Ahead of Print
How effective are dietary interventions for prevention and management of chronic wounds in individuals with diabetes: a systematic review protocol
Hailey R Donnelly, Clare E Collins, Erin D Clarke, Natalie Gilbertson-Viljevac, Prudence I Morrissey, Peta E Tehan
Keywords systematic review, Diabetes, wounds, dietary intake, dietary intervention
For referencing Donnelly HR et al. How effective are dietary interventions for prevention and management of chronic wounds in individuals with diabetes: a systematic review protocol. Wound Practice and Research 2023; 31(3):to be assigned.
DOI
https://doi.org/10.33235/wpr.31.3.to be assigned
Submitted 5 July 2023
Accepted 30 August 2023
Abstract
Background Adequate nutrition is essential for individuals with diabetes and wounds to optimise both wound healing and blood glucose control. Previous systematic reviews have evaluated the effectiveness of nutrition supplementation for wound healing in individuals with diabetes. However, none have reported comprehensively on the range of dietary interventions utilised in this population, despite these being common within clinical practice. Therefore, the aim of this systematic review is firstly to evaluate the effectiveness of dietary interventions for wound prevention and management in people with diabetes, and secondly to describe intervention characteristics.
Methods Using PRISMA-P to guide the review, five databases will be searched for intervention studies (Medline, Embase, CINAHL, Scopus, Cochrane Library) as well as clinical trials registries. The Rob‑2 and ROBINS tools will assess risk of bias. Studies will be described narratively, and a meta-analysis conducted if adequate levels of homogeneity exist between included studies and outcome variables, including study types, and characteristics of the sample including sample size. An appropriate model will be chosen to undertake meta-analysis.
Discussion The systematic review results will inform clinicians on the most effective dietary interventions to optimise wound healing in individuals with diabetes. Study results will inform design and conduct of future nutrition interventions in wound healing.
Introduction
Chronic wounds are a silent epidemic – the pooled prevalence of ulcers of various aetiologies is 2.21 per 1000 population, accounting for A$3.5 billion of economic burden to the Australian healthcare system annually1,2. Chronic wounds are defined as hard-to-heal wounds that do not progress through timely healing despite optimal care in 4–6 weeks3, and include pressure injuries (PI), venous leg ulcers (VLU), arterial ulceration and diabetes-related foot ulcers (DFU)2. DFU is a broad term which encompasses three subtypes of wounds – neuropathic, ischaemic and neuro-ischaemic ulcers4. Diabetes is a major contributor to impaired or delayed healing of chronic wounds5,6. Currently, 10.5% (537 million) of the world’s population aged 20–79 years are living with diabetes7. In 2021, 6.7 million deaths worldwide were estimated to have been attributed to diabetes (all types) and related complications7, with one of the most common complications being chronic wounds8.
Individuals with diabetes can develop a variety of different chronic wounds, including DFU, PI and VLU, with an estimated 19–34% of those living with diabetes developing a DFU in their lifetime9. The prevalence of other chronic wound types in individuals with diabetes is less clear9,10. Three previous systematic reviews demonstrated the increased risk of PI in individuals with diabetes11–13, with one review concluding that a person living with diabetes had a 1.77 times increased likelihood of developing a PI compared to an individual without diabetes12. The prevalence of VLU in individuals with diabetes has also not been quantified, with previous research postulating that between 17–21.7% of VLU patients also have a diagnosis of diabetes14,15.
The underlying pathophysiology behind diabetes and wound healing is complex, with a combination of neuropathic, vascular, immune and biochemical factors impairing the wound healing process16,17. Peripheral sensory neuropathy secondary to diabetes is particularly relevant, as reduced innervation is proposed to impact on wound healing18. Further, peripheral arterial disease results in a reduction of blood flow to lower extremities, reducing nutrient, oxygen and immune cell delivery to foot and leg wounds, further inhibiting the wound healing processes19. Finally, hyperglycaemia can negatively impact wound healing10 through impairing angiogenesis and endothelial nitric oxide synthesis, decreasing leukocyte function, and the accumulation of advanced glycation end-product in tissues is also detrimental to wound healing as it can result in apoptosis20–22.
