Volume 24 Number 3
Combining pressure injury and incontinence-associated dermatitis prevalence surveys: an effective protocol?
Jill L Campbell, Sandra Gosley, Kerrie Coleman and Fiona M Coyer
Keywords pressure injury, Incontinence-associated dermatitis, incontinence, prevalence
Abstract
Incontinence-associated dermatitis (IAD) is a largely preventable skin injury that can occur following chronic skin exposure to urine and faeces as a result of incontinence. Limited data is available about the prevalence of IAD in the Australian acute care setting. In 2011, the facility combined the annual pressure injury (PI) prevalence survey with a survey to determine the prevalence of IAD. This paper examines the PI and IAD prevalence results from surveys before and after the introduction of the combined survey protocol. The surveys were conducted in a major acute care Australian hospital between 2009 and 2013, with PI only surveys conducted between 2009 and 2010, and the combined PI and IAD surveys undertaken from 2011 to 2013. Overall, PI point prevalence decreased from 12.8% (n = 500) in 2009 to 6.3% (n = 444) in 2013. IAD prevalence was first reported in the 2011 survey. IAD prevalence decreased from 10% (n = 376) in 2011, to 2.7% (n = 444) in 2013. Combining the PI and IAD survey protocols provided valuable and previously unknown IAD data. In addition, combining the surveys was accomplished without increased financial or staff resources, nor increased survey participation burden for patients. Key aspects of the combined protocol have subsequently been adopted by the facility as standard procedures for ongoing PI prevalence surveys.
Introduction
Pressure injuries (PIs) are localised injuries to the skin and/or underlying tissue, usually over a bony prominence resulting from sustained pressure (including pressure with shear). Largely preventable, PIs, if sustained while in hospital care, are considered to be nosocomial skin injuries1. Prevalence surveys are a common and well-established method for determining the number of existing PIs in the acute care setting2. Prevalence data provides a snapshot of the burden of a condition at the time of the survey, and can provide data to assist in evaluating clinical and preventative practices, benchmarking, and resource allocation2-5. Incontinence-associated dermatitis (IAD) is skin injury that can occur following chronic skin exposure to urine and faeces as a direct result of incontinence6-8. Therefore, the primary risk factor for IAD is incontinence9. Moreover, IAD can predispose patients to serious complications such as superficial PI and/or superimposed infections6,9,11-13. Like PIs, IAD is largely preventable. IAD and PIs commonly co-exist, are often co-located, and are frequently misclassified by clinicians9,14. If misclassification occurs within the context of a PI prevalence survey, it is possible that IAD may be classified as a stage I or II PI, thereby erroneously increasing PI prevalence2,9. Patients at risk of skin injury due to pressure and shear are also likely to be vulnerable to injury from moisture, friction, and irritants found in urine and/or faeces12,14-16. Unlike the extensive understanding of PI prevalence in the acute care setting, there is a gap in the understanding of IAD prevalence in this setting. Furthermore, established protocols or agreement on the ideal methods to conduct prevalence surveys are lacking9,15. Given the potentially serious complications of IAD, understanding the scope of this condition in the acute care setting is imperative for maintaining skin integrity and the broader mandate of patient safety17.
In Australia, the prevalence of PIs in the acute care setting has decreased steadily over the last decade from 26% in 2003 to 2.5% in 201218-21. Internationally, PI prevalence has also declined over the last decade. Prevalence in the United States ranged from 13% in 2003 to 11% in 200922. An appreciation of incontinence is required to understand IAD23, with the prevalence of incontinence providing a guide as to the proportion of patients at risk of IAD24. The prevalence of IAD in the acute care setting is reported to range between 20% and 42%13,23,25. A 2014 Australian study23 found the prevalence of incontinence to be 24%, with 42% of those incontinent patients having IAD, reflecting the significant extent of the condition in this setting.
