Volume 24 Number 3
The diabetic foot ulcer periwound: a comparison of visual assessment and a skin diagnostic device
Alexandra Rowledge, Nicoletta Frescos, Charne Miller, Elizabeth Perry and William McGuiness
Keywords wound, Diabetes-related foot ulcers, periwound, hydration, erythema.
Abstract
Background: This study aimed to evaluate the relationship between visual and objective periwound assessment and explore how these assessments relate to diabetes-related foot ulcer (DRFU) healing.
Methods: Seventeen people with DRFU were recruited from a foot clinic. The periwound of each participant’s DRFU was assessed at baseline and fortnightly for 6 weeks. Assessment included visual appraisal by podiatrists and objective evaluation using the SD202 skin-measuring device (C.K. Electronic). Wound healing measures included whether or not the wound healed, the number of days to healing and wound healing rates. Pearson’s correlation coefficients were obtained for relationships between: (i) clinician and SD202 device periwound assessments; (ii) clinician periwound assessment and wound healing measures; and (iii) SD202 device measures and wound healing measures.
Results: Relationships between clinician and SD202 hydration assessments were consistently strong and positive but only significant at baseline (r=0.565 p=0.035) and 2 weeks (r=0.611 p=0.035). No significant relationships between clinician and SD202 periwound erythema assessments were detected at any time point. Clinician-observed maceration (for example, baseline r=-0.901, p<.001) and SD202 appraised erythema (for example, baseline r=0.648, p=0.023) related significantly to wound healing.
Conclusions: This pilot study highlights that further research is required to establish valid and reliable periwound assessment approaches.
Background
Diabetes-related foot ulcers (DRFU) represent a major global health concern, affecting up to 25% of people with diabetes mellitus (DM)1. These ulcers not only reduce the quality of life and physical functioning of those affected but also have a significant economic impact on communities and the health care system as a whole1,2. Prompt, appropriate wound assessment informs optimal DRFU management and can prevent the development of severe infection and consequent lower extremity amputation1.
At present, there are no standardised DRFU assessments; however, there is generalised agreement regarding which aspects of the wound should be examined, including the wound bed, exudate type and levels, wound edges and surrounding skin2-4. It is considered important to assess the periwound, being the skin within 4 cm of the wound edge5.
Periwound skin is subject to damage caused by adhesive dressing removal6,7, excess, uncontrolled wound exudate5,8, and pressure-induced callus formation. An impaired periwound can exacerbate pain and contribute to protracted healing9. It appears wound healing is impacted by a number of specific periwound impairments such as overhydration, dryness and lipid deficiency.
Overhydrated, macerated periwound skin can increase the risk of infection10,11, precipitate inflammation12 and result in wound enlargement11,13. Studies demonstrate that maceration is a risk factor for the development of pressure injuries14,15 and that excessively moist skin is more susceptible to breakdown when subjected to compressive and shearing forces16. Neuropathic DRFU are commonly bordered by a macerated callus17, which is thought to be of detriment to wound healing, acting as a reservoir for bacteria and restricting drainage of wound exudate18. Dry periwound skin may also impair healing. Dry skin is relatively inelastic and more susceptible to breakdown; it is less able to withstand physical forces and can crack and fissure19. Skin dryness is commonly observed in people with diabetes as nerve changes can affect sudomotor function, decreasing sweat gland activity. When skin lipid levels are low skin barrier function is compromised, which results in decreased ability to maintain optimal skin hydration and increased susceptibility to infection20. In addition, in vitro testing has demonstrated that skin lipids play a role in the innate immune defence against bacterial colonisation and infection21.
The pigment melanin contributes to skin colour. There is evidence to suggest that the epidermal barrier is stronger and more rapidly repaired in darkly pigmented skin, which has higher concentrations of melanin22. It is hypothesised that melanin can inhibit the proliferation of infectious organisms of the skin, through interaction with microbial peptides23,24. Given that melanin has a role in the body’s immune defence system, periwound melanin levels may influence wound healing. Haemoglobin levels also contribute to skin colour. An increase in capillary blood flow and subsequent rise in haemoglobin concentration results in skin redness or erythema25. When concurrent with other clinical signs, an erythematous periwound can be indicative of infection, which significantly delays wound healing26. There is evidence to suggest that chronic DRFU exhibit a prolonged inflammatory response27,28. Since redness is a sign of inflammation, extended periwound erythema may suggest wound healing impairment. Additionally, highly variable periwound erythema may reflect repeated ischaemia and reperfusion, which is thought to disrupt normal wound healing processes29.
Given that periwound hydration, lipids, melanin and erythema are implicated in wound repair and regeneration, the valid and reliable assessment of these skin parameters could guide effective DRFU management, improve treatment outcomes and reduce associated costs.
To date, periwound assessment has been largely subjective, with clinicians employing various descriptors such as erythematous, macerated, callused and fragile to depict periwound condition4. The scales against which these factors are measured or described, be they dichotomous, a Likert scale rating, or purely qualitative, lack evidence of validity or reliability.