In populations with diabetes and chronic wounds (VLU, DFU and PIs), previous studies have identified that poor diet quality, micronutrient deficiencies and malnutrition are highly prevalent23–30. Nutrition is an important factor in wound healing, with inadequate intakes of energy, macronutrients, and specific micronutrients, including protein, zinc, vitamin C and vitamin D, delaying timely tissue repair31–34. Further, nutrition can also influence the prevention of wounds35. A recent systematic review identified specific nutritional deficiencies associated with development of DFU and further described that the micronutrient status of individuals with diabetes and active foot ulcers was significantly different compared to someone living with diabetes without a foot ulcer36. Specifically, vitamin E supplementation has been demonstrated to assist in the prevention of wounds in individuals with diabetes, as well as assisting to delay the progression of existing wounds35.
Whilst the relationship between nutrition and wound healing is well established, particularly in PI23,32,37, it is not currently clear as to the best way to clinically intervene, particularly in patients with diabetes who have more complex nutritional needs. One previous randomised control trial which included participants with diabetes demonstrated a positive association between medical nutrition therapy and PI healing37. It is well established that individuals with PI have substantial protein and nitrogen losses due to high levels of wound exudate. Therefore, ensuring adequate intake of these nutrients is important to facilitate timely wound healing processes32.
Dietitian intervention, nutrition counselling and nutrition education are commonly employed in practice to help individuals optimise their dietary intakes, with multiple studies demonstrating effectiveness24,37–41. Previous systematic reviews and meta-analyses have investigated the effectiveness of specific nutrition supplementation approaches for wound healing in DFU23,42–50. However, none to date have explored the effectiveness of different dietary interventions (behavioural and/or supplementation) for either the prevention and/or management of a range of chronic wound aetiologies (VLU, DFU, PI) in individuals with diabetes.
Objectives
Therefore, this systematic review aims to:
- Evaluate the effectiveness of dietary interventions for wound prevention and management in the healing and incidence of wounds in individuals with diabetes.
- Describe the characteristics of dietary interventions used for the prevention and management of wounds in those living with diabetes.
Methods
This systemic review protocol will be reported in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols checklist (PRISMA-P)51.
Participants/setting
Studies will be included:
- That include individuals of any age, sex and ethnicity, in any healthcare setting (i.e., hospital, community, residential aged care), with any co-morbidities, with type 1 or 2 diabetes with a wound will be included.
- If wounds include DFU, pressure ulcerations/PIs, arterial/ischaemic ulcers, VLU or mixed leg ulcerations.
- If wound classification systems include Texas Wound Classification System, WIfI Classification System or Wagner’s Classification52–54. Acute surgical wounds will be excluded.
- If all participants have diabetes, or if studies which are not specifically in a diabetes cohort report outcomes of those with diabetes separately.
Intervention(s)
Studies will be included:
- That test a dietary intervention for the prevention and/or treatment of wounds in people living with diabetes.
- If dietary interventions include: supplementation (oral, enteral or parenteral) of any type (food pattern, macronutrient, vitamin, mineral or multi-nutrient), dose, mode, duration; any special diet; nutrition education; dietitian intervention; and/or nutrition counselling.
- If preventative dietary interventions target preventing wounds in those living with diabetes who are at risk of ulceration and who are currently without an active wound.
Studies will not be included:
- If the participants with diabetes are part of a larger group and are not able to be evaluated as a sub-group.
- If the dietary intervention is a part of a multi-component intervention or bundle of care, where the results of the dietary intervention cannot be evaluated individually.
Comparator(s)
- Control group with no dietary intervention OR
- Standard care/diet OR
- Comparison of two or more types of wound prevention/treatment dietary interventions.
Outcome measures for wound prevention studies
Primary outcomes:
- Incidence of ulcers/wounds during the study intervention.
Secondary outcomes:
- Changes to eating behaviours, diet quality, food and/or nutrient intake.
- Any biochemistry assessed but not limited to: HbA1c, fasting glucose, inflammatory markers (e.g., CRP, IL‑6, TNF‑α), vitamin/mineral status, cholesterol levels, urinalysis or any other objective measures reported.
- Acceptability/satisfaction with intervention.
- Cost of intervention.
- Quality of life – validated scale.
- Adherence/dropout rate.
- Physical activity levels.
Outcome measures for wound management studies
Primary outcomes:
- Measures of wound healing, including time to healing, changes in healing rate, changes in wound length, width and depth, total number of wounds, time to complete healing, or proportion of wounds healed at completion of study.
