Volume 42 Number 2
Fluid handling dynamics and durability of silver-containing gelling fibre dressings tested in a robotic wound system
Aleksei Orlov, Adi Lustig, Angela Grigatti and Amit Gefen
Keywords Wound care, biomechanical model, exudate management, laboratory testing, sorptivity, tissue phantom
For referencing Orlov A et al. Fluid handling dynamics and durability of silver-containing gelling fibre dressings tested in a robotic wound system. WCET® Journal 2022;42(2):15-23
DOI https://doi.org/10.33235/wcet.42.2.15-23
Abstract
Objective To develop a robotic phantom system containing multiple simulated wound replicates to determine the synergy in fluid absorbency and retention (sorptivity) performances and the post-simulated-use mechanical durability of silver-containing gelling fibre primary dressings when used with a secondary dressing, as per clinical practice.
Methods Using a robotic system containing six identical wound simulators, the authors tested the sorptivity performances of the Exufiber Ag+ (Mölnlycke Health Care, Gothenburg, Sweden) primary dressing (ExAg-polyvinyl alcohol [PVA]) against a market-leading comparator product, when used with a secondary foam dressing. The durability of the primary dressings after simulated use was further investigated through tensile mechanical testing.
Results The ExAg-PVA primary dressing delivered greater fluid amounts for absorbency and retention by the secondary foam dressing, approximately 2- and 1.5-fold more than the comparator dressing pair after 10 and 15 hours, respectively. The ExAg-PVA dressing was also substantially less sensitive to the direction of pulling forces and, accordingly, exhibited post-use mechanical strength that was approximately four- and six-times greater than that of the other primary dressing (when the latter dressing was tested out-of-alignment with its visible seams) after 10 and 15 hours, respectively.
Conclusions The dynamics of the sorptivity and fluid sharing between primary and secondary dressings and the effect of directional preference of strength of the primary dressings for adequate durability, resulting in safe post-use removals, have been described. The comparative quantification of these capabilities should help clinical and nonclinical decision-makers select dressings that best meet their patient needs.
Copyright ©2022 the Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Introduction
Wounds of all types, including chronic cavity wounds such as pressure injuries, are one of the most impactful, expensive, and common medical problems. Nearly 2.5% of the US population experiences wounds, which is similar, for example, to the prevalence of stroke in the US.1–4 As the population ages and the prevalences of diabetes and obesity increase, there is a sharp growth in demand for advanced and cost-effective wound care to deliver improved patient outcomes. Treatment dressings play a pivotal role in all aspects of wound healing. Designed, in essence, as a temporary artificial substitute for intact skin, treatment dressings protect the wound and manage the exudates that result from periwound inflammatory processes. The presence and composition of exudates typically support the function of tissue-repairing cells and newly generated tissues; thus, in a normal tissue-repair process, the rate of exudate and its constituents will match the healing phase.5–7 However, abnormal prolongation of the inflammatory stage, such as in chronic wounds, may disrupt the physiologic control of exudate production, leading to excessive exudate amounts or altered ratios of protein content which causes the fluid to be thick, highly viscous, or even sticky.5
Effective wound dressings are required to manage exudate fluids secreted at varying rates and viscosities, in the same wound at different times, or for wounds of the same etiology but different patients. A common clinical practice is to insert a primary wound dressing through the wound opening to form the first-line reservoir for fluid absorption and retention on the wound bed. A secondary dressing is then placed above the cavity (and the primary dressing) for mechanical and biological protection, as well as to make a second vessel for the accumulating fluids. Gelling fibre dressings composed of polyvinyl alcohol (PVA) fibres or sodium carboxymethyl cellulose (CMC) are widely used as primary dressings because of their ability to trap fluids by transformation into a gel phase8 (examples of these commercial primary wound dressing products are listed in the woundsource.com resource database9 under the “Gelling Fiber Dressings” wound dressing product category). A variant of these primary gelling fibre dressings are those that contain silver ions to further induce an antimicrobial effect. To deliver effective treatments, a primary dressing and a secondary dressing must work in synergy; that is, neither dressing should approach its maximum fluid absorption capacity within the timeframe indicated for use.3,10–12
The performance of a wound dressing depends primarily on the specific material composition, structure, and manufacturing technology of the dressing. These characteristics, in combination with the relevant clinical protocol, specific wound environment, and the type of paired secondary dressing, impact the safety and efficacy of the primary dressing product. Two fluid-structure interaction properties that are relevant to the function of primary dressings are “sorptivity,” the capacity of a dressing structure to transfer excessive exudate away from the wound bed and onward to the secondary dressing through capillary action,10,13 and “durability,” the ability of a dressing to withstand patient body weight and other mechanical forces applied during changes and remain intact within the wound over the period of use or upon removal.10,13
In the 1970s, it was reported that the presence of gauze dressing particles in wounds “act as foreign bodies and may delay healing. Their removal has been associated with accelerated healing.”14 More recently, Chakravarthy and colleagues15 documented grossly visible disintegration of modern (hydrocolloid) dressings in wounds. Dressing disintegration was associated with inflammatory giant cells—pathologic evidence for a foreign-body reaction—which highlights the importance of durability at both the macroscale and microscale. Specifically, Chakravarthy et al15 evaluated the density of giant cells in histologic tissue sections that were extracted from wounds in a pig model of wound healing and subsequently stained by hematoxylin and eosin. They documented the presence of foreign material (ie, dressing debris) in the vicinity of the giant cells in the histologic slides, which correlated with their observed fragmentation of some of the tested hydrocolloid dressings upon removal during dressing changes.15
Accordingly, poorly performing wound dressings or dressing pairs may cause suboptimal moisture balance, mechanical damage to tissues, foreign-body reactions, or a combination of these adverse events. Such dressing failure modes should be identified through methodological bioengineering laboratory testing so that medical claims can be controlled by regulatory bodies and dressing failure in clinical settings can be avoided. In this context, the authors continue to invest research efforts into the development and improvement of preclinical and laboratory testing methods for objective, quantitative, and standardised evaluation of wound dressing performance.3,10,11,13 Here, the authors present a novel robotic phantom system that contains multiple simulated wound replicates for the simultaneous testing of dressings. The system was developed and used to determine the synergy in fluid absorbency and retention performances of two silver-containing gelling fibre primary dressing products. The methods, equipment, and protocols reported herein form the basis for the next level of clinically relevant performance testing for wound dressings, focused on both safety and efficacy.
Methods
Robotic Exuding Wounds
The researchers developed and used a robotic phantom system comprising six wound replicates. Each wound unit in this system simulated an exuding, 2.5 cm-deep cavity wound (Figure 1). All six wound units included three layers of synthetic soft-tissues simulants (Figure 1). The top layer, representing the peri-wound skin, consisted of 5 mm-thick transparent silicone rubber (RTV615, Momentive Performance Materials Inc, Waterford, New York). An 8-mm-thick layer of paraffin gel (“candle-gel”; Ziv Chemicals Ltd., Holon, Israel) was placed below this “skin” layer, to represent adipose tissue. The inferior layer (with thickness of 12 mm) representing skeletal muscle was again made of silicone rubber (identical to that used as the skin simulant). The elastic modulus of the aforementioned silicone rubber material, measured through uniaxial tensile testing using an electromechanical material-testing machine (Instron model 5944; Instron Co, Norwood, Massachusetts) following ASTM D412-06,16 is 1.5 MPa, which is characteristic of both skin and muscle tissues under large deformations.17–19 The elastic modulus of the paraffin gel used to represent adipose tissue was measured through previously reported indentation tests20 and found to be 5 kPa, which is similar to the reported stiffness of native adipose tissues.21 To simulate continuous secretion of exudate from the above wound constructs, a spiral perforated irrigation tube was laid in each simulated wound bed and tunneled through the phantom structure to connect to a multichannel, programmable syringe pump (NE-1600; New Era Pump Systems Inc, Farmingdale, New York; Figure 1). The effective wet surface formed in the simulated wound beds through this irrigation element was approximately 24 cm2, corresponding to an effective irrigation depth of approximately 2.5 cm. The multichannel syringe pump provided precise control over the flow volume and release rate of an exudate-substitute fluid delivered into the simulated wound beds. The safe and reproducible exudate substitute fluid formula is based on Xanthan gum (C35H49O29); it was previously developed by the authors’ research group for use with the robotic wound phantom systems. This formula facilitates control of the fluid viscosity and pH level to adequately represent the physical characteristics of native exudates.10-13,22 Two exudate solutions were prepared, with high and low viscosities of 0.23 Pa×s and 0.06 Pa×s, respectively; the fluid density was 1.03 g/mL for both. An infrared heating lamp was positioned above the six wound replicas to maintain them at a temperature of 33 ± 2° C;23 the temperatures were monitored via a digital thermometer (Newtron TM-5005-SINGLE I/P; MRC Laboratory Equipment Ltd, Holon, Israel).
