Volume 44 Number 3
Examining the association of immunosuppressants and wound healing: a narrative review
Aria Appoo, Brandon L Christensen, Ranjani Somayaji
Keywords wound healing, Calcineurin inhibitors, glucocorticoids, immunosuppressants, monoclonal antibodies, mTOR inhibitors
For referencing Appoo A, Christensen BL, Somayaji R. Examining the association of immunosuppressants and wound healing: a narrative review. WCET® Journal 2024;44(3):12-19.
DOI 10.33235/wcet.44.3.12-19
Abstract
Objective To review how different classes of immunosuppressants affect wound healing.
Data Sources A literature search was conducted in PubMed, Google Scholar, and the University of Calgary Health Sciences Library.
Study Selection The researchers initially screened article titles using key words such as “immunosuppressive medication,” “wound healing,” and “immunosuppression.” Articles in which the title and/or abstract contained these key words, that addressed wound healing related to immunosuppressant medications, and were published after 2000 were included in the review. When human data were not available for an immunosuppressant (class), animal studies were included.
Data Extraction The 61 included articles underwent full text review and summarisation.
Data Synthesis: All included studies were summarised descriptively including immunosuppressive mechanism of action, study participants or subjects, and evidence of effects on wound healing.
Conclusions Corticosteroids and mechanistic target of rapamycin inhibitors most consistently demonstrate detrimental effects on wound healing. For other classes of immunosuppressants, evidence is limited with varying effects on wound healing described. Larger, high-quality studies are required to better understand the effects of immunosuppressants, including those with new mechanisms of action, to identify those with the most impact on wound healing.
Introduction
Immunosuppressants are medications with a variety of indications including in solid organ and hematopoietic transplants and autoimmune diseases. They function by suppressing the activity of various components of the adaptive immune system, thus diminishing the cascade of inflammatory response to normal host tissue or modulating the natural rejection response to transplanted materials.1 The main classes of immunosuppressants are corticosteroids/glucocorticoids,2,3 calcineurin inhibitors (CNIs),2,4,5 mechanistic target of rapamycin (mTOR) inhibitors,2,4 monoclonal antibodies (mAbs),2,4 polyclonal antibodies (pAbs),2,4 and antiproliferative agents.2 For the purpose of this review, wounds are defined as an opening in the skin as a result of surgery, trauma, or disease that is susceptible to infection.
The immune system plays an important role in infection prevention as well as the healing process of wounds; inflammatory effects lead to cellular proliferation and secretion of important intracellular and extracellular components.6 With immunosuppressants, the immune system is modulated, thus potentially affecting a wound’s healing time and susceptibility to infection.7 With a growing number of patients on immunosuppressing medications, particularly patients postsurgical transplant, the effect of immunosuppressants on wound healing is an important issue. This review article aims to provide clinicians with an understanding of how different classes of immunosuppressants affect wound healing.
Methods
The authors conducted a literature search using the generic names of several common immunosuppressants (glucocorticoids/corticosteroids, mTOR inhibitors, methotrexate, mAbs, pAbs, CNIs, mycophenolate, azathioprine), as well as the terms “wound healing” and “immunosuppression.” The primary database searched was PubMed, supplemented by Google Scholar and the University of Calgary Health Science Library database. When possible, the search formatted as follows: “immunosuppressant name [MeSH Terms] AND “wound healing [MeSH Terms].” If the immunosuppressant name was not available as a MeSH term, then the term was searched with no restriction applied. The search was limited to articles in English published between 2000 and 2021.
The researchers screened article titles and abstracts for relevance. Articles were considered relevant if they compared various immunosuppressants, discussed their effects on wound healing, and measured wound healing or reported deleterious effects on wounds. If search terms did not identify any studies with human participants, the authors then included studies that used animals to evaluate the immunosuppressive effects of a given drug class on wound healing. When no data were available from 2000 onward, researchers conducted a historic search for the relevant immunosuppressive medications.
All included studies were summarised descriptively including immunosuppressive mechanism of action, study participants/subjects, and evidence of effects on wound healing.
Results
