Volume 24 Number 3
The characteristics of wound pain associated with diabetes-related foot ulcers: a pilot study
Ashlea M Dickinson, Nicoletta Frescos, Julia C Firth and Peter S Hamblin
Keywords Chronic pain; diabetes complications; diabetic foot; foot ulcer; pain measurement;
Abstract
There is increasing evidence that people with diabetes-related foot ulcers (DRFU) can experience pain related to their wound. A cross-sectional pilot study was conducted to examine and compare the prevalence, intensity and nature of DRFU pain in people with neuropathic, neuroischaemic and ischaemic wound aetiologies. A questionnaire incorporating pain assessment tools, the Short-form McGill Pain Questionnaire and the Short-form Brief Pain Inventory was used to interview 15 patients with DRFU. Descriptive analyses were conducted. The mean age was 64 years, 60% had neuropathic ulcers and 40% had neuroischaemic ulcers. No ischaemic DRFU were observed in the study sample. Formal assessment tools had a higher reported pain prevalence (53%) compared with a single question asked by the researcher (33%). Low scores for pain intensity and effect of pain on health-related quality of life were reported for both aetiologies. This study indicates people with DRFU can experience wound pain, despite analgesia usage. It also highlights that clinical pain assessment and management techniques appear inadequate. A larger study is warranted to investigate characteristics of DRFU pain, to determine if statistical differences in pain experiences exist between wound aetiologies and to develop guidelines for assessment and management of wound pain.
Introduction
Wound-related pain is often underestimated and undertreated in people with diabetes-related foot ulcers (DRFU), resulting in poor treatment outcomes. Many health professionals have believed that people with neuropathy do not experience pain1. There is little research in this area, yet the consequences of misdiagnosing or ignoring the pain associated with DRFU is perilous. DRFU pain has often been attributed to ischaemia, infection and/or Charcot arthropathy; whilst the presence of peripheral neuropathy is considered by many clinicians to be not associated with wound-specific pain1. Recent prevalence studies have questioned this, finding that up to 86% of patients with peripheral neuropathy reported wound-specific pain2-5. Furthermore, these studies report much higher pain prevalence rates in DRFU when using formal assessment tools rather than clinician questioning5.
Wound pain has detrimental consequences to an individual’s health-related quality of life (HRQoL) and is associated with depression, elevated stress, a diminished ability to perform activities of daily living, relationship issues and fatigue6. Recently many studies have investigated the association between chronic pain and delayed wound healing. While the causal relationship still remains unclear, elevated stress levels, depression, anxiety and pain all have been significantly associated with longer healing times in chronic wounds and poorer overall healing outcomes7. Persistent, recalcitrant ulceration not only has been associated with progressively negative effects on HRQoL but it also increases the risk of infection and amputation in DRFU8,9. Therefore, assessment of pain should be a key component in providing holistic wound management.
A literature review of the Cochrane Library, CINAHL, EMBASE and PubMed using the keywords diabetes, foot, wound, pain, neuropathies and ischaemia was completed. From these databases, only four studies were retrieved which investigated the presence of wound-specific pain with findings that individuals with DRFU can experience wound pain2-5. Overall, the methodologies in these studies were heterogeneous, particularly with regard to the choice of pain assessment tools. The study by Ribu et al.2 focused on the effect of DRFU pain on HRQoL; however, they did not categorise ulcers into aetiological types. Similarly, Obilor et al.3 focused on DRFU pain on QoL but used an adapted, non-validated wound-related pain questionnaire. Bradbury and Price4 questioned the validity of their findings due to the use of a non-validated modified assessment tool, while the study by Bengtsson et al.5 used a non-validated questionnaire with multiple interviewers. The inclusion of confounding factors osteomyelitis and Charcot osteoarthropathy in two out of three of these studies also makes it difficult to determine the independent effect of neuropathic or neuroischaemic foot ulcers or DRFU on pain experience2,4. However, all of these studies found that people with neuropathic wounds or neuroischaemic wounds experienced wound-related pain, indicating that a much higher prevalence of DRFU pain is evident than previously considered.