Secondary outcomes:
- Changes to eating behaviours, diet quality, food and/or nutrient intake.
- Any biochemistry assessed but not limited to: HbA1c, fasting glucose, inflammatory markers (e.g., CRP, IL‑6, TNF‑α), vitamin/mineral status, cholesterol levels, urinalysis or any other objective measures reported.
- Acceptability/satisfaction with intervention.
- Cost of intervention.
- Quality of life – validated scale.
- Adherence/dropout rate.
- Physical activity levels.
- Admission and length of stay (if in hospital).
- Development of new ulcers.
- Amputation rate.
- Surgical interventions.
Studies will also be included if wound healing is included as a secondary outcome.
Study design
Randomised control trials, pseudo-randomised control trials, quasi-experimental studies and pre- and post-studies of any timeframe, in English and any publication year will be included.
The following study designs will be excluded – cross-sectional studies, cohort studies, case studies, case series, systematic reviews and meta-analysis, and conference abstracts.
Information sources
We will utilise five databases to maximise the inclusion of all studies relevant to the review aims. The databases to be searched are Medline, Embase, CINAHL, Scopus and Cochrane Library. Reference lists of included studies will also be searched for articles relevant to the aims of this study. Study authors will be contacted if key data is not presented in the relevant format. Experts in the field will be contacted to confirm all relevant studies are included in this review. Moreover, the clinical trials registries (Europe, Australia and New Zealand, the World Health Organization and the United States of America trials database) will be searched for relevant registered studies. Trial full texts will be sought after on other databases. If trials have not been published, authors will be contacted so see if there has been any progress.
Search strategy
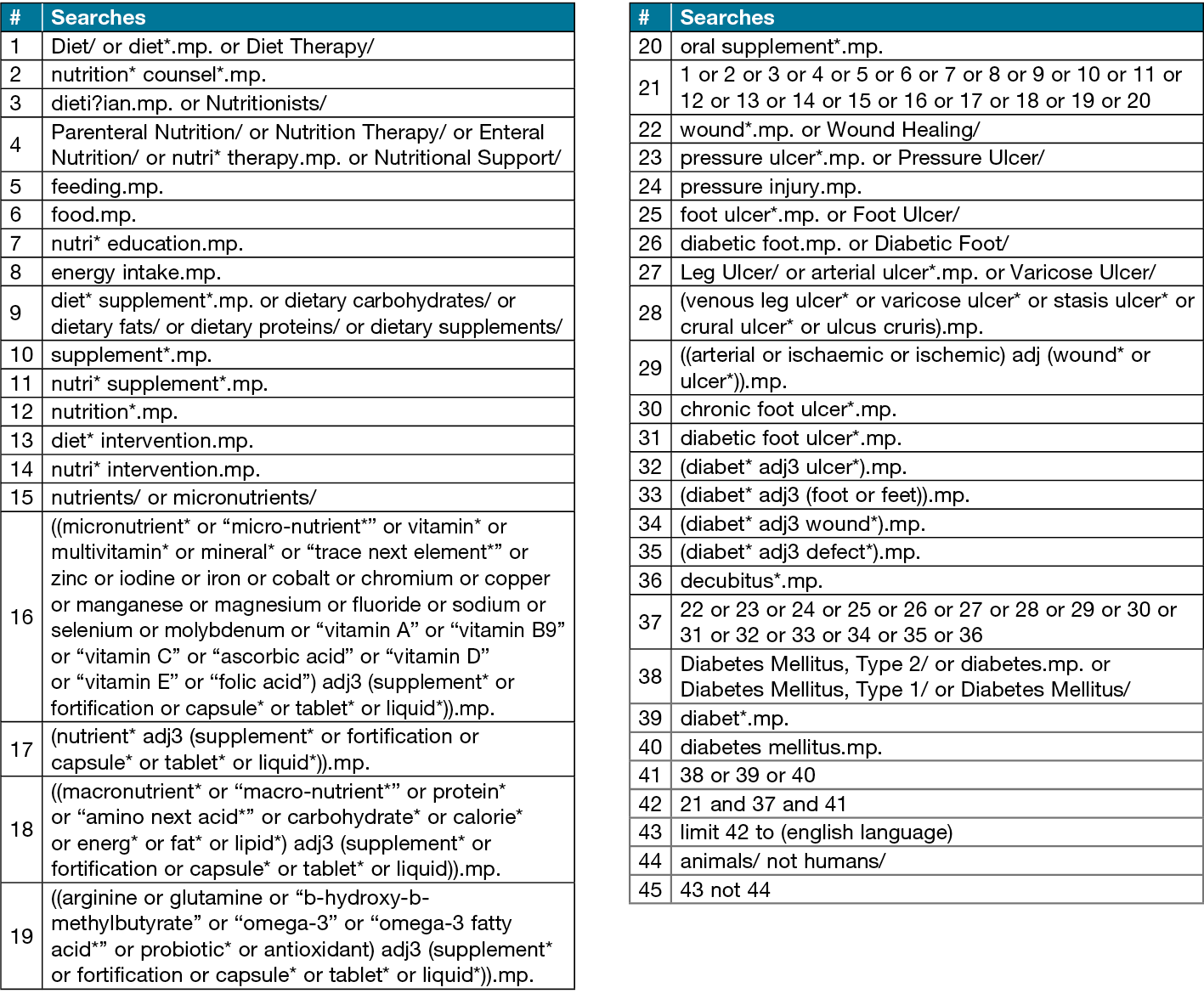
The search strategy was developed within the research team and assistance from an experienced senior health and medical research librarian. An initial search was conducted on Medline to confirm the search strategy identifies relevant articles. The Medline search strategy is presented in Table 1. Potentially eligible articles identified in the initial search will be screened for key words that can be used to strengthen the search strategy. The search will be limited to human subjects and studies published in English. To identify articles eligible for inclusion in the systematic review, search terms with appropriate truncation and indexing will be utilised. The search strategy will be modified to suit each database used. The search strategy aims to identify experimental studies investigating the effectiveness of dietary interventions on wound healing in those living with diabetes.