The authors screened 200 article titles and abstracts, and of these, 61 articles were included in the review. Table 1 highlights the results of select clinical and animal studies. The specific indications for various immunosuppressants including their possible impacts on wounds are outlined in Table 2.
Table 1. Select studies comparing the effects of different immunosuppressants on wound healing
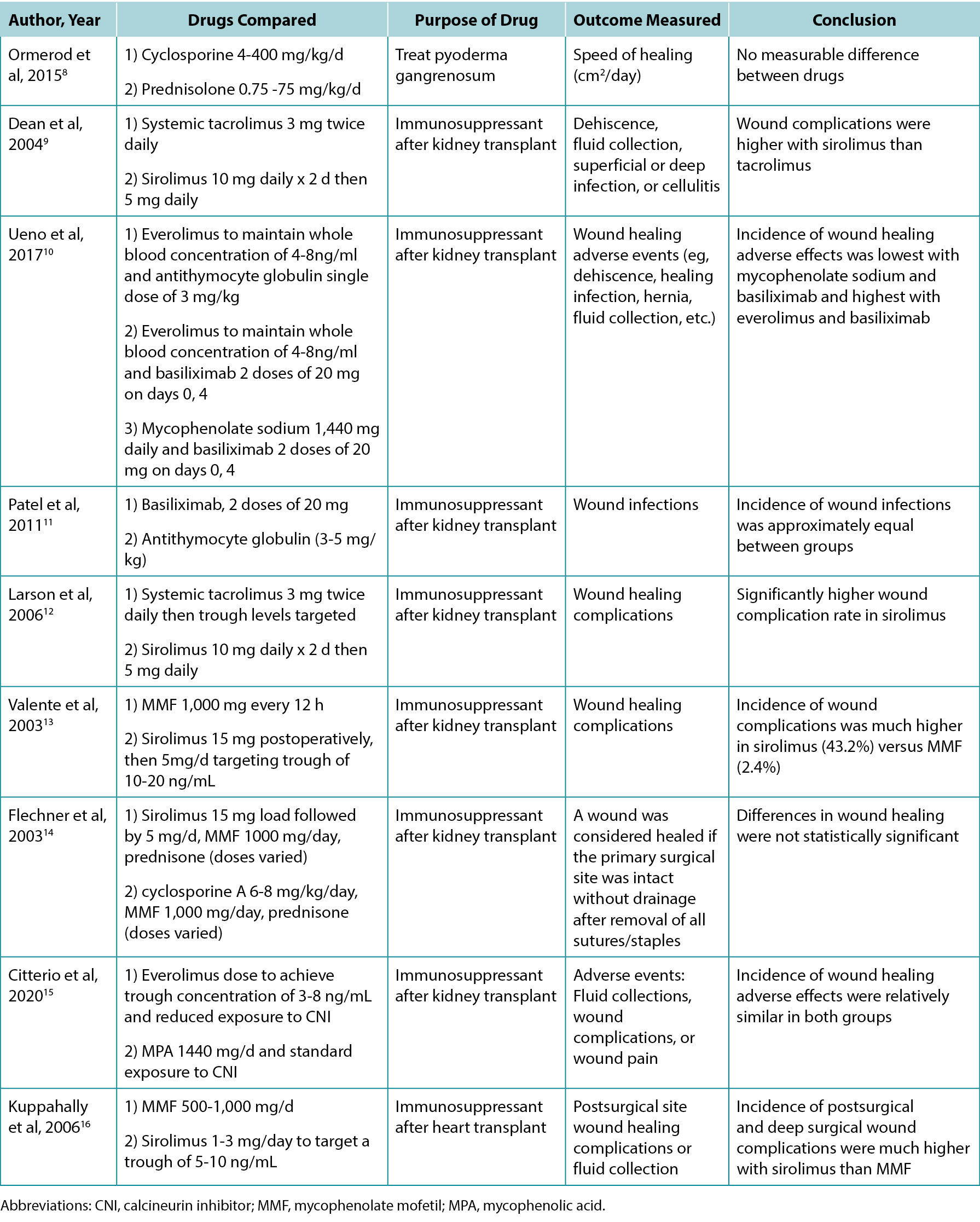
Table 2. Overview of indications and wound effects of various immunosuppressants
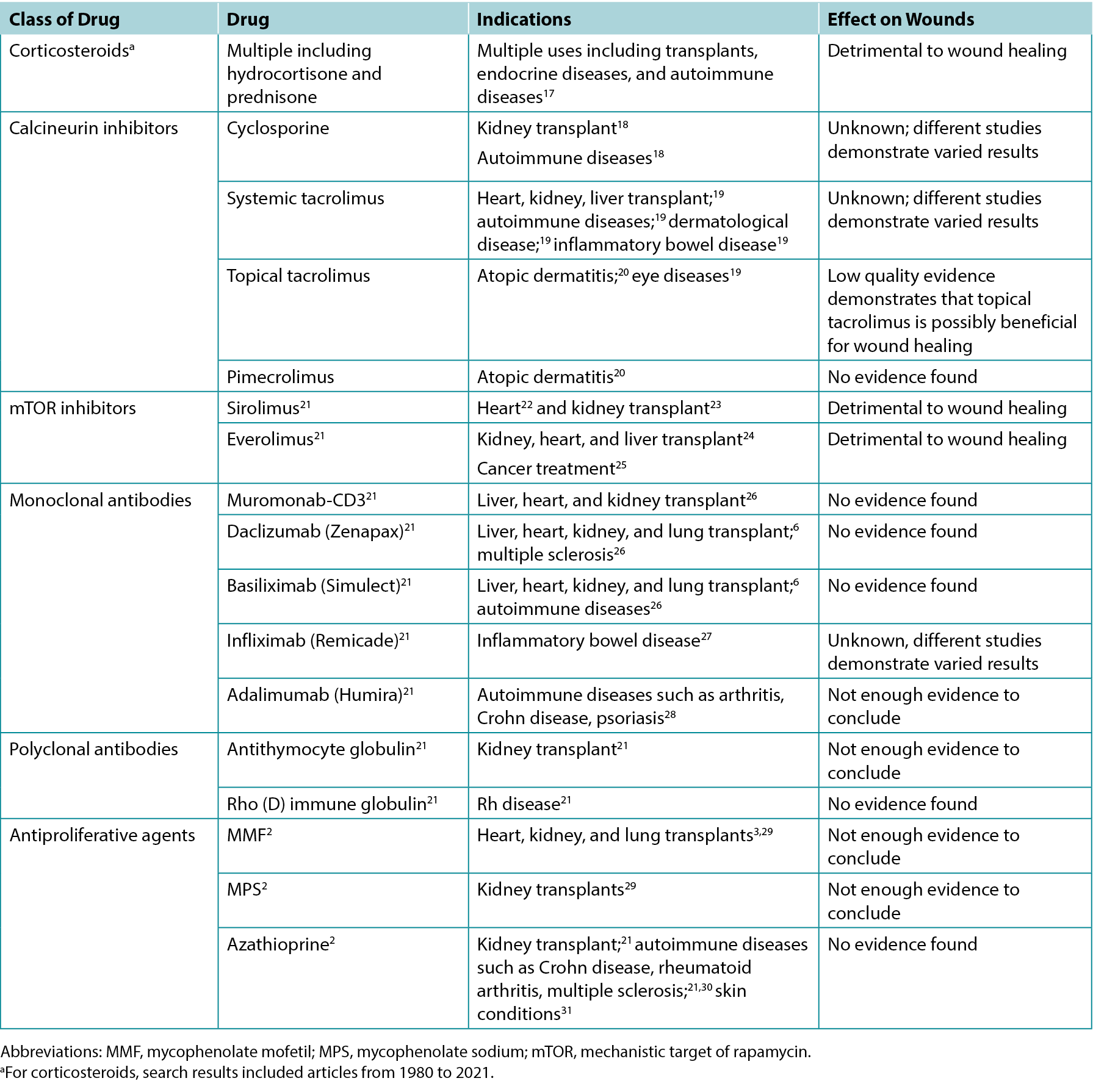
Calcineurin inhibitors
Calcineurin inhibitors are used for a variety of autoimmune diseases, organ transplants, dermatologic conditions, and chronic wounds.32 There are three main types of CNIs: cyclosporine (systemic), tacrolimus (systemic and topical), and pimecrolimus (topical).33 They work by binding to part of the calcineurin molecule found in human cells, thus stopping the release of certain cytokines that are responsible for activating T cells.32 Therefore, CNIs disable one of the main arms of the body’s adaptive immune response.
Systemic. Few studies have focused on the effects of CNIs on wound healing in humans; however, many basic science studies on animals have been performed. Two such studies using rats compared the effect of various doses of systemic tacrolimus versus a control, testing the breaking strengths of the wounds created through surgery.7,34 Willems et al7 concluded that tacrolimus does not affect wound healing, whereas Schäffer et al34 concluded that tacrolimus is detrimental to wound healing. In a case report using systemic tacrolimus as a treatment for ulcers in a person with lichen planus and pyoderma gangrenosum, Miller35 demonstrated treatment success with this therapy.
No recent human studies have investigated the effect of cyclosporine on wound healing, and two studies using rat models yielded contradictory results. These rat studies focused on the effects of cyclosporine on different markers in the body that signify effective wound healing.36,37 Nemlander et al36 compared cyclosporine to methylprednisolone and found that cyclosporine A had no suppressive effect on various inflammatory and biochemical markers in comparison with the glucocorticoid therapy. In contrast, Petri et al37 found that cyclosporine A had a negative effect on other markers within granulation fibroblasts, most notably activin A among procollagen 1, integrin 1, interleukin 6, transforming growth factor 1, and keratinocyte growth factor. In another animal study, Goldberg et al38 assigned dogs to one of three groups – no immunosuppression, methylprednisolone plus azathioprine, or cyclosporine A – after a lung transplant. They found that cyclosporine A had no significant effect on the healing of the surgical wound as measured by breaking strength in comparison with glucocorticoid and azathioprine immunosuppression. Overall, the literature on systemic CNIs and wound healing is limited with a heterogeneity of comparators and mixed results on wound healing.
Topical. Topical CNIs (tacrolimus and pimecrolimus) are often used for dermatologic conditions such as atopic dermatitis or pyoderma gangrenosum.20,39,40 Some case studies have shown that tacrolimus is effective at healing complex leg ulcers in the context of venous insufficiency or necrobiosis lipoidica when regular treatment strategies have been ineffective.41,42 Further, a rat-based study with acute cutaneous injury demonstrated that wounds treated with topical tacrolimus versus control (petrolatum) did not differ in healing speed.43
Monoclonal antibodies
There are a variety of different mAb therapies with indications in transplants and autoimmune disorders such as rheumatoid arthritis and psoriasis.21 In general, mAbs work by binding to different receptors and antigens to inhibit the effect of cytokines and other signal pathways that activate the immune system.21 In a small prospective cohort study among patients with rheumatoid arthritis undergoing orthopedic surgery, Bibbo and Goldberg44 found that there was no increased risk of surgical wound infections or healing complications in patients on infliximab versus conventional therapy. Further, Streit et al45 reported a case in which topical infliximab was helpful in healing leg ulcers that were resistant to standard treatment, suggesting that inhibiting tumor necrosis factor α is helpful for wound healing. Similarly, there was a case report that treated pyoderma gangrenosum with infliximab resulting in ulcer improvement.46 Inhibiting tumor necrosis factor α was further associated with wound healing in venous leg ulcers through the systemic use of adalimumab.47 However, a study that used infliximab on rat abdominal wounds found that tensile strength was significantly lower in the wounds of rats who had been given infliximab versus control.48 Overall, the literature is limited but suggests clinical outcomes may be favourable in terms of wound healing.