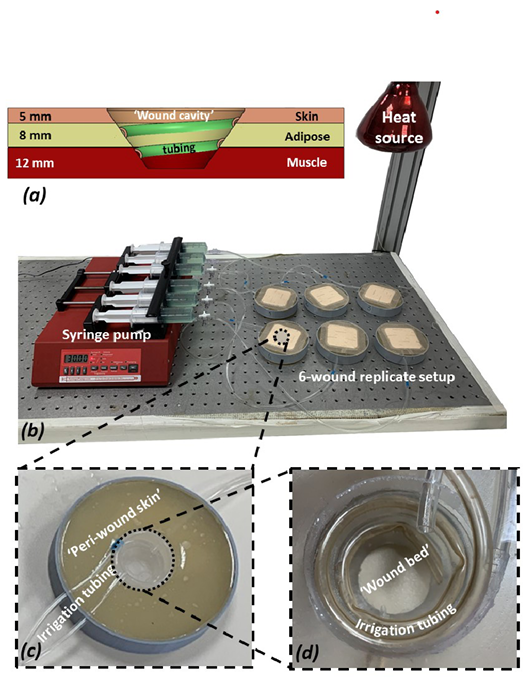
Figure 1. The experimental setup and its components
a) A scheme of a cross-section through a single “wound” simulator unit showing the different tissue simulant layers and their respective thicknesses, the shape and depth of the ‘wound cavity’ and the configuration of the spiral irrigation tubing. (b) A photograph of the robotic phantom system including the six wound replicates and the control unit. (c) A close-up view of a single simulated wound. (d) Zoom on the simulated exuding “wound bed.”
Simulated Treatments
Two types of 10×10 cm primary gelling fibre dressings were investigated: Exufiber Ag+ (Mölnlycke Health Care AB, Gothenburg, Sweden) with PVA fibres (ExAg-PVA) and an equivalent, commercially available, and market-leading silver ion dressing product containing sodium CMC as the gelling material (Ag-CMC). The Ag-CMC dressing is a soft, sterile, nonwoven pad composed of sodium CMC hydrocolloid fibre material that is impregnated with 1.2% ionic silver.24-26 It is indicated for clinical use in various wound types, both acute and chronic, and its physical and antibacterial properties have been described in detail elsewhere.24-26 Of note, although the two types of primary dressings selected for this study—PVA-based and CMC-based—are made of distinct materials and produced through different manufacturing technologies, both are clinically indicated for use as cavity wound fillers, which was the rationale for the current comparison. Mepilex Border Flex (MBF-Foam; Mölnlycke Health Care), a multilayer bordered silicone-foam dressing, was used as the secondary dressing to cover the simulated wounds in all the tests. The MBF-Foam is a five-layer dressing that includes (from the outer dressing surface to its wound-facing aspect): backing film, a retention layer, a spreading layer, an absorptive foam layer (which transports exudate to the spreading layer), and a wound contact layer.
Prior to applying the dressing products onto the simulated wounds, each dressing (primary or secondary) was weighed. The dressings were then applied according to manufacturer instructions. The simulated wounds were positioned facing upward, so that the tested dressings were required to absorb and retain the exudate substitute through capillary action (ie, against gravity). The robotic system was activated with a flow rate of 2 mL/h. To determine the time course of the absorbency performances of the tested dressings and, importantly, the dynamics of the fluid distribution between the primary and secondary dressings, the products were tested for multiple durations of simulated use: 5, 10, and 15 hours.
Dressing Studies
Fluid Retention and Distribution Between the Primary and Secondary Dressings. Following simulated use in the robotic phantom system, the dressings were reweighed to calculate the net mass gain in each dressing due to fluid absorption; any nonretained, residual fluids were carefully collected from the wound cavities and also weighed. After converting the measured fluid masses to volumes (by dividing the absorbed and residual fluid masses by the fluid density), the total exudate volume was calculated separately for each test as the sum of the fluid volumes in the primary and secondary dressings plus the volume of the residual fluid. Next, the distribution of fluid volumes between the primary and secondary dressings was calculated for each test as the percentage of fluid retained in the primary versus the secondary dressing, with respect to the corresponding total exudate volume (the theoretical value of which is also calculable, as the product of the predetermined flow rate and the time of operation of the robotic wound system).10
Fiber Directionality. For gelling fibre dressings, the directionality of the fibres, which typically is related to the manufacturing technology of these primary dressings (ie, PVA vs CMC), likely affects the performances of the dressing product, such as the sorptivity (the fibres become the structural conductors for the capillary action) and the durability (the fibres provide structural support and mechanical tolerance against forces that are aligned with their primary direction). Accordingly, the researchers assessed the directionality of the fibres in the two primary dressing types by digital image processing of microscopy images of the dressing surfaces, which were acquired using a light optical stereo microscope (Axiolab A450909; Carl Zeiss AG, Oberkochen, Germany). The micrographs of the dressing surfaces were acquired in transmitted light mode using a 1.25× magnification objective, a C-mounted digital camera (Swift Cam SC1803; Swift Optical Instruments Inc, Schertz Texas) and the Swift Imaging software (version 3.0, Swift Optical Instruments Inc.). Five fields of view (FOVs) were captured at consistent surface locations from two primary dressing specimens of each type: One FOV was located at the center of the dressing, and the other four FOVs formed a cross around the dressing center, with each such peripheral FOV located at a distance of 2.5 cm from the dressing edges. This resulted in a total of 15 digital micrographs of the FOVs per dressing type, each with dimensions of 4,912 × 3,684 pixels (1 square pixel = 0.919 µm2). For the purpose of the fibre directionality analyses, the acquired FOVs were further divided into three rectangular sub-FOVs, each with dimensions of 1445×3288 pixels. The fibre directionality analyses were conducted using the postacquisition plugin “OrientationJ” of the ImageJ software suite (version 1.X),27,28 which segments the fibres in the digital micrographs and calculates the probability function for their planar orientation in each analyzed sub-FOV. After calibrating this code and visually verifying its performances, the normalised histograms of the fibre orientations in the studied primary dressings were extracted.
Strength of the Primary Dressings After Simulated Use. Immediately after the simulated use sessions, the tensile strength of each primary dressing specimen was tested using an electromechanical testing machine (Instron model 5944; Instron Co) equipped with a 2kN load cell, following a protocol that is based on ASTM D882-02.29 Primary dressing specimens prepared according to the above testing standard were stretched at a deformation rate of 50 mm/min until ultimate failure occurred. Based on the resulting force-deformation data, stress-strain curves of the dressings after simulated use were plotted and the area under the stress-strain curve, which is the strain energy density (SED) to failure, was calculated for each test using a dedicated MATLAB computer code (ver. R2019a; MathWorks, Inc, Natick, Massachusetts). Based on these microscopy analyses of the fibre orientations, the ExAg-PVA dressing was treated as a structure without a specific directional preference (ie, test specimens from this dressing type were prepared and mechanically tested at random directions). However, the Ag-CMC silver-containing primary dressing, which has a specific directional preference of its fibres (as further detailed in the Results), was tested in two different configurations: where its principal fibre direction was fully aligned with the loading axis of the material testing machine, and where the fibres were out of such alignment.
Statistical analyses
All the experiments reported above were conducted in replicates of six and descriptive statistics of means and SDs were calculated for the retained fluid volumes, the distribution of fluid contents between the primary and secondary dressings per each simulated use duration (5, 10, and 15 hours), and the SED to failure of the primary dressings (depending on the directionality of the fibres with respect to the loading axis, as noted previously). Next, analyses of variance (ANOVA), followed by post-hoc Tukey-Kramer multiple pairwise comparisons, were run to identify potential differences between the dressing performances in the previously described fluid management (sorptivity) and material strength tests. Specifically, two-way ANOVAs for the factors of the usage time and the primary dressing type were conducted for the fluid retention and fluid distribution data. In addition, a three-way ANOVA for the factors of the usage time, the primary dressing type, and the directionality of the tensile test with respect to the fibre orientation (in the primary dressing that exhibited directional preference of fibre orientation) was performed for the material strength data. Only the longer exposure times of 10 and 15 hours, for which substantial fluid mass had accrued in the tested primary dressings, were considered for the latter three-way ANOVA. With respect to the directionality factor, based on the microscopy analyses, the last ANOVA considered the ExAg-PVA dressing, which did not show any specific and consistent directional preference of its fibres, as having a single strength (SED-to-failure) property. However, the Ag-CMC had two strength properties, namely, the strength measured (1) when the fibres were fully aligned with the loading axis of the material testing machine and, (2) when they were out of such alignment. P <.05 was considered statistically significant.
Results
When examined in isolation, the absorbency of the ExAg-PVA primary dressing was lower (approximately 11-22%) than that of the Ag-CMC primary silver dressing (Figure 2a). However, this difference was due to a more effective transfer of the exudate-like fluid from the ExAg-PVA dressing to the secondary dressing, as evident when examining the data for the fluid distribution between the primary and secondary dressings (Figure 2b). Specifically, when functioning in a pair with the ExAg-PVA dressing, the secondary dressing contained approximately twice the amount of fluid at the 10-hour time point and 1.5-times the amount of fluid after 15 hours with respect to the comparator pair (Figure 2b). The dynamics of the fluid distribution between the primary and secondary dressings over time (Figure 2b) further revealed that the reservoir of the secondary dressing began to receive fluid no earlier than 5 hours from the time of the dressing application (Figure 2b). After 15 hours, the secondary dressing shared approximately 54.2% of the retained fluid when the primary dressing was ExAg-PVA but only 36.7% when the Ag-CMC primary dressing was used (P < .05; Figure 2b). Importantly, these results represent the performances of the dressing pairs and therefore, better reflect real-world clinical practice, as opposed to assessments of the function of wound dressings in isolation (Figure 2b).
