Volume 44 Number 1
Wound bed preparation 2024: Delphi consensus on foot ulcer management in resource-limited settings
Hiske Smart, R Gary Sibbald, Laurie Goodman, Elizabeth A Ayello, Reneeka Jaimangal, John H Gregory, Sadanori Akita, Afsaneh Alavi, David G Armstrong, Helen Arputhanathan, Febe Bruwer, Jeremy Caul, Beverley Chan, Frans Cronje, Belen Dofitas, Jassin Hamed, Catherine Harley, Jolene Heil, Mary Hill, Devon Jahnke, Dale Kalina, Chaitanya Kodange, Bharat Kotru, Laura Lee Kozody, Stephan Landis, Kimberly LeBlanc, Mary MacDonald, Tobi Mark, Carlos Martin, Dieter Mayer, Christine Murphy, Harikrishna Nair, Cesar Orellana, Brian Ostrow, Douglas Queen, Patrick Rainville, Erin Rajhathy, Gregory Schultz, Ranjani Somayaji, Michael C Stacey, Gulnaz Tariq, Gregory Weir, Catharine Whiteside, Helen Yifter, Ramesh Zacharias
Keywords Diabetes, wound bed preparation, low-resource settings, foot ulcer, rural, delphi consensus, low- and middle-income countries, innovation
For referencing Smart H et al. Wound bed preparation 2024: Delphi consensus on foot ulcer management in resource-limited settings. WCET® Journal 2024;44(1):13-35.
DOI 10.33235/wcet.44.1.13-35
Abstract
Background Chronic wound management in low-resource settings deserves special attention. Rural or underresourced settings (ie, those with limited basic needs/healthcare supplies and inconsistent availability of interprofessional team members) may not be able to apply or duplicate best practices from urban or abundantly resourced settings.
Objective The authors linked world expertise to develop a practical and scientifically sound application of the wound bed preparation model for communities without ideal resources.
Methods A group of 41 wound experts from 15 countries reached a consensus on wound bed preparation in resource-limited settings.
Results Each statement of 10 key concepts (32 substatements) reached more than 88% consensus.
Conclusions The consensus statements and rationales can guide clinical practice and research for practitioners in low-resource settings. These concepts should prompt ongoing innovation to improve patient outcomes and healthcare system efficiency for all persons with foot ulcers, especially persons with diabetes.
General purpose
To review a practical and scientifically sound application of the wound bed preparation model for communities without ideal resources.
Target audience
This continuing education activity is intended for physicians, physician assistants, nurse practitioners, and nurses with an interest in skin and wound care.
Learning objectives/putcomes
After participating in this educational activity, the participant will:
- Summarise issues related to wound assessment.
- Identify a class of drugs for the treatment of type II diabetes mellitus that has been shown to improve glycemia, nephroprotection, and cardiovascular outcomes.
- Synthesise strategies for wound management, including treatment in resource-limited settings.
- Specify the target time for edge advancement in chronic, healable wounds.
Introduction
A framework for wound bed preparation (WBP) was introduced in 2000 to emphasise treating the whole person as a foundation of optimal local wound care.1 As this has evolved into an international framework, it became clear that not all wounds are healable. These concepts of maintenance and nonhealable wounds led to revisions of local wound care principles and WBP expansion. The importance of integrated coordinated care with nurses, doctors, and allied healthcare professionals working together to optimise patient care outcomes and healthcare system utilisation was paramount in further WBP developments.
This article focuses on applying the WBP framework to manage foot-related wounds, particularly in persons with diabetes (PWDs), leprosy-related neuropathic foot ulcers, and other complications that include neuropathy and vascular disease. Several parameters are critical to PWDs, including poor glycemic control, BP changes, high cholesterol, inadequate plantar pressure redistribution, infections, and lack of exercise. The effects of smoking are also particularly detrimental to saving limbs and lives of PWDs.
Critical for this article is a set definition for resource-restricted settings, including low resource availability; lacking or restricted funding; remote, isolated, or rural settings; and Indigenous populations. These terms all relate to healthcare settings that may have challenges accessing supplies, equipment, specialists, and advanced wound care competencies and skills. Low-resource settings can be present anywhere in the world and are not limited to lower-income or developing countries.
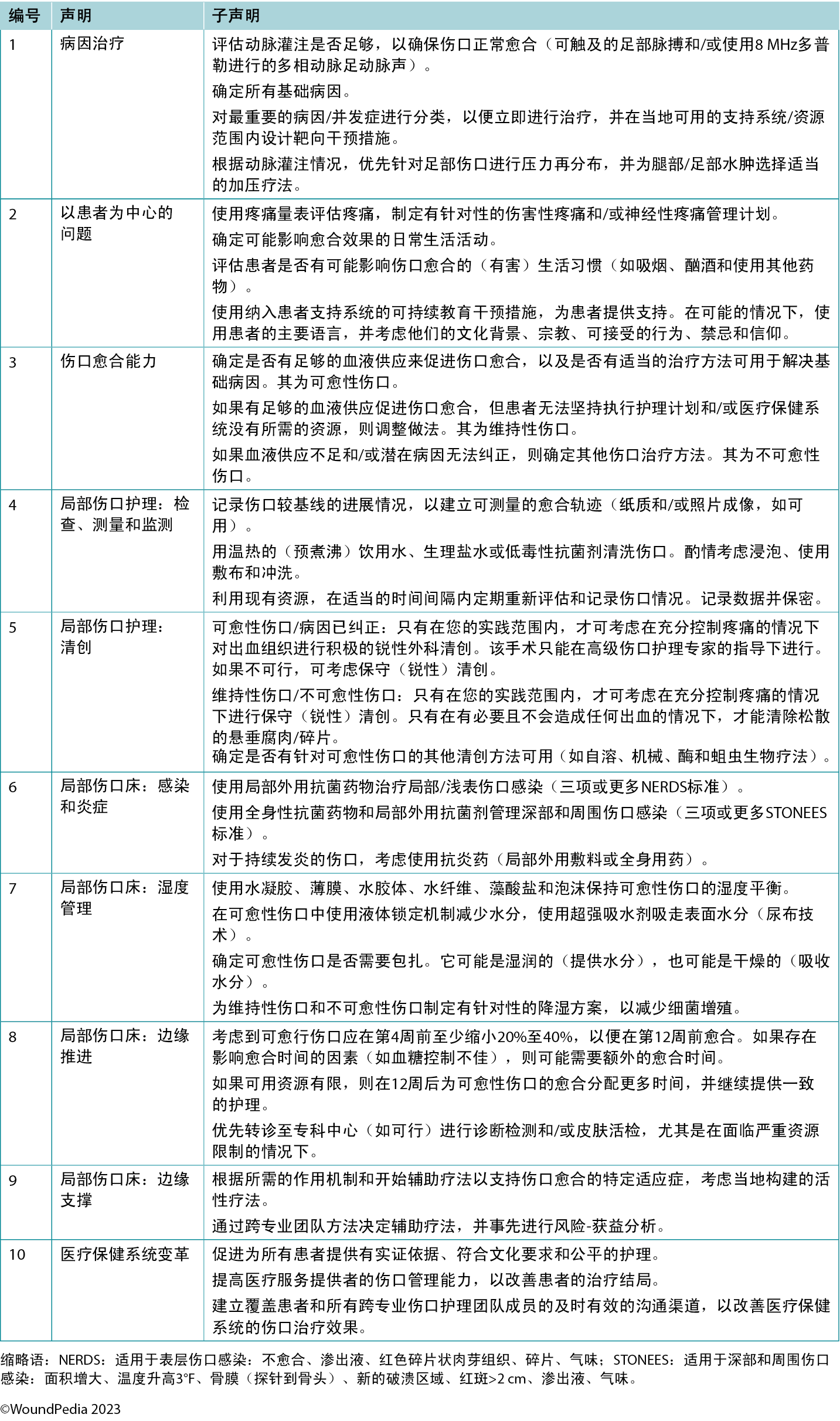
The Delphi process underpinning this work expanded and developed the WBP framework in its current format. Forty-one authors drawn from 15 countries took part in the Delphi process, which occurred over two rounds using a four-part Likert-type scale (1, strongly agree; 2, agree; 3, disagree; 4, strongly disagree). The first round consisted of 29 statements. Although all statements exceeded the desired 80% consensus level, there were 299 comments considered by the lead group of authors. A professional editor was employed to improve the comprehension and grammatical accuracy of the statements before a Delphi round 2 was deployed with 32 constructed statements. All statements exceeded 88% consensus level.
In round 2, 14 statements achieved 100% consensus. One statement stood out and was rated as “strongly agree” by all Delphi panel members: 10C. Establish timely and effective communication that includes the patient and all interprofessional wound care team members for improved healthcare system wound outcomes. In parallel, each of the international wound experts worked in groups to develop the manuscript content. Refer to Supplemental Table 1 (http://links.lww.com/NSW/A176) for the consensus statements.
The consensus aimed to set a scientifically based minimal standard of care that could be optimised for available resources. Refer to Table 1 for the 10 key steps and 32 substatements for wounds in limited-resource environments. This consensus process updated the framework for WBP to be applicable regardless of resource availability. In addition, it also includes the concepts of healing trajectories and healthcare system change for the first time (Figure 1).
The remainder of this report will highlight the 10 consensus statements and discuss the rationale for each statement.
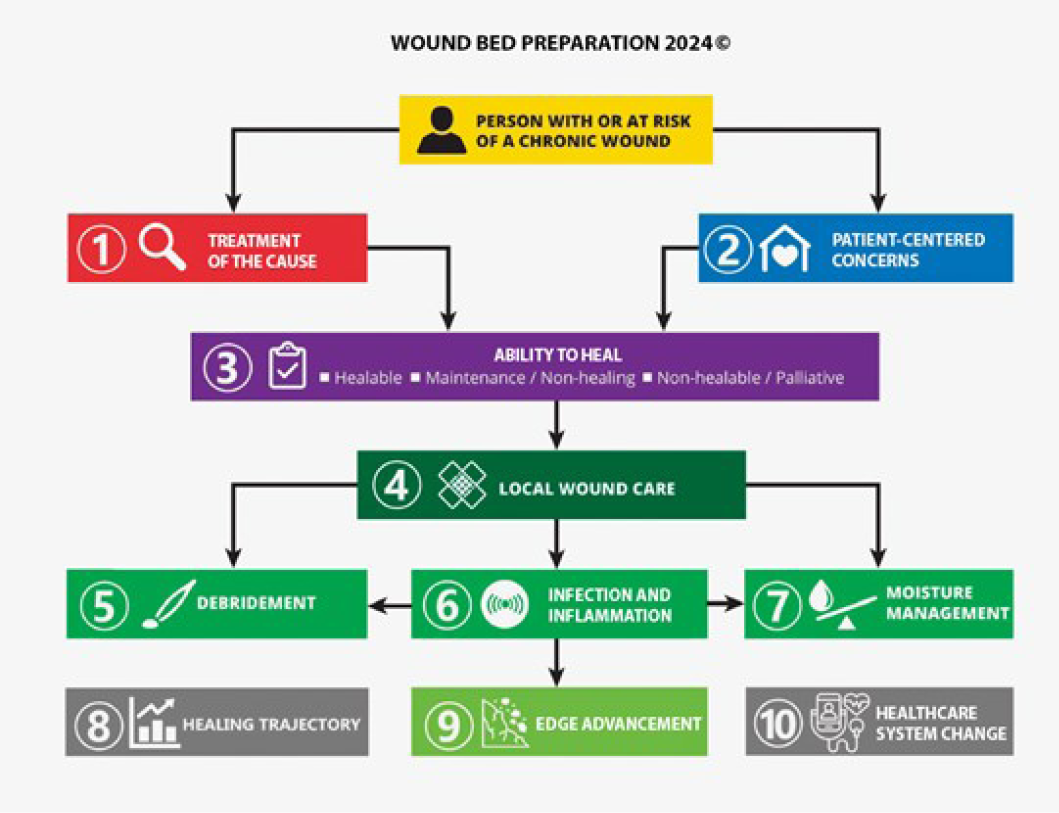
Figure 1. Wound bed preparation 2024. ©WoundPedia 2023
Table 1. Wound bed preparation for wounds below the knee in environments with limited resource availability
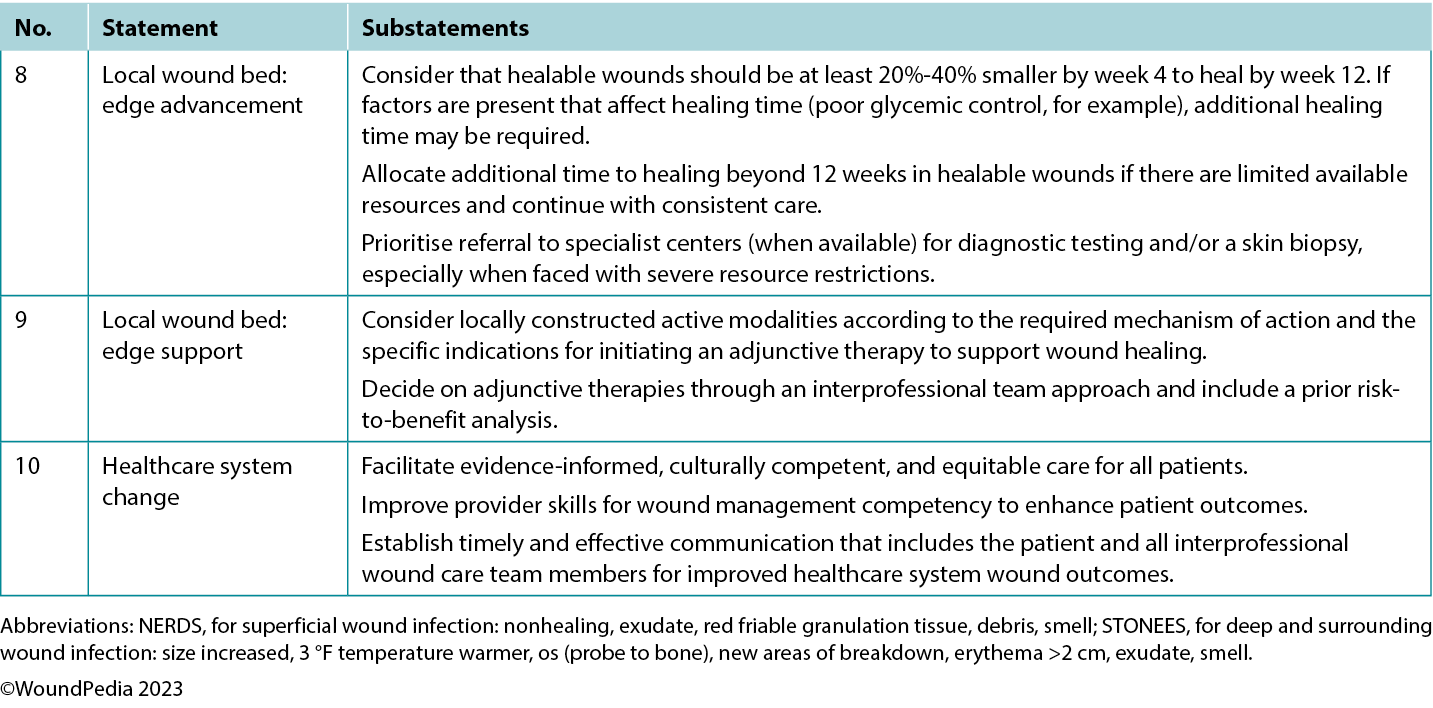
Statement 1: treatment of the cause
1A. Assess for adequate arterial perfusion to ensure proper wound healing (palpable foot pulse[s] and/or multiphasic arterial foot artery sounds with an 8-MHz Doppler)
To determine lower-limb blood flow, find a palpable foot pulse(s) as a vital first action. Start with the dorsalis pedis and/or the posterior tibial pulses. If an 8-MHz handheld Doppler is available, confirm multiphasic flow patterns (biphasic/triphasic). Refer to vascular specialists when a monophasic or absent Doppler sound is observed or foot pulses are not palpable. Other signs of inadequate arterial perfusion are lower limb pain at rest and ischemic limb changes (cold extremity with dependent rubor that blanches on elevation).
Persons with diabetes are susceptible to microvascular issues (peripheral neuropathy, Charcot foot changes) and macrovascular complications including peripheral arterial disease (PAD). These conditions contribute to calluses, foot ulcers, and mixed tissue loss. Because up to 50% of susceptible populations experience both diabetes and PAD,2 timely identification of PAD is critical (using physical examination and vascular tests) because it is a major risk factor for poor ulcer healing and amputation. If detected, immediate revascularisation (angioplasty or vascular bypass) is vital to restore proper arterial flow to the foot. Additional assessment may include capillary refill time, a Buerger blanching test (pale on elevation, bright red rubor on dependency), and claudication with walking.
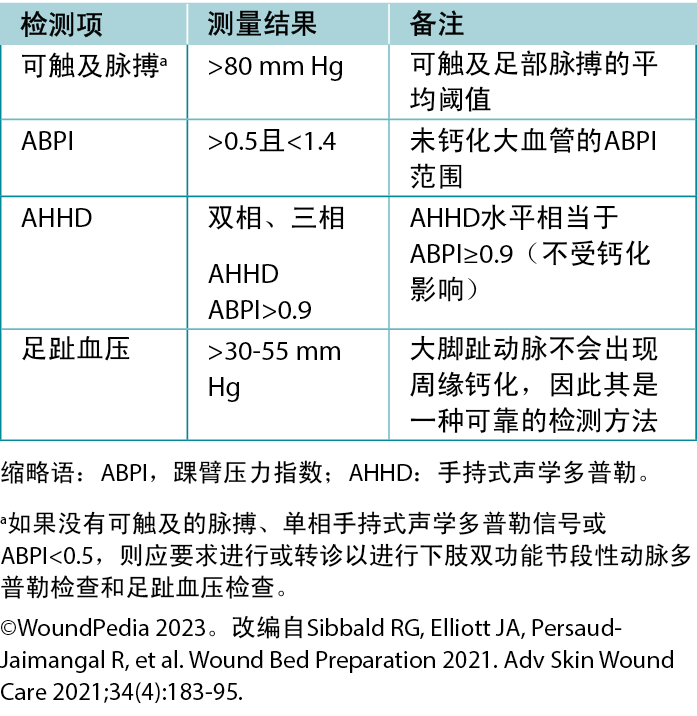
Vascular examination. In cases of arterial insufficiency, the extremity is often cool to the touch because of insufficient nutrient and oxygen supply (Table 2). Severe cases may manifest tissue necrosis presenting as ulcers, macerated toe webs (often with secondary infection), fissures, or gangrene. Other severe case indicators include pallor upon leg elevation, exercise-induced claudication that resolves with rest, dependent cyanosis or rubor, and muscle atrophy. Notably, lower extremity edema is more indicative of venous rather than arterial issues. Arterial lesions are generally punched out, with a deep base that often contains tendons, whereas venous ulcers show irregular border morphology with a shallow granulation tissue base.3
Table 2. Vascular supply needed for tissue healing
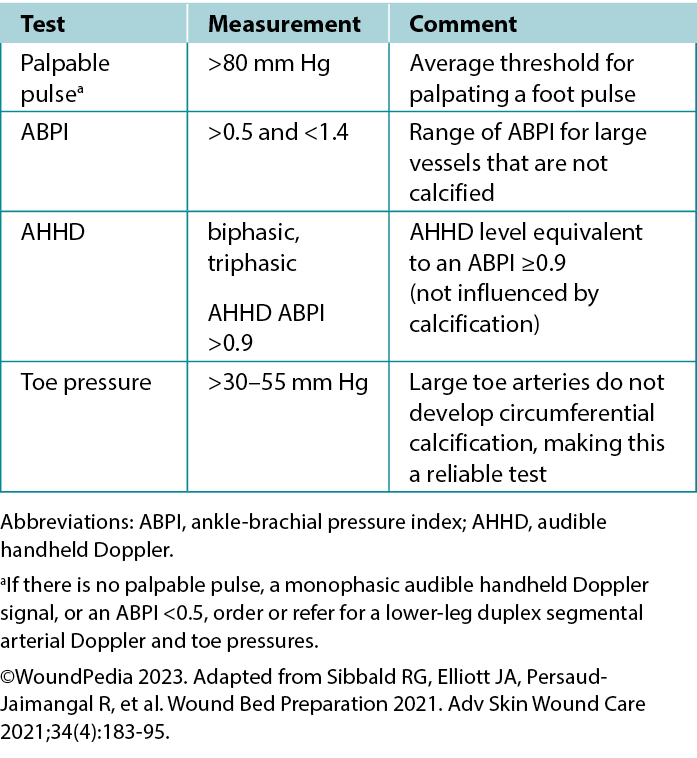
Ankle-brachial pressure index (ABPI) examination. The ABPI measures the ratio of ankle systolic BP divided by the brachial systolic BP using an 8-MHz Doppler. The procedure involves using a BP cuff and recording systolic BP when arterial sounds reappear after cuff inflation. However, factors such as edema, inflammation, and arterial calcification can affect its accuracy. If an 8-MHz Doppler cannot be afforded/obtained, early referral to a tertiary assessment center becomes the priority in cases where the absent foot pulses are present. In certain healthcare settings, an ABPI is still required as vital quantification assessment before beginning any lower limb intervention.
Audible handheld Doppler (AHHD) examination. An AHHD can easily be added as an additional parameter in certain settings, should providers choose a simpler and faster test (not influenced by calcification, no need to squeeze a painful calf, and no need to be recumbent for 20 minutes). The AHHD assessment also can provide accurate results in case of large toe amputation and be recorded as an MP3 or MP4 file and transferred for remote verification of the signal interpretation.
Healthcare professionals should apply gel on the appropriate foot pulse sites with an 8-MHz Doppler probe positioned at a 45° angle to the skin on the dorsalis pedis, posterior tibial, and peroneal arteries. The acquired Doppler signals/waveforms can then be analysed (either by audible sound or visual tracings): A comprehensive tutorial on the AHHD procedure is available online at https://journals.lww.com/aswcjournal/Pages/videogallery.aspx?videoId=20. Optimise signal quality with careful repositioning of the probe to obtain the loudest or most multiphasic signal.
A monophasic or absent waveform warrants a comprehensive vascular assessment, including a Duplex segmental lower leg arterial Doppler in the vascular laboratory. A multiphasic waveform typically indicates the absence of peripheral vascular disease.4 In PWDs, interpret the ABPI ratio with caution (due to arteriosclerosis or arterial calcification); AHHD multiphasic findings are a preferable choice to confirm adequate blood supply for wound healing. A multiphasic waveform (biphasic, triphasic) suggests an AHHD value equivalent to a normal ABPI ≥0.9.
Although AHHD is effective in excluding arterial disease, it may not identify existing segmental perfusion deficits—islands of ischemia or angiosome defects.5 Therefore, physical examination of the foot and lower limb is vital for a conclusive diagnosis. Healthcare professionals can record AHHD signals and transmit them to specialists, facilitating either synchronous or asynchronous assessments remotely. Synchronous assessments enable real-time patient involvement and prompt decision-making.