Polyclonal Antibodies
Polyclonal antibodies are very similar to mAbs in function, with slightly varied mechanism of action.49,50 Unlike mAbs, a group of pAbs is created from many different lines of B cells, and different pAbs bind to different epitopes of an antigen. In contrast, mAbs come from a single line of B cells and can bind to only one antigen.49,50
There are two main pAbs: antithymocyte globulin (also known as antihuman thymocyte globulin) and rho (ρ) immune globulin.21 Antithymocyte globulin is most commonly used as an immunosuppressant after kidney transplantation and works by binding to a variety of lymphocytes and depleting the number of T cells in the body.21 Rho immune globulin is used in pregnancies in which the gestational parent is Rh- and the fetus is Rh+ to essentially stop the formation of anti-Rh+ antibodies in the mother.21
Few studies have investigated the effects of pAbs on wound healing. However, two studies outlined the effects of antithymocyte globulin and basiliximab. Ueno et al10 investigated the use of these drugs combined with everolimus in patients with renal transplants. They reported higher rates of adverse effects on wound healing with basiliximab.10 Patel et al11 demonstrated that the incidence of wound infections was equal in patients taking basiliximab versus antithymocyte globulin after renal transplant. White blood cells play a key role in wound healing by secreting necessary cytokines and preventing infection51; thus, it is reasonable to hypothesise that antithymocyte globulin would affect wound healing because it reduces the number of white blood cells and their regulatory mechanisms.
mTOR Inhibitors
Mechanistic target of rapamycin inhibitors interact with proteins in complex signaling pathways to prevent cells from moving into the S phase of the cell cycle and therefore suppressing proliferation.3,21 Although mTOR predominantly targets T cells, it can also affect B cells.3 Interestingly, mTOR can increase production of certain inflammatory cytokines such as interleukin 6 and decrease production of interleukin 10, an anti-inflammatory cytokine.3 There are two main mTOR inhibitors: sirolimus and everolimus.3,21 In general, mTOR inhibitors have a variety of applications including cancer therapy and after transplants.3,21 Everolimus inhibits the proliferation of fibroblasts in in vitro models,52 suggesting that it could have negative consequences for wound healing because fibroblasts are essential for creating an extracellular matrix and scaffolding other cells.53 In a study comparing sirolimus and systemic tacrolimus, sirolimus had a wound complication rate of 47%, whereas the rate with tacrolimus was only 8%.9 This is consistent with another study by Larson et al12 demonstrating more frequent wound complications with sirolimus compared with tacrolimus. Those authors found that patients with obesity who were on sirolimus had very high rates of wound complications; as a result, the authors excluded all patients with obesity from the study.12 In line with previous findings, a study on rats showed that increased sirolimus doses decreased breaking strength.54 The authors hypothesised that this effect may be caused by lower levels of vascular endothelial growth factor and nitric oxide in rats receiving higher doses of sirolimus.54 In a review article, Nashan and Citterio55 concluded that mTOR inhibitors are harmful to wound healing in high doses, but seem to have a neutral effect in low does. Given early concerns with mTOR inhibitors and wound healing, regimens using these immunosuppressive agents have evolved with lower doses of the mTOR inhibitors and combination therapy. In the large TRANSFORM (Advancing renal TRANSplant eFficacy and safety Outcomes with an eveRoliMus-based regimen) randomised controlled trial, Citterio et al15 compared everolimus plus reduced-dose CNI with mycophenolic acid plus standard-dose CNI (standard care) in patients with renal transplants. They found that wound-related adverse events did not differ between groups (20.6% vs 17.3%; risk ratio, 1.19; 95% CI, 0.99 to 1.43).15 One limitation of this study was close monitoring of everolimus concentrations and difficulties achieving the targeted plasma concentrations C0 between 3 and 8 ng/mL.15 Overall, the current literature suggests that mTOR inhibitors have a detrimental effect on wound healing, especially at higher doses, and that improved dosing regimens may lessen or mitigate this risk.
Antiproliferative agents
There are three regularly used antiproliferative agents: mycophenolate mofetil (MMF), mycophenolate sodium (MPS), and azathioprine.2,29 Both MMF and MPS are inosine monophosphate dehydrogenase inhibitors. They have a similar effect to mTOR inhibitors in terms of their mechanism of immunosuppression. In the body, MMF and MPS are converted into mycophenolic acid, which blocks a portion of a pathway that is crucial for DNA synthesis to decrease proliferation of T and B cells.2,5,29 Whereas MMF is used for its immunosuppressive effect in heart, kidney, and lung transplants,3,29 MPS is used for kidney transplants.29 Azathioprine is used as an immunosuppressive drug for kidney transplants as well as autoimmune diseases, including rheumatoid arthritis, Crohn disease, and multiple sclerosis.21,30 Azathioprine reacts with glutathione in the body and is converted into 6-mercaptopurine. Additional metabolites are then generated, ultimately blocking purine synthesis and T-cell stimulation.3,21
In a study comparing two different doses of MMF in kidney transplant recipients, Flechner et al56 found no significant difference in the incidence of wounds requiring surgical intervention, similarly for wounds treated with local wound care. In analysing article titles for the present review, the authors did not find any studies regarding the sole effect of azathioprine on external wound healing in humans. However, Ginestal et al57 compared the effects of azathioprine versus placebo in a rat study. They found that the wounds of the rats who were on azathioprine took longer to heal than those on the placebo, suggesting that azathioprine may have detrimental effects on wound healing, but the extent that it would affect humans is unclear.57
Antimetabolite
Methotrexate is a commonly used folate antagonist with indications in many rheumatologic disorders. It also has antineoplastic activity in higher doses. Upon absorption, it enters the cell and is converted to methotrexate polyglutamates where it competes for dihydrofolate reductase, thus preventing the transformation of folic acid for its use in the building of nucleic acids.6 Experimental in vitro animal studies suggest that methotrexate may impair wound healing, but these effects have not been borne out in clinical studies, particularly in postsurgical wounds.6 Thus, it is recommended that this drug be continued postoperatively.
Corticosteroids/Glucocorticoids
Glucocorticoids prevent the formation of inflammatory chemicals such as cytokines, cell-adhesion molecules, and complement factors.3 By inhibiting interleukin 2 formation, glucocorticoids also prevent T-cell proliferation and activation.21 They also impair monocytes and B cells.3,21 Glucocorticoids were the first antirejection drugs created; however, there has been a movement to phase them out because of their serious adverse effects.3,21 Glucocorticoids are highly detrimental to wound healing because they interfere with many key stages, such as collagen deposition and synthesis, angiogenesis, fibroblast proliferation, growth factors, and phagocytosis, among others.2,3,58,59
Practical considerations for healthcare providers
Persons with compromised immune systems (due to medications, comorbidities, or age) require additional considerations for chronic wound management. Specific to immunosuppressive medications, healthcare providers should take a careful history not only of the medications and dosing (including changes in dosing), but also of the underlying conditions requiring these medications (eg, autoimmune disorders, organ transplantation). Because many immunosuppressive medications can impair wound healing, it is crucial for healthcare providers to assess healing potential early on to set and manage patient expectations. Early referral to medical or surgical specialists to assist with wound care and a team-based approach is essential, given the increased complexity of caring for these individuals. In cases when wounds are not healing, set alternate goals of care for the wound with the patient (eg, maintenance or nonhealable) if immunosuppressive doses cannot be reduced (assuming it is contributing to poor healing); undertake changes in consultation with the patient’s primary or specialist care providers. As individuals and populations with comorbidities live longer, caring for persons with chronic wounds on immunosuppressive medications will become increasingly common and wound care clinicians must be proactive in managing these patients.
