Volume 24 Number 3
The impact of exposure time on biophysical parameters of the wound environment and patient comfort during dressing changes: a descriptive study
Tamara Page, Judy Magarey and Rick Wiechula
Keywords wound, dressing, temperature, TEWL, pH.
Abstract
Wound healing is a complex milieu that affects millions of people around the world every day. This descriptive correlational study investigated the impact of prolonged exposure through delays in dressing changes, on the biophysical wound bed parameters (wound temperature, transepidermal water loss (TEWL) and pH) and possible contamination of the wound. In addition, the effect delays have on patient pain, comfort and activities of daily living (ADLs), were investigated.
The results identified that the participants’ wounds were hypothermic as well as alkaline on dressing removal and throughout the period of exposure. The mean wound temperature increased throughout the total duration of the down time, which was contrary to expectation, although despite this, all wounds remained hypothermic and the wounds became more alkaline (p=0.0079). Agar plates placed in proximity to the exposed wounds grew pathogens, which could potentially contaminate the wound and participants were unable to perform some ADLs.
The impact of delayed wound dressing changes on the patient’s ADLs and pain are important in patient-centred care and the poor state of the wounds’ microenvironment immediately following removal of the dressing needs further investigation.
Introduction
The skin protects the body from possible mechanical, physical and chemical injury from the external environment and maintains the internal haemostatic environment. Maintaining skin integrity is paramount to it being able to carry out its major functions of protection, thermoregulation, sensation, metabolism and communication1.
The make-up of the skin, its functions and the sequence of events which occur when it is wounded are central to healing. A new wound initially classified as an ‘acute’ wound may be closed with sutures, tape or staples; hence stated as healing by primary intention; whereas wounds that are not able to be directly closed, heal by secondary intention1.
Acute wounds normally follow a sequence of events in response to injury; however, if there is an interruption to any phase and a subsequent delay in healing, the wounds are classified as ‘chronic’. Each wound will have its own distinct characteristics and key attributes, which, in addition, could impact on healing.
Wound healing is a complex milieu that affects millions of people around the world every day. Reports indicate that 433,000 Australians, 6.5 million Americans, and between 3.55 and 4.5 per 1000 people in countries such as the United Kingdom and India suffer from chronic wounds2-3. The burden of chronic wounds is an ever-growing issue due to the ageing population and increasing associated co-morbidities and health care costs2-3.
There are a range of wound bed parameters including; temperature, transepidermal water loss (TEWL) and pH that have been identified as important to maintain within an optimal range to aid healing (Table 1). The literature (derived mainly from animal research) considers a temperature above 36°C to be optimum for healing with a temperature below 33°C deeming epithelialisation absent4-6. There are numerous studies that have reported the benefits of a warm, moist environment, which allows newly formed skin cells to move freely across the wound bed, accelerating healing time7-9.
Table 1: Suggested temperature, pH and TEWL parameters
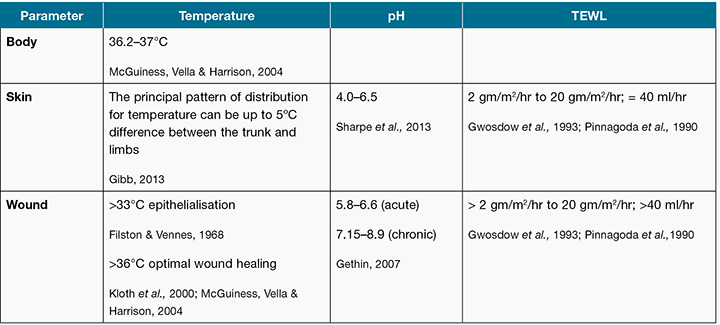
Cells and enzymes function optimally in a moist environment at normal body temperature; hence a loss of skin integrity or development of a wound enables TEWL via evaporation to increase and convection, which, in turn, cools the tissue10-11. The normal skin range of TEWL is reported to be anywhere from 2 gm/m2/hr to 20 gm/m2/hr; this is equivalent to less than 40 ml/hr10-11.