Chronic venous insufficiency. Chronic venous insufficiency may coexist in PWDs and those with foot ulcers. It mainly affects the lower limbs and impairs the return of deoxygenated blood to the heart and lungs. This condition often arises from venous valve dysfunction that can be triggered by factors including pregnancy or weight gain. Symptoms commonly include varicose veins, edema, skin discoloration from hemosiderin, lipodermatosclerosis, and venous ulcers.6 These ulcers can be any size (from small to circumferential) and form quickly over areas of venous pooling, usually on the medial aspects of the lower limbs.
The cornerstone of treatment for venous ulcers is compression therapy. This compensates for valve dysfunction by enhancing the peristaltic action of the calf muscle pump. Additional measures include leg elevation and walking. Venous ablative procedures may be considered when ulcers are attributed to superficial veins. Untreated venous edema can delay foot ulcer healing.7
For the medical optimisation of PAD, key strategies include optimal BP control, initiating cholesterol medication, and, often, starting statin therapy. Recent studies also recommend treating patients with PAD and concurrent coronary artery disease or carotid disease using a combination of low-dose aspirin (91-100 mg PO daily) and low-dose rivaroxaban (2.5 mg PO BID).8 Additional modifiable factors are smoking cessation and walking or exercise programs. For patients with a foot ulcer (especially in PWDs), appropriate plantar pressure redistribution or offloading is essential.
1B. Identify all underlying causes
Foot complications are of great concern to PWDs and represent a significant burden of disease to healthcare systems. A holistic approach is represented by the mnemonic AIM (assessment, identification, management) and focuses on treating or mitigating the underlying cause of diabetic foot issues, especially neuropathy. The application of a focused assessment approach as a baseline standard of care (VIPS: vascular, infection, pressure, surgical debridement) is crucial in preventing severe complications, including foot ulcerations, lower limb amputations, and an increased incidence of early/preventable death. Some critical foot-related elements in resource-challenged settings include late presentations to formal care, delayed diagnosis,9 barefoot walking, neglected wounds, and the absence of preventive foot care. A hospital-based observational analysis conducted in Ethiopia identified several factors contributing to diabetic foot complications;10 these included high humidity, foot deformity, neuropathy, unidentified active ulcers, inadequate or ill-fitting footwear, poor foot hygiene (eg, foot and toenail fungus), and lack of foot care awareness. A systematic review on plantar ulcers among patients with leprosy (n = 7 studies) identified the following risk factors for developing ulcers: inability to feel a 10 g monofilament on sensory testing, severe foot deformities or hyperpronation, lower education, and unemployment.11 Addressing these challenges in any resource-challenged environment requires a multipronged approach that may include patient and healthcare professional education, early detection, access to care, footwear programs, and community engagement.
Regular and thorough foot assessments include detecting neuropathy (loss of protective sensation), vascular issues (poor or absent lower limb blood circulation), signs of infection, areas of high pressure (callus formation), and friction (blisters, often with hemorrhagic components) to facilitate timely interventions. The simplified 60-second screening tool may be a valuable means to quickly assess, stratify, and follow up patients based on their risk levels without significant cost.12 Patients with a history of ulceration, amputation, peripheral vascular surgery, or Charcot neuroarthropathy are at the highest risk for skin breakdown and should receive additional attention to prevent ulceration and further complications.
To detect infection, the NERDS (superficial wound infection: nonhealing, exudate, red friable granulation tissue, debris, smell) or STONEES (deep and surrounding wound infection: size increased, 3 °F temperature warmer, os [probe to bone], new areas of breakdown, erythema >2, exudate, smell) criteria along with using noncontact infrared thermometry can be helpful.13 Elevated temperatures of 3 °F compared with the opposite limb may signal inflammation and a foot at a higher risk of ulceration.13 A comparative change of 1.67 °C is difficult to measure clinically. This same finding in a person with a lower leg or foot ulcer is eight times more likely to signify deep and surrounding infection when accompanied by two or more additional STONEES criteria.13
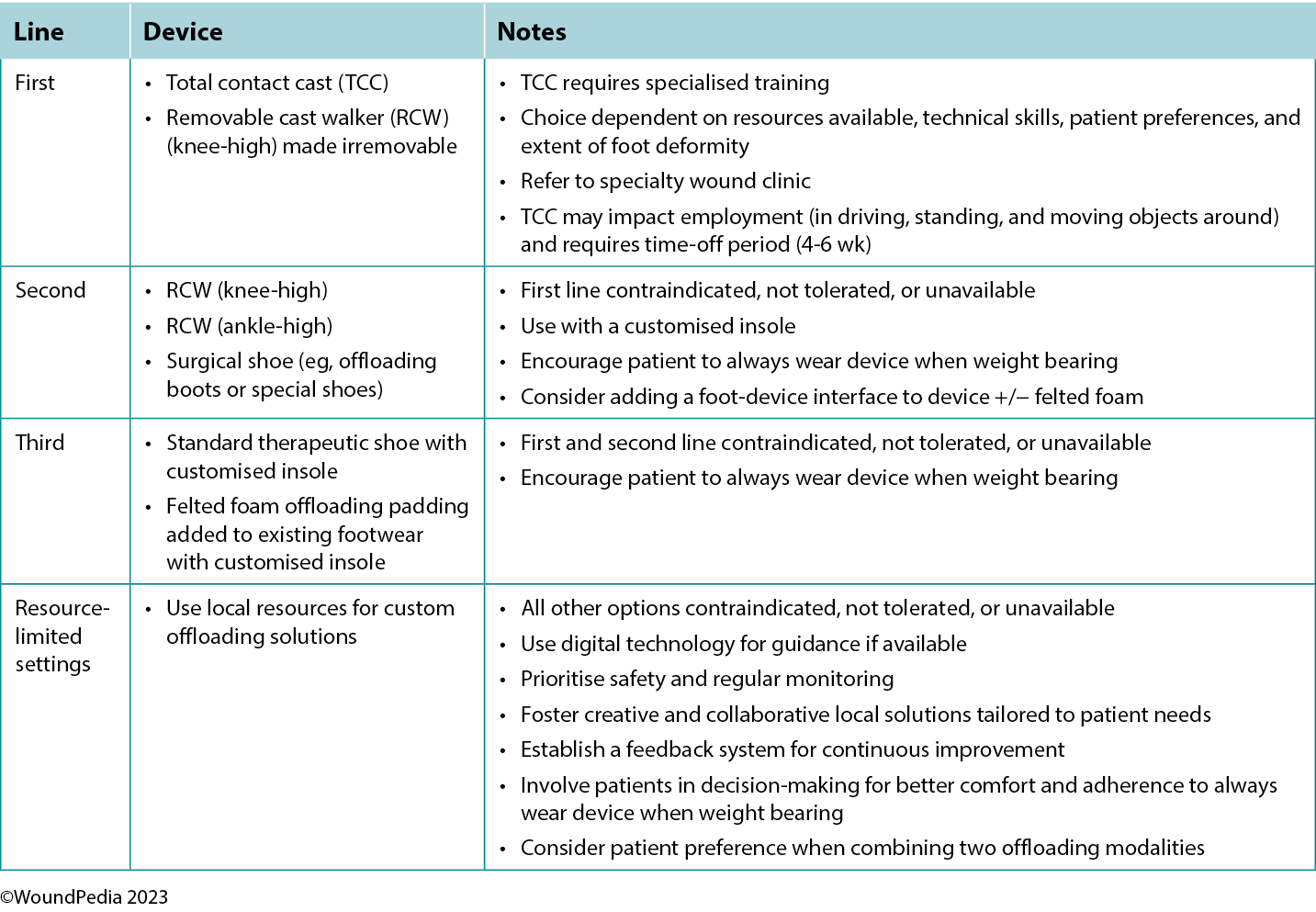
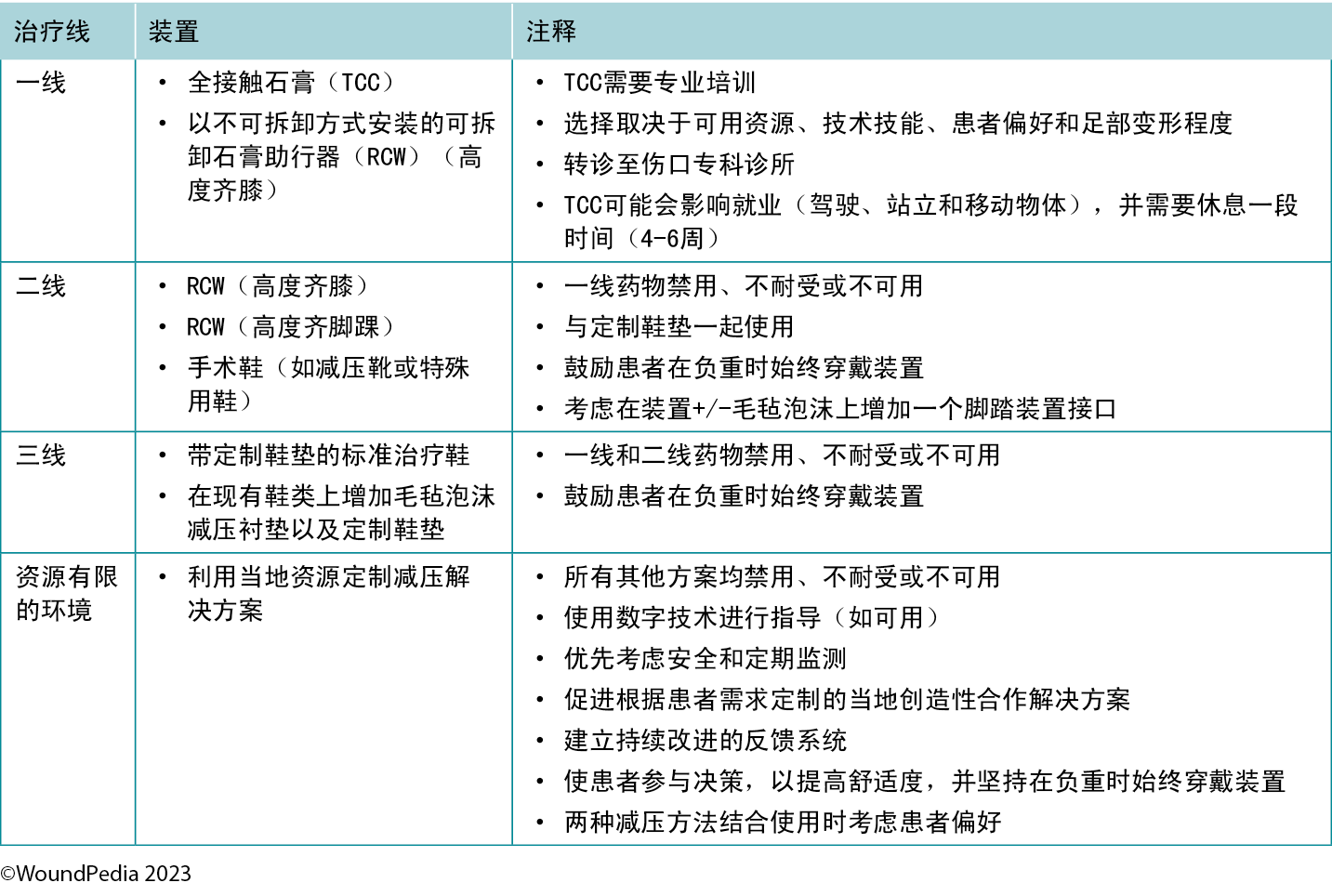
Patients with neuropathy without an ulcer and presenting with a hot swollen foot could have acute Charcot neuroarthropathy. Infrared thermometry is a valuable assessment tool in these cases: acute Charcot feet may be 8 to 15 °F warmer than the mirror image on the opposite foot. These patients require a comprehensive medical history, physical examination, and radiographic images to facilitate early detection. Further actions include the application of a total contact cast for stabilisation and complete plantar pressure offloading with a wheelchair to prevent further bone deterioration and prevent lower limb amputation (Table 3).
Table 3. Offloading interventions for a plantar diabetic/neuropathic foot wound28
Callus formation in PWDs is positively correlated with pressure and shear stress. In individuals with diabetic neuropathy, various factors including foot deformities, limited joint mobility, repetitive stress during walking, and poorly fitting shoes can increase the risk of callus formation.14 Moreover, the presence of calluses can pose a significant risk because repetitive trauma may lead to subcutaneous hemorrhage and eventually progress to ulceration. Provide tailor-made pressure redistribution devices (soft insoles, cobbler interventions to adapt shoes, and reduced barefoot walking) to prevent subsequent callous-to-ulcer progression.
Different etiologic causes may lead to foot ulcers in PWDs: neuropathic because of peripheral neuropathy, ischemic associated with PAD, or a combination of both—neuroischemic foot complications. The presence of diabetic neuropathy is established from medical history; physical examination (5.07/10 g monofilament test); altered sensation distributed symmetrically in both limbs (stocking and glove distribution); and burning, stinging, shooting, or stabbing pain.
1C. Triage the most important causes/comorbidities to treat immediately and design targeted intervention(s) within locally available support systems/resources
A diabetic foot ulcer (DFU) occurs in 25% to 34% of PWDs and is one of the most feared complications, potentially leading to lower limb amputation, severe disability, and reduced life expectancy.15 Multifactorial in origin, diabetes-related peripheral neuropathy and PAD leave feet highly vulnerable to traumatic injury. Access to timely diagnosis and intervention are determinant factors in effective management and limb preservation.
Chronic hyperglycemia measured by elevated hemoglobin A1c (HbA1c) is a major risk factor for sensory, motor, and autonomic neuropathy. Both PAD and dry skin increase foot vulnerability to infection and delayed healing, all contributing to poor outcomes. Chronic kidney disease adds to the risk.16 Recent studies of continuous glucose monitoring report that high glucose variability (reduced time to target range levels) may additionally contribute to long-term complications.17
Up to 50% of PWDs will develop neuropathy with no known cure. Management includes proper daily foot inspections for evidence of trauma or infection, foot care, and effective glucose control.18 In addition to neuropathy, PAD contributes equally to DFUs; PAD is largely asymptomatic and may remain underdiagnosed and untreated for a prolonged period. Individuals with diabetes have more than a twofold increased prevalence of PAD compared with those without.19 A systematic review of community-based studies for global prevalence and risk factors for PAD ranked diabetes highest next to smoking.20
The European Society of Hypertension recommends providers and patients target a systolic BP less than 130 mm Hg and diastolic BP less than 80 mm Hg. In PWDs, systolic BP should not fall less than 120 mm Hg to prevent reduced blood flow to vital organs and lower limbs.21 Although diuretics, calcium-channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and β-blockers can all be used, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers reduce cardiovascular events.22,23 Recently, SGLT2 inhibitors have shown excellent results in improving glycemia, nephroprotection, and cardiovascular outcomes.24 In addition, DFU incidence is reduced by early detection of PAD with lifestyle modifications.17
The complexity of managing a DFU requires an interprofessional team approach to identify biological, social, geographic, and cultural determinants of health. In Denmark, PWDs are registered by region with access to specialised clinics with interprofessional wound care teams. Lower limb amputation rates have declined significantly due to improved diabetes care, regular foot inspection, better self-care, and timely treatment.25 In more geographically dispersed diverse populations (eg, Ontario, Canada), significant disparities in amputation rates exist, highest in rural regions where timely prevention such as revascularisation surgery and foot care specialists are underserviced or absent.
The most vulnerable persons for diabetic foot-related amputations in Canada are Indigenous people, immigrants, and persons living in rural and northern regions.26 The Indigenous Diabetes Health Circle provides a culturally sensitive approach to local Ontario First Nations communities with education and knowledge about diabetes, wellness, and self-management. Its holistic foot care program supports a continuum of services that connects community members to Indigenous agency partners and local healthcare professionals and is proven to reduce DFU incidence and prevent amputations.27
1D. Prioritise redistribution of pressure for foot wound(s) and choose appropriate compression for leg/foot edema based on arterial perfusion
The criterion standard for plantar pressure redistribution devices is the total contact cast or the removable cast walker made irremovable.28 Even in healthcare systems in which these offloading modalities are readily available, less than 10% of eligible patients are fitted for and adherent to the use of these devices.29
Offloading is paramount to healing foot ulcers (Table 3). The aim is to select the best device for the patient incorporating patient-centered concerns, the goals of care, and best practice evidence. Consider creative solutions to repurpose local materials such as soft felted inserts for offloading in resource-limited settings. It is important to establish an early surveillance program between the patient and healthcare professional to monitor and ensure the desired offloading outcomes. Healthcare professionals need to evaluate the effectiveness of the devices and continually make modifications as needed through established follow-up. In regions lacking specialised practitioners, empowering healthcare workers with competencies in basic offloading can bridge this gap.
Statement 2: patient-centred concerns
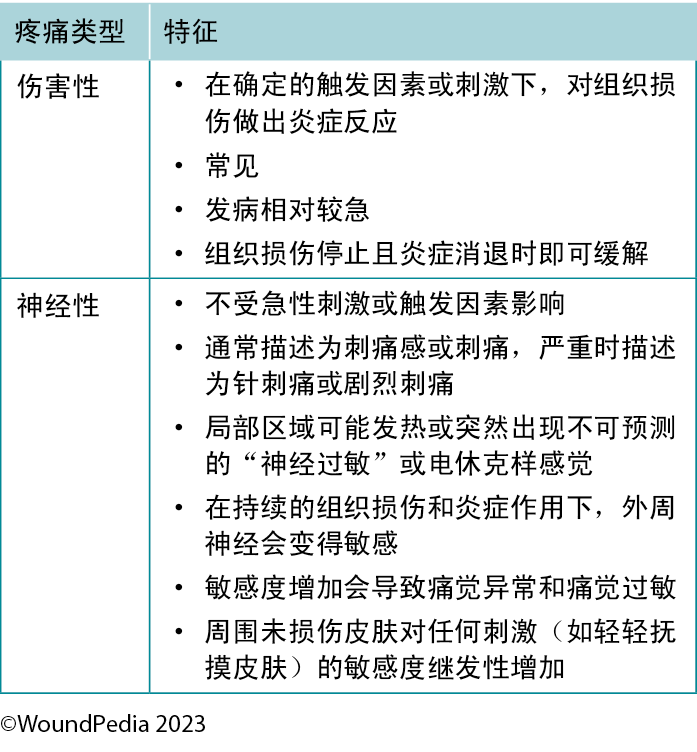
2A. Assess pain using a pain scale with a targeted plan for nociceptive and/or neuropathic pain management
The perception of pain involves a stimulus that may be physical or chemical. There are two main types of pain: nociceptive and neuropathic (Table 4). Wound-related pain is an important component of patient-centered concerns that is often undervalued by healthcare professionals. By the late 1990s, wound pain was a focus for healthcare professionals with the launch of a pivotal position document focused on wound pain.30 The document recognised and focused on the distress of chronic wound pain and its influence on patients’ health-related quality of life. The management of wound pain was then integrated into the WBP framework.1,31
Table 4. Pain types and responses30
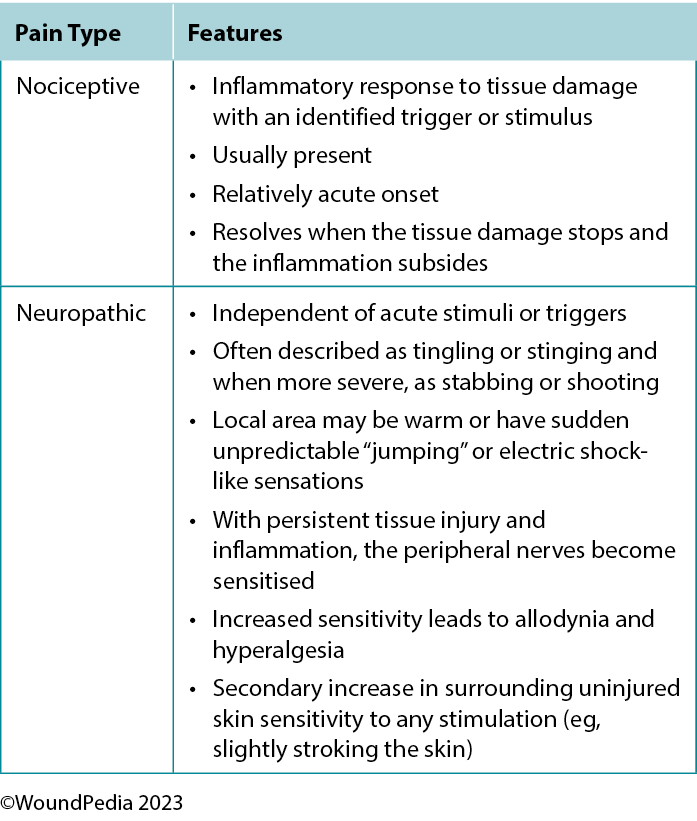
Pain can play a significant role in the total management of persons with wounds and their ultimate healing success.32 Pain signals associated with injury play an important function in patient wellness and as such must be acknowledged through adequate assessment and management. For example, pain or any change in pain is a key predictor of wound infection and one of the four cardinal signs of inflammation.1 Unresolved pain is often associated with delayed wound closure.
Assessment. A pain history is essential to wound pain management.32 Assessment must include the nature, onset, duration, and exacerbating and relieving factors. This will help determine the cause of the pain and direct its management. Pain intensity can be reliably measured using validated pain scales. A verbally administered 0- to 10-point numerical rating scale is a good first choice for measuring pain numerical intensity. Most patients can function with a pain level of 3 to 4 out of 10.33
Patients with persistent pain should be reassessed regularly for improvement, deterioration, and adherence to medication regimens. The use of a pain diary with entries regarding pain intensity, medications used, mood, and response to treatment may be a good management strategy. For individuals with communication limitations, the pain scale should incorporate pictures for easy recognition.
Management. The management of wound pain can be integrated into the WBP framework: treat the cause and address local wound factors and patient-centered concerns.3 Treating the cause should determine the correct diagnosis and initiate treatment of the wound pain. Patient-centered concerns must focus on what the patient sees as the primary reasons and resolutions for the pain. Patient anticipatory pain and suffering can be just as disruptive to quality of life as the actual experience of pain.