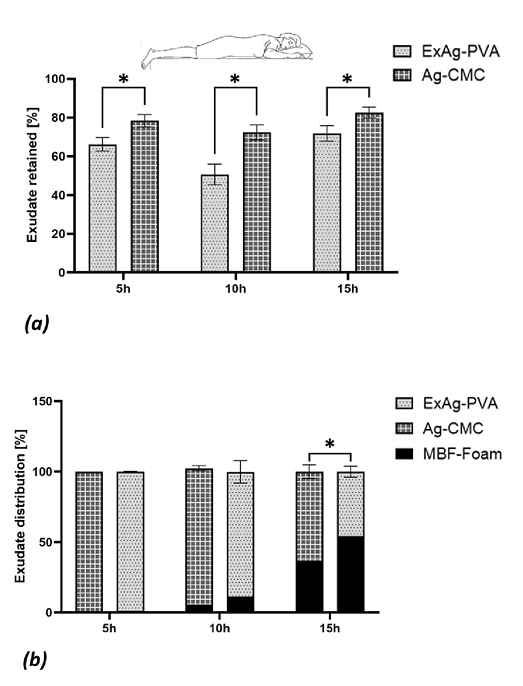
Figure 2. Fluid absorption and retention performances of the tested dressings
(a) The fluid retained in the primary dressings, reflecting the sorptivity of these dressings (reported as percentage of the total fluid volume retained in the primary and secondary dressings plus the residual fluid in the simulated wound bed). (b) The fluid distribution between the primary and secondary dressings after 5, 10, and 15 hours of simulated use in offloaded wounds (as in a prone position). The error bars are the SDs from the mean values of six test repetitions per test configuration and an asterisk indicates a statistically significant difference in the relevant outcome measure (P < .01).
The digital microscopy image analyses of the primary dressings indicated that the ExAg-PVA dressing had no distinguishable directional fibre preference. Specifically, to verify the relative lack of directional preference of the ExAg-PVA dressing with respect to that of the Ag-CMC primary silver-containing dressing, the researchers calculated the integral bounded between the fibre orientation histogram curve and the 0.5 (midpoint) level for the two primary dressings. This integral is defined here as the fibre orientation index (FOI). When a dressing does not exhibit a directional preference of its fibres, the positive and negative areas between the aforementioned histogram curve and the 0.5 level approximately cancel each other out, which results in a relatively low FOI value. The analyses of the FOI data indicated that the ExAg-PVA dressing had a statistically significant, approximately 3.9-fold lower FOI (15.6 ± 11.8) with respect to that of the Ag-CMC primary silver-containing dressing (60.8 ± 48.8; P < .05 for five different microscope FOVs on each dressing type). This FOI property ratio quantitatively demonstrates the strong preference of the fibre alignment in the Ag-CMC primary dressing toward the direction of the visible reinforcing seams in that dressing (the 90° direction marked in Figure 3a). Of note, the fibre orientation histogram of the latter (Ag-CMC) dressing was always above the midpoint level, which again indicates a strong directional preference (Figure 3a). Visual inspection of the microscopy data confirmed that the majority of the fibres in the Ag-CMC primary dressing were aligned and grouped together to connect to the vertical (90°-oriented) visible seams. The micrographs further demonstrated numerous elliptically shaped voids with characteristic maximum length dimensions of approximately 400 to 600 µm in the Ag-CMC primary dressing (Figure 3a). These findings, of a preferred fibre orientation and abundant presence of noncircular voids, justified the selection of the Ag-CMC primary silver-containing dressing as having a specific directional preference (ie, along its visible seams) for the purpose of further mechanical testing, as follows.
For the mechanical testing of the primary dressings post simulated use, the later time points of 10 and 15 hours of fluid exposure were selected because the previous results indicated that at 10 hours and afterward, both primary dressing types had transferred fluid to their paired secondary dressings (Figure 2b). Thus, at the 10- and 15-hour timepoints, both primary dressing types used their fluid reservoirs in a manner indicating that they were indeed tested at their “wet” state. Further, based on the results of the microscopy analyses, the ExAg-PVA dressing was tested as a structure without a specific directional preference (ie, irrespective of the direction by which test specimens were cut from this dressing type), whereas the Ag-CMC dressing was tested in two different configurations: fully aligned with the primary fibre orientation (90° direction), which is the direction of the visible seams, and out of such alignment (ie, randomly selected but different from the 90° direction).
The SED-to-failure data for the two primary dressing types are shown in Figure 3b and demonstrate considerable differences in mechanical behaviors of the post-use dressing types, particularly concerning the ductility of the ExAg-PVA dressing versus the Ag-CMC product. Ductility is the degree to which a material or structure can sustain plastic/irreversible deformations and continue to absorb strain energy under tensile loading before catastrophic failure occurs. Interestingly, the ExAg-PVA dressing appeared to gain ductility as it absorbed more fluid and gelled and accordingly, at 15 hours, it had 1.7-times greater SED-to-failure than it did at 10 hours. In contrast, gelling transformation did not translate into greater ductility for the Ag-CMC primary dressing; its SED-to-failure data were indistinguishable for the 10- and 15-hour time points (Figure 3b). Moreover, the aforementioned strength tests clearly indicated that the main loadbearing structure in the Ag-CMC primary dressing was, indeed, the reinforcing (visible) seams and the (near) 90°-oriented fibres. When tested out of alignment, the strength of the Ag-CMC primary dressing dropped significantly, by more than 8-fold (P < .05). With respect to the mechanical strength of the ExAg-PVA dressing, the Ag-CMC primary dressing had an out-of-alignment strength that was approximately four and six times lower for the 10- and 15-hour time points, respectively (P < .05).
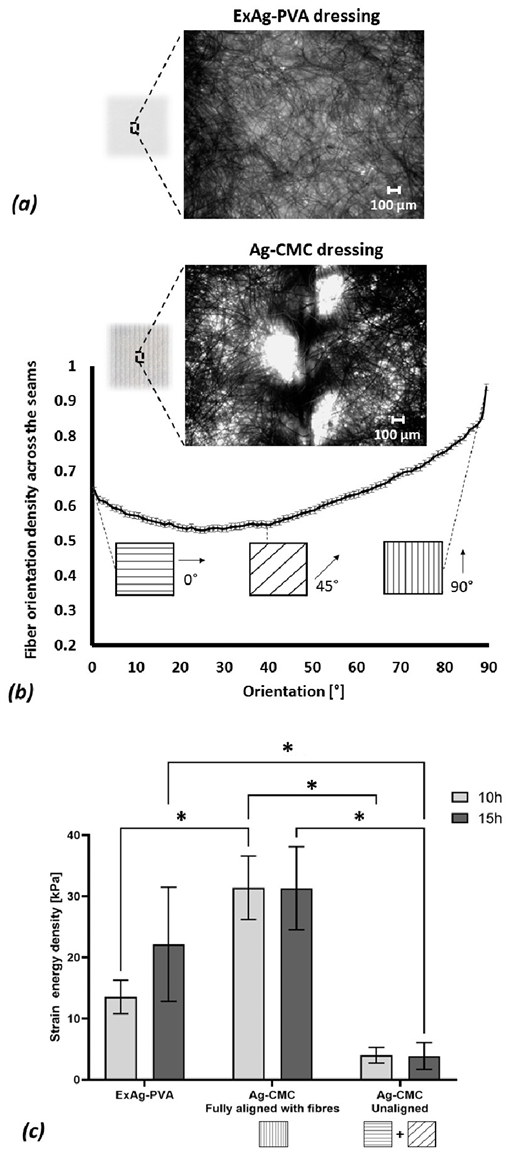
Figure 3. Structure-function analyses of the tested primary wound dressings
(a) An example of a microscopic image of the Exufiber Ag+ (ExAg-PVA) dressing. (b) Representative microscopy image analysis of the fiber orientation in the Ag-sodium carboxymethyl cellulose (CMC) dressing, showing the normalised planar distribution of fiber orientations in this primary dressing type. (c) The strain energy density to failure of the two tested primary dressing types post simulated use of 10 and 15 hours, where the Ag-CMC product has been tested in two configurations, either in full alignment of the principal direction of the fibers of the dressing with the loading axis of the testing machine, or out of such alignment. The error bars are the SDs from the mean values of six test repetitions per test configuration and an asterisk indicates a statistically significant difference in the relevant outcome measure (P < .01).