Presently, no studies have shown in rigorous clinical or statistical analyses whether there is a significant difference in pain experience between neuropathic, neuroischaemic and ischaemic wound aetiologies. It is important to be able to identify whether the pain is of neuropathic, ischaemic or nociceptive origin, as this will alter management practices of DRFU pain. Determining the underlying aetiology of the presence of pain enables better pain management which has implications for improving wound healing times as well as patient HRQoL10.
This pilot study aimed firstly to determine the prevalence of wound pain in DRFU, and secondly to assess whether there was a difference in the intensity and nature of DRFU pain in people with neuropathic, neuroischaemic and ischaemic wound aetiologies.
Methods
Study design
This was a descriptive cross-sectional study that used convenience sampling to recruit participants referred to a multidisciplinary Diabetes Foot Service within a major hospital over a three-month period. The study data was collected using a structured questionnaire. A draft questionnaire was piloted prior to commencing data collection with minor amendments being made. The questionnaire elicited basic demographic information, wound characteristics, pain assessments and pain management.
All inpatients and outpatients (n=80) who were referred to the Diabetes Foot Service were screened for eligibility.
Inclusion criteria were: patients ≥18 years of age with a diagnosis of either type 1 or type 2 diabetes mellitus confirmed by medical records. Participants also were required to have up to three DRFU, defined as a below ankle wound which penetrates through the dermis in an individual with diabetes11.
Exclusion criteria: individuals with a cognitive impairment confirmed by medical records or another morbidity which restricted them from being able to provide informed consent as well as verbally articulate their pain. Patients with greater than three foot ulcers were excluded due to possible associated health complications which could detract from the validity of the study.
As there is a current lack of cross-culturally valid assessment tools, individuals who were unable to speak English fluently were also ineligible to participate in this study. The focus of this study was to compare differences between neuropathic, neuroischaemic and ischaemic foot wounds; therefore, healed wounds as well as other possible confounding factors of wound pain including cutaneous infection, gout, confirmed osteomyelitis or an active Charcot foot were excluded from this study as well.
The presence of infection was determined from the medical file. An independent podiatrist used the PEDIS classification system, which stands for perfusion, extent, depth, infection and sensation, to identify the presence of infection by clinical signs and symptoms. This validated classification system was developed by the International Working Group on the Diabetic Foot12. Culture, x-ray and magnetic resonance imaging results were also used to determine infection presence.
Eligible consenting participants were interviewed prior to their multidisciplinary assessment using a structured questionnaire. The researcher was blinded to neurological and vascular assessment results until after the interview.
Variables and measures
Foot ulcer status data, which included longest duration of ulcer, ulcer location(s), ulcer aetiology and category, were collected from the participant’s medical file. Foot ulcers were graded using the University of Texas Wound Classification System (UTWCS) based on National Health and Medical Research Council recommendations as it is a well-validated tool for use13,14.
Wound aetiology categories of neuropathic, neuroischaemic and ischaemic wounds were determined by vascular and neurological tests conducted by an independent podiatrist during each participant’s initial appointment with the Diabetes Foot Service. Participants were tested with a 10 g monofilament at three plantar sites on the foot of: hallux, first metatarsal head, and fifth metatarsal head. A participant was considered to have peripheral neuropathy when two out of the three sites were undetected or they were unable to detect the vibration of a 128 Hz tuning fork on the first toe11. The presence of peripheral vascular disease was determined firstly by angiography if one had been performed within the preceding three months. Angiography is considered the gold standard for diagnosing peripheral vascular disease15. Duplex ultrasound results, Ankle Brachial Pressure Index (ABPI) or toe pressures were used secondarily to indicate ischaemia, if no angiogram had been performed within this period. An ABPI< 0.9 or toe pressures < 30 mmHg was indicative of ischaemia15.