Multiple pain management strategies exist and are not always pharmaceutical (Table 5). Consider total patient management, including all aspects of WBP, in choosing a management strategy. Remember: pain is what the patient says it is.34
Table 5. Pain management strategies
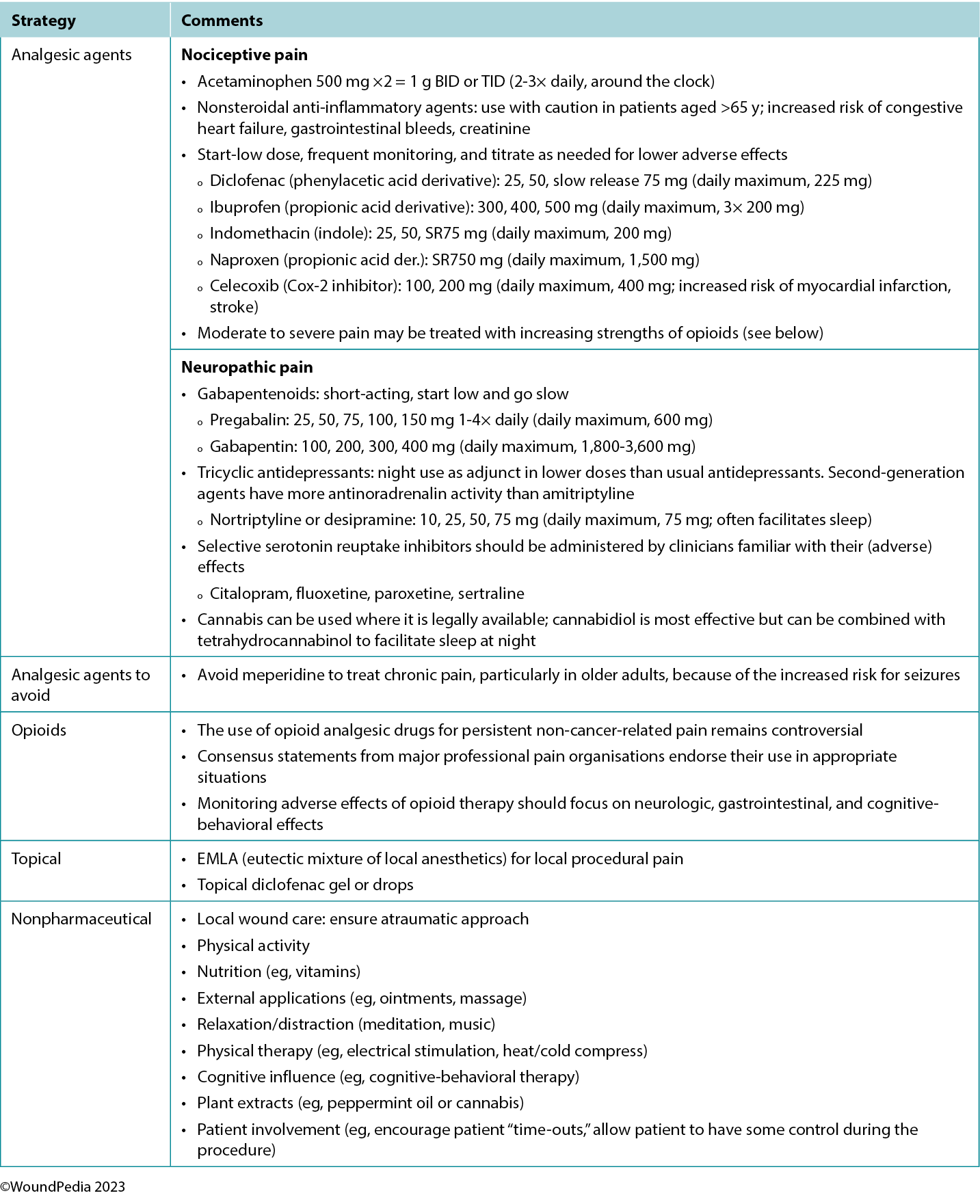
Assessment and management given limited resources. In regions with limited resources, assessment remains possible because it requires no costly tools. Most pain scales are freely available despite some initial cost relative to education and training of practitioners.31 Treatment options for pain in resource-restricted environments may be more practical if driven with nonpharmacologic methods (Table 5). Some of these options may also be more socially acceptable or already practiced culturally (eg, meditation, plant-based treatments) and particularly applicable in nociceptive pain management. Implementing such strategies can then preserve pharmaceutical management for those with pain that cannot be managed by nonpharmacologic means (eg, neuropathic pain).35
2B. Identify activities of daily living that may affect healing outcomes
2C. Assess patients for (harmful) lifestyle habits that may impact wound healing (eg, smoking, alcohol, and other substances)
2D. Empower patients using sustainable educational interventions that include their support system. When possible, use the patient’s primary language and consider their cultural background, religion, accepted behaviors, taboos, and beliefs
In resource-limited environments, patient-centered concerns and barriers to clinical outcomes are crucial for providers to understand and address within the context of cultural, spiritual, and religious beliefs of the respective community.
Wound healing disparities exist among Indigenous populations across the globe. These disparities are often rooted in historical and ongoing socioeconomic, cultural, and healthcare-related factors. Cultural diversity and societal pressure also often dictate formal resource allocation processes to certain health sectors. Some key barriers to wound healing among Indigenous populations include historical trauma, socioeconomic disparities, limited access to healthcare, cultural barriers, chronic health conditions, cultural healing practices, geographic isolation, and healthcare system bias.36 Many cultures have an honor system in managing their sick, older adult, and chronically ill members. These principles are easier upheld if sufficient resources are available to maintain them.
Many studies have identified a major medical illness as one of the core reasons a family can incur large debts.37,38 Even in government-run clinics, additional dressing materials or medicines may be charged to the family. Patients and family members may therefore resort to alternative treatment methods (nonallopathic medicine) from local/Indigenous/traditional healers. Although often less costly, these healers may not have the necessary skills or expertise in managing chronic wounds, leading to deterioration.
Loss of independent mobility because of wound chronicity is another major factor that impacts attendance and regular follow-up. Transport availability varies in resource-restricted environments, and walking may be the only way to access a public transport pick-up point. Secluded rural environments are often affected the most and require traveling significant distances to reach a formal healthcare facility.
The social health of the family environment and willingness to incorporate a person with healthcare needs can drive the quality of care/self-care rendered as well as patient safety within a home environment. Because of the social structure and milieu in various countries, family support and social support may differ by culture. Often, as time passes, the financial burden increases; patient independence and activities of daily living (ADLs) achievement deteriorates; dressing changes become more challenging; and caregivers become stressed, fatigued, and exhausted.39 Late referrals and critical/terminal patient conditions when eventually presenting to formal care are additional aggravating social and behavioral factors that can compound poor wound healing outcomes.
Individuals with wounds treated at home need a designated space to themselves. Wound odor, persistent pain, and a different routine are the main stressors on both the patient and family in settings where space is at a premium. The mere presence of a major skin wound already negatively impacts the patient’s own social interactions, relationships, sexuality, and self-confidence. This leads to progressive anxiety and depression that may bring about pessimism regarding perceived treatment benefits. Subsequently, patients have lowered self-efficacy that is often associated with further wound deterioration including eventual lower-extremity amputations.40
The real challenge occurs when lifestyle modifications are needed. This often requires additional resources or tailor-made education to construct self-care plans.40,41 The need for health education and lifestyle modification interventions to have clear rationales cannot be overemphasised. Lifestyle interventions may become a financial, social, and logistical challenge, first to acquire and then to maintain within any restricted living domain. Further, it is a vital step to ensure that any financial help received is correctly allocated to the family member with a wound.
A biopsychosocial approach is necessary in managing wound care for patients in resource-restricted environments. The wound care team, beyond managing the wound, must address the social stressors/factors affecting the patient. Each patient will need a unique, mutually agreed-upon management plan that fits their constraints (medical, financial, family, social, and emotional support).40
Statement 3: ability to heal
3A. Healable wound: Determine if adequate blood supply is present for wound healing and that appropriate treatment is available to address the underlying cause(s)
3B. Maintenance wound: Adjust practice if adequate blood supply is present for wound healing but the patient cannot adhere to the plan of care and/or the healthcare system does not have the required resources
3C. Nonhealable wound: Determine alternative wound treatment(s) if blood supply is inadequate and/or the underlying cause cannot be corrected
The process of determining the healing classification of a wound begins with a thorough patient history and physical examination. Identifying the underlying cause(s) of the wound is important. Addressing the underlying cause(s) is the first step to developing an achievable management plan.
In some cases, chronic wounds may also become stalled and fail to achieve the wound edge advancement over the set time; these are known as hard-to-heal wounds. They often fall into the maintenance category but, with additional assessment, may be healable with a re-evaluation of the patient, history, causes, and treatment plan.3,42
Patient-centered concerns and expectations need to be identified, compared with, and aligned with institutional/clinician resource availability, skills, and immediate intervention options that are available.3,42 The management plan is based on the assigned healing classification, which may change.
Protect nonhealable wounds against tissue loss, deep and surrounding wound infection, and general deterioration from a wet wound environment. Decreasing moisture is a priority. Maintenance wounds also need protection against further tissue losses with dry wound bed management and local infection controls as the mainstay. Tissue protection may also be a temporary measure until resources become available and additional patient factors are controlled to achieve full optimisation.
Stalled but healable wounds (hard-to-heal) need a second chance to achieve edge advancement with urgency in reassessment and interprofessional team intervention as the highest priorities.3,42
Statement 4: local wound care: examine, measure and monitor
4A. Document wound progress from baseline to establish measurable healing trajectories (paper-based and/or photoimaging, if available).
4B. Cleanse wounds with tepid (preboiled) potable water, saline, or low-toxicity antiseptic agents. Consider soaks, compresses, and irrigation, where appropriate.
4C. Reassess and document wounds regularly at appropriate intervals over time with available resources. Document and maintain confidentiality of the data.
Wound assessment documentation is an integral component of healthcare practice. It is instrumental in ensuring the delivery of high-quality patient care, monitoring wound status, and providing direction for any changes in wound interventions. The comprehensive and accurate recording of wound assessments is essential to ensure improved patient outcomes, effective communication among healthcare professionals, and legal and regulatory compliance.43
Monitoring progress. Wound assessment documentation serves as a historical record of the wound’s progression over time. By regularly documenting wound characteristics, including size, depth, color, exudate, infection, tissue type, and so on, healthcare professionals can track changes, identify potential complications, and adjust treatment plans. If photo documentation is used, obtain patient consent according to organisational policy, ensure appropriate technique with adequate lighting and appropriate distance from the wound, and include a measuring guide in the photo.44 This ongoing evaluation is critical for evaluating the effectiveness of interventions and making informed decisions regarding wound management. When measuring surface area or volume, it is imperative to use a consistent technique regardless of technology.
Communication. Accurate wound assessments facilitate effective communication among healthcare professionals. When all team members have access to consistent and up-to-date wound documentation, they can work together to develop and implement a coordinated care plan, ensuring patients receive the best possible treatment. It is vital to use consistent terminology that is common to all disciplines. The International Classification of Diseases medical classification system is an example of a globally applicable standard.45
Legal and regulatory compliance. Proper wound assessment and documentation are essential to meet legal and regulatory requirements. Inaccurate or incomplete records can lead to legal issues and negatively impact a healthcare professional’s practice, even in resource-limited environments.
Reimbursement. In many healthcare systems, proper wound assessment documentation is tied to reimbursement-based funding models. Accurate and detailed records are often necessary to justify the use of specific wound care products or procedures and ensure organisations receive adequate reimbursement for services rendered.
Research and quality improvement. Wound assessment data are a valuable resource for research and quality improvement initiatives. This continuous learning process helps improve global wound care and patient outcomes.
Patient-centered care. Proper wound assessment and documentation are an essential component of patient-centered care. It ensures that patients’ conditions are thoroughly evaluated and their treatment plans are tailored to their specific needs. When patients observe that their healthcare provider is committed to documenting and monitoring their wounds, it can enhance trust and patient satisfaction.
To promote language consistency and enhance data clarity, an increasing number of enterprises are creating electronic wound assessment software and applications to reduce the time required for thorough documentation.43 These programs and applications (some more affordable than others) can provide the healthcare team with a structured set of parameters to ensure all clinical features are documented, thereby enhancing overall communication.46 Although these tools are an asset to healthcare professionals, they are not without risk, such as incorrect copying and pasting from previous reports/consult notes or patient data security risks. In areas where such programs and applications are not available, updating
the patient and providing written assessment and intervention plans may be warranted.
Statement 5: local wound care: debridement
5A. Healable wounds/cause corrected: Consider active sharp surgical debridement to bleeding tissue with adequate pain control only if it is within your scope of practice. This is undertaken with guidance from advanced wound care expertise only. If not available, consider conservative (sharp) debridement
5B. Maintenance wounds/nonhealable wounds: Consider conservative (sharp) debridement with adequate pain control only if it is within your scope of practice. Only remove loose hanging slough/debris when indicated and without causing any bleeding
5C. Determine if alternative debridement modalities for healable wounds are available (eg, autolytic, mechanical, enzymatic, and maggot biological options)
Debridement is an important process in the WBP paradigm to remove necrotic tissue and other biomaterials, including biofilm, in healable wounds and prevent odor and infection in maintenance wounds. For all wounds located below the knee, communicate the outcomes of any vascular testing (eg, ABPI, waveform) to all members of the interprofessional team, and document accordingly before attempting debridement because many types may be deleterious to the wound bed with reduced vascular supply.
For healable wounds, local wound bed interventions are best determined via the WBP paradigm based on the patient and wound characteristics. Consider surgical debridement (to bleeding tissue) as a first-line intervention. However, in many rural and remote regions, access to a skilled healthcare professional with the necessary education, knowledge, and judgment for this procedure may not exist.
Conservative (sharp) debridement (without causing bleeding) requires advanced knowledge and skills and is more suitable for nonacute care or specialty clinic settings. Only remove loose hanging or unattached debris from the wound without causing trauma to the wound bed.
Clinical sterile maggot debridement therapy is a limited treatment modality in resource-restricted environments, except in cases of accidental exposure. Maggot infestation is often discovered during dressing changes. Good debridement outcomes may result from accidental maggot therapy, particularly if the larvae are from the highly selective Lucilia sericata/cuprina bottlefly source, as they focus on devitalised tissue as their food source.47,48 Detrimental outcomes may present if the infestation is from the ordinary housefly (Musca domestica) or other invader species, as those larvae may indiscriminately destroy healthy tissue.47,48
Healthcare professionals should assess alternative methods of debridement (eg, autolytic, enzymatic, mechanical) for community sectors, including primary care, home care, and long-term care. Within available resources it is vital to consider patient safety, assess environmental factors, and identify barriers to wound healing before initiating debridement.
Appropriate debridement protocols for maintenance and nonhealable wounds differ significantly from those for healable wounds. Although moist wound healing provides an optimal environment for wound healing, it is a form of debridement (autolytic) that may be detrimental to nonhealable and maintenance wounds. Debridement is generally not a suitable intervention for stable maintenance or nonhealable wounds, because the goal is to keep those dry and free of infection.49 Consider debridement only when a maintenance or nonhealable wound becomes unstable to remove infected or necrotic wound debris in the most atraumatic manner possible.
Patient goals typically include enhancing comfort, minimising wound-related odor, reducing pain, and improving ADLs. Keeping the wound dry enables the formation of a protective layer, whereas debridement carries the risk of removing this protective layer and introducing pathogenic organisms.
Statement 6: local wound care: infection and inflammation
6A. Treat local/superficial wound infection (three or more NERDS criteria) with topical antimicrobials
6B. Manage deep and surrounding wound infection (three or more STONEES criteria) with systemic antimicrobials and concurrent topical antiseptics
6C. Consider anti-inflammatory agents in wounds with persistent ongoing inflammation (topical dressings or systemic medication)
The validated NERDS and STONEES criteria can guide the assessment and treatment of infection and inflammation in chronic wounds.50 Base the diagnosis of infection on clinical signs and symptoms rather than superficial wound swabs, which should be used only to guide antimicrobial selection in the event of an infection. If deep and surrounding tissue infection is suspected, identify the bacterial species and their sensitivity to commonly used antibacterial agents to help guide systemic antimicrobial use. This is especially true if a deep and surrounding infection is not responding to the empiric treatment.
The best tissue samples for wound bed culture swabs are obtained after cleaning the wound with potable water or saline and sampling the wound base without debris. A culture from tissue samples using a curette or other biopsy technique is most likely to represent the organisms in the wound tissue. Alternately, a semiquantitative swab technique using the Levine method can correlate to tissue biopsies.51 The swab is placed on granulation tissue and pressed enough to extract wound exudate and then rotated 360° to cover all surfaces of the swab. Placing the swab in the transport media to premoisten it prior to placing the swab on the skin may increase the bacterial yield for patients with low-exudate wounds.52
Pathogenic bacteria may infiltrate to the bone and cause osteomyelitis, which derails healing potential and is challenging to cure. A bone biopsy is the criterion standard to diagnose suspected osteomyelitis, because superficial cultures do not access the deep bone tissue, and imaging is limited by variable specificity.53 However, a bone biopsy may be uncomfortable, is dependent on skilled clinicians, and may extend the tissue damage. For these reasons, it frequently is not a feasible option, even more so if resources are scarce.
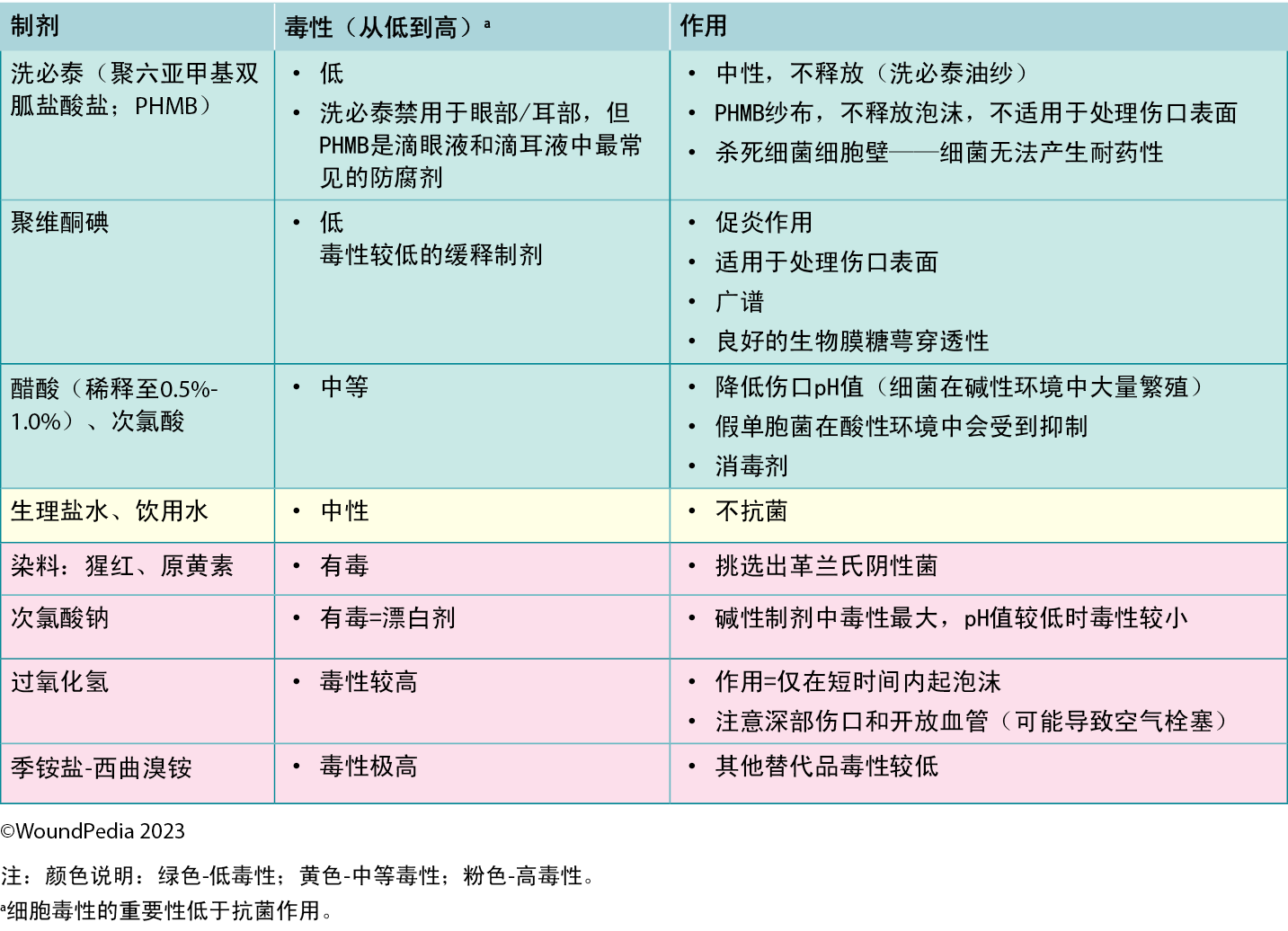
When deep wound infection is confirmed, antimicrobial dressings are appropriate to locally support systemic antibiotic therapy and prevent spread to the deep and surrounding compartment from surface bacteria. The five most common choices of antimicrobial dressings are silver, polyhexamethylene biguanide, iodine, methylene blue/crystal violet, and honey. Of these, silver and honey have additional anti-inflammatory properties.54
In some wounds, broad-spectrum antiseptics are applied for short-term rapid bacterial burden reduction to support systemic antibiotics. When the risk of infection outweighs the cytotoxic properties, low-cost povidone-iodine-moistened gauze changed daily over exposed bone can reduce surface bacteria. This is a short-term strategy accompanied by clinical evaluation for serum thyroid function levels especially when wound surfaces are large. However, with the newer low-toxicity antiseptics, other options are now available that are less aggressive but equally effective.
In general, wounds in immunocompetent patients that present for less than 1 month are treated with agents for Gram-positive coverage. Polymicrobial infections (typically seen in PWDs) or wounds longer than 1 month in duration call for broad-spectrum agents with Gram-positive, Gram-negative, and anaerobic coverage because these patients are often also immunocompromised.55
Cytotoxic agents may be appropriate for nonhealable wounds if the need for topical antimicrobial action is greater than the tissue toxicity. Antiseptics are generally preferred over topical antibiotics as part of antibiotic stewardship because of a lower risk of systemic bacterial resistance and adverse effects associated with contact irritant or contact allergic dermatitis.54
6D. Gently cleanse the wound with low-toxicity solutions (water, saline, noncytotoxic antiseptic agents).
The cleansing solution used depends on the characteristics of the wound and availability in practice. There is poor consensus in the literature on wound cleansing recommendations. An updated 2021 Cochrane review on cleansing solutions of venous leg ulcers concluded that there is a lack of randomised controlled trial evidence “to guide decision-making about the effectiveness of wound cleansing compared with no cleansing and the optimal approaches to cleansing of venous leg ulcers.”56 However, general wound care principles involve low-toxicity solutions including potable or preboiled water, saline, and other wound-friendly antiseptic agents.57 This avoids cytotoxic effects and damage to healthy granulation tissue in healable wounds.
Acetic acid diluted to 0.5% to 1.0% or hypochlorous acid can also be used in some cases in which an acidic environment is preferred (eg, for topical treatment of Pseudomonas aeruginosa).58 Based on the healability classification of the wound, antiseptic agents with some tissue cytotoxicity may be used after a positive risk-benefit assessment. This includes agents such as low-concentration chlorhexidine or its derivative polyhexamethylene biguanide and povidone-iodine. This may be beneficial in cases of maintenance and nonhealable wounds that have a high potential for infection. Further, these agents can be used to manage odor and exudate in addition to controlling bioburden. In resource-limited environments, consider wound hygiene measures and how solutions are prepared, stored, and distributed to patients to prevent cross-contamination.