Discussion
Wound exudates are critical for tissue repair—they facilitate cell mobility and transport of signaling molecules and growth factors across the wound bed. However, excess exudate production may lead to maceration, become a medium for infections, or prolong the inflammation period.30,31 Thus, excess exudate amounts should be absorbed and retained in therapeutic dressings to support the natural wound-healing process. Clinical practice in treating cavity wounds as well as other highly exuding wounds (such as venous leg ulcers and burns) is to use a nonadherent wound filler as the primary dressing to induce a moist wound-healing environment while maximising dressing contact with the wound bed for effective absorbency. A secondary dressing is then applied to close the wound and protect it from potential mechanical traumas and pathogen invasion while also allowing evaporation of the exudate and release of byproduct gases. The secondary dressing also potentially provides an additional reservoir for absorbency and retention of the wound fluids, but it strongly depends on the sorptivity of the primary dressing for effective exudate management. Hence, for effective treatments, the primary and secondary dressings must work in synergy; both dressings should share the retained fluid mass as equally as possible and not approach their maximum fluid absorption capacity until a dressing change is indicated.3,10,11 Of note, for clinical realism, sorptivity should be assessed by testing a wound dressing pair using a relatively viscous, not watery, test fluid, which was the approach of the current study.10,11 New exudate cannot enter a primary dressing if there is no space for it at the wound-contacting aspect of the dressing; however, the existence of such available space depends on adequate transport of existing exudate from the primary to the secondary dressing.10,11 The capillary action that enables this fluid movement is inversely proportional to the square root of the viscosity of the transferred fluid (see Equation 2 in Lustig et al10); that is, the more viscous the fluid, the more difficult it is for it to be transported against gravity upward to the secondary dressing in any offloaded wound.10 Of note, nonoffloaded wounds may occur in real-world clinical practice and include, for example, wounds that are subjected to body weight forces such as plantar diabetic foot ulcers or sacral pressure injuries in patients who are ventilated supine, or wounds that are compressed by a medical device such as compression stockings applied on venous leg ulcers. The current study and configuration of the robotic exuding wounds apply to off-loaded wounds only; the additional biophysical complexity that arises from the action of bodyweight or external (eg, medical device-related) forces on a wound or its vicinity was not taken into account.
Gelling fibre dressings based on PVA or CMC fibres are used as primary dressings on a variety of highly exuding wound types. These dressings are meant to form a soft, cohesive gel when in contact with exudate, which induces the necessary moisture in the wound. However, such dressings must exhibit sufficient capillary action (sorptivity) to effectively transfer any excess exudate fluids away from the wound bed and prevent their pooling at the wound-dressing interfaces or within any undermining or tunneling spaces.3,10,11 In this work, a robotic phantom system of multiple simulated wound replicates was developed and used to evaluate the synergy in fluid absorbency and retention performances (facilitated through sorptivity) of two market-leading silver-containing gelling fibre primary dressing products when used with a secondary foam dressing, as per clinical practice. The ability of these primary dressings to stay intact while being subjected to pulling forces post simulated use sessions (ie, to exhibit clinically relevant durability) was further tested. These pulling forces mimic the mechanical effect of the forces that a clinician would apply with his/her gloved fingers or forceps when removing a used dressing and replacing it with a new one.
The ExAg-PVA was substantially more effective in transferring exudate simulants to the secondary foam dressing compared with the Ag-CMC. The ExAg-PVA dressing contained less fluid at each time point, and its paired secondary dressing accepted that fluid and retained it at increasing amounts over time. The latter results are particularly innovative because they reveal, for the first time in the literature, that the sharing process initiates between 5 and 10 hours after application of the dressings and amplifies thereafter. Specifically, the ExAg-PVA primary dressing delivers greater fluid amounts for absorbency and retention by the secondary dressing, approximately 2- and 1.5-fold the amounts of fluid at the 10- and 15-hour time points, respectively, with reference to the comparator dressing pair. The more fluid that is transferred to the secondary dressing, the greater the available capacity of the primary dressing to manage new inflowing exudates. Thus, laboratory evaluations must assess the function of wound dressings in the relevant clinical context (ie, measuring the function of the primary-secondary dressing pair as opposed to testing dressing products in isolation). Importantly, these experimental results demonstrate that the extent and rate of fluid sharing depend on the dressing materials and composition, indicating that there are more and less optimal choices of primary-secondary dressing combinations.
Nevertheless, sorptivity and the associated absorbency and retention performances are only one aspect to consider when assessing the safety and effectiveness of wound dressings through bioengineering laboratory testing. The mechanical strength of a primary dressing must be sufficient to endure the forces that occur throughout the life cycle of the dressing, including under the extraction forces that a clinician applies when the dressing is removed. Despite being exposed to the aggressive chemical and thermodynamic environment of the wound, a dressing must not disintegrate or leave debris or particles in the wound. Any debris (even microparticles) that originate from the primary dressing and spread onto the wound surface may initiate a foreign-body reaction whereby the immune system attempts to form granuloma (aggregation of macrophages and fibroblasts around each particle to isolate it from the body tissues). Such events consume valuable inflammatory and tissue repair efforts, which detract from the local biologic healing resources (eg, the potential numbers of the immune and fibroblast cells that are available for the tissue repair task).3,10,15,32
The microscopy image analyses did not show a consistent, strong directional preference of the fibres in the ExAg-PVA dressing, indicating low directional strength preference compared with the Ag-CMC, which did demonstrate a strong directional preference associated with its weave structure, specifically toward the visible reinforcing seams of the dressing. The durability testing results were consistent with the aforementioned microscopy findings for the Ag-CMC. Specifically, the Ag-CMC demonstrated poor mechanical strength when the direction of the pulling forces did not fully align with that of the reinforcing seams. Moreover, the current mechanical testing indicated that the strength of the Ag-CMC dressing is dominantly provided by the visible reinforcing seams and the closely aligned fibres; this imposes a real-world requirement (not presented by the manufacturer) that a clinician removing the dressing should be aware of the orientation of the seams and attempt to pull in that specific direction so as to avoid accidental (partial or complete) tearing. Yet, when removing a primary dressing from the wound cavity, the likelihood that a nurse would (blindly) pull the dressing at a specific orientation that exactly matches the primary fibre orientation of the dressing approaches zero. Moreover, removing a dressing in line with its primary fibre orientation is made even harder if the dressing takes on the same color as the exudate while in the wound. Because dressings are typically folded in the wound cavity and most of their structure is invisible, even if a nurse would hypothetically attempt to pull the dressing in a specific direction (to conform with the dressing’s optimal mechanical energy absorption to failure properties), then still, from a cost-effectiveness perspective, a dressing requiring removals at specific orientation and extra attention to that aspect adds to the clinical workload. The above implies that in real-world, clinically relevant scenarios, the Ag-CMC would have a four to six times lower strength (ie, mechanical energy absorption to failure) than that of the ExAg-PVA dressing, and the Ag-CMC dressing is therefore much more likely to leave debris or particles in a wound. The likelihood of such dressing failure events increases further when a wound has undermining, is tunneled, or has sticky or rough surfaces, all of which may be associated with greater pullout forces required to release the used dressing to be changed.
As with any experimental study, there are limitations in the ability to mimic in vivo processes and the large variety of clinical scenarios. Accordingly, in future work, additional experiments focusing on typically nonoffloaded wounds such as venous leg ulcers, are warranted. There is also a need to test additional wound dressing types and technologies from various manufacturers and include the aspect of cost-effectiveness versus the measured performance parameters. Other sensors and post analyses can potentially be added to the apparatus (and to each wound simulant unit) or be integrated in the testing methodology, such as intrawound continuous pH monitoring or mass spectrometry of the residual fluids following dressing usage periods. Further progress can be achieved by introducing exudate substitutes that contain specific microbiomes that may represent different wound etiologies, such as infected pressure injuries or diabetic foot ulcers. All of these future improvements would require additional validation and reliability studies before informing clinical practice.
Conclusions
Using an automated robotic phantom system of multiple open cavity wounds, the authors evaluated the absorbency and retention and therefore the sorptivity performances of silver-containing gelling fibre dressings paired with a secondary foam dressing. The mechanical durability of the primary silver-containing dressings was tested after simulated use. The current experimental system and protocol were designed with emphasis on the clinical relevance of the bioengineering laboratory testing, to reproduce how wound dressings are used in practice and consider the real-world scenarios that may be associated with their failure. Consistent with the previous published work of the authors, sorptivity and durability were again identified as critical factors that should be assessed when evaluating wound dressings in laboratory testing.3,10,11 The present findings further underpin that wound dressings belonging to a certain product category, such as silver-containing gelling fibre dressings, are not all the same, and the specific absorption, retention, and, importantly, the ability to synergistically work with a secondary dressing (which good sorptivity enables) differ across products, depending on the specific materials and composition of the dressing.3,10,32,33 The present experimental data revealed that the ExAg-PVA dressing has better sorptivity and durability than the comparator Ag-CMC product. Moreover, the authors described the dynamics of the fluid sharing between primary and secondary dressings and identified the importance of not having a specific fibre directionality in a primary dressing for durability and safe post-use removals. The comparative quantification of these capabilities should help both clinical and nonclinical decision-makers to assess and select the wound dressings that best meet the needs of their patients. As with any preclinical work, the current laboratory findings need to be validated against randomised controlled trials with appropriate sample sizes of different wound etiologies. The role of the laboratory work reported here is primarily to guide such potential clinical research to focus on the novel aspects of fluid sharing between the primary and secondary wound dressings, and the ability of the primary dressings to remain intact during removals, topics that have been poorly addressed in the literature thus far.
