Ahead of Print
An integrative review of pulsed electromagnetic field therapy (PEMF) and wound healing
John Helmy, Manuel Valdebran
Keywords wound healing, pulsed electromagnetic field therapy, PEMF, skin lesions
For referencing Helmy J & Valdebran M. An integrative review of pulsed electromagnetic field therapy (PEMF) and wound healing. Wound Practice and Research 2023; 31(3):to be assigned.
DOI
https://doi.org/10.33235/wpr.31.3.to be assigned
Submitted 22 June 2023
Accepted 28 August 2023
Abstract
This literature review assesses the most recent data to summarise the emerging potential uses, benefits and risks of pulsed electromagnetic field therapy (PEMF) in wound healing. Electromagnetic fields have mainly been used as an adjunct therapy for osteoarthritis and other diseases involving joints. However, PEMF has also been shown to influence various signalling molecules involved in wound healing, including MMP‑2, IL‑6 and TGF‑β. Therefore, studies have begun to explore the use of PEMF in other diseases, such as incision wound repair, diabetes-related foot ulcers (DFU) and pressure ulcers. However, the cellular response to PEMF is highly variable and likely influenced by multiple factors – frequency, duration, tissue type, stage of wound repair and field strength. This high degree of variability may explain why PEMF seems to promote cell proliferation under certain conditions and inhibit cell growth with different parameters. This review highlights the need for further research to determine precisely how different variables influence PEMF therapy. Before PEMF can be implemented widely in clinical practice, this review provides a starting point for further controlled trials. This review might also provide a solid base to propose standardised experimental guidelines to investigate PEMF efficacy in wound healing, ulcer treatment and type 2 diabetes.
Introduction
For decades, pulsed electromagnetic field therapy (PEMF) has been used as a non-invasive alternative therapy for bone, musculoskeletal and joint diseases. Currently, PEMF is mainly used as an adjunct therapy to alleviate pain symptoms; however, PEMF has been shown to increase cartilage proliferation, synthesis and differentiation while also increasing the production of growth factors1,2. As a result, PEMF is often considered as an option for patients who are unable to tolerate medications or surgery such as the elderly or immunosuppressed3.
Due to its effect on proliferation and growth factors, PEMF has a wide variety of clinical applications such as pain relief for osteoarthritis and lower back injuries1. As the knowledge and usage of PEMF grow, its potential benefits are being explored for depression, nervous reparation, diabetes, ischaemia, metabolic disorders, benign prostatic hyperplasia, dysmenorrhoea, organ stimulation and wound healing1,4. Although the evidence for such treatments is lacking, dermatologic diseases have been treated previously through interventions that impact growth factors and cell differentiation5. Therefore, PEMF has the potential to be an effective non-invasive treatment option in this field. The aim of this literature review is to highlight available evidence for PEMF efficacy in the treatment of dermatologic conditions, specifically in wound healing. In doing so, the potential modalities for PEMF may be expanded beyond the treatment of bone and joint diseases.
Wound healing is not a linear process. It involves multiple concurrent pathways working together to achieve successful wound repair. Not only do multiple signalling pathways occur simultaneously, the pathways and functional goal change as time passes6. Stages of the healing process are dynamic, often overlap and differ depending on the type of wound. Homeostasis, collagen synthesis, proliferation, inflammation, new tissue formation and tissue remodelling are a few examples of the various pathways that exhibit mutual influence on each other to tightly regulate the wound repair process7. Therefore, in order to accurately determine the effect of PEMF on wound healing, multiple studies are required to measure how each variable is affected and how these variables interact with each other at different phases of the healing process.
Background and technology
A basic understanding of PEMF and its components is critical to appreciate the potential effectiveness of PEMF as a medical therapy. The mechanistic basis of PEMF is to manipulate magnetic energy in order to influence cellular processes. PEMF involves exposing the body to a changing magnetic field, which in turn induces an electric field that produces a current at the site of interest8. PEMF applies low-frequency magnetic fields with specific amplitudes, waveforms and frequencies that range between 6–500Hz9. Electromagnetic fields can be applied non-invasively to specific areas of the body or through total body stimulation by using single or paired Helmholtz coils2. Also, the use of magnetic fields allows for deep tissue penetration8.
The Helmholtz coils are connected to a generator of continuous electrical current. The current passing through the coils generates an electromagnetic field which can be applied to areas of interest2. The electric field is measured in volts (V) or millivolts (mV) and the magnetic field in Gauss (G) or Tesla (T) where 1G=104T2. Exposure to magnetic fields increases the movement of ions within cells, causing hyperpolarisation and higher levels of aerobic metabolic cellular processes8. These cellular changes are thought to be accomplished by increasing the synthesis of tissue-specific extracellular matrix proteins and specific signalling molecules involved with wound healing3. These cellular changes allow PEMF to influence rates of cellular proliferation and apoptosis. As a result, PEMF is being investigated to aid in tissue proliferation and differentiation. Furthermore, the type of magnetic field generator, as well as its frequency (Hertz, (Hz)), amplitude, duration and duty cycle (the interval between trains of pulses), can be manipulated based on the target tissue and stage of disease8. Aligning all these variables with particular therapeutic purposes is vital for PEMF to be applied to a clinical setting.