There is emerging interest in the use of surfactants to remove biofilms that often exist within wound debris and have two surfaces of different viscosity (Supplemental Table 2, http://links.lww.com/NSW/A177). Wound irrigation remains a controversial topic for use in chronic wounds. However, expert opinion is not to irrigate wounds if the base of the wound is not visible to avoid accumulation of the irrigation solution in closed spaces and accidental enlargement of the wound.57 Irrigate wounds with an adequate solution volume (50-100 mL per centimeter of wound length).59
Statement 7: local wound care: moisture management
7A. Maintain moisture balance in healable wounds with hydrogels, films, hydrocolloids, hydrofibers, alginates, and foams
7B. Institute moisture reduction with fluid-lock mechanisms in healable wounds using superabsorbents to wick moisture away from the surface (diaper technology)
7C. Determine if wound packing is needed for healable wounds. It could be wet (donate moisture) or dry (absorb moisture)
7D. Establish a targeted moisture reduction protocol in maintenance and nonhealable wounds to reduce bacterial proliferation
Maintaining moisture balance is complex and depends on the wound type and healing classification. Consider moisture balance, infection control, and patient education in selection of dressing materials.60 Incorporating and tailoring moisture management strategies to the specific wound type and the resources available can optimise patient outcomes and minimise complications (Table 6).3,54 Further research and clinical studies should continue to refine our understanding of wound moisture management to enhance wound care practices in the future.3,54
Table 6. Goals of moisture management based on wound healability3,42,54
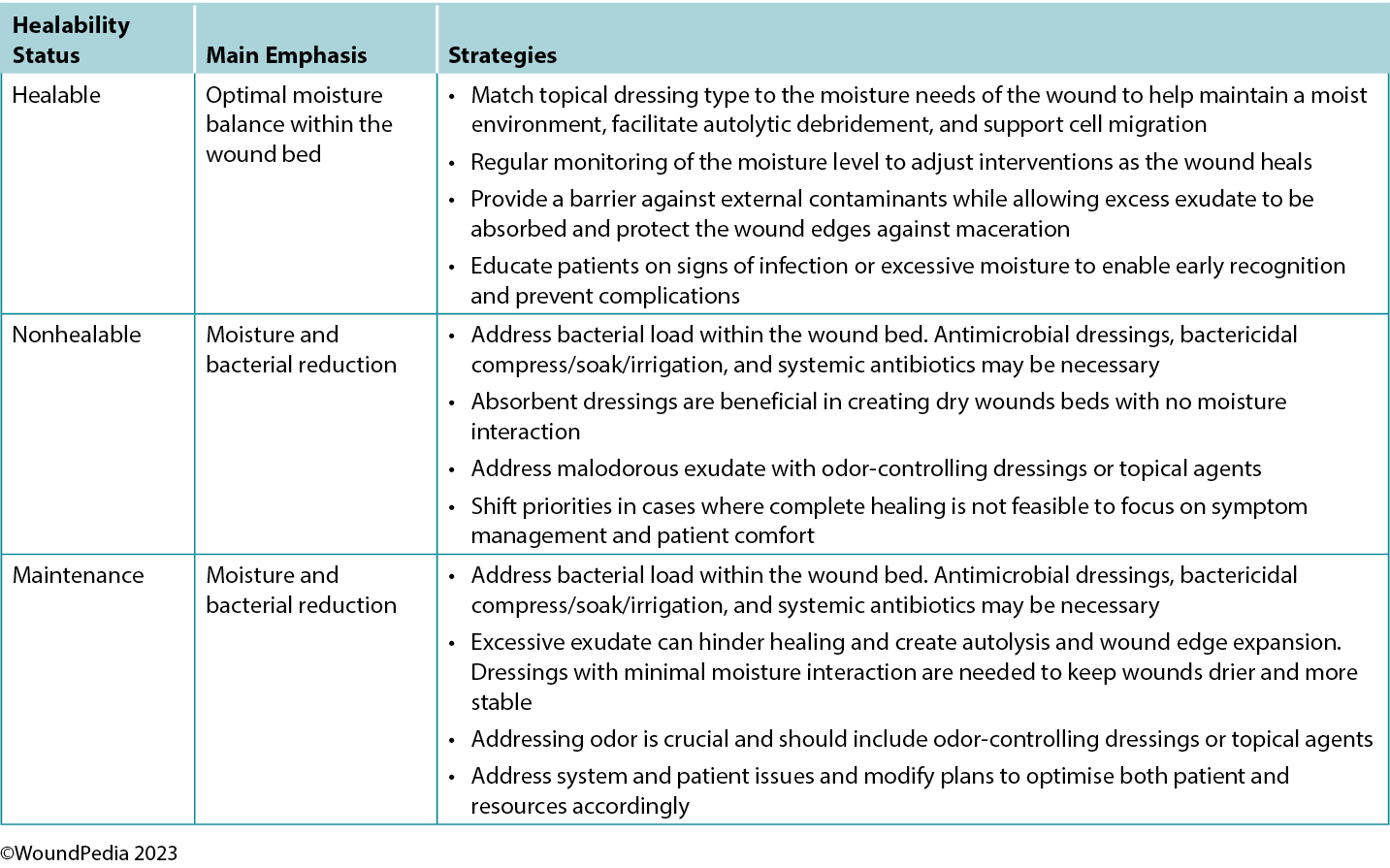
In general, as the level of exudate increases, so should the moisture absorbency or transferring capability of the dressing.3 Moisture-balance dressing options can be combined with antibacterial and anti-inflammatory properties to further meet the wound needs.
The choice of dressing for maintenance and nonhealable wounds should prioritise comfort while avoiding fluid donation to the wound bed and maceration of the wound edges. These wounds require regular reassessment for any progress or deterioration and should be managed to reduce bacterial load. Depending on wound evolution and characteristics over time, dressings may need to be adapted.
Statement 8: Healing trajectory
8A. Consider that healable wounds should be at least 20% to 40% smaller by week 4 to heal by week 12. If factors are present that affect healing time (poor glycemic control, for example), additional healing time may be required
8B. Allocate additional time to healing beyond 12 weeks in healable wounds if there are limited available resources, and continue with consistent care
8C. Prioritise referral to specialist centers (when available) for diagnostic testing and/or a skin biopsy, especially when faced with severe resource restrictions
Wound healing trajectories are useful and necessary for evaluating the needed time to heal, especially using clinical data (measurements) for both acute and chronic wounds. The healing trajectory is based on accurate and consistent wound measurements that determine the wound surface area closure over time. This helps to distinguish wound progression, stalled wounds, and worsening wound status.
Healable acute wounds should be completely closed within 30 days. Expect chronic healable wounds to have a 20% to 40% edge advancement at 30 days (4 weeks) and to close within 12 weeks.3,42,61,62 Nonhealable wounds have no defined time allocated to close, no edge advancement is expected, and all initiated steps are to prevent further deterioration. Maintenance wounds are not expected to heal nor deteriorate, and wound healing may progress slowly without a fixed time expectation unless patient/institution/system optimisation is achieved.
Re-evaluate hard-to-heal wounds periodically for alternate diagnoses. In these cases, consider wound biopsy, further investigation, and/or referral to an interprofessional assessment team. A healing trajectory can be assessed in the first 4 to 8 weeks to predict if a wound is likely to heal by week 12, provided there are no new complicating factors.63 Changes in the wound, the individual, or the environment may necessitate the reclassification of a wound to the maintenance or nonhealable category.
Statement 9: Edge advancement
9A. Consider locally constructed active modalities according to the required mechanism of action and the specific indications for initiating an adjunctive therapy to support wound healing
9B. Decide on adjunctive therapies through an interprofessional team approach and include a prior risk-to-benefit analysis
Select adjunctive therapies according to healability. Initiate these as soon as possible after injury in persons with major trauma to prevent sequelae of long chronicity. Select modalities using an interprofessional team approach based on what is available and the physical mechanism required (while ensuring trauma tissue healability). In hard-to-heal wounds, the wound may need a second chance to heal after reassessment.42 Ensure informed consent is a part of adjunctive therapy decisions.
A risk-to-benefit and cost-effectiveness analysis is helpful and will add in system sustainability. The key to effective adjunctive modality decisions is based on the risk it poses to initiate the therapy (physical discomfort, financial hardship, patient adherence) versus the benefit it will provide (tissue oxygenation, wound contraction, edema reduction, cellular reactivation).42,64 The best risk-benefit decisions are made within an interprofessional team including the patient, ensuring group commitment and treatment completion within optimal use of resources.
Optimising resources, or the slight repurposing thereof, may lead to creative strategies in the hands of interprofessional teams to tailor-make solutions to ensure all patients are optimally treated despite resource restrictions.
Surgery. Even in the most restricted environments, this is the one modality that is mostly available within a medical catchment area, often in tertiary care settings with referrals received from primary care clinics to provide debridement, general surgery with primary/tertiary intention closure, skin grafts, and/or amputations.
Skin grafts may be available in resource-restricted environments as an advanced modality to achieve tissue closure to reduce healing times and prevent recurrent deep wound infections. The procedure can minimise the extensive use of dressing materials over a prolonged period and reduce primary care chronic wound workload. Skin graft decisions are often made to preserve body functionality and promote early wound closure above aesthetic outcomes.65 However, avoid skin grafts for ischemic wounds and most venous stasis ulcers; consider them only in wounds where a favorable outcome could be expected.66,67
Electrical stimulation. Wound healing can be accelerated by enhancing the natural electrical current present in injured skin. In resource-limited environments, this modality should be investigated for wound healing because of its high-level evidence base and availability of devices of this nature (direct current, alternating current, low-intensity direct current, pulsed electromagnetic fields, high-voltage pulsed current, and transcutaneous electrical nerve stimulation devices). The evidence supports increased cellular proliferation and increased microcapillary perfusion, as well as a reduction in bacterial burden and infection on treated wound beds.68
Wall-mounted negative-pressure wound therapy. This can give the healthcare professional control over exudate management and accuracy in fluid replacement for inpatients with high exudate and large tissue defects. Start with the lowest possible pressure (minus 50-80 mm Hg). The patient becomes bedbound for the modality to be maintained, and the base layer (often gauze or petrolatum-impregnated dressings) needs to be replaced at least daily. This, together with starting on the lowest possible negative-pressure setting, will prevent mechanical trauma to the wound bed from tissue adherence and traumatic dressing removal in cases where nonadherent base layers are not available.69 There are now disposable negative-pressure wound therapy devices designed for use in the community as well as do-it-yourself options that can render acceptable clinical outcomes.70,71
Breathing high-flow, highly concentrated oxygen. Oxygen is often overlooked as a legitimate wound healing modality.72 Ironically, it is rarely used, although nearly all formal healthcare settings have copious amounts of oxygen available, even in relatively resource-poor environments. The availability of oxygen concentrators has also increased because of the COVID-19 pandemic, making oxygen administration also a possibility on an outpatient basis in home-based settings.73 Although hyperbaric oxygenation is the most effective means of increasing plasma oxygen concentration and tissue oxygen delivery, this modality is not always readily available. However, providing normobaric (ward) oxygenation still produces a 7.5-fold increase in plasma-borne oxygen (ie, from 0.3 mL/dL to 2.3 mL/dL; Table 7).74 Moreover, intermittent inhalation of 100% oxygen in patients without chronic obstructive pulmonary disorder (eg, 6 hours on/2 hours off by nonrebreather mask) over 3 to 4 days is not harmful to the lungs and may provide significant additional oxygen substrate, while the intermittent increase and decrease in oxygen delivery activate the expression of hypoxia-inducible factor (a strong stimulus for angiogenesis).75 This would suggest that in lieu of hyperbaric oxygen therapy, normobaric oxygen is justified for mitigating conditions on the FDA-approved list of indications for hyperbaric oxygen (Table 7).76 This may include tissue reperfusion injuries (eg, crush injuries, compartment syndromes before and after surgical release); bacterial toxin inhibition (ie, infective myonecrosis or other anaerobic infections); or large-tissue defects (after debridement to maintain the reactivated inflammatory response for up to 48 hours). Normobaric oxygen provides 50% of the Po2 of typical hyperbaric oxygen therapy (at 2 atmospheres); however, many of the pharmacologic effects of oxygen are achieved only under hyperbaric dosages.77,78
Table 7. Normobaric oxygen therapy mechanisms and delivery systems
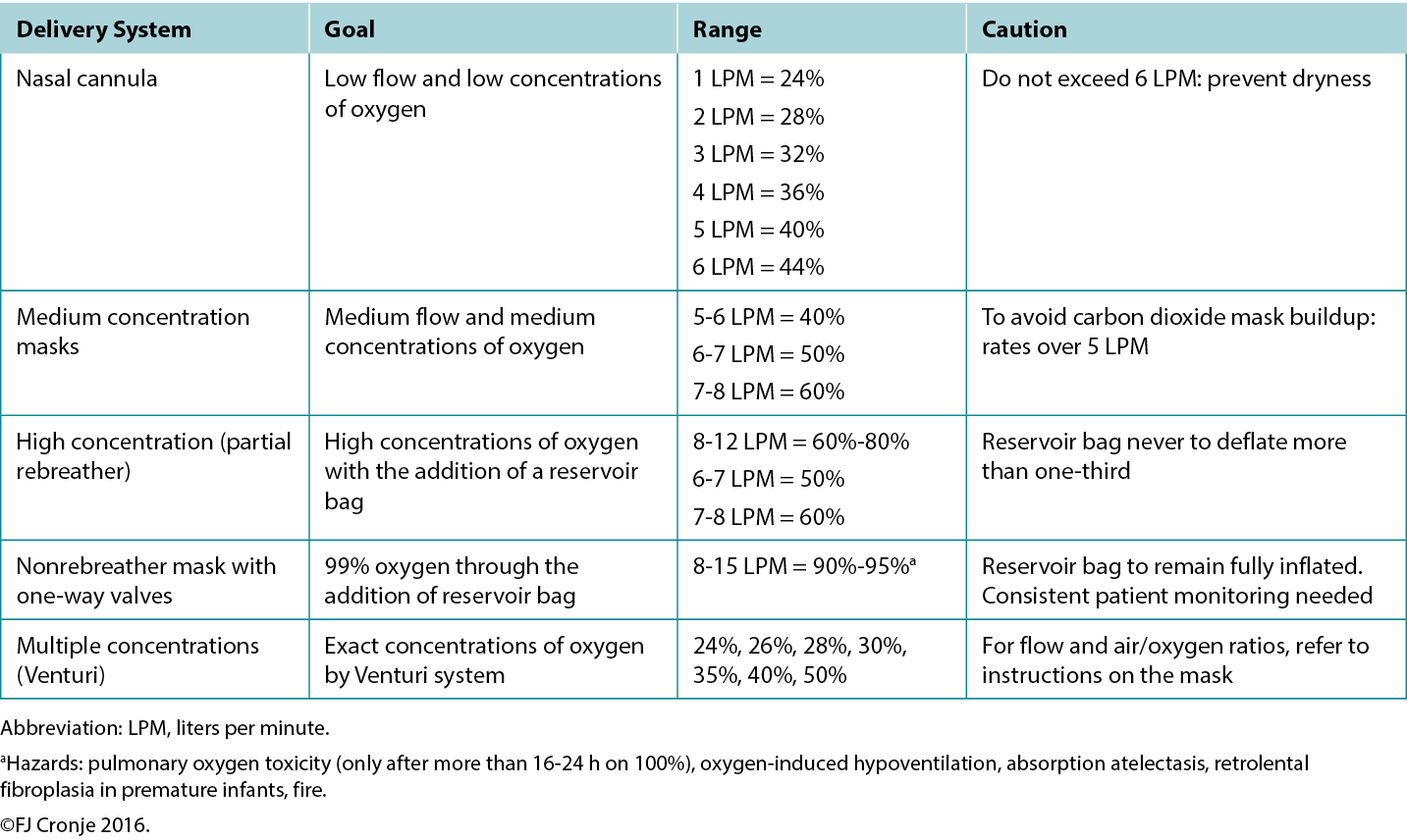
Note that topical oxygen administration directly to a wound does not have the same physiologic effects attributed to systemic oxygen delivery.79 Topical oxygen delivery systems remain subject to ongoing research to determine their beneficial actions beyond improved epithelialisation and possibly a mild antiseptic effect.64 The latter effect has also been attributed to ozone therapy.80 A systematic review completed in 2018 indicated that these therapies could potentially elicit mild oxidative stress or disinfection but that the risk of toxicity due to uncontrolled reactive oxygen species is high.81 Within the limited-resource environment, these devices and use thereof are likely uncontrolled.
Statement 10: Healthcare system change
10A. Facilitate evidence-informed, culturally competent, and equitable care for all patients
10B. Improve provider skills for wound management competency to enhance patient outcomes
10C. Establish timely and effective communication that includes the patient and all interprofessional wound care team members for improved healthcare system wound outcomes
Globally, chronic wound management consumes a significant portion of healthcare resources; preventive care represents the most cost-effective strategy for reducing healthcare system expenditures. There are numerous approaches to integrating preventive care into practice. For instance, DFUs are notorious for leading to high amputation rates and morbidity worldwide, even though many DFUs are preventable. Although diabetes was initially believed to be most prevalent in developed countries, it is noteworthy that 80% of diabetes-related deaths occur in countries with limited resources.82 A validated screening tool, an educated and available interprofessional team, and the implementation of prevention algorithms can be used in countries regardless of the availability of resources.83-85 Other organisational strategies that could be implemented include the following.
Patient navigation. Managing a DFU requires continual support from a circle of care that includes family members and healthcare professionals (including home care and wound care) working as a team. Timely access to both health and social services is often necessary to prevent and heal DFUs. Regionalised, community-based integrated diabetes services linked to interprofessional wound care clinics are proving to be most successful.86
One effective way to ensure optimal and timely care is patient navigation. This concept is adaptable to all healthcare sectors and can improve the timely diagnosis and treatment of wound infections, optimise pain management, and increase access to specialised care, thereby expediting wound closure rates.87 Patient navigation is becoming a vital component of integrated care, facilitating seamless transition between sectors and enhanced clinical outcomes. Further, it is accompanied by a boost in morale for both patient and healthcare professional, decreased nonacute hospital admissions or readmissions, enhanced patient quality of life, and improved adherence to treatment protocols. All these factors combined generate significant cost savings to healthcare systems.87,88
A critical element of successful patient navigation programs is the inclusion of a comprehensive and systematic approach to guide healthcare professionals in assessing and delivering care to each individual patient (eg, the WBP framework). These pathways do not need to be complicated or time consuming, but should ensure specific criteria are met, including regular foot care for those at high risk of amputation, glycemic control to an HbA1c less than 9%, and a BP less than 130/90 mm Hg.23,89
Policy interventions. Organisational policies that detail and provide guidance on best practice interventions and pathways are crucial for the successful and sustainable implementation of wound care protocols. Base these policies on current published guidelines for each specific wound type and translate them into the environment they need to serve. The institution needs to accept them as standards of practice and be approved as such to serve as evidence-based care in environments in which other guidelines may not be successfully adopted because of cultural or language translation challenges. Further, these policies must clearly outline the process for entering and using data, as ongoing quality improvement initiatives are based on data to improve and maintain effective care processes.
Adapted wound care. Although the delivery of care should be adapted to the unique needs of each healthcare sector, certain concepts should be standard, particularly those that enhance effective communication both within and across sectors. The increased adoption of digital technology expedites assessments, enhances access to specialised care, and optimises the allocation of limited resources.90 Where adaptations are implemented as care processes, these practices should be well documented as care standards and be easily accessible to ensure consistency and continuity of care within respective institutions.
Delphi consensus highlights
Some important comments from the panel:
- The ability to heal (healability) is a changeable modality and not to be seen as a static classification as patient condition, circumstances, and choices drive the classification allocation process (3A).
- The adjustment of practice as required in maintenance wounds incorporates the establishment of a conservative wound bed approach, with attention to the allowances the patient is willing to make within their life choices to slowly ensure patient optimisation and subsequently increase host competence (3B).
- When the method of patient documentation is agreed upon within an institution, it should be uniformly used to prevent communication gaps between providers that may inadvertently cause a negative impact on patient care outcomes (4A).
- Antiseptic mouth washes suitable for mucosa are often also friendly for use on a wound bed (4B). This may be considered off-label use.
- The choice for topical bacterial burden control should be on topical (low) toxicity antiseptics (the five most important ones), depending on the ability to heal and bacterial burden priorities to be addressed. Refrain from using topical antibiotic preparations, ointments, and creams on chronic wounds because those preparations are often focused only on Gram-positive organisms and would allow Gram-negative and anaerobic organisms on the wound bed to multiply freely. Further, topical antibiotic preparations need only one mutation to create systemic resistance to the targeted organism. Topical antibiotics are often in a carrier medium that is associated with contact irritant or contact allergic dermatitis (6A and 6B).
- When a healable wound has a significant moisture burden and remains in need of superabsorbent dressings to control the moisture balance of the wound bed, the wound needs thorough reassessment to ensure all underlying causes have been corrected for (7B).
- In establishing an interprofessional team, use any means of communication to build and maintain such a team as that could significantly optimise local capacity despite distances between providers and specialists to increase clinical outcomes despite local limitations (9B).
Conclusions
Optimise chronic wound care in resource-limited environments by initiating small adaptations and creative interventions without compromise to the core principles of care required. Interprofessional wound care teams can serve as a virtual resource to isolated and remote communities to improve clinical outcomes. All of the critical elements for (diabetic) foot management and care can easily be incorporated into diverse environments by increasing local capacity and providing clinician education and culturally appropriate patient empowerment.
Practice pearls
- The holistic care of PWDs includes the optimisation of HbA1c levels, BP, cholesterol levels, and drugs with cardiac- and renal-protective properties.
- The audible handheld 8-MHz Doppler pulse signal is a suitable bedside test for arterial blood supply to the lower limbs. It can be performed as a modification of the traditional ABPI, and the pulse sounds are easy verifiable by remote expert team members using MP3 or MP4 recordings from a cellphone.
- The NERDS and STONEES mnemonics can guide diagnosis and treatment of local/deep and surrounding infection, including an oral antibiotic indication for osteomyelitis.
- Plantar pressure redistribution can be accomplished from innovative, less costly alternatives such as soft felted simple inserts, to the total contact cast and removable cast walker made irremovable if the latter are unavailable or unsuitable for patient preferences or ADLs.
- Integrated coordinated care teams can connect with virtual expertise by equipping healthcare professionals with skills in patient navigation.