Skin-measuring devices have been widely used in the cosmetic industry and in skin health surveillance30 to measure certain skin parameters. The SD202 Skin Diagnostic is an example of one such device. It consists of a Corneometer, Sebumeter and Mexameter, which measure skin hydration, lipids levels and melanin and erythema respectively. The SD202 Skin Diagnostic could be applied to the field of wound management, enabling quantitative assessment of periwound skin parameters and thereby enhancing understanding of visual evaluation validity and improving wound assessment and management.
The aim of this study was to explore the relationships between: (i) observational clinician periwound assessment and SD202 periwound assessment; (ii) SD202 periwound assessment and DRFU healing; and (iii) observational clinician assessment and DRFU healing.
Methods
Design
In this prospective, longitudinal study, a skin diagnostic device was used to assess epidermal hydration, erythema, lipids and melanin in the DRFU periwound. For the purposes of this study, the SD202 was used as the “standard” to compare with clinical data. Measures were obtained at baseline then fortnightly for a six-week data collection period.
Setting, sample and recruitment
Study participants were recruited from a podiatry department in a major Melbourne metropolitan hospital between January and April 2014. Ethical approval was obtained from The Alfred (ID number: 587/13) and La Trobe University (ID number: 13/261) human research ethics committees. Informed consent was obtained from all study participants prior to the instigation of any study procedures. Patients with a confirmed diagnosis of DM (either type 1 or type 2) and one or more foot ulcers were eligible for study inclusion. Where participants presented with multiple foot ulcers, the largest wound was denoted the ‘study wound’ and was the wound assessed at each time point. Patients with interdigital and cancerous wounds were excluded from the study.
Data collection procedures
Demographic, diabetes and DRFU data were obtained from participants’ health records and through patient interview. Participants’ wounds were assessed four times: at baseline, then at 2, 4 and 6 weeks or less if their DRFU healed (Figure 1). Assessment consisted of periwound evaluation and wound size measures. The study protocol had no bearing on wound management; the clinicians continued to follow the organisation’s wound treatment protocol.
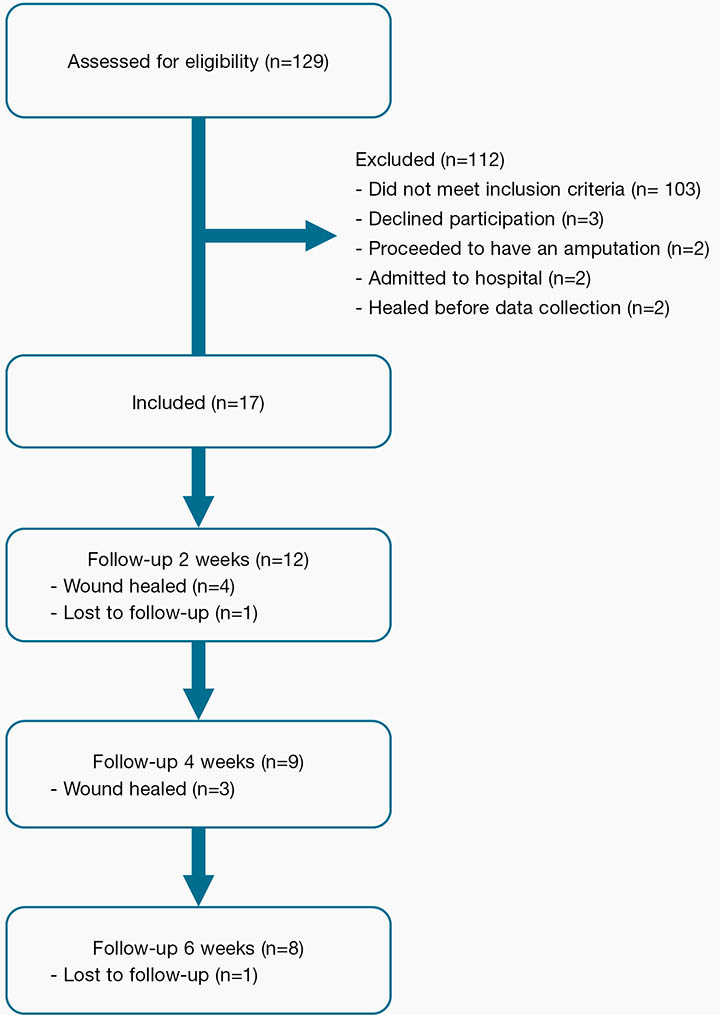
Figure 1: Flow of participants through study from screening to 6-week follow-up
Periwound assessment procedure
Periwound skin was assessed in two ways: objectively through use of the SD202 skin diagnostic device and subjectively through clinician observation. At each time point, periwound assessment was performed before and after wound cleansing. Wound cleansing involved a sterile saline flush of the wound and, where indicated, conservative sharp debridement of the wound bed and/or the periwound. Results presented in this study are derived from post-cleansing data.