Although no side effects have been noted due to the use of magnetic field therapies, electromagnetic fields have been classified as a potential carcinogenic factor with long-term exposure8. Furthermore, magnetic energy has been shown to be important for normal human function. Studies have shown that humans who lack exposure to a magnetic field for extended periods of time, such as astronauts in space, present with insomnia, fatigue and an increased risk of osteoporosis8. Therefore, the potential risks and benefits of PEMF must be carefully assessed when considering its use in clinical settings.
Methods
We utilised PubMed to review the current literature. Search terms and query criteria are listed in Table 1. We limited our search to English language studies from the past 10 years – January 2013 through January 2023 – and utilised focused PubMed keyword search criteria including “PEMF therapy” “pulsed electromagnetic therapy” and “wound healing”. Including Boolean text query strategies “AND” and “OR” with these PubMed searches yielded additional sources. We expanded our search by using the following PubMed keyword search criteria: “pulsed electromagnetic therapy” AND “wound healing” OR “PEMF” OR “skin” OR “ulcers”.
Table 1. Literature search criteria
Furthermore, a citation search yielded additional sources. We found 347 publications, of which 45 were included in this systematic review. Factors used to determine the quality of manuscripts used in this review include the type of study, study design, and the relative strengths of measured outcomes. Studies that were found to not be relevant to the research question after an in-depth review were excluded. During the screening process, studies that did not acknowledge and attempt to address potential biases and confounding variables were also excluded. With regards to study design, observational studies were excluded from this review due to the inability to correct for confounding variables. Also, studies using mouse models or human cells in vitro were only included if they followed an experimental design with clear control and experimental groups and a stated null hypothesis. Finally, the strength of measured outcomes was determined based on statistical significance, sample size and study design. Because the use of PEMF in wound healing is a fairly new area of research, the majority of studies in this review were performed using mouse models or in vitro human cells. The few available studies with human participants were mostly pilot studies with low sample size and power. Only the results of well-controlled studies that attempt to account for bias were included in this review. The strategies used to search through the existing literature are summarised in Figure 1.
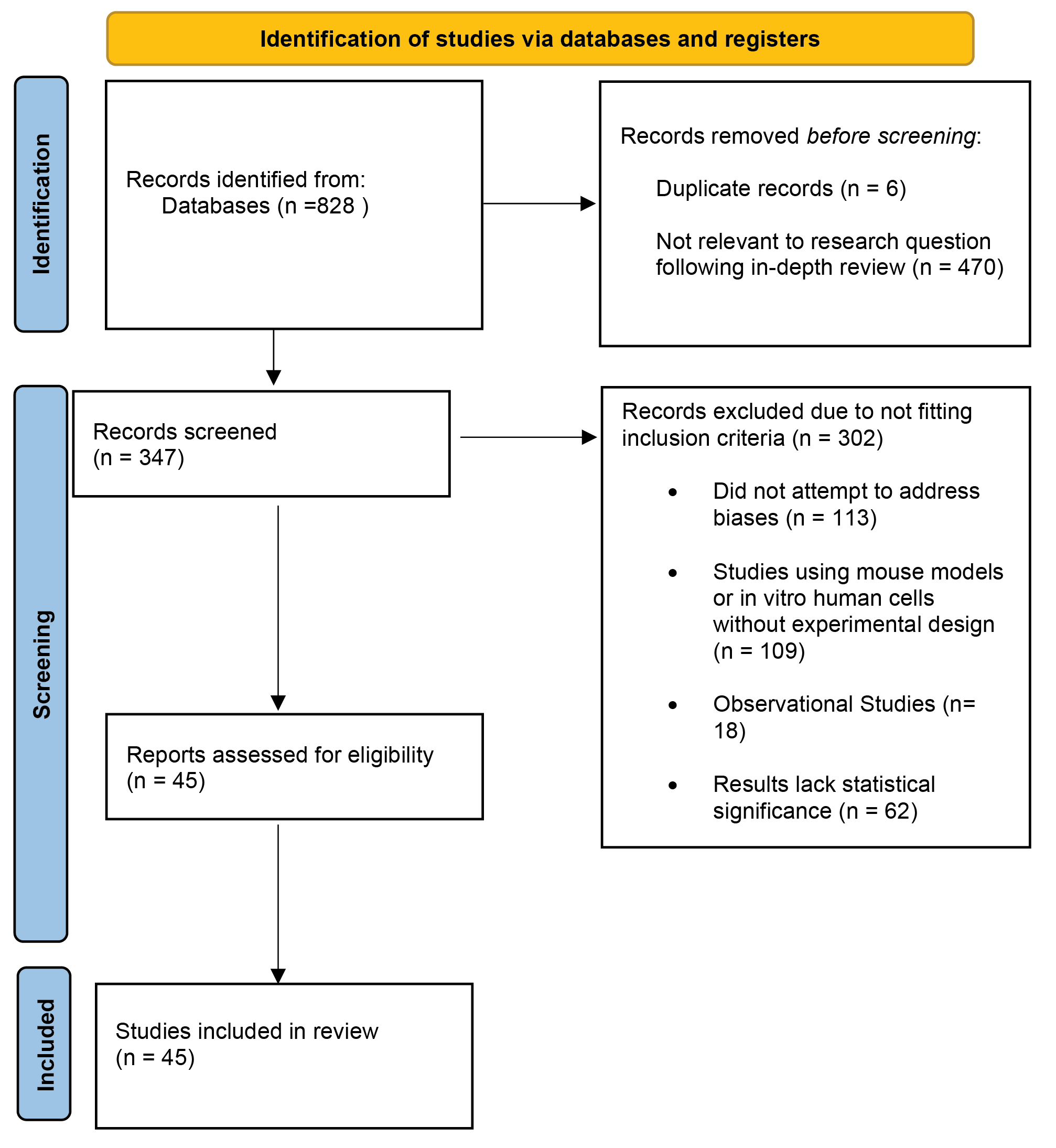
Figure 1. Search strategy flow chart
Results
Emerging dermatologic uses for wound healing
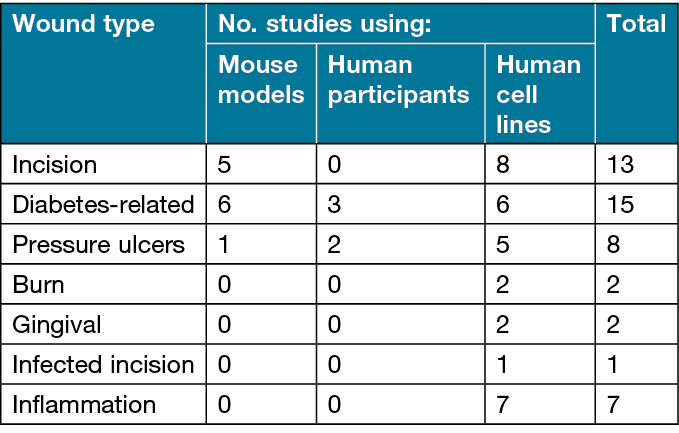
Table 2 shows how the studies were categorised following a review of the existing literature. From the publications included in this review, PEMF on incision wounds, diabetes-related wounds and pressure ulcers were the most well-studied. Burn, gingival and infected incision wounds had very little existing research regarding the use of PEMF. Table 2 shows that the amount of evidence supporting PEMF therapy for each wound type varies widely. However, even in cases more heavily researched, such as incision wounds and pressure ulcers, additional investigations are needed to determine key PEMF variables for manipulation based on particular pathologic features.
