Volume 44 Number 3
The role of standardised product terminology in product development and clinical practice
Greg Czaplewski, Kim Smitka
Keywords clinical practice, standardised product terminology, convexity, ostomy product development, patient-centred.
For referencing Czaplewski G, Smitka K. The role of standardised product terminology in product development and clinical practice. WCET® Journal Supplement. 2024;44(3)Sup:s3-5.
DOI 10.33235/wcet.44.3.sup.s3-5
Abstract
Convexity products for ostomy care have been available many years. However, there still remains confusion regarding both nomenclature regarding convexity products and their clinical application. This gap creates a non-scientific approach to patient care as the clinician often relies on personal experiences creating more of an ‘art-based’ approach rather than care delivered from evidence.
Recent publications are helping to improve the base knowledge of the clinician with new evidence that supports such clinical decision making in clinical practice. Ultimately, this evidence-based approach can improve patient outcomes by facilitating quality care while assisting product developers to create products that are more humanistic and patient-centered.
This article explores some of this recent evidence, some clinical applications substantiated by evidence, and the potential for future product developments and ostomy product standardisation.
Introduction
In the evolving landscape of ostomy care, clinicians face a challenge arising from the inconsistent use of terminology associated with ostomy products, specifically soft convex, firm convex, or deep convex. These commonly used terms, though familiar to clinicians, lack standardised definitions, resulting in variations in product attributes across manufacturers. For instance, a convex product from one manufacturer will have differences in fit and performance compared to a convex product from another manufacturer. This lack of uniformity poses a challenge to clinicians when prescribing products. While product performance uniformity across manufacturers isn’t the goal, uniformity of terminology used to describe these products would be of great benefit to clinicians. That way, the clinician can make a more informed decision when prescribing