Discussion
With the ongoing advances in medicine, the need for immunosuppression in the context of transplant, autoimmune disease, and malignancy has increased. This review highlights the paucity of robust studies in this field and the mixed effects of various immunosuppression on wound healing. High-quality evidence exists with respect to the deleterious effects of glucocorticoid therapy and mTOR therapy (particularly sirolimus) on wound healing. Four studies compared sirolimus with either MMF or systemic tacrolimus, and all four demonstrated that sirolimus was associated with an increased incidence of wound complications.9,12,13,16
The literature on agents such as systemic CNIs is mixed, with some suggesting adverse effects on wounds and others suggesting benefits; additional research focusing on this question is needed. Newer topical CNIs have shown little impact on delayed wound healing and, in some cases, may benefit healing, but additional investigation is warranted for their use in chronic wounds directly. Studies indicate that antiproliferative agents, antimetabolites, and newer mABs do not negatively impact wound healing. However, additional research is needed, given the lack of evidence on wound healing in mAB therapy.
Overall, the evidence in this area is limited and draws variable conclusions surrounding the effects of immunosuppressants on wound healing. In particular, few studies have included human participants. In general, when immunosuppressives are prescribed after transplants to prevent rejection, patients take more than one drug to effectively prevent rejection. Therefore, challenges exist in performing human studies evaluating the effects of individual drugs in isolation.
Because this was not a systematic review, the authors may not have identified all relevant articles. However, as one form of validation, the authors identified a few key reviews before conducting the literature search and then ensured these articles appeared in the search as expected. Given the paucity of literature in this area, particularly as it relates to the wound care field, a narrative review adds value to educate and increase awareness when working with individuals on these medications.
With the growing need for immunosuppression, additional study in this field is critical. Future research should investigate newer classes of immunosuppressants in animal models to identify potential pathways to delayed wound healing and potential ways to mitigate such effects. Further, additional high-quality human studies that evaluate both individual and combination immunotherapies are required to better understand the risks and how different immunosuppressants may impact wound healing. To explore immunosuppressants as a potential treatment for chronic or complex wounds, it is important for future studies to be conducted on a large scale and control for confounding clinical factors, such as through randomised controlled trials.
Conclusions
Immunosuppressants range from possibly beneficial to clearly deleterious in terms of wound healing. There is little conclusive evidence in this field, and the effects of immunosuppressants on wound healing are worth exploring further to better tailor immunosuppression to patients at risk for or experiencing chronic, nonhealing wounds. Some immunosuppressants may offer benefits in wound treatment when conventional therapies have failed, opening up the possibility of a new treatment option for wounds.