Intact skin releases sebum, which provides an acidic coating with a pH of between 4 and 6.5. This slightly acidic and natural antibacterial substance provides protective properties, which retards the growth of micro-organisms and promotes epithelial growth12-13. When the skin is wounded and initially debrided, the pH increases and the wound tends to be neutral or somewhat alkaline with wound surface pH reported as ranging from 5.8 to 6.613. Gethin further delineates between acute and chronic wounds, stating that chronic wounds have a pH of 7.15 to 8.912. As wound epithelialisation is associated with a decrease in pH towards an acidic level, it can be proposed that enabling the surface pH of a chronic wound to be more acidic is beneficial to wound healing12-13.
Advancements in contemporary wound dressing products have resulted in products which actively contribute to the wound healing process as many are designed to maintain wound bed parameters and assist in providing an optimum healing environment14. Dressing materials that maintain a wound at or near body temperature are associated with significantly higher mitotic activity, with the number of dividing cells increased by 108%15; therefore an optimal environment will accelerate healing and promote tissue growth.
There are a number of wound dressing products which are described throughout the literature as having the ability to decrease the wound surface pH, with a mild antibacterial effect as acidic values are reached13,16. Gethin, Cowman and Conroy reported a decrease in wound size as the wound became more acidic following the application of honey which had a pH of 3.5 units17.
The type of dressing chosen by the practitioner can influence the wound bed parameters, thermoregulatory and protective functions normally provided by the skin. Each wound dressing also has its own characteristics that influence choice, including the frequency of the dressing change.
The frequency of a dressing change is an important factor in the maintenance of the wounds’ biophysical parameters, as a temperature decrease of 2ºC is sufficient to affect biological processes4,18,19. In vitro studies have concluded that 33ºC is the critical level at which neutrophil, fibroblast and epithelial cell activity decreases19. It has been reported that following removal of a dressing for wound cleansing and assessment, that leukocytes only regain their normal mitotic activity after four hours, and that wound temperature can take up to four hours to return to normal15.
The temperature recovery time reported above is similar to that reported by McGuiness, Vella and Harrison19, where it is stated that the average recovery time of wound temperature following a wound dressing change was only 23 minutes; however, the maximum time recorded was over three hours19. The average dressing change in their study took 11 minutes, which would imply that there was no delay in redressing the wound, that is, the dressing was removed from the wound, the wound cleaned and then the wound redressed.
However, in contrast, a study by Page found the average length of time it took to complete a wound dressing change was 103 minutes20. There were significant delays in many of the wound dressing changes, as the wound dressings were removed to allow an assessment of the wound by health professionals and then they were redressed following the review20. The combination of the frequency of dressing changes and the time taken to redress the wound may delay wound healing20.
The impact of the wound dressing change on these biophysical wound bed parameters was identified as a gap in the literature and hence the focal point of this study. In addition, potential contamination of the wound and the impact of the wound dressing changes on patient pain, comfort and activities of daily living (ADLs), important aspects of the holistic approach to patient care, were also considered.
Method
The study was conducted in a South Australian tertiary institution, in non-critical care units, with adult patients who had an open wound requiring assessment, in line with a dressing change. Ethical approval was obtained from the Human Research Ethics Committee and the instruments utilised within the data collection process were validated to ensure reliability of the data collected. The data collection were quite complex with repeated measures needing to be taken on each participant (Figure 1).
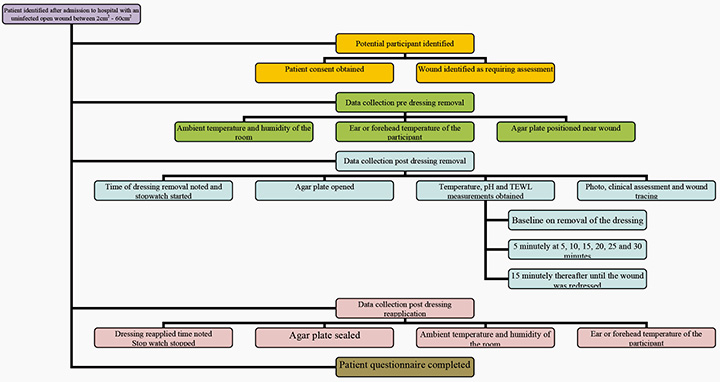
Figure 1
Descriptive statistics were used for patient demographic data and frequency distributions to organise the data and show how it was distributed amongst the different variables. Generalised estimating equations (GEE) were used to analyse this correlated data. Outcome variables that are normally distributed can be modelled and analysed using a ‘linear’ GEE. Outcome variables which were not normally distributed nor had a normally distributed logarithmic function required a ‘logistic’ GEE model. A P value of <0.05 was considered to be statistically significant.