products, using the terminology as guidance. Recognising this challenge, the Hollister research and development team aims to continue down the path of a trailblazing journal article published in 2021,1 which initiated a shift in clinical practice by introducing a standardised framework defining ostomy products based on certain characteristics—specifically flexibility, compressibility, depth, slope, and tension location.
Five characteristics of convexity and clinical application statements
Published in 2021, the five characteristics of convexity and clinical application statements (Table 1) have been instrumental in how clinicians and manufacturers frame discussions about convexity products.1
Twelve nurse panelists from eleven countries convened to define and establish consistency in convex skin barrier characteristics and associated clinical application of the products based on these defined characteristics.1 Setting common nomenclature will be influential for both research opportunities and novice nurse education, as clinicians can now describe specific convexity attributes and the clinical decision making for correct product selection.1 Adopting new nomenclature and incorporating it earlier into clinical practice seems a fundamental shift in ostomy care, however if stoma care is to advance, it should become the norm.2
Table 1. Definitions and clinical application statements for the five characteristics of convexity1
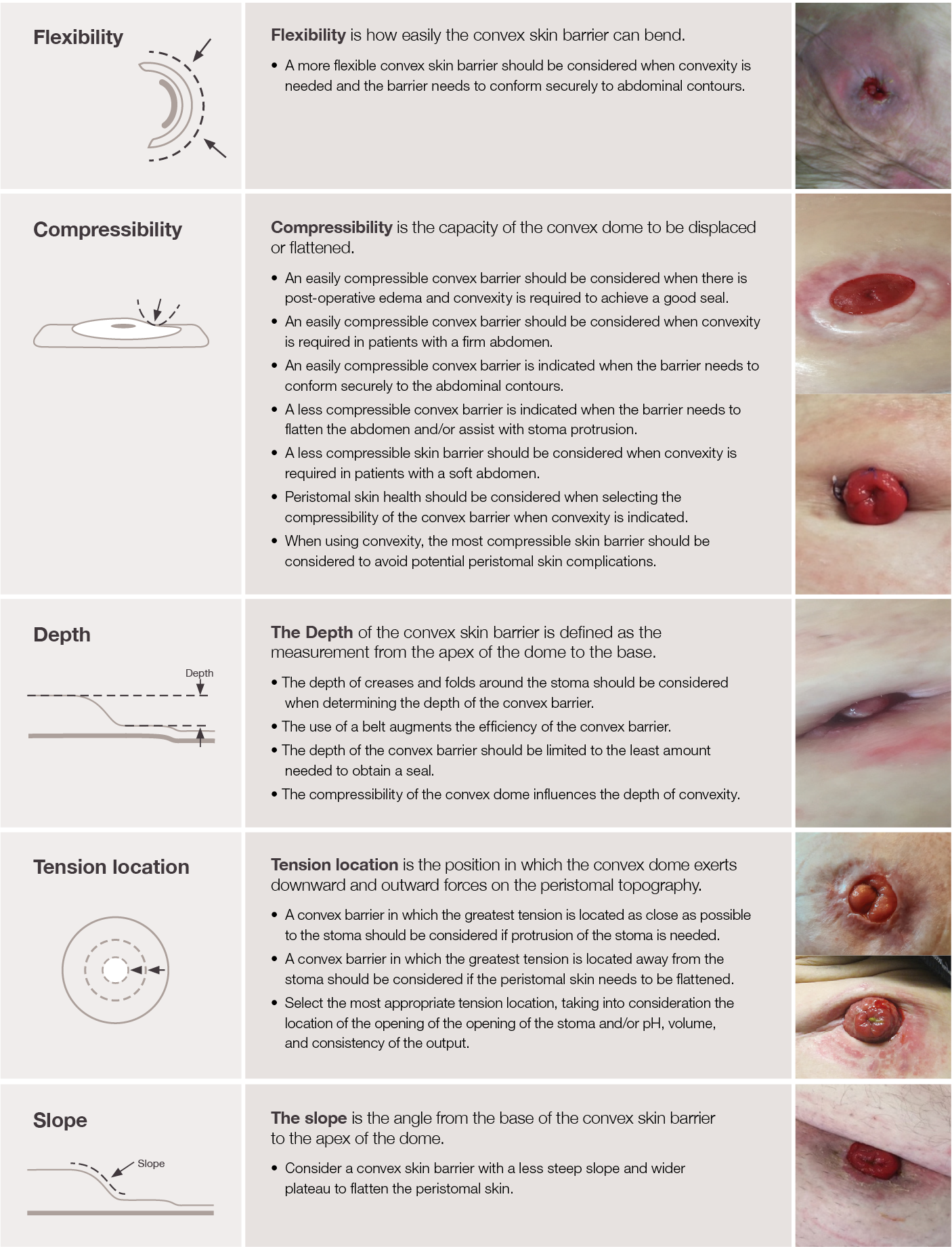
SOURCE: McNichol L, Cobb T, Depaifve Y, et al. Characteristics of convex skin barriers and clinical application:
Results of an international consensus panel. JWOCN. 2021;48(6):524–532.
Copyright permission was obtained through Wolters Kluwer for use of the clinical images in Table 1.
Incorporating standardised characteristics into ostomy product development
The goal for research and development is to incorporate these characteristics into new products. Moving away from conventional labels of firm, soft, and deep convex, our emphasis is on continuing to explore these five product characteristics as we continue to innovate in the world of convexity. Before we can incorporate the characteristics into designs, we must standardise how we measure them and how we think about their impact to the end user.