Table 2. Categorisation of included reports based on wound type and study design
Incision wound healing
Due to the relatively recent focus on using electromagnetic fields in medical practice, most studies investigating the potential benefits of PEMF have been conducted in vitro or on rats. Data on human participants is limited; therefore, the safety and risks associated with these newer methodologies are still being determined. Yet the current data suggests a significant benefit from using PEMF for the treatment of incision wounds. Specifically, studies suggest that PEMF accelerates early stages of wound closure4,6,10,11, increases tensile strength12,13, promotes tissue remodelling and collagen synthesis6,14, promotes epithelialisation and myofibroblast migration12,13, and enhances cytokines involved in anti-inflammatory responses to promote wound healing11,15–18.
Multiple studies have explored the effects of PEMF by treating rats with incision wounds. Based on the most recent data, PEMF seems to have different effects depending on the specific stage of the healing process in which PEMF is administered15. PEMF delivered at higher field strengths (10mT) and for shorter durations seems to improve processes involved in the early stages of wound closure (less than 14 days post-wounding), such as energy absorption capacity and maximum load15. However, at later stages of wound healing (more than 14 days post-wounding), PEMF seems to have the opposite effect and may inhibit tissue repair15. One study found that PEMF increased collagen deposition and promoted proliferation but did not affect the quality or alignment of the fibres15.
Furthermore, the beneficial effects of PEMF on proliferation and collagen deposition were seen exclusively during the early stages of the wound healing processes, when the site of injury prioritises the recruitment of structural properties15. Further studies suggest that PEMF can also increase de novo collagen synthesis, in addition to the above-mentioned effects on proliferation and deposition19. An increase in epithelialisation and decrease in contraction during the early stages of wound repair has also been observed upon treatment of incision wounds with PEMF12. If applied during the early stages of the wound repair process, one study found that PEMF can result in up to a 60% increase in tensile strength over control groups6. On the other hand, during the late phase of wound healing, there was a significant decrease in maximum stress and Young’s modules, reflecting a negative effect on wound healing15. Also, these findings suggest that, in the case of treating incision wounds, PEMF may increase the tensile biomechanical strength of the wound during early stages only, and should be applied for no more than 14 days to avoid the risk of inhibiting the healing process during later stages of the wound response15.
Diabetes-related wound healing
Diabetes mellitus is a chronic disorder with multiple complications. Due to a slower metabolism, diabetes mellitus reduces the effectiveness and speed of the body’s natural wound repair process4. Patients with diabetes have reduced vascular growth, wound tensile strength and proliferation compared to patients without diabetes4. Therefore, there has been great interest in enhancing the healing process in patients with diabetes through medical treatment. PEMF has been shown to have similar effects to those observed in other types of wounds when used to treat diabetes-related wounds.