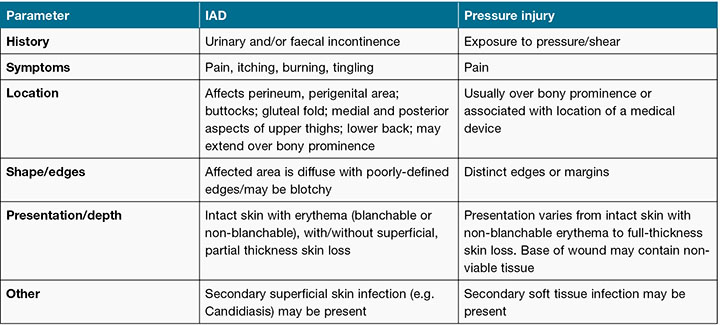
Awareness of the importance of IAD as a significant skin injury has been growing over the last decade8,15. One of the challenges that persists in regard to IAD is the difficulty clinicians face in accurately differentiating between IAD and PI5,14,26,27. Several factors may contribute to this difficulty. Firstly, while there is an agreed categorisation system for PIs1, at the time of the study, there was no internationally agreed categorisation system for IAD. PIs are classified or staged according to a classification system that includes stage I–IV injuries, as well as unstageable and suspected deep tissue injury categories1. While several IAD severity categorisation systems have been proposed9,28-30, use of these categorisation systems in clinical practice is limited. This may, in part, be due to the lack of evidence regarding improvements to clinical decision making and care when these systems are used9. Secondly, accurate classification is complicated by similarities in clinical presentation and location of PIs and IAD (Table 1), with particular challenges found in differentiating IAD from category/stage I and II PI9.
Table 1: PI and IAD differentiation. Reproduced with permission from Beeckman et al., 2015
In 2011, as a result of a growing appreciation of the significance of IAD as nosocomial skin injury, the role of IAD and incontinence as risk factors for PI, and the imperative for accurate and reliable PI data, the facility conducted a combined PI and IAD prevalence survey. This combined prevalence survey was a facility-wide quality improvement activity, and also formed a component of the first author’s higher research degree, (results of the research have been published elsewhere)23. Combining PI and IAD prevalence surveys into a single protocol is not routine practice in most facilities. The potential benefits of combining PI and IAD surveys may include: improved PI data accuracy as a result of improved surveyor education; access to new and valuable IAD and incontinence data, allowing for a more comprehensive understanding of skin integrity risks and injuries within the facility; and, importantly, value adding to costly PI surveys by means of simultaneously capturing IAD and PI data at minimal or no extra cost to the facility, or burden to all stakeholders, including ward routine and clients being surveyed.
Poor understanding of the prevalence of both PIs and IAD, as well as lesion misclassification can have implications for patient outcomes, delivery of quality care, resource allocation, PI prevalence data accuracy and skin integrity benchmarking1,2,9,15. An opportunity exists to improve the understanding of these skin injuries in the acute care setting, by way of combining PI and IAD prevalence surveys and simultaneously obtaining valuable PI and IAD prevalence data.
Objective
The objectives of this paper were to review PI prevalence before (2009–2010) and after (2011–2013) the commencement of the protocol combining PI and IAD surveys, and to review IAD prevalence data after the commencement of the protocol combining PI and IAD surveys (2011–2013).
Methods
Design
PI prevalence results from surveys conducted between 2009 and 2013 were examined. IAD prevalence results from surveys conducted between 2011 and 2013 were examined.
Setting and sample
This study was conducted at a 929-bed major acute care teaching hospital in Australia. Hospitalised adults aged 18 years or older admitted to the facility on the days of the surveys were eligible for inclusion. Patients were surveyed from Internal Medicine, Surgery, Critical Care, Cancer Care, and Women’s and Newborns admitting services (only non-obstetric patients were included from Women’s and Newborns admitting service). Patients from the Mental Health admitting service were excluded from the surveys as per the standard facility PI prevalence survey protocol.
PI and IAD prevalence formulae
PI prevalence was calculated as the total number of participants with one or more PIs detected on skin inspection on the survey day, divided by the total number of participants. In 2011, the prevalence of IAD amongst incontinent patients was calculated as the total number of IAD cases in the sample divided by the total number of incontinent participants as per the protocol for the research conducted in 2011 by the first author23. In 2012 and 2013, incontinence prevalence was not calculated, as the prevalence of incontinence was not recorded in these surveys. Therefore, for these years, the prevalence of IAD was calculated by the total number of IAD cases in the sample, divided by the total number of participants in the sample.
Measures
PI classification
All PIs were staged according to the PI staging guidelines accepted for use in Australia at the time of each survey31,32.
IAD classification
IAD was classified in 2011 according the Skin Assessment Tool30. This tool was designed to provide a cumulative IAD severity score and was used for the research component of the project. In the 2012 and 2013 surveys no IAD severity instrument was used; IAD was reported as present or absent. The presence of superimposed fungal infection was not recorded in the 2012–2013 surveys.
Procedures
PI prevalence survey methodology used by Queensland Health for the surveys 2009–2013, was based on methodology developed by Prentice, Stacey and Lewin in 200333. IAD prevalence survey methodology followed best practice guidelines available at the time13,26,33-35.