SD202 device
The SD202 is a skin diagnostic device that consists of a Corneometer, Mexameter and a Sebumeter that measure skin hydration, colour and lipids respectively (Figure 2). To date, it has been used widely in cosmetology and dermatology, but is yet to be used in DRFU assessment.
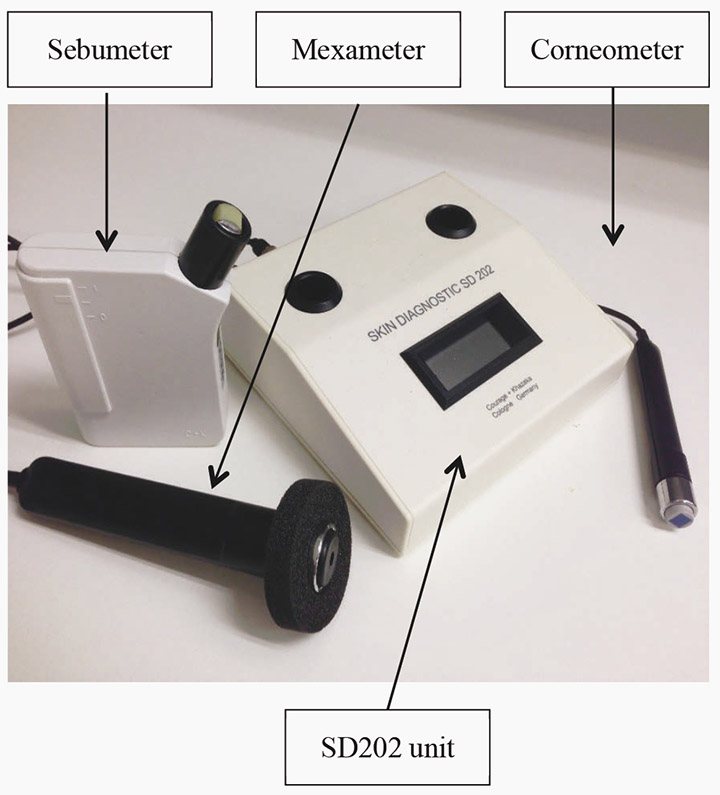
Figure 2: SD202 device
The Corneometer employs the capacitance method of epidermal hydration assessment. It consists of a main housing unit and a measuring probe that works as a capacitor when held against the skin. Water within the stratum corneum acts as a dielectric, thus an increase or decrease in skin hydration levels results in proportional changes to the capacitance of the system. The Corneometer outputs hydration readings as arbitrary units on a scale of 0–99.
The Mexameter is a narrow-band reflectance spectrometer. It consists of a photodetector and 16 light emitting diodes that emit light at three defined wavelengths: 568 nm (green), 660 nm (red), and 880 nm (infrared). Haemoglobin, which gives the skin a red colouration, exhibits high absorption of green light and minimal absorption of red light, and melanin, responsible for brown skin pigmentation, absorbs red and infrared light. Measuring the intensity of light (at different wavelengths) reflected by the skin enables the Mexameter to determine haemoglobin and melanin levels on a scale of 0–99.
The Sebumeter is considered a photometric device in that it measures transmission of light through collected samples. Samples are obtained by placing a plastic strip against the test site for 15 seconds. As the plastic strip absorbs sebum from the skin it becomes translucent. Light is passed through the strip and its translucency is measured by a photocell contained within the main unit of the Sebumeter. A microprocessor provides an estimate of total skin lipids based on the measure of translucency. The estimate is presented in arbitrary units on a scale of 0–99.
Objective SD202 periwound assessment
SD202 measurements were taken at four sites on the foot: within 5 mm of the top, bottom, right and left of the wound edge. This protocol was extrapolated from the SD202 manufacturer’s guidelines. In accordance with the manufacturer’s recommendations, skin lipids were measured first, followed by skin hydration and then skin colour. Contact between SD202 measuring probes and the skin can reduce skin surface lipids; therefore, to ensure accuracy skin lipids were measured prior to other periwound parameters. Postural changes impact haemoglobin levels and can alter mexameter readings31, thus all participants were assessed in a reclined, seated position that remained unchanged throughout the consultation. As recommended by the manufacturers of the SD202, lighting within the clinic rooms was maintained at a constant level. A single assessor performed the SD202 assessment at all data collection points to ensure consistency and optimise reliability.
Subjective clinician periwound assessment
Staff employed in the clinic completed a standard observational periwound assessment for all wounds included in the study. The periwound was inspected and, in line with the podiatry department’s wound assessment protocol, its appearance was characterised as normal, fragile, erythematous, oedematous, callused and macerated, using a categorical (yes/no) measure
Wound healing assessment procedure
Wounds were measured at each time point using the Visitrak digital planimetry system (Smith & Nephew Pty Ltd). Visitrak is an easy, quick and reliable tool which has been shown to provide precise and objective information in comparison to other methods such as acetate tracing only or linear measurements32. Wounds were traced onto Visitrak acetate grids and annotated on the Visitrak digital pad which calculates the wound size (cm2). Detection of changes in size is indicator of wound healing; monitoring the changes in wound area allows assessment on the effectiveness of wound treatment and detection of deterioration or stasis of wounds.