- Toolkits containing enablers for practice along with 8-MHz Dopplers and infrared thermometers can facilitate care in resource-limited settings.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
伤口床准备2024:在资源有限的环境中进行脚部溃疡管理的Delphi共识
Hiske Smart, R Gary Sibbald, Laurie Goodman, Elizabeth A Ayello, Reneeka Jaimangal, John H Gregory, Sadanori Akita, Afsaneh Alavi, David G Armstrong, Helen Arputhanathan, Febe Bruwer, Jeremy Caul, Beverley Chan, Frans Cronje, Belen Dofitas, Jassin Hamed, Catherine Harley, Jolene Heil, Mary Hill, Devon Jahnke, Dale Kalina, Chaitanya Kodange, Bharat Kotru, Laura Lee Kozody, Stephan Landis, Kimberly LeBlanc, Mary MacDonald, Tobi Mark, Carlos Martin, Dieter Mayer, Christine Murphy, Harikrishna Nair, Cesar Orellana, Brian Ostrow, Douglas Queen, Patrick Rainville, Erin Rajhathy, Gregory Schultz, Ranjani Somayaji, Michael C Stacey, Gulnaz Tariq, Gregory Weir, Catharine Whiteside, Helen Yifter, Ramesh Zacharias
DOI: 10.33235/wcet.44.1.13-35
摘要
背景 低资源环境中的慢性伤口管理值得特别关注。农村或资源不足的环境(即基本需求/医疗保健供应有限、跨专业团队成员不稳定的环境)可能无法应用或复制城市或资源丰富的环境所采用的最佳实践。
目的 作者汇集了世界范围内的专业知识,为缺乏所需资源的社区开发一种具有科学性的实用伤口床准备模式。
方法 来自15个国家的41位伤口专家组成的小组就资源有限环境下的伤口床准备工作达成了共识。
结果 关于10个关键概念的每项声明(32项子声明)均达成了88%以上的共识。
结论 共识声明和基本原理可以指导低资源环境中从业人员的临床实践和研究。这些概念应促使我们不断创新,改善所有脚部溃疡患者,尤其是糖尿病患者的治疗效果,提高医疗保健系统的效率。
总体目的
回顾一种具有科学性的实用伤口床准备模式在缺乏所需资源的社区中的应用情况。
目标受众
本次持续教育活动的预期对象是对皮肤和伤口护理感兴趣的医生、医生助理、执业护士和护士。
学习目标/结果
参加本次教育活动后,参与者将:
1. 总结与伤口评估相关的问题。
2. 确定一类经证明可改善血糖、肾脏保护和心血管结局的II型糖尿病治疗药物。
3. 整合伤口管理策略,包括在资源有限的情况下进行治疗。
4. 说明慢性可愈性伤口边缘推进的目标时间。
引言
2000年推出了伤口床准备(WBP)框架,以强调将全人治疗作为最佳局部伤口护理的基础1。随着这一框架发展成为一个国际框架,就能明确并非所有伤口均能愈合。这些关于维持性伤口和不可愈性伤口的概念导致需对当地伤口护理原则进行修订,以及对WBP进行扩展。护士、医生和综合医疗保健人员携手合作,优化患者护理效果和医疗保健系统利用率,这种综合协调护理对于进一步开发WBP至关重要。
本文重点介绍如何应用WBP框架来管理足部相关伤口,尤其是对于患有糖尿病、麻风相关神经性脚部溃疡以及包括神经病和血管疾病在内的其他并发症的患者。以下几个参数对PWD十分重要:血糖控制不佳、血压变化、高胆固醇、足底压力再分布不足、感染和缺乏运动。吸烟对挽救PWD的肢体和生命也特别有害。
本文的重点在于资源有限环境的一系列定义,包括资源可用性较低;缺乏资助或资助有限;偏远、隔离或农村环境;以及土著人口。这些术语均与医疗保健机构相关,这些机构在获取用品、设备、专家以及高级伤口护理能力和技能方面可能会遇到困难。低资源环境可能存在于世界任何地方,并不局限于低收入国家或发展中国家。
作为这项工作的基础,Delphi过程促进了当前形式WBP框架的扩展和开发。来自15个国家的41位作者参加了Delphi过程,该过程分为两轮,采用李克特四级量表(1级,完全同意;2级,同意;3级,不同意;4级,完全不同意)。第一轮共有29项声明。虽然所有声明均超过了所期望的80%的共识水平,但主要作者小组仍考虑了299条意见。在进行Delphi过程第二轮讨论之前,我们聘请了一位专业编辑来提高声明的可理解性和语法准确性,共构建了32项声明。所有声明均达到了超过88%的共识水平。
在第二轮中,有14项声明达成了100%的共识。其中一项声明非常突出,Delphi小组的所有成员均将其评为“完全同意”:10C。建立覆盖患者和所有跨专业伤口护理团队成员的及时有效的沟通渠道,以改善医疗保健系统的伤口治疗效果。与此同时,每一位国际伤口专家均以小组为单位编写手稿内容。有关共识声明,请参阅补充表1(http://links.lww.com/NSW/A176)。
该共识旨在制定一个以科学为基础的最低护理标准,以优化可用资源。在资源有限的环境中处理伤口的10个关键步骤和32项子声明请参阅表1。这一共识过程更新了WBP框架,使其适用于任何环境,不论其资源自用性。此外,它还首次纳入了愈合轨迹和医疗保健系统变革的概念(图1)。

图1.伤口床准备2024.˝WoundPedia 2023
表1.在资源可用性有限的环境中为膝下伤口准备伤口床
本报告的其余部分将重点介绍10项共识声明,并讨论每项声明的原理。
声明1:病因治疗
1A. 评估动脉灌注是否足够,以确保伤口正常愈合
(可触及的足部脉搏和/或使用8 MHz多普勒进行的多相动脉足动脉声)
要测定下肢血流量,首先需找到可触及的足部脉搏。从足背动脉和/或胫后脉搏开始。如果可进行8 MHz手持式多普勒检查,则应确认多相血流模式(双相/三相)。当观察到单相或无多普勒声或无法触及足部脉搏时,请转诊至血管专科医生。动脉灌注不足的其他体征包括静息时下肢疼痛和肢体缺血性改变(肢端发冷伴抬高时发白的下垂部位红紫)。
糖尿病患者容易出现微血管问题(周围神经病、Charcot足部病变)和大血管并发症,包括外周动脉疾病(PAD)。这些情况会导致胼胝、脚部溃疡和混合组织损失。由于多达50%的易感人群同时患有糖尿病和PAD2,及时确认PAD至关重要(通过体格检查和血管试验),该疾病是导致溃疡愈合不良和截肢的主要风险因素。检测到该疾病后,必须立即进行血管重建(血管成形术或血管搭桥术),以恢复足部正常的动脉血流。其他评估可包括毛细血管再充盈时间、Buerger肢体发白试验(抬高时皮肤苍白,下垂时皮肤红紫)和行走时的跛行。
血管检查。在动脉功能不全的情况下,由于营养和氧气供应不足,肢体触感通常较为冰凉(表2)。重度病例可能会出现组织坏死,表现为溃疡、趾蹼浸渍(通常伴有继发性感染)、龟裂或坏疽。其他重度病例指标包括抬腿时皮肤苍白、运动引发的跛行(静息后缓解)、下垂部位发绀或红紫以及肌肉萎缩。值得注意的是,下肢水肿大多指示静脉问题,而非动脉问题。动脉溃疡一般呈穿凿样,基底较深,通常含有肌腱,而静脉溃疡的边界形态不规则,基底肉芽组织较浅3。
表2.组织愈合所需的血管供应
踝臂压力指数(ABPI)检查。ABPI使用8 MHz多普勒测量踝关节收缩压除以肱动脉收缩压的比值。该程序包括使用血压袖带,并在袖带充气后动脉声再次出现时记录收缩压。不过,水肿、炎症和动脉钙化等因素会影响其准确性。如果无法负担/无法进行8 MHz多普勒,在出现足部脉搏消失的情况下,应优先考虑尽早转诊至三级评估中心。在某些医疗保健机构中,在开始进行任何下肢干预之前,仍需要进行ABPI作为重要的定量评估。
手持式声学多普勒(AHHD)检查。如果医疗服务提供者选择更简单、更快捷的检测方法(不受钙化影响,无需挤压疼痛的小腿,也无需斜卧20分钟),那么在某些情况下,可以很容易地将AHHD添加为附加参数。在大脚趾截肢的情况下,AHHD评估也能提供准确的结果,并能以MP3或MP4文件格式进行记录和传输,以便对信号解释进行远程验证。
医疗保健人员应使用8 MHz多普勒探头将凝胶涂抹在脚背动脉、胫后动脉和腓动脉的适当足部脉搏部位,探头与皮肤成45度角。然后可以对获取的多普勒信号/波形进行分析(通过可听声或视觉轨迹):请访问https://journals.lww.com/aswcjournal/Pages/videogallery.aspx?videoId=20获取有关AHHD程序的全面教程。通过仔细调整探头位置来优化信号质量,以获得最响亮或最多相的信号。
对于单相或无波形的情况,需进行全面的血管评估,包括在血管实验室进行双功能节段性小腿动脉多普勒检查。多相波形通常表示不存在外周血管疾病4。在PWD中,需谨慎解释ABPI比值(由于动脉硬化或动脉钙化);AHHD多相检查结果是确认伤口愈合所需血液供应充足的首选参数。多相波形(双相、三相)表明AHHD值相当于ABPI正常值,即≥0.9。
虽然AHHD可有效排除动脉疾病,但其可能无法识别现有的节段性灌注缺陷Å\Å\缺血区或血管区域缺损5。因此,足部和下肢的体格检查对于确诊至关重要。医疗保健专业人员可以记录AHHD信号,并将其传输给专家,以便进行同步或异步远程评估。同步评估可使患者实时参与并迅速做出决策。
慢性静脉功能不全。PWD和脚部溃疡患者可能同时患有慢性静脉功能不全。其主要影响下肢,阻碍脱氧血液返回心肺。该疾病通常由静脉瓣膜功能障碍引起,而妊娠或体重升高等因素均可能诱发静脉瓣膜功能障碍。症状通常包括静脉曲张、水肿、血铁黄素导致的皮肤变色、脂肪皮肤硬化症和静脉溃疡6。这些溃疡大小不一(从小尺寸向圆周扩散),在静脉汇集区域迅速形成,通常位于下肢内侧。
加压疗法的治疗静脉溃疡的基石。其可以通过増强小腿肌肉泵的蠕动作用来补偿瓣膜功能障碍。其他措施包括抬高腿部和步行。如果溃疡由浅静脉引起,可以考虑进行静脉消融术。未经治疗的静脉水肿会延迟脚部溃疡愈合7。
对于PAD的医疗优化,关键策略包括最佳血压控制、开始胆固醇药物治疗,通常还包括开始他汀类药物治疗。最近的研究还建议对PAD并发冠状动脉疾病或颈动脉疾病的患者采用低剂量阿司匹林(每日91-100 mg PO)和低剂量利伐沙班
(2.5 mg PO BID)联合治疗8。其他可调整因素包括戒烟和步行或运动计划。适当的足底压力再分布或减轻压力对于脚部溃疡患者(尤其是PWD)至关重要。
1B. 确定所有基础病因
足部并发症是PWD极为关注的问题,也是医疗保健系统的一项重大疾病负担。助记符AIM(评估、识别、管理)是一种整体方法,其重点是治疗或减轻糖尿病足问题(尤其是神经病)的基础病因。采用重点评估方法作为基线护理标准(VIPS:血管、感染、压力、手术清创),对于预防重度并发症(包括足部溃疡、下肢截肢以及早期/可预防死亡发生率的増加)至关重要。在资源匮乏的环境中,与足部相关的一些关键因素包括:接受正式治疗的时间较晚、诊断延迟9、赤足行走、伤口被忽视以及缺乏预防性足部护理。在埃塞俄比亚进行的一项基于医院的观察性分析确定了导致糖尿病足并发症的几个因素10;这些因素包括湿度过高、足部变形、神经病、未识别的活动性溃疡、鞋袜不足或不合脚、足部卫生状态较差(如足部和趾甲真菌)以及缺乏足部护理意识。一项关于麻风患者足底溃疡的系统性综述(n=7项研究)确定了以下溃疡发生的风险因素:在感觉测试中无法感知到10 g单丝、重度足部变形或足旋前过度、教育程度较低以及无业11。在任何资源匮乏的环境中应对这些挑战,均需采取多管齐下的方法,其中可能包括患者和医疗保健专业人员教育、早期检测、提供护理、鞋类项目和社区参与。
定期和全面的足部评估包括检测神经病(保护性感觉丧失)、血管问题(下肢血液循环不良或缺失)、感染迹象、高压区域(胼胝形成)和摩擦(水泡,通常伴有出血成分),以便及时采取干预措施。这种简化的60秒钟筛查工具可能是根据患者的风险水平进行快速评估、分层和随访的重要手段,而且成本较低12。有溃疡病史、截肢史、外周血管手术史或Charcot神经性关节病史的患者发生皮肤破溃的风险最高,应给予更多关注,防止溃疡和进一步的并发症。
NERDS(浅表伤口感染:不愈合、渗出液、红色碎片状肉芽组织、碎片、气味)或STONEES(深部和周围伤口感染:面积増大、温度升高3ÅãF、骨膜[探针到骨头]、新的破溃区域、红斑>2、渗出液、气味)标准以及使用非接触式红外测温仪均对检测感染有所帮助13。与对侧肢体相比,温度升高3ÅãF可能预示着炎症和足部溃疡风险较高13。1.67ÅãC的比较变化在临床上难以测量。在小腿或脚部溃疡患者中,如果同时符合两个或更多STONEES标准,那么同样的检查结果意味着深部和周围感染的可能性要高出八倍13。
未出现溃疡但表现出热肿足的神经病患者可能患有急性Charcot神经性关节病。在这些病例中,红外测温仪是一种极具价值的评估工具:急性Charcot足的温度可能比对侧足的镜像温度高8-15ÅãF。此类患者需进行全面的病史、体格检查和放射影像检查,以便及早检测到相关症状。进一步的措施包括使用全接触石膏进行稳定,以及使用轮椅完全减轻足底压力,以防止骨骼进一步恶化和下肢截肢(表3)。
表3.对足底糖尿病性/神经病变性足部伤口进行减压干预28
PWD中的胼胝形成与压力和剪切应力呈正相关。对于糖尿病神经病变患者而言,足部变形、关节活动受限、行走时重复受力以及鞋子不合适等各种因素均会増加胼胝形成的风险14。此外,胼胝的存在也会带来重大风险,因为重复性外伤可能会导致皮下出血,最终进展为溃疡。提供量身定制的压力再分布装置(软鞋垫、鞋匠干预调整鞋子、减少赤足行走),以防止胼胝后续进展为溃疡。
不同的病因可能导致PWD出现脚部溃疡,具体如下:周围神经病引起的神经性溃疡、与PAD相关的缺血性溃疡,或两者结合的神经缺血性足部并发症。根据病史、体格检查(5.07/10 g单丝测
试)、双侧肢体对称分布的感觉改变(袜套和手套式分布)以及烧灼感、刺痛、剧烈刺痛或针刺痛,可确定是否存在糖尿病神经病变。
1C. 对最重要的病因/并发症进行分类,以便立即进行治疗,并在当地可用的支持系统/资源范围内设计靶向干预措施
25%至34%的PWD会出现糖尿病足溃疡(DFU),这是最令人担忧的并发症之一,可能导致下肢截肢、重度残疾和预期寿命缩短15。糖尿病相关周围神经病和PAD可由多种因素导致,会使足部极易受到外伤性损伤。及时接受诊断和干预是有效管理和保护肢体的决定性因素。
以血红蛋白A1c(HbA1c)升高进行衡量的慢性高血糖症是感觉、运动和自主神经病变的主要风险因素。PAD和皮肤干燥均会増加足部感染和延迟愈合的可能性,从而导致不良结局。慢性肾脏疾病会増加患病风险16。最近关于持续血糖监测的研究报告称,较高的血糖水平变异性(达到目标范围水平的时间缩短)也可能导致长期并发症17。
多达50%的PWD会出现神经病,但目前尚无治愈方法。管理措施包括每天适当检查足部是否有外伤或感染迹象、足部护理和有效控制血糖18。除神经病外,PAD也同样会导致DFU;PAD大多无症状,可能长期未得到诊断和治疗。与未患糖尿病的人群相比,糖尿病患者的PAD患病率増加了两倍多19。一项对基于社区的PAD全球患病率和风险因素研究进行的系统性回顾显示,糖尿病位居首位,其次为吸烟20。
欧洲高血压学会建议医疗服务提供者和患者旨在将收缩压控制在130 mm Hg以下,舒张压控制在80 mm Hg以下。PWD的收缩压不应低于120 mm Hg,以防止重要器官和下肢的血流量减少21。虽然利尿剂、钙通道阻滞剂、血管紧张素转换酶抑制剂、血管紧张素受体阻滞剂和É¿阻滞剂均可使用,但血管紧张素转换酶抑制剂和血管紧张素受体阻滞剂可减少心血管事件的发生率22,23。最近,SGLT2抑制剂在改善血糖、肾脏保护和心血管结局方面显示出良好的效果24。此外,通过改变生活方式及早发现PAD可降低DFU的发生率17。
管理DFU十分复杂,需要采用跨专业团队方法,以识别健康状况的生物、社会、地理和文化决定因素。在丹麦,PWD按地区进行登记,并可前往设有跨专业伤口护理团队的专科诊所就诊。由于糖尿病护理的改善、定期足部检查、自我护理质量提高和及时治疗,下肢截肢率已显著下降25。在地理位置较为分散的不同人群中(如加拿大安大略省),截肢率存在显著差异,其中农村地区的截肢率最高,因为在这些地区,血管重建术和足部护理专家等及时预防服务不足或缺乏此类服务。
在加拿大,最易发生糖尿病足相关截肢的人群包括土著人、移民以及生活在农村和北部地区的人群26。土著糖尿病健康圈为安大略省当地的第一民族社区提供了一种具有文化敏感性的方法,提供有关糖尿病、健康和自我管理的教育和知识。其整体足部护理计划支持一系列服务,将社区成员与土著机构合作伙伴和当地医疗保健专业人员联系起来,经证明可降低DFU发生率并防止截肢27。
1D. 根据动脉灌注情况,优先针对足部伤口进行压力再分布,并为腿部/足部水肿选择适当的加压疗法
标准足底压力再分布装置为全接触石膏或以不可拆卸方式安装的可拆卸石膏助行器28。即使在可随时获得这些减压装置的医疗保健系统中,也只有不到10%的合格患者适合安装并坚持使用这些装置29。
减轻压力对脚部溃疡的愈合至关重要(表3)。目的是结合以患者为中心的问题、护理目标和最佳实践证据,为患者选择最佳装置。考虑创造性解决方案,重新利用当地材料,如在资源有限的环境中使用软毡垫进行减压。重要的一点是,需在患者和医疗保健专业人员之间制定早期监测计划,以监测和确保达到所需的减压效果。医疗保健专业人员需要对装置的有效性进行评价,并通过既定的随访不断进行必要的修改。在缺乏专科从业人员的地区,使医护人员具备基础减压能力可以填补这一需求空白。
声明2:以患者为中心的问题
2A. 使用疼痛量表评估疼痛,制定有针对性的伤害性疼痛和/或神经性疼痛管理计划
对疼痛的感知涉及物理或化学刺激。疼痛主要有两种类型:伤害性疼痛和神经性疼痛(表4)。在以患者为中心的问题中,伤口相关疼痛是一个重要组成部分,但医护人员往往对其关注过少。到20世纪90年代末期,随着一份聚焦于伤口疼痛的关键立场文件的发布,伤口疼痛成为医疗保健专业人员的关注重点30。该文件认可并关注慢性伤口疼痛带来的痛苦及其对患者健康相关生活质量的影响。随后,将伤口疼痛管理纳入了WBP框
架1,31。
表4.疼痛类型和反应30
疼痛在伤口患者的整体管理和最终成功愈合方面起着重要作用32。损伤相关疼痛信号在确保患者健康方面发挥着重要作用,因此必须通过适当的评估和管理加以确认。例如,疼痛或疼痛的任何变化均为伤口感染的关键预测因素,也是炎症的四大主要体征之一1。疼痛无法缓解往往与伤口闭合延迟相关。
评估。疼痛史对于伤口疼痛管理至关重要32。评估必须包括疼痛的性质、发作时间、持续时间、恶化和缓解因素。这将有助于确定疼痛的原因并指导管理。疼痛强度可通过有效的疼痛量表进行可靠测量。0-10分的口头数字评分表是测量疼痛数字强度的首选。大多数患者的疼痛程度为3分至4分(满分10分),即可正常工作33。
应定期重新评估持续疼痛患者的病情改善、恶化情况以及对药物治疗方案的依从性。使用疼痛日记来记录疼痛强度、用药情况、情绪和对治疗的反应可能是一种较好的管理策略。对于有交流障碍的个体,疼痛量表应包含图片,以便于识别。
管理。可将伤口疼痛的处理纳入WBP框架:治疗病因,解决局部伤口因素和以患者为中心的问题3。病因治疗应确定正确的诊断结果,并开始治疗伤口疼痛。以患者为中心的问题必须侧重于患者认为疼痛的主要原因和解决方案。患者预期的疼痛和痛苦与实际经历的疼痛一样会影响生活质量。
目前存在多种疼痛管理策略,但均非药物治疗(表5)。在选择管理策略时,需考虑全面的患者管理,包括WBP的各个方面。请记住:疼痛是患者的感知34。
表5.疼痛管理策略
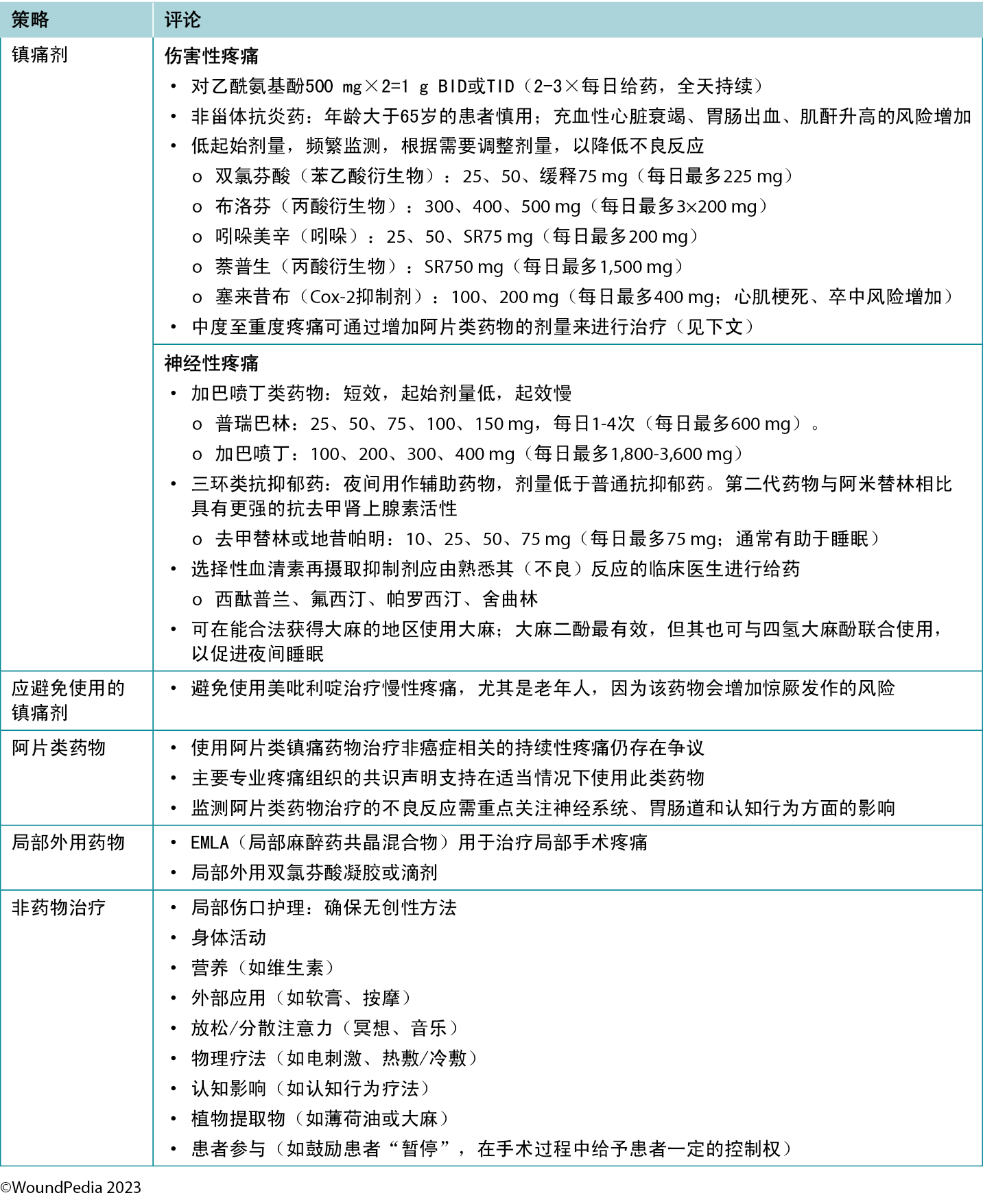
在资源有限的情况下进行评估和管理。在资源有限的地区,评估仍然是可行的,因为它无需借助昂贵的工具。虽然初始成本与从业人员的教育和培训有关,但大多数疼痛量表均可免费获取31。在资源有限的环境中,采用非药物方法治疗疼痛可能会更加实际(表5)。其中一些治疗方案可能更容易被社会接受,或在文化方面已得到实践(如冥想、植物疗法),并且尤其适用于管理伤害性疼痛。实施此类策略可以为那些无法通过非药物手段控制的疼痛患者(如神经性疼痛)保留药物管理方案35。
2B. 确定可能影响愈合效果的日常生活活动
2C. 评估患者是否有可能影响伤口愈合的(有害)生活习惯(如吸烟、酗酒和使用其他药物)
2D. 使用纳入患者支持系统的可持续教育干预措施,为患者提供支持。在可能的情况下,使用患者的主要语言,并考虑他们的文化背景、宗教、可接受的行为、禁忌和信仰
在资源有限的环境中,医疗服务提供者必须在相应社区的文化、精神和宗教信仰的背景下,了解和解决以患者为中心的问题以及影响临床效果的障碍。