Table 1. Medline search strategy
Study records
Data management
For consistency and efficiency, the Covidence® systematic review software (Veritas Health Innovation, Melbourne, Australia) will be used to store eligible studies and facilitate research team-based screening of titles and abstracts, full texts and data extraction. The literature search, uploading of studies to Covidence® and removal of duplicates will be conducted by one author (HD).
Selection process
Three researchers will utilise Covidence® to independently screen titles and abstracts of all studies identified in the search, with two researchers assessing each title and abstract. A third researcher will resolve conflicts in title and abstract screening. For studies that are considered potentially relevant, full text records will be retrieved and independently assessed by two researchers for inclusion. For studies excluded during the full text screening, a reason for exclusion will be recorded. If there is conflict between the two researchers for inclusion or exclusion of a study during full text screening, this will be resolved by discussion. Studies deemed to meet inclusion criteria will be included.
Data collection process
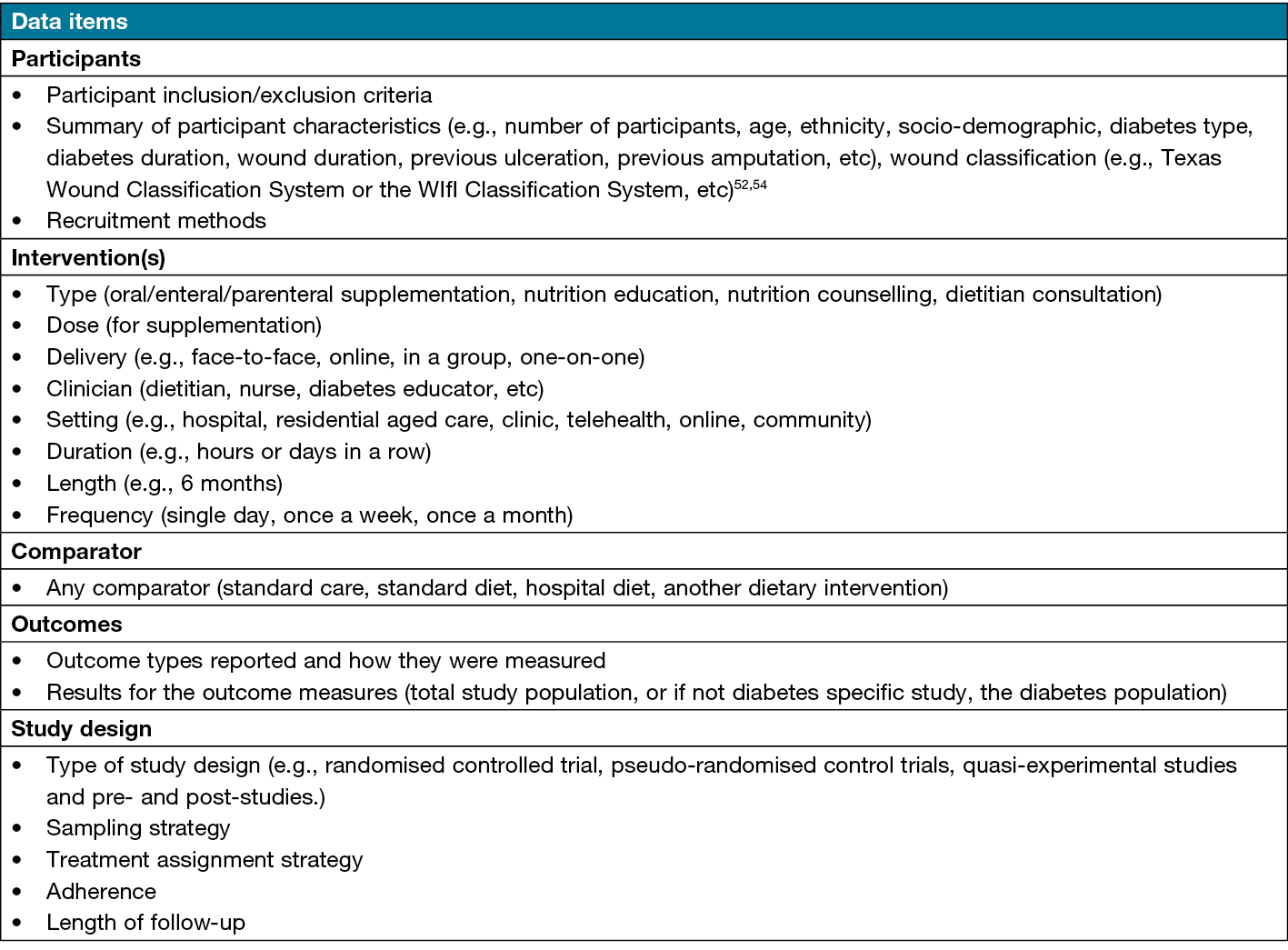
Data will be extracted from eligible studies independently by a single researcher using a standardised data extraction criterion developed by the research team (Table 2). A 10% check of data extraction will be completed by an independent person (second reviewer). Data will be extracted using Covidence® software. The data extraction criterion will be pilot tested with five studies randomly selected. Included studies that contain missing data or unclear information will be contacted (where available).
Table 2. Data items. Data to be extracted from eligible studies will include authors, year of publication, journal title and country of study as well as the following data
Risk of bias in individual studies
We will assess the risk of bias in the individual studies included by using Rob‑2 and ROBINS Tools55,56. The tool will be utilised by two independent reviewers (HD, PT), and a third reviewer will address any disagreements that arise (EC).