Acknowledgments
Alberta Innovates High School Youth Research Summer (HYRS) Program, which provided an educational stipend to the lead author for her work on this and other projects.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors have disclosed no financial relationships related to this article.
探索免疫抑制剂与伤口愈合的关系:叙述性综述
Aria Appoo, Brandon L Christensen, Ranjani Somayaji
DOI: 10.33235/wcet.44.3.12-19
摘要
目的 探讨不同类别的免疫抑制剂对伤口愈合的影响。
数据来源 在PubMed、Google Scholar和卡尔加里大学健康科学图书馆进行了文献检索。
研究选择 研究人员使用“免疫抑制药物”、“伤口愈合”和“免疫抑制”等关键词对文章标题进行了初步筛选。标题和/或摘要包含这些关键词的文章,涉及与免疫抑制剂药物相关的伤口愈合,并且发表于2000年以后的文章均被纳入综述。当没有免疫抑制剂(类)的人类数据时,则纳入动物研究。
数据提取 对纳入的61篇文章进行了全文审阅和总结。
数据整合:描述性总结了所有纳入的研究,包括免疫抑制作用机制、研究参与者或受试者以及对伤口愈合的影响证据。
结论 皮质类固醇和雷帕霉素机械靶蛋白抑制剂最常表现出对伤口愈合的不利影响。对于其他类别的免疫抑制剂,证据有限,对伤口愈合的影响各不相同。为了更好地了解免疫抑制剂(包括具有新作用机制的免疫抑制剂)的效果,确定对伤口愈合影响最大的免疫抑制剂,需要进行更大规模、高质量的研究。
引言
免疫抑制剂是一种具有多种适应症的药物,其适用范围包括实体器官移植、造血器官移植以及自身免疫性疾病等。它们通过抑制适应性免疫系统各种成分的活性发挥作用,从而减少对正常宿主组织的一连串炎症反应,或调节对移植材料的自然排斥反应。1免疫抑制剂的主要种类包括皮质类固醇/糖皮质激素2,3、钙调神经磷酸酶抑制剂(CNI)2,4,5、雷帕霉素机制靶点抑制剂(mTOR)2,4、单克隆抗体(mAb)2,4、多克隆抗体(pAb)2,4和抗増殖剂。2 在本综述中,伤口被定义为手术、创伤或易感染疾病导致的皮肤开口。
免疫系统在预防感染和伤口愈合过程中发挥着重要作用;炎症效应会导致细胞増殖并分泌重要的细胞内和细胞外成分。6使用免疫抑制剂,可以调节免疫系统,进而影响伤口的愈合时间和易感染性。7随着越来越多的患者(尤其是手术移植后的患者)服用免疫抑制药物,免疫抑制剂对伤口愈合的影响成为一个重要问题。本综述文章旨在使临床医生了解不同类别的免疫抑制剂如何影响伤口愈合。
方法
作者使用多种常见免疫抑制剂(糖皮质激素/皮质类固醇、mTOR抑制剂、甲氨蝶呤、单克隆抗体、pAb、CNI、霉酚酸酯、硫唑嘌呤)的通用名称以及术语“伤口愈合”和“免疫抑制”进行了文献检索。主要检索数据库为PubMed,其次是Google Scholar和卡尔加里大学健康科学图书馆数据库。在可能的情况下,检索格式如下:“免疫抑制剂名称[MeSH术语]”和“伤口愈合[MeSH术语]”。如果没有作为MeSH术语的免疫抑制剂名称,则不加限制地检索该术语。检索仅限于2000年至2021年间发表的英文文章。
研究人员对文章标题和摘要进行了相关性筛选。如果文章比较了各种免疫抑制剂,讨论了它们对伤口愈合的影响,并衡量了伤口愈合或报告了对伤口的不利影响,则被视为相关文章。如果检索词没有识别出任何人类参与的研究,作者就会纳入使用动物来评估给定药物类别对伤口愈合的免疫抑制作用的研究。如果没有2000年以后的数据,研究人员就对相关的免疫抑制药物进行了历史检索。
描述性总结了所有纳入的研究,包括免疫抑制作用机制、研究参与者/受试者以及对伤口愈合的影响证据。
结果
作者筛选了200篇文章的标题和摘要,其中61篇文章被纳入综述。表1重点介绍了部分临床和动物研究的结果。表2列出了各种免疫抑制剂的具体适应症,包括它们对伤口可能产生的影响。
表1.选择比较不同免疫抑制剂对伤口愈合影响的研究
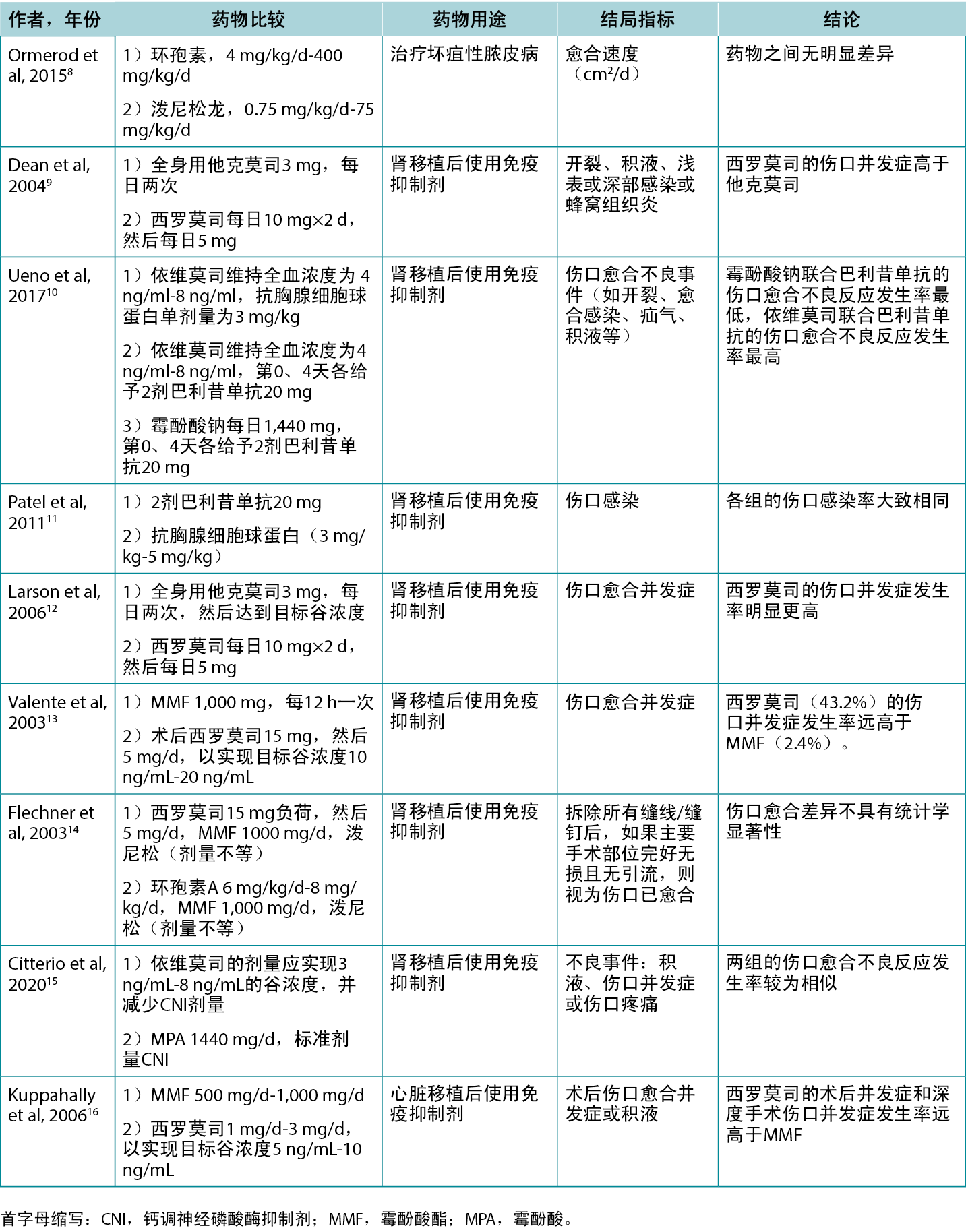
表2.各种免疫抑制剂的适应症和伤口影响概览
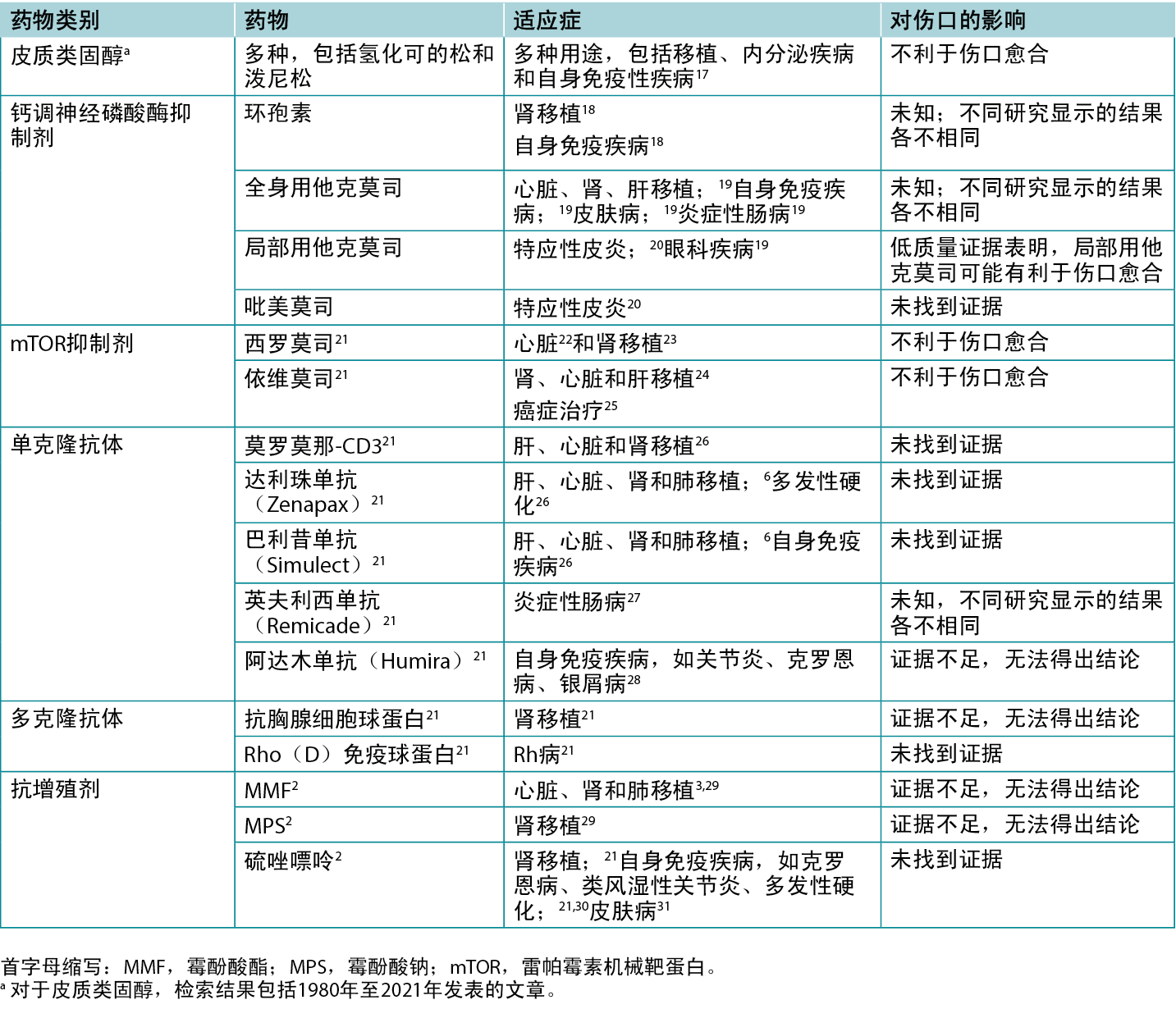
钙调神经磷酸酶抑制剂
钙调神经磷酸酶抑制剂可用于治疗各种自身免疫性疾病、器官移植、皮肤病和慢性伤口。32CNI主要有三种类型:环孢素(全身用药)、他克莫司(全身用药和局部用药)和吡美莫司(局部用药)。33它们通过与人体细胞中的部分钙调神经磷酸酶分子结合,从而阻止某些细胞因子的释放,这些细胞因子负责激活T细胞。32因此,CNI会使人体适应性免疫反应的主要机制之一失效。
全身用药。很少有研究关注CNI对人体伤口愈合的影响,但对动物进行了许多基础科学研究。有两项此类研究使用大鼠,比较了不同剂量全身用他克莫司与对照组的效果,测试了通过手术造成的伤口的破损强度。7,34Willems等人7认为他克莫司不会影响伤口愈合,而Schäffer等人34则认为他克莫司不利于伤口愈合。Miller35在一份使用全身用他克莫司治疗扁平苔藓和坏疽性脓皮病患者溃疡的病例报告中证明,这种疗法取得了成功。
最近没有人类研究调查环孢素对伤口愈合的影响,两项使用大鼠模型的研究得出了相互矛盾的结果。这些大鼠研究的重点是环孢素对体内标志伤口有效愈合的不同标志物的影响。36,37Nemlander等人36比较了环孢素和甲泼尼龙,研究发现,与糖皮质激素疗法相比,环孢素A对各种炎症和生化指标没有抑制作用。相反,Petri等人37发现环孢素A对肉芽组织成纤维细胞内的其他标志物有负面影响,其中最明显的是促胶原蛋白1、整合素1、白细胞介素6、转化生长因子1和角质细胞生长因子中的活化素A。在另一项动物研究中,Goldberg等人38将接受肺移植手术的犬分为三组Å\Å\即不使用免疫抑制剂、使用甲泼尼龙加硫唑嘌呤或使用环孢素A。他们发现,与糖皮质激素和硫唑嘌呤免疫抑制剂相比,环孢素A对手术伤口愈合的影响(以断裂强度衡量)并不明显。总体而言,有关全身用CNI和伤口愈合的文献十分有限,比较对象也不尽相同,对伤口愈合的影响结果也不尽相同。
局部用药。局部用CNI(他克莫司和吡美莫司)常用于治疗特应性皮炎或坏疽性脓皮病等皮肤病。20,39,40一些病例研究表明,当常规治疗策略无效时,他克莫司可有效治愈静脉功能不全或类脂坏死引起的复杂腿部溃疡。41,42此外,一项基于大鼠急性皮肤损伤的研究表明,与对照组(凡士林)相比,局部用他克莫司治疗伤口在愈合速度上没有差异。43
单克隆抗体
