Volume 42 Number 3
Pressure injury management in critically ill patients with COVID-19 in a makeshift hospital in Indonesia: A report of two cases
Kezia Eveline, Hemma W Indirayani, Rachmaniar Pramanasari, Firas F Alkaff
Keywords pressure injury, COVID-19, Case report, ICU, Indonesia
For referencing Eveline K et al. Pressure injury management in critically ill patients with COVID-19 in a makeshift hospital in Indonesia: A report of two cases. WCET® Journal 2022;42(3):23-29
DOI
https://doi.org/10.33235/wcet.42.3.23-29
Submitted 9 January 2022
Accepted 15 March 2022
Abstract
Patients who are critically ill with COVID-19 need ventilation support in the ICU. However, ICU patients are at higher risk of developing a pressure injury (PI). Unfortunately, PI prevention is not optimally implemented in Indonesia, especially in the makeshift hospitals created during the COVID-19 pandemic.
Here, the authors report two cases of critically ill COVID-19 patients who developed large sacral PIs during hospitalisation in a makeshift hospital in Indonesia. The first patient developed a grade III, 7 cm × 7 cm sacral PI on the 14th day of hospitalisation. The second patient developed a grade IV, 12 cm × 8 cm sacral PI on the 16th day of hospitalisation. Both patients had elevated D-dimer levels and used a noninvasive ventilator for one week.
The wounds were treated with surgical debridement, silver hydrogel dressing, and hydrocolloid dressing, and complemented with static air mattress overlay.
The authors recommend that in situations where there is a shortage of healthcare workers, the government should provide pressure-redistribution devices and silicone foam dressings for all critically ill patients to prevent PI development and lighten the workload of healthcare workers.
Copyright Advances in Skin & Wound Care and the World Council of Enterostomal Therapists®.
Introduction
COVID-19 has a wide clinical spectrum.1 Although the majority of those infected with COVID-19 are asymptomatic or have only mild symptoms, older adults and those with comorbidities are more likely to become critically ill.2 Among patients who are critically ill with COVID-19, the majority develop acute respiratory distress syndrome (ARDS), a life-threatening form of respiratory failure with a high mortality rate.3,4 Patients who develop ARDS require ventilation support in the ICU. However, patients in the ICU are also at higher risk of pressure injury (PI) development due to prolonged immobilisation.5
Unfortunately, PI prevention measures are not optimally implemented in Indonesia,8 especially in the makeshift hospitals created during the COVID-19 pandemic. Here, the authors report two cases of critically ill COVID-19 patients who developed large PIs during hospitalisation in a makeshift hospital in one of the largest cities in Indonesia. Written informed consent was provided by the legal guardian (for case 1) and by the subject of the case (for case 2) to publish the case details and associated images. The authors highlight challenges in PI management during the COVID-19 pandemic and propose several suggestions to aid PI prevention.
Case report
Case 1
A 40-year-old man with a medical history of schizophrenia and intellectual disability presented to the ER with the chief complaints of irritability, fever, dry cough, and shortness of breath. Three days before admission, the patient developed a fever of 38° C at home. On physical examination at the ER, the patient’s temperature was 37.8° C, BP was 120/80 mmHg, heart rate was 102 beats per minute, respiratory rate was 24 breaths per minute, and oxygen saturation was 85% without any oxygen support. Lung auscultation revealed rales in the left lung and chest X-ray revealed pulmonary infiltrates in the left lung (Figure 1). Laboratory evaluation showed elevated D-dimers (>20.000 ng/mL FEU) and hypoalbuminemia (2.2 mg/dl). Based on the initial evaluation, COVID-19 infection was suspected. A nasopharynx specimen was taken for COVID-19 reverse transcriptase-polymerase chain reaction evaluation to confirm the COVID-19 diagnosis.
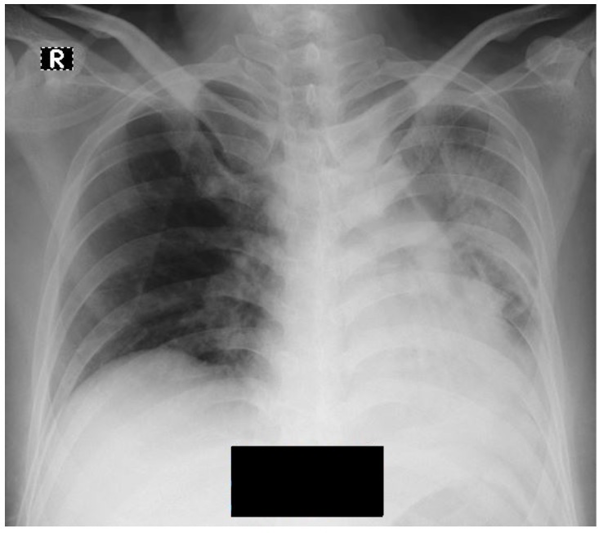
Figure 1. Chest x-ray in case 1,X-ray showing infiltrate in left lung.
While waiting for the test result, the patient was given 15 liters per minute oxygen via nonrebreathing mask and treated with intravenous moxifloxacin 400 mg daily, intravenous acetylcysteine 500 mg daily, intravenous albumin 50 grams daily, and 40 mg subcutaneous enoxaparin twice daily. To treat the symptoms of schizophrenia, the patient was given oral clozapine 50 mg twice daily, oral quetiapine 200 mg twice daily, and oral trihexyphenidyl 2mg twice daily. Because the patient was uncooperative, the patient was restrained on the bed after his legal guardian provided written consent. A foley catheter was then inserted and a diaper was used. The following day, the patient’s COVID-19 test result came back positive. The patient was then given additional treatment of intravenous oseltamivir 75 mg twice daily.
