Volume 44 Number 3
Translating the evidence into clinical practice – a journey through change
Rosemary Hill
Keywords clinical practice, evidence, convexity, change, skin barrier.
For referencing Hill R. Translating the evidence into clinical practice – a journey through change. WCET® Journal Supplement. 2024;44(3)Sup:s11-14.
DOI 10.33235/wcet.44.3.sup.s11-14
Abstract
Driving changes to clinical practice can be a daunting task. However, if meaningful change is to occur, the use of evidence to support the decision for change can be crucial in gaining agreement with stakeholders. This article describes the journey taken at one institution by a clinician following the trail of evidence that has recently been developed regarding the use of convexity with a barrier ring, earlier in the patient journey for creating positive impacts in patient outcomes.
Introduction
In today’s rapidly changing healthcare environment, innovation can challenge nurses in many ways. Switching from mercury thermometers to electronic could be considered an easy change as the benefit can clearly be seen. Sometimes, however, the benefits may not be as easily recognised at first. Additionally, implementing a change in practice can produce anxiety or fear of failure leading to resistance.1 In the 1940s Kurt Lewin introduced a change model involving three steps: unfreezing, changing, and refreezing.2 (Figure 1) Unfreezing relates to recognising the need for change; changing implements the transformation and demonstrates its benefits, and refreezing reinforces the change in behaviour and helps sustain it.2 The goal is to make changes that create minimal impact on people, yet ensure better outcomes.2 Utilising Lewin’s theory can lead to a greater understanding of how change can affect an organisation and an individual, help to recognise the barriers and solutions to successful implementation, and to identify opposing forces that act on human behaviour during change.
This paper discussses the use of recent evidence to implement change in clinical practices throughout an entire organisation. This change concerned moving from flat ostomy skin barriers to using convexity products earlier in the patient journey with the primary objective of improving their outcomes.

Figure 1. Lewin’s Change Model
Source: https://www.change-management-coach.com/kurt_lewin.html
Unfreezing
Any surgery can create a level of anxiety in the prospective surgical patient. Ostomy surgery is particularly fraught with challenges including medical, psychological, and social issues,3 as well as depression, which occurs in almost 50% of ostomy patients.4 This experience can be worsened with the onset of leakage in the first instance for the person with a newly fashioned ostomy. We have observed at our hospital that the language around this leakage can play a profound impact on person’s adjustment after surgery. For example, the patient may experience this first leakage after surgery in the hospital bed and have the attending nurse pronounce what they assume to be reassuring, passing references regarding the leakage. Comments such as ‘this happens often’ or ‘don’t worry you will get used to it’ can unintentionally create negative expectations around their ostomy management. While clinicians may be used to the experience, it should be recognised that the new patient is not. A more appropriate response, for example, might be ‘well that should not be happening, and we can fix this.’
In my practice I have recently become cognisant that within the 24–48 hours post-operative period, leakage of effluent was frequently occurring under recently applied flat ostomy skin barriers post-operatively (Figure 2). Additionally, speaking with colleagues at various conferences, I heard that they had noticed similar occurrences and reported that they were beginning to use soft convex skin barriers in the initial post-operative period as a prevention mechanism for reducing leakage on patient discharge. This message to me was compounded when some recent publications around the use of convexity earlier in the journey were described at different educational events, as well as professional conferences. Of note, two of the more recent publications, each resulting from consensus statements, provided supportive evidence around the early post-operative use of convexity. One article describes the characteristics of convexity which recommends the use of a more compressible convex skin barrier in the immediate post-operative period5 and the second reported on the ability to use convex skin barriers at any point along the patient journey.6 One of the chief objections in the past regarding the use of convexity post-operatively was the risk of mucocutaneous separation.6 However, the evidence in the literature does not support this as a risk7 and some studies have shown that the convexity can be used in the post-operative period.6,8 I felt comfortable in the evidence supporting me in changing my practice.