Acknowledgment
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant was also partially supported by the Israeli Ministry of Science & Technology (Medical Devices Program grant no. 3-17421, awarded to Prof Amit Gefen in 2020) and by Mölnlycke Health Care (Gothenburg, Sweden). The authors thank Ms Orel Belo, the Laboratory Engineer at the Gefen Laboratory, for supporting the experimental work. The authors have disclosed no other financial relationships related to this article. Submitted July 23, 2021; accepted in revised form November 1, 2021; published online ahead of print March 14, 2022. Copyright Advances in Skin & Wound Care and the World Council of Enterostomal Therapists.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
在机器人伤口系统中测试含银凝胶纤维敷料的液体处理动力学和耐久性
Aleksei Orlov, Adi Lustig, Angela Grigatti and Amit Gefen
DOI: https://doi.org/10.33235/wcet.42.2.15-23
摘要
目的 根据临床实践,开发包含多个模拟伤口复制品的机器人体模系统,以确定含银凝胶纤维接触性敷料与外层敷料一起使用时,液体吸收和保留(吸附性)性能的协同作用以及模拟使用后的机械耐久性。
方法 作者使用包含六个相同伤口模拟装置的机器人系统,测试了Exufiber Ag+(Mölnlycke Health Care,Gothenburg,瑞典)接触性敷料(ExAg-聚乙烯醇[PVA])与市场领先的对比产品在与外层泡沫敷料一起使用时的吸附性能。通过拉伸力学测试进一步研究了模拟使用后接触性敷料的耐久性。
结果 借助外层泡沫敷料,ExAg-PVA接触性敷料能够实现更多的液体量的吸收和保留,在10小时和15小时后,分别比对比敷料组合多约2倍和1.5倍。ExAg-PVA敷料对拉力方向的敏感性也大大降低,因此,在使用10小时和15小时后,其机械强度大约分别为其他接触性敷料的4倍和6倍(当后一种敷料与其可见接缝未对齐时)。
结论 描述了接触性敷料和外层敷料之间的吸附性和液体共享动力学,以及接触性敷料强度的方向性偏好对足够耐久性的影响,从而实现使用后的安全移除。对这些能力的比较量化应有助于临床和非临床决策者选择最能满足患者需求的敷料。
版权所有©2022 作者。本文是一篇根据知识共享署名-非商业性使用-禁止演绎(CCBY-NC-ND)4.0许可协议的条款发布的开放获取类文章,允许下载本文,并允许通过适当引用分享本文。未经杂志许可,不得以任何方式更改本论文或将其用于商业用途。
引言
各种类型的伤口,包括慢性腔洞伤口(如压力性损伤),是最具影响、最昂贵和最常见的医疗问题之一。近2.5%的美国人口都经历过伤口,这与美国的中风发病率相似。1-4随着人口老龄化以及糖尿病和肥胖症患病率的增加,对先进且具有成本效益的伤口护理的需求急剧增长,以提供更好的患者结局。治疗敷料在伤口愈合的各个方面都发挥着关键作用。本质上,治疗敷料是作为完整皮肤的临时人工替代品而设计的,可以保护伤口并控制伤口周围炎症过程产生的渗出液。渗出液的存在和成分通常支持组织修复细胞和新生成组织的功能;因此,在正常的组织修复过程中,渗出液及其成分的渗出率将与愈合阶段相匹配。5-7然而,炎症阶段的异常延长(如慢性伤口),可能会破坏对渗出液分泌的生理控制,导致渗出量过多或蛋白质含量比例改变,使液体变稠、高粘度,甚至是粘稠5。
需要使用有效的伤口敷料来处理在同一伤口的不同时间,或针对相同病因但不同患者的伤口处,以不同速率和粘度分泌的渗出液。临床上常见的做法是通过伤口开口插入接触性伤口敷料,形成伤口床上的一线储液器,用于液体吸收和保留。然后在腔洞(和接触性敷料)上方放置外层敷料,以提供机械和生物保护,并作为积聚液体的第二个容器。由聚乙烯醇(PVA)纤维或羧甲基纤维素钠(CMC)组成的凝胶纤维敷料被广泛用作接触性敷料,因为它们能够通过转化为凝胶相来截留液体8(这些商业接触性伤口敷料产品的示例列于woundsource.com资源数据库9的“凝胶纤维敷料”伤口敷料产品类别下)。这些接触性凝胶纤维敷料的一种变体是含有银离子的敷料,用于进一步起到抗菌效果。为了提供有效的治疗,接触性敷料和外层敷料必须协同工作;也就是说,两种敷料都不应在规定的使用时间内达到其最大液体吸收量。3,10–12
伤口敷料的性能主要取决于敷料的具体材料成分、结构和制造技术。这些特征,再加上相关的临床方案、特定的伤口环境和配对的外层敷料的类型,就会影响接触性敷料产品的安全性和有效性。与接触性敷料功能相关的两种液体-结构相互作用特性是“吸附性”(即敷料结构通过毛细管作用将多余渗出液从伤口床转移到外层敷料的能力10,13)和“耐久性”(即敷料能够承受患者体重和在更换过程中施加的其他机械力,并在使用期间或移除时在伤口内保持完整的能力。10,13)
20世纪70年代,据报告称,伤口中纱布敷料颗粒“像异物一样存在,可能会延缓愈合。将其移除能够加速愈合。”14最近,Chakravarthy和同事15记录了现代(水胶体)敷料在伤口中的明显分解。敷料分解与炎症性巨细胞(异物反应的病理证据)有关,突出了宏观和微观层面上耐久性的重要性。具体而言,Chakravarthy等人15评价了组织学组织切片中的巨细胞密度,这些组织切片是从猪伤口愈合模型的伤口中提取的,随后用苏木精和伊红染色。他们记录了组织切片中巨细胞附近存在异物(即敷料碎屑),这与在更换敷料过程中移除一些受试水胶体敷料后观察到的碎片有关。15
因此,性能不佳的伤口敷料或敷料组合可能导致水分平衡欠佳、组织机械损伤、异物反应,或以上这些不良事件的同时出现。应通过方法学上的生物工程实验室测试来确定此类敷料失效模式,以便监管机构能够控制医疗索赔,避免临床上的敷料失效。在此背景下,作者继续投入研究工作,开发和改进临床前和实验室测试方法,以客观、定量和标准化评估伤口敷料性能。3,10,11,13在此,作者介绍了一种新型机器人体模系统,该系统包含多个模拟伤口复制品,用于同时测试敷料。该系统被开发并用于确定两种含银凝胶纤维接触性敷料产品在液体吸收和保留性能方面的协同作用。本文报告的方法、设备和方案构成了伤口敷料下一阶段临床相关性能测试的基础,重点关注安全性和有效性。
方法
机器人模拟渗出性伤口
研究人员开发并使用了一个包含六个伤口复制品的机器人体模系统。该系统中的每个伤口装置都模拟了一个2.5cm深的渗出性腔洞伤口(图1)。所有六个伤口装置都包括三层合成软组织模拟物(图1)。顶层代表伤口周围皮肤,由5 mm厚的透明硅橡胶(RTV615,Momentive Performance Materials Inc,Waterford,纽约)组成。在该“皮肤”层下方放置一层8 mm厚的石蜡凝胶(“蜡烛凝胶”;Ziv Chemicals Ltd.,Holon,以色列),代表脂肪组织。代表骨骼肌的下层(厚度为12 mm)也由硅橡胶制成(与用作皮肤模拟物的硅橡胶相同)。根据ASTM D412-06,16使用机电材料测试机(Instron型号5944;Instron Co,Norwood,Massachusetts)通过单轴拉伸测试测得上述硅橡胶材料的弹性模量为1.5 MPa,这是皮肤和肌肉组织在大幅度变形下的特征。17–19通过先前报告的压痕测试20测得用于代表脂肪组织的石蜡凝胶的弹性模量为5 kPa,与报告的天然脂肪组织硬度相似。21为了模拟上述伤口结构中渗出液的持续分泌,在每个模拟伤口床中放置一根螺旋穿孔灌注管,并穿过模型结构,连接到一个多通道可编程注射泵(NE-1600;New Era pump Systems Inc,Farmingdale,纽约;图1)。通过该灌注元件在模拟伤口床中形成的有效湿润表面约为24 cm2,对应于约2.5 cm的有效灌注深度。多通道注射泵可精确控制流入模拟伤口床的渗出液替代液的流量和释放速率。安全且可再现的渗出液替代液配方基于黄原胶(C35H49O29);它之前由作者的研究小组开发,用于机器人伤口体模系统。该配方有助于控制液体粘度和pH值水平,以充分代表天然渗出液的物理特性。10-13,22制备了两种渗出液溶液,高粘度和低粘度分别为0.23 Pa×s和0.06 Pa×s;两者的液体密度均为1.03 g/mL。将红外加热灯放置在六个伤口复制品上方,以将其保持在33±2°C的温度下;23通过数字温度计(Newtron TM-5005-SINGLE I/P;MRC Laboratory Equipment Ltd,Holon,以色列)监测温度。

图1.实验装置及其组件
a)通过单个“伤口”模拟装置的横截面图,显示不同的组织模拟层及其各自的厚度、“伤口腔洞”的形状和深度以及螺旋灌洗管的配置。(b)机器人体模系统(包括六个伤口复制品和控制装置)的照片。(c)单个模拟伤口的特写视图。(d)放大模拟渗出型“伤口床”
模拟治疗
研究了两种类型的10×10 cm接触性凝胶纤维敷料:含PVA纤维的Exufiber Ag+(Mölnlycke Health Care AB, Gothenburg, 瑞典)(ExAg-PVA)以及等效、市售且市场领先的银离子敷料产品(含有CMC钠作为凝胶材料)(Ag-CMC)。Ag-CMC敷料是一种柔软、无菌的无纺布垫,由浸有1.2%离子银的钠CMC水胶体纤维材料组成。24-26它适用于各种急性和慢性伤口类型的临床使用,其物理和抗菌特性已在别处详细描述。24-26值得注意的是,尽管本研究中选择的两种接触性敷料(基于PVA的敷料和基于CMC的敷料)由不同的材料制成,并通过不同的制造技术生产,但这两种敷料均在临床上都可作为腔洞伤口填充物使用,这是当前比较的基本原理。Mepilex Border Flex(MBF泡沫;Mölnlycke Health Care)是一种多层压边型硅胶泡沫敷料,在所有测试中用作覆盖模拟伤口的外层敷料。MBF泡沫是一种五层敷料,包括(从敷料外表面到其面向伤口的面):背衬膜、保留层、扩散层、吸收泡沫层(将渗出液输送到扩散层)和伤口接触层。