Prior to data collection surveyors (registered nurses, occupational therapists, physiotherapists and medical practitioners seconded from the facility) were trained in the use of the survey instruments, skin inspection procedures, and assessment and classification of PIs. In the 2011–2013 surveys, surveyor education was expanded to include accurate differentiation between IAD, clinical presentation of fungal infections, as well as identification and staging of PIs. Inter-rater reliability was established at the conclusion of all education sessions by scores on written multiple-choice tests, and tests using clinical photographs. The surveys conducted between 2009 and 2010 included photographs of a variety of PIs. The surveys conducted between 2011 and 2013 included clinical photographs of IAD, fungal infections and a variety of PIs. The tests required participants to accurately identify and stage PIs (for the tests conducted for the 2009–2010 surveys), and differentiate between IAD, clinical evidence of fungal infection and PI, as well as accurately stage PIs (for surveys conducted in 2011–2013). In all years, surveyors were required to achieve a score of 85% to participate in the survey. The use of photographs to test inter-rater reliability has been used previously5,36,37.
The surveys were conducted over two days. Teams of two surveyors conducted skin inspections on all eligible, consenting patients. Any loss of skin integrity in the pelvic region was classified as either PI or IAD. PI risk assessment, skin integrity documentation, the use of pressure redistributing equipment and demographic data were collected for all participants. In 2011 (as a component of the first author’s research), data were collected on continence status, stool frequency and quality, and IAD severity. When patients were off the ward at the time of data collection, the surveyors returned later to collect data for those patients where possible. To ensure accuracy, expert skin integrity nurses conducted independent skin inspections on the same day, on all patients reported by the surveyors to have a PI, IAD or clinical evidence of a fungal infection.
Results
Between 2009 and 2013, 2126 patients participated in the facility-wide PI prevalence surveys (Table 2). Overall, PI point prevalence decreased from 12.8% in September 2009 to 6.3% in October 2013 (Figure 1). Hospital-acquired PI point prevalence decreased from 12.7% in June 2010 to 4.0% in October 2013, with the community-acquired PI point prevalence ranging from 3% to 2.2% over the period (Table 2 and Figure 1). No IAD or incontinence data were recorded in 2009 and 2010. The research component of the project was conducted in 2011, with fewer patients consenting to participate in the research than participated in the PI prevalence survey. This explains the difference in the denominator between the IAD data and the PI data for 2011 (Table 2). In 2011, the prevalence of incontinence was 24% (n = 376) and the prevalence of IAD for the entire sample was 10% (n = 376), with the prevalence of IAD for incontinent patients being 42% (n = 91)23. The prevalence of IAD in 2012 was 3.6% (n = 273) and in 2013, 2.7% (n = 444).
Table 2: Pressure injury survey data summary, 2009–2013
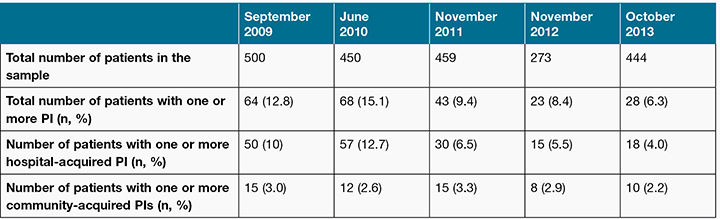
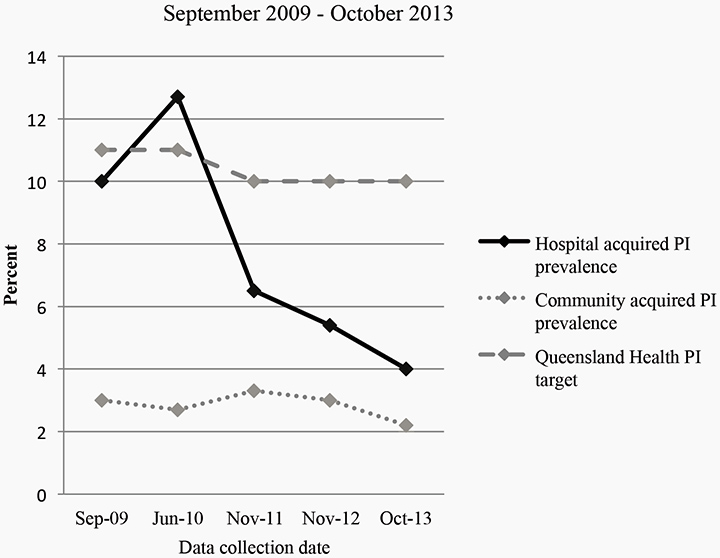
Figure 1: Pressure injury prevalence 2009–2013
Discussion
The requirement for accurate, valid and reliable skin integrity prevalence data within the acute care setting is essential for understanding the scope of a problem38, for evaluation of skin injury prevention protocols, and increasingly prevalence data is being used as an indicator of quality care2. The survey data demonstrate a sustained decrease in PI prevalence from the 2010 survey through to 2013 inclusive of the 2011 survey, where the PI and IAD prevalence surveys were combined into a single procedure. The facility PI prevalence is consistent with national and international downward trends, including hospital-acquired PI prevalence19-22,39-41. Survey data also demonstrated a sustained decrease in IAD prevalence. The downward PI prevalence trend from 2011 may be explained in part by improved differentiation of PI and IAD as a result of more comprehensive surveyor education, which may ultimately improve prevalence data accuracy and result