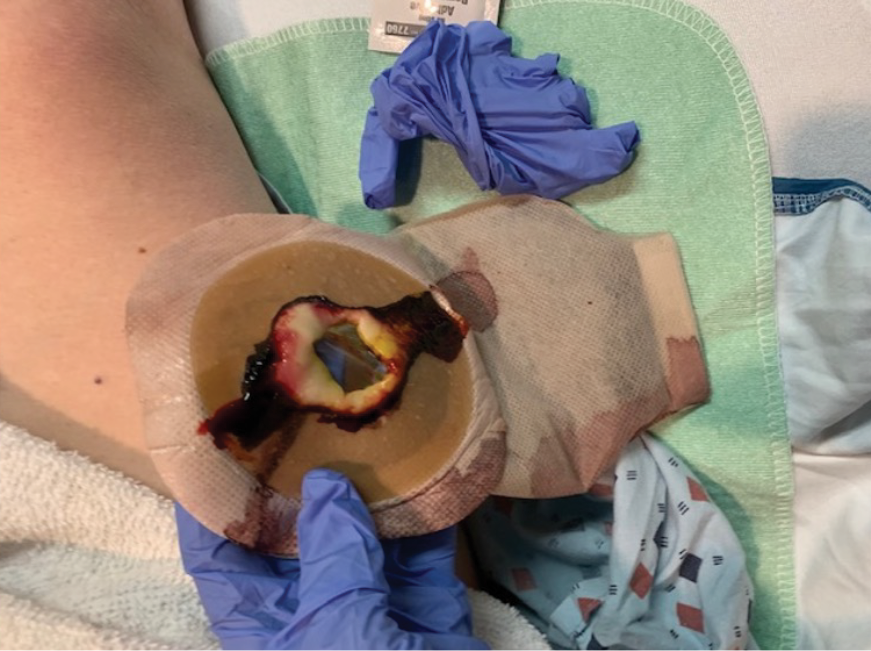
Figure 2. Flat skin barrier less than 24 hours post-operatively
There were several concerns for our patients with the current situation in our organisation. Ostomies are often created close to an incision point based on the stoma site marking process and this is frequently the midline. This means that effluent from leakage could come into contact with the newly created surgical incision. It was presumed that there would be increased risk of surgical site infection as a result.
Leakage of stomal effluent is also a significant risk factor in the development of peristomal skin complications (PSCs), such as peristomal moisture associated skin damage (PMASD).8 PSCs occur in up to 80% of patients with an ostomy 6 and are associated with impairments in physical function, multiple components of health-related quality of life, failure to adjust, and higher costs.6 As described previously, leakage also can also have dire consequences for the confidence of the newly ostomised patient.6
During my thought processes, I questioned the use of a flat skin barrier at all during the post-operative period. Why would I not consider changing the flat skin barrier used in the operating room to that of a soft convex skin barrier given this new evidence and the results? In terms of unfreezing, there was high recognition that an opportunity for change was apparent, and such change had the potential to afford real patient benefits if successful.
Making the Change
The standard practice at our organisation included the application of flat ostomy skin barriers intraoperatively for patients undergoing ileostomy, colostomy and urostomy surgery. Additionally, flat ostomy skin barriers were used to manage patients on the surgical wards post-operatively. I conducted a retrospective review of my patients with this method of management and discovered that many experienced leakages once discharged with these types of products. In some instances, the patient record described that ‘probable use of convexity in the future will be required’. This provided me with further information that proactive change was required.
Initially, a case series of seven patients (two urostomy, four ileostomy, and one colostomy) were selected to provide directional data regarding the success (or failure) of the proposed change. One surgeon was identified, and an operating room (OR) education nurse provided support and guidance to help facilitate the process (Figures 3 and 4). Patient ages ranged from 55 to 85 years old with varying aetiologies, including Crohn’s, diverticular disease, rectal cancer, bladder cancer and pancreatic cancer. OR staff also recognised the role of barrier rings and suggested that they would be able to apply these to the patients in the OR, as well, as they understood the risk of leakage and the potential associated challenges. Under my supervision, all patients had a two-piece soft convex skin barrier and flat barrier ring (slim) applied to their newly created stoma in the OR while on the operating table (Figures 5, 6 and 7).
All patients in this series had successful pouching application using the new products and none experienced leakage, including one patient with a high output stoma (Figures 8 and 9). This provided further evidence that allowed me to continue with the change. As they say, ‘the proof is in the pudding.’