Having standardised measurements creates a foundational structure for product development. Furthermore, it guides new convexity designs, anchoring innovative concepts in these five principal characteristics that govern fit performance. In measuring the characteristics, two styles of measurement become apparent. On one hand, we have slope, depth, and tension location. These three characteristics are more self-apparent or clearly visible when a clinician is handling a convex product. These three characteristics can be defined through readily available metrological techniques, like using a 3D light scanner to understand the exact depth, slope, or tension location of the product. On the other hand, we have flexibility and compressibility. These two characteristics are less self-apparent and not easily defined by merely looking at a product. They are dynamic, as opposed to static, in that the properties of flexibility and compressibility are better defined using time as an element. For example, these two characteristics become more apparent as the clinician handles the product and applies force to it by either flexing the product or compressing it with their hands. Another example is the end user who will wear a convex product for an extended period of time. Over time, they will feel the flexibility and compressibility of the product differently as they create different motions with their bodies. Therefore, these two characteristics are best measured in a laboratory using force and time as inputs.
Innovative solutions for future product development
In current products on the market today, slope, depth, and tension location are all geometrically focused and relatively static, with product shape and size playing a key role in defining the three characteristics. Conversely, flexibility and compressibility are more dynamic in that they both incorporate the element of time. While product shape and size are still important, how the product behaves while in contact with the patient is highlighted by these two dynamic characteristics.
Flexibility refers to the product’s ability to adapt and conform to the abdominal contours of the body. Clinicians recognise its importance to ensure a good fit that supports a patient’s abdominal topography and abdominal tone. For example, a soft convex barrier will provide a better fit than a firm convex barrier on an abdomen with deep creases, where the barrier needs to flex into those contours.3 Therefore, selection of the right convex skin barrier hinges on this dynamic barrier characteristic.
Another dynamic component is compressibility. This refers to how the product responds to pressure and deformation during wear. Clinicians evaluate compressibility of the ostomy barrier to ensure a good fit around the stoma and for the barrier to adapt to the abdominal contour changes. For instance, an easily compressible barrier should be considered when the patient presents with a firm abdomen so less pressure is exerted on the abdomen.1 A less compressible barrier should be considered when the abdomen is soft or if the stoma needs additional support to protrude.1
While the dynamic characteristics of flexibility and compressibility significantly influence how a convex product behaves while in contact with the patient, it’s crucial to recognise that all five characteristics are interrelated and collectively contribute to getting an optimal fit. For instance, consider the clinical application of a convex skin barrier. Here, compressibility directly affects the depth of convexity provided.1 An easily compressible convex skin barrier will provide less depth when compared to a less compressible convex skin barrier. This can be seen when comparing a soft convex barrier to a firm convex barrier. Understanding how these two characteristics impact the convex barrier’s ability to provide the right amount of depth is essential for achieving an effective seal around the stoma using the right barrier.
Conclusion
In new product development, the focus extends beyond terminology and measuring the characteristics. The five characteristics will play a pivotal role in identifying potential gaps in current convex products, guiding strategies to address challenges posed by diverse end user topographies encountered by clinicians daily. Tailoring products to meet the specific needs of individual users, with a particular emphasis on achieving a better fit, is a key focus. This approach is called human-centered design, focusing on true unmet needs, and designing products to meet those needs.4 Continued research of avenues to enhance the adaptability of products will potentially allow adjustments to these five characteristics of convexity during wear. While representing an exploratory direction, this solution may offer a more personalised and comfortable experience.5 This research remains grounded in the principles of measurement precision, nomenclature standardisation, and user-centered designs, ensuring that future products meet and exceed the expectations of clinicians and patients alike.
Conflict of interest
The authors are employees of Hollister Incorporated.
Funding
Apart from being employees of Hollister, the authors received no funding for this paper.