全球各地的土著人口在伤口愈合方面存在差异。这些差异往往源于历史和当前的社会经济、文化和医疗保健相关因素。此外,文化多样性和社会压力往往决定了某些卫生部门的正式资源分配过程。土著人口中伤口愈合的一些关键障碍包括历史创伤、社会经济差异、获得医疗保健的机会有限、文化障碍、慢性疾病、文化相关愈合实践、地理隔离以及医疗保健系统偏倚36。在许多文化背景下,会采用荣誉制度来管理患者、老年人和慢性疾病患者。如果有足够的资源来维护这些原则,就更容易坚持这些原则。
许多研究表明,重大疾病是一个家庭产生巨额债务的核心原因之一37,38。即使在政府开办的诊所,也可能会向家庭收取额外的敷料或药品费用。因此,患者和家属可能会采用当地/土著/传统治疗者提供的替代治疗方法(非对抗疗法药物)。虽然这些治疗者收取的费用通常较低,但他们可能不具备处理慢性伤口的必要技能或专业知识,从而导致伤口恶化。
因伤口长期存在而失去独立行动能力是影响就诊和定期随访的另一个主要因素。在资源有限的环境中,交通工具的可用性各不相同,步行可能是到达公共交通上车点的唯一方式。偏僻的农村环境往往受影响最大,需要长途跋涉才能到达正规医疗保健机构。
家庭环境的社会健康状况以及将有医疗保健需求的个体纳入其中的意愿,均会影响所提供的护理/自我护理的质量,以及患者在家庭环境中的安全。由于各国的社会结构和环境有所不同,家庭支持和社会支持也因文化而异。通常情况下,随着时间的推移,经济负担会加重;患者的独立性和日常生活活动(ADL)能力会有所下降;更换敷料变得更具挑战性;护理者会感到压力、疲劳和筋疲力尽39。转诊时间过晚以及患者最终接受正式护理时病情危重/处于终末状态,均为进一步导致伤口愈合效果不佳的额外加重社会和行为因素。
在家治疗伤口的患者需要特定的私人空间。在空间有限的情况下,伤口异味、持续疼痛和不同的生活习惯是患者和家属面临的主要压力源。仅仅是严重皮肤伤口就已经对患者自身的社会互动、人际关系、性能力和自信心带来了负面影响。这会导致进行性焦虑和抑郁,并可能会使人对所感知到的治疗获益产生悲观情绪。随后,患者的自我效能感降低,这往往与伤口进一步恶化有关,包括最终的下肢截肢40。
当需要改变生活方式时,真正的挑战才开始。这往往需要额外的资源或量身定制的教育来制定自我保健计划40,41。健康教育和生活方式调整干预措施必须具有明确的原理,这一点怎么强调都不为过。生活方式干预措施可能会成为经济、社会和后勤方面的挑战,首先是获取此类干预措施,然后是在任何受限的生活领域内维持。此外,这是确保将获得的任何经济帮助正确分配给出现伤口的家庭成员的重要步骤。
在资源有限的环境中,有必要采用生物心理社会学方法来管理患者的伤口护理。伤口护理团队除了管理伤口外,还必须解决影响患者的社会压力源/因素。每例患者均需要一个独一无二的、相互协商一致的、适合其限制因素(医疗、经济、家庭、社会和情感支持)的管理计划40。
声明3:愈合能力
3A. 可愈性伤口:确定是否有足够的血液供应来促进伤口愈合,以及是否有适当的治疗方法可用于解决基础病因
3B. 维持性伤口:如果有足够的血液供应促进伤口愈合,但患者无法坚持执行护理计划和/或医疗保健系统没有所需的资源,则调整做法
3C. 不可愈性伤口:如果血液供应不足和/或基础病因无法纠正,则确定其他伤口治疗方法
在确定伤口愈合分类的过程中,首先要对患者进行全面的病史和体格检查。确定伤口的基础病因非常重要。解决基础病因是制定可实现的管理计划的第一步。
在某些情况下,慢性伤口也可能会停止愈合,无法在规定时间内实现伤口边缘的推进;这些伤口被称为难愈性伤口。这些症状通常属于维持性症状,但经过额外评估,即对患者、病史、病因和治疗计划进行重新评价后,可能会愈合3,42。
需要确定以患者为中心的问题和期望,并将其与机构/临床医生资源可用性、技能和可用的即时干预方案进行比较和调整3,42。管理计划以指定的愈合分类为基础,而愈合分类可能会发生变化。
不可愈性伤口需要保护,防止组织损失、伤口深部和周围感染以及伤口潮湿环境造成的整体恶化。当务之急是降低湿度。维持性伤口也需要保护,防止组织进一步损失,主要是进行干伤口床管理和局部感染控制。在有可用资源且其他患者因素得到控制以实现全面优化之前,组织保护也可能是一项临时措施。
停止愈合的可愈性伤口(难愈性伤口)需要第二次治疗来实现边缘推进,重新评估和跨专业团队干预的紧迫性是优先级最高的事项3,42。
声明4:局部伤口护理:检查、测量和监测
4A. 记录伤口较基线的进展情况,以建立可测量的愈合轨迹(纸质和/或照片成像,如可用)。
4B. 用温热的(预煮沸)饮用水、生理盐水或低毒性抗菌剂清洗伤口。酌情考虑浸泡、使用敷布和冲洗。
4C. 利用现有资源,在适当的时间间隔内定期重新评估和记录伤口情况。记录数据并保密。
伤口评估记录是医疗保健实践中不可或缺的组成部分。它有助于确保为患者提供高质量的护理、监测伤口状态,并为伤口干预措施的任何变化提供指导。全面准确地记录伤口评估结果对于确保改善患者结局、医疗保健专业人员之间的有效沟通以及遵守法律法规至关重要43。
监测进展。伤口评估文件可作为伤口随时间进展的历史记录。通过定期记录伤口特征,包括大小、深度、颜色、渗出液、感染、组织类型等,医疗保健专业人员可以跟踪伤口变化、识别潜在并发症并调整治疗计划。如果使用照片记录,则应根据组织政策征得患者同意,确保采用适当的技术、充足的照明并与伤口保持适当的距离,还需在照片中提供测量指南44。这种持续评价对于评价干预措施的有效性和做出有关伤口管理的明智决定至关重要。在测量表面积或体积时,无论采用何种技术,使用的技术必须一致。
沟通。准确的伤口评估有助于促进医疗保健专业人员之间的有效沟通。当所有团队成员均获得一致且最新的伤口记录时,他们就能共同制定并执行协调一致的护理计划,确保患者得到可能的最佳治疗。使用所有学科通用的一致术语非常重要。《国际疾病分类》医学分类系统是全球适用标准的一个范例45。
遵守法律法规。正确的伤口评估和记录对于符合法律法规要求至关重要。即使在资源有限的环境中,不准确或不完整的记录也可能导致法律问题,并对医疗保健专业人员的实践产生负面影响。
报销。在许多医疗保健系统中,正确的伤口评估记录与基于报销的资助模式息息相关。为了证明使用特定伤口护理产品或程序的合理性,并确保机构因其提供的服务而获得足够的补偿,通常需要提供准确且详细的记录。
研究与质量改进。伤口评估数据是研究和质量改进举措的宝贵资源。这种持续的学习过程有助于改善全球伤口护理和患者结局。
以患者为中心的护理。正确的伤口评估和记录是以患者为中心的护理的重要组成部分。它可确保对患者的病情进行全面评价,并根据他们的具体需求制定治疗计划。当患者看到他们的医疗服务提供者致力于记录和监测他们的伤口时,就会増强信任感和患者满意度。
为了促进语言的一致性并提高数据的清晰度,越来越多的企业正在开发电子伤口评估软件和应用程序,以减少全面记录所需的时间43。这些计划和应用程序(有些比其他程序更经济实惠)可为医疗保健团队提供一套结构化参数,以确保记录所有临床特征,从而加强全面沟通46。虽然这些工具是医疗保健专业人员的宝贵财富,但它们也并非没有风险,例如,从先前的报告/会诊记录中错误复制和粘贴或患者数据安全风险。在没有此类计划和应用软件的地区,可能需要向患者提供最新信息,并提供书面评估和干预计划。
声明5:局部伤口护理:清创
5A. 可愈性伤口/病因已纠正:只有在您的实践范围内,才可考虑在充分控制疼痛的情况下对出血组织进行积极的锐性外科清创。该手术只能在高级伤口护理专家的指导下进行。如果不可行,可考虑保守(锐性)清创
5B. 维持性伤口/不可愈性伤口:只有在您的实践范围内,才可考虑在充分控制疼痛的情况下进行保守(锐性)清创。只有在有必要且不会造成任何出血的情况下,才能清除松散的悬垂腐肉/碎片
5C. 确定是否有针对可愈性伤口的其他清创方法可用(如自溶、机械、酶和蛆虫生物疗法)
清创是WBP范例中的一个重要过程,可清除可愈性伤口中的坏死组织和其他生物材料,包括生物膜,并防止维持性伤口产生异味和感染。对于膝关节以下的所有伤口,在尝试清创之前,应将任何血管检测(如ABPI、波形)结果告知跨专业团队的所有成员,并进行相应记录,因为许多类型的检测可能会对血管供应减少的伤口床造成损害。
对于可愈性伤口,最好根据患者和伤口特征,通过WBP范例确定局部伤口床的干预措施。考虑将手术清创(清除出血组织)作为一线干预措施。然而,在许多农村和偏远地区,可能没有受过必要教育、具备必要知识和判断力的熟练的医疗保健专业人员来进行此类手术。
保守(锐性)清创(不会导致出血)需要先进的知识和技能,更适合非急性护理或专科门诊环境。只能清除伤口上松散的悬垂或未附着碎片,不能对伤口床造成外伤。
在资源有限的环境中,临床无菌蛆虫清创疗法是一种有限的治疗方式,意外暴露的情况除外。通常会在更换敷料时发现蛆虫侵染。意外蛆虫治疗可能会产生良好的清创效果,特别是如果幼虫来自选择性较强的丝光绿蝇/铜绿蝇,因为它们的食物来源主要是坏死的组织47,48。如果是普通家蝇(Musca domestica)或其他入侵物种造成的侵染,可能会产生有害结果,因为这些幼虫可能会肆意破坏健康组织47,48。
医护人员应评估社区部门的替代清创方法(如自溶、酶解、机械),包括初级护理、家庭护理和长期护理。在可用范围内,必须考虑患者安全、评估环境因素,并在开始清创前识别伤口愈合的障碍。
维持性伤口和不可愈性伤口的适当清创方案与可愈性伤口的清创方案有很大不同。虽然湿性伤口愈合为伤口愈合提供了最佳环境,但它是一种清创(自溶)形式,可能对不可愈性伤口和维持性伤口有不利影响。一般而言,清创不适合用于稳定的维持性或不可愈性伤口,因为其目的是保持伤口干燥,不受感染49。只有当维持性或不可愈性伤口变得不稳定时,才可考虑进行清创,尽可能以无创方式清除感染或坏死的伤口碎片。
患者目标通常包括提高舒适度、尽可能减少伤口相关异味、减轻疼痛和改善ADL。保持伤口干燥有助于形成保护层,而清创则会带来去除保护层和引入致病微生物的风险。
声明6:局部伤口护理:感染和炎症
6A. 使用局部外用抗菌药物治疗局部/浅表伤口感染(三项或更多NERDS标准)
6B. 使用全身性抗菌药物和联合使用局部外用抗菌剂管理深部和周围伤口感染(三项或更多STONEES标准)
6C. 对于持续发炎的伤口,考虑使用抗炎药(局部外用敷料或全身用药)
经验证的NERDS和STONEES标准可用于指导慢性伤口感染和炎症的评估和治疗50。感染诊断依据是临床体征和症状,而不是浅表伤口拭子,后者应仅在发生感染时用于指导抗菌药物的选择。如果怀疑发生深部和周围组织感染,则应确定细菌种类及其对常用抗菌药物的敏感性,以帮助指导全身性抗菌药物的使用。如果经验疗法对深部感染和周围感染无效,则更应进行上述操作。
用饮用水或生理盐水清洗伤口并在伤口底部取样(不取碎片)后,可获得最佳的伤口床培养拭子组织样本。使用刮匙或其他活检技术获得的组织样本培养物最有可能代表伤口组织中的生物体。另外,使用Levine方法的半定量拭子技术也可用于组织活检51。将拭子置于肉芽组织上,充分按压以提取伤口渗出液,然后旋转360度以覆盖所有拭子的表面。在将拭子放置在皮肤上之前,先将拭子放置在转运培养基中进行预湿润,该操作可提高渗出液较少的伤口患者的细菌收率52。
致病菌可能会渗入骨骼,引起骨髓炎,从而破坏愈合潜力,并且难以治愈。骨活检是诊断可疑骨髓炎的标准,因为浅表培养物无法进入深部骨组织,而影像学检查又因其特异性不一而受到限
制53。不过,骨活检可能使患者感觉不适,并且依赖于熟练的临床医生,还可能会扩大组织损伤。考虑到这些原因,骨活检往往不是一个可行的选项,如果资源匮乏,就更加不可行。
确认发生伤口深部感染时,可使用抗菌敷料为全身性抗生素治疗提供局部支持,并防止表面细菌扩散到深部和周围区域。五种最常见的抗菌敷料分别为银、聚六亚甲基双胍盐酸盐、碘、亚甲蓝/结晶紫和蜂蜜。其中,银和蜂蜜具有额外的抗炎特性54。
在某些伤口中,使用广谱抗菌剂可在短期内迅速减少细菌负担,以支持全身性抗生素的使用。当感染风险大于细胞毒性特性时,每天更换所暴露骨骼上的低成本聚维酮碘湿纱布可以减少表面细菌。这是一种短期策略,同时还需要对血清甲状腺功能水平进行临床评价,尤其是在伤口表面积较大的情况下。不过,随着新型低毒性抗菌剂的问世,有其他侵蚀性较小但同样有效的治疗方案可供选择。
一般而言,伤口愈合时间少于1个月的免疫功能正常患者可使用能覆盖革兰氏阳性菌的药物进行治疗。多种微生物感染(通常见于PWD)或伤口持续时间超过1个月的患者需要使用能覆盖革兰氏阳性菌、革兰氏阴性菌和厌氧菌的广谱制剂,因为此类患者通常也会出现免疫力受损55。
如果对局部抗菌作用的需求大于组织毒性,则细胞毒性药物可能适用于不可愈性伤口。作为抗生素管理的一部分,抗菌剂通常比局部外用抗生素更受青睐,因为抗菌剂产生全身性细菌耐药性的风险较低,并且其导致的与接触性刺激性或接触性过敏性皮炎相关的不良反应也较少54。
6D. 用低毒性溶液(水、生理盐水、无细胞毒性抗菌剂)轻轻清洗伤口。
所用的清洁溶液取决于伤口的特性和实际可用性。有关伤口清洁建议的文献尚未达成共识。2021年更新的Cochrane关于腿部静脉溃疡清洁溶液的综述得出结论,缺乏随机对照试验证据“来指导有关伤口清洁与不清洁相比的有效性以及腿部静脉溃疡最佳清洁方法的决策”56。不过,一般的伤口护理原则涉及低毒性溶液,包括饮用水或预煮沸水、生理盐水和其他伤口友好型抗菌
剂57。这可避免细胞毒性效应和对可愈性伤口中健康肉芽组织的损害。
稀释至0.5%至1.0%的醋酸或次氯酸也可用于某些需要酸性环境的病例(如铜绿假单胞菌局部治疗)58。根据伤口的可愈合性分类,在进行积极的风险-获益评估后,可使用具有一定组织细胞毒性的抗菌剂。这包括低浓度洗必泰或其衍生物聚六亚甲基双胍盐酸盐和聚维酮碘等制剂。使用此类药物可能对伤口感染可能性较高的维持性伤口和不可愈性伤口有利。此外,除了控制生物负荷外,这些制剂还可用于控制异味和渗出液。在资源有限的环境中,需考虑伤口卫生措施以及如何配制、储存和向患者分发溶液,以防止交叉感染。
当前,人们开始关注使用表面活性剂清除生物膜的应用,生物膜通常存在于伤口碎片中,并有两个粘度不同的表面(补充表2,http://links.lww.com/NSW/A177)。对慢性伤口进行伤口冲洗仍是一个具有争议的话题。然而,专家认为,如果无法看到伤口基底,建议不对伤口进行冲洗,以避免冲洗溶液在封闭空间内积聚,意外扩大伤口57。冲洗伤口时,溶液体积需足够(每厘米伤口长度50-100 mL)59。
声明7:局部伤口护理:湿度管理
7A. 使用水凝胶、薄膜、水胶体、水纤维、藻酸盐和泡沫保持可愈性伤口的湿度平衡
7B. 在可愈性伤口中使用液体锁定机制减少水分,使用超强吸水剂吸走表面水分(尿布技术)
7C. 确定可愈性伤口是否需要包扎。它可能是湿润的(提供水分),也可能是干燥的(吸收水分)
7D. 为维持性伤口和不可愈性伤口制定有针对性的降湿方案,以减少细菌増殖
保持湿度平衡非常复杂,取决于伤口类型和愈合分类。在选择敷料时,应考虑湿度平衡、感染控制和患者教育等因素60。根据特定伤口类型和可用资源纳入和调整湿度管理策略,可优化患者治疗效果并将并发症风险降至最低(表6)3,54。进一步的研究和临床研究应继续完善我们对伤口湿度管理的理解,以改进未来的伤口护理实践3,54。
表6.基于伤口愈合能力的湿度管理目标3,42,54

一般而言,随着渗出液的増加,敷料的吸收能力或转移能力也应増加3。湿度平衡敷料可同时具有抗菌和消炎特性,以进一步满足伤口需求。
在为维持性伤口和不可愈性伤口选择敷料时,应将舒适度放在首位,同时避免伤口床积液和伤口边缘浸渍。需要定期重新评估这些伤口的进展或恶化情况,并对其进行管理以减少细菌负荷。根据伤口随时间推移而发生的变化和特性,可能需要对敷料进行调整。
声明8:愈合轨迹
8A. 考虑到可愈行伤口应在第4周前至少缩小20%至40%,以便在第12周前愈合。如果存在影响愈合时间的因素(如血糖控制不佳),则可能需要额外的愈合时间
8B. 如果可用资源有限,则在12周后为可愈性伤口的愈合分配更多时间,并继续提供一致的护理
8C. 优先转诊至专科中心(如可行)进行诊断检测和/或皮肤活检,尤其是在面临严重资源限制的情况下
伤口愈合轨迹对于评价伤口愈合所需的时间非常有用且具有必要性,尤其是在利用急性和慢性伤口的临床数据(测量值)的情况下。伤口愈合轨迹基于精确一致的伤口测量结果,这些测量结果可确定伤口表面积随时间的闭合情况。这有助于区分伤口进展、停止愈合的伤口和恶化的伤口状态。
急性可愈性伤口应在30天内完全闭合。预计慢性可愈性伤口在30天(4周)时边缘会向前推进20%至40%,并在12周内愈合3,42,61,62。不可愈性伤口没有明确分配的闭合时间,预计边缘不会向前推进,所有启动的步骤均旨在防止伤口进一步恶化。预计维持性伤口不会愈合,也不会恶化,伤口愈合可能进展缓慢,没有固定的预期时间,除非患者/机构/系统达到最佳状态。
定期重新评价难愈性伤口,以确定其他诊断结果。在此类病例中,应考虑进行伤口活检、进一步检查和/或转诊至跨专业评估团队。如果不存在新的并发症因素,可以在前4至8周内评估伤口愈合轨迹,以预测伤口是否有可能在第12周前愈合63。伤口、个体或环境发生变化时,可能需要将伤口重新归类为维持性或不可愈性类别。
声明9:边缘推进
9A. 根据所需的作用机制和开始辅助疗法以支持伤口愈合的特定适应症,考虑当地构建的活性疗法
9B. 通过跨专业团队方法决定辅助疗法,并事先进行风险-获益分析
根据可治愈性选择辅助疗法。在重大外伤患者受伤后,应尽快开始这些治疗,防止出现长期慢性后遗症。根据现有条件和所需的物理机制(同时确保外伤组织的可愈合性),采用跨专业团队方法选择治疗方式。对于难愈性伤口,在重新评估后,伤口可能需要进行二次愈合42。确保在做出辅助疗法相关决策时获得患者的知情同意。
风险-获益和成本效益分析会有所帮助,并将増加系统的可持续性。有效辅助疗法决策的关键在于将开始该治疗所带来的风险(身体不适、经济困难、患者依从性)与该治疗带来的获益(组织氧合、伤口收缩、水肿消退、细胞再激活)进行比较42,64。最佳的风险-获益决策是由包括患者在内的跨专业团队共同商讨做出的,以确保在最大程度利用资源的情况下,做出团队承诺并完成治疗。
优化资源或稍微改变资源用途有助于跨专业团队掌握创造性策略,量身定制解决方案,确保所有患者在资源有限的情况下得到最佳治疗。
外科手术。即使是在资源最受限制的环境中,外科手术也是医疗服务覆盖区内最常见的一种治疗方式,通常在三级医疗机构进行,由初级护理诊所转诊,提供清创、带有初级/三级意向性闭合的普通外科手术、皮肤移植和/或截肢手术。
在资源有限的环境中,皮肤移植可作为实现组织闭合的先进方法,以缩短愈合时间并防止深部伤口反复感染。该手术可最大限度地减少长期大量使用敷料的情况,并减轻慢性伤口初级护理的工作负担。决定进行皮肤移植往往是为了保持身体功能,促进伤口尽早愈合,从而达到美观的效
果65。不过,缺血性伤口和大多数静脉郁血性溃疡应避免皮肤移植;仅预期效果良好的伤口才可考虑皮肤移植66,67。
电刺激。通过増强受伤皮肤中的天然电流,可以加速伤口愈合。在资源有限的环境中,应对这种伤口愈合方法进行研究,因为它具有高水平证据基础,并且可以获得具有此类性质的装置(直流电、交流电、低强度直流电、脉冲电磁场、高压脉冲电流和经皮神经电刺激装置)。有证据表明,该方法可増加细胞増殖和微毛细血管灌注,并可减少经处理的伤口床的细菌负担和感染68。
壁挂式负压伤口治疗。此类治疗有助于医疗保健专业人员对渗出液管理进行控制,并准确地为渗出液较多和组织缺损较大的住院患者补充液体。从尽可能低的压力(负50-80 mm Hg)开始。患者需要卧床才能维持此类疗法,而且至少需要每天更换基底层(通常是纱布或凡士林浸没敷料)。此类治疗,如果从尽可能低的负压环境开始,可以防止组织粘连对伤口床造成的机械性外伤,并可在没有非粘连基底层的情况下,防止敷料脱落造成的外伤69。目前,可提供专为社区使用而设计的一次性负压伤口治疗装置,也可提供能带来可接受临床效果的自助选择方案70,71。
呼吸高流量、高浓度氧气。氧气作为一种合法的伤口愈合方法常常被忽视72。具有讽刺意味的是,虽然几乎所有正规医疗保健机构均存有大量氧气,甚至在资源相对匮乏的环境中也是如此,但氧气却鲜少使用。由于COVID-19大流行,氧气浓缩器的供应量也有所増加,这使得门诊供氧在家庭环境中也成为可能73。虽然高压氧治疗是提高血浆氧浓度和组织供氧最有效的方法,但这种方法并不总是随时可用。然而,提供常压(病房)氧治疗仍可使血浆氧含量増加7.5倍(即从0.3 mL/dL増加至2.3 mL/dL;表7)74。此外,在无慢性阻塞性肺病的患者中,间歇性吸入100%氧气(如使用非再吸入型面罩,吸入6小时/停用2小时)3至4天对肺部无害,并可提供大量额外的氧基质,而间歇性増加和减少氧气输送可激活缺氧诱导因子(对血管生成有强烈刺激作用)的表达75。这表明,常压氧治疗可以代替高压氧治疗,用于缓解FDA批准的高压氧适应症列表中的疾病(表7)76。这可能包括组织再灌注损伤(如挤压伤、手术松解前后的骨筋膜室综合征);细菌毒素抑制(即感染性肌坏死或其他厌氧菌感染);或大组织缺损(清创后维持重新激活的炎症反应长达48小时)。常压氧提供的Po2是典型高压氧治疗(2个大气压)的50%;然而,氧的许多药理作用只有在高压氧剂量下才能实现77,78。
表7.常压氧治疗机制和输送系统
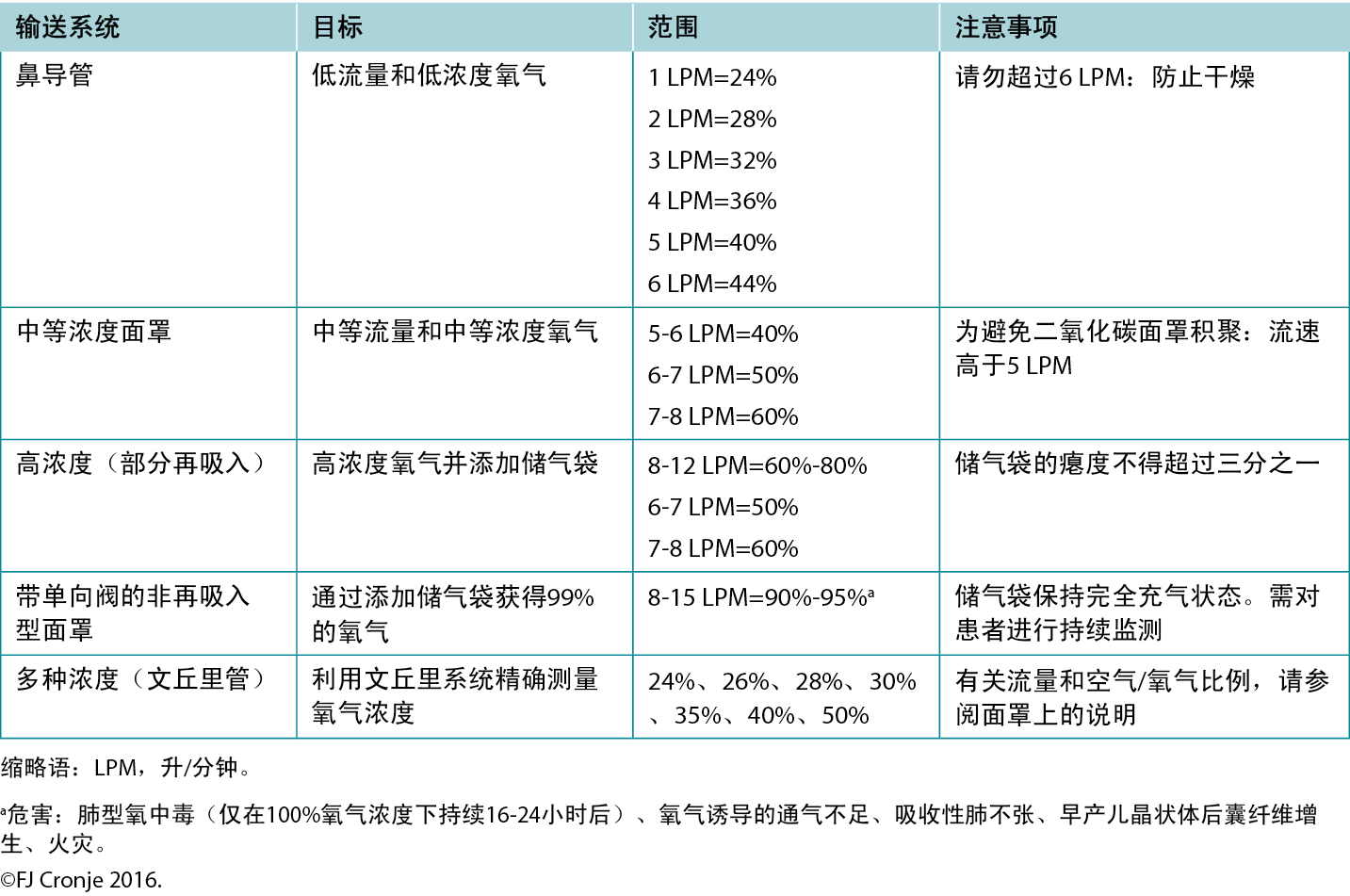