有多种不同的mAb疗法,适用于移植和自身免疫性疾病(如类风湿性关节炎和银屑病)。21一般来说,mAb通过与不同的受体和抗原结合来抑制激活免疫系统的细胞因子和其他信号通路的作用。21在一项针对接受骨科手术的类风湿性关节炎患者的小型前瞻性队列研究中,Bibbo和Goldberg44发现,与传统疗法相比,使用英夫利西单抗的患者发生手术伤口感染或愈合并发症的风险并没有増加。此外,Streit等人45还报告了一例局部用英夫利西单抗有助于治愈对标准治疗无效的腿部溃疡的病例,这表明抑制肿瘤坏死因子Éø有助于伤口愈合。同样,有一份病例报告称,使用英夫利西单抗治疗坏疽性脓皮病后,溃疡得到了改善。46通过全身使用阿达木单抗,抑制肿瘤坏死因子Éø进一步促进了下肢静脉性溃疡的伤口愈合。47然而,一项使用英夫利西单抗治疗大鼠腹部伤口的研究发现,与对照组相比,服用英夫利西单抗的大鼠伤口的抗张强度明显降低。48总体而言,文献资料有限,但结果表明,在伤口愈合方面,临床结局可能是有利的。
多克隆抗体
多克隆抗体的功能与mAb非常相似,但作用机制略有不同。49,50与mAb不同的是,一组pAb由许多不同的B细胞系产生,不同的pAb与抗原的不同表位结合。相反,mAb来自单系B细胞,只能与一种抗原结合。49,50
pAb主要有两种:抗胸腺细胞球蛋白(又称抗人胸腺细胞球蛋白)和Rho(Éœ)免疫球蛋白。21抗胸腺细胞球蛋白最常用作肾移植后的免疫抑制剂,它通过与多种淋巴细胞结合,消耗体内T细胞的数量来发挥作用。21Rho免疫球蛋白用于妊娠父母为Rh-而胎儿为Rh+的孕妇,从根本上阻止母亲体内抗Rh+抗体的形成。21
很少有研究调查pAb对伤口愈合的影响。但是,有两项研究概述了抗胸腺细胞球蛋白和巴利昔单抗的效果。Ueno等人10研究了这些药物与依维莫司联合用于肾移植患者的情况。他们报告称,巴利昔单抗对伤口愈合的不良反应率较高。10Patel等人11证实,肾移植后服用巴利昔单抗和抗胸腺细胞球蛋白的患者伤口感染发生率相同。白细胞通过分泌必要的细胞因子和防止感染,在伤口愈合中发挥着关键作用51;因此,有理由假设抗胸腺细胞球蛋白会影响伤口愈合,因为它减少了白细胞的数量及其调节机制。
mTOR抑制剂
雷帕霉素机械靶蛋白抑制剂与复杂信号通路中的蛋白质相互作用,阻止细胞进入细胞周期的S期,从而抑制细胞増殖3,21虽然mTOR主要针对 T 细胞,但它也会影响B细胞。3有趣的是,mTOR可以増加某些炎症细胞因子(如白细胞介素6)的产生,而减少白细胞介素10(一种抗炎细胞因子)的产生。3目前有两种主要的mTOR抑制剂:西罗莫司和依维莫司。3,21一般来说,mTOR抑制剂有多种用途,包括癌症治疗和移植后治疗。3,21依维莫司在体外模型中会抑制成纤维细胞的増殖,52这表明它可能会对伤口愈合产生负面影响,因为成纤维细胞对于创建细胞外基质和为其他细胞搭建支架至关重要。53在一项比较西罗莫司和全身用他克莫司的研究中,西罗莫司的伤口并发症发生率为47%,而他克莫司的伤口并发症发生率仅为8%。9这与Larson等人12的另一项研究结果一致,即西罗莫司的伤口并发症发生率高于他克莫司。这些作者发现,服用西罗莫司的肥胖症患者的伤口并发症发生率非常高;因此,作者将所有肥胖症患者排除在研究之外。12这与之前的研究结果一致,一项对大鼠的研究表明,増加西罗莫司剂量会降低断裂强度。54作者推测这种效应可能是由于接受较高剂量西罗莫司的大鼠体内血管内皮生长因子和一氧化氮水平较低所致。54Nashan和Citterio55在一篇综述文章中总结道,mTOR抑制剂在高剂量下对伤口愈合有害,但在低剂量下似乎没有影响。鉴于早期对mTOR抑制剂和伤口愈合的担忧,使用这些免疫抑制剂的治疗方案已发展为较低剂量的mTOR抑制剂和联合疗法。在大型TRANSFORM(以依维莫司为基础的方案提高肾移植的有效性和安全性结果)随机对照试验中,Citterio等人15比较了依维莫司加低剂量CNI与霉酚酸加标准剂量CNI(标准护理)对肾移植患者的治疗效果。他们发现,与伤口相关的不良事件在不同组间并无差异(20.6% vs 17.3%;风险比1.19;95% CI,0.99-1.43)。15这项研究的局限性之一是对依维莫司浓度的密切监测,以及难以达到3 ng/mL-8 ng/mL的目标血浆浓度C0。15总体而言,目前的文献表明,mTOR抑制剂会对伤口愈合产生不利影响,尤其是在较高剂量时,并且改进的给药方案可以减少或减轻这种风险。
抗増殖剂
常用的抗増殖剂有三种:霉酚酸酯(MMF)、霉酚酸钠(MPS)和硫唑嘌呤。2,29MMF和MPS都是次黄嘌呤单核苷酸脱氢酶抑制剂。它们的免疫抑制机制与mTOR抑制剂相似。在人体内,MMF和MPS会转化为霉酚酸,而霉酚酸会阻断DNA合成关键途径的一部分,从而减少T细胞和B细胞的増殖。2,5,29MMF在心脏、肾脏和肺移植中具有免疫抑制作用,3,29而MPS则用于肾脏移植。29硫唑嘌呤是一种免疫抑制剂,可用于肾移植和自身免疫性疾病,包括类风湿性关节炎、克罗恩病和多发性硬化症。21,30硫唑嘌呤会与体内的谷胱甘肽发生反应,并转化为6-巯基嘌呤。然后产生额外的代谢物,最终阻断嘌呤合成和T细胞刺激。3,21
在一项比较肾移植受者两种不同剂量MMF的研究中,Flechner等人56发现,需要手术干预的伤口发生率没有显著差异,同样,采用局部伤口护理的伤口发生率也没有显著差异。在为本综述分析文章标题时,作者没有发现任何关于硫唑嘌呤对人体外部伤口愈合的唯一作用的研究。不过,Ginestal等人57在一项大鼠研究中比较了硫唑嘌呤和安慰剂的效果。他们发现,与服用安慰剂的大鼠相比,服用硫唑嘌呤的大鼠伤口愈合的时间更长,这表明硫唑嘌呤可能会对伤口愈合产生不利影响,但对人类的影响程度尚不清楚。57
抗代谢药
甲氨蝶呤是一种常用的叶酸拮抗剂,适用于多种风湿病。在较高剂量下,它还具有抗肿瘤活性。吸收后,它进入细胞并转化为甲氨蝶呤多聚谷氨酸,在细胞内与二氢叶酸还原酶竞争,从而阻止叶酸转化为核酸。6体外动物实验研究表明,甲氨蝶呤可能会影响伤口愈合,但这些影响尚未在临床研究中得到证实,特别是在手术后伤口中。6因此,建议术后继续使用这种药物。
皮质类固醇/糖皮质激素
糖皮质激素能阻止细胞因子、细胞粘附分子和补体因子等炎症化学物质的形成。3通过抑制白细胞介素2的形成,糖皮质激素还能阻止T细胞的増殖和活化。21此外,它们还会损害单核细胞和B细胞。3,21糖皮质激素是最早出现的抗排斥药物;然而,由于其严重的不良反应,已经出现了逐步淘汰糖皮质激素的趋势。3,21糖皮质激素对伤口愈合极为不利,因为它们会干扰许多关键阶段,如胶原沉积和合成、血管生成、成纤维细胞増殖、生长因子和吞噬作用等。2,3,58,59
医疗服务提供者的实际考虑因素
对于免疫系统受损的个体(由于药物、并发症或年龄),在进行慢性伤口管理时需要考虑更多因素。具体到免疫抑制药物,医疗服务提供者不仅要仔细记录药物和剂量(包括剂量变化),还要了解需要使用这些药物的基础状况(如自身免疫性疾病、器官移植)。许多免疫抑制药物会影响伤口愈合,因此医疗服务提供者必须尽早评估伤口愈合的可能性,并据此设定和管理患者期望。鉴于此类患者的护理更加复杂,应尽早将其转诊至内科或外科专家,以协助进行伤口护理并采取团队合作的方式,这一点至关重要。在伤口没有愈合的情况下,如果不能减少免疫抑制药物的剂量(假设免疫抑制药物导致伤口愈合不良),则应与患者共同设定伤口护理的替代目标(例如,维持现状或接受无法愈合的状态);与患者的初级或专科护理人员协商后进行更改。随着患有合并症的个人和群体寿命的延长,使用免疫抑制药物治疗慢性伤口患者的情况将愈加普遍,伤口护理临床医生必须采取积极主动的态度来管理这些患者。
讨论