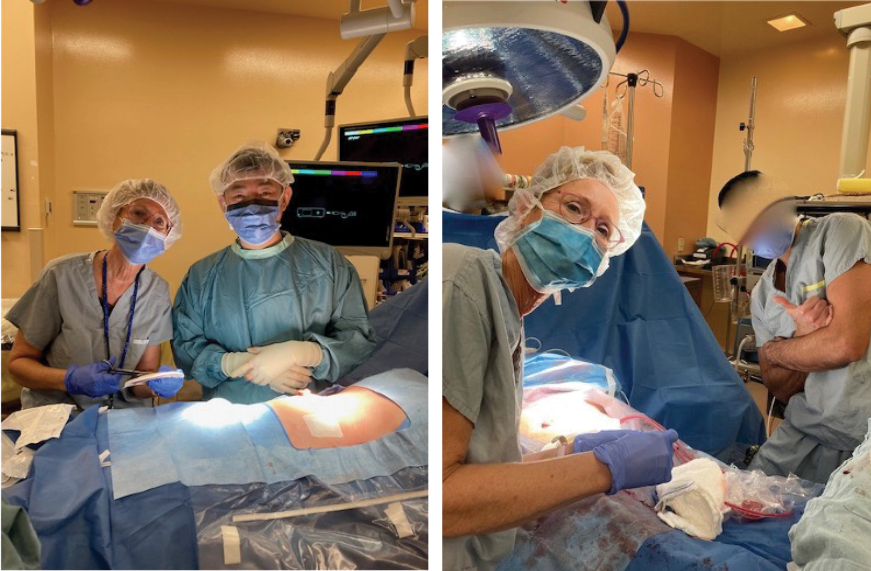
Figures 3 and 4. In our hospital operating room with the colorectal surgeons
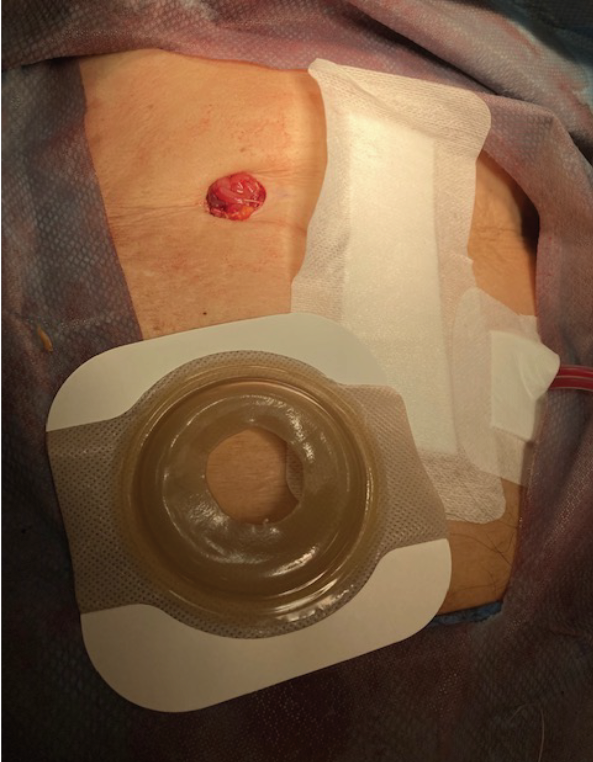
Figure 5. Two piece soft convex skin barrier cut to size of colostomy
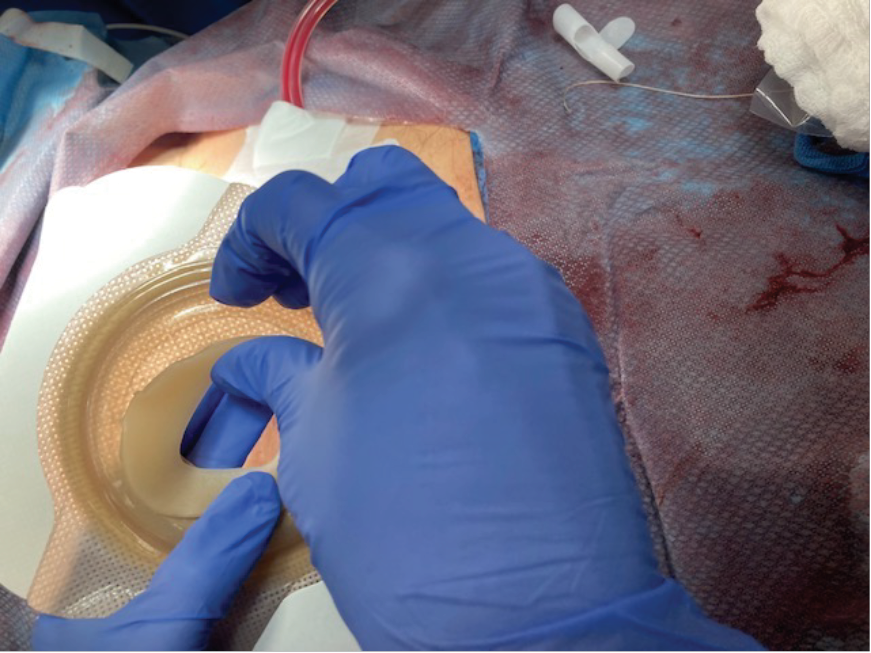
Figure 6. Application of skin barrier ring
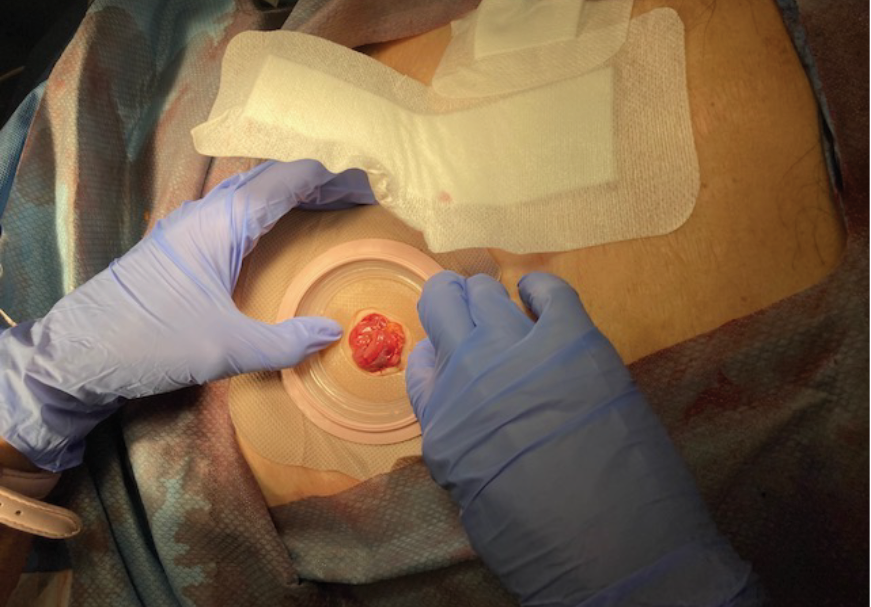
Figure 7. Application of skin barrier to stoma
(note midline dressing applied over the skin barrier edges).
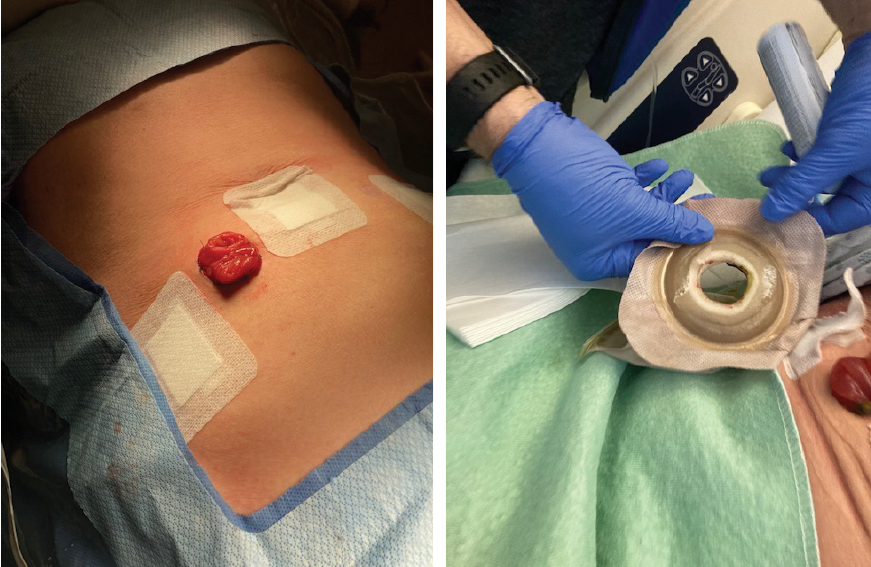
Figures 8 and 9. High output loop ileostomy (note flat skin while supine),
skin barrier appearance after 24 hours (note skin topography when semi-recumbent)
Refreezing
Maintaining and entrenching the change meant engagement with all OR staff and surgeons to relay all the evidence and its purpose. Additionally, ward staff were educated on the new products to manage the patients post-operatively. Lastly, engaging with procurement to ensure supply of the correct products in each area. This also meant active removal of all the current flat products to ensure these patients all received the new, more compressible, convex products and barrier rings. Education with all staff was ongoing. There was minimal upheaval and there were real patient benefits from this change, which made it easy for all to accept. We now have this as standard practice in our organisation.
Outcomes
While the case series and the supporting evidence helped prove my case for change, it is important to continuously monitor such a change, particularly after long held process has been altered. To ensure the ongoing success for our patients is maintained, a retrospective chart review was undertaken. One of the biggest fears many clinicians expressed was about the increased potential for convexity to contribute to muco-cutaneous separation. This concept was monitored specifically in 21 patients during this retrospective review. Only one experienced any muco-cutaneous separation. However, this patient had risk factors including high BMI (body mass index) and challenging stoma construction that was under tension with low stoma height. These factors have been shown to predispose patients to the development of peristomal skin complications including mucocutaneous separation.9,10 All indications are favourable thus far, based on this review and another publication with all the data is underway to illustrate the patient outcomes.
Conclusion
Change for some is never easy. However, real, and impactful change can be made when there is solid evidence and a willingness to approach change with an open mind. Knowing that our patients will experience better outcomes in their journey because of this change, is testament to our belief that they deserve better outcomes. We know our patients face significant challenges in their journey. If we can reduce some of their stressors by making changes that give better results, we can give them one less thing to worry about and their overall adjustment and quality of life after surgery can be improved.
We have found that the soft convex skin barrier with a slim ring provides a customised and secure skin seal against leakage. The characteristics of a compressible and flexible skin barrier provides just the right amount of tension to address peristomal topographies and stomal challenges. I would urge fellow clinicians to review the evidence and see where change can make a positive difference for the patients in their practices.
Conflict of Interest
The author declares no conflicts of interest.
Funding
The author received no funding for this article.
将证据转化为临床实践——变革之旅
Rosemary Hill
DOI: 10.33235/wcet.44.3.sup.s11-14
摘要
推动临床实践变革是一项艰巨的任务。然而,若要实现有意义的变革,依靠证据来支持决策对赢得利益相关者的支持至关重要。本文讲述了一位临床医生在一家医疗机构中的工作经历。这位医生基于最新获得的证据,在患者治疗的早期阶段采用了带有防漏环的凸面装置。这一新技术的应用显著改善了患者结局。
引言
