; and keep the wound free from infection. Conservative sharp debridement was risky to undertake given the anatomical location of the wound and the proximity of large vessels at the sternocleidomastoid region, hence autolytic debridement was selected together with an antimicrobial product which continued to reduce the bioburden on the wound bed4.
With Mr B, the wound clinicians witnessed the early and delay effects of radiation therapy on wound healing, given its proximity to the sternocleidomastoid wound. The redness that Mr B had immediately post-radiation (shown in Figures 5 and 6 in the periwound region), was due to the dry desquamation caused by radiation therapy11. There was also alteration in all layers of tissue post-radiation therapy in which the dermis and subcutaneous tissue were progressively replaced by dense and fibrotic tissue. This was due to the irregularity in collagen fibrils caused by abnormalities in collagen production formed by fibroblasts and myofibroblasts11.
Cadexomer iodine ointment was selected for its antimicrobial and autolytic properties12. The occlusive nature of hydrocolloid dressings helped facilitate granulation and inhibited bacterial growth4. Mr B preferred the conformability and exudate absorption capability of hydrocolloid dressings. He was advised to apply moisturiser on his periwound skin as it was dry and caused him some itch and discomfort. Though the wound size is small, it took almost a year before Mr B could achieve full epithelialisation of his wound.
The triangle of wound assessment tool provided wound clinicians with a comprehensive look at the assessment of a wound so a suitable management plan could be developed. The tool was used in combination with the understanding of wound healing in irradiated areas or wound beds devoid of blood supply. It was also noted that it would be costly for patients if the clinicians were unable to select a dressing product for its intended purpose4. Armed with this information, the wound clinician and the patient were able to set realistic treatment goals and timeframes for healing.
Conclusion