Results
A total of 12 participants from the 20 prospective participants consented to be included in the study. The other eight participants initially recruited were excluded after consent was obtained as the inclusion criteria (prolonged down time) were not achieved.
The participants’ ages ranged from 21 to 77 years, with an equal number of male and female participants, all Caucasian. Their wounds were located in various anatomical locations, with six participants having wounds located on the lower legs, three on the abdomen, one on the hip, one on the heel and one in the groin (Table 2). Of the 12 participants, three had no co-morbidities. Of the remaining nine a number of co-morbidities were documented which may impact on the wound healing process: namely circulatory disease; respiratory disease; and oncologic-related disease.
Table 2: Participant demographics
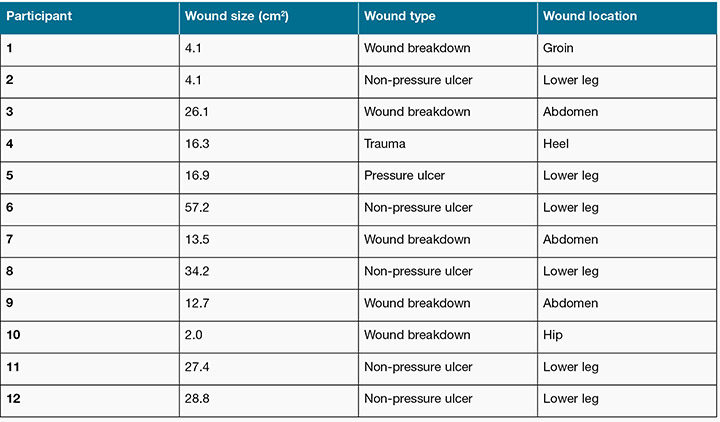
The participants presented with four types of wounds: wound breakdown (n=5); non-pressure ulcers (n=5); pressure ulcer (n=1); and traumatic wound (n=1) with the wound surface area ranging from 2 cm2 to 57.2 cm2. The researcher measured the time in minutes that the wound was ‘exposed’ (the time the primary dressing was removed from the participants’ wound and the temporary cover applied to the time the primary dressing was reapplied to the wound). The time in minutes was then described as the ‘down time’, and ranged from 22 to 209 minutes (mean 123 minutes (SD 63)).
The individual results for wound temperature, TEWL and pH were collected every five minutes for the first 30 minutes and thereafter every 15 minutes until the wound was redressed. The minimum number of measurements taken on any one participant was five (20 minute duration), with the most measurements being 17 (209 minute duration). A total of 145 measurements were collected from the 12 participants collectively.
Temperature
The temperature for each of the participant’s wounds for the length of exposure at each of the designated time periods is shown in Figure 2; with the minimum temperature recorded 27.2°C for Participant 7 and the maximum 36°C for Participant 1. The changes in temperature were small; however, the results are clinically important as 83% (n=10) of the participants’ wounds at the baseline measurement remained well below the critical 33°C required for mitotic activity to assist the healing process, with 100% of participants having a wound temperature less than 36°C.
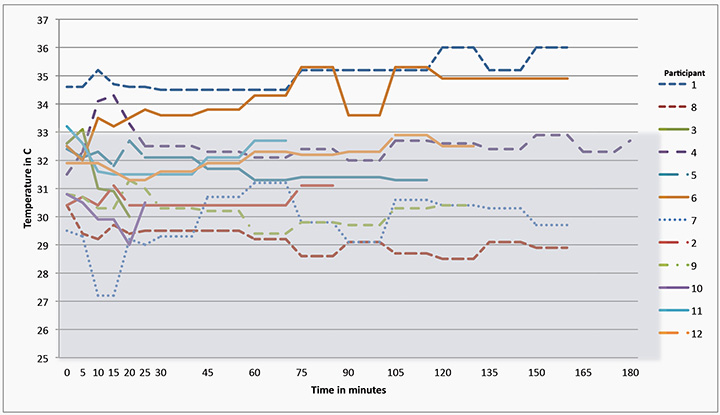
Figure 2: Participants’ wound temperatures throughout the data collection process
After 20 minutes only two participants’ wound temperatures were above 33°C, with one achieving a temperature of 36°C at two time points. Wound temperatures fluctuated in 91% (n=11) of participants throughout the time they were without their primary dressing, with a slight overall mean temperature increase of 0.24°C.