Pain experience data was collected using the Short-form McGill Pain Questionnaire (SF-MPQ)16 which measures quality and intensity of pain and the Short-form Brief Pain Inventory (SF-BPI)17 which measures impact on Health Related Quality of Life (HRQoL). These assessment tools have been validated for similar populations and were used to gather data on pain intensity and nature as well as effect on HRQoL17-21. Pain management data was collected on use of analgesics from the clinical records.
Data analysis
De-identified data was entered into IBM SPSS Statistics Version 21. Due to the nature of the pilot study, statistical tests were unable to be performed. Descriptive statistics were completed on all study variables to obtain the frequency and range of categorical variables as well as the mean and standard deviation of continuous variables. Where continuous data was not of normal distribution, median and range were obtained. Patient characteristics, foot ulcer status and pain experience were tabulated and compared between the aetiological populations. The SF-MPQ and SF-BPI were analysed using these tools’ own prescribed analytical methods16,17.
Ethical considerations
Ethical approvals were gained from one university (FHEC13/027), and one health care institutional (HREC/12/WH/173) committee. Informed consent was obtained from all participants.
Results
Sample
A total of 80 patients were screened for eligibility to participate in this study. Of those who met the eligibility criteria a total of 15 participants were recruited. The participant recruitment is outlined in Figure 1. Age ranged between 49 and 83 years, with a mean age of 64.1 ± 10.4 years. Participant characteristics are displayed in Table 1. Due to data not being recorded in medical files or being outdated, glycaemic control (HbA1c) and body mass index was excluded from the study results.
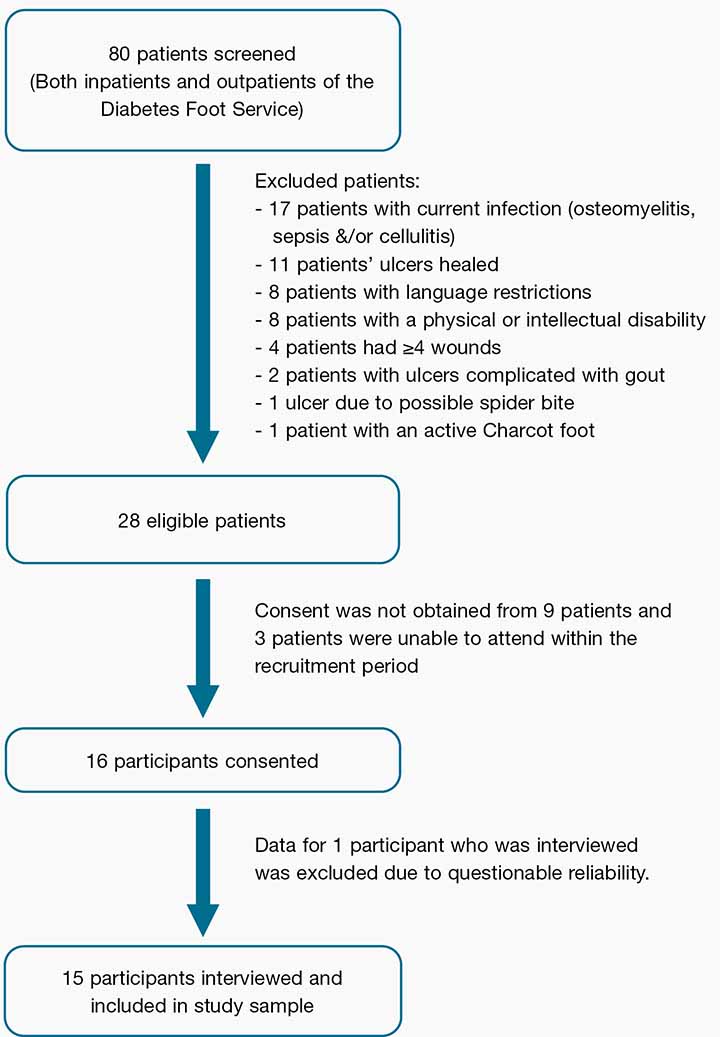
Figure 1: A flowchart of the participant recruitment
Table 1: Characteristics of patients with neuropathic DRFU versus neuroischaemic DRFU
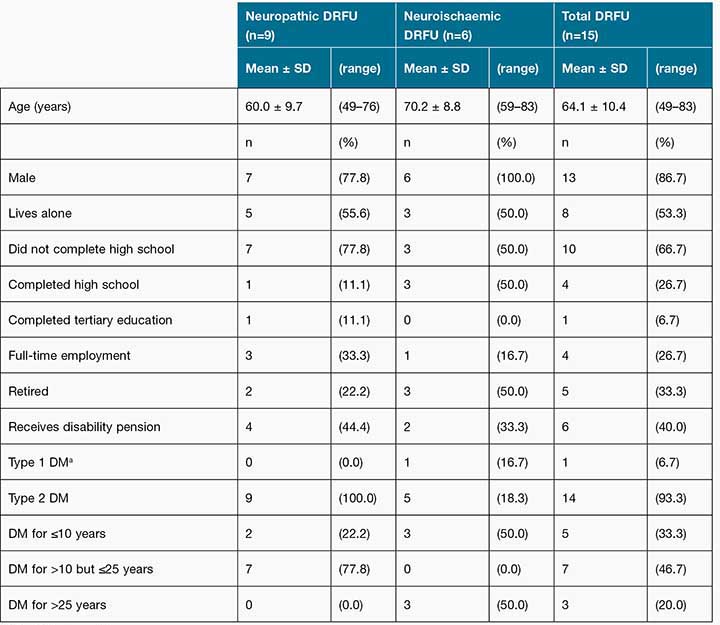
aDM = Diabetes mellitus
Diabetes-related complications are presented in Table 2. Key findings were that diabetes-related complications were common across the sample: 87% had a previous foot ulcer, with 47% of these having undergone some kind of lower limb amputation. A high proportion of the sample, 80% were prescribed analgesia, including drugs for neuropathic pain while 60% (n=9) were currently prescribed antibiotics at the time of interview. Despite the high usage of antibiotics there was no clinical evidence of DRFU infection amongst the sample.
Table 2: A) Diabetes-related complications and B) Prescribed medications by neuropathic DRFU versus neuroischaemic DRFU
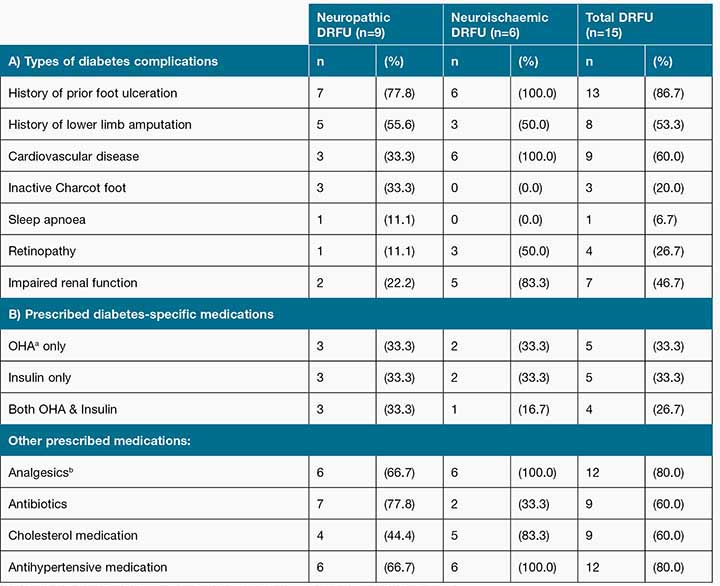
aOHA= oral hypoglycaemic agent bAnalgesics were not prescribed for wound-specific pain
Foot ulcer characteristics
Sixty per cent of participants (n=9) had neuropathic ulcers and 40% (n=6) had neuroischaemic ulcers. There were no ischaemic ulcers within the sample group. Forty per cent of participants (n=6) had ulcers present for more than six months.