Effects measures
The effect measure of mean difference will be utilised in the result synthesis.
Data synthesis
Studies included in data synthesis will be characterised by the intervention type and outcome measures. The synthesising of the data will be guided by the research aims. Extracted findings will be presented in tables. The findings will be discussed in relation to the current literature and implications for research, policy and practice. Descriptive data will be reported for the number of studies and reported in a table.
If there is sufficient homogeneity amongst included studies, a meta-analysis will be performed. Depending on the number of included studies, the study types and characteristics of the sample, including sample size, an appropriate model will be chosen to undertake meta-analysis.
Reporting bias assessment
Data will only be obtained from the publication, and authors will be contacted for missing data.
Confidence in cumulative evidence
The Grading of Recommendations Assessment, Development and Evaluations (GRADE) system will be used to assess the certainty and strength of the body of evidence57.
Discussion
This systematic review and meta-analysis will summarise the scientific literature and determine the overall effectiveness of dietary interventions for the prevention and treatment of wounds in those living with diabetes. The findings from this systematic review will help inform clinicians and healthcare services on the most effective dietary interventions to optimise wound healing in those with diabetes, whilst emphasising the importance of dietetic input in the care cycle for this population. The results also have the potential to influence wound clinicians’ practice for this population and the need to consider nutrition in clinical assessment and subsequent dietitian referral. The findings will postulate appropriate design for future trial designs in this field. Moreover, this systematic review may also highlight the paucity of dietary interventions in those living with a DFU, and emphasise the need for more high quality research in this critical area of patient care.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
HD is undertaking this systematic review as part of her PhD (Nutrition and Dietetics) at the University of Newcastle and is supported by a University of Newcastle research training program scholarship. CC is supported by an NHMRC Leadership Research Fellowship (L3, APP2009340).