During hospitalisation, the patient’s oxygen saturation was not improving. On day 10, blood gas analysis showed uncompensated respiratory acidosis (pH 7.28, PO2 122 mmHg, PCO2 51.8 mmHg, and HCO3 24.9 mEq/L) with Jackson Reese circuit oxygen support. The patient also developed ARDS (PaO2/FiO2 ratio 122). Because this makeshift hospital lacked mechanical ventilators, a noninvasive ventilator was used. The patient had mean arterial pressure of 60 mmHg and Glasgow Coma Scale of 8, indicating ongoing septic shock. Thus, intravenous norepinephrine (0.1 mcg/kg/min) was administered continuously using syringe pump. Because the patient was immobilised, 30 minutes right-oblique and 30 minutes left-oblique passive mobilisation was undertaken every 8 hours. The restraints were released prior to mobilisation and retied after mobilisation to the new position. To maintain nutrition intake, a nasogastric feeding tube was inserted. The required nutrition was calculated by the dietitian and given as milk feeding.
Due to the limited number of healthcare workers (HCWs) available every shift and their high workloads, PI risk assessment was not performed. However, the patient’s skin was inspected every 8 hours during diaper changes. On day 14, a 7 cm × 7 cm bilateral sacral wound was noted with epithelial surface and superficial wound bed (Figure 2a). The wound was cleansed using normal saline and wound irrigation solution and a silver hydrogel dressing was applied to the wound bed. A hydrocolloid foam dressing was placed on top of the silver hydrogel dressing and extended 2 cm around the wound to protect the peri-wound skin from maceration. To prevent urine or fecal contamination, nonsterile gauze was placed above the hydrocolloid foam dressing and fixed with retention tape. In addition, a static air mattress overlay was placed on the bed as a pressure-redistribution device (PRD). The wound dressing was changed every 3 days, or earlier if contaminated with urine or feces.
On day 18, the sacral wound enlarged to 15 cm × 10 cm with slough and suspicion of “deeper ulceration” (Figure 2b). Surgical debridement was then performed by the nurses at the bedside. After surgical debridement, fascia was seen as the wound bed (Figure 2c). According to the 2016 National Pressure Ulcer Advisory Panel (NPUAP) staging system,10 the wound was classified as grade III. Because of the deep cavity wound, sterile gauze was placed in the cavity to fill the space after applying a silver hydrogel dressing onto the wound bed. The wound dressing was changed every 2 days instead of every 3 days, or earlier if contaminated with urine or feces. When needed, surgical debridement was performed again. On day 26, granulation began to appear from the wound (Figure 2d). The patient was discharged on day 39. Nurses followed up with weekly home visits until the wound was completely closed to evaluate the wound-healing process, perform surgical debridement if needed, change the wound dressing, and teach family members how to change the wound dressing in between home visits.
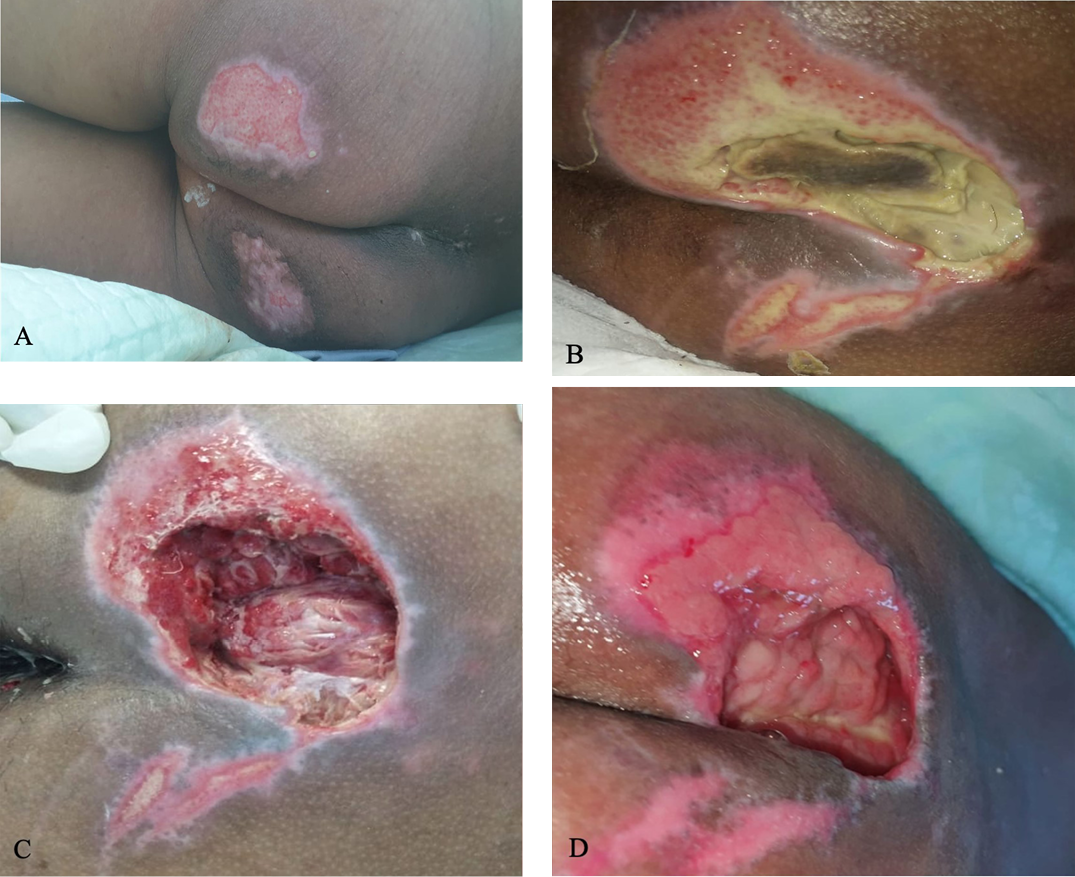
Figure 2. Sacral ulcer in case 1
A) Sacral ulcer with epithelial wound bed measuring 7 cm × 7 cm on day 14. B) Covered by slough and suspicion of “deeper ulcer,” measuring 15 cm × 10 cm on day 18. C) Minimal slough and fascia in the wound bed after surgical debridement and wound dressing, 15 cm x 10 cm with depth of 6 cm on day 22. D) Granulation tissue on day 26, measuring 10 cm × 6 cm.