TEWL
The measurement of the TEWL for each of the participant’s wounds for the length of exposure at each of the designated time points is shown in Figure 3; with the minimum TEWL recorded as six units for Participant 3. All wounds had a measurable TEWL at all data collection time points, with 67% of participants’ wounds having the maximum TEWL of 20 units at dressing removal and 33% of participants’ wounds losing the maximum (20 units) moisture throughout the downtime of the wound. A third of the participants’ wounds had a decreased rate of moisture loss throughout the downtime of the wound.
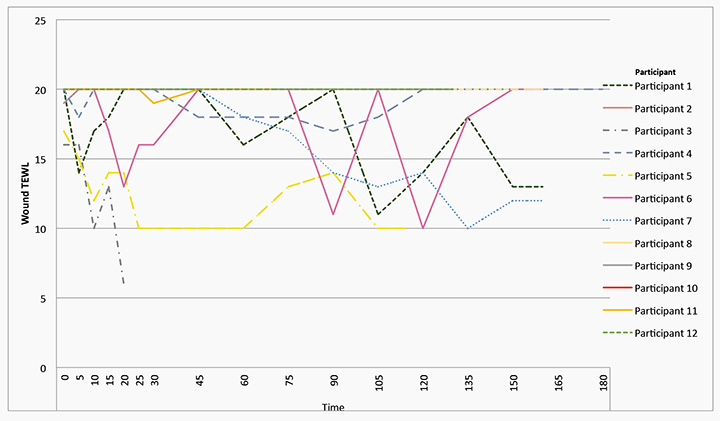
Figure 3: Graphical representation of all participants’ wound TEWL
pH
The measurement of the pH for each of the participant’s wounds for the length of exposure at each of the designated time periods is shown in Figure 4. The most acidic pH recorded was 6.71 for Participant 6 and the most alkaline was 9.3 for Participant 2. Figure 4 also demonstrates the range of wound pH (5.8–6.6) which can be expected to retard the growth of micro-organisms and promote epithelial growth. All participants’ wounds were alkaline at baseline, with 83% of participants’ wounds becoming more alkaline the longer they were exposed without their primary dressing (mean 8.25, SD 0.66). There was a significant association found between pH of wound and wound exposure in the first 20 minutes of exposure (logistic GEE model accounting for repeated measures over time: P value=0.0079). For every increase of one minute in wound exposure time, the odds of having a pH>8.5 is 12% greater (odds ratio=1.12, 95% confidence interval: 1.03, 1.21).
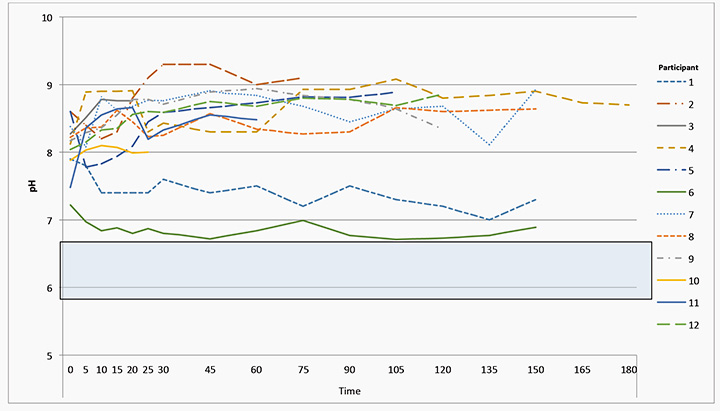
Figure 4: Graphical representation of all participants’ wound surface pH
Comfort
Participants were asked to identify if the wound assessment process had impacted on any of their ADLs. Toileting was the most common of these impacted upon by the delay in the wound dressing change (46%); with only one participating stating their ADLS were not impacted upon (9%). Analgesia was only offered in 30% of cases, with some participants requesting analgesia after the procedure had commenced.
