Volume 24 Number 1
Treatment of early localised infection of the Omniflow® II prosthesis with negative pressure wound therapy
Miroslav Krejčí, Robert Staffa and Pavel Gladiš
Introduction
A biosynthetic vascular prosthesis is a special type of vascular graft. Implantation of this type of prosthesis can be considered for patients with limb ischaemia, diabetes mellitus, insufficient saphenous vein, and trophic ulcers.
We present a rare case of successful healing of an early Szilagyi grade III vascular prosthetic infection in the groin using a topical negative pressure device and moist wound healing materials.
Case report
We present a polymorbid hypersthenic female, 68 years old, body mass index (BMI) 25.7. Her personal history included type 2 diabetes mellitus, ischaemic heart disease, hypertension, chronic renal failure, hyperlipidaemia, atrial fibrillation, anaemia, varicose veins and gonarthrosis. She had undergone right open lumbar sympathectomy and laparoscopic cholecystectomy.
The patient had chronic limb ischaemia, Fontain stage IV, or Rutherford grade 5. A left distal femoropopliteal prosthetic bypass had been implanted 16 months earlier. We used the straight aldehyde denatured bovine collagen prosthesis Omniflow® II (Bio Nova International Pty Ltd, Australia) due to the absence of a suitable autologous venous graft. The prosthesis developed an early silent thrombosis as a result of poor run-off, with no accompanying signs of trophic impairment or prosthetic infection.
Considering the trophic ulcers on the patient’s right foot (cultivation beta-lactamase-producing Klebsiella pneumoniae) we performed a distal femoropopliteal reconstruction using the same type of Omniflow® II biosynthetic prosthesis. Graft patency was confirmed intraoperatively by peripheral angiography. Antibiotic prophylaxis was administered and the postoperative course was uneventful.
Three weeks after the surgery, an abscess formation appeared in the right groin. The graft remained patent and good pulsation was detectable on the popliteal artery. We performed an incision and drainage of the right groin. A second incision was performed to drain a superficial abscess cavity in the middle of the thigh. During the revision surgery, an exposed proximal part of the prosthesis was found in the groin wound (Figure 1).
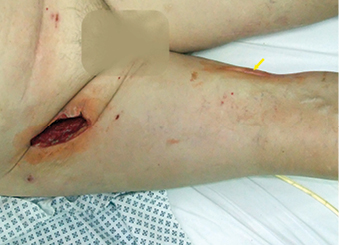
Figure 1: Incision in the right groin and on the thigh (arrow)
The cultivation from the groin revealed haemolytic Escherichia coli. The patient was treated with antibiotics (ciprofloxacin). An antiseptic dressing was applied to the wounds. The superficial wound on the thigh completely healed with the aid of moist wound healing (MWH) materials.
Twelve days after the drainage procedure, the Vivano® negative pressure wound therapy (NPWT) device (Hartmann, Germany) was applied to the right groin (Figure 2).
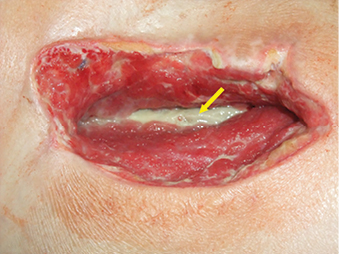
Figure 2: Right groin at the time of the application of negative pressure therapy system. Exposed vascular prosthesis Omniflow® II on the wound floor (arrow)
A layer of polyvinyl alcohol dressing: VAC® WhiteFoam (KCI®) was employed onto the prosthesis and covered with VivanoMed® black polyurethane foam. We applied a continuous suction regime at 100 mmHg (Figure 3). The therapy lasted 16 days, including four bedside dressing changes. Afterwards, the residual wound was treated with standard MWH materials: Actisorb® Plus and later Inadine® (Systagenix). The wound in the right groin completely healed (Figure 4).