Some participants (n=4) had multiple DRFU; the total number of ulcers was 22 in the 15 study participants. The locations of the ulcers were equally distributed: 20% in the toe region only; 20% in the metatarsal area only; 20% in the midfoot/hindfoot area only; 13% ulcers in multiple locations; and 27% located at an amputation site. The most frequent wound type was Grade I-A (UTWCS) being a superficial wound, not involving tendon, capsule or bone with 10 ulcers (46%).
Wound-related pain
Pain prevalence
Of the 15 participants, 33% (n=5) reported pain when asked the question by a researcher “Do you feel pain in your wound?” However, this increased to 53% (n=8) when both the SF-MPQ and SF-BPI were used. Interestingly, all outpatients (n=11) denied the presence of wound pain when questioned by a treating podiatry practitioner during their routine appointment (see Table 3).
Table 3: Prevalence of A) Reported pain and B) Where pain is located by neuropathic DRFU versus neuroischaemic DRFU
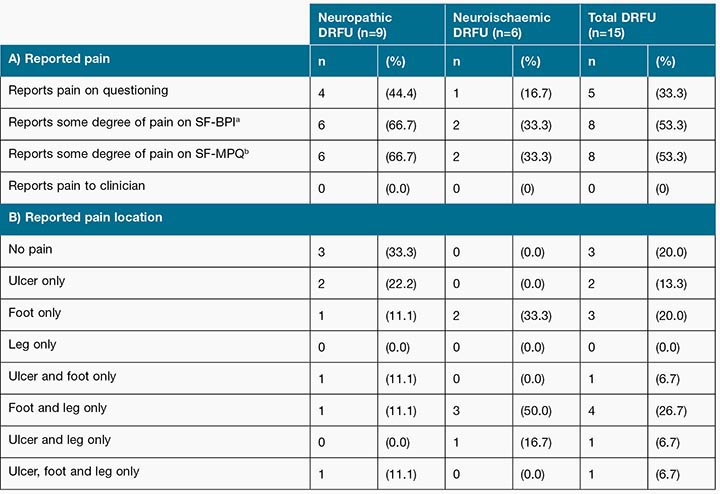
aSF-BPI = Short form-Brief Pain Inventory
bSF-MPQ = Short form-McGill Pain Questionnaire
Pain intensity
Present pain intensity VAS scores ranged 0–60 mm out of 100 mm in the total sample, with a median score of 2 mm. Seventy-nine per cent (n=12) of participants reported a score below or equal to 5 mm. One participant did not complete the VAS due to poor eyesight. For the neuropathic ulcer group, VAS scores ranged from 0 to 60 mm, with a median score of 5. The neuroischaemic ulcer group had a median score of 0, with a range of 0–3 mm. Only one participant reported a VAS score above 40 mm, a level considered to indicate moderate, poorly managed pain22 (see Table 4).
Table 4: Present pain intensity using the Short form-McGill Pain Questionnaire
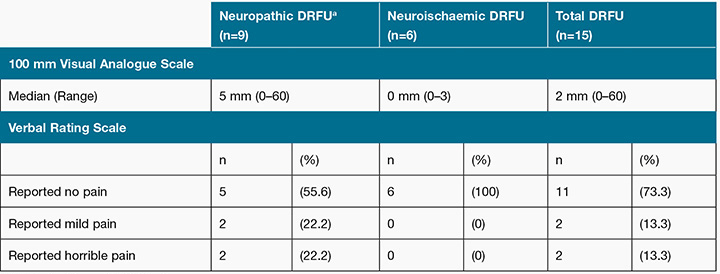
aDRFU = diabetes-related foot ulcer
The VRS had similar findings with 73% (n=11) of participants reporting no present pain; the entire neuroischaemic ulcer group reported an absence of present pain. For the neuropathic ulcer group, 22% (n=2) of participants described their present pain as “mild” and another 22% (n=2) of participants described their current pain as “horrible” (see Table 4).