随着医学的不断进步,移植手术、自身免疫性疾病及恶性肿瘤的治疗对免疫抑制药物的需求也在不断増加。本文综述强调了该领域内研究的不足,并指出不同免疫抑制药物对伤口愈合的影响存在差异。已有高质量证据表明,糖皮质激素治疗和mTOR疗法(尤其是西罗莫司)对伤口愈合具有不利影响。四项研究比较了西罗莫司与霉酚酸酯(MMF)或全身用他克莫司的效果,结果表明西罗莫司会増加伤口并发症的发生率。9,12,13,16
有关全身用CNI等制剂的文献资料存在分歧,部分研究认为会对伤口产生不利影响,而其他研究则认为会带来益处;针对此问题需开展更多研究。新型局部用CNI对伤口愈合延迟的影响很小,在某些情况下还可能有利于伤口愈合,但仍需进一步研究其在慢性伤口治疗中的直接应用。研究表明,抗増值剂、抗代谢药和新型mAB不会对伤口愈合产生负面影响。然而,由于缺乏关于mAB疗法对伤口愈合影响的证据,还需要进行更多的研究。
总体而言,关于免疫抑制剂对伤口愈合影响的证据有限且结论不一致,尤其是涉及人类参与者的研究甚少。一般而言,移植手术后使用免疫抑制药物来预防排斥反应时,患者需要服用一种以上的药物才能有效预防排斥反应。因此,在进行人体研究以评估单独药物的效果时存在挑战。
由于本文并非系统性综述,作者可能未能涵盖所有相关文献。但是,作为一种验证手段,作者在文献检索前设定了若干关键综述,并确保这些文章如期出现在检索结果中。鉴于该领域内文献资料的稀缺,尤其是与伤口护理领域相关的文献,叙述性综述提升了教育价值,并増强了对使用这些药物个体的关注。
随着对免疫抑制药物的需求日益増长,在这一领域开展更多研究至关重要。未来的研究应在动物模型中研究更新的免疫抑制剂类别,以确定导致伤口愈合延迟的潜在途径以及减轻此类影响的潜在方法。此外,还需要开展更多高质量的人体研究,对单独和联合免疫疗法进行评估,以便更好地了解风险以及不同的免疫抑制剂对伤口愈合的影响。为了探索免疫抑制剂作为慢性或复杂伤口的潜在治疗方法,未来的研究必须以大规模进行,并通过随机对照试验等方式控制混杂临床因素。
结论
免疫抑制剂对伤口愈合的影响各不相同,可能有益,也可能明显不利。鉴于该领域的确凿证据匮乏,有必要进一步探讨免疫抑制剂对伤口愈合的影响,以便更好地为有慢性伤口愈合困难风险或正在经历慢性伤口愈合困难的患者制定个性化的免疫抑制治疗方案。当传统治疗方法无效时,某些免疫抑制剂可能在伤口治疗中带来益处,为伤口治疗提供新的选择。
致谢
感谢阿尔伯塔创新高中青年暑期研究计划(HYRS)为本项目及其他项目的主要作者提供了教育津贴。
利益冲突声明
作者声明无利益冲突。
资助
作者未披露与本文相关的经济利益关系。
Author(s)
Aria Appoo
Medical Student, University of Oxford, Oxford, United Kingdom
Brandon L Christensen*
MD
Resident Physician, Division of Infectious Diseases
University of Calgary, Alberta, Canada
Ranjani Somayaji
BScPT MD MPH
Assistant Professor, Departments of Medicine, Microbiology
Immunology and Infectious Disease and Community Health Sciences
* Corresponding author
References
- Fireman M, DiMartini AF, Armstrong SC, Cozza KL. Immunosuppressants. Psychosomatics 2004;45(4):354-60.
- Taylor AL, Watson CJE, Bradley JA. Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol 2005;56(1):23-46.
- Weltz A, Scalea J, Popescu M, Xu J, Bromberg JS. Mechanisms of immunosuppressive drugs. In: Kidney Transplantation. New York: Springer; 2014:127-42.
- Subramanian S, Trence DL. Immunosuppressive agents: effects on glucose and lipid metabolism. Endocrinol Metab Clin North Am 2007;36(4):891-905.
- Mika A, Stepnowski P. Current methods of the analysis of immunosuppressive agents in clinical materials: a review. J Pharm Biomed Anal 2016;127:207-31.
- Pountos I, Giannoudis P V. Effect of methotrexate on bone and wound healing. Expert Opin Drug Saf 2017;16(5):535-45.
- Willems MCM, van der Vliet JA, Lomme RMLM, Hendriks T. Tacrolimus does not affect early wound healing in a rodent model of bowel anastomoses and abdominal wall closure. PLoS One 2013;8(9):e76348.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ 2015;350:h2958.
- Dean PG, Lund WJ, Larson TS, et al. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004;77(10):1555-61.
- Ueno P, Felipe C, Ferreira A, et al. Wound healing complications in kidney transplant recipients receiving everolimus. Transplantation 2017;101(4):844-50.
- Patel S, Pankewycz O, Kohli R, et al. Obesity in renal transplantation: the role of induction therapy on long-term outcomes. Transplant Proc 2011;43(2):469-71.
- Larson TS, Dean PG, Stegall MD, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant 2006;6(3):514-22.
- Valente JF, Hricik D, Weigel K, et al. Comparison of sirolimus vs. mycophenolate mofetil on surgical complications and wound healing in adult kidney transplantation. Am J Transplant 2003;3(9):1128-34.