Case 2
A 59-year-old man with a medical history of type 2 diabetes mellitus, hypertension, and cerebrovascular accident (CVA) presented to the ER with the chief complaints of fever, cough, and shortness of breath. Five days earlier, the patient had developed a cough and fever of 38.5° C at home. The patient tested positive for COVID-19 3 days before presenting to the ER. On physical examination at the ER, the temperature was 37.5° C, BP was 140/90 mmHg, heart rate was 110 beats per minute, respiratory rate was 24 breaths per minute, and oxygen saturation was 75% without any oxygen support. The patient had left-side hemiplegia due to the CVA. Lung auscultation revealed rales in both lungs. Chest X-ray revealed bilateral pulmonary opacities and cardiomegaly (Figure 3). Laboratory evaluation showed elevated D-dimers (2.100 ng/mL FEU), elevated interleukin-6 level (120 pg/mL), and hypoalbuminemia (2.8 mg/dl). Based on the initial evaluation, the patient was diagnosed with COVID-19 and recent CVA.
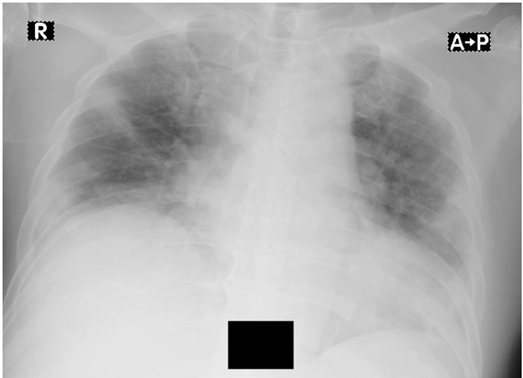
Figure 3. Chest x-ray in case 2
X-ray showing infiltrate in both lungs and cardiomegaly with cardio-thoracic ratio of 60%.
The patient was given 15 liters per minute oxygen via Jackson Rees circuit and treated with intravenous moxifloxacin 400 mg daily, intravenous acetylcysteine 500 mg daily, intravenous albumin 50 grams daily, subcutaneous heparin 5000 International Unit (IU), intravenous dexamethasone 6 mg daily, 400 mg intravenous Tocilizumab single dose, and oral oseltamivir 75 mg twice daily. To treat the hypertension, the patient was given oral amlodipine 10 mg once daily and oral candesartan 16 mg once daily. The patient also received subcutaneous long actin insulin 20 IU once daily and rapid acting insulin 16 IU thrice daily to treat the diabetes mellitus.
During hospitalisation, the patient’s oxygen saturation was not improving. On day 7, blood gas analysis showed uncompensated respiratory acidosis (pH 7.3, PO2 150 mmHg, PCO2 50.2 mmHg, and HCO3 23.5 mEq/L) with Jackson Reese circuit oxygen support. The patient developed ARDS (PaO2/FiO2 ratio 150) and was given a noninvasive ventilator due to the lack of mechanical ventilators. Because the patient was immobilised, 30 minutes right-oblique and 30 minutes left-oblique passive mobilisation was undertaken every 8 hours. To maintain nutritional intake, a nasogastric feeding tube was inserted; milk feeding was calculated by a dietician.
Due to the limited number of HCWs every shift and their high workloads, PI risk assessment was not performed. However, the patient’s skin was inspected every 8 hours during diaper changes. On day 16, a 12 cm × 8 cm sacral wound with suspicion of “deeper ulceration” was found on both sides of the sacrum (Figure 4a). On day 18, central necrotic tissue was present (Figure 4b). Nurses performed surgical debridement at the bedside (Figure 4c) and the wound was cleansed using normal saline and wound irrigation solution. A silver hydrogel dressing was applied on the wound bed and a hydrocolloid foam dressing was placed on top of the silver hydrogel dressing and extended 2 cm around the wound to protect the peri-wound skin from maceration. To prevent urine or fecal contamination, nonsterile gauze was placed above the hydrocolloid foam dressing and fixed with retention tape. Further, a static air mattress overlay was placed on the bed as a PRD. The wound dressing was changed every 3 days, or earlier if contaminated with urine or feces. When needed, surgical debridement was performed again.
Two days after treatment, the wound bed was more profound than the initial wound with bone and tendon exposure (Figure 4d). According to the 2016 NPUAP staging system,10 the wound was grade IV. Because of the deep cavity wound, the cavity was filled with sterile gauze after applying a silver hydrogel dressing onto the wound bed. The wound dressing was changed every 2 days instead of every 3 days, or earlier if contaminated with urine or feces. On day 25, granulation tissue presented on the ulcer (Figure 4e) and by day 30, the ulcer had narrowed (Figure 4f). The patient was discharged on day 35. Nurses followed up with weekly home visits until the wound was completely closed to evaluate the wound-healing process, perform surgical debridement if needed, change the wound dressing, and teach family members how to change the wound dressing in between the scheduled home visits.