Low pain intensity scores were also reported for the SF-BPI severity score that measured wound pain intensity within the past 24 hours, with a median score of 0 (range=0–5.8) out of a highest possible score of 10. A SF-MPQ sensory pain intensity median score of 1.0 (range=0–17) out of 33 was calculated for the total sample (see Table 5). This was indicative of participants rating the severity of most of wound pain qualities described in the SF-MPQ as “none”.
Nature of pain
One-third (n=5) of participants reported they experienced some sort of pain during debridement or probing of ulceration (see figure 2). Twenty per cent (n=3) experienced pain during dressing changes, 13% (n=2) when attempting to sleep at night, 20% (n=3) when resting during the day, 13% (n=2) when standing and 27% (n=4) when walking. Of the total participants, 13% (n=2) described their wound pain as “brief”, 40% (n=6) described the pain as “intermittent”, 7% (n=1) described the pain as continuous, while 40% (n=6) stated that pain “doesn’t exist” in their wound.
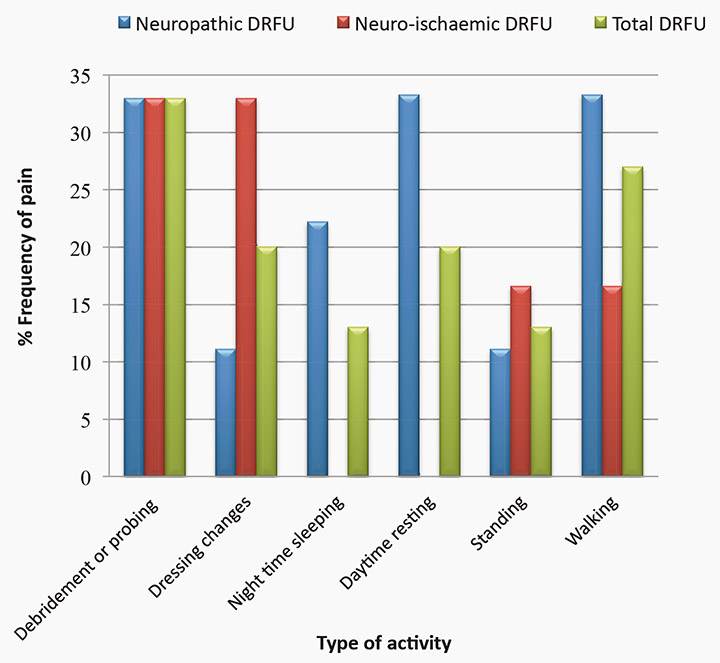
Figure 2: Reported frequency of foot ulcer pain experienced by type of activity
Table 5 compares the effect of pain on HRQoL; the SF-BPI interference score that measures the effect pain had on HRQoL in the last 24 hours had a median score of 0.3 (range=0–6.4) out of a highest possible score of 10. The SF-MPQ affective pain intensity score which measures how unpleasant wound pain is on HRQoL had a median score of 0 (range=0–7) out of a possible highest score of 12.
Table 5: The median A) Pain intensity score and B) Effect of pain on HRQoLa
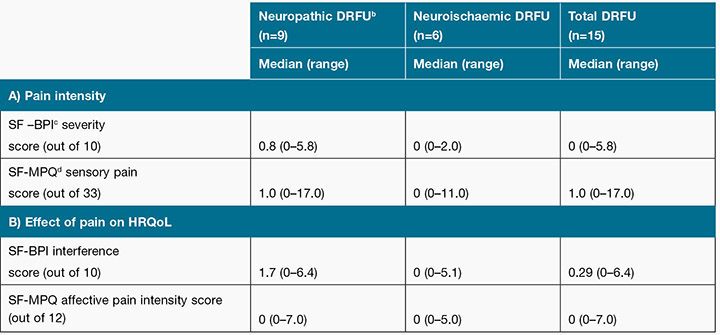
aDRFU = diabetes-related foot ulcer, bHRQoL= Health Related Quality of Life,
cSF-BPI = Short-form Brief Pain Inventory, dSF-MPQ = Short-form McGill Pain Questionnaire
Figure 3 compares the frequency of SF-MPQ pain descriptors selected by participants with neuropathic and neuroischaemic ulcers. The most common wound pain quality chosen by participants was the descriptor “tender”. Approximately half selected this term to describe their wound pain. Of these seven participants, four rated the descriptor of their wound pain as mild, one rated the descriptor as moderate and two rated the pain as severe.