in lower PI prevalence. However, the effect of the combined survey protocols on PI prevalence cannot be quantified. Other factors that may have contributed to the downward PI prevalence trend during that period include: PI education programs for clinicians; ongoing utilisation of pressure injury prevention champions in clinical areas; and the introduction of a system whereby financial penalty was applied to Queensland hospitals by the Queensland Department of Health if a patient developed a preventable stage III or IV hospital-acquired PI42. Improved surveyor education, effective collection of data without the need to increase personnel, material or financial resources and, importantly, no increased survey participation burden experienced by patients demonstrated the utility of combining the surveys.
Overall, the unique surveys provided important data on both PI and IAD prevalence. Subsequently, key aspects of the combined protocol, that is, enhanced surveyor education and recording the presence of IAD as a component of data collection was adopted as standard practice for ongoing PI prevalence surveys within the facility.
PI and IAD prevalence
The sustained decrease in the facility’s PI prevalence demonstrated in the surveys is consistent with downward national and international PI prevalence trends. Wound prevalence surveys conducted in Western Australian public hospitals between 2007 and 201121 found PI prevalence ranged between 12% and 9%, with hospital-acquired PI prevalence ranging between 7% and 9%. In Victoria, Australia, statewide PI prevalence surveys conducted in 2003, 2004 and 2006 found the prevalence of PIs ranged between 26% and 18% respectively, with approximately two-thirds of PIs sustained in hospital in all three surveys19. Surveys conducted at a metropolitan hospital in Queensland20 reported hospital-acquired PI prevalence rates ranging from 8% in 2009, 4% in 2012, with overall PI prevalence ranging from 12% in 2009 to 6% in 2012.
Internationally, a large American study, conducted over eight years39, reported PI prevalence data from 78 acute care hospitals (nearly 260,000 patients). This study found the hospital-acquired PI rate decreased from 10% in 2003 to 2% in 2010. Another study22 in the United States found PI prevalence in acute care hospitals in 2008–2009 to be 13% and 12% respectively, with the hospital-acquired prevalence being 6% and 5% respectively. A Belgian study41 found PI prevalence of 12% in 143 hospitals. Consistent with the national and international downward PI prevalence trends, the facility data demonstrates continuing reductions in PI and hospital-acquired PI prevalence.
It can be speculated that a portion of the PI prevalence reduction between 2011 and 2013 may be attributed to improved surveyor accuracy regarding differentiation of PIs and IAD as a result of the more comprehensive surveyor education provided prior to participation in these surveys. Traditionally, PI surveys focused on establishing inter-rater reliability with regard to accurate identification and staging of PIs only. While difficulty with differentiating between PI and IAD is recognised5,36,37, the impact of misclassified lesions on PI prevalence data has not been reported in the literature, nor quantified in the facility.
The majority of published IAD data comes from the aged care or critical care environment. The IAD prevalence in critical care is reported to be 36%25, with the prevalence of IAD ranging between 6% and 22% in aged care43,44. The 2011 IAD survey (detailed results published elsewhere23), found that 24% of participants were incontinent and of those who were incontinent, 42% had IAD23. A 2007 study conducted in the acute care setting in the United States reported the prevalence of incontinence to be 19%, with IAD present in 20% of those who were incontinent. IAD trend data is not available nationally or internationally to enable comparison with the facility IAD data. The facility downward IAD prevalence trend between 2011 and 2013 may be due to the comprehensive surveyor education requiring accuracy in differentiation between PI, IAD and fungal infections as well as facility-wide ongoing education for clinicians regarding IAD. Similar to PI prevalence data, prospective IAD data is necessary for benchmarking and tracking quality care over time.