PEMF treatment in patients without diabetes has resulted in improved vascular growth, enhanced blood circulation, greater myofibroblast proliferation, higher tensile strength and increased collagen deposition15. Overall, there were statistically significant reductions (p<0.01) in recovery and repair times for both diabetes-related incision wounds and diabetes-related foot ulcers (DFU) when treated with PEMF11,20. The slower repair time of diabetes-related wounds may be due to a reduced rate of collagen deposition and decreased recovery of tensile strength during early wound stages21. PEMF may be especially useful in the treatment of diabetes-related wounds due to its potential to increase tensile strength and re-epithelisation21. PEMF may be able to enhance the healing process in diabetes-related wounds to be similar to that of non-diabetes-related wounds22. When treated with PEMF, incision wound healing in patients with diabetes was found to have oxygen tensions and vascularity comparable to those of incision wounds in patients without diabetes22. Furthermore, both diabetes-related and non-diabetes-related incision wounds had similar levels of increased FGF‑2, promoting angiogenesis and preventing necrosis in response to ischaemic injury22,23.
By preventing necrosis, PEMF can potentially be used to reduce the incidence of ulcer formation and amputation in patients with diabetes. DFU are often difficult to treat effectively, leading to poor outcomes such as amputation or a severely prolonged wound repair process24. However, when treated with PEMF, DFU had improved microcirculation and decreased total wound repair times20,25,26. One case study on elderly patients with diabetes and persistent DFU showed significant improvement from PEMF treatment25. The participants were at risk for amputation due to the persistence of the ulcers despite treatment with multiple medications in an attempt to halt or slow ulcer progression; however, after daily PEMF treatment, all ulcers had healed25. However, despite the significant clinical indications for the use of PEMF in the treatment of DFU, the number of studies conducted on this topic is still small. More robust studies using different frequencies, field strengths and wound stages are required to determine potential harmful effects, as well as ideal treatment parameters24–26.
Similar to other wound types, the beneficial effects of PEMF seem to be observed only during the early stages of wound repair in diabetes-related wounds. Increased collagen fibre deposition21,27, myofibroblast populations28,29, tensile strength and rate of wound closure30 in patients with diabetes were measured during and following PEMF treatment for comparison to control groups with diabetes. These positive therapeutic effects were only observed at 10 days or less post-wounding21. No significant differences were observed during the later stages of the wound healing process (greater than 10 days post-wounding)21. These results support both the use of PEMF in the treatment of diabetes-related incision wounds and also the idea that PEMF acts through multiple signalling pathways to increase the early proliferative wound healing processes. The results also found that PEMF has little effect on later wound healing processes focused on alignment and remodelling.
Pressure ulcer treatment
Another cutaneous injury that can benefit from treatment with PEMF is pressure ulcers. A double-blind, placebo-controlled clinical trial followed 24 patients over the course of 12 weeks31. This trial found that 50% of pressure ulcers treated with PEMF healed completely or showed significant improvement (classified as a lower stage pressure ulcer), while 0% of the ulcers in the placebo group showed any sign of improvement31. Additionally, 54% of the ulcers in the placebo group worsened (classified as a higher stage pressure ulcer), whereas 0% of the ulcers in the PEMF treatment group worsened31. The clinical trial also found that PEMF treatment was associated with decreased wound depth, reduced pain intensity, and no reported adverse events31.
Another randomised double-blind study underscores the importance of fine-tuning specific parameters to better understand the effects of PEMF32. The study found that PEMF may be more effective at treating less severe pressure ulcers, specifically ulcers of stage II and below32. PEMF had the most prominent effect on reducing the median time to complete resolution in earlier stage II pressure ulcers compared to later stage III ulcers32. Furthermore, this study found that PEMF reduced the symptoms of pressure ulcers through three mechanisms: modification of cytokine profile to promote the transition from a chronic inflammatory state to an anti-inflammatory state; promotion of angiogenesis by increasing epithelial cell proliferation and circulating levels of FGF‑2; and the upregulation of collagen synthesis32.
One study looking at PEMF treatment for pressure ulcers found no significant difference between the PEMF treatment group and the control group33. Of note, there were two participants in the PEMF group whose ulcers fully resolved, but that number was not sufficient to be statistically significant33. Furthermore, the study utilised a low frequency of 1Hz, a short duration of exposure, and included only patients with severe (stage III and IV) ulcers33. Almost all studies that have measured a beneficial effect from PEMF treatment on cutaneous wounds have indicated that PEMF primarily benefits early stage wounds3–6,14,15,19,21,22. In fact, one of the studies discussed above that investigated the effects of PEMF on pressure ulcers found that PEMF was effective at treating stage II and below ulcers32. Therefore, the fact that this study only included participants with stage III and above ulcers may have contributed to the lack of significant results. Also, the study may not have used a high enough frequency or long enough duration for PEMF treatment to obtain significant results. Nevertheless, this study highlights the need for a better understanding of how PEMF parameters influence the biological response to treatment.
As previously noted, further studies are needed to accurately determine the ideal parameters of PEMF for different cutaneous wounds. PEMF treatment seems to affect pressure ulcers through multiple interconnecting mechanisms, including cytokine modification, a decrease in total wound resolution time, reduced wound depth, lower pain intensity, and increased cell proliferation32,33. As more studies are conducted to better understand the safety risks and ideal parameters associated with PEMF treatment, PEMF may become a widely used clinical tool for the treatment of pressure ulcers.