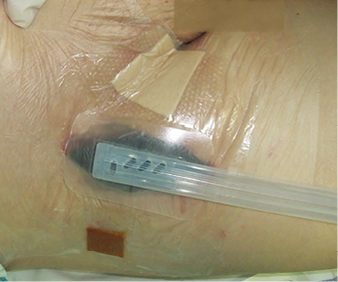
Figure 3: Negative pressure wound healing system in the right groin. Polyurethane foam in the wound. Medially, mild intertrigo healed with Mepilex® Border (Mölnlycke Health Care) and laterally a small skin excoriation covered with Inadine® (Systagenix)
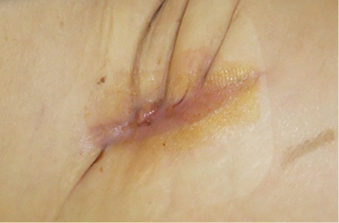
Figure 4: Subsequent moist wound healing therapy with Actisorb® Plus and later Inadine® (Systagenix). Completely healed wound in the right groin
When being treated in our outpatient ward, the patient demonstrated no signs of infection. To verify the result of the treatment, duplex scan and CT angiography were performed three months after the surgery. There was no abscess formation around the graft and no sign of floating prosthesis. Scar tissue was described near the proximal anastomosis of the patent bypass (Figure 5). The level of inflammatory markers was low (Leukocytes 6.0 10e9/L, CRP 14.0 mg/L). Pulsation was detectable on the right popliteal artery.
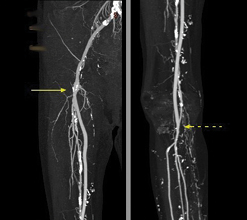
Figure 5: CT angiography, reconstruction. Anastomoses of the patent Omniflow® II prosthesis marked with arrows. Department of Imaging Methods, St Anne’s University Hospital, Brno
During the 18-month follow-up care, there were no systemic or local signs of prosthetic infection. The treatment of trophic ulcers on the patient’s heel continued and the wound closed gradually.
Discussion
The biosynthetic prosthesis Omniflow® II had been reported to have better long-term patency than other grafts in distal revascularisations, even in cases with poor run-off1-3. The prosthesis is recommended where an autologous vein is not available4.
The material of the biosynthetic prosthesis was proven to have a higher resistance to infection than other types of artificial grafts in earlier studies. In 1997, Koch et al. reported a 0% infection rate; in our 2012 study, the infection rate reached 2.8%2,3. The use of grafts has, therefore, been considered in cases of trophic ulcer and venous insufficiency, as well as to replace an infected vascular graft2,5,6. However, we would now suggest using an autologous femoral vein or an arterial or venous allograft7.
Neufang et al. used a biosynthetic prosthesis as the proximal part of a sequential distal composite bypass revascularisation in a case of critical limb ischaemia. They experienced one early infection and no late infections of the prosthesis. The management of the infection was not clarified by the authors8.
A recent animal study was performed to determine whether the expanded polytetrafluoroethylene (ePTFE) graft or Omniflow® II prosthesis was more resistant to infection caused by Staphylococcus aureus (as the most frequently cultivated bacteria in prosthetic infection). They recommend strict perioperative antibiotic administration and concluded that the Omniflow® II prosthesis was more susceptible to infection in the early postoperative period than ePTFE graft. These results should be included in the indication criteria for application of the prosthesis, though the authors recommended further studies9.
In this case report we described a polymorbid female who was at a high risk for disturbed wound healing due to obesity, diabetes and trophic limb ulcer10. Based on a physical examination followed by CT angiography, distal femoropopliteal bypass procedure was recommended. The patient was informed of the expected outcomes of the treatment and informed consent was obtained prior to surgery using standard department protocols. Due to varicose veins and trophic ulcer, we performed the surgery using the biosynthetic prosthesis Omniflow® II. The postoperative period was disturbed by an abscess formation in the groin and additionally on the thigh. Haemolytic Escherichia coli was found in the wounds and antibiotics were administered. On the thigh, only the subcutaneous tissue was exposed and the wound healed completely using the MWH materials.
In the groin, the infection involved the prosthetic graft (that is, Szilagyi grade III). There are more options in the treatment of a localised infection in the groin, including complete or partial graft replacement. The patient was stable, with no signs of sepsis, bleeding or acute limb ischaemia. Therefore, we decided to use NPWT, which has presented a valuable treatment modality in the therapy of localised vascular prosthetic infection11,12. The Vivano® system (Hartmann, Germany) was applied into the wound with bedside dressing changes. The residual wound was treated with standard MWH materials.
There were no clinical, laboratory, ultrasonographic or CT signs of infection during the follow-up care. To our knowledge, a successful treatment of recent infection of the biosynthetic prosthesis using NPWT has not yet been published in a peer-reviewed scientific journal.
Conclusion
Contemporary study data have mainly dealt with the uses of biosynthetic prostheses as grafts for primary or revision bypass surgery. In our case report, we focused on the treatment of a recent localised primary infection of the Omniflow® II prosthesis. The infection was managed successfully with the use of NPWT and MWH materials. However, lifelong follow-up care is necessary due to the risk of the recurrence of the graft infection.