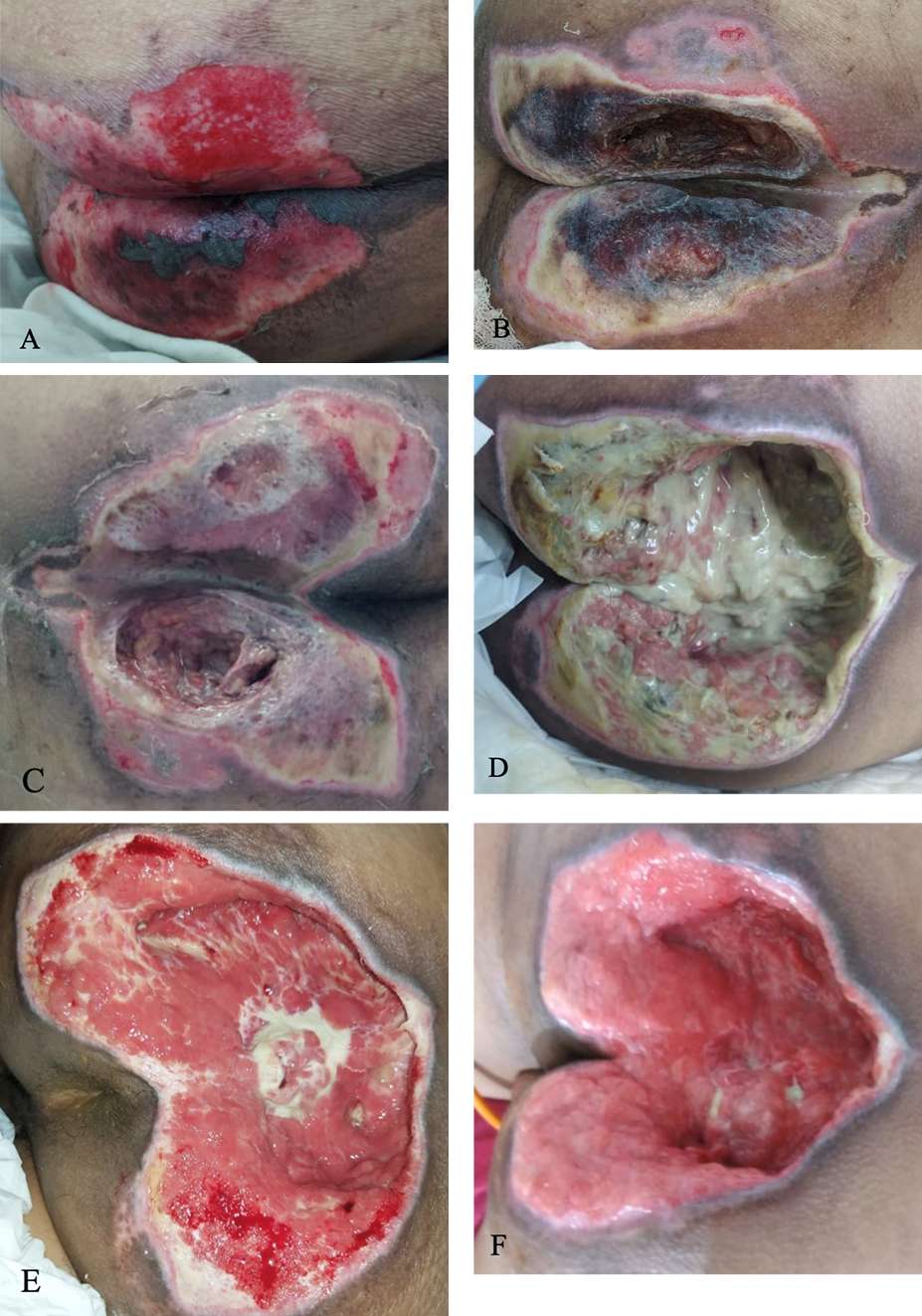
Figure 4. Sacral ulcer in case 2
A) Sacral ulcer on both side of buttocks with epithelial wound bed and necrotic tissue on central side, measuring 12 cm x 8 cm on day 16. B) Covered with both slough and necrotic tissue, measuring 12 cm x 10 cm on day 18. C) After surgical debridement on day 18. D) Covered with massive slough, depth of 7 cm on day 20. E) Granulation tissue and minimal slough on day 25. F) Granulation tissue on day 30.
Discussion
As reflected in the latest NPUAP definition of PI, the main factors influencing PI development are pressure, shear, and soft tissue tolerance.10 Pressure refers to sustained pressure on a local point, whereas shearing force occurs when two opposing surfaces slide in opposite directions. When pressure and shear disrupt blood flow and local tissue hypoxia lasts for a critical period of time, tissue damage results. However, at what intensity and duration of force tissue damage occurs depends on the tissue tolerance.12 Pressure injuries usually occur in bony areas such as the sacrum.13
Researchers have identified a number of PI risk factors, including immobility, being confined to bed, malnutrition, dehydration, infection, urinary and bowel incontinence, diabetes, vascular disease, and vasopressor use.14-16 Sedated patients are particularly prone to PI because they do not perceive painful stimuli from intense and prolonged pressure and are not able to actively change their position in bed.7,17,18 Recently, it has been reported that patients who are critically ill with COVID-19 are also at higher risk of PI.19 These patients have reduced perfusion and are more likely to develop systemic coagulopathy, which leads to decreased tissue tolerance.19 In the first case, the patient was critically ill with COVID-19 and required a vasopressor. This patient had impaired mental health as a comorbidity. People with severe mental health impairment such as schizophrenia tend to have poor diet and may neglect their personal hygiene, causing similar conditions to having urinary and bowel incontinence.13 In the second case, the patient was also critically ill with COVID-19 and had CVA and diabetes mellitus as comorbidities.
Pressure injury is associated with prolonged hospitalisation.20-22 The length of stay was 39 days for the first case report patient and 35 days for the second patient. The first patient could have been discharged 9 days earlier if there were no PI present; the second patient could have been discharged 5 days earlier. In a previous case series of three severely ill patients with COVID-19, PIs were first noted between 7 and 19 days after hospitalisation.19 For the patients described herein, PI was noted on day 14 of hospitalisation for the first patient, and on day 16 for the second patient.
According to the latest guidelines, comprehensive PI risk assessment, preventive skin care, and early mobilisation and repositioning are some of the PI management strategies that should be considered.23 In terms of patients who are critically ill with COVID-19, Tang et al24 found that PIs can be managed by improving the underlying contributing factors, providing PRD with proper positioning, improving mobility, minimising excessive moisture, correcting malnutrition, and performing close daily monitoring of the PI including the dressing, surrounding skin, and any possible complications.