PEMF use for other cutaneous wounds
Multiple types of cutaneous wounds have been studied as targets for PEMF treatment, including burn, gingival and infected incision wounds34–37. Although the literature on these wounds is sparse compared to ulcer, diabetes-related or incision wounds, the results are promising. A study conducted on the effectiveness of PEMF treatment on Staphylococcus aureus infection wounds found that PEMF inhibited the growth rate of S. aureus34. All groups treated with PEMF had lower measured colony forming unit (CFU) levels when compared to control groups, suggesting a beneficial effect of PEMF treatment on bacterially infected incision wounds34.
Another study looked at the effects of PEMF on the burn wounds of 47 participants. Burn wounds are difficult to treat because they are a result of coagulation necrosis from severe tissue damage. Although many products are available to assist in burn wound resolution, a lack of solid area for grafting or the poor general condition of patients often results in a poor prognosis. Furthermore, the high cost of burn products acts as an additional barrier, preventing their widespread usage35. This study aimed to determine how PEMF at different doses and durations would influence the healing process of cutaneous burn wounds. Groups were either treated with nothing, saline or PEMF for two lengths of time (7 or 14 days) at 1.5mT and 40Hz35. Groups who were treated with PEMF had statistically significant increased levels of vascularisation compared to control groups. Also, in the group that was treated with PEMF for longer (14 days rather than 7 days), 75% of burn wounds exhibited increased epithelialisation35.
Studies have also attempted to use PEMF to treat gingival wounds. When gingival wounds were exposed to PEMF, one study measured an increased expression of various signalling molecules involved in proliferation including IL‑6, TGF‑β and iNOS36,37. The same study also found increased levels of MMP‑2, MCP‑1 and HO‑1 expression, all of which are thought to increase wound repair rate36,37. The study argues that PEMF resulted in increased proliferation, migration and metabolism of fibroblasts in injured gingival tissue compared to control groups36,37.
All the studies discussed above conducted trials on rats only, not humans. Also, for each of the cutaneous wounds discussed in this section, there have not been enough studies on PEMF treatment to conclude with certainty that the results are reproducible. Therefore, further studies are critical to determine reproducibility, accuracy, dose parameters and safety in humans. On the other hand, it is also important to note the similarity of the effects of PEMF on lesser studied wounds to those on more extensively studied wounds. In either case, statistically significant increases were observed in multiple signalling molecules36,37, epithelialisation36 and cell proliferation34,35. The similarity of findings supports the notion that PEMF does indeed have a beneficial effect on the core mechanisms of wound healing. With further research, PEMF may become a more affordable, non-invasive treatment option for a wide variety of cutaneous wounds.
Inflammation
PEMF has also been shown to reduce inflammation in chronic wounds through both intracellular and extracellular effects. Extracellularly, PEMF treatment may cause a reduction in the number and activity of inflammatory cytokines at the target tissue site10. Multiple studies have measured reductions in inflammatory cytokines (IL‑1β, IL‑6, TNF‑α) following PEMF treatment9,14,38,39. Intracellularly, PEMF treatment has been found to influence multiple cell signalling pathways. The inhibition of MMP‑9 expression via the Akt/ERK pathway may be one method by which PEMF exhibits its anti-inflammatory effects17. PEMF may also reduce inflammation through the upregulation of inflammatory mediators like nitrogen oxide synthase, while simultaneously downregulating Cox‑2 and PGE2 expression18. Additionally, treatment with PEMF may affect endogenous cellular mechanisms such as resting membrane potential and voltage-gated calcium channels to reduce inflammatory cell signals like NF-κB40. The anti-inflammatory effects of PEMF may lead to a downstream downregulation of inflammatory cells including mesenchymal stem cells and macrophages38,39.
Clearly, PEMF’s influence on the wound repair process is highly dynamic, affecting multiple congruent pathways, with differing effects depending on the stage and morphology of the wound. A logical proposed progression of action is that PEMF functions by first stopping the inflammatory processes, then enhancing the restorative cellular pathways to improve and accelerate the body’s natural wound healing ability10. Further research is still needed in this area to determine if the beneficial effects are reproducible in humans, as well as to fine-tune the ideal intensity and duration of PEMF for the treatment of inflammatory wounds.
Late stage wounds and variability of PEMF effects
Much of the literature under review focuses on the usage of PEMF as an enhancer of cell proliferation and organisation to treat various early stage cutaneous wounds. However, when PEMF is used on late stage cutaneous wounds, multiple studies have noted an inhibitory effect on wound healing15,21. This difference in impact is likely due to a transition from a focus on tissue proliferation to tissue remodelling during the healing process15.