需要注意的是,直接向伤口局部供氧并不会产生与全身供氧相同的生理效应79。局部供氧系统仍需不断研究,以确定其除改善上皮化和可能的轻度抗菌作用之外的有益作用64。后一种作用也归因于臭氧治疗80。2018年完成的一项系统性综述表明,这些治疗有可能引起轻度氧化性应激或消毒,但由于活性氧物质不受控制而产生毒性的风险较高81。在资源有限的环境中,这些装置及其使用很可能不受控制。
声明10:医疗保健系统变革
10A. 促进为所有患者提供有实证依据、符合文化要求和公平的护理
10B. 提高医疗服务提供者的伤口管理能力,以改善患者的治疗结局
10C. 建立覆盖患者和所有跨专业伤口护理团队成员的及时有效的沟通渠道,以改善医疗保健系统的伤口治疗效果
在全球范围内,慢性伤口管理消耗了大量医疗保健资源;预防性护理是降低医疗保健系统支出的最具成本效益的策略。将预防性护理纳入实践的方法多种多样。例如,虽然许多DFU均具有可预防性,但DFU因其导致的高截肢率和高发病率而在世界范围内广为人知。虽然最初认为糖尿病在发达国家最为患病率最高,但值得注意的是,80%与糖尿病相关的死亡发生在资源有限的国家82。无论资源是否充足,各国均可以使用经验证的筛查工具以及受过教育且可用的跨专业团队,并执行预防算法83-85。可实施的其他组织策略包括以下内容。
患者导航。管理DFU需要护理圈的持续支持,护理圈包括作为一个团队工作的家庭成员和医疗保健专业人员(包括家庭护理和伤口护理)。预防和治愈DFU通常需要及时获得医疗和社会服务。事实证明,与跨专业伤口护理诊所相联系的区域化社区糖尿病综合服务最为成功86。
确保最佳和及时护理的一个有效方法是患者导航。这一概念适用于所有医疗保健部门,可以改善伤口感染的及时诊断和治疗,优化疼痛管理,増加获得专业护理的机会,从而加快伤口愈合率87。患者导航正成为综合护理的重要组成部分,促进各部门之间的无缝衔接,提高临床效果。此外,它还能提高患者和医疗保健专业人员的士气,减少非急诊入院或再入院情况,提高患者的生活质量,并改善对治疗方案的依从性。所有这些因素相结合,为医疗保健系统节省了大量成本87,88。
成功的患者导航计划的一个关键因素是采用全面系统的方法来指导医疗保健专业人员评估每例患者的情况并为其提供护理服务(如WBP框架)。这些路径不需要太过复杂或耗时,但应确保符合特定标准,包括对截肢风险较高的人群进行定期足部护理,控制血糖,使HbA1c低于9%,并保持血压低于130/90 mm Hg23,89。
政策干预。对最佳干预措施和路径进行详细说明并提供指导的组织政策,对于伤口护理方案的成功和持续实施至关重要。这些政策以当前公布的针对每种特定伤口类型的指导原则为基础,并对其进行翻译以适应需服务的环境。机构需要将其作为实践标准并获得批准,以便在因文化或语言翻译困难而无法成功采用其他指导原则的环境中用作循证护理。此外,这些政策必须明确概述输入和使用数据的流程,因为持续的质量改进举措基于数据来改进和维持有效的护理流程。
调整伤口护理。虽然护理的提供应适应每个医疗保健部门的独特需求,但某些概念应成为标准,特别是那些能加强部门内部和部门之间有效沟通的概念。数字技术的广泛使用加快了评估速度,増加了获得专业护理的机会,并优化了有限资源的分配90。如果将调整作为护理流程来实施,这些实践应作为护理标准进行记录,并易于获取,以确保相应机构内护理的一致性和连续性。
Delphi共识要点
小组成员提出了一些重要意见:
- 愈合能力(愈合性)是一种可变化的概念,不能将其视为一种静态分类,因为患者的病情、环境和选择会推动分类分配过程(3A)。
- 维持性伤口所需的实践调整包括制定保守的伤口床方法,同时关注患者在其生活选择中愿意做出的让步,以慢慢确保患者处于最佳状态,并随之提高患者的主体能力(3B)。
- 当机构内部就患者记录方法达成一致时,应统一使用这种方法,以防止医疗服务提供者之间出现沟通漏洞,避免无意中对患者护理结局造成负面影响(4A)。
- 适用于粘膜的抗菌漱口水通常也适合用于伤口床(4B)。这可能被视为超说明书使用。
- 局部细菌负担控制应选择局部外用(低)毒性抗菌剂(最重要的五种),这取决于愈合能力和需优先处理的细菌负担。避免对慢性伤口使用局部外用抗生素制剂、软膏和乳霜,因为此类制剂通常仅针对革兰氏阳性菌,会让伤口床上的革兰氏阴性菌和厌氧菌自由繁殖。此外,如果局部外用抗生素制剂发生一次突变,其便会对靶向生物产生全身耐药性。局部外用抗生素通常使用与接触性刺激性或接触性过敏性皮炎有关的载体介质(6A和6B)。
- 如果可愈性伤口有明显的水分负担,仍然需要使用超强吸收敷料来控制伤口床的湿度平衡,则需要对伤口进行全面的重新评估,以确保所有基础病因均已得到纠正(7B)。
- 在建立跨专业团队时,需利用一切沟通手段来建立和维持这一团队,因为虽然医疗服务提供者和专家之间距离遥远,但团队合作可以极大地优化当地护理能力,从而提高临床效果(9B)。
结论
在资源有限的环境中优化慢性伤口护理,在不损害所需核心护理原则的前提下,进行微小调整和创造性干预。跨专业伤口护理团队可以为隔离和偏远社区提供虚拟资源,以改善临床效果。通过提高当地护理能力、提供临床医生教育,并在文化方面给予适当的患者授权,(糖尿病)足部管理和护理的所有关键因素均可以很容易地融入不同的环境中。
实践要点
- PWD的整体护理包括优化HbA1c水平、血压、胆固醇水平以及使用具有心脏和肾脏保护作用的药物。
- 手持式声学8 MHz多普勒脉冲信号是一种适用于床边检测下肢动脉血液供应的方法。它可以作为传统ABPI的改良版进行,远程专家团队成员使用手机的MP3或MP4录音功能即可轻松验证脉搏声。
- NERDS和STONEES助记符可以指导局部/深部和周围感染的诊断和治疗,包括口服抗生素适应症骨髓炎。
- 足底压力再分布可以通过创新、成本较低的替代方法来实现,如软毛毡简易衬垫,以及全接触石膏和以不可拆卸方式安装的可拆卸石膏助行器(如果后者不可用或不适合患者偏好或ADL)。
- 综合协调护理团队可以通过使医疗保健专业人员具备患者导航技能来与虚拟专业知识建立联系。
- 工具包中包含的实践辅助工具以及8 MHz多普勒仪和红外测温仪可为资源有限环境中的护理工作提供便利。
利益冲突声明
作者声明无利益冲突。
资助
作者未因该项研究收到任何资助。
Author(s)
Hiske Smart*
MA RN PG Dip (UK)
Clinical Nurse Specialist: Wound Specialist Services King Hamad American Mission Hospital, A’Ali, Kingdom of Bahrain
R Gary Sibbald
MD Med FRCPC (Med Derm) FAAD MAPWCA JM
Professor of Medicine and Public Health, Dalla Lana School of Public Health, University of Toronto, Ontario, Canada
Laurie Goodman
MHScN RN Advanced Practice Nurse
WoundPedia, Mississauga, Ontario, Canada
Elizabeth A Ayello
PhD RN CWON ETN MAWPCA FAAN
President, Ayello Harris & Associates, Inc, Copake, New York, USA
Reneeka Jaimangal
MD MDcCH IIWCC
Project Manager, Project ECHO Ontario Skin and Wound Care, WoundPedia, Mississauga, Ontario, Canada
John H Gregory
BEng
President, Opencity Inc, Kitchener, Ontario, Canada
Sadanori Akita
MD PhD
Professor, Fukushima Medical University, Fukuoka, Japan
Afsaneh Alavi
MD FRCPC
Professor of Dermatology, Mayo Clinic, Rochester, Minnesota, USA
David G Armstrong
DPM MD PhD
Professor of Surgery, Keck School of Medicine, University of Southern California, Los Angeles, California, USA
Helen Arputhanathan
MSc RN NSWOC WOCC(C)
Manager, Patient Services & Clinical Wound Care, Home and Community Care Support Services, Hamilton Niagara Haldimand Brant, Waterloo, Ontario, Canada
Febe Bruwer
PhD (UFS) MSocSci MSc
Advanced Nurse Specialist and Unit Manager of the Wound Clinic, Life Roseacres Hospital, Germiston, South Africa
Jeremy Caul
MClSc-WH RN WOCC(c)
Nurse Advisor, Home and Community Care, Ontario Region for Indigenous Services Canada/First Nations and Inuit Health Branch, Ontario, Canada
Beverley Chan
MD MSc,FRCSC
Vascular Surgeon and Division Lead, Halton Healthcare, Oakville, Ontario, Canada
Frans Cronje
MBChB MSc
Aerospace Medicine, PGDOccMed, Fellow EHM (Duke), Aviation and Aerospace Medical Specialist, Head of Department Baromedicine, King Hamad American Mission Hospital, A’ali, Kingdom of Bahrain
Belen Dofitas
MD PhD
Associate Professor, Department of Dermatology, College of Medicine & Philippine General Hospital, University of the Philippines, Manila
Jassin Hamed
MD
Consultant Internal Medicine, COO Global Care Hospital, Abu Dhabi, United Arab Emirates
Catherine Harley
eMBA RN
Chief Executive Officer, Nurses Specialized in Wound, Ostomy and Continence Canada, Ottawa, Ontario, Canada
Jolene Heil
MclSc-WH RN NSWOC
Advanced Practice Nurse and Clinical Nurse Specialist—Wound Care, Providence Care, Kingston, Ontario, Canada
Mary Hill
MN BScN RN NSWOC WOCC(C)
NSWOC Educator/Consultant CAT Team, Integrated Home Care—Calgary Zone, Alberta, Canada
Devon Jahnke
DCh MCISc-WH
Chiropodist, Complex Centre for Diabetes Care, Health Sciences North, Sudbury Outpatient Centre, Ontario, Canada
Dale Kalina
MD MBA FRCPC (ID)
Chief Medical Information Officer, Brant Community Healthcare System, Brantford, Ontario, Canada
Chaitanya Kodange
MBBS DMM DHA MD (Psy) IIWCC-UAE
Consultant Diving, Hyperbaric and Wound Care & Psychiatrist, King Hamad University Hospital, Kingdom of Bahrain
Bharat Kotru
PhD MSc DPM
Podiatrist and Wound Care Specialist, Advance Foot & Wound Care Centre, Amandeep Group of Hospitals, Amritsar, Punjab, India
Laura Lee Kozody
DCh
Chiropodist, Toronto Regional Wound Healing Clinic, Mississauga, Ontario, Canada
Stephan Landis
MD FRCPC
Consultant, Guelph General Hospital Ambulatory Wound Clinic, Guelph, Ontario, Canada
Kimberly LeBlanc
PhD RN NSWOC WOCC(C) FCAN
Academic Chair, Nurses Specializing in Wound, Ostomy and Continence Canada, Ottawa, Ontario, Canada
Mary MacDonald
MD PhD FRCSC
Assistant Professor, School of Medicine, NOSM University, and Vascular Surgeon, Thunder Bay Regional Health Sciences Centre, Thunder Bay, Ontario, Canada
Tobi Mark
BSc DCh
Associate Professor of Chiropody, The Michener Institute of Education at UHN, Toronto, Ontario, Canada
Carlos Martin
DM PG-Dip MBBS
Consultant, Vascular Surgery, Georgetown Public Hospital Corporation, Guyana
Dieter Mayer
MD FAPWCA Consultant, Institute for Advanced Wound Care & Education, Hausen am Albis, Switzerland
Christine Murphy
PhD MClSc-WH RN NSWOC WOCC(C)
Advanced Practice Nurse, The Ottawa Hospital, Ontario, Canada
Harikrishna Nair
MD PhD FRCPI FRCPE FCWCS
Head and Senior Wound Care Physician, Wound Care Unit, Department of Internal Medicine, Kuala Lumpur Hospital, and Professor, Faculty of Medicine, Lincoln University College, Malaysia
Cesar Orellana
MD FRCPC FACP
Infectious Diseases Consultant, Grand River Hospital and St Mary’s General Hospital, Kitchener, Ontario, Canada
Brian Ostrow
MD BSc FRCS(C)
Adjunct Assistant Professor (retired), Department of Surgery, University of Toronto, Ontario, Canada
Douglas Queen
PhD MBA
Chief Executive Officer, Medicalhelplines.com Inc, Toronto, Ontario, Canada
Patrick Rainville
DCh,
Owner, Rainville Foot Health, Timmins, Ontario, Canada
Erin Rajhathy
MclSc-WH RN NSWOC WOCC(C)
Doctoral Student, Swedish Centre for Skin and Wound Research, Nursing Science Unit, School of Health Sciences, Faculty of Medicine and Health, Örebro University, Örebro, Sweden
Gregory Schultz
PhD
Professor Emeritus, University of Florida, Gainesville, Florida, USA
Ranjani Somayaji
MD MPH FRCPC
Associate Professor, University of Calgary, Calgary, Canada
Michael C Stacey
DS MBBS FRACS
Vascular Surgeon and Professor of Surgery, McMaster University, Hamilton, Ontario, Canada
Gulnaz Tariq
MSc (UK) RN PG Dip (PAK)
Director of Wound Care & Education, Global Care Hospital, Abu Dhabi, United Arab Emirates
Gregory Weir
MBChB Mmed (Ch)
Vascular Surgeon, Life Eugene Marais Hospital, Pretoria, South Africa
Catharine Whiteside
MD PhD CM FRCPC
Professor Emerita and Former Dean of Medicine, University of Toronto, Ontario, Canada
Helen Yifter
MD
Associate Professor of Medicine, University of Rwanda, Kigali, Rwanda
Ramesh Zacharias
MD FRCS(C)
Assistant Clinical Professor, Anesthesia, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada
* Corresponding author
References
- Sibbald RG, Williamson D, Orsted HL, et al. Preparing the wound bed—debridement, bacterial balance and moisture balance. Ostomy Wound Manage 2000;46(11):14-35.
- Fitridge R, Chuter V, Mills J, et al. The intersocietal IWGDF, ESVS, SVS guidelines on peripheral artery disease in people with diabetes mellitus and a foot ulcer. J Vasc Surg 2023;78(5):1101-31.
- Sibbald RG, Elliott JA, Persaud-Jaimangal R, et al. Wound Bed Preparation 2021. Adv Skin Wound Care 2021;34(4):183-95.
- Alavi A, Sibbald RG, Nabavizadeh R, Valaei F, Coutts P, Mayer D. Audible handheld Doppler ultrasound determines reliable and inexpensive exclusion of significant peripheral arterial disease. Vascular 2015;23(6):622-9.