Wound healing rate was measured as a percentage of change in the wound surface area. The healing rate (%) for each time interval was calculated by subtracting the current wound area from the previous wound area, dividing by the previous wound area then multiplying by 100. In addition to fortnightly healing rates, an overall, study duration healing rate was determined using measurements from baseline and 6 weeks. Positive healing rates were obtained when the wound area decreased and negative healing rates reflected an increase in wound area. The maximum possible healing rate of positive 100 indicated that the wound in question healed completely, that is, reduced in size by 100% during the specified time period. In addition to wound healing rates, a categorical measure of whether or not a wound had healed was included as a component of the wound healing assessment.
Statistical analysis
Data were entered into an SPSS database and screened for normality, linearity and the presence of outliers. In theory, SD202 hydration and erythema measures could exhibit a curve linear relationship with wound healing rates; very high and very low periwound hydration or erythema is expected to impair wound healing. However, the scatterplots for these variables demonstrated that they did not relate in a curve linear fashion and did not violate the Pearson’s correlation assumption of linearity. Pearson’s correlation coefficients (r) were determined for the relationships between: (i) clinician periwound assessment and SD202 device measures; (ii) clinician periwound assessment and wound healing measures; and (iii) SD202 periwound assessment and wound healing measures. Statistical significance was assessed at p<0.05.
Results
The participant population comprised 3 females and 14 males with a mean age of 58.88 (SD=12.79). Of the 17 participants, 5 (29.4%) had type 1 DM and 12 (70.6%) had type 2 DM. The mean duration of diabetes was 18.96 years (SD=15.09) and the average wound duration was 6.76 months (SD=5.96). Wounds were categorised according to their aetiology: 8 wounds were neuropathic; 1 neuroischaemic; 5 slow healing amputation sites; and 3 a result of other causes.
At baseline, the wound area varied from 0.10 to 7.40 cm2 with a mean of 2.00 cm2 (SD=2.57). During the study time frame, 7 of the 17 wounds healed. Wound healing rates were highly variable as demonstrated in Table 1.
Table 1: The range, mean and standard deviation of wound healing rates (HR)
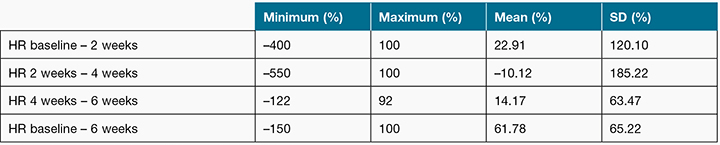
Note: Negative values indicates the wound increased in size
The clinical staff who undertook the observational periwound assessment, comprised of five podiatrists whose length of experience ranged from 4 to 22 years (M=11.8).
The wounds included in the study exhibited a range of periwound characteristics. Table 2 outlines the number and percentage of wounds with normal, fragile, erythematous, macerated and callused periwound skin as assessed by clinicians. The range and mean of SD202 values for periwound hydration, erythema and melanin are shown in Table 3. The SD202 device did not detect periwound skin lipids; all SD202 sebumeter readings were zero. Thus, periwound skin lipid measurements could not be considered in further analyses.
Table 2: Periwound characteristics as assessed through clinician observation at baseline, 2-week, 4-week, and 6-week follow-up
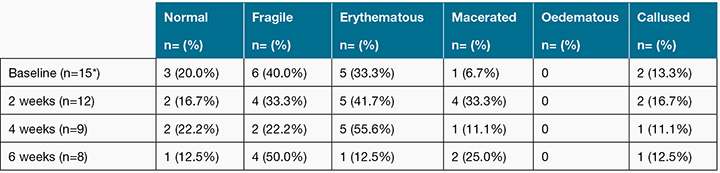
* Two participants data missed due to data collection protocols applied only after wound cleansing. These data represent post-cleansing assessment
Table 3: Periwound characteristics as assessed by the SD202 device
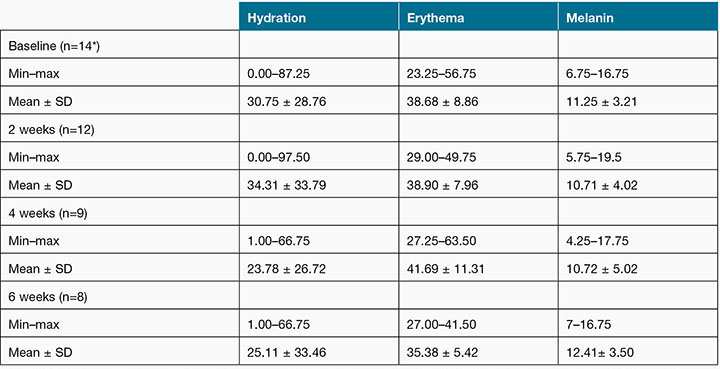
Note: SD202 hydration, erythema and melanin measurements are on a scale of 0–99, with higher values representing greater levels of hydration, erythema and melanin.