A favourable outcome in wound closures for complex wounds by secondary intention is determined by understanding the challenges a complex wound can pose. The wound clinician can develop a realistic, patient-centric treatment plan and select the appropriate dressing product which is suitable for its intended purpose.
Conflict of Interest
The authors declare that there is no conflict of interest involved in the studies.
Funding
The authors received no funding for this study.
颞肌和胸锁乳突肌受累的复杂头部伤口管理:病例系列
Angela Yi Jia Liew and Yee Yee Chang
DOI: https://doi.org/10.33235/wcet.39.4.41-44
摘要
使用全面的伤口评估工具和适当的敷料选择可以帮助临床医生处理复杂的伤口。本文介绍了两例头部受伤患者的护理。第一例患者右颞皮瓣移植失败,广泛切除后骨膜暴露。第二例探讨了在头颈癌放射治疗期间发生的胸锁乳突肌脓肿的管理。本文概述了二期愈合方法的选择如何防止患者接受可能产生不利结果的其他手术。
前言
头颈癌管理方面的医学进步使患者可以灵活选择治疗方案。但是,主动免疫治疗或既往手术切除史等因素可能会影响愈合结果或导致不健康的伤口床1。此外,患者可能不适合手术,或者可能更倾向于保守治疗。
本案例系列将探讨两个利用常规敷料产品来帮助伤口闭合的病例。第一例为右颞肌分层皮肤移植失败的患者,该患者为治疗基底细胞癌进行了广泛切除,随后颅骨暴露。第二例重点关注化疗期间,在胸锁乳突肌区域出现颈部脓肿并进行了切开引流的患者。两个病例均使用伤口评估三角进行了回顾,该评估方法为临床医生提供了全面评价的指南2。
病例一
A先生是一名77岁的中国患者,于2016年4月29日因右颞肌病灶在一家三级急症医院的头颈专科门诊就诊。该病灶自出生起就存在;但是,他注意到两年前在一次理发中意外受伤后,病灶尺寸增至1×3 cm。该病灶无接触性出血,无分泌物,无压痛。头颈检查未发现明显的颈部淋巴结肿大。A先生于2016年5月接受了右颞肿块切除活检。组织学检查表明,其患有皮脂腺痣囊肿引起的顶浆分泌腺癌和基底细胞癌。正电子发射断层成像(PET)扫描显示在其他区域没有明显的氟代脱氧葡萄糖(FDG)摄取,但是A先生要求进行更广泛的切除,以便充分清除肿瘤。
2016年5月23日,A先生进行了广泛切除,其中使用右颞皮瓣覆盖暴露的骨膜,然后通过分层皮肤移植覆盖伤口床。分层皮肤移植物用聚氨酯泡沫敷料固定,2天后出院。10天后对右颞皮瓣进行初步伤口检查,结果显示分层皮肤移植物的情况良好。但是,一周后的伤口检查显示分层皮肤移植物的边缘脱落,并且骨膜暴露。对患者使用含银的亲水纤维Aquacel® Ag(英国)进行伤口管理,同时建议其接受另一个皮瓣覆盖手术,因为尽管每隔一天更换敷料,但伤口愈合情况仍然处于停滞状态。他的主治外科医师将他转诊至伤口护理团队,因为A先生拒绝接受手术,而是要求采取保守的治疗方法。
经检查,右颞伤口的整体尺寸为 3×5 cm,深度为1 cm(图 1)。伤口床包含暴露的骨膜,骨膜尺寸为2×3cm,伤口边缘和已移植皮肤的区域存在脱落现象。未检测到局部感染的迹象。对伤口周围区域备皮,使边缘便于敷贴敷料。伤口临床医生对伤口边缘的失活组织进行了保守性锐器清创。使用生理盐水清洗伤口后,使用Heliaid®胶原薄片敷料进行初步包扎。告知患者在包扎材料变色时更换外层纱布,以便进行渗出液控制。
在初诊1个月后的检查中,患者伤口床下方显示出易碎的肥厚肉芽组织(图2)。每周使用刀片进行剃刮切除,以处理肥厚肉芽组织,如果胶原蛋白敷料没有降解,则不进行更换,因为它充当了使肉芽组织迁移并覆盖裸露骨膜的支架3。
3个月后,外科医生进行了一次检查,结果显示伤口的边缘收缩,覆盖了骨膜(图3)。伤口边缘仍存在肥厚肉芽,需要定期剃刮切除。使用胶原蛋白敷料,直至与伤口护理团队进行初步咨询后5个月完全上皮化(图4)。
病例研究2
B先生是一名71岁的中国患者,已因右扁桃体鳞状细胞癌(SCC)进行了放射治疗,现因右胸锁乳突肌区域出现脓肿而送往急诊科。颈部的计算机断层成像(CT)扫描显示,其整个右胸锁乳突肌区域出现多处脓肿,并且上覆蜂窝织炎。他接受了紧急脓肿切开和引流。由于B先生当时正在接受积极放射治疗,无法通过手术切除SCC,因此他被转诊至伤口护理团队进行手术部位的后续管理。
于2016年8月15日在一家三级急症医院的伤口专科门诊进行了初步评估。右胸锁乳突肌伤口的基部存在脱落,其尺寸为3×4.5 cm,深度为1 cm,从12点至2点钟逐渐减小到长度为1 cm(图5)。患者出现伤口周围红斑、急性放射治疗导致的色素沉着过度,以及压痛伴少量渗出液。此外,由于伤口床位于主要颈部血管附近,因此不建议进行锐器清创。由于患者需要完成预定的放射治疗,因此制定了使用局部抗菌剂初步治疗伤口的计划。因此,患者每天接受聚维酮碘敷料治疗,因为它具有成本效益,并可减少伤口床上的生物负载4。