在将敷料产品应用于模拟伤口之前,对每个敷料(接触性敷料/外层敷料)进行称重。然后根据制造商说明施用敷料。将模拟伤口朝上放置,以便受试敷料按照需要,通过毛细血管作用(即对抗重力)吸收和保留渗出液替代物。以2mL/h的流速启动机器人系统。为了确定受试敷料吸收性能的时间过程,重要的是为了确定接触性敷料和外层敷料之间液体分布的动力学,对产品进行了多次不同时间的模拟使用测试:5、10和15小时。
敷料研究
接触性和外层敷料之间的液体保留和分布。在机器人体模系统中模拟使用后,重新对敷料进行称重,以计算由于液体吸收导致的每次敷料中的净质量增加;从伤口腔洞中小心收集所有未保留的残余液体,并称重。将测量的液体质量转换为体积后(通过将吸收和残余液体质量除以液体密度),将每次测试的总渗出液体积分别计算为接触性和外层敷料中的液体体积加上残余液体体积之和。接下来,计算每次测试中接触性敷料和外层敷料之间的液体体积分布,作为接触性敷料与外层敷料中保留液体占相应总渗出液体积的百分比(其理论值也可计算,为机器人伤口系统预定流速和运行时间的乘积)。10
纤维方向性。对于凝胶纤维敷料,纤维的方向性可能会影响敷料产品的性能,这通常与这些接触性敷料(即PVA与CMC)的制造技术有关,例如吸附性(纤维成为毛细作用的结构导体)和耐久性(纤维提供结构支撑和机械耐受力,以抵抗与其主要方向对齐的力)。因此,研究人员通过使用光学立体显微镜(Axiolab A450909;Carl Zeiss AG,Oberkochen,德国)获取的敷料表面显微图像的数字图像处理,评估了两种接触性敷料类型中纤维的方向性。使用1.25倍放大物镜、C型数码相机(Swift Cam SC1803;Swift Optical Instruments Inc,Schertz Texas)和Swift成像软件(版本3.0,Swift Optical Instruments Inc.)在透射光模式下获取敷料表面的显微照片。从每种类型的两个接触性敷料试样的同一表面位置捕获五个视场(FOV):一个FOV位于敷料中心,其他四个FOV围绕敷料中心形成十字,每个外围FOV位于距敷料边缘2.5 cm的距离处。最终每种敷料类型总共15张FOV的数字显微照片,每张尺寸为4912×3684像素(1平方像素=0.919 µm2)。为了进行纤维方向性分析,将获得的FOV进一步划分为三个矩形子FOV,每个子FOV的尺寸为1445×3288像素。使用ImageJ软件套件(版本1.X)27,28的采集后插件“OrientationJ”进行纤维方向性分析,该插件在数字显微照片中分割纤维,并计算其在每个分析子FOV中的平面方向的概率函数。校准该代码并目视验证其性能后,提取所研究接触性敷料中纤维取向的标准化直方图。
模拟使用后接触性敷料的强度。在模拟使用阶段后,按照基于ASTM D882-02的方案,使用配有2 kN称重传感器的机电测试机(Instron型号5944;Instron Co)测试每个接触性敷料试样的拉伸强度。29按照上述测试标准制备的接触性敷料试样以50 mm/min的变形率拉伸,直至发生最终失效。根据产生的力-变形数据,绘制了模拟使用后敷料的应力-应变曲线,并使用专用的MATLAB计算机代码(版本R2019a;MathWorks,Inc,Natick,Massachusetts)计算了每次测试的应力-应变曲线下面积,即失效应变能密度(SED)。基于这些纤维取向的显微镜分析,ExAg-PVA敷料被视为没有特定方向偏好的结构(即,制备这种敷料类型的试样,并在随机方向上进行力学测试)。然而,含银的Ag-CMC接触性敷料,其纤维具有特定的方向偏好(如结果中的进一步详述),在两种不同的配置中进行了测试:其主要纤维方向与材料测试机的载荷轴完全对齐,以及纤维未对齐。
统计分析
以上报告的所有实验均使用六个复制品进行,计算了每个模拟使用持续时间(5、10和15小时)内保留液体体积、接触性和外层敷料之间液体含量分布的平均值和SD的描述性统计数据,以及接触性敷料的失效SED(取决于纤维相对于载荷轴的方向性,如前所述)。接下来,进行方差分析(ANOVA),然后进行事后Tukey-Kramer多重成对比较,以确定之前描述的液体管理(吸附性)和材料强度测试中的敷料性能之间的潜在差异。具体而言,针对液体保留和液体分布数据,对使用时间和接触性敷料类型等因素进行了双因素方差分析。此外,针对材料强度数据,在纤维取向方面(在显示纤维取向的方向偏好的接触性敷料中),对使用时间、接触性敷料类型和拉伸测试等因素进行了三因素方差分析。在后一个三因素方差分析中,仅考虑更长的暴露时间(10小时和15小时),在此期间受试的接触性敷料中累积了大量液体。关于方向性因素,根据显微镜分析,最后一次方差分析认为ExAg-PVA敷料没有显示其纤维的任何特定和一致性的方向偏好,具有单一强度(失效SED)特性。然而,Ag-CMC具有两种强度特性,即(1)当纤维与材料测试机的载荷轴完全对齐时测得的强度,以及(2)当纤维未对齐时测得的强度。P<.05被认为具有统计学意义。
结果
单独检查时,ExAg-PVA接触性敷料的吸收率低于(约11-22%)Ag-CMC接触性银敷料(图2a)。然而,这种差异是由于渗出液样液体从ExAg-PVA敷料更有效地转移到外层敷料,这在检查接触性敷料和外层敷料之间的液体分布数据时很明显(图2b)。具体而言,当与ExAg-PVA敷料成对使用时,外层敷料在10小时时间点所含液体量约为对照组的两倍,15小时后所含液体量约为对照组的1.5倍(图2b)。随着时间的推移,接触性敷料和外层敷料之间的液体分布动态变化(图2b)进一步显示,外层敷料的储液器在敷料施用5小时后开始接收液体(图2b)。15小时后,当接触性敷料为ExAg-PVA时,外层敷料接收约54.2%的保留液体,但当使用Ag-CMC接触性敷料时,外层敷料仅接收36.7%(P<.05;图2b)。重要的是,这些结果代表了敷料组合的性能,因此更好地反映了实际的临床实践,而不是单独评估伤口敷料的功能(图2b)。

图2.受试敷料的液体吸收和保留性能
(a)保留在接触性敷料中的液体,反映这些敷料的吸附性(体现为保留在接触性和外层敷料中的液体总体积加上模拟伤口床中的残余液体的百分比)。(b)在非受力性伤口(如俯卧位)中模拟使用5、10和15小时后,接触性敷料和外层敷料之间的液体分布。误差条是每个测试配置六次重复测试平均值的标准差,星号表示相关结果测量值存在统计学显著差异(P<0.01)。
接触性敷料的数字显微镜图像分析表明,ExAg-PVA敷料没有明显的定向纤维偏好。具体而言,为了验证ExAg-PVA敷料相对于Ag-CMC接触性含银敷料的方向偏好相对不足,研究人员计算了两种接触性敷料的纤维取向直方图曲线和0.5(中点)水平之间的积分界限。该积分在此定义为纤维取向指数(FOI)。当敷料未表现出其纤维的方向偏好时,上述直方图曲线和0.5水平之间的正负区域大致相互抵消,从而导致FOI值相对较低。FOI数据分析表明,ExAg-PVA敷料的FOI(15.6±11.8)较Ag-CMC接触性含银敷料(60.8±48.8)低约3.9倍,具有统计学意义(对于每种敷料类型的五种不同显微镜FOV,P<0.05)。该FOI性能比定量地表明,Ag-CMC接触性敷料中的纤维取向强烈倾向于该敷料中可见加固接缝的方向(图3a中标记的90°方向)。值得注意的是,后者(Ag-CMC)敷料的纤维取向直方图始终高于中点水平,这再次表明了强烈的方向偏好(图3a)。对显微镜数据的目视检查证实,Ag-CMC接触性敷料中的大多数纤维已对齐并组合在一起,以连接到垂直(90°取向)的可见接缝。显微照片进一步显示,在Ag-CMC接触性敷料中,存在大量椭圆形空隙,其特征最大长度尺寸约为400至600 μm(图3a)。这些发现(纤维取向偏好和大量非圆形空隙的存在)证明Ag-CMC接触性含银敷料的选择具有特定的方向偏好(即沿着其可见接缝),以便进行进一步的力学测试,如下所示。
对于模拟使用后接触性敷料的力学测试,选择了较晚的10小时和15小时的液体暴露时间点,因为之前的结果表明,在10小时和之后,两种类型的接触性敷料都已将液体转移到其配对的外层敷料中(图2b)。因此,在10小时和15小时的时间点,两种接触性敷料类型使用其储液器的方式表明,它们确实在“湿润”状态下进行了测试。此外,根据显微镜分析的结果,ExAg-PVA敷料作为一种没有特定方向偏好的结构进行测试(即,无论从该敷料类型中切取试样的方向如何),而Ag-CMC敷料则以两种不同的配置进行测试:完全与主要纤维方向(90°方向)对齐,即可见接缝的方向;以及不对齐(即随机选择,但非90°方向)。
两种接触性敷料类型的失效SED数据如图3b所示,表明使用后敷料类型的机械性能存在很大差异,尤其是关于ExAg-PVA敷料与Ag-CMC产品的延展性。延展性是指材料或结构在发生灾难性失效之前能够承受塑性/不可逆变形并在拉伸载荷下继续吸收应变能的程度。有趣的是,ExAg-PVA敷料似乎获得了延展性,因为它吸收了更多的液体并形成凝胶,因此,在15小时的时候,它的失效SED是10小时时的1.7倍。相比之下,Ag-CMC接触性敷料的凝胶化转变并没有转化为更大的延展性;在10小时和15小时的时间点内,其失效SED数据无法区分(图3b)。此外,上述强度测试清楚地表明,Ag-CMC接触性敷料中的主要承重结构实际上是加固(可见)接缝和(接近)90°取向的纤维。当测试不对齐时,Ag-CMC接触性敷料的强度显著下降,下降了8倍以上(P<0.05)。就ExAg-PVA敷料的机械强度而言,Ag-CMC接触性敷料未对齐时的强度在10小时和15小时的时间点分别降低了约四倍和六倍(P<0.05)。

图3.受试接触性伤口敷料的结构-功能分析
(a)Exufiber Ag+(ExAg-PVA)敷料的显微图像示例。(b)Ag-羧甲基纤维素钠(CMC)敷料中纤维取向的代表性显微图像分析,显示了该接触性敷料类型中纤维取向的标准化平面分布。(c)模拟使用10小时和15小时后,两种受试接触性敷料类型的失效应变能密度,其中Ag-CMC产品在两种配置中进行了测试,一种是在敷料纤维主方向与测试机载荷轴完全对齐的情况下,一种是在不对齐的情况下。误差条是每个测试配置六次重复测试平均值的标准差,星号表示相关结果测量值存在统计学显著差异(P<0.01)。
讨论