However, proper PI management translates to an extra workload for nurses. Unfortunately, due to significant staffing shortages, HCWs’ workload during the COVID-19 pandemic in Indonesia is already high.9 In this makeshift hospital, there are only one doctor and three nurses on every shift, resulting in a HCW-to-patient ratio of 1:5. In addition, most of the patients with COVID-19 were critically ill. With a very unbalanced HCW-to-patient ratio and resultant high workload for the HCWs, it is not possible to follow the guidelines for proper PI management. As a result, PI risk assessment was not performed when patients arrived at the makeshift hospital; mobilisation and skin assessment were performed every 8 hours (once per HCW’s working shift) instead of being individualised according to risk assessment.
With regard to PRDs, a recent study found that PRD use was associated with an 88% reduced risk of PI development in high risk ICU patients.25 Similarly, a previous systematic review also concluded that PRD should be provided to patients who are at high risk of PI development.26 Pressure-redistribution devices help prevent PIs by decreasing the magnitude and duration of pressure as well as reducing the shear and friction between the patient and the bed surface.10,25 Thus, providing PRDs for all critically ill patients with COVID-19 would be a beneficial strategy for PI prevention. However, in the makeshift hospital, only two PRDs are available, and thus are allocated for PI treatment and not for PI prevention.
Based on the difficulties experienced during the COVID-19 pandemic, the authors recommend several PI prevention strategies for hospitals with HCW shortages:23,28
Re-educate HCWs about PI management
Conduct skin assessments as often as possible
Ensure patients have adequate nutrition and hydration
Use PRDs for all patients who are critically ill with COVID-19
Apply silicone foam dressings over the bony prominences that serve as the main pressure points in all critically ill patients with COVID-19.
Conclusions
Patients who are critically ill with COVID-19 are also at greater risk of PI development during hospitalisation. In a situation where there is a shortage of HCWs, governments should compensate by providing additional PRDs and silicone foam dressings for all critically ill patients to prevent PI development and lighten the workload of HCWs.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
印度尼西亚一家临时医院中COVID-19重症患者的压力性损伤管理: 两个病例的报告
Kezia Eveline, Hemma W Indirayani, Rachmaniar Pramanasari, Firas F Alkaff
DOI: https://doi.org/10.33235/wcet.42.3.23-29
摘要
COVID-19重症患者需要在重症监护室(ICU)进行通气支持。然而,ICU患者发生压力性损伤(PI)的风险较高。遗憾的是,PI预防在印度尼西亚,尤其是在COVID-19大流行期间建立的临时医院中没有得到最佳实施。
在此,作者报告了两例COVID-19重症患者,他们在印度尼西亚一家临时医院住院期间出现大面积骶骨PI。第一名患者在住院第14天出现Ⅲ级7 cmÅ~7 cm的骶骨PI。第二名患者在住院第16天出现IV级12 cmÅ~8 cm的骶骨PI。两名患者的D-二聚体水平均升高,均使用无创呼吸机一周。
采用外科清创、银水凝胶敷料和水胶体敷料治疗伤口,并辅以静态气垫覆盖。
作者建议,在医护人员短缺的情况下,政府应为所有重症患者提供压力再分配装置和硅酮泡沫敷料,以预防PI的发生,减轻医护人员的工作量。
《皮肤与伤口护理进展》和世界造口治疗师委员会®版权所有。
引言
COVID-19具有广泛的临床表现。1虽然大多数感染COVID-19的患者无症状或仅有轻微症状,但老年人和有合并症的患者更有可能成为重症患者。2大多数COVID-19重症患者会发生急性呼吸窘迫综合征(ARDS),这是一种危及生命的呼吸衰竭,死亡率较高。3,4发生ARDS的患者需要在ICU接受通气支持。然而,由于长期不活动,ICU患者发生压力性损伤(PI)的风险也较高。5
不幸的是,PI预防措施在印度尼西亚没有得到最佳实施,8特别是在COVID-19大流行期间建立的临时医院。在此,作者报告了两例COVID-19重症患者,他们在印度尼西亚最大城市之一的临时医院住院期间发生了大面积PI。法定监护人(病例1)和病例受试者(病例2)提供了书面知情同意书,允许发表病例详情和相关图像。作者强调了COVID-19大流行期间PI管理面临的挑战,并提出了帮助PI预防的几点建议。