*Data for n=2 missed due to data collection protocols applied only after wound cleansing, and missed for n=1 due to the wound being cleansed before objective measures could be attended
Correlations between clinician and SD202 periwound assessments
A statistically significant, positive correlation was detected between clinician assessment of maceration and SD202 hydration measures at baseline (r=0.565, p=0.035) and 2 weeks (r=0.611, p=0.035). The strength and direction of this correlation remained consistent at 4 weeks (r=0.652, p=0.057) and 6 weeks (r=0.505, p=-0.201); however, statistical significance was not reached. There were no significant correlations between clinician-assessed erythema and SD202 erythema measures at any of the four time points. Clinicians do not appraise periwound melanin levels, thus, a correlation with the SD202 melanin measure could not be established.
Correlations between SD202 periwound assessment and DRFU healing
The majority of correlations between SD202 hydration scores and wound healing outcomes (wound healing rates and categorical wound healing measure) were negative, indicating that as skin hydration increased, poorer healing outcomes were observed. The strength of the coefficients ranged from r=–0.516 (p=0.086) to r=–0.016 (p=0.962); none of these correlations were statistically significant.
SD202 erythema values were positively related to the categorical (yes/no) wound healing measure at baseline (r=0.648, p=0.023), 2 weeks (r=0.782, p=0.004) and 4 weeks (r=0.773, p=0.015), indicating that better wound healing was observed in more erythematous wounds. A correlation could not be obtained for the 6-week time point, as the wound healing variable was constant; none of the remaining wounds healed within the 4- to 6-week time interval. No statistically significant associations between SD202 melanin values and wound healing measures were identified.
Correlations between clinician periwound assessments and DRFU healing
Clinician assessment of maceration had a strong negative association to the overall study healing rate at baseline (r=–0.901, p<0.001). At 4 weeks, clinician-assessed maceration was strongly related to the healing rate for the 4- to 6-week time interval (r=-0.868, p=0.005) as well as the overall study healing rate (r=-0.932, p<0.001). The correlation between study healing rate and maceration at 2 weeks (r=–0.464, p=0.151) and 6 weeks (r=–0.567, p=0.143) was moderately strong but not statistically significant. No statistically significant relationships were detected between clinician assessment of maceration and categorical wound healing measures and statistically significant correlations were not detected between clinician erythema assessments and wound healing measures at any time point.
Discussion
This study addressed two deficits in the body of literature pertaining to DRFU assessment: firstly, the lack of evidence to support current periwound assessment practices; and secondly, the relationship between periwound skin condition and wound healing. The use of the objective SD202 skin-measuring device enabled evaluation of current periwound assessment and exploration of periwound hydration, melanin, lipid and erythema measures as predictors of wound healing.
Clinician and SD202 periwound assessments
Periwound skin assessed as macerated by clinicians obtained higher SD202 hydration readings than periwound skin that was not considered macerated. Consistently strong, positive correlations between clinician-assessed maceration and SD202 hydration measures were detected across all time points; however, statistical significance was reached at baseline and 2 weeks only. This finding suggests that visual appraisal alone may be a good indicator of periwound hydration properties. This, however, is contrary to reports in the literature, where it is thought that macerated skin can be overlooked or mistaken for fungal infections and newly epithelialised tissue33. In this study, the clinicians responsible for periwound assessment were experienced hospital podiatrists specialising in the assessment of foot wounds. While this study suggests that visual appraisal is adequate in gauging periwound hydration properties, results may not be generalisable to the wider clinician population; thus, further studies are needed to verify the validity and reliability of visual periwound hydration assessment with different assessors of varying experience or training.
In clinical practice, periwound erythema is assessed as an indicator of increased capillary haemoglobin concentration, which is an element of the pathophysiological response to various skin insults such as trauma and infection34. Clinically assessed erythema and SD202 erythema values were not significantly correlated at any time point, indicating that visual assessment of periwound erythema may not be an accurate gauge of actual periwound redness. The literature supports the study finding that subjective assessment of skin colour may lack reliability. A multitude of factors influence human colour perception, including room lighting and angle of observation25, as well as the observer’s emotional state, visual acuity and previous colour experience35. Individuals draw upon different references when distinguishing colours and even with coloured reference samples, visual assessment of skin that is not smooth and regularly pigmented lacks reliability36. Given the obvious limitations of subjective, visual colour assessment, the SD202 skin-measuring device may prove a valuable adjunct to current practice, enabling more reliable, objective measurement of periwound erythema. Supporting the inclusion of an objective measure of periwound erythema, the SD202 erythema measures were found in this study to be associated with indicators of wound healing.