Drivers for combining surveys
The impetus for combining the PI and IAD prevalence surveys into a single procedure in 2011 was based on the facility’s need to understand the prevalence of incontinence and IAD in its population. Parallels between PI and IAD survey procedures such as common patient populations, the requirement for comparable surveyor education, establishment of inter-rater reliability and the requirement for participants to undergo a pelvic skin inspection1,13,23,45, meant that combining the survey protocols was straightforward. In addition, the logistical requirements for an IAD prevalence survey are almost identical to the requirements for the routine PI survey conducted each year in the facility.
It is accepted that direct skin inspection is the gold standard for obtaining PI prevalence data, with the caveat that surveyors have adequate skill in classifying PIs and differentiating them from other lesions such as IAD2,46,47. The rationale for the recommendation of skin inspection as the primary data source is based on the understanding that documentation in regard to PI is often inadequate2. It would therefore be consistent that data obtained from direct skin inspection would also be the gold standard practice for IAD prevalence survey methodology. In light of the fact that a thorough pelvic skin inspection is required for both PI and IAD prevalence data collection, combining the survey protocols does not result in any further survey participation burden for the patient.
PIs and IAD have different aetiologies and as such require different prevention and management strategies. Subsequently, misclassification can have a significant impact on patient outcomes, data accuracy, benchmarking, and resource allocation9,14,26,27. In 2007, the NPUAP48 issued a statement with the description of stage II PI, stating that this category should not be used to describe “skin tears, tape burns, perineal dermatitis, maceration or excoriation” (p. 40). This raised awareness of the fact that until that time most superficial pelvic lesions were classified as a stage II PI16. At the time the surveys were conducted, if a patient developed a stage III or IV PI, a funding penalty was applied to hospitals by the Queensland Department of Health. This meant that a stage III hospital-acquired PI incurs a funding penalty of $30,000, and a stage IV hospital-acquired PI incurs a penalty of $50,00042. Accordingly, a misclassified pelvic lesion (for example IAD erroneously classified as a stage III PI) has the potential to attract a penalty of $30,000 for the health care provider. Accurate differentiation of PIs and IAD is, therefore, of utmost importance for patients and health care providers alike.
The utility of combining the PI and IAD surveys was persuasive. The combined survey used the same number of surveyors and support staff, meaning no increase in personnel or financial resources was necessary. In addition, the data collection was completed in the same time as the previous annual PI surveys, that is, over two days. Therefore, combining the PI and IAD protocols maximised value from the costly and resource-intensive annual PI prevalence survey. While a cost-effectiveness analysis was not undertaken in the 2011–2013 surveys, any minor cost increase as a result of extra time taken recording IAD data was offset by the benefits of access to the additional data. A further positive outcome is that, anecdotally, IAD awareness is improved for staff that participate as surveyors, and subsequently champion IAD awareness in their respective clinical areas. As a result of the combined protocol, valuable data regarding the burden of IAD and incontinence became available to the facility, enabling comprehensive understanding of the burden of these conditions.
Prevalence studies are difficult and costly to perform, and require a significant number of adequately trained personnel. In view of the high cost of conducting these studies, and the financial and clinical imperative that resultant data are accurate, it is logical to combine the PI and IAD surveys into a single protocol. Further, documenting and reporting of these valuable metrics is crucial given the appreciation of the association between incontinence and IAD as risk factors for PI development. Utilising protocols that aid in the understanding of the burden of incontinence and IAD in patients vulnerable to PI is surely the next logical step in quality improvement.
Limitations
As discussed, no international agreement exists as to an IAD prevalence survey methodology, or methodology combining PI and IAD surveys, which leads to study limitations. An IAD severity instrument was not utilised for the 2012 and 2013 surveys, rather, the presence or absence of IAD was reported. PI data were reported in all surveys by stage and location as individual totals. Therefore, it was not possible to report the prevalence of stage I or II pelvic PIs, or to investigate the prevalence of these PIs when IAD was included in data collection. An appreciation of incontinence is essential for understanding the epidemiology of IAD. The 2012 and 2013 surveys did not collect incontinence data; therefore IAD is reported as a percentage of the entire sample rather than a percentage of incontinent participants. Opportunities exist to improve collection and reporting of survey data, as well as reaching agreement in regard to survey protocols, particularly formulae for calculating the prevalence of IAD23.