在当今瞬息万变的医疗环境中,创新可能会在许多方面对护士提出挑战。从水银温度计到电子温度计的转变可以说是轻而易举,因为其益处显而易见。然而,有时候,这种益处可能初期并不明显。此外,在实践中实施变革可能引发焦虑或对失败的恐惧,从而导致抵触情绪。120世纪40年代,Kurt Lewin提出了一种变革模型,该模式包含三个步骤:解冻、变革以及再冻结。2(图1)解冻与认识到变革的必要性有关;变革指实施变革并展示变革带来的益处;再冻结则是加强行为的改变并帮助维持这种改变。2其目标是做出对人们影响最小的改变,同时确保获得更好的结局。2利用Lewin的理论,可以更好地理解变革如何影响组织和个人,有助于认识到成功实施变革的基础和解决方案,并且能够识别出在变革过程中作用于人类行为的对立力量。
本文讨论了如何利用最新证据,在整个组织内实施临床实践变革。这一变革涉及到从使用平面造口皮肤底盘到在患者就医早期使用凸面装置产品,其主要目的是改善患者结局。

图1.Lewin变革模型
来源:https://www.change-management-coach.com/kurt_lewin.html
解冻
任何手术都会让潜在的手术患者产生一定程度的焦虑。造口手术尤其充满挑战,包括医疗、心理和社会问题,3以及抑郁症,几乎50%的造口患者都患有抑郁症。4这种体验在新造口患者首次遇到渗漏时可能会变得更糟。在医院中我们观察到,围绕这种渗漏所产生的声音会对患者术后的适应情况产生极为深远的影响。例如,患者可能会在医院病床上经历第一次渗漏,并听到主治护士说出他们认为具有宽慰性的关于渗漏的话语。诸如“这种情况经常发生”或“别担心,你会习惯的”之类的话语会让患者无意中对造口管理产生负面期望。虽然临床医生可能习惯了这种经历,但应该认识到新患者并不习惯。例如,更恰当的回答可能是“这种情况不应该发生,我们可以解决这个问题”。
在实践中,我最近认识到,在术后24-48小时内,术后近期应用的造口皮肤底盘经常出现排泄物渗漏(图2)。此外,在各种会议上与同事交谈时,我听说他们也注意到了类似的情况,并报告称他们开始在术后初期使用柔软的凸面皮肤底盘装置,作为减少患者出院时渗漏的预防机制。在不同的教育活动以及专业会议上,有人介绍了最近发表的一些关于病程早期使用凸面装置的文章,这让我更加确信了这一点。值得注意的是,最近发表的两份共识声明都围绕术后早期使用凸面装置提供了支持性证据。其中一篇文章介绍了凸面装置的特征,建议在术后立即使用可压缩性更强的凸面皮肤底盘5,第二篇文章则报道了在患者的任何阶段都可以使用凸面皮肤底盘。6过去对术后使用凸面装置的主要反对意见之一是皮肤黏膜分离的风险。6不过,文献中的证据并不支持这是一种风险7,一些研究表明,凸面装置可以在术后使用。6,8鉴于有证据支持实践变革,我感到十分放心。

图2.术后24小时内的平面皮肤底盘
对于我们组织目前的状况,患者有几种担忧。根据造口部位的标记过程,通常在靠近切口点的地方创建造口,而切口点通常是中线。这意味着渗漏的排泄物可能会接触到新创建的手术切口。据推测,手术部位感染的风险会因此増加。
造口排泄物的渗漏也是造口周围皮肤并发症(PSC)的一个重要风险因素,如造口周围潮湿相关性皮肤损伤(PMASD)。8多达80%的造口患者会出现PSC,6并可能导致身体功能受损、健康相关生活质量的多个方面受损、适应失败和费用更高。6如前所述,渗漏也会对新造口患者的信心造成灾难性打击。6
在思考的过程中,我对术后是否使用平面皮肤底盘提出了质疑。既然有了这些新的证据和结果,我为什么不考虑将手术室使用的平面皮肤底盘改成软凸面皮肤底盘呢?在解冻过程中,人们深刻认识到,变革的机会显而易见,如果这种变革成功实施,将可能为患者带来显著的益处。
实施变革
我们机构的标准实践是在接受回肠造口术、结肠造口术和尿道造口术的患者术中应用平面造口皮肤底盘。此外,管理手术病房的术后患者时,也会应用平面造口皮肤底盘。我对采用这种管理方法的患者进行了回顾性审查,发现很多使用这类产品的患者在出院后都出现了渗漏。在某些案例中,患者病历中记录了“今后可能需要使用凸面装置”。这为我提供了进一步的信息,即需要进行积极的变革。
最初,我们选择了7例患者(2例尿道造口术患者、4例回肠造口术患者和1例结肠造口术患者)的病例系列,以提供有关拟议变革成功(或失败)的方向性数据。拟定了一名外科医生,并由一名手术室(OR)教育护士提供支持和指导,以帮助促进这一过程(图3和图4)。患者年龄从55岁到85岁不等,病因各异,包括克罗恩病、憩室病、直肠癌、膀胱癌和胰腺癌。OR人员也认识到了防漏环的作用,并表示他们也可以将其应用于OR的患者,因为他们了解渗漏的风险和潜在的相关挑战。在我的监督下,OR内所有手术台上的患者都在新造口部位应用了两件式软凸面皮肤底盘和平面防漏环(细长型)(图5、图6和图7)。
该系列中的所有患者都成功使用了新产品造口袋,无一出现渗漏,其中包括一名高输出造口患者(图8和图9)。这为我继续实施变革提供了进一步的证据。正如人们所说,“实践是检验真理的唯一标准”。

图3和4.在我们医院的手术室里,与结直肠外科医生一起完成手术

图5.按结肠造口大小切割的两件式软凸面皮肤底盘

图6.应用皮肤防漏环

图7.将皮肤底盘应用于造口(注意中线敷料应用于皮肤底盘边缘)。

图8和9.高输出回肠袢造口术(注意仰卧时的皮肤平整度,
24小时后的皮肤底盘外观(注意半卧位时的皮肤形态)
再冻结
要维持和巩固这一变革,就必须让所有OR人员和外科医生都参与进来,转达所有证据及其目的。此外,病房工作人员也接受了有关新产品的教育,以便对患者进行术后管理。最后,与采购部门合作,确保为每个地区提供正确的产品。这也意味着要主动移除目前所有的平面产品,以确保这些患者都能用上新的、可压缩更强的凸面产品和防漏环。对所有工作人员的教育仍在继续。这种变革带来的动荡微乎其微,患者也能从中真正受益,因此大家都很容易接受。现在,这已成为我们组织的标准实践。
结局