- Dworak M, Andraska EA, Gharacholou SM, Myers M, Chapman SC. Fluorescent angiography used as a tool to guide angiosome-directed endovascular therapy for diabetic foot ulcers. J Vasc Surg Cases Innov Tech 2021;7(1):159-63.
- Sassaki VS, Fukaya E. Varicose veins: approach, assessment, and management to the patient with chronic venous disease. Med Clin North Am 2023;107(5):895-909.
- Armstrong DG, Boulton AJM, Sicco AB. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376(24):2367-75.
- Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377(14):1319-30.
- Levin ME. An overview of the diabetic foot: pathogenesis, management and prevention of lesions. Int J Diab Dev Countries 1994;14:39-47.
- Bekele F, Chelkeba L, Fekadu G, Bekele K. Risk factors and outcomes of diabetic foot ulcer among diabetes mellitus patients admitted to Nekemte referral hospital, Western Ethiopia: prospective observational study. Ann Med Surg (Lond) 2020;51:17-23.
- Govindasamy K, Darlong J, Watson SI, Gill P. Prevalence of plantar ulcer and its risk factors in leprosy: a systematic review and meta-analysis. J Foot Ankle Res 2023;16(1):77.
- Sibbald RG, Ayello EA, Alavi A, et al. Screening for the high-risk diabetic foot: a 60-Second Tool. Adv Skin Wound Care 2012;25(10):465-76.
- Sibbald RG, Mufti A, Armstrong DG. Infrared skin thermometry: an underutilized cost-effective tool for routine wound care practice and patient high-risk diabetic foot self-monitoring. Adv Skin Wound Care 2015;28(1):37-44.
- Amemiya A, Noguchi H, Oe M, et al. Factors associated with callus formation in the plantar region through gait measurement in patients with diabetic neuropathy: an observational case-control study. Sensors 2020;20:4863.
- Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res 2020;13(1):16.
- Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2018;42(Suppl 1):S1-325.
- Zhang X, Yang X, Sun B, et al. Perspectives of glycemic variability in diabetic neuropathy: a comprehensive review. Commun Biol 2021;4:1366.
- Bril V, Breiner A, Perkins BA, Zochodne D. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Neuropathy. Can J Diabetes 2018;42:S217-21.
- Soyoye DO, Abiodun OO, Ikem RT, Kolawole BA, Akintomide AO. Diabetes and peripheral artery disease: a review. World J Diabetes 2021;12(6):827-38.
- Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health 2019;7:e1020-30.
- Williams B, Mancia G. Ten commandments of the 2018 ESC/ESH HTN guidelines on hypertension in adults. Eur Heart J 2018;39:3007-8.
- Dagenais GR. Vascular protection: telmisartan in the ONTARGET trial programme. Eur Heart J Suppl 2009;11(suppl_F):F47-53.
- Gerstein HC. Diabetes and the HOPE study: implications for macrovascular and microvascular disease. Int J Clin Pract Suppl 2001;(117):8-12.
- Fonseca-Correa JI, Correa-Rotter R. Sodium-glucose cotransporter 2 inhibitors mechanisms of action: a review. Front Med 2021;8:777861.
- Rasmussen BSB, Yderstraede KB, Carstensen B, et al. Substantial reduction in the number of amputations among patients with diabetes: a cohort study over 16 years. Diabetologia 2016;59:121-9.
- de Mestral C, Hussain MA, Austin PC, et al. Regional health care services and rates of lower extremity amputation related to diabetes and peripheral artery disease: an ecological study. CMAJ Open 2020;8(4):E659-66.
- Baird R, Cosh L, Bruser G, Rowe R, Walker J. Indigenous Diabetes Health Circle: Foot Care Evaluation Program. April 2022. https://idhc.life/wp-content/uploads/2023/02/IDHC_Foot-Care-Research-Report.pdf. Last accessed January 3, 2024.
- Bus SA, Armstrong DG, Crews RT, et al. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev 2023:e3647.
- Withers RV, Perrin BM, Landorf KB, et al. Offloading effects of a removable cast walker with and without modification for diabetes-related foot ulceration: a plantar pressure study. J Foot Ankle Res 2023;16:27.
- Moffatt CJ, Franks PJ, Hollinworth H. Understanding wound pain and trauma: an international perspective. In: Pain at Wound Dressing Changes: A Position Document. European Wound Management Association; 2002:2-7.
- Queen D, Woo K, Shulz VN, Sibbald RG. Chronic wound pain and palliative cancer care. Ostomy Wound Manage 2003;49(10):16-8.
- Reddy M, Kohr R, Queen D, Keast D, Sibbald RG. Practical treatment of wound pain and trauma: a patient-centered approach. An overview. Ostomy Wound Manage 2003;49(4A):2-15.
- Boonstra AM, Stewart RE, Köke AJ, et al. Cut-off points for mild, moderate, and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal pain: variability and influence of sex and catastrophizing. Front Psychol 2016;7:1466.
- Byma EA, Wheeler H. The experience of new graduate registered nurses as managers of pain. Pain Manage Nurs 2021;22(3):429-35.
- D’Souza RS, Barman R, Joseph A, Abd-Elsayed A. Evidence-based treatment of painful diabetic neuropathy: a systematic review. Curr Pain Headache Rep 2022;26(8):583-94.
- Sibbald RG, Hastings-Truelove A, DeJong P, Ayello EA. Reconciliation and diversity for educators: the medicine wheel, Bloom’s taxonomy, and CanMEDS competencies. Adv Skin Wound Care 2023;36(2):64-6.
- Murphy A, McGowan C, McKee M, et al. Coping with healthcare costs for chronic illness in low-income and middle-income countries: a systematic literature review. BMJ Global Health 2019;4:e001475.
- Okediji PT, Ojo AO, Ojo AI, Ojo AS, Ojo OE, Abioye-Kuteyi EA. The economic impacts of chronic illness on households of patients in Ile-Ife, South-western Nigeria. Cureus 2017;9(10):e1756.
- Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA 2014;311(10):1052-60.
- Kodange C. Screening for depression in patients with chronic wounds. Adv Skin Wound Care 2021;34(9):502-3.
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022;45(11):2753-86.
- Boersema GC, Smart H, Giaquinto-Cilliers MGC, et al. Management of nonhealable and maintenance wounds: a systematic integrative review and referral pathway. Adv Skin Wound Care 2021;34(1):11-22.
- Ebbers T, Kool RB, Smeele LE, et al. The impact of structured and standardized documentation on documentation quality; a multicenter, retrospective study. J Med Syst 2022;46(7):46.
- Onuh OC, Brydges HT, Nasr H, Savage E, Gorenstein S, Chiu E. Capturing essentials in wound photography past, present, and future: a proposed algorithm for standardization. Adv Skin Wound Care 2022;35:483-92.
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 2023. www.who.int/standards/classifications/classification-of-diseases. Last accessed January 3, 2024.
- Wurster F, Fütterer G, Beckmann M, et al. The analyzation of change in documentation due to the introduction of electronic patient records in hospitals—a systematic review. J Med Syst 2022;46(8):54.
- Nair HK, Ahmad NW, Ismail AA, et al. Maggot debridement therapy to treat hard-to-heal diabetic foot ulcers: a single-centre study. J Wound Care 2021;30(Sup12):S30-6.
- Williams KA, Cronje FJ, Avenant L, Villet MH. Identifying flies used for maggot debridement therapy. S Afr Med J 2008;98(3):196-9.
- Tran DL, Huang RW, Chiu ES, et al. Debridement: technical considerations and treatment options for the interprofessional team. Adv Skin Wound Care 2023;36(4):180-7.
- Woo KY, Sibbald RG. A cross-sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manage 2009;55(8):40.
- Angel DE, Lloyd P, Carville K, Santamaria N. The clinical efficacy of two semi‐quantitative wound‐swabbing techniques in identifying the causative organism(s) in infected cutaneous wounds. Int Wound J 2011;8(2):176-85.
- Rawlinson S, Ciric L, Cloutman-Green E. How to carry out microbiological sampling of healthcare environment surfaces? A review of current evidence. J Hosp Infect 2019;103(4):363-74.
- Hatzenbuehler J, Pulling TJ. Diagnosis and management of osteomyelitis. Am Fam Physician 2011;84(9):1027-33.
- Sibbald RG, Elliott JA, Ayello EA, Somayaji R. Optimizing the moisture management tightrope with Wound Bed Preparation 2015©. Adv Skin Wound Care 2015;28(10):466-76.
- Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med 1999;341(25):1906-12.
- McLain NE, Moore ZE, Avsar P. Wound cleansing for treating venous leg ulcers. Cochrane Database Syst Rev 2021;3(3):CD011675.
- Sibbald RG, Goodman L, Woo KY, et al. Special considerations in Wound Bed Preparation 2011: an update©. Adv Skin Wound Care 2011;24(9): 415-36.
- Block MS, Rowan BG. Hypochlorous acid: a review. J Oral Maxillofacial Surg 2020;78(9):1461-6.
- International Wound Infection Institute. Wound infection in clinical practice: principles of best practice. 3rd ed. Wounds Int 2022:1-60.
- Giaquinto-Cilliers MGC. Classification of dressings: a framework adapted to the Wound Bed Preparation Paradigm. Wound Heal South Afr 2023;16(2):31-3.
- Berezo M, Budman J, Deutscher D, Hess CT, Smith K, Hayes D. Predicting chronic wound healing time using machine learning. Adv Wound Care 2022;11(6):281-96.
- Pavlovčič U, Diaci J, Možina J, et al. Wound perimeter, area, and volume measurement based on laser 3D and color acquisition. Biomed Eng Online 2015;14(39).
- Laporte M, Keller HH, Payette H, et al. Validity and reliability of the new Canadian Nutrition Screening Tool in the ‘real-world’ hospital setting. Eur J Clin Nutr 2015;69(5):558-64.
- Feldmeier JJ, Hopf HW, Warriner R3, Fife CE, Gesell LB, Bennett M. UHMS position statement: topical oxygen for chronic wounds. Undersea Hyperb Med 2005;32(3):157-68.
- Guzman KJ, Gemo N, Martins DB, et al. Current challenges of plastic surgical care in sub-Saharan Africa (Maputo, Mozambique). Plast Reconstr Surg Glob Open 2018;6(8):e1893.
- Jones JE, Nelson EA. Skin grafting for venous leg ulcers. Cochrane Database Syst Rev 2007;(2):CD001737.
- Kirketerp-Møller K, Doerfler P, Schoefmann N, et al. Biomarkers of skin graft healing in venous leg ulcers. Acta Derm Venereol 2022;102:adv00749.
- Thakral G, Lafontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabetes Foot Ankle 2013;4.
- Chaput B, Garrido I, Eburdery H, Grolleau JL, Chavoin JP. Low-cost negative-pressure wound therapy using wall vacuum: a 15 dollars by day alternative. Plast Reconstr Surg Glob Open 2015;3(6):e418.
- Estillore KM, Quevedo GL, Bonifacio LR. Improvised suction apparatus for closure of large soft tissue deficit. Malaysian Orthop J 2013;7(2):29.
- Farré R, Rodríguez-Lázaro MA, Gonzalez-Martin J, et al. Device for negative pressure wound therapy in low-resource regions: open-source description and bench test evaluation. J Clin Med 2022;11(18):5417.
- Rose S, Sardar S, Sasi S, Al Mohanadi DH, Al-Mohammed AA, Zahid M. Time for change in practice of in-patient oxygen therapy: a period-limited, multidimensional approach to improve oxygen prescription compliance: quality improvement project at Hamad General Hospital, Qatar. BMJ Open Qual 2021;10(4):e001574.
- McAllister S, Thorn L, Boladuadua S, et al. Cost analysis and critical success factors of the use of oxygen concentrators versus cylinders in sub-divisional hospitals in Fiji. BMC Health Serv Res 2021;21(1):1-7.
- Jain KK. Physical, physiological, and biochemical aspects of hyperbaric oxygenation. In: Jain KK, ed. Textbook of Hyperbaric Medicine. 6th ed. Springer; 2017:11-22.
- Chang AJ, Bargmann CI. Hypoxia and the HIF-1 transcriptional pathway reorganize a neuronal circuit for oxygen-dependent behavior in Caenorhabditis elegans. Proc Natl Acad Sci USA 2008;105(20):7321-6.
- Moon RE. Hyperbaric Oxygen Therapy Indications. North Palm Beach, FL: Best Publishing Company; 2019.
- Chazalviel L, Blatteau JE, Vallée N, Risso JJ, Besnard S, Abraini JH. Effects of normobaric versus hyperbaric oxygen on cell injury induced by oxygen and glucose deprivation in acute brain slices. Med Gas Res 2016;6(3):169-73.
- Velej V, Cankar K, Vidmar J. The effects of normobaric and hyperbaric oxygenation on MRI signal intensities in T1 -weighted, T2 -weighted and FLAIR images in human brain. Radiol Oncol 2023;57(3):317-24.
- UHMS position statement: topical oxygen for chronic wounds. Undersea Hyperb Med 2018;45(3):379-80.
- Rapone B, Ferrara E, Santacroce L, et al. The gaseous ozone therapy as a promising antiseptic adjuvant of periodontal treatment: a randomized controlled clinical trial. Int J Environ Res Public Health 2022;19(2):985.
- Fitzpatrick E, Holland OJ, Vanderlelie JJ. Ozone therapy for the treatment of chronic wounds: a systematic review. Int Wound J 2018;15(4):633-44.
- Woodbury M, Sibbald RG, Ostrow B, Persaud R, Lowe J. Tool for rapid & easy identification of high-risk diabetic foot: validation & clinical pilot of the simplified 60 second diabetic foot screening tool. PLoS One 2015;10(6):1-3.
- Abbas ZG, Lutale JK, Bakker K, Baker N, Archibald LK. The ‘step by step’ diabetic foot project in Tanzania: a model for improving patient outcomes in less-developed countries. Int Wound J 2011;8:169-75.
- Abbas ZG. Reducing diabetic limb amputations in developing countries. Expert Rev Endocrinol Metab 2015;10:425-34.
- Ousey K, Chadwick P, Jawien A, et al. Identifying and treating foot ulcers in patients with diabetes: saving feet, legs and lives. J Wound Care 2018;27(5 Suppl 5b).
- Heerschap C, Nicholas A, Whitehead M. Wound management: investigating the interprofessional decision-making process. Int Wound J 2019;16(1):233-42.
- Arputhanathan H, Hyde J, Atiloa T, Queen D, Elliott J, Sibbald RG. A patient navigation model to improve complex wound care outcomes. Adv Skin Wound Care 2022;35(9):499-508.
- Doucet S, Luke A, Anthonisen G, et al. Hospital-based patient navigation programmes for patients who experience injury-related trauma and their caregivers: a scoping review protocol. BMJ Open 2022;12:e055750.
- Narayan KMV, Zhang P, Kanaya AM, et al. Diabetes: the pandemic and potential solutions. In: Jamison DT, Breman JG, Measham AR, et al, eds. Disease Control Priorities in Developing Countries. 2nd ed. Washington, DC: World Bank; 2006:591-603.
- Kostovich CT, Etinggen B, Wirth M, et al. Outcomes of telehealth for wound care: a scoping review. Adv Skin Wound Care 2022;35(7):394-403.
Supplemental Table 1. Delphi consensus achieved among 41 contributors on 32 wound bed preparation (wbp) 2024 statements
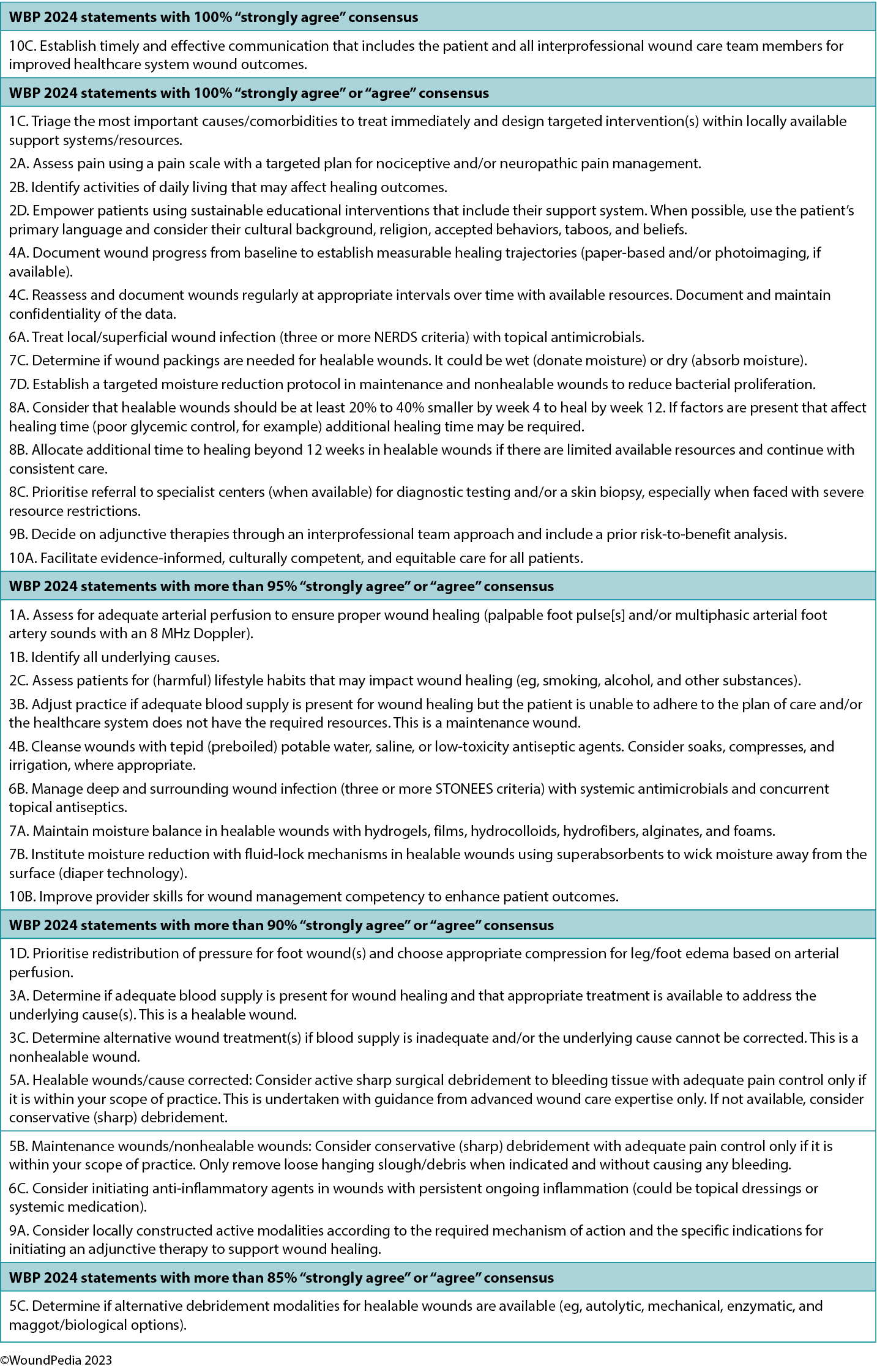
Supplement Table 2. Common antiseptic agents for nonhealable wounds
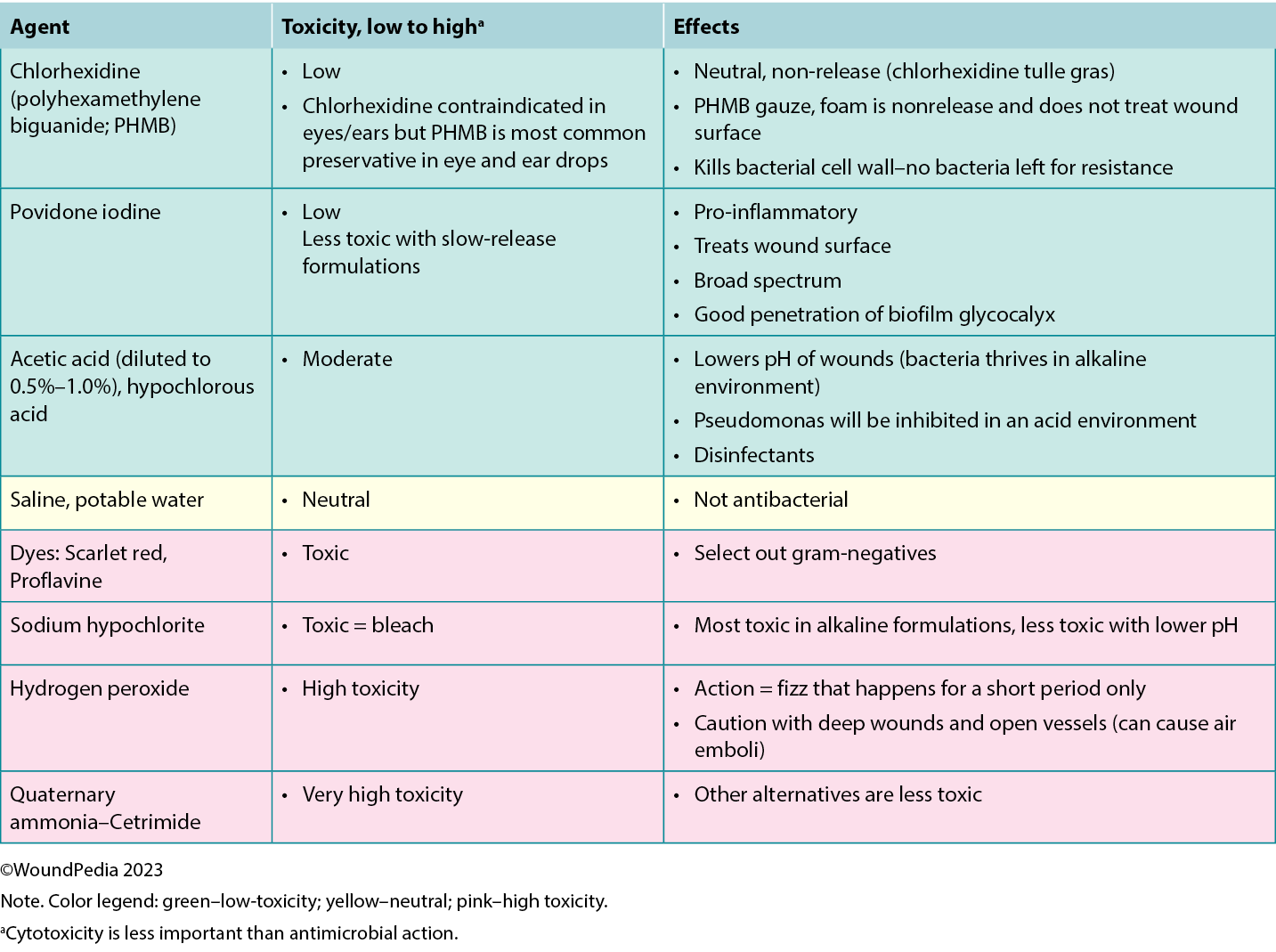
补充表1.41位贡献者就32项伤口床准备(wbp)2024声明达成Delphi共识

补充表2.常用于不可愈合伤口的抗菌剂