Conclusion
This paper has proposed a unique protocol for conducting combined PI and IAD prevalence surveys. The data from the combined surveys reveals downward trends in both PI and IAD prevalence. In addition, the combined protocol has been shown to be effective, practicable and achievable, without incurring additional costs to the facility, or placing additional burdens on patients to participate. The resultant IAD data is the first of its kind in Australia, and provides previously unknown IAD trend data for the acute care setting.
PIs and IAD are both largely preventable skin injuries. During the last decade, there is a wider appreciation of the potentially serious complications of IAD, particularly IAD as a risk factor for superficial PI12. Therefore, from a patient safety perspective, understanding the prevalence of IAD and incontinence constitutes a vital component of maintaining skin integrity17. Prevalence studies are difficult and costly to perform and require a significant number of adequately trained personnel. In view of the financial burden to the facility in conducting these studies, and the financial as well as clinical imperative that resultant data are accurate, combining PI and IAD surveys into a single protocol has multiple benefits. While this research could not quantify the extent of the influence of the combined protocol on PI prevalence data, a sustained downward trend in PI prevalence was demonstrated. It is feasible, therefore, to attribute a portion of the reduction in PI prevalence to improved accuracy in the classification of pelvic lesions. Recommendations for future surveys include determining agreement as to the definition of incontinence for IAD surveys, agreement as to the minimum data set required for a combined PI and IAD survey protocol, formulae for calculating and reporting IAD prevalence and, finally, agreement as to an IAD severity classification instrument. Further research is required to evaluate PI and IAD prevention programs within the facility. In the future, PI prevalence surveys may evolve into broader, comprehensive skin integrity prevalence surveys, providing a rich source of data that will ultimately inform and guide skin integrity care and outcomes in acute care patients.
Acknowledgements
The corresponding author is a recipient of a Royal Brisbane and Women’s Hospital (RBWH) Foundation Research Project Grant, a RBWH Foundation Research Scholarship and Centaur Memorial Fund for Nurses Scholarship.
Author(s)
Jill L Campbell*
RN, BAppSc(Nurs), GradDip(Wound Care), PhD(c)
Clinical Nurse, Skin Integrity Service
Royal Brisbane and Women’s Hospital
Metro North Hospital & Health Service
Butterfield St, Herston, Qld 4029, Australia
School of Nursing
Queensland University of Technology
Tel +617 3138 1746
Fax +617 3138 3814
Email Jill.campbell@hdr.qut.edu.au
Sandra Gosley
RN, BNurs
Clinical Nurse Consultant, Safety and Quality Unit; Pressure Injury Prevention
Royal Brisbane and Women’s Hospital
Metro North Hospital & Health Service
Butterfield St, Herston, Qld 4029, Australia
Email Sandra.Gosley@health.qld.gov.au
Kerrie Coleman
DipAppSc, BNSc, MN Clinical (Wound),
MN Chronic Disease
Nurse Practitioner Complex Wound Management
Critical Care & Clinical Support Services
Royal Brisbane & Women’s Hospital
Metro North Hospital & Health Service
Butterfield St, Herston, Qld 4029, Australia
Email Kerrie.Coleman@health.qld.gov.au
Fiona M Coyer
RN, PGCEA, MSc(Nurs), PhD
Professor of Nursing, Joint Appointment
Metro North Hospital Health Service
Royal Brisbane and Women’s Hospital and School of Nursing, Queensland University of Technology
Butterfield St, Herston, Qld 4029, Australia
Visiting Professor University of Huddersfield, UK
Email f.coyer@qut.edu.au
* Corresponding author
References
- National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: Clinical Practice Guideline. Osborne Park, Australia: Cambridge Media; 2014.
- Baharestani M, Black J, Carville K, Clark M, Cuddigan J, Dealey C et al. Dilemmas in measuring and using pressure ulcer prevalence and incidence: an international consensus. Int Wound J 2009;6(2):97–104.
- Amlung S, Miller W, Bosley L. The 1999 National Pressure Ulcer Prevalence Survey: a benchmarking approach. Adv Skin Wound Care 2001;14(6):297–301.
- Baumgarten M. Methodology. Designing prevalence and incidence studies. Adv Wound Care 1998;11(6):287–93.
- Beeckman D, Schoonhoven L, Fletcher J, Furtado K, Gunningberg L, Heyman H et al. EPUAP classification system for pressure ulcers: European reliability study. J Adv Nurs 2007;60(6):682–91.