病例报告
病例1
患者男,40岁,有精神分裂症和智力障碍病史,因主诉易怒、发热、干咳、呼吸短促到急诊室(ER)就诊。入院前三天,患者在家中发烧38ÅãC。在ER进行体格检查时,患者体温为37.8ÅãC,血压(BP)为120/80 mmHg,心率为102次/分钟,呼吸频率为24次/分钟,血氧饱和度为85%,无任何氧气支持。肺部听诊发现左肺有啰音,X线胸片显示左肺存在浸润(图1)。实验室评价显示D-二聚体升高(>20.000 ng/mL FEU)和血白蛋白过少(2.2 mg/dl)。根据初步评价,怀疑患者感染了COVID-19。取鼻咽标本进行COVID-19逆转录聚合酶链反应评价,以确认COVID-19诊断。

图1.病例1的x线胸片,显示左肺浸润。
等待检查结果时,患者通过非再吸入型面罩每分钟吸氧15 L,并接受静脉注射莫西沙星400 mg/天、静脉注射乙酰半胱氨酸500 mg/天、静脉注射白蛋白50 g/天,皮下注射依诺肝素40 mg两次/天。为治疗精神分裂症的症状,患者口服氯氮平50 mg、喹硫平200 mg和苯海索2 mg,各每日两次。因患者不合作,经其法定监护人书面同意后,将患者束缚在床上。然后插入foley导尿管,并使用尿布。次日,患者的COVID-19检测结果呈阳性。随后患者每日进行两次75 mg奥司他韦静脉注射作为额外治疗。
住院期间,患者的血氧饱和度未改善。第10天,血气分析显示,Jackson Reese回路氧气支持下出现失代偿性呼吸性酸中毒(pH值为
7.28,PO2为122 mmHg,PCO2为51.8 mmHg,HCO3为24.9 mEq/L)。患者还出现了ARDS(PaO2/FiO2比值为122)。因为这家临时医院没有机械呼吸机,所以使用了无创呼吸机。患者的平均动脉压为60 mmHg,格拉斯哥昏迷评分为8分,表明患者为持续性感染性休克。因此,使用注射泵持续静脉注射去甲肾上腺素(0.1 É g/kg/min)。由于患者被固定,所以每8小时进行30分钟右斜位和30分钟左斜位被动运动。运动前解除患者束缚,运动至新位置后重新固定。为维持营养摄入,为患者插入鼻饲管。由营养师计算所需营养,并以牛奶的形式喂食。
由于每次轮班可用的医护人员(HCW)数量有限,且工作量较大,因此未进行PI风险评估。但是,在换尿布期间,每8小时检查一次患者的皮肤。第14天,观察到7 cmÅ~7 cm的双侧骶骨伤口和上皮表面和浅表伤口床(图2a)。使用生理盐水和伤口冲洗液清洁伤口,并在伤口床敷用银水凝胶敷料。将水胶体泡沫敷料置于银水凝胶敷料的顶部,并向伤口周围延伸2 cm,以保护伤口周围皮肤免受浸渍。为防止尿液或粪便污染,将非无菌纱布置于水胶体泡沫敷料上方,并用保留胶带固定。此外,将静态气垫放置在床上,作为压力再分配装置(PRD)。每3天更换一次伤口敷料,如果敷料被尿液或粪便污染,则提前更换。
第18天,骶骨伤口扩大至15 cmÅ~10 cm,有腐肉,怀疑为“深层溃疡”(图2b)。然后由护士在床旁进行外科清创。外科清创后,筋膜被视为伤口床(图2c)。根据2016年美国国家压疮咨询委员会(NPUAP)分期系统,10该伤口分类为III级。由于腔洞伤口较深,在伤口床上敷用银水凝胶敷料后,在腔洞内放置无菌纱布以填充空间。伤口敷料每2天更换一次,而不是每3天一次,如果受到尿液或粪便污染,则提前更换。需要时再次进行外科清创。第26天,伤口开始出现肉芽(图2d)。患者在第39天出院。护士每周进行家访,直至伤口完全闭合,以评估伤口愈合过程,必要时进行外科清创,更换伤口敷料,并教导家属如何在家访之间更换伤口敷料。

图2.病例1中的骶骨溃疡
A)第14天的骶骨溃疡,上皮伤口床测量尺寸为7 cmÅ~7 cm。B)第18天,覆盖有腐肉,怀疑为“深层溃疡”,测量尺寸为15 cmÅ~10 cm。C)第22天,进行外科清创和伤口敷料包扎后,伤口床内的腐肉和筋膜极少,尺寸为15 cmÅ~10 cm,深度为6 cm。D)第26天的肉芽组织,测量尺寸为10 cm Å~6 cm。
病例2
患者男,59岁,有2型糖尿病、高血压和脑血管意外(CVA)病史,因主诉发热、咳嗽和呼吸短促到ER就诊。五天前患者在家曾出现咳嗽,发烧38.5ÅãC。在ER就诊前3天,患者检测出COVID-19阳性。在ER进行体格检查时,患者体温为37.5ÅãC,BP为140/90 mmHg,心率为110次/分钟,呼吸频率为24次/分钟,血氧饱和度为75%,无任何氧气支持。患者因CVA出现左侧偏瘫。肺部听诊显示双肺有啰音。X线胸片显示双肺存在阴影,心脏肥大(图3)。实验室评价显示D-二聚体升高(2.100 ng/mL FEU)、白细胞介素-6水平升高(120 pg/mL)以及血白蛋白过少(2.8 mg/dl)。根据初步评价,患者确诊为COVID-19和近期CVA。

图3.病例2的x线胸片
胸片显示双肺浸润和心脏肥大,心胸比为60%。
患者通过Jackson Rees回路每分钟吸氧15 L,并接受静脉注射莫西沙星400 mg/天、静脉注射乙酰半胱胺酸500 mg/天、静脉注射白蛋白50 g/天,皮下注射肝素5000国际单位(IU)、静脉注射地塞米松6 mg/天,单次静脉注射400 mg托珠单抗,以及口服75 mg奥司他韦每日两次。为治疗高血压,患者每日口服氨氯地平10 mg和坎地沙坦16 mg各一次。患者还接受皮下注射长效胰岛素20 IU(每日一次)和速效胰岛素16 IU
(每日三次)以治疗糖尿病。
住院期间,患者的血氧饱和度未改善。第7天,血气分析显示,Jackson Reese回路氧气支持下出现失代偿性呼吸性酸中毒(pH值为
7.3,PO2为150 mmHg,PCO2为50.2 mmHg,HCO3为23.5 mEq/L)。患者出现ARDS(PaO2/FiO2比值为150),因缺乏机械呼吸机而使用无创呼吸机。由于患者被固定,所以每8小时进行30分钟右斜位和30分钟左斜位被动运动。为维持营养摄入,为患者插入鼻饲管;由营养师计算营养并以牛奶喂食。
由于每次轮班的医护人员数量有限且工作量较大,因此未进行PI风险评估。但是,在换尿布期间,每8小时检查一次患者的皮肤。第16天,在骶骨两侧发现12 cmÅ~8 cm的骶骨伤口,怀疑为“深层溃疡”(图4a)。第18天,出现中央坏死组织(图4b)。护士在床旁进行外科清创(图4c),使用生理盐水和伤口冲洗液清洁伤口。将银水凝胶敷料敷在伤口床上,并将水胶体泡沫敷料置于银水凝胶敷料顶部,并向伤口周围延伸2 cm,以保护伤口周围皮肤免受浸渍。为防止尿液或粪便污染,将非无菌纱布置于水胶体泡沫敷料上方,并用保留胶带固定。此外,将静态气垫放置在床上作为PRD。每3天更换一次伤口敷料,如果敷料被尿液或粪便污染,则提前更换。需要时再次进行外科清创。
治疗两天后,伤口床比最初的伤口更加深,骨头和肌腱暴露(图4d)。根据2016 NPUAP分期系统,10该伤口为IV级。由于腔洞伤口较深,在伤口床上敷用银水凝胶敷料后,用无菌纱布填充腔洞。伤口敷料每2天更换一次,而不是每3天一次,如果受到尿液或粪便污染,则提前更换。第25天,溃疡上出现肉芽组织(图4e),到第30天,溃疡变窄(图4f)。患者在第35天出院。护士每周进行家访,直至伤口完全闭合,以评估伤口愈合过程,必要时进行外科清创,更换伤口敷料,并教导家属如何在安排的家访之间更换伤口敷料。

图4.病例2中的骶骨溃疡
A)第16天臀部两侧的骶骨溃疡,中心侧存在上皮伤口床和坏死组织,测量尺寸为12 cmÅ~8 cm。B)第18天,覆盖有腐肉和坏死组织,测量尺寸为12 cmÅ~10 cm。C)第18天,进行外科清创后。D)第20天,覆盖有大量腐肉,深度为7 cm。E)第25天的肉芽组织和极少腐肉。F)第30天的肉芽组织。
讨论