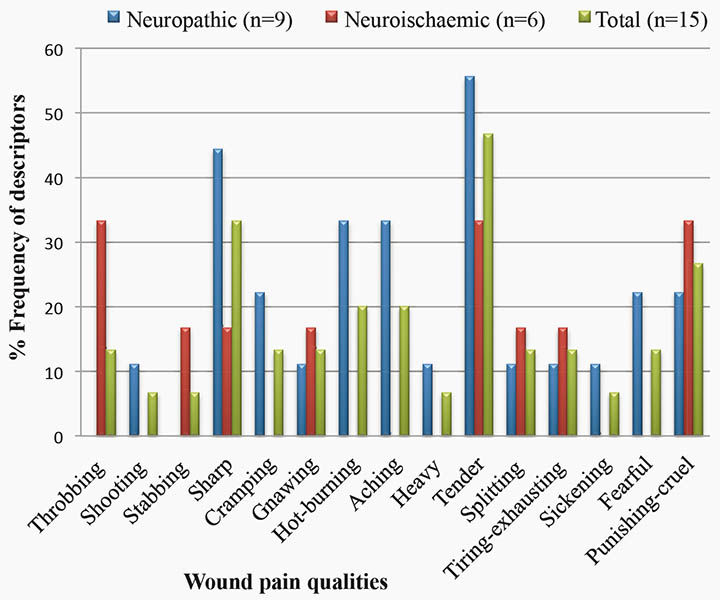
Figure 3: Comparison of the frequency Short form-McGill Pain Questionnaire pain qualities selected by participants to describe their wound pain within aetiological groups.
Wound pain management
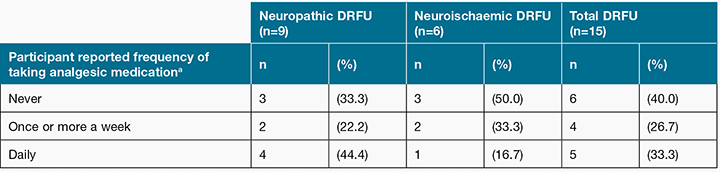
Of the 12 participants (80% of the sample) who were prescribed oral analgesia, only nine (75%) reported actually taking it (see Table 6). Out of these nine participants, four reported using analgesia once or more a week; while five reported using analgesia on a daily basis. In the neuropathic ulcer group, 100% of participants who were prescribed analgesia reported using analgesia, compared to 50% in the neuroischaemic ulcer group. Use of analgesia was not prescribed specifically for DRFU pain.
Table 6: Participant reported frequency of taking analgesic medication
While data regarding other pain management strategies was collected, it was difficult to interpret due to the small sample size using each modality. Of those (n=13) wearing offloading footwear, 46% reported wound pain relief. Three of the 11 wearing offloading felt padding reported wound pain relief, while two of the five wearing a total contact cast reported wound pain relief. Of the participants who reported wound pain and the use of pain treatments, the average degree of benefit from using pain relief modalities in the preceding 24 hours was 55% (n=6, range=20–100%).
Discussion
These results indicate that patients with DRFU can experience wound pain in the presence of peripheral neuropathy. In our study, 33% (n=5) of participants reported pain when asked the specific question by the researcher “Do you have pain in your wound?” while 53% (n=8) reported some degree of pain on both the SF-MPQ and SF-BPI. This is an important finding as 80% of participants were taking analgesia. The differences between the results in this study are consistent with previous studies which report much higher pain prevalence rates using formal assessment tools compared with a singular question2,4,5. The discrepancy between prevalence statistics poses the question: How accurately are we able to measure patient’s wound pain? While these pain assessment tools are validated within the patient demographic, there are no pain tools as yet which have been specifically validated for use with DRFU. Therefