Volume 44 Number 1
WHAM evidence summary: fish skin for treating burns
Emily Haesler
Keywords burns, Traditional wound management, fish skin, tilapia fish, evidence summary
For referencing Haesler E. WHAM evidence summary: fish skin for treating burns. WCET® Journal 2024;44(1):44-48.
DOI 10.33235/wcet.44.1.44-48
Clinical question
What is the best available evidence for fish skin for treating burns?
Summary
In low and middle resource settings, fish skin has been used as a low cost, traditional biological dressing for treatment of burns and other wounds. The high collagen concentration and tensile strength1-4 of fish skin has led to its use as a xenograft. There is insufficient clinical evidence on healing outcomes to make a recommendation on using fish skin for treating burns. Level 1 evidence5-7 at high risk of bias suggests that complete healing might be faster with a fish skin dressing compared to the local standard care (most frequently, silver sulfadiazine cream replaced every two days), but the time to healing difference was negligible in most studies and may not be clinically significant. Level 1 evidence5-7 on effectiveness in achieving better control of pain intensity showed mixed results. However, no studies reported that fish skin dressings were inferior to local standard care, adverse events were not reported to be an issue and some low level evidence indicated people receiving fish skin dressings were satisfied with the outcomes.
Clinical practice reccommendations
All recommendations should be applied with consideration to the wound, the person, the health professional and the clinical context.
|
There is insufficient evidence to make a recommendation on the use of fish skin dressings to promote healing in burns. |
Sources of evidence: search and appraisal
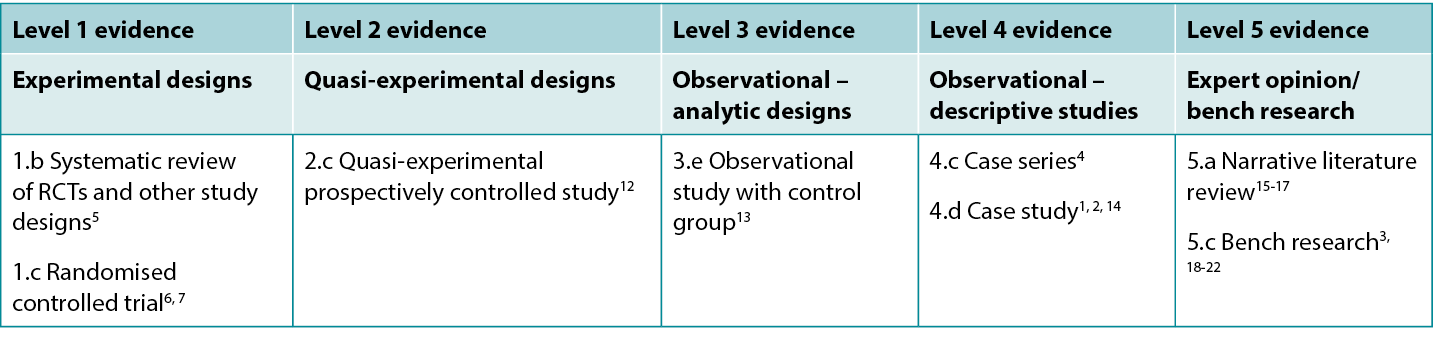
This summary was conducted using methods published by the Joanna Briggs Institute.8-11 The summary is based on a systematic literature search combining search terms related to fish skin, burns and healing. Searches were conducted for evidence reporting use of fish skin in human burns published up to 31 January 2024 in English in the following databases: Cumulative Index to Nursing and Allied Health Literature (CINAHL), Medline (Ovid), Google Scholar, Embase (Ovid), AMED, Global Health, Health Internetwork Access to Research Initiative (Hinari, access via Research4Life) and Cochrane Library. Levels of evidence for intervention studies are reported in Table 1.
Table 1. Levels of evidence for clinical studies
Background
Some types of fish skin have been used as a wound dressings in low resource communities due to their similarities to human skin. Fish skin has high collagen concentration, high resistance, and high tensile strength.1-4 Fish skin also has anti-viral, anti-bacterial and anti-oxidative properties, and is rich is unsaturated fatty acids, which might contribute to efficacy as a burn treatment.4, 16
The clinical research in this evidence summary is focused on the use of natural fish skin that is applied directly to burns (usually after a sterilisation process). The fish skin adheres to the wound bed as a xenograft, protecting the wound bed during healing and reducing the number of dressing changes that are required. This has potential to reduce healthcare resources and to reduce wound-related pain.1, 2, 4, 5, 12, 15
Bench research has also described the extraction and use of collagen from fish skin in commercial wound dressing products, including sponges, hydrogels and topical powders3,17-22 but no clinical research on the use of these products for human burns was identified in the literature search.
Clinical evidence on fish skin for wound healing
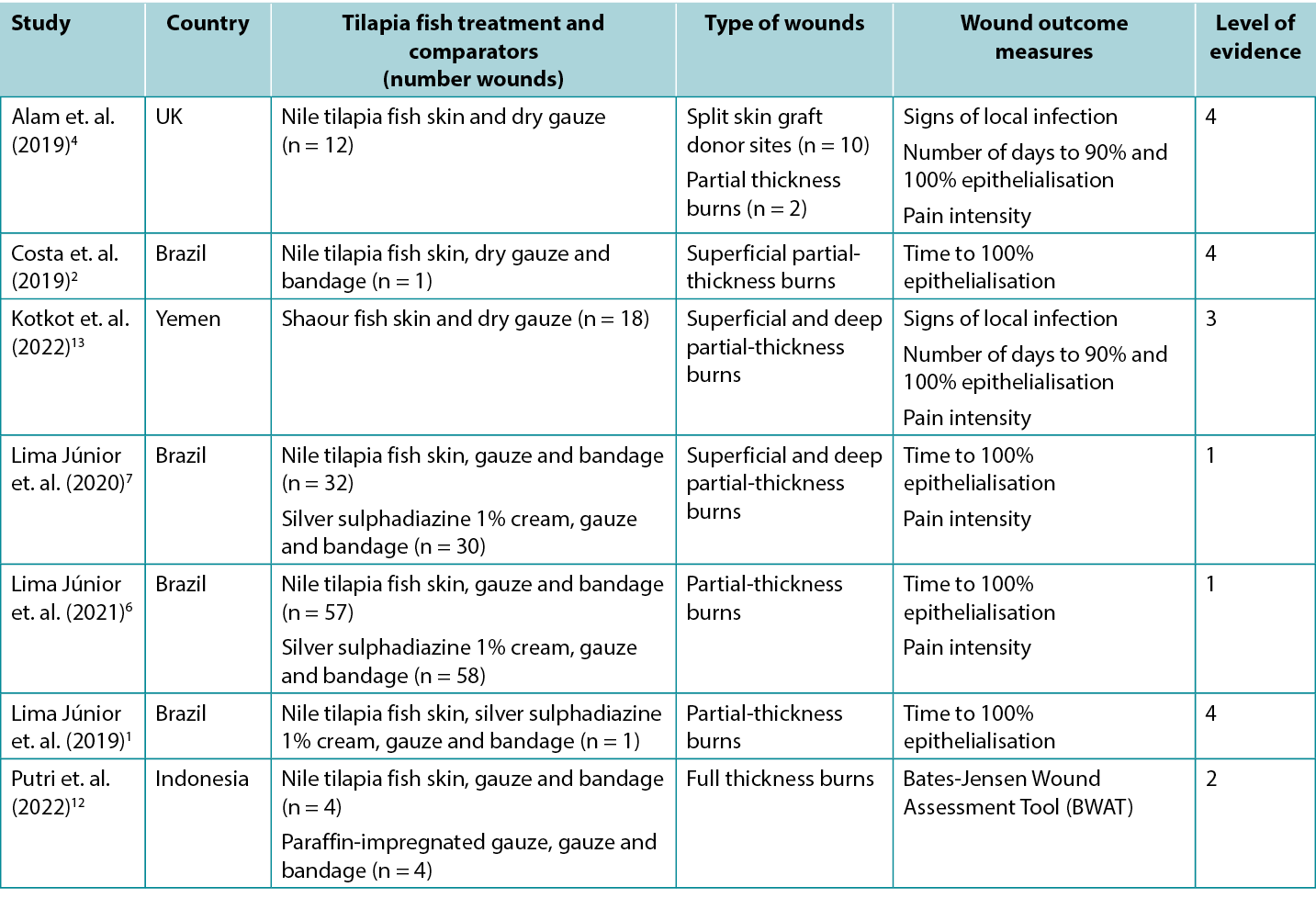
Studies reporting clinical outcomes for human burns treated with tilapia fish skin dressing and shaour fish skin dressing are summarized in Table 2. Half of the published evidence1, 2, 6, 7 was produced by one team in Brazil.
Table 2. Summary of the primary evidence for tilapia fish skin dressing for wound management
Fish skin for promoting healing in burns
The highest level of evidence comes from a meta-analysis5 at high risk of bias that included three studies1, 6, 7 (reported below). Pooled results showed tilapia fish skin dressing was associated with shorter time for partial-thickness burns to reach complete epithelialisation (standard mean difference [SMD] –0.903, 95% confidence interval [CI] −1.45 to −0.355, p<0.001) (Level 1). The primary studies all showed positive outcomes for healing with fish skin dressings:
- One RCT7 at moderate risk of bias, compared tilapia fish skin dressing with silver sulphadiazine 1% cream for treating partial thickness burns. The study had three arms based on the depth and extent of the participants burns (arm A: superficial second-degree burns to less than 10% of the body [n = 23]; arm B: superficial second-degree burns to 10–20% of the body [n = 19] and arm C: deep second-degree burns to 5–15% of the body [n = 20]). After light debridement and cleansing with a topical antimicrobial, the treatment group in each arm received a tilapia fish skin dressing, gauze and a bandage. Every 48 hours the secondary dressing was removed to check the fish skin dressing was correctly adhered. The control group in each arm received the local standard care regimen (silver sulphadiazine 1% cream, gauze and a bandage, changed every 48 hours). In all three study arms, complete epithelialisation was achieved significantly faster in burns treated with the tilapia fish dressing (mean difference between treatment arm and control arm ranged from 1.43 to 3.20 days, p < 0.05 in all arms)7 (Level 1).
- In an RCT6 at high risk of bias the same research team extended their research in individuals with partial thickness burns. Individuals with burns to up to 10% of the body that occurred no more than 72 hours prior and had not yet received treatment were eligible for the trial if they had no product sensitivity or significant co-morbidities. The treatment and control dressing regimens were the same as in the RCT reported above. The treatment group experienced faster healing (mean days: 10.2 ± 0.9 versus 9.7 ± 0.6; p = 0.001) Although the outcomes were statistically significant, the difference between the two regimens could not be considered clinically significant6 (Level 1).
- In a comparative study12 at high risk of bias, tilapia fish skin was applied to acute, non-infected full thickness limb burns (n = 4) following surgical sharp debridement. The fish skin dressings were changed every five days. The outcomes were compared to those for burns on the contralateral limbs that received the local standard care (paraffin-impregnated gauze changed every three days). The mean Bates-Jensen Wound Assessment Tool (BWAT) scores on day 10 were not different (fish skin: 18.75 ± 1.25 versus control: 30.5 18.75 ± 0.9) (Level 2).
- An observational study (n = 18)13 at high risk of bias, reported the effectiveness of shaour fish skin for treating partial-thickness burns. After preparation, the fish skin was applied to the burn area and secured with dry gauze. The fish skin dressing was replaced at day seven and day 15. The mean time to 90% epithelialisation was 11.05 ± 2.57 days (range 7–15) and the mean time to 100% epithelialisation was 17.27 ± 2.05 days (range 13–21). No cases experienced signs of local infection or allergic reaction13 (Level 3).
- A case series4 at high risk of bias reported the use of tilapia fish skin in split-skin graft donor sites for people (n =10) who had experienced burns. The fish skin was soaked in saline and applied directly to the donor sites, held in place with gauze. Dressings were changed on day 7 and then every three days. The average time to complete epithelialisation was 11.5 days (range 10–16) and the mean pain score on a VRS (0–10) at day 7 was 2.3 (range 1–4). In this study, an additional two participants received tilapia skin dressing applied to partial thickness burns, with complete epithelialisation observed at two weeks. No cases experienced signs of local infection (Level 4).
- Several case reports at high risk of bias describe the successful use of fish skin to treat partial thickness burns,1,2 including burns in babies and young children.2 In these case reports, healing occurred without complication in 10 to 17 days1, 2 (Level 4).
Wound-related pain outcomes with fish skin dressing
Findings on the impact of tilapia fish skin dressing on wound-related pain are inconclusive. First, pain was only reported on unidimensional scales measuring pain intensity, and in many studies it was not clear when the pain assessment was conducted. When the results from three studies1, 6, 7 were pooled in a meta-analysis,5 tilapia fish dressing was associated with lower pain intensity but the result was not significant (standard mean difference on a 10cm visual analogue scale (VAS) –0.608, 95% CI −0.885 to −0.331, p = 0.54) (Level 1). The following results were reported in the primary research:
- Participants in the three-armed RCT7 reported pain intensity using a 10cm VAS. There was no significant difference between pain intensity for tilapia fish skin dressing and silver sulphadiazine 1% cream in the arm in which participants had superficial second-degree burns to less than 10% of the body (p > 0.05). In the arms in which participants had superficial second-degree burns to 10–20% of the body or deep second-degree burns to 5–15% of the body, those receiving tilapia fish skin dressing reported lower pain intensity immediately after dressing change than those receiving silver sulphadiazine 1% cream (p < 0.005 for all wound dressing changes in both arms)7 (Level 1).
- Participants in the second RCT6 reported more rapid reduction in burn-related pain intensity (p < 0.001) with a tilapia fish skin dressing compared to a control group receiving silver sulphadiazine 1% cream (Level 1).
- In the observational study (n = 18),13 the mean pain rating on a Verbal Rating Scale (VRS, 0–10) was 6.94 ± 0.72 (range 6–8) at day 7, and this decreased statistically significantly (p < 0.001) to 5.22 ± 0.64 (range 4–6) at day 15 (Level 3).
- Individuals treated with fish skin dressing in other studies reported the dressing was comfortable.4, 12
Considerations for use
Consider local policies, procedures, and licensing before implementing traditional wound treatments.
Preparation
In the clinical studies,1, 2, 6 the fish skin was sterilised using a chemical process followed by gamma irradiation and stored in sterile packaging under refrigeration prior to use. After preparation, the product can be stored in refrigerated sterile packaging for up to two years.2
Clinical use
- In clinical use,1,6,13 burns were lightly debrided (if indicated) and then cleansed in sterile saline or a topical antimicrobial solution before fish skin was applied. The fish skin covered the entire wound or burn, including approximately 1cm of healthy peri-wound skin. The fish skin was covered with dry gauze with or without additional bandaging. In one study2 the fish skin was washed in sterile 0.9% saline for 5 minutes three times immediately before its application to the burn.
- In most clinical reports, the fish skin dressing was checked every few days to ensure the fish skin adhered to the burn, but the fish skin was not replaced.1,6,7 As the fish skin dries, it sloughs from the wound bed. At this stage, moistening the area (e.g., in the shower or using a cleansing solution) can assist in lifting the fish skin, revealing new epithelisation.1 In other reports, the fish skin dressing was replaced after 5 to 7 days.4,12,13
- Fish skin dressing may be inappropriate for some anatomical regions, including the face, neck and groin, due to difficulty achieving adequate adherence on skin folds.2,7,15
Cost effectiveness
- Several sources1,7,12 suggested that fish skin dressing is cost effective because the dressing does not need frequent replacement. In most reports in this evidence summary the fish skin was not replaced; in one study the fish skin dressing change was changed weekly13 In the pooled results5 from three studies1,6,7, tilapia skin dressing was associated with fewer dressings (SMD −4.195, 95% CI −5.615 to −2.774, p = 0.074) but the result was not significant (Level 1).
- In an RCT6, there were significantly lower costs associated with using tilapia fish skin dressings compared with silver sulphadiazine cream ($11 ± $1 versus Brazilian $19± $1; dollars in 2020), related to lower costs for dressing materials and analgesia (Level 1).
Adverse effects
Most of the research1,2,5-7 included in this evidence summary reported no adverse events associated with fish skin dressings. In one small study12, two of the participants died due to septic shock deemed not related to either the fish skin dressing or the comparison paraffin-impregnated gauze dressing they were receiving.
Conflicts of interest
The author declares no conflicts of interest in accordance with International Committee of Medical Journal Editors (ICMJE) standards.
About WHAM evidence summaries
WHAM evidence summaries provide a summary of the best available evidence on specific topics and make suggestions that can be used to inform clinical practice. Evidence contained within this summary should be evaluated by appropriately trained professionals with expertise in wound prevention and management, and the evidence should be considered in the context of the individual, the professional, the clinical setting and other relevant clinical information.
WHAM evidence summaries are developed using methodology consistent with that published by Joanna Briggs Institute8-11. Evidence underpinning a WHAM recommendation is identified via a PICO search strategy, assigned a level of evidence and evaluated for risk of bias. All WHAM evidence summaries are peer-reviewed by an international Expert Reference Group. For more information on the methods and the WHAM Expert Reference Group, visit the website: www.WHAMwounds.com.
Copyright © Wound Healing and Management Collaborative, Curtin University, and the authors.
WHAM证据总结:鱼皮用于治疗烧伤
Emily Haesler
DOI: 10.33235/wcet.44.1.44-48
临床问题
鱼皮用于治疗烧伤的最佳可用证据是什么?
概述
在中低资源环境中,鱼皮一直被用作治疗烧伤和其他伤口的低成本传统生物敷料。鱼皮具有较高的胶原蛋白浓度和抗张强度1-4,因此用作异种移植材料。当前尚无足够的临床证据证明其愈合效果,无法为使用鱼皮治疗烧伤的建议提供支持。存在高偏倚风险的1级证据5-7表明,与当地标准护理(最常见的是磺胺嘧啶银乳霜,每两天更换一次)相比,使用鱼皮敷料可能会更快实现完全愈合,但在大多数研究中,愈合时间的差异可以忽略不计,并且可能不具有临床意义。与更好地控制疼痛强度的有效性相关的1级证据5-7显示结果不一。然而,没有研究报告称鱼皮敷料劣于当地标准护理,也没有报告称不良事件是一个问题,一些低等级证据表明接受鱼皮敷料的患者对治疗效果感到满意。
临床实践建议
采用任何建议时,应考虑伤口、患者、专业医护人员和临床环境。
| 尚无足够的证据支持使用鱼皮辅料来促进伤口愈合的建议。 |
证据来源:检索和评价
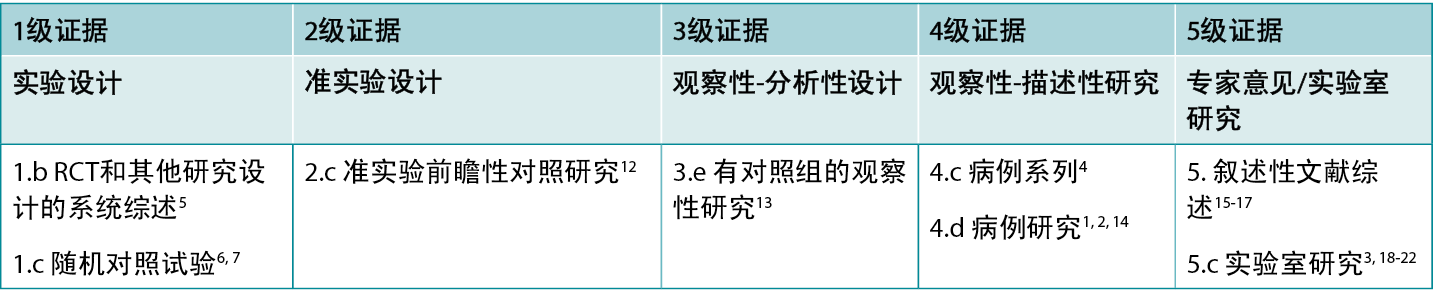
本总结是采用乔安娜 布里格斯研究所公布的方法进行的8-11。本总结基于系统性文献检索,结合了与鱼皮、烧伤和愈合相关的检索词。在以下数据库中搜索了截至2024年1月31日用英语发表的有关鱼皮用于治疗人体烧伤的证据:护理与联合卫生文献累积索引(CINAHL)、Medline(Ovid)、谷歌学术、Embase(Ovid)、AMED和卫生互联网共享研究成果倡议(Hinari,通过Research4Life访问)和Cochrane图书馆。。表1报告了干预研究的证据等级。
表1.临床研究的证据等级
背景
在低资源社区,某些类型的鱼皮因与人体皮肤相似而被用作伤口敷料。鱼皮具有高胶原蛋白浓度、高抗性和高抗张强度1-4。此外,鱼皮还具有抗病毒、抗菌和抗氧化特性,并含有丰富的不饱和脂肪酸,这可能有助于増强烧伤治疗的疗效4, 16。
本证据总结中的临床研究重点关注天然鱼皮直接应用于烧伤部位的用途(通常经过灭菌处理)。鱼皮以异种移植的形式附着在伤口床上,在伤口愈合期间保护伤口床,减少所需敷料更换次数。这有可能减少医疗保健资源,减轻伤口相关疼痛1, 2, 4, 5, 12, 15。
实验室研究也描述了从鱼皮中提取胶原蛋白并将其用于商业伤口敷料产品的情况,包括海绵、水凝胶和局部外用粉末3,17-22,但在文献检索中未发现关于将这些产品用于人体烧伤的临床研究。
鱼皮用于伤口愈合的临床证据
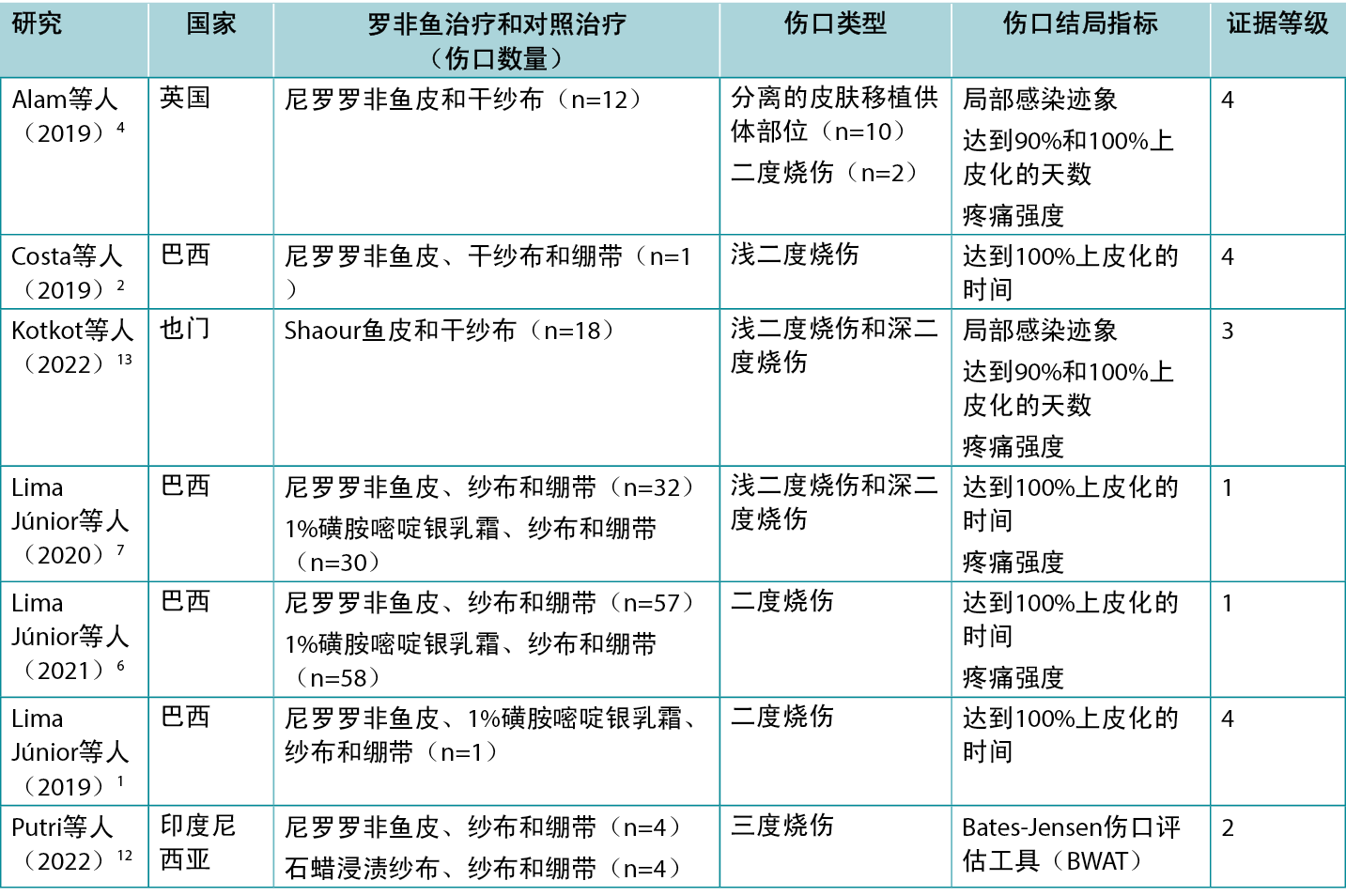
使用罗非鱼皮敷料和Shaour鱼皮敷料治疗人体烧伤的临床效果请参见表2。已发表的证据1, 2, 6, 7中有一半由一个巴西团队提供。
表2.罗非鱼皮敷料用于管理伤口的主要证据总结
鱼皮用于促进烧伤愈合
最高级别的证据来自一项偏倚风险较高的荟萃分析5,其中包括三项研究1, 6, 7(报告如下)。汇总结果显示,罗非鱼皮敷料可缩短二度烧伤达到完全上皮化的时间(标准均值差[SMD]为-0.903,95%置信区间[CI]为-1.45至-0.355,p<0.001)(1级)。初级研究均显示,鱼皮敷料对伤口愈合有积极作用:
- 一项存在中度偏倚风险的RCT7比较了罗非鱼皮敷料和1%磺胺嘧啶银乳霜治疗二度烧伤的效果。该研究根据受试者烧伤的深度和范围将其分为三组(A组:身体10%以下浅二度烧伤[n=23];B组:身体10%-20%浅二度烧伤[n=19];C组:身体5%-15%深二度烧伤[n=20])。在用局部外用抗菌剂进行轻度清创和清洁后,上述每个组中的治疗组均需接受罗非鱼皮敷料、纱布和绷带。每隔48小时移除二次敷料,以检查鱼皮敷料是否正确粘附。每组的对照组接受当地标准护理方案(1%磺胺嘧啶银乳霜、纱布和绷带,每48小时更换一次)。在所有三个研究组中,使用罗非鱼敷料治疗的烧伤达到完全上皮化的速度明显更快(治疗组与对照组之间的均值差为1.43天至3.20天不等,p<0.05)7(1级)。
- 在一项偏倚风险较高的研究6中,同一研究团队对二度烧伤患者进行了扩展研究。烧伤面积不超过身体的10%、发生时间不超过72小时且尚未接受治疗的患者,如果未出现产品敏感性或严重合并症,则有资格参加试验。治疗和对照敷料方案与上文报告的RCT中使用的方案相同。治疗组的伤口愈合速度更快(平均天数:10.2±0.9 vs 9.7±0.6;p=0.001),虽然结果具有统计学意义,但认为两种治疗方案之间的差异不具有临床意义6(1级)。
- 在一项偏倚风险较高的对比研究12中,罗非鱼皮用于手术锐性清创后的急性、非感染性三度肢体烧伤(n=4)。每五天更换一次鱼皮敷料。将其结局与接受当地标准护理(石蜡浸渍纱布,每三天更换一次)的对侧肢体烧伤的结局进行了比较。第10天的Bates-Jensen伤口评估工具(BWAT)平均得分无差异(鱼皮:18.75±1.25 vs对照:30.5 18.75±0.9)(2级)。
- 一项偏倚风险较高的观察性研究(n=18)13报告了Shaour鱼皮治疗二度烧伤的疗效。准备工作完成后,在烧伤部位应用鱼皮,并用干燥纱布固定。在第7天和第15天更换鱼皮敷料。达到90%上皮化的平均时间为11.05±2.57天(范围:7-15天),达到100%上皮化的平均时间为17.27±2.05天(范围:13-21天)。所有病例均未出现局部感染或过敏反应迹象13(3级)。
- 一份偏倚风险较高的病例系列4报告称,在烧伤患者(n=10)的分皮移植供体部位使用了罗非鱼皮。鱼皮浸泡在生理盐水中,直接应用于供体部位,并用纱布固定。在第7天更换敷料,之后每三天更换一次。达到完全上皮化的平均时间为11.5天(范围:10-16天),第7天VRS(0-10分)疼痛评分的平均值为2.3(范围:1-4分)。在这项研究中,另有两例受试者接受了罗非鱼皮敷料,用于二度烧伤,两周时观察到完全上皮化。所有病例均未出现局部感染迹象(4级)。
- 多份偏倚风险较高的病例报告描述了使用鱼皮治疗二度烧伤1,2(包括婴幼儿烧伤)的成功案例2,在这些病例报告中,10至17天内伤口痊愈,且无并发症1, 2(4级)。
鱼皮敷料治疗伤口相关疼痛的效果
罗非鱼皮敷料对伤口相关疼痛的影响尚无定论。首先,仅采用测量疼痛强度的单维度量表报告了疼痛,并且许多研究中并未明确何时进行了疼痛评估。在一项荟萃分析中汇总三项研究1, 6, 7的结果时5,发现罗非鱼敷料可降低疼痛强度,但结果并不显著(10 cm视觉模拟量表(VAS)的标准均值差为-0.608,95% CI为-0.885至-0.331,p=0.54)(1级)。初级研究报告了以下结果:
- 三臂RCT7的受试者使用10 cm VAS报告了疼痛强度。在身体10%以下浅二度烧伤的受试者组中,罗非鱼皮敷料和1%磺胺嘧啶银乳霜治疗在疼痛强度方面不存在显著差异(p>0.05)。在身体10%-20%浅二度烧伤或身体5%-15%深二度烧伤的受试者组中,接受罗非鱼皮敷料治疗的患者在更换敷料后立即报告的疼痛强度低于接受1%磺胺嘧啶银乳霜治疗的患者(对于两组中的所有伤口敷料更换,p<0.005)7(1级)。
- 与接受1%磺胺嘧啶银乳霜的对照组相比,第二项RCT6的受试者报告称,使用罗非鱼皮敷料能更快减轻与烧伤有关的疼痛强度(p<0.001)(1级)。
- 在该观察性研究(n=18)13中,第7天时,口头评定量表(VRS,0-10)的平均疼痛评分为6.94±0.72(范围:6-8),第15天时,疼痛评分出现具有统计学意义的降低(p<0.001),降至5.22±0.64(范围:4-6)(3级)。
- 在其他研究中,接受鱼皮敷料治疗的患者报告称敷料较为舒适4, 12。
使用注意事项
进行传统伤口治疗前,考虑当地政策、程序和许可情况。
制备
在临床研究中1, 2, 6,先采用化学方法并随后用伽马射线照射,对鱼皮进行灭菌,使用前在冷藏条件下将其储存在无菌包装中。制备完成后,产品可在冷藏无菌包装中储存长达两年2。
临床使用
- 在临床应用中1,6,13,烧伤部位需进行轻度清创(如需要),然后用无菌生理盐水或局部外用抗菌溶液清洗,最后应用鱼皮。鱼皮覆盖了整个伤口或烧伤部位,包括约1 cm的伤口周围健康皮肤。用干燥纱布覆盖鱼皮或不进行额外包扎。在一项研究2中,在烧伤部位应用鱼皮之前,先用0.9%无菌生理盐水清洗5分钟,重复清洗三次,然后应用至烧伤部位。
- 在大多数临床报告中,会每隔几天对鱼皮敷料进行检查,以确保鱼皮紧贴烧伤部位,但不会更换鱼皮1,6,7。鱼皮变干时,会从伤口床脱落。在这一阶段,润湿患处(如淋浴或使用清洁溶液)有助于揭开鱼皮,露出新的上皮1。在其他报告中,鱼皮敷料均在5至7天后更换4,12,13。
- 鱼皮敷料很难在皮肤褶皱处达到足够的粘附性,因此其可能不适用于某些解剖部位,包括面部、颈部和腹股沟2,7,15。
成本效益
- 一些资料来源1,7,12表明,鱼皮敷料无需经常更换,因此具有成本效益。在本证据总结的大多数报告中,鱼皮未进行更换;在一项研究中,鱼皮敷料每周更换一次13。在三项研究1,6,7的汇总结果5中,罗非鱼皮敷料与敷料减少相关(SMD -4.195,95% CI -5.615至-2.774,p=0.074),但结果不显著(1级)。
- 在一项RCT6中,使用罗非鱼皮敷料的相关成本与磺胺嘧啶银乳霜相比明显更低(11±1美元 vs 19±1美元[巴西];基于2020年美元货币价值),这与敷料材料和镇痛的成本较低有关(1级)。
不良反应
本证据总结中包含的大多数研究1,2,5-7均未报告与鱼皮敷料相关的不良事件。在一项小型研究12中,有两例受试者因感染性休克而死亡,认为这两起事件与受试者接受的鱼皮敷料或对照石蜡浸渍纱布敷料不相关。
利益冲突
根据国际医学期刊编辑委员会(ICMJE)的标准,作者声明无利益冲突。
关于WHAM证据总结
WHAM证据总结提供了关于特定主题的最佳可用证据的总结,并提出了可用于指导临床实践的建议。本总结中包含的证据应由经过适当培训的具有伤口预防和管理专业知识的专业人士进行评价,并应根据个人、专业人士、临床环境以及其他相关临床信息考虑证据。
WHAM证据总结的编写方法与乔安娜 布里格斯研究所8-11公布的方法一致。通过PICO检索策略识别支持WHAM建议的证据,划分证据等级并评价偏倚风险。所有WHAM证据总结均经过国际专家参考小组的同行评审。有关该方法和WHAM专家参考小组的更多信息,请访问以下网站:www.WHAMwounds.com。
版权所有˝ 科廷大学伤口愈合和管理协作组织以及相关作者。

Author(s)
Emily Haesler
PhD P Grad Dip Adv Nurs (Gerontics) BN FWA
Adjunct Professor, Curtin University, Curtin Health Innovation Research Institute, Wound Healing and Management (WHAM) Collaborative
References
- Lima-Júnior EM, de Moraes Filho MO, Costa BA, Fechine FV, de Moraes MEA, Silva-Junior FR, Soares MFAdN, Rocha MBS, Leontsinis CMP. Innovative treatment using tilapia skin as a xenograft for partial thickness burns after a gunpowder explosion. J Surg Case Rep, 2019; 6: rjz181.
- Costa BA, Lima Júnior EM, de Moraes Filho MO, Fechine FV, de Moraes MEA, Silva Júnior FR, do Nascimento Soares MFA, Rocha MBS. Use of tilapia skin as a xenograft for pediatric burn treatment: A case report. J Burn Care Res, 2019; 40(5): 714-7.
- Ge B, Wang H, Li J, Liu H, Yin Y, Zhang N, Qin S. Comprehensive assessment of Nile tilapia skin (Oreochromis niloticus) collagen hydrogels for wound dressings. Marine Drugs. 2020; 18(4).
- Alam K, Jeffery SLA. Acellular Fish skin grafts for management of split thickness donor sites and partial thickness burns: A case series. Mil Med, 2019; 184(Suppl 1): 16-20.
- Cadri S, Elrosasy A, Al Mawla AM, Albakri K, Abdelwahab OA, Soliman A, Jaradat B, Cadri N, Alabdallat YJ, Negida A. The efficacy of Nile tilapia skin xenograft for treating superficial partial-thickness burn versus the standard of care: a meta-analysis of published trials. Arch Dermatol Res, 2023; 316(1): 33.
- Lima Júnior EM, de Moraes Filho MO, Costa BA, Fechine FV, Vale ML, Diógenes AKL, Neves KRT, Uchôa A, Soares M, de Moraes MEA. Nile tilapia fish skin-based wound dressing improves pain and treatment-related costs of superficial partial-thickness burns: A phase III randomized controlled trial. Plast Reconstr Surg, 2021; 147(5): 1189-98.
- Lima Júnior EM, De Moraes Filho MO, Costa BA, Rohleder AVP, Sales Rocha MB, Fechine FV, Forte AJ, Alves A, Silva Júnior FR, Martins CB, Mathor MB, Moraes MEA. Innovative burn treatment using tilapia skin as a xenograft: A phase II randomized controlled trial. J Burn Care Res, 2020; 41(3): 585-92.
- Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. https://synthesismanual.jbi.global: Joanna Briggs Institute, 2020.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. New JBI Grades of Recommendation. Adelaide, Australia: Joanna Briggs Institute, 2013.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. Supporting Document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation. Adelaide, Australia: Joanna Briggs Institute, 2014.
- Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: A streamlined rapid review approach. Worldviews Evid Based Nurs, 2015;12(3):131-8.
- Putri N, Kreshanti P, Syarif A, Duhita G, Johanna N, Wardhana A. Efficacy of tilapia skin xenograft compared to paraffin-impregnated gauze as a full-thickness burn dressing after excisional debridement: A case series. Int J Surg Case Rep, 2022; 95(107240): 107240.
- Kotkot A, Ghabisha S, Ahmed F, Al-wageeh S, Al-shami E, Al-hajri A, Aljbri W, Mohammed F. Fish skin as a biological dressing for burn injuries. Journal of Emergency Medicine, Trauma and Acute Care, 2022; 2022(4).
- Riaz Z. Treatment of human skin burns through using tilapia skin. Bull. Biol. All. Sci. Res., 2021;6:24.
- Luze H, Nischwitz SP, Smolle C, Zrim R, Kamolz LP. The use of acellular fish skin grafts in burn wound management. A systematic review. Medicina (Kaunas), 2022; 58(7).
- Esmaeili A, Biazar E, Ebrahimi M, Heidari Keshel S, Kheilnezhad B, Saeedi Landi F. Acellular fish skin for wound healing. Int Wound J, 2023; 20(7): 2924-41.
- Afifah A, Suparno O, Haditjaroko L, Tarman K. Utilisation of fish skin waste as a collagen wound dressing on burn injuries: a mini review. IOP Conference Series: Earth and Environmental Science, 2019; 335(1): 012031.
- Lima-Verde MEQ, Parthiban SP, Júnior AECF, De Barros Silva PG, Junior EML, De Moraes MO, De Paulo Aragão Sabóia V, Bertassoni LE, Alves APNN. Nile tilapia fish skin, scales, and spine as naturally derived biomaterials for tissue regeneration. Current Oral Health Reports, 2020;7(4):335-43.
- Li D, Sun WQ, Wang T, Gao Y, Wu J, Xie Z, Zhao J, He C, Zhu M, Zhang S, Wang P, Mo X. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Materials Science and Engineering: C, 2021; 127: 112202.
- Wang T, Yang L, Wang G, Han L, Chen K, Liu P, Xu S, Li D, Xie Z, Mo X, Wang L, Liang H, Liu X, Zhang S, Gao Y. Biocompatibility, hemostatic properties, and wound healing evaluation of tilapia skin collagen sponges. Journal of Bioactive and Compatible Polymers, 2020; 36(1): 44-58.
- Yang L, Chen K, Liu P, Kang Y, Shen S, Qu C, Gong S, Liu Y, Gao Y. Preparation of Nile tilapia skin collagen powder by low-temperature and comprehensive evaluation of hemostasis and wound healing. Int J Artif Organs, 2023; 46(2): 99-112.
- Zhou T, Wang N, Xue Y, Ding T, Liu X, Mo X, Sun J. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids and Surfaces B: Biointerfaces, 2016; 143: 415-22.