Author contribution
HRD, PET and CEC designed the systematic review protocol. The search strategy was developed within the research team and with assistance from a senior research librarian with experience in health and medical research.
Author(s)
Hailey R Donnelly1,2*, Clare E Collins1,2, Erin D Clarke1,2, Natalie Gilbertson-Viljevac1, Prudence I Morrissey1, Peta E Tehan1,3
1School of Health Sciences, College of Health, Medicine and Wellbeing, University of Newcastle, NSW, Australia
2Food and Nutrition Research Program, Hunter Medical Research Institute, Rankin Park, NSW, Australia
3School of Clinical Sciences, Monash University, Melbourne, VIC, Australia
*Corresponding author email hailey.donnelly@uon.edu.au
References
- Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol 2019;29:8–15. doi:10.1016/j.annepidem.2018.10.005
- McCosker L, Tulleners R, Cheng Q, et al. Chronic wounds in Australia: a systematic review of key epidemiological and clinical parameters. Int Wound J 2019;16(1):84–95. doi:10.1111/iwj.12996
- Lavery LA, Barnes SA, Keith MS, Seaman JW, Jr., Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care 2008;31(1):26–9. doi:10.2337/dc07-1300
- Yotsu RR, Pham NM, Oe M, et al. Comparison of characteristics and healing course of diabetic foot ulcers by etiological classification: neuropathic, ischemic, and neuro-ischemic type. J Diabetes Complications 2014;28(4):528–35. doi:10.1016/j.jdiacomp.2014.03.013
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366(9498):1736–43. doi:10.1016/s0140-6736(05)67700-8
- Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg 2003;30(1):37–45. doi:10.1016/s0094-1298(02)00066-4
- IDF Diabetes Atlas. IDF diabetes atlas. 10th ed. International Diabetes Federation; 2021:141. Available from: www.diabetesatlas.org
- Sorber R, Abularrage CJ. Diabetic foot ulcers: epidemiology and the role of multidisciplinary care teams. Semin Vasc Surg 2021;34(1):47–53. doi:10.1053/j.semvascsurg.2021.02.006
- Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376(24):2367–2375. doi:10.1056/NEJMra1615439
- Reardon R, Simring S, Kim B, Mortensen J, Williams D, Leslie A. The diabetic foot ulcer. Aust J Gen Prac 2020;49(5). doi:10.31128/AJGP-11-19-5161
- Kang ZQ, Zhai XJ. The association between pre-existing diabetes mellitus and pressure ulcers in patients following surgery: a meta-analysis. Sci Rep 2015;5:13007. doi:10.1038/srep13007
- Liang M, Chen Q, Zhang Y, et al. Impact of diabetes on the risk of bedsore in patients undergoing surgery: an updated quantitative analysis of cohort studies. Oncotarget 2017;8(9):14516–14524. doi:10.18632/oncotarget.14312
- Liu P, He W, Chen HL. Diabetes mellitus as a risk factor for surgery-related pressure ulcers: a meta-analysis. J Wound Ostomy Continence Nurs 2012;39(5):495–9. doi:10.1097/WON.0b013e318265222a
- Finlayson K, Miaskowski C, Alexander K, et al. Distinct wound healing and quality-of-life outcomes in subgroups of patients with venous leg ulcers with different symptom cluster experiences. J Pain Symptom Manage. 2017;53(5):871–879. doi:10.1016/j.jpainsymman.2016.12.336
- Finlayson KJ, Parker CN, Miller C, et al. Predicting the likelihood of venous leg ulcer recurrence: the diagnostic accuracy of a newly developed risk assessment tool. Int Wound J 2018;15(5):686–694. doi:10.1111/iwj.12911
- Sharp A, Clark J. Diabetes and its effects on wound healing. Nurs Stand 2011;25(45):41–7. doi:10.7748/ns2011.07.25.45.41.c8626
- Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The treatment of impaired wound healing in diabetes: looking among old drugs. Pharmaceuticals (Basel) 2020;13(4).doi:10.3390/ph13040060
- Nowak NC, Menichella DM, Miller R, Paller AS. Cutaneous innervation in impaired diabetic wound healing. Translat Res 2021;236:87–108. doi:10.1016/j.trsl.2021.05.003
- Mills SJ, Hofma BR, Cowin AJ. Pathophysiology of wound healing. In: Fitridge R, editor. Mechanisms of vascular disease: a textbook for vascular specialists. Springer International Publishing; 2020:541–561.