In summary, results indicate that clinician assessment of maceration and SD202 hydration measures relate strongly, whereas visual erythema assessment and SD202 erythema values appear largely unrelated. While clinician assessment of maceration appears to be a good indicator of excess skin hydration, it is lacking in that the dry or desiccated periwound is not considered. The SD202 device may mitigate discrepancies between different clinicians’ assessments and in doing so improve treatment consistency, enhancing clinician agreement around management goals.
Clinician periwound assessment and DRFU healing
Further analysis was undertaken to explore the association between clinician periwound assessments and wound healing measures. Evidence suggests a macerated periwound is detrimental to wound healing11, a contention that is supported by the study findings; statistically significant negative correlations between clinician assessment of maceration and wound healing rates were detected at baseline and at 4 weeks. While study results for some time points indicate that macerated wounds may heal more slowly, as a whole these results lacked consistency; the strength of the relationship between maceration and wound healing was variable. The inconsistency may be explained in part by the categorical nature of the clinician’s assessment. Clinical periwound assessment indicated whether or not maceration was present, but did not reflect the extent of maceration. It would be expected that the greater the extent of the maceration, the greater the reduction in wound healing rate. The development of clinician periwound visual assessment scales may improve the reliability and validity of periwound hydration assessment.
Other clinician periwound observations (normal, fragile and callus) did not relate significantly to wound healing measures at any of the four assessment time points.
Overall, the results reflect that current clinical periwound assessment plays a negligible role in the prediction of wound healing. It is possible that with a refined assessment tool and adequate clinician training the ability of observational periwound assessment to predict wound healing may increase. Importantly, these results do not negate the importance of clinician appraisal of periwound parameters, as assessment of this nature is key in evaluating treatment responses, informing dressing selection and, in some cases, highlighting the need for offloading intervention.
SD202 periwound assessment and DRFU healing
The SD202 device was a novel application in the wound management field to enable objective measurement of the periwound skin parameters: hydration, lipids, melanin and erythema, which were assessed against wound healing measures to determine their value as predictors of wound healing.
SD202 hydration measures and wound healing rates were not significantly correlated at any other time point. The literature implies that periwound hydration and wound healing rates may be related in a curve linear manner as excess hydration of periwound skin can impair skin barrier function5,8,10-13 and a lack of periwound hydration can also impact skin function and potentially delay wound healing19. A relationship of this nature was not evident upon examination of relevant scatterplots. Further studies with a larger sample size are needed to explore the nature of relationship between periwound skin hydration and wound healing.
Results indicate that, compared to SD202 hydration measures, SD202 erythema values were more strongly and consistently associated with wound healing. A categorical (yes/no) measure of wound healing related significantly to SD202 erythema measures at 2 weeks and 4 weeks, reflecting that in DRFU that healed the periwound was generally more erythematous than in those that did not. Given that degree of erythema rises with increased capillary intake, diameter and blood flow (or haemoglobin concentration) it is plausible that higher SD202 erythema scores signified superior tissue perfusion and oxygenation with wound oxygenation paramount to wound healing37–39. Evidence has focused on the variability and duration of erythema, rather than the extent of periwound erythema as a predictor of wound healing in DRFU. It has been postulated that the DRFU healing process is impaired by repeated ischaemia and reperfusion manifesting as variable periwound erythema29. In our study, changes in periwound erythema were recorded; however, data were only available across all multiple time points for three wounds that healed, so comparisons of variability in wounds that healed versus those that did not was limited. While the study results reflect that the SD202 erythema measures and wound healing were related, the clinical significance or implications of periwound erythema for the wounds in the current study is unclear and erythema is multifactorial.
The study was unable to assess the relationship between skin lipids and wound healing, as the SD202 Sebumeter failed to detect lipids at any of the periwound sites tested. The plantar and dorsal surfaces of the foot lack sebaceous glands40 and removal of adhesive dressings can strip the periwound of lipids21,41. Also, studies have demonstrated that, compared to healthy controls, people with diabetes have a lower skin surface lipid content42. Together these factors may account for the inability of the SD202 device to detect lipids in DRFU periwound skin.
Limitations
There were limitations associated with equipment used in the study. The size of SD202 probes precluded examination of the skin surrounding interdigital wounds, which meant these wounds were excluded from the study. Literature suggests that the Visitrak device used in calculating wound area may lack accuracy when wounds are less than 2–2.5 cm2 in area43,44. Given that the average baseline study wound area was 2 cm2, wound area measurements and, therefore, wound healing rate calculations may have lacked precision.
The study was limited in its generalisability. Clinicians performing periwound assessments in this study may not be representative of the general clinician population. Clinicians were qualified and experienced podiatrists specialising in the assessment and management of foot wounds. Additionally, clinical periwound assessment was performed according to the podiatry department’s wound assessment form and may differ to assessment protocols used in other settings.