- Flechner SM, Zhou L, Derweesh I, et al. The impact of sirolimus, mycophenolate mofetil, cyclosporine, azathioprine, and steroids on wound healing in 513 kidney-transplant recipients. Transplantation 2003;76(12):1729-34.
- Citterio F, Henry M, Kim DY, et al. Wound healing adverse events in kidney transplant recipients receiving everolimus with reduced calcineurin inhibitor exposure or current standard-of-care: insights from the 24 month TRANSFORM study. Expert Opin Drug Saf 2020;19(10):1339-48.
- Kuppahally S, Al-Khaldi A, Weisshaar D, et al. Wound healing complications with de novo sirolimus versus mycophenolate mofetil-based regimen in cardiac transplant recipients. Am J Transplant 2006;6(5Pt1)986-92.
- Kapugi M, Cunningham K. Corticosteroids. Orthop Nurs 2019;38(5):336-9.
- Ponticelli C. Cyclosporine: from renal transplantation to autoimmune diseases. Ann N Y Acad Sci 2005;1051(1):551-8.
- Akar Y, Yucel G, Durukan AH, Yucel I, Arici G. Systemic toxicity of tacrolimus given by various routes and the response to dose reduction. Clin Exp Ophthalmol 2005;33(1):53-9.
- Frazier W, Bhardwaj N. Atopic dermatitis: diagnosis and treatment. Am Fam Physician 2020;101(10):590-8.
- Khan MM. Immunopharmacology. New York, NY: Springer US; 2008.
- Kurian K, Addisu A. Sirolimus: a novel immunosuppressive drug in heart transplantation. Recent Pat Cardiovasc Drug Discov 2009;4(3):187-91.
- Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 2003;35(3):S7-14.
- Van Gelder T, Fischer L, Shihab F, Shipkova M. Optimizing everolimus exposure when combined with calcineurin inhibitors in solid organ transplantation. Transplant Rev 2017;31(3):151-7.
- Falkowski S, Woillard JB. Therapeutic drug monitoring of everolimus in oncology: evidences and perspectives. Ther Drug Monit 2019;41(5):568-74.
- National Institutes of Diabetes and Digestive and Kidney Diseases. Clinical and Research Information on Drug-Induced Liver Injury. LiverTox. 2012. February 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK547852/. Last accessed January 25, 2024.
- Lichtenstein L, Ron Y, Kivity S, et al. Infliximab-related infusion reactions: systematic review. J Crohns Colitis 2015;9(9):806-15.
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008;117(2):244-79.
- Staatz CE, Tett SE. Clinical Pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet 2007;46(1):13-58.
- Casetta I, Iuliano G, Filippini G. Azathioprine for multiple sclerosis. Cochrane Database Syst Rev 2007;2007(4):CD003982.
- Chavez-Alvarez S, Herz-Ruelas M, Villarreal-Martinez A, Ocampo-Candiani J, Garza-Garza R, Gomez-Flores M. Azathioprine: its uses in dermatology. An Bras Dermatol 2020;95(6):731-6.
- Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol 2013;191(12):5785-91.
- Safarani OA, Patel J. Calcineurin Inhibitors. StatPearls Publishing; 2021.
- Schäffer MR, Fuchs N, Proksch B, Bongartz M, Beiter T, Becker HD. Tacrolimus impairs wound healing: a possible role of decreased nitric oxide synthesis. Transplantation 1998;65(6):813-8.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Wound Manag Prev 2008;54(4):36-42.
- Nemlander A, Ahonen J, Wiktorowicz K, et al. Effect of cyclosporine on wound healing an analysis with viscous cellulose sponges. Transplantation 1983;36(1):1-5.
- Petri J, Schurk S, Gebauer S, Haustein U. Cyclosporine A delays wound healing and apoptosis and suppresses activin beta-A expression in rats. Eur J Dermatol 1998;8(2):104-13.
- Goldberg M, Lima O, Morgan E, et al. A comparison between cyclosporin A and methylprednisolone plus azathioprine on bronchial healing following canine lung autotransplantation. J Thorac Cardiovasc Surg 1983;85(6):821-6.
- Sussman G. The use of topical calcineurin inhibitors in chronic wound management. Wound Pract Res 2000;26(3):140-5.
- Lyon C, Stapleton M, Smith A, Mendelsohn S, Beck M, Griffiths C. Topical tacrolimus in the management of peristomal pyoderma gangrenosum. J Dermatol Treat 2001;12(1):13-7.
- Ginocchio L, Draghi L, Darvishian F, Ross FL. Refractory ulcerated necrobiosis lipoidica: closure of a difficult wound with topical tacrolimus. Adv Skin Wound Care 2017;30(10):469-72.
- Mackelfresh J, Soon S, Arbiser JL. Combination therapy of doxycycline and topical tacrolimus for venous ulcers. JAMA Dermatol 2005;141(11):1476-7.
- Namkoong S, Chung J, Yoo J, et al. Topical tacrolimus does not negatively impact acute skin wound healing. Exp Dermatol 2013;22(5):369-71.
- Bibbo C, Goldberg JW. Infectious and healing complications after elective orthopaedic foot and ankle surgery during tumor necrosis factor-alpha inhibition therapy. Foot Ankle Int 2004;25(5):331-5.
- Streit M, Beleznay Z, Braathen LR. Topical application of the tumour necrosis factor-alpha antibody infliximab improves healing of chronic wounds. Int Wound J 2006;3(3):171-9.
- Hewitt D, Tait C. Use of infliximab in pyoderma gangrenosum. Australas J Dermatol 2007;48(2):95-8.
- Fox JD, Baquerizo-Nole KL, Keegan BR, et al. Adalimumab treatment leads to reduction of tissue tumor necrosis factor-alpha correlated with venous leg ulcer improvement: a pilot study. Int Wound J 2016;13(5):963-6.
- De Lopes JV, Freitas LAM, Marques RD, Bocca AL, de Sousa JB, de Oliveira PG. Analysis of the tensile strength on the healing of the abdominal wall of rats treated with infliximab. Acta Cir Bras 2008;23(5):441-6.
- Larrañaga MD, Lewis S, Richard J, Robert A, eds. Polyclonal antibodies. In: Hawley’s Condensed Chemical Dictionary. 16th ed. Hoboken, NJ: Wiley; 2016.
- Johnson M. Monoclonal antibodies. In: Longe JL, ed. Gale Encyclopedia of Nursing and Allied Health. 4th ed. Credo Reference; 2018.
- Kordestani SS. Wound healing process. In: Atlas of Wound Healing. Abyaneh MS, Fayyazbakhsh F, eds. Elsevier; 2019:11-22.
- Azzola A, Havryk A, Chhajed P, et al. Everolimus and mycophenolate mofetil are potent inhibitors of fibroblast proliferation after lung transplantation. Transplantation 2004;77(2):275-80.
- Bainbridge, P. Wound healing and the role of fibroblasts. J Wound Care, 2013;22(8):407-412.
- Schäffer M, Schier R, Napirei M, Michalski S, Traska T, Viebahn R. Sirolimus impairs wound healing. Langenbecks Arch Surg 2007;392(3):207-303.
- Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation. Transplantation 2012;94(6):547-61.
- Flechner SM, Feng J, Mastroianni B, et al. The effect of 2-gram versus 1-gram concentration controlled mycophenolate mofetil on renal transplant outcomes using sirolimus-based calcineurin inhibitor drug-free immunosuppression. Transplantation 2005;79(8):926-34.
- Ginestal R, Pérez-Köhler B, Pérez-López P, et al. Comparing the influence of two immunosuppressants (fingolimod, azathioprine) on wound healing in a rat model of primary and secondary intention wound closure. Wound Repair Regen 2019;27(1):59-68.
- Wicke C. Effects of steroids and retinoids on wound healing. Arch Surg 2000;135(11):1265-70.
- Anstead G. Steroids, retinoids, and wound healing. Adv Wound Care (New Rochelle) 1998;11(6):277-85.