Conflicts of interest
We declare no conflicts of interest with the subject matter or materials discussed in this manuscript.
Acknowledgements
This work was supported by a grant from the Czech Health Research Council, Ministry of Health of the Czech Republic, Education Influence on the Selected Psychosomatic Factors in Patients Indicated for the Vascular Prosthesis Implantation, No. 15-33437A. The patient was informed of the expected outcomes of the treatment and approval was obtained using standard protocols of the 2nd Department of Surgery, St Anne’s University Hospital, Brno, Czech Republic.
Author(s)
Miroslav Krejčí *
MD, PhD
2nd Department of Surgery, Center for Vascular Disease, St Anne’s University Hospital and Faculty of Medicine, Masaryk University
Pekařská 53, Brno 65691, Czech Republic, EU
Email: miroslav.krejci@fnusa.cz
Robert Staffa
MD, PhD
2nd Department of Surgery, Center for Vascular Disease, St Anne’s University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic, EU
Pavel Gladiš
MD, CSc
2nd Department of Surgery, Center for Vascular Disease, St Anne’s University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic, EU
* Corresponding author
References
- Guardascione G, Florio A, Sassi O et al. Femoropopliteal Vascular Reconstruction Below Knee when Greater Saphenous Vein is unavailable: ePTFE or Biosynthetic Prosthesis (Omniflow II). Presented at the 59th International Congress of European Society for Cardiovascular Surgery (ESCVS), Warsaw, Poland, 2009. Online: http://icvts.oxfordjournals.org/content/8/Supplement_1/S1.full.pdf+html. [cited 30 March, 2015].
- Vlachovsky R, Staffa R, Kriz Z. Our experience with biosynthetic graft implantation — prospective study of 35 patients. Presented at the 16th Slovak Congress of Vascular Surgery with International Participation, Jasna, Slovakia, 2012. [Abstract]. Online: http://cjmedical.com/cjmcms/ulimages/ MDd7CGzGysVlachovsky%20&%20Staffa%20Our%20experience%20with%20biosynthetic%20graft%20 implantation%20Abstract%202012.pdf. [cited 30 March, 2015].
- Koch G, Gutschi S, Pascher O et al. Analysis of 274 Omniflow Vascular Prostheses implanted over an eight-year period. Aust N Z J Surg 1997;67(9):637–9.
- Omniflow™ II. The Biosynthetic Vascular Prosthesis. For peripheral revascularisation and arteriovenous access. Online: http://www.bionova.com.au/wp-content/uploads/Omniflow-II-brochure-BN-9-60-1LK.pdf. [cited 30 March, 2015].
- Fellmer PT, Wiltberger G, Tautenhahn HM et al. Early Results after Peripheral Vascular Replacement with Biosynthetic Collagen Prosthesis in Cases of Graft Infection. [Article in German]. Zentralbl Chir 2014;139(5):546–551.
- Wiltberger G, Matia I, Schmelzle M et al. Mid- and long-term results after replacement of infected peripheral vascular prosthetic grafts with biosynthetic collagen prosthesis. J Cardiovasc Surg (Torino) 2014;55(5):693–698.
- Staffa R, Kriz Z, Vlachovsky R et al. Autogenous superficial femoral vein for replacement of an infected aorto-ilio-femoral prosthetic graft. [Article in Czech]. Rozhl Chir 2010;89(1):39–44.
- Neufang A, Dorweiler B, Espinola-Klein C et al. Outcomes of complex femorodistal sequential autologous vein and biologic prosthesis composite bypass grafts. J Vasc Surg 2014;60:1543–53.
- Bozoglan O, Mese B, Eroglu E et al. Which prosthesis is more resistant to vascular graft infection: polytetrafluoroethylene or Omniflow II biosynthetic grafts? Surgery Today [serial on the Internet]. 2015, Mar 6 [cited 30 March, 2015]. Available from: MEDLINE Complete.
- Vikkunen J, Heikkinen M, Lepäntalo M et al. Diabetes as an independent risk factor for early postoperative complications in critical limb ischemia. J Vasc Surg 2004;40:761–767
- Diener H, Larena-Avellaneda A, Debus ES. Postoperative Komplikationen in der Gefässchirurgie. Chirurg 2009;80(9):814–826.
- Domingos Hadamitzky C, Schulte S, Horsch S. Vacuum assisted wound closure in postoperative periprosthetic groin infections: a new gold standard? J Cardiovasc Surg 2007;48:477–483.