The small sample size imposed limitations on statistical analyses, reduced the chance of detecting important relationships and likely influenced the consistency of the results across time points. Due to the limited number of study participants, only simple linear correlations between periwound parameters and wound healing could be established. Other factors known to influence wound healing, for example: blood glucose control and co-morbidities — peripheral vascular disease and neuropathy — and different wound management, such as off-loading, were not controlled for or standardised in this study and, as such, the effects of these on the strength of correlations were not taken into account.
Conclusion
This study suggests that visual and objective assessments of periwound erythema are largely unrelated. Conversely, visual and objective hydration measures appear reasonably well correlated. Neither clinician nor SD202 periwound assessments are consistently and strongly associated with wound healing; however, elements of each of these methods of assessment — visual appraisal of hydration and SD202 evaluation of erythema — show promise as predictors of wound healing. Thus, based on study findings, it appears that a composite periwound assessment incorporating clinician assessment of maceration and objective erythema measures may best reflect the periwound condition as it relates to wound healing.
Further research is required to corroborate study findings and to advance an understanding of the relationship between DRFU periwound skin status and wound healing. It is recommended that subsequent studies incorporate larger samples, enabling more complex statistical analysis and identification of the unique contribution that quantitative periwound assessment can make to the prediction of wound healing. A larger sample size would also facilitate evaluation of the relationship between periwound erythema variability and wound healing. Future studies may focus on the development and validation of an approach to periwound assessment that harnesses the strengths of multiple modes of skin assessment, including clinician appraisal and objective skin-measurement devices.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
The authors thank the participants who contributed to this study and the Podiatry Department, Alfred Health, for their great support of individuals and this endeavour more broadly. Reviewers of this work are acknowledged for their advice that has enhanced the clarity and transparency of the research.
Author(s)
Alexandra Rowledge
BHlthSc, MPodPrac(Hons)
Discipline of Podiatry, College of Science,
Health and Engineering, La Trobe University, Bundoora, Vic 3086, Australia
Email Alexandra.Rowledge@svha.org.au
Nicoletta Frescos*
BAppSc(Pod), MPH
Discipline of Podiatry, College of Science,
Health and Engineering, La Trobe University, Bundoora, Vic 3086, Australia
Tel +61 3 9479 5832
Email n.frescos@latrobe.edu.au
Charne Miller
PhD
Alfred Clinical School, La Trobe University, Level 4, The Alfred Centre, 99 Commercial Road, Prahran, Vic 3181, Australia
Email c.miller@latrobe.edu.au
Elizabeth Perry
BAppSc(Pod)
Podiatry Department, The Alfred Hospital,
55 Commercial Road, Prahran, Vic 3181, Australia
Email l.perry@alfred.org.au
William McGuiness
PhD
Alfred Clinical School, La Trobe University, Level 4, The Alfred Centre, 99 Commercial Road, Prahran, Vic 3181, Australia
Alfred Health, 55 Commercial Road, Prahran,
Vic 3181, Australia
Email w.mcguiness@latrobe.edu.au
* Corresponding author
References
- National Institute for Health and Clinical Excellence (NICE). Diabetic foot problems: inpatient management of diabetic foot problems. Clinical Guideline 119. London: NICE; 2011.
- International Best Practice Guidelines: Wound Management in Diabetic Foot Ulcers. Wounds International 2013. Available from: www.woundsinternational.com.
- Ayello E. The TIME Principles of Wound Bed Preparation. Adv Skin Wound Care 2009;22(S1):S2–4.
- Keast DH, Bowering CK, Evans AW, Mackean GL, Burrows C, D’Souza L. MEASURE: A proposed assessment framework for developing best practice recommendations for wound assessment. Wound Repair Regen 2004;12(S1):S1–S17.
- Colwell JC, Ratliff CR, Goldberg M, Baharestani MM, Bliss DS, Gray M et al. MASD Part 3: Peristomal moisture-associated dermatitis and periwound moisture-associated dermatitis: A consensus. J Wound Ostomy Continence Nurs 2011;38(5):541–53.
- Dykes PJ, Heggie R, Hill SA. Effects of adhesive dressings on the stratum corneum of the skin. J Wound Care 2001;10(2):7–10.
- Tokumura F, Umekage K, Sado M, Otsuka S, Suda S, Taniguchi M et al. Skin irritation due to repetitive application of adhesive tape: the influence of adhesive strength and seasonal variability. Skin Res Technol 2005;11(2):102–6.
- Lawton S. Assessing and managing vulnerable periwound skin 2009. Available from: http://www.worldwidewounds.com/2009/October/Lawton-Langoen/vulnerable-skin-2.html#1.
- Cutting KF. Impact of adhesive surgical tape and wound dressings on the skin, with reference to skin stripping. J Wound Care 2008;17(4):157–62.
- Cutting KF, White RJ. Maceration of the skin and wound bed: its nature and causes. J Wound Care 2002;11(7):275–8.
- Rogers A, Watret L. Maceration and its effect on periwound margins. Diabet Foot 2003;6(3):S2.