- Berlanga-Acosta J. Diabetic lower extremity wounds: the rationale for growth factors-based infiltration treatment. Int Wound J 2011;8(6):612–20. doi:10.1111/j.1742-481X.2011.00840.x
- Boulton AJM, Whitehouse RW. The diabetic foot. In: Feingold KR, Anawalt B, Blackman MR, et al, editors. MDText.com Inc; 2000.
- Shaikh-Kader A, Houreld NN, Rajendran NK, Abrahamse H. The link between advanced glycation end products and apoptosis in delayed wound healing. Cell Biochem Funct 2019;37(6):432–442. doi:10.1002/cbf.3424
- Barber GA, Weller CD, Gibson SJ. Effects and associations of nutrition in patients with venous leg ulcers: a systematic review. J Adv Nurs 2018;74(4):774–787. doi:10.1111/jan.13474
- Basiri R, Spicer MT, Munoz J, Arjmandi BH. Nutritional intervention improves the dietary intake of essential micronutrients in patients with diabetic foot ulcers. Curr Dev Nutr 2020;4:8.
- Green SM, Winterberg H, Franks PJ, Moffatt CJ, Eberhardie C, McLaren S. Nutritional intake in community patients with pressure ulcers. J Wound Care 1999;8(7):325–30. doi:10.12968/jowc.1999.8.7.25900
- Lauwers P, Dirinck E, Van Bouwel S, et al. Malnutrition and its relation with diabetic foot ulcer severity and outcome: a review. Acta Clin Belg 2020:1–7. doi:10.1080/17843286.2020.1800315
- McDaniel J, Kemmner K, Rusnak S. Nutritional profile of older adults with chronic venous leg ulcers: a pilot study. Geriatr Nurs 2015;36(5):381–386. doi:10.1016/j.gerinurse.2015.05.005
- Pena G, Kuang B, Cowled P, et al. Micronutrient status in diabetic patients with foot ulcers. Adv Wound Care (New Rochelle) 2020;9(1):9–15. doi:10.1089/wound.2019.0973
- Sajid N, Miyan Z, Zaidi SIH, Jaffri SSA, AbdeAli M. Protein requirement and its intake in subjects with diabetic foot ulcers at a tertiary care hospital. Pak J Med Sci 2018;34(4):886–890. doi:10.12669/pjms.344.15399
- Zhang SS, Tang ZY, Fang P, Qian HJ, Xu L, Ning G. Nutritional status deteriorates as the severity of diabetic foot ulcers increases and independently associates with prognosis. Exp Ther Med 2013;5(1):215–222. doi:10.3892/etm.2012.780
- Burkiewicz CJ, Guadagnin FA, Skare TL, do Nascimento MM, Servin SC, de Souza GD. Vitamin D and skin repair: a prospective, double-blind and placebo controlled study in the healing of leg ulcers. Rev Col Bras Cir 2012;39(5):401–7. doi:10.1590/s0100-69912012000500011
- Munoz N, Posthauer ME, Cereda E, Schols J, Haesler E. The role of nutrition for pressure injury prevention and healing: the 2019 international clinical practice guideline recommendations. Adv Skin Wound Care 2020;33(3):123–136. doi:10.1097/01.ASW.0000653144.90739.ad
- Quain AM, Khardori NM. Nutrition in wound care management: a comprehensive overview. Wounds 2015;27(12):327–35.