Contamination
Potential contamination of the wound while the primary dressing was not in place was assessed by situating agar plates within close proximity of the wound. The agar plates were sent for analysis at the state pathology service, with three participants (27%) having Aspergillus fumigatus detected and 10 participants (91%) mixed non-pathogens during the time the wounds were exposed.
Conclusion
Analysis of the data on the impact of the wound assessment process on the wound bed parameters: temperature, TEWL and pH demonstrated three main points. All wounds were hypothermic at baseline (below 36°C) with 10 of the 12 wounds below the critical temperature of 33°C deemed necessary for epithelial growth and this continued throughout the time the wounds were without their primary dressings5-7. All wounds had maximum TEWL measurements at baseline, indicating either a strained barrier or critical moisture loss, with 67% continuing to have critical moisture loss and the other 33% having a reduced moisture loss; therefore impacting on the attainment of a moist wound environment that enables healing15. All of the wounds were alkaline at baseline (above 7.0) with 83% increasing in alkalinity throughout the time the wounds were without their primary dressings, and as all wounds were alkaline the natural antibacterial protective properties that retard the growth of micro-organisms and promote epithelial growth were diminished1,13.
The impact of the wound assessment procedure on the patients demonstrated that they were unable to attend to their nutritional, toileting and hygiene needs; in addition the offer and administration of adequate analgesia was not conducted as per best practice. The environmental assessment found a number of organisms present in the environment that could have contaminated the wounds throughout the assessment procedure. It was beyond the capacity of the study to follow participants to establish if their wounds became infected.
Limitations
A power analysis using data from a previous study which reviewed wound temperatures during the dressing change was used to determine the sample size required19. A sample size of 12 was determined to have 90% power to detect a difference in means of 2.7°C, assuming a standard deviation of 2.53°C. The power analysis was not conducted using the other wound bed parameters as there were no additional studies reporting pH and TEWL data. The small numbers of participants therefore require some of the results to be viewed with due caution. The variables with multiple measures such as temperature had sufficient power; however, where the data were stratified for wound types, type of temporary dressing and size of the wound this was not the case. Thus results relating to these parameters must be considered with caution and further research is required to investigate any possible relationships.
Recommendations for practice
The recommendations for practice are based on consideration of three main issues arising from the results. The first is the suboptimal condition of the wound at baseline, the second is the deteriorating condition of the wound with exposure and finally the negative impact on the patient in regard to their ADL and pain/comfort.
There is cause for concern as the wounds in this study were found to be hypothermic and alkaline on removal of the primary dressing. In contemporary wound management the choice of dressing is primarily dictated by the need to maintain an appropriate moisture level. Although different primary dressings will have diverse thermoregulatory qualities these are often secondary concerns.
While it may not be possible to use an alternative primary dressing there are options with regard to the secondary dressing used. For example, the addition of a simple combination dressing held in place by a crepe bandage may assist in keeping the wound surface temperature at an appropriate level. There are dressings that by their nature promote a more acidic environment; however, they must also have the appropriate moisture management qualities.
The next issue to consider is the use of a temporary cover during wound assessment. Clinicians should plan for dressings to be removed so that the timing and duration results in the minimum amount of exposure; however, there is always the potential for delay in assessment. Covering the wound with a temporary dressing is always necessary. Ideally the covering needs to provide a reasonable seal and have some thermal qualities while still being able to be removed quickly to examine the wound bed without discomfort to the patient. There are some options that could assist with this, such as when removing the dressing prior to assessment, only removing the outer dressing or bandage and leaving the primary dressing in situ until the medical team arrives to review the wound. This may allow for a more accurate assessment of the wound bed parameters and the ability of the chosen primary dressing in maintaining thermoregulation, moisture and acidity of the wound bed. However, the complexity of wound care and the abundance of product combinations and applications may not facilitate this. This does not address the patient impact either in regard to appropriate analgesia and ability to attend to ADLs.
Alternatively, other organisations have progressed to routinely applying plastic wraps to wounds once the primary dressing has been removed for assessment. There have been concerns about the sterility of plastic wrap use; however, research by Heinle and Clopton indicated 39% of samples had no bacterial growth and 81% of samples had three or fewer colonies of typical flora normally found on the human skin21. Additional research has been conducted more recently by Liao et al. with no clinically significant micro-organism growth found on the samples tested; reaffirming the potential for infection as extremely low22.