- Voegeli D. Moisture-associated skin damage: an overview for community nurses. Br J Community Nurs 2013;18(1):6–12.
- Hollingworth H. Challenges in protecting periwound skin. Nurs Stand 2009;24(7):53–62.
- Jordan MM, Clark M. Report on incidence of pressure sore in the patient community of the Greater Glasgow Health Board Area. Glasgow: University of Strathclyde; 1977.
- Thyagoragan C, Silver JR. Aetiology of pressure sores in patients with spinal cord injury. Br Med J 1984;289:1487–9.
- Cochrane G. The Severely Disabled. In: Bader DL, editor. Pressure Sores — Clinical Practice and Scientific Approach. London: Macmillan; 1990.
- Edmonds ME, Foster A, Sanders LJ. A Practical Manual of Diabetic Foot Care. 2nd ed. Oxford UK: Blackwell Publishing Ltd; 2008.
- Baker N. Debridement of the Diabetic Foot: A Podiatric Perspective. Int J Lower Extrem Wounds 2002;1:87–92.
- Fore-Pfliger J. The epidermal skin barrier: Implications for the wound care practitioner, part II. Adv Skin Wound Care 2004;17(9):480–8.
- Fore-Pfliger J. The epidermal skin barrier: implications for the wound care practitioner, part I. Adv Skin Wound Care 2004;17(8):418–25.
- Smith KR, Thiboutot DM. Thematic review series: Skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res 2007;49:271–81.
- Reed JT, Ghadially R, Elias PM. Skin type, but neither race nor gender, influence epidermal permeability barrier function. Arch Dermatol 1995;131:1134–8.
- Burkhart CG, Burkhar CN. The mole theory: primary function of melanocytes and melanin may be antimicrobial defense and immunomodulation (not solar protection). Int J Dermatol 2004;44:340–2.
- Mackintosh JA. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J Theor Biol 2001;211:101–13.
- Kollias N. The physical basis of skin colour and its evaluation. Clin Dermatol 1995;13:361–7.
- Sibbald RG. Increased bacterial burden and infection: The story of NERDS and STONES. Adv Skin Wound Care 2007;19(8):462–3.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–43.
- Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers verses acute wounds. J Invest Dermatol 1998;111(5):850–7.
- Mustoe TA, O’Shaugnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Reconstr Surg 2006;117(S7):S35–S41.
- Serup J, Jemac GBE. Handbook of Non Invasive Methods and the Skin. Boca Raton: CRC Press; 1995.
- Takiwaki H, Serup J. Measurement of Erythema and Melanin Indices In: Serup J, Jemec GBE, editors. Handbook of Non Invasive Methods and the Skin. Boca Raton: CRC Press; 1995.
- Gethin G, Cowman S. Wound measurement comparing the use of acetate tracings and Visitrak™ digital planimetry. J Clin Nurs 2006;15(4)422–427
- Colwell JC, Ratliff CR, Goldberg M, Baharestani MM, Bliss DZ, Gray M et al. MASD part 3: peristomal moisture-associated dermatitis and periwound moisture-associated dermatitis: a consensus. J Wound Ostomy Continence Nurs 2011;38(5):541–553.
- Kollias N. The Physical Basis of Skin Colour and its Evaluation. Clin Dermatol 1995;13:361–7.
- McGuiness W, Dunn SV, Jones MJ. Developing an accurate system of measuring colour in a venous leg ulcer in order to assess healing. J Wound Care 2005;14(6):249–54.
- Pierard GE. EEMCO guidance for the assessment of skin colour. J Eur Acad Dermatol Venereol 1998;10(1):1–11.
- Bishop A. Role of oxygen in wound healing. J Wound Care 2008;17:399–402.
- Rodriguez PG, Felix FN, Woodley DT, David T, Shim EK. The role of oxygen in wound healing. Dermatol Surg 2008;34(9):1159–69.
- Stadelmann WK, Digenis AG, Tobin GR. Impediments to wound healing. Am J Surg 1998;176(S1):S39–S47.
- Wojcik A, Budzisz E, Rotsztejn H. Skin surface lipids and their measurements. Postepy Dermatol Alergol 2011;28(6):498.
- Zettersten EM, Ghadially R, Feingold KR, Elias PM. Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. J Am Acad Dermatol 1997;37:403–8.
- Seirafi H, Farsinejad K, Firooz K, Davoudi SM, Robati RM, Hoseini MS et al. Biophysical characteristic of skin in diabetes: a controlled study. Eur Acad Dermatol Venereol 2009;23:146–9.
- Foltynski P, Ladyzynski P, Sabalinska S, Wojcicki JM. Accuracy and Precision of Selected Wound Area Measurement Methods in Diabetic Foot Ulceration. Diabetes Technol Ther 2013;15(8):711–20.
- Shaw J, Hughes CM, Lagan KM, Bell PM, Stevenson MR. An evaluation of three wound measurement techniques in diabetic foot wounds. Diabetes Care 2007;30(10):2641–2.