正如最新的NPUAP PI定义所反映的,影响PI发生的主要因素是压力、剪切力和软组织耐受性。10压力指局部点上的持续压力,而当两个相对的表面以相反的方向滑动时,则会产生剪切力。当压力和剪切力破坏血流,局部组织缺氧持续一段关键时间时,会导致组织损伤。然而,在何种力的强度和持续时间下会发生组织损伤取决于组织耐受性。12压力性损伤通常发生在骶骨等骨区域。13
研究人员已经确定了许多PI风险因素,包括不活动、卧床、营养不良、脱水、感染、大小便失禁、糖尿病、血管疾病和使用血管加压药。14-16镇静患者极易发生PI,因为他们感觉不到来自强烈和长期压力的疼痛刺激,并且无法主动改变他们在床上的位置。7,17,18最近据报告,COVID-19重症患者也有较高的PI风险。19这些患者的灌注减少,更可能出现全身性凝血病,从而导致组织耐受性降低。19在第一个病例中,患者为COVID-19重症患者,需要使用血管加压药。该患者的心理健康受损是一种合并症。重度精神健康障碍(如精神分裂症)患者往往饮食较差,可能会忽视个人卫生,从而出现与大小便失禁相似的情况。13在第二个病例中,患者还患有重症COVID-19,并伴有CVA和糖尿病等合并症。
压力性损伤与住院时间延长相关20-22。第一个病例报告患者的住院时间为39天,第二名患者的住院时间为35天。如果没有PI,第一名患者本可以提前9天出院;第二名患者本可以提前5天出院。在之前的三名COVID-19重症患者的病例系列中,在患者住院后7-19天首次观察到PI。19对于本文描述的患者,第一名患者在住院第14天观察到PI,第二名患者在第16天观察到PI。
根据最新指南,综合PI风险评估、预防性皮肤护理以及早期活动和重新定位是应考虑的一些PI管理策略。23对于COVID-19重症患者,Tang等人24发现,PI可通过改善潜在影响因素、为PRD提供适当的定位、改善移动性、尽量减少过度潮湿、纠正营养不良以及日常密切监测PI
(包括敷料、周围皮肤及任何可能的并发症)进行管理。
但是,适当的PI管理会转化为护士的额外工作量。不幸的是,由于人员严重短缺,在印度尼西亚COVID-19大流行期间,医护人员的工作量已经很大。9在这家临时医院,每次轮班只有一名医生和三名护士,导致医护人员与患者的比例为1:5。此外,大多数COVID-19患者都是重症患者。医护人员与患者的比例非常不平衡,导致医护人员工作量大,因此不可能按照指南进行适当的PI管理。因此,患者到达临时医院时,未进行PI风险评估;每8小时进行一次活动和皮肤评估(每个医护人员轮班进行一次),而不是根据风险评估进行个性化评估。
关于PRD,最近的一项研究发现,使用PRD可使ICU高危患者的PI发生风险降低88%25。同样,之前的系统综述也得出结论,应向PI发生风险较高的患者提供PRD26。压力再分配装置通过降低压力的幅度和持续时间以及减少患者和床面之间的剪切力和摩擦力来帮助预防PI10,25。因此,为所有COVID-19重症患者提供PRD将是预防PI的有益策略。然而,临时医院只有两个PRD可用,因此PRD被分配用于PI治疗,而不是用于PI预防。
根据COVID-19大流行期间遇到的困难,作者为医护人员短缺的医院推荐了几种PI预防策略:23,28
对医护人员进行PI管理的再教育
尽可能频繁地进行皮肤评估
确保患者有足够的营养和水分
将PRD用于所有COVID-19重症患者
在所有COVID-19重症患者中,在作为主要压力点的骨突处敷用硅酮泡沫敷料。
结论
COVID-19重症患者住院期间发生PI的风险更高。在医护人员短缺的情况下,政府应通过为所有重症患者提供额外的PRD和硅酮泡沫敷料来进行补偿,以预防PI的发生,减轻医护人员的工作量。
利益冲突声明
作者声明无利益冲突。
资助
作者未因该项研究收到任何资助。
Author(s)
Kezia Eveline MD
Plastic Reconstructive and Aesthetic Surgery Resident, Faculty of Medicine Universitas Airlangga, Dr Soetomo General Academic Hospital, Surabaya, Indonesia
Hemma W Indirayani MD
Internist, Menur Mental Hospital, Surabaya, Indonesia
Rachmaniar Pramanasari MD
Plastic Reconstructive and Aesthetic Surgeon, Airlangga University Hospital, Surabaya, Indonesia
Firas F Alkaff* MD
Lecturer, Division of Pharmacology and Therapy, Department of Anatomy, Histology and Pharmacology, Faculty of Medicine Universitas Airlangga
PhD Researcher, Division of Nephrology, Department of Internal Medicine, University Medical Centre, Groningen, The Netherlands
* Corresponding author
Kezia Eveline, MD is Plastic Reconstructive and Aesthetic Surgery Resident at Faculty of Medicine Universitas Airlangga, Dr. Soetomo General Academic Hospital, Surabaya, Indonesia. Hemma W. Indriyani, MD is Internist at Menur Mental Hospital, Surabaya. Rachmaniar Pramanasari, MD, is Plastic Reconstructive and Aesthetic Surgeon at Airlangga University Hospital, Surabaya. Firas F. Alkaff, MD is Lecturer at Division of Pharmacology and Therapy, Department of Anatomy, Histology, and Pharmacology, Faculty of Medicine Universitas Airlangga and PhD Researcher at Division of Nephrology, Department of Internal Medicine, University Medical Center Groningen, Groningen, The Netherlands
Kezia Eveline(MD)是印度尼西亚苏腊巴亚Dr Soetomo综合学术医院,艾尔朗加大学医学院的整形重建和美容外科住院医师。Hemma W. Indriyani(MD)是苏腊巴亚Menur精神病院的内科医生。Rachmaniar Pramanasari(MD)是苏腊巴亚艾尔朗加大学医学院的整形重建和美容外科医生。Firas F. Alkaff(MD)是艾尔朗加大学医学院,解剖学、组织学和药理学系,药理学和治疗学部的讲师,以及荷兰格罗宁根大学医学中心内科系肾脏内科部的博士研究员
References
- Ramos-Casals M, Brito-Zeron P, Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol 2021;17:315-32.
- Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy 2021;76:428-55.
- Xu W, Sun NN, Gao HN, et al. Risk factors analysis of COVID-19 patients with ARDS and prediction based on machine learning. Sci Rep 2021;11:2933.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81.
- Challoner T, Vesel T, Dosanjh A, Kok K. The risk of pressure ulcers in a proned COVID population. Surgeon 2021;S1479-666X(21)00121-9.
- He M, Tang A, Ge X, Zheng J. Pressure ulcers in the intensive care unit: an analysis of skin barrier risk factors. Adv Skin Wound Care 2016;29:493-8.
- Keller BP, Wille J, van Ramshorst B, van der Werken C. Pressure ulcers in intensive care patients: a review of risks and prevention. Intensive Care Med 2002;28:1379-88.
- Amir Y, Lohrmann C, Halfens RJ, Schols JM. Pressure ulcers in four Indonesian hospitals: prevalence, patient characteristics, ulcer characteristics, prevention and treatment. Int Wound J 2017;14:184-93.
- Mahendradhata Y, Andayani N, Hasri ET, et al. The capacity of the Indonesian healthcare system to respond to COVID-19. Front Public Health 2021;9:649819.
- Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M. Revised National Pressure Ulcer Advisory Panel pressure injury staging system: revised pressure injury staging system. J Wound Ostomy Continence Nurs 2016;43:585-97.
- Defloor T. The risk of pressure sores: a conceptual scheme. J Clin Nurs 1999;8:206-16.
- Mervis JS, Phillips TJ. Pressure ulcers: Pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol 2019;81:881-90.
- Bhattacharya S, Mishra RK. Pressure ulcers: current understanding and newer modalities of treatment. Indian J Plast Surg 2015;48(1):4-16.
- Cox J, Roche S. Vasopressors and development of pressure ulcers in adult critical care patients. Am J Crit Care 2015;24:501-10.
- Ahn H, Cowan L, Garvan C, Lyon D, Stechmiller J. Risk factors for pressure ulcers including suspected deep tissue injury in nursing home facility residents: analysis of national minimum data set 3.0. Adv Skin Wound Care 2016;29:178-90; quiz E1.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allergy Asthma Rep 2020;20(12):75.
- Nedergaard HK, Haberlandt T, Toft P, Jensen HI. Pressure ulcers in critically ill patients - preventable by non-sedation? A substudy of the NONSEDA-trial. Intensive Crit Care Nurs 2018;44:31-5.
- Sasabe Y, Niitani M, Teramoto C, et al. Deep sedation predicts pressure injury in patients admitted to intensive care units. Nurs Crit Care 2022;DOI: 10.1111/nicc.12753.
- Young S, Narang J, Kumar S, et al. Large sacral/buttocks ulcerations in the setting of coagulopathy: a case series establishing the skin as a target organ of significant damage and potential morbidity in patients with severe COVID-19. Int Wound J 2020;17:2033-7.
- Triantafyllou C, Chorianopoulou E, Kourkouni E, Zaoutis TE, Kourlaba G. Prevalence, incidence, length of stay and cost of healthcare-acquired pressure ulcers in pediatric populations: a systematic review and meta-analysis. Int J Nurs Stud 2021;115:103843.
- Gupta P, Shiju S, Chacko G, et al. A quality improvement programme to reduce hospital-acquired pressure injuries. BMJ Open Qual 2020;9(3):e000905.
- Allman RM, Goode PS, Burst N, Bartolucci AA, Thomas DR. Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Adv Wound Care 1999;12:22-30.
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. EPUAP/NPIAP/PPPIA; 2019.
- Tang J, Li B, Gong J, Li W, Yang J. Challenges in the management of critical ill COVID-19 patients with pressure ulcer. Int Wound J 2020;17:1523-4.
- Bai DL, Liu TW, Chou HL, Hsu YL. Relationship between a pressure redistributing foam mattress and pressure injuries: an observational prospective cohort study. PLoS One 2020;15(11):e0241276.
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev 2015;9:CD001735.
- Shi C, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention: a network meta-analysis. PLoS One 2018;13(2):e0192707.
- Team V, Team L, Jones A, Teede H and Weller CD (2021) Pressure Injury Prevention in COVID-19 Patients With Acute Respiratory Distress Syndrome. Front. Med. 7:558696. doi: 10.3389/fmed.2020.558696