One study attempted to treat diabetes-related amputee stump wounds with PEMF but failed to measure any significant differences in the wound healing processes41–43. The fact that PEMF seems to primarily benefit early stage wounds offers a possible explanation for the lack of improvement in this study. Amputation usually occurs much later in the wound treatment process after multiple attempts to resolve the issue have failed43. Although wounds are usually cut beyond the old wound tissue during the amputation process, the tissue area has most likely been exposed to increased levels of inflammatory mediators such as Il‑6 and TNF‑β due to its proximity to the original wound site43. Therefore, the remaining tissue at the amputation site has most likely been involved in wound healing/repair for a significant amount of time prior to amputation43. The lack of beneficial results from the treatment of diabetes-related stump wounds with PEMF may be due to the fact that amputation is usually performed later in the treatment plan of severe wounds, resulting in a late stage healing process upon initiation of PEMF treatment.
This inhibitory effect on remodelling has been studied as a treatment for dermatologic cancers44. Electrochemotherapy mediated by PEMF was found to have a 2-fold increase in drug uptake compared to traditional electrochemotherapy in rat melanoma models45. Furthermore, electrochemotherapy with PEMF was found to have comparable tumour growth delays to traditional chemotherapy45. The main advantage of PEMF-mediated electrochemotherapy over traditional chemotherapy is its painless and contactless application45.
The frequency, duration and target tissue type seem to influence the effect of PEMF. Depending on the parameters used, PEMF is capable of both inhibition and stimulation of tissue proliferation. For example, tissue exposed to PEMF at 50Hz, 1mT for 1 hour had increased keratinocyte proliferation compared to control groups, while the same tissue exposed to PEMF at 60Hz, 1.5mT for 144 hours had reduced cell proliferation33. Furthermore, different signalling pathways are stimulated based on the PEMF parameters, which may contribute to varying therapeutic effects. Excitatory effects were associated with increased activation of Akt/PI3k and ERK; however, inhibitory effects resulted from an increase in ATM-Chk2-p21 signalling44. At higher frequencies (6–7mT), an increase in DNA double-strand breaks, apoptosis and levels of reactive oxygen species (ROS) were measured, contributing to the inhibition of cell proliferation. Yet tissues exposed to lower frequencies of PEMF (1mT) had decreased ROS levels44. Higher amplitudes of PEMF promoted tissue regeneration and increased pro-inflammatory cytokines such as IL‑6, IL‑10 and TGF‑β42,43. However, lower PEMF amplitudes generated opposite effects and inhibited the same inflammatory mediators39. These results highlight the high degree of variability of the effects of PEMF on target tissues. While the study above proposed a frequency-dependent explanation for the variability in PEMF effects44, it is more likely a combination of overlapping influences from multiple factors such as frequency, duration, tissue type and field strength.
Current limitations for clinical application of PEMF
A major limitation of using PEMF therapeutically is its variable effects on molecular and biological mechanisms. This limitation is likely due to a lack of understanding of how PEMF parameters influence its effects. Definitive guidelines or conclusions can’t be drawn without further research on how frequency, amplitude, duration, tissue type and field strength influence the biological response to PEMF. Although further research is needed to determine how various parameter combinations influence the cellular response to PEMF, PEMF seems to have the potential to serve as a non-invasive treatment for skin wounds, ulcers and even types of skin cancer. Thus, further research on ideal PEMF parameters for precise therapeutic uses is warranted.
Conclusion
In conclusion, PEMF is a relatively novel medical technology that has many exciting potential uses in the field of dermatology. However, due to the novelty of this technology, further research is critical. PEMF has been shown to have variable effects, promoting cell growth or cell death depending on the circumstances. This variability allows PEMF to have a wide variety of potential uses including wound repair, type 2 diabetes treatment and skin cancer treatment. However, in order to safely administer PEMF and achieve the appropriate response, the influence of multiple overlapping variables on the effects of PEMF must first be carefully determined. Additionally, the majority of studies aimed at exploring new uses for PEMF have been conducted on rats. For new uses of PEMF to integrate into clinical practice, the safety and reproducibility of measured results must be determined in human participants as well. This review provides a solid base to develop standardised experimental guidelines to investigate PEMF efficacy in wound healing, diabetes and ulcer treatment in future controlled trials.
Author contribution
Writing – original draft preparation, review and editing JH; visualisation JH; supervision and draft approval MV. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
The authors received no funding for this study.
Author(s)
John Helmy1*, Manuel Valdebran2,3
1College of Medicine, Medical University of South Carolina, Charleston, SC 29425, USA
2Department of Dermatology and Dermatological Surgery, Medical University of South Carolina, Charleston, SC 29425, USA
3Department of Pediatrics, Medical University of South Carolina, Charleston, SC 29425, USA
*Corresponding author email jeh308@musc.edu
References
- Anbarasan S, Baraneedharan U, Paul SF, Kaur H, Rangaswami S, Bhaskar E. Low dose short duration pulsed electromagnetic field effects on cultured human chondrocytes: an experimental study. Ind J Orthopaed 2016;50(1):87–93.
- Di Bartolomeo M, Cavani F, Pellacani A, Grande A, Salvatori R, Chiarini L, Nocini R, Anesi A. Pulsed electro-magnetic field (PEMF) effect on bone healing in animal models: a review of its efficacy related to different type of damage. Biology (Basel) 2022;11(3).
- Roza S, Syful AF, Izzuna MG. Pulsed electromagnetic field therapy – an update. Technology Review. Ministry of Health Malaysia: Malaysian Health Technology Assessment Section 2020;38.