Another concern expressed regarding the use of plastic wrap is its potential toxicity due to the use of certain plasticisers; however, Heinle indicated that a patient’s exposure to the plastic wrap from a wound dressing is only for a brief period of time, lasting a few hours at most, so the risk would be minimal21.
The final major issue that needs to be considered is the time and timing of the dressing removal and assessment. Wounds need to be assessed to ensure achievement of identified outcomes; whether it is for healing or maintenance care and ensuring suitable wound management continues, such as the appropriate choice of a dressing9. Wound care requires regular monitoring through assessment and the only way to undertake this is for the dressing to be removed23. The concern with this process is the timing and duration of the assessment when the patient is an inpatient or attends the outpatient department.
It was noted at the commencement of the data collection that wound dressings were routinely removed at approximately 0715 to ensure wounds were ready to be assessed by the time the medical team reviewed the patient on a particular ward round. This timing of the dressing removal changed to 0600 during the course of the study. The rationale provided by the nursing staff was to minimise the ‘rushed’ workload following handover. This decision did not appear to take into account the impact on the patient or the patients’ wounds and would also increase the exposure time with subsequent impact on the wound microenvironment20,24-27. In addition, this situation illustrates a lack of involvement of the patients in decisions about their care26.
No doubt the timing of the wound assessment can be a complex affair. Medical staff will have only certain times of the day that they are available to review wounds. They will be committed to operating lists and outpatient clinics. The nursing staff will be providing care that is not directly related to wounds. A recent study recommended that medical ward rounds should be conducted reasonably early in the morning to plan the necessary care for the day27. This timing, however, should take into account the ADLs to reduce the impact of the wound assessment process on the patient being able to eat, toilet and bathe, whilst at the same time accounting for procedures within the environment.
Consideration should be given to rostering, workflow and staff allocation. If wounds do need to be assessed on morning ward rounds then the staff should be managed to insure the least disruption to the patients’ ADLs. Patient allocation to nursing staff could allow dressing activities to be staggered or shared between the nursing staff rostered on for the shift or alternatively having additional nursing resources available to assist with the wound dressing rounds, which may reduce the time of wound exposure.
The timing of analgesia also needs to be considered prior to the wound assessment process, with analgesia being offered 30 minutes prior to the dressing removal, ensuring staff adhere to evidence-based guidelines related to analgesia administration. If the initial offer was declined, ensuring repeated offers are also made as required throughout the procedure.
There should also be consideration of how often wounds need to be viewed. An ongoing issue for wound management is accurate and detailed documentation of the wound28. Advances in clinical photography could assist here. There is an abundance of literature on the use of wound surface photography; however, this has its own challenges, which may lead to misinterpretation of the condition of the wound28,29. Patients are required to consent to photography, and a number of factors that can impact on the quality and consistency of the image including the distance, background, lighting, focus and exposure28,29. Despite this there is considerable potential in using photography and telehealth to reduce the number of times the wound needs to be observed and subsequently the duration of exposure without a primary dressing, as identified in the AWMA Telehealth Framework document30.
The planning of care including administering pain relief, ensuring medical officers attend in a timely fashion to assess the wound and the nurse removing the primary dressing to cause the least disruption to the patient will always be difficult. To reduce the amount of time that wounds are exposed this challenge needs to be accepted.
Acknowledgements
Thank you to the nursing staff and participants involved in the study.
Author(s)
Tamara Page*
RN, BN, GradDip(HighDep), MNSc,
GradDipEd(HighEd), PhD
University of Adelaide, SA, Australia
Tel 0408 812 307
Email tamara.page@adelaide.edu.au
Judy Magarey
RN, BNurs, MN(Research), DNurs
A/Professor, University of Adelaide, SA, Australia
Rick Wiechula
RN, BA, DNurs
University of Adelaide, SA, Australia
* Corresponding author
References
- Gibb M. Skin integrity and wound care. In: Crisp J, Taylor C, Douglas C, Rebeiro G, editors. Potter & Perry’s Fundamentals of Nursing. 4th ed. Australia: Elsevier; 2013.
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17(6):763–71.
- Macdonald J, Kingsley A. WAWLC: World Alliance for Wound and Lymphedema Care. Wounds 2010;22(3):55–9.
- Esclamado RM, Damiano GA, Cummings CW. Effect of local hypothermia on early wound repair. Arch Otolaryngol Head Neck Surg 1990;116(7):803–8.