标准化产品术语在产品开发和临床实践中的作用
Greg Czaplewski, Kim Smitka
DOI: 10.33235/wcet.44.3.sup.s3-5
摘要
用于造口护理的凸面产品已问世多年。然而,关于凸面产品的术语及其临床应用仍存在混淆。这种差距导致患者护理方法极不科学,因为临床医生常常依赖个人经验,从而形成了更多偏向“艺术性”的护理方式,而非基于证据的护理模式。
最近发表的论文有助于提高临床医生的基础知识,为临床实践中的临床决策提供新的证据支持。最终,这种循证方法可以通过促进优质护理来改善患者结局,同时帮助产品开发者创造更人性化、以患者为中心的产品。
本文探讨了一些最新的证据、经证据证实的一些临床应用,以及未来产品发展和造口产品标准化的潜力。
引言
在造口护理不断发展的过程中,临床医生面临着一个挑战,即造口产品(特别是软凸、硬凸或深凸)术语使用的一致性问题。这些常用术语尽管为临床医生所熟知,但由于缺乏标准化的定义,导致不同制造商的产品属性存在差异。例如,某个制造商生产的凸面产品与另一制造商生产的凸面产品相比,在贴合度和性能方面存在差异。这种不一致性给临床医生开具处方带来了挑战。虽然并不要求所有制造商的产品性能一致,但统一描述这些产品的术语将对临床医生大有裨益。由此,临床医生在开具产品处方时,便能以这些术语为指导,做出更明智的临床决策。认识到这一挑战,Hollister研发团队继续沿着2021年发表的一篇开创性期刊文章的路径前进,1该文通过引入一个基于某些特征的标准化框架来定义造口产品,从而推动了临床实践的变革Å\Å\具体而言,这些特征包括柔韧性、可压缩性、深度、坡度和张力位置。
凸面装置的五大特征及其临床应用声明
2021年发表的凸面装置的五大特征及其临床应用声明(表1)对临床医生和制造商讨论凸面产品时具有重要意义。1
来自11个国家的12名护士小组成员齐聚一堂,依据定义的特征,共同对凸面皮肤底盘的特征以及相关产品的临床应用予以定义,进而建立起一致性。1制定通用术语将对研究机会和护士新手教育产生影响,因为临床医生现在可以描述特定的凸面装置属性和正确选择产品的临床决策。1采用新术语并将其提前纳入临床实践似乎是造口护理的一个根本性转变,但如果造口护理要取得进展,就应将其作为规范。2
表1.凸面装置五大特征的定义及其临床应用声明1
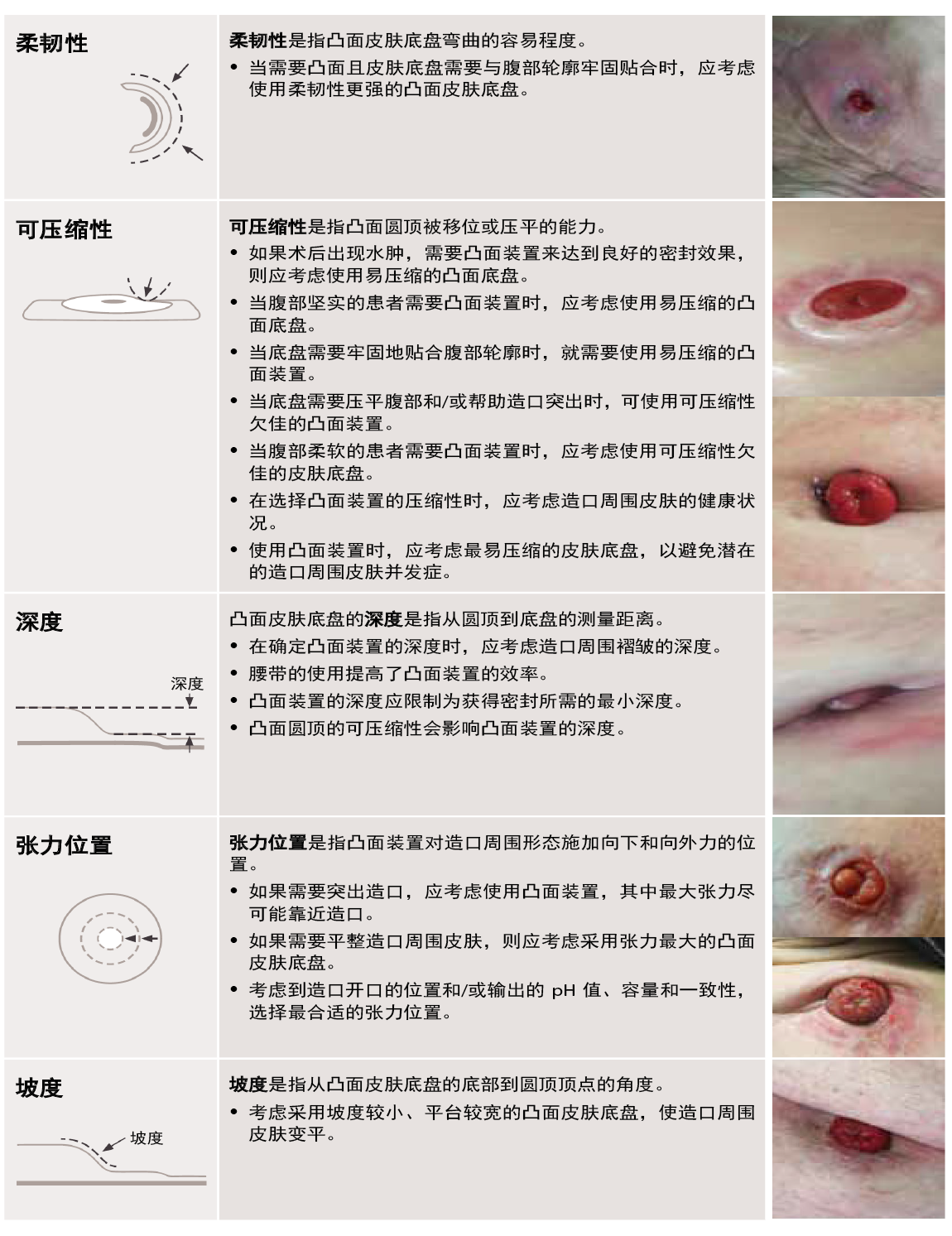
来源:McNichol L, Cobb T, Depaifve Y, et al. Characteristics of convex skin barriers and clinical application:
Results of an international consensus panel. JWOCN. 2021;48(6):524–532.
表1中的临床图片已通过Wolters Kluwer获得版权许可。
将标准化特征融入造口产品开发
研发的目标是将这些特征融入新产品中。我们摒弃了传统的坚硬、柔软和深凸等标签,强调在凸面装置领域不断创新的同时,继续对这五种产品特征展开探索。在将这些特征融入设计之前,我们必须首先规范如何衡量这些特征,以及如何考量它们对最终用户的影响。