伤口渗出液是组织修复的关键—它们促进细胞移动以及信号分子和生长因子在伤口床上的运输。然而,分泌过多的渗出液可能会导致浸渍,成为感染介质,或延长炎症期。30,31因此,多余的渗出液应被吸收并保留在治疗性敷料中,以支持自然的伤口愈合过程。治疗腔洞伤口和其他高渗出性伤口(如下肢静脉性溃疡和烧伤)的临床做法是使用非粘附性伤口填料作为接触性敷料,以诱导湿润的伤口愈合环境,同时最大限度地增加敷料与伤口床的接触,以实现有效吸收。然后使用外层敷料来闭合伤口,防止潜在的机械创伤和病原体入侵,同时允许渗出液蒸发和副产物气体释放。外层敷料还可能为伤口液体的吸收和保留提供一个额外的储液器(但这在很大程度上取决于接触性敷料的吸附性),以实现有效的渗出液管理。因此,为了有效治疗,接触性和外层敷料必须协同工作;两种敷料应尽可能均匀地共享保留的液体质量,并且在指示敷料更换之前,不要接近其最大液体吸收量。3,10,11值得注意的是,为了实现临床真实性,应通过使用相对粘稠而非含水的测试液测试伤口敷料组合,以评估其吸附性,这是当前研究使用的方法。10,11如果敷料的伤口接触面没有空间容纳新渗出液,则新渗出液不能进入接触性敷料;然而,这种是否有可用容量取决于现有渗出液从接触性敷料到外层敷料的充分运输。10,11使液体运动的毛细管作用与转移液体粘度的平方根成反比(见Lustig等人10中的方程式2);也就是说,液体的粘性越大,就越难抵抗重力向上输送到任何非受力性伤口的外层敷料中。10值得注意的是,在现实的临床实践中可能会发生受力性伤口,包括承受体重力的伤口,如仰卧通气患者的糖尿病足溃疡或骶骨压力损伤,或由医疗器械压缩的伤口,如应用于下肢静脉性溃疡的压力袜。目前机器人渗出性伤口的研究和配置仅适用于非受力伤口;未考虑体重或外力(如医疗器械相关)对伤口或其附近的作用所产生的额外生物物理复杂性。
基于PVA或CMC纤维的凝胶纤维敷料用作多种高渗出性伤口类型的接触性敷料。这些敷料会在与渗出液接触时形成柔软的有粘性凝胶,从而在伤口中产生必要的水分。然而,此类敷料必须具有足够的毛细作用(吸附性),以有效地将任何多余的渗出液从伤口床转移出去,并防止其在伤口-敷料接触面或任何潜行腔洞或隧道伤口空间内聚集。3,10,11在这项工作中,根据临床实践,开发了一个包含多个模拟伤口复制品的机器人体模系统,用于评估两种市场领先的含银凝胶纤维接触性敷料产品在与外层泡沫敷料一起使用时在液体吸收性和保留性能(通过吸附性促进)方面的协同作用。进一步测试了这些接触性敷料在模拟使用后承受拉力时保持完整的能力(即表现出与临床相关的耐久性)。这些拉力模拟了临床医生在取出用过的敷料并更换新敷料时,用戴手套的手指或镊子施加的力的机械效应。
与Ag-CMC相比,ExAg-PVA在将渗出液模拟液转移到外层泡沫敷料方面明显更有效。ExAg-PVA敷料在每个时间点所含液体较少,其配对的外层敷料接受该液体,并随时间推移而保留更多的液体。后者的结果尤其具有创新性,因为它们首次在文献中揭示,共享过程在施用敷料后5到10个小时开始,然后扩大。具体而言,ExAg-PVA接触性敷料提供更大的液体量供外层敷料吸收和保留,相对于参考对照敷料组合,前者在10小时和15小时时间点的液体量分别约为后者的2倍和1.5倍。转移到外层敷料的液体越多,接触性敷料处理新流入渗出液的可用容量就越大。因此,实验室评价必须在相关的临床环境中评估伤口敷料的功能(即,测量接触性-外层敷料组合的功能,而不是单独测试敷料产品)。重要的是,这些实验结果表明,液体共享的程度和速率取决于敷料的材料和成分,表明接触性-外层敷料组合的最佳选择越来越少。
然而,在通过生物工程实验室测试评估伤口敷料的安全性和有效性时,吸附性以及相关的吸收和保留性能只是需要考虑的一个方面。接触性敷料的机械强度必须足以承受敷料整个生命周期内出现的力,包括临床医生在取出敷料时施加的拔出力。尽管暴露于伤口的侵蚀性化学和热力学环境中,敷料不得分解或在伤口中留下碎屑或颗粒。来自接触性敷料并扩散到伤口表面的任何碎屑(甚至微粒)都可能引发异物反应,然后免疫系统试图形成肉芽肿(巨噬细胞和成纤维细胞聚集在每个颗粒周围,将其与身体组织隔离)。这种情况会耗费宝贵的炎症和组织修复工作,从而损耗局部生物修复资源(例如,可用于组织修复任务的免疫细胞和成纤维细胞的潜在数量)。3,10,15,32
显微镜图像分析未显示ExAg-PVA敷料中纤维的一致、强烈的方向性偏好,表明与Ag-CMC相比,纤维的方向性强度偏好较低,Ag-CMC确实显示出与其编织结构相关的强烈方向性偏好,特别是对敷料的可见加固接缝。耐久性测试结果与上述Ag-CMC的显微镜检查结果一致。具体而言,当拉力方向与加固接缝方向不完全对齐时,Ag-CMC的机械强度较差。此外,目前的力学测试表明,Ag-CMC敷料的强度主要由可见的加固接缝和紧密排列的纤维提供;这就提出了一个实际的要求(制造商未提出),即移除敷料的临床医生应了解接缝的方向,并尝试朝该特定方向拉动,以避免意外(部分或完全)撕裂。然而,当从伤口腔洞中取出接触性敷料时,护士(盲目地)以与敷料主纤维方向完全匹配的特定方向拉动敷料的可能性接近于零。此外,如果敷料在伤口中呈现的颜色与渗出液的颜色相同,则按照其主要纤维方向去除敷料会变得更加困难。由于敷料通常折叠在伤口腔洞中,并且其大部分结构都是看不见的,即使假设护士会试图将敷料拉向特定方向(以符合敷料对失效性能的最佳机械能吸收),从成本效益的角度来看,需要在特定方向移除敷料以及对这方面的额外关注增加了临床工作量。上述情况表明,在实际的临床相关场景中,Ag-CMC的强度(即机械能吸收到失效)比ExAg-PVA敷料低4到6倍,因此Ag-CMC敷料在伤口中留下碎屑或颗粒的可能性更大。当伤口有潜行腔洞、隧道伤口或表面有粘性或粗糙表面时,此类敷料失效事件的可能性进一步增加,所有这些都可能与释放待更换的已用敷料所需的更大拔出力有关。
与任何实验研究一样,模拟体内过程和大量临床场景的能力方面也存在局限性。因此,在未来的研究中,有必要对下肢静脉性溃疡等典型的受力性伤口进行额外的实验。还需要测试来自不同制造商的其他伤口敷料类型和技术,并包括成本效益与测量的性能参数的对比。其他传感器和后期分析可潜在地添加到设备(以及每个伤口模拟装置)中,或集成到测试方法中,例如伤口内持续pH监测或敷料使用阶段后残留液体的质谱分析。通过引入含有可能代表不同伤口病因的特定微生物组的渗出液替代液,可以取得进一步的进展,例如感染性压力损伤或糖尿病足溃疡。所有这些未来的改进都需要在通知临床实践之前进行额外的验证和可靠性研究。
结论
作者使用多个开放性腔洞伤口的自动机器人体模系统,评价了含银凝胶纤维敷料与外层泡沫敷料的吸收率和保留率,从而评估其吸附性能。模拟使用后,测试了接触性含银敷料的机械耐久性。当前的实验系统和方案的设计重点是生物工程实验室测试的临床相关性,旨在再现伤口敷料在实践中的使用方式,并考虑可能与其失效相关的现实情况。与作者先前发表的论文一致,吸附性和耐久性再次被确定为在实验室测试中评价伤口敷料时应评估的关键因素。3,10,11目前的研究结果进一步证明,属于某一产品类别的伤口敷料,如含银凝胶纤维敷料,并不完全相同,不同产品的具体吸收、保留率不同,并且重要的是与外层敷料(具有良好的吸附性)协同工作的能力不同,这取决于敷料的具体材料和成分。3,10,32,33目前的实验数据表明,ExAg-PVA敷料比对照Ag-CMC产品具有更好的吸附性和耐久性。此外,作者描述了接触性敷料和外层敷料之间液体共享的动力学,并确定了在接触性敷料中不具有特定纤维方向性对于耐久性和使用后安全去除的重要性。这些能力的比较量化应有助于临床和非临床决策者评估和选择最能满足患者需求的伤口敷料。与任何临床前研究一样,当前的实验室研究结果需要根据不同伤口病因的适当样本量进行随机对照试验进行验证。本文报告的实验室研究的作用主要是指导此类可能的临床研究,将重点放在接触性和外层伤口敷料之间液体共享的新方面,以及接触性敷料在移除过程中保持完整的能力,这些主题迄今为止在文献中很少涉及。
致谢
本项目得到了欧盟“地平线2020”研究与创新计划Marie Skłodowska-Curie奖学金的的资助,部分支持来自于以色列科学技术部(医疗器械项目授予编号3-17421,于2020年授予Amit Gefen教授)和Mölnlycke Health Care(Gothenburg,瑞典)。感谢Gefen实验室的实验室工程师Orel Belo女士对实验工作的支持。未公开与本文相关的其他财务关系。提交日期:2021年7月23日;修回日期:2021年11月1日;在线发表早于印刷,发表日期:2022年3月14日。版权所有《皮肤与伤口护理进展》和世界造口治疗师委员会(World Council of Enterostomal Therapists)。
利益冲突声明
作者声明无利益冲突。
资助
作者未因该项研究收到任何资助。
Author(s)
Aleksei Orlov MSc
Graduate Student, Department of Biomedical Engineering, Tel Aviv University, Israel
Adi Lustig MSc
Graduate Student, Department of Biomedical Engineering, Tel Aviv University, Israel
Angela Grigatti MSc
Graduate Student, Department of Biomedical Engineering, Tel Aviv University, Israel
Amit Gefen* PhD
Professor of Biomedical Engineering and the Herbert J Berman Chair in Vascular Bioengineering,
Department of Biomedical Engineering, Tel Aviv University, Israel
At Tel Aviv University, Department of Biomedical Engineering, Tel Aviv, Israel, Aleksei Orlov, MSc; Adi Lustig, MSc; and Angela Grigatti, MSc, are Graduate Students at the research group of Professor Gefen; and Amit Gefen, PhD, is Professor of Biomedical Engineering and the Herbert J. Berman Chair in Vascular Bioengineering.
* Corresponding author
References
- Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care 2019;8:39-48.
- Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open 2020;10:e045253.