- Patiño O, Grana D, Bolgiani A, Prezzavento G, Miño J, Merlo A, Benaim F. Pulsed electromagnetic fields in experimental cutaneous wound healing in rats. J Burn Care Rehab 1996;17(6).
- Liani R, La Torre S, Liani V, Melchiorre A, D’Ettorre D, Tripaldi R, Lattanzio S, Di Luzio R, Coli M, Velussi C. Study of pulsed electrostatic field (PESF) in the perfusion of peripheral tissues: microangiopathy, nutrition and quality of perceiver life. PLoS One 2022;15:17.
- Strauch B, Patel MK, Navarro JA, Berdichevsky M, Yu H-L, Pilla AA. Pulsed magnetic fields accelerate cutaneous wound healing in rats. Plast Reconstr Surg 2007;120(2).
- Gualdi G, Costantini E, Reale M, Amerio P. Wound repair and extremely low frequency-electromagnetic field: insight from in vitro study and potential clinical application. Int J Mol Sci 2021;22.
- Lv H, Liu J, Zhen C, Wang Y, Wei Y, Ren W, Shang P. Magnetic fields as a potential therapy for diabetic wounds based on animal experiments and clinical trials. Cell Prolif 2021;54(3).
- Hong E, Lee G, Hwang S, Kim J, Jo M, Kang H, Yoo H, Kim S, Lee Y, Rhee J. Pulsed electromagnetic field (PEMF) treatment ameliorates murine model of collagen-induced arthritis. Int J Mol Sci 2023;24(2).
- Gordon GA. Designed electromagnetic pulsed therapy: clinical applications. J Cell Physiol 2007;212.
- Kwan RL-C, Lu S, Choi HM-C, Kloth LC, Cheing GL-Y. Efficacy of biophysical energies on healing of diabetic skin wounds in cell studies and animal experimental models: a systematic review. Int J Mol Sci 2019;20.
- Milgram J, Shahar R, Levin-Harrus T, Kass P. The effect of short, high intensity magnetic field pulses on the healing of skin wounds in rats. Bioelectromagnetics 2004;25.
- Athanasiou A, Karkambounas S, Batistatou A, Lykoudis E, Katsaraki A, Kartsiouni T, Papalois A, Evangelou A. The effect of pulsed electromagnetic fields on secondary skin wound healing: an experimental study. Bioelectromagnetics 2007;28(5).
- Gómez-Ochoa I, Gómez-Ochoa P, Gómez-Casal F. Pulsed electromagnetic fields decrease proinflammatory cytokine secretion (IL‑1β and TNF-α) on human fibroblast-like cell culture. Rheumatol Int 2011;31.
- Choi HC, Cheung AK, Ng GF, Cheing GY. Effects of pulsed electromagnetic field (PEMF) on the tensile biomechanical properties of diabetic wounds at different phases of healing. PLoS One 2018;13(11).
- Pesce M, Patruno A, Speranza L, Reale M. Extremely low frequency electromagnetic field and wound healing: implication of cytokines as biological mediators. Eur Cytokine Network 2013;24(1).
- Patruno A, Ferrone A, Costantini E, Franceschelli S, Pesce M, Speranza L, Amerio P, D’Angelo C, Felaco M, Grilli A, Reale M. Extremely low-frequency electromagnetic fields accelerates wound healing modulating MMP‑9 and inflammatory cytokines. Cell Prolif 2018;51(2).
- Patruno A, Amerio P, Pesce M, Vianale G, Di Luzio S, Tulli A, Franceschelli S, Grilli A, Muraro R, Reale M. Extremely low frequency electromagnetic fields modulate expression of inducible nitric oxide synthase, endothelial nitric oxide synthase and cyclooxygenase‑2 in the human keratinocyte cell line HaCat: potential therapeutic effects in wound healing. Br J Dermatol 2010;162.
- Ahmadian S, Zarchi SR, Bolouri B. Effects of extremely-low-frequency pulsed electromagnetic fields on collagen synthesis in rat skin. Biotech App Biochem 2006;43.
- Kwan RL-C, Cheing GL-Y, Vong SK-S, Lo SK. Electrophysical therapy for managing diabetic foot ulcers: a systematic review. Int Wound J 2013;10.
- Cheing GL-Y, Li X, Huang L, Kwan RL-C, Cheung K-K. Pulsed electromagnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats. Bioelectromagnetics 2014, 35.
- Callaghan MJ, Chang EI, Seiser N, Aarabi S, Ghali S, Kinnucan ER, Simon BJ, Gurtner GC. Pulsed electromagnetic fields accelerate normal and diabetic wound healing by increasing endogenous FGF‑2 release. Plastic Reconstr Surg 2008;121(1).
- Goto T, Fujioka M, Ishida M, Kuribayashi M, Ueshima K, Kubo T. Noninvasive up-regulation of angiopoietin‑2 and fibroblast growth factor‑2 in bone marrow by pulsed electromagnetic field. J Orthoped Sc 2010;15.
- Kwan RL, Wong WC, Yip SL, Chan KL, Zheng YP, Cheing GL. Pulsed electromagnetic field therapy promotes healing and microcirculation of chronic diabetic foot ulcers: a pilot study. Adv Skin Wound Care 2015;28(5).