- Gray M, Bliss D, Doughty D, Ermer-Seltun J, Kennedy-Evans K, Palmer M. Incontinence-associated dermatitis: a consensus. J Wound Ostomy Continence Nurs 2007;34(1):45–56.
- Gray M, Black J, Baharestani M, Bliss D, Colwell J, Goldberg M et al. Moisture-associated skin damage: overview and pathophysiology. J Wound Ostomy Continence Nurs 2011;38(3):233–41.
- Voegeli D. Incontinence-associated dermatitis: new insights into an old problem. British J Nurs 2016;25(5):256–62.
- Beeckman D, Campbell J, Campbell K, Chimentao D, Coyer F, Domansky R et al. Proceedings of the Global IAD Expert Panel. Incontinence-associated dermatitis: Moving prevention forward. Wounds International; 2015. Accessed from www.woundsinternational.com
- Doughty D, Junkin J, Kurz P, Selekof J, Gray M, Fader M et al. Incontinence-associated dermatitis: consensus statements, evidence-based guidelines for prevention and treatment, and current challenges. J Wound Ostomy Continence Nurs 2012;39(3):303–15.
- Furlanetto K, Emond K. “Will I come home incontinent?” A retrospective file review: Incidence of development of incontinence and correlation with length of stay in acute settings for people with dementia or cognitive impairment aged 65 years and over. Collegian 2016:23:79–86.
- Gefen A. From incontinence-associated dermatitis to pressure ulcers. J Wound Care 2014;23(7):345.
- Junkin J, Selekof J. Prevalence of incontinence and associated skin injury in the acute care inpatient. J Wound, Ostomy Continence Nurs 2007;34(3):260–9.
- Voegeli D. Pressure ulcer or moisture lesion — what’s the difference? Nurs Residential Care 2011;13(5):222–7.
- Beeckman D. A decade of research on Incontinence-associated dermatitis (IAD): Evidence, knowledge gaps and next steps. J Tissue Viability 2016. doi: 10.1016/j.tv.2016.02.004
- Doughty D. Differential assessment of trunk wounds: pressure ulceration versus incontinence-associated dermatitis versus intertriginous dermatitis. Ostomy Wound Manage 2012;58(4):20.
- Campbell J, Coyer F, Osborne S. The Skin Safety Model: Reconceptualizing skin vulnerability in older patients. J Nurs Scholarsh 2016;48(1):14.
- Antonio T, Conrad K. Clinical and economic improvements in pressure injury care at Ballarat Health Services. Wound Practice Research 2013; 21(1):4–10
- Victorian Quality Council. Pressure ulcer point prevalence surveys (PUPPS): State-wide PUPPS 3 report 2006. 2008. Accessed from https://www2.health.vic.gov.au/about/publications/researchandreports/pressure-ulcer-prevalence-survey
- Miles S, Fulbrook P, Nowicki T, Franks C. Decreasing pressure injury prevalence in an Australian general hospital: a 10-year review. Wound Practice and Research 2013; 21(4):148–56.
- Mulligan S, Prentice J, Scott L. WoundsWest Wound prevalence survey 2011 State-wide Overview Report. Perth, Western Australia: Ambulatory Care Services, Department of Health, 2011.
- Vangilder C, Amlung S, Harrison P, Meyer S. Results of the 2008–2009 International Pressure Ulcer Prevalence Survey and a 3-year, acute care, unit-specific analysis. Ostomy Wound Manage 2009;55(11):39–45.
- Campbell J, Coyer F, Osborne S. Incontinence-associated dermatitis: A cross-sectional prevalence study in the Australian acute care hospital setting. Int Wound J 2014; doi: 10.111/iwj.12322
- Ersser S, Getliffe K, Voegeli D, Regan S. A critical review of the inter-relationship between skin vulnerability and urinary incontinence and related nursing intervention. Int J Nurs Stud 2005;42(7):823–35.
- Bliss D, Savik K, Thorson M, Ehman S, Lebak K, Beilman G. Incontinence-associated dermatitis in critically ill adults: time to development, severity, and risk factors. J Wound Ostomy Continence Nurs 2011;38(4):433–45.
- Defloor T, Schoonhoven L, Katrien V, Weststrate J, Myny D. Reliability of the European Pressure Ulcer Advisory Panel classification system. J Adv Nurs 2006;54(2):189–98.