完成肿瘤治疗2周后,对伤口进行了重新评估(图6)。测量的伤口尺寸为3×4.5 cm,深度为1 cm。观察到的肉芽区域极少,大部分伤口床被粘着的腐肉覆盖。使用生理盐水清洗伤口。B先生最初使用Iodosorb®(新西兰)卡地姆碘粉末,随后改为卡地姆碘软膏,并使用Duoderm® CGF®(丹麦)水胶体片作为二级敷料,以促进形成湿润的伤口愈合环境4。由于伤口进展缓慢,3-4个月后将治疗模式更改为含银胶体Askina® Calgitrol(英国),但1个月后因更多纤维化组织沉积在伤口床处而停止使用(图7)。随后,B先生重新接受卡地姆碘软膏和水胶体片治疗。鉴于伤口床周围主要血管的解剖结构,患者每周两次在伤口护理门诊接受治疗,以便对松散的失活组织进行仔细清创。由于伤口周围皮肤干燥,建议B先生每天使用保湿霜。
初始治疗和检查后六个月,测量的伤口床尺寸为3×4 cm,伤口深度为0.5 cm(图8)。B先生每周在伤口门诊对Iodosorb®卡地姆碘软膏和Duoderm® CGF®水胶体(丹麦)敷料进行两次更换,以便对松散的失活组织进行清创。清除所有纤维化组织并且伤口床变为浅层后,患者继续使用水胶体片作为初级敷料,以促进肉芽形成4。初诊后1年实现完全上皮化。
讨论
Winter关于猪模型中伤口上皮化的研究5显示,如需伤口愈合,必须综合考虑三个因素,即减少伤口床上的细菌负载、实现血运重建和保持伤口湿润。此外,最近的一篇论文将清创和水肿处理作为伤口愈合中需要考虑的关键因素6。了解上述原理后,结合使用伤口评估三角工具,有助于伤口临床医生制定以患者为中心的治疗计划,从而为两个病例的敷料产品选择提供指导7。
对于A先生,其伤口床是暴露的骨膜,没有血液供应8。该伤口床上的渗出液水平为伤口愈合提供了足够的水分,并且没有过量。A先生的伤口没有出现任何局部、扩散或全身感染的迹象。伤口边缘由肥厚组织组成3。被头发覆盖的颞区为伤口周围区域。伤口周围皮肤状况健康,没有湿疹、浸软或脱水的迹象。因此,管理计划是:从边缘促进肉芽形成以闭合伤口;切除伤口边缘处的肥大组织;并保护伤口床免受外部感染源的侵害(可能由无粘性的二级辅料导致)。该伤口情况将敷料产品的选择范围缩小到可以促进血管生成和肉芽形成的产品,例如胶原蛋白2。
骨膜的外层上细胞贫乏;因此,由于缺乏血液供应和胶原合成所需的活组织,在此类伤口床上实现上皮化非常具有挑战性9。此类难愈合伤口中经常存在激活基质金属蛋白酶(MMP)的弹性蛋白酶3。它通过结合和消耗伤口床内的胶原蛋白水平来抑制弹性蛋白和胶原蛋白的细胞外基质成分。这导致细胞外基质降解和成纤维细胞水平降低,而这两种物质在伤口愈合的增生期至关重要3。使用胶原蛋白敷料可以破坏弹性蛋白酶水平,通过结合生长因子来降低MMP水平,并进一步使伤口床上的MMP失活3。同样,使用胶原蛋白敷料可以改善伤口床中新胶原蛋白的沉积,因为成纤维细胞和巨噬细胞能够稳固地依附在三维胶原蛋白结构上。这有助于伤口从炎症阶段进入增生阶段,从而开始血管生成3。最后,它通过在伤口床内提供湿润的伤口环境和液体平衡来刺激细胞迁移3。
定期对伤口边缘的肥厚组织进行保守性锐器清创,便于细胞横向迁移2。二级敷料的应用具有挑战性;因此,必须对颞区进行备皮,以便进行观察,并防止头发等异物污染伤口床10。毛囊的皮脂也使二级敷料难以附着。因此,在使用任何二级敷料之前,应在伤口周围区域使用一层无痛隔离喷雾。
至于B先生,由于持续接受放射治疗,其伤口情况有所不同。最初的伤口表现是伤口床干燥;切开和引流后的最初治疗目标是减少伤口床上的生物负载。放射治疗后,其伤口的后续评估结果为伤口床纤维化、干燥,以及存在脱落现象。伤口边缘和伤口周围皮肤也很干燥。管理计划是:为伤口床、伤口边缘和伤口周围皮肤补水,以促进形成湿润的伤口愈合环境;对失活组织进行清创;并保持伤口不受感染。考虑到伤口的解剖学位置和胸锁乳突肌区域附近的大血管,进行保守锐器清创存在一定风险,因此选择了自溶性清创,并使用抗菌产品持续减少伤口床的生物负载4。
在B先生的病例中,伤口临床医生见证了放射疗法对伤口愈合的早期和延迟效果,因为治疗区域靠近胸锁乳突肌伤口。B先生在接受放射治疗后皮肤立即发红(图5和图6所示的伤口周围区域),这是由于放射疗法引起皮肤干燥脱皮11。放射治疗后,组织全层都发生了变化,其中真皮和皮下组织逐渐被致密和纤维化组织所代替。这是由于成纤维细胞和肌成纤维细胞形成的胶原蛋白产生异常,从而导致胶原蛋白纤维的不规则性11。
选择卡地姆碘软膏的原因是其具有抗菌和自溶特性12。水胶体敷料的闭塞性有助于促进肉芽形成并抑制细菌生长4。B先生更喜欢水胶体敷料的顺应性和渗出液吸收能力。建议他对伤口周围皮肤使用保湿霜,因为此处皮肤比较干燥,并且会引起瘙痒和不适。尽管伤口很小,但B先生花了将近一年的时间才实现伤口完全上皮化。
伤口评估三角工具为伤口临床医生提供了伤口评估的全面信息,有助于制定适当的管理计划。该工具与对辐照区域的了解或无血液供应伤口床的伤口愈合情况结合使用。还应注意的是,如果临床医生无法选择适合其预期用途的敷料产品,则对患者而言,费用将非常昂贵4。获得这些信息后,伤口临床医生和患者就能够设定切合实际的治疗目标和时间表。