- Filston H, Vennes GJ, Jr. Temperature as a factor in wound healing. Surg Gynecol Obstet 1968 Mar;126(3):572–84.
- Kokate JY, Leland KJ, Held AM, Hansen GL, Kveen GL, Johnson BA et al. Temperature-modulated pressure ulcers: a porcine model. Arch Phys Med Rehabil 1995;76(7):666–73.
- Winter G, Scales J. Effect of air drying and dressings on the surface of a wound. Nature 1963;197:91–2.
- Koupil J, Brychta P, Horky D, Smola J, Prasek J. The influence of moisture wound healing on the incidence of bacterial infection and histological changes in healthy human skin after treatment of interactive dressings. Acta Chir Plast 2003;45(3):89–94.
- Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(2):S1–28.
- Gwosdow AR, Cunningham JJ, Lydon M, Rascati R, Berglund LG. Evaporative water losses through a temporary wound dressing under simulated wound conditions. J Burn Care Rehabil 1993;14(4):450–4.
- Pinnagoda J, Tupker R, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. Contact Dermatitis 1990;22:164–78.
- Gethin GT. The significance of surface pH in chronic wounds. Wounds UK 2007;3(3):52–6.
- Sharpe JR, Booth S, Jubin K, Jordan NR, Lawrence-Watt DJ, Dheansa BS. Progression of wound pH during the course of healing in burns. J Burn Care Res 2013;34(3):201–8.
- Cutting K. Wound dressings: 21st century performance requirements. J Wound Care 2010;Suppl:1–9.
- Lock P, editor. The effects of temperature on mitotic activity at the edge of experimental wounds. Wound Healing Plastic, Surgical and Dermatological Aspects: Symposium — Papers and Discussion. Finland: ESPOO; 1979.
- Romanelli M, Schipani E, Piaggesi A, Barachini P. Evaluation of surface pH on Venous Leg Ulcers under Allevyn Dressings. London: The Royal Society of Medicine Press; 1997.
- Gethin GT, Cowman S, Conroy RM. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int Wound J 2008;5(2):185–94.
- Kloth LC, Berman JE, Dumit-Minkel S, Sutton CH, Papanek PE, Wurzel J. Effects of a normothermic dressing on pressure ulcer healing. Adv Skin Wound Care 2000;13(2):69–74.
- McGuiness W, Vella E, Harrison D. Influence of dressing changes on wound temperature. J Wound Care 2004;13(9):383–5.
- Page T, McCutcheon H. Indecent exposure: a descriptive study of wound exposure times associated with dressing changes. Primary Intention 2004;12:170–9.
- Heinle J, Clopton E. Plastic Wrap is a Warm, Comfortable, Economical Pre-hospital Dressing for Burn Wounds. J Burn Care Res 2001;22:S172.
- Liao A, Andresen D, Martin H, Harvey J, Holland A. The infection risk of plastic wrap as an acute burns dressing. Burns 2014;40(3):443–5.
- Keast D, Bowering K, Evans W, Mackean G, Burrows C, D’Souza L. Measure: A proposed assessment framework for developing best practice recommendations for wound assessment. Wound Repair Regen 2004;12(3S):1–17.
- Harding K. How long can a wound be left exposed between dressing changes? J Wound Care 2000;10(5):146–7.
- Gimbel N, Farris W. The influence of surface temperature in the epithelialisation rate of split thickness donor sites. Arch Surg 1966;92:554–65.
- West E, Barron DN, Reeves R. Overcoming the barriers to patient-centred care: time, tools and training. J Clin Nurs 2005 Apr;14(4):435–43.
- Rowlands C, Griffiths S, Blencowe N, Brown A, Hollowood A, Hornby S et al. Surgical ward rounds in England: a trainee-led multi-centre study of current practice. Patient Saf Surg 2014;8(11):5.
- Ahn C, Salcido R. Advances in wound photography and assessment methods. Adv Skin Wound Care 2008;21(2):85–93.
- Hamilton A. Digital photography in wound management. In: WoundsWest, editor: Government of Western Australia, Department of Health 2010.
- Education and Professional Development Subcommittee (EPDSC). Telehealth Framework Document. Australian Wound Management Association; 2013.