- Gefen A, Ousey K. Safe and effective wound care during the COVID-19 pandemic. J Wound Care 2020;29:622-3.
- Sen CK. Human wound and its burden: updated 2020 compendium of estimates. Adv Wound Care (New Rochelle) 2021;10:281-92.
- How To Guide: Managing High Exudate Wounds - A Wounds UK Publication. https://www.wounds-uk.com/resources/details/how-to-guide-managing-high-exudate-wounds. Last accessed February 18, 2022.
- Cutting KF. Wound exudate: composition and functions. Br J Community Nurs 2003;8(Supp3):S4-9.
- Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(Supp1):S1-28.
- Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv Wound Care 2013;2:348-56.
- Woundsource Resource Database. https://www.woundsource.com/product-category/dressings/gelling-fiber-dressings. Last accessed March 11, 2022.
- Lustig A, Alves P, Call E, Santamaria N, Gefen A. The sorptivity and durability of gelling fibre dressings tested in a simulated sacral pressure ulcer system. Int Wound J 2021;18:194-208.
- Gefen A, Santamaria N. Saturation of a dressing applied to an exuding wound: The gap between clinical judgment and laboratory testing. Wounds Int J 2021;12:20-6.
- Lustig A, Gefen A. Fluid management and strength post-simulated use of primary and secondary dressings for treating diabetic foot ulcers: robotic phantom studies. Int Wound J 2022;19:305-15.
- Lustig A, Gefen A. Three-dimensional shape-conformation performances of wound dressings tested in a robotic sacral pressure ulcer phantom. Int Wound J 2021;18:670-80.
- Wood RA. Disintegration of cellulose dressings in open granulating wounds. Br Med J 1976;1:1444-5.
- Chakravarthy D, Rodway N, Schmidt S, Smith D, Evancho M, Sims R. Evaluation of three new hydrocolloid dressings: retention of dressing integrity and biodegradability of absorbent components attenuate inflammation. J Biomed Mater Res 1994;28:1165-73.
- ASTM D412 - 06, Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers - Tension, ASTM International, West Conshohocken, PA, 2006, www.astm.org.
- Xu F, Lu TJ. Chapter 3 Skin Biothermomechanics: modeling and experimental characterization. Adv Appl Mech 2009;43:147-248.
- Kuthe CD, Uddanwadiker RV. Investigation of effect of fiber orientation on mechanical behavior of skeletal muscle. J Appl Biomater Funct Mater 2016;14:e154-62.
- Graham HK, McConnell JC, Limbert G, Sherratt MJ. How stiff is skin? Exp Dermatol 2019;28:4-9.
- Friedman R, Haimy A, Epstein Y, Gefen A. Evaluation of helmet and goggle designs by modeling non-penetrating projectile impacts. Comput Methods Biomech Biomed Engin 2019;22:229-42.
- Gefen A, Haberman E. Viscoelastic properties of ovine adipose tissue covering the gluteus muscles. J Biomech Eng 2007;129:924-30.
- Gefen A. Not all superabsorbent wound dressings are born equal: theory and experiments. J Wound Care 2021;30:738-50.
- Dini V, Salvo P, Janowska A, Di Francesco F, Barbini A, Romanelli M. Correlation between wound temperature obtained with an infrared camera and clinical wound bed score in venous leg ulcers. Wounds 2015;27:274-8.
- Barnea Y, Weiss J, Gur E. A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther Clin Risk Manag 2010;6:21-7.
- Walker M, Parsons D. Hydrofiber® Technology: its role in exudate management. Wounds UK 2010;6:31-38.
- Hurlow J. AQUACEL® Ag dressing with Hydrofiber® technology. Adv Wound Care (New Rochelle) 2012;1:104-7.
- Rezakhaniha R, Agianniotis A, Schrauwen JT, et al. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol 2012;11:461-73.
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671-5.
- ASTM D882-02, Standard Test Method for Tensile Properties of Thin Plastic Sheeting, ASTM International, West Conshohocken, PA, 2002, www.astm.org.
- Expert working group; Satellite expert working group. Wound exudate and the role of dressings. A consensus document. Int Wound J 2008;5(Suppl 1):iii-12.
- Harding K, Carville K, Chadwick P, et al. World Union of Wound Healing Societies. World Union of Wound Healing Societies (WUWHS) Consensus Document. Wound exudate: effective assessment and management. Wounds Int 2019. https://www.woundsinternational.com/download/resource/7732.
- Grande A, Sivakumaran D. Assessment of the wet strength and log reduction capacity of a silver alginate dressing. 2016 Symp Adv Wound Care (SAWC) April 13-17. 2016:5.
- Gefen A, Santamaria N, Creehan S, Black J. Patient safety may be compromised if study conclusions are generalized to products that make similar claims but have no equivalent research evidence. J Patient Saf Risk Manag 2019;24:37-45.