虽然系列案例和辅助证据支持了我的变革理由,但在长期坚持的流程被改变后,持续监测变革的效果至关重要。为确保患者持续获得成功,我们进行了一次回顾性病历审查。许多临床医生表示,他们最担心的问题之一就是凸面装置导致皮肤黏膜分离的可能性増加。在这次回顾性审查中,对21例患者专门监测了这一情况。仅一例患者出现皮肤黏膜分离。但是,这例患者本身存在一些风险因素,包括体重指数(BMI)较高和造口结构具有挑战性,处于张力状态下且造口高度较低。这些因素已被证明容易导致患者出现造口周围皮肤并发症,包括皮肤黏膜分离。9,10基于此次回顾和另一篇即将发表的全面展示患者结局的文章,目前所有迹象都是积极的。
结论
对一些人而言,变革绝非易事。然而,当有坚实的证据基础和支持以开放心态面对变革的态度时,真正的、有影响力的变革是可以实现的。我们的患者会因为这种变革而在他们的旅程中获得更好的结局,这是对我们信念的证明,即他们值得更好的结局。我们知道患者在其旅程中面临重大挑战。如果我们能通过变革来减轻他们的一些压力,从而取得更好的效果,我们就能让他们少操心一件事,他们术后的整体适应情况和生活质量就能得到改善。
我们发现,带有纤细防漏环的软凸面皮肤底盘可提供定制的安全皮肤密封,防止渗漏。可压缩和柔软皮肤底盘的特征可提供恰到好处的张力,以应对造口周围形态和造口挑战。我敦促同行临床医生审阅证据,看看哪些方面的变革可以为其实践中的患者带来积极的改变。
利益冲突声明
作者声明无利益冲突。
资助
本文作者未收到任何资助。
Author(s)
Rosemary Hill
RN BScN NSWOC WOCC(C)
Lions Gate Hospital
Vancouver Coastal Health, Canada
Email rosemary.hill@vch.ca
References
- Lock, D, 2023. The psychology of fear of change: Unpacking organizational resistance. Daniel Lock Consulting. Accessed January 2024. https://daniellock.com/fear-of-change-organization
- Raza, M. Lewin’s 3 stage model of change explained. BMC Software Inc. 2019. Accessed January 2024. https://www.bmc.com/blogs/lewin-three-stage-model-change/
- Sceats LA, Dehghan MS, Rumer KK, et al. Surgery, stomas, and anxiety and depression in inflammatory bowel disease: a retrospective cohort analysis of privately insured patients. Colorectal Dis. 2020;22(5):544–553.
- Tang WSW, Chiang LLC, Kwang KW, Zhang MWB. Prevalence of depression and its potential contributing factors in patients with enterostomy: A meta-analytical review. Front. Psychiatry. 2022; 13:1001232.
- McNichol L, Cobb T, Depaifve Y, et al. Characteristics of convex skin barriers and clinical application: Results of an international consensus panel. JWOCN. 2021;48(6):524–532.
- Colwell J, Stoia Davis, J, Emodi, K. et al. Use of a convex pouching system in the post operative period. A national consensus. JWOCN. 2022:49(3):240–246.
- Hoeflok J, Kittscha J, Purnell P. Use of convexity in pouching – A comprehensive review. JWOCN. 2013:40(5):506–512.
- Hoeflok J, Salvadalena S, Pridham S. et al. Use of convexity in ostomy care – Results of an international consensus meeting. JWOCN. 2017:44(1):55–62.
- Braumann C, Muller V, Knies M, et al. Complications after ostomy surgery: Emergencies and obese patients are at risk. World J Surgery. 2018:43(3):751–757.
- Shiraishi T, Ogawa H, Naomi S, et al. Surgical techniques and stoma-related complications associated with emergency stoma creation. Anticancer Res. 2023:43(9):4189–4195.