- Guerriero F, Botarelli E, Mele G, Polo L, Zoncu D, Renati P, Sgarlata C, Rollone M, Ricevuti G, Maurizi N, Francis M, Rondanelli M, Perna S, Guido D, Mannu P. Effectiveness of an innovative pulsed electromagnetic fields stimulation in healing of untreatable skin ulcers in the frail elderly: two case reports. Case Rep Dermatol Med 2015.
- Stiller MJ, Grace H, Pak JL, Shupack S, Thaler CK, Lorrie J. A portable pulsed electromagnetic field (PEMF) device to enhance healing of recalcitrant venous ulcers: a double-blind, placebo-controlled clinical trial. Br J Dermatol 1992;127(2).
- Rosso F, Bonasia E, Marmotti A, Cottino U, Rossi R. Mechanical stimulation (pulsed electromagnetic fields “PEMF” and extracorporeal shock wave therapy “ESWT”) and tendon regeneration: a possible alternative. Front Aging Neurosci 2015,7:211.
- Liu M, Lee C, Laron D, Zhang N, Waldorff I, Ryaby T, Feeley B, Liu X. Role of pulsed electromagnetic fields (PEMF) on tenocytes and myoblasts-potential application for treating rotator cuff tears. J Orthoped Res 2017;35(5).
- Choi MC, Cheung KK, Li X. Pulsed electromagnetic field (PEMF) promotes collagen fibre deposition associated with increased myofibroblast population in the early healing phase of diabetic wound. Arch Dermatol 2016.
- Goudarzi I, Hajizadeh S, Salmani ME, Abrari K. Pulsed electromagnetic fields accelerate wound healing in the skin of diabetic rats. Bioelectromagnetics 2010;31(4).
- Costin GE, Birlea SA, Norris DA. Trends in wound repair: cellular and molecular basis of regenerative therapy using electromagnetic fields. Current Molec Med 2012;12(1).
- Salzberg CA, Cooper-Vastola SA, Perez F, Viehbeck MG, Byrne DW. The effects of non-thermal pulsed electromagnetic energy on wound healing of pressure ulcers in spinal cord-injured patients: a randomized, double-blind study. Ostomy Wound Manage 1995;41(3).
- Gupta A, Taly AB, Srivastava A, Kumar S, Thyloth M. Efficacy of pulsed electromagnetic field therapy in healing of pressure ulcers: a randomized control trial. Neurol India 2009;57.
- Ahmed I, Istivan T, Cosic I. Evaluation of the effects of extremely low frequency (ELF) pulsed electromagnetic fields (PEMF) on survival of the bacterium Staphylococcus aureus. EPJ Nonlin Biomed Phys 2013;1:5.
- Ibrahim T, Ahmet UT, Bora T, Işıl O, Aykut P. The healing effect of pulsed magnetic field on burn wounds. Burns 2022;48(3).
- Costantini E, Sinjari B, D’Angelo C, Murmura G, Reale M, Caputi S. Human gingival fibroblasts exposed to extremely low-frequency electromagnetic fields: in vitro model of wound-healing improvement. Int J Mol Sci 2019;20(9).
- Dogru AG, Tunik S, Akpolat V, Dogru M, Saribas EE, Kaya FA, Nergiz Y. The effects of pulsed and sinusoidal electromagnetic fields on E-cadherin and type IV collagen in gingiva: a histopathological and immunohistochemical study. Adv Clin Exper Med 2013;22(2).
- Ross L, Zhou Y, McCall E, Soker S, Criswell L. The use of pulsed electromagnetic field to modulate inflammation and improve tissue regeneration: a review. Bioelectricity 2019;1(4).
- Ross L, Ang C, Almeida-Porada G. Targeting mesenchymal stromal cells/pericytes (MSCs) with pulsed electromagnetic field (PEMF) has the potential to treat rheumatoid arthritis. Frontiers Immunol 2019;10.
- Funk H. Coupling of pulsed electromagnetic fields (PEMF) therapy to molecular grounds of the cell. Am J Translat Res 2018;10(5).
- Kwok T, Ting PT, Wong EK, Brassard A. Peripheral neuropathy for dermatologists: what if not diabetic neuropathy? J Cutan Med Surg 2013;1.
- Musaev AV, Guseinova SG, Imamverdieva SS. The use of pulsed electromagnetic fields with complex modulation in the treatment of patients with diabetic polyneuropathy. Neurosci Behav Physiol 2003;33.
- Isakov E, Ring H, Mendelevich I, Boduragin N, Susak Z, Kupfert Y, Marchetti N. Electromagnetic stimulation of stump wounds in diabetic amputees. J Rehab Sci 1996;9(2).
- Nezamtaheri MS, Goliaei B, Shariatpanahi SP, Ansari AM. Differential biological responses of adherent and non-adherent (cancer and non-cancerous) cells to variable extremely low frequency magnetic fields. Scientific Rep 2022;12(1).
- Kranjc S, Kranjc M, Scancar J, Jelenc J, Sersa G, Miklavcic D. Electrochemotherapy by pulsed electromagnetic field treatment (PEMF) in mouse melanoma B16F10 in vivo. Radiol Oncol 2016;50(1).