标准化衡量方法为产品开发奠定了基础。此外,它还为新的凸面装置设计提供指导,将创新概念固定在这五个影响贴合性能的主要特征之上。在衡量特征时,有两种明显的衡量方式。一方面,可以从坡度、深度和张力位置方面进行衡量。当临床医生操作凸面装置时,这三个特征会更加明显或清晰可见。这三个特征可以通过现有计量技术来确定,比如使用3D光扫描仪来了解产品的确切深度、坡度或张力位置。另一方面,可以从柔韧性和可压缩性方面进行衡量。这两个特征并不直观,仅凭观察产品难以定义。它们是动态的,而非静态的,柔韧性和可压缩性的性质最好通过考虑时间因素来定义。例如,当临床医生操作产品并通过弯曲产品或用手压缩产品对其施力时,这两个特征会变得更加明显。再如,最终用户需要长时间佩戴凸面产品。随着时间的推移,当他们用身体做出不同的动作时,就会感受到产品的柔韧性和可压缩性的差异。因此,这两个特征最好在实验室中使用力和时间作为输入进行测量。
未来产品开发的创新解决方案
目前市场上的产品,坡度、深度和张力位置具有几何聚焦且相对静态的特点,产品的形状和尺寸在定义这三个特征时起着关键作用。与之相反,柔韧性和可压缩性则更具动态性,因为它们会随着时间变化。虽然产品的形状和尺寸仍然很重要,但在考量产品与患者接触时的表现时,这两个动态特征则显得尤为突出。
柔韧性是指产品适应和符合人体腹部轮廓的能力。临床医生认识到确保良好贴合以支持患者腹部形状和腹部张力的重要性。例如,在有深皱褶的腹部,柔软的凸面装置要比坚硬的凸面装置贴合度更高,因为在深皱褶处,底盘需要弯曲以适应这些轮廓。3因此,选择合适的凸面皮肤底盘装置取决于这一动态底盘特征。
另一个动态部分是可压缩性。它是指产品在佩戴过程中对压力和变形的反应。临床医生会对造口底盘的可压缩性进行评估,以确保造口周围的贴合度良好,并使底盘适应腹部轮廓的变化。例如,当患者腹部坚实时,应考虑使用易压缩的底盘,以减少对腹部施加的压力。1当腹部较柔软或造口需要额外的支撑才能突出时,应考虑使用可压缩性欠佳的底盘。1†
虽然柔韧性和可压缩性的动态特征对凸面装置在与患者接触时的表现有着重大影响,但是关键在于要认识到所有五个特征都是相互关联的,都有助于实现最佳的贴合效果。例如,考虑凸面皮肤底盘的临床应用。可压缩性直接影响所提供的凸面装置的深度。1与可压缩性欠佳的凸面皮肤底盘相比,易压缩的凸面皮肤底盘提供的深度较小。将软凸面装置与硬凸面装置进行比较,即可看出这一点。了解这两个特征如何影响凸面装置提供适当深度的能力,对于使用正确的底盘实现造口周围的有效密封至关重要。
结论
在新产品开发中,关注的重点不仅仅是术语和特征的衡量。这五种特征将在确定当前凸面产品的潜在差距方面发挥关键作用,并为应对临床医生日常遇到的不同最终用户体型所带来的挑战提供指导策略。定制产品以满足个体用户的特定需求,特别强调更好的贴合度,是一项重点工作。这种方法被称为“以人为本的设计”,它关注真正未被满足的需求,并设计出满足这些需求的产品。4继续研究提高产品适应性的途径,将有可能在佩戴过程中调整凸面装置的这五个特征。虽然这是一个探索性的方向,但这一解决方案可以提供更加个性化和舒适的体验。5这项研究始终以测量精度、术语标准化和以用户为中心的设计原则为基础,确保未来的产品可以满足乃至超越临床医生和患者的期望。