- Mahoney M, Rozenboom B, Doughty D, Smith H. Issues related to accurate classification of buttocks wounds. J Wound Ostomy Continence Nurs 2011;38(6):635–42.
- Junkin J. Incontinence-Associated Dermatitis Intervention Tool (IADIT). 2008. Accessed from http://www.sageproducts.com/lit/21239.pdf
- Borchert K, Bliss DZ, Savik K, Radosevich DM. The incontinence-associated dermatitis and its severity instrument: development and validation. J Wound Ostomy Continence Nurs 2010;37(5):527–35.
- Kennedy K, Lutz L, editors. Comparison of the efficacy and cost-effectiveness of three skin protectants in the management of incontinent dermatitis. Proceedings of the European Conference on Advances in Wound Management; 1996.
- National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: Clinical Practice Guideline. Washington DC: National Pressure Ulcer Advisory Panel; 2009.
- Australian Wound Management Association (AWMA). Pan Pacific Clinical Practice Guideline for the prevention and management of pressure injury. Osborne Park, Western Australia: AWMA; 2012.
- Prentice J, Stacey M, Lewin G. An Australian model for conducting pressure ulcer prevalence surveys. Primary Intention 2003;11(2):87–88, 90–91, 93–96,98–100,102–109.
- Beeckman D, Woodward S, Rajpaul K, Vanderwee K. Clinical challenges of preventing incontinence-associated dermatitis. Br J Nurs 2011;20(13):784–90.
- Baharestani M, Black JM, Carville K, Clark M, Cuddigan J, Dealey C et al. International Guidelines. Pressure ulcer prevention: prevalence and incidence in context. A consensus document. Wounds International; 2009. Accessed from; http://www.woundsinternational.com/consensus-documents/view/international-review-pressure-ulcer-prevention-pressure-shear-friction-and-microclimate-in-context-1
- Beeckman D, Schoonhoven L, Boucque H, Van Maele G, Defloor T. Pressure ulcers: e-learning to improve classification by nurses and nursing students. J Clin Nurs 2008;17(13):1697–707.
- Defloor T, Schoonhoven L. Inter-rater reliability of the EPUAP pressure ulcer classification system using photographs. J Clin Nurs 2004;13(8):952–9.
- Coggon D, Barker DJP, Rose G. Epidemiology for the uninitiated. London: BMJ; 2003.
- Stotts NA, Brown DS, Donaldson NE, Aydin C, Fridman M. Eliminating hospital-acquired pressure ulcers: within our reach. Adv Skin Wound Care 2013;26(1):13–8.
- Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. J Eval Clin Pract 2007;13(2):227–35.
- Vanderwee K, Defloor T, Beeckman D, Demarre L, Verhaeghe S, Van Durme T et al. Assessing the adequacy of pressure ulcer prevention in hospitals: a nationwide prevalence survey. BMJ Qual Saf 2011;20(3):260–7.
- Queensland Government. Health Funding Principles and Guidelines 2013–14. Brisbane, Queensland: Queensland Government; 2013. Accessed from: https://publications.qld.gov.au/storage/f/2014-06-06T04:24:00.515Z/health-fund-pples-n-guidelines-13-14.pdf
- Bliss D, Savik K, Harms S, Fan Q, Wyman J. Prevalence and correlates of perineal dermatitis in nursing home residents. Nurs Res 2006;55(4):243–51.
- Beeckman D, Vanderwee K, Demarre L, Paquay L, Van Hecke A, Defloor T. Pressure ulcer prevention: development and psychometric validation of a knowledge assessment instrument. Int J Nurs Stud 2010;47(4):399–410.
- Arnold-Long M, Reed L, Dunning K, Ying J. Incontinence-associated dermatitis (IAD) in a long-term acute care (LTAC) facility: findings from a 12 week prospective study. J Wound Ostomy Continence Nurs 2011;38(3S):S7–S.
- Defloor T, Clark M, Witherow A, Colin D, Lindholm C, Schoonhoven L et al. EPUAP statement on prevalence and incidence monitoring of pressure ulcer occurrence in 2005. European Pressure Ulcer Advisory Panel 2005;6(3):74–80.
- Pieper B, editor. National Pressure Ulcer Advisory Panel. Pressure ulcers: prevalence, incidence, and implications for the future. Washington DC: NPUAP; 2012.
- Black J, Baharestani M, Cuddigan J, Dorner B, Edsberg L, Langemo D et al. National Pressure Ulcer Advisory Panel’s updated pressure ulcer staging system. Adv Skin Wound Care 2007;20(5):269–74.