- Razzaghi R, Pourbagheri H, Momen-Heravi M, et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complications 2017;31(4):766–772. doi:10.1016/j.jdiacomp.2016.06.017
- Baburao Jain A, Anand Jain V. Vitamin E, its beneficial role in diabetes mellitus (DM) and its complications. J Clin Diagn Res 2012;6(10):1624–8. doi:10.7860/jcdr/2012/4791.2625
- Kurian SJ, Baral T, Unnikrishnan MK, et al. The association between micronutrient levels and diabetic foot ulcer: a systematic review with meta-analysis. Front Endocrinol (Lausanne) 2023;14:1152854. doi:10.3389/fendo.2023.1152854
- Banks MD, Ross LJ, Webster J, et al. Pressure ulcer healing with an intensive nutrition intervention in an acute setting: a pilot randomised controlled trial. J Wound Care 2016;25(7):384–92. doi:10.12968/jowc.2016.25.7.384
- Basiri R, Spicer MT, Levenson CW, Ormsbee MJ, Ledermann T, Arjmandi BH. Nutritional supplementation concurrent with nutrition education accelerates the wound healing process in patients with diabetic foot ulcers. Biomedicines. 2020;8(8).doi:10.3390/biomedicines8080263
- Mohammad NA, Khresheh RM. Evaluate the effect of education interventions in the prevention of diabetic foot ulcers through knowledge of the disease and self-care practices in Saudi Arabia. Open Access Maced J Med Sci 2018;6(11):2206–2213. doi:10.3889/oamjms.2018.439
- Roberts S, Desbrow B, Chaboyer W. Feasibility of a patient-centred nutrition intervention to improve oral intakes of patients at risk of pressure ulcer: a pilot randomised control trial. Scand J Caring Sci 2016;30(2):271–80. doi:10.1111/scs.12239
- Sung JA, Gurung S, Lam T, et al. A ‘speed-dating’ model of wound care? Rapid, high-volume assessment of patients with diabetes in a multidisciplinary foot wound clinic. Exp Clin Endocrinol Diabetes 2020. doi:10.1055/a-1151-4731
- Bechara N, Gunton JE, Flood V, Hng TM, McGloin C. Associations between nutrients and foot ulceration in diabetes: a systematic review. Nutrients 2021;13(8). doi:10.3390/nu13082576
- Cereda E, Neyens JCL, Caccialanza R, Rondanelli M, Schols J. Efficacy of a disease-specific nutritional support for pressure ulcer healing: a systematic review and meta-analysis. J Nutr Health Aging 2017;21(6):655–661. doi:10.1007/s12603-016-0822-y
- Cheshmeh S, Hojati N, Mohammadi A, et al. The use of oral and enteral tube-fed arginine supplementation in pressure injury care: a systematic review and meta-analysis. Nurs Open 2021;9(6):2552–2561. doi:10.1002/nop2.974
- Langer G, Fink A. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev 2014;(6):CD003216. doi:10.1002/14651858.CD003216.pub2
- Liu P, Shen WQ, Chen HL. Efficacy of arginine-enriched enteral formulas for the healing of pressure ulcers: a systematic review. J Wound Care 2017;26(6):319–323. doi:10.12968/jowc.2017.26.6.319
- Maier HM, Ilich JZ, Kim JS, Spicer MT. Nutrition supplementation for diabetic wound healing: a systematic review of current literature. Skinmed 2013;11(4):217–24.
- Moore ZE, Corcoran MA, Patton D. Nutritional interventions for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev 2020;7:CD011378. doi:10.1002/14651858.CD011378.pub2
- Song YP, Wang L, Yu HR, et al. Zinc therapy is a reasonable choice for patients with pressure injuries: a systematic review and meta-analysis. Nutr Clin Pract 2020;35(6):1001–1009. doi:10.1002/ncp.10485
- Wilkinson EA. Oral zinc for arterial and venous leg ulcers. Cochrane Database Syst Rev 2014;(9):CD001273. doi:10.1002/14651858.CD001273.pub3
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:10.1136/bmj.n71
- Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21(5):855–9. doi:10.2337/diacare.21.5.855
- Jalilian M, Shiri S. The reliability of the Wagner Scale for evaluation the diabetic wounds: a literature review. Diabetes Metab Syndr 2022;16(1):102369. doi:10.1016/j.dsx.2021.102369
- Mills JL, Sr., Conte MS, Armstrong DG, et al. The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59(1):220–34.e1–2. doi:10.1016/j.jvs.2013.08.003
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. doi:10.1136/bmj.i4919
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. doi:10.1136/bmj.l4898
- The GRADE Working Group. GRADE; 2023 [cited 2023, March 13]. Available from: https://www.gradeworkinggroup.org/