利益冲突声明
作者为Hollister Incorporated的员工。
资助
作者除身为Hollister的员工外,并未因本文而获得任何其他资助。
Author(s)
Greg Czaplewski1
Senior Engineer Research & Development
Kim Smitka1*
Senior Manager, Global Clinical Education
Email kim.smitka@hollister.com
1Hollister Incorporated, Libertyville, Illinois, USA
* Corresponding author
References
- McNichol L, Cobb T, Depaifve Y. et al. Characteristics of convex skin barriers and clinical application: Results of an international consensus panel. JWOCN. 2021;48(6):524–532.
- McCarroll J. Proactive use of new convexity consensus statements and patient assessment tools in achieving positive patient outcomes. White paper. Hollister Incorporated, 2000 Hollister Drive, Libertyville, Illinois, 60048, USA; 2023.
- Hoeflok J, Salvadalena G, Pridham S, et al. Use of convexity in ostomy care: results of an international consensus meeting. JWOCN. 2017; 44(1): 1–8.
- Melles M, Albayrak A, Goossens R. Innovating health care: Key characteristics of human-centered design. Int J Qual Health Care. 2021; 33(S1): 37–44.
- Bourke R, Davis E, Dunne S, et al. Making sense of convexity. White paper. Hollister Incorporated, 2000 Hollister Drive, Libertyville, Illinois, 60048, USA; 2007.