结论
通过了解复杂伤口可能带来的挑战,可以确定二期伤口愈合中复杂伤口闭合的良好效果。伤口临床医生可以制定出切合实际、以患者为中心的治疗计划,并选择适合其预期用途的合适敷料产品。
利益冲突
作者声明本研究中没有任何利益冲突。
资助
作者未因该项研究收到任何资助。
Author(s)
Angela Yi Jia Liew*
RN, CWOCN
Assistant Nurse Clinician, Division of Nursing, Speciality Nursing (Wound Care), Singapore General Hospital, Singapore
Email Angela.liew.y.j@sgh.com.sg
Yee Yee Chang
MN, RN, CWOCN
Nurse Clinician, Division of Nursing, Speciality Nursing (Wound Care), Singapore General Hospital, Singapore
* Corresponding author
References
- Coskun H, Erisen L, Basut O. Factors affecting wound infection rates in head and neck surgery. Otolaryngol Neck Surg [Internet]. 2000;123(3):328–33.
- Dowsett C, Protz K, Drouard M, Harding K. Triangle of wound assessment made easy. Wounds Asia [Internet]. 2015;1–6.
- Fleck CA, Simman R. Modern collagen wound dressings: function and purpose. J Am Col Certif Wound Spec [Internet]. 2010;2(3):50–4.
- Vowden K, Vowden P. Wound dressings: principles and practice. Surg (UK) [Internet]. 2017;35(9):489–94.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability [Internet]. 2006;16(2):20–3.
- Jones J. Winter’s concept of moist wound healing: a review of the evidence and impact on clinical practice. J Wound Care 2005;14(6):273–6.
- Baranoski S, Ayello EA. Wound dressings: an evolving art and science. Adv Ski Wound Care 2012;25(2):87–92.
- Dwek JR. The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol 2010;39(4):319–23.
- Agarwal A, Andrew LE, Ayello EA, Baranoski S, Bates-Jensen BM, Bauer C, et al. Chapter two: wound healing. In: Doughty DB, McNichol L, editors. Wound, Ostomy and Continence Nurses SocietyTM core curriculum: wound management. 1st ed. Philadelphia: Wolters Kluwer; 2016. p. 24–68.
- Sebastian S. Does preoperative scalp shaving result in fewer postoperative wound infections when compared with no scalp shaving? A systematic review. J Neurosci Nurs [Internet]. 2012;44(3):149–56.
- Wang J, Boerma M, Fu Q, Hauer-Jensen M. Radiation responses in skin and connective tissues: effect on wound healing and surgical outcome. Hernia 2006;10(6):502–6.
- Sandoz H, Swanson T, Weir D, Schultz G. Biofilm-based wound care with cadexomer iodine. Wounds Int [Internet]. 2017;(November):1–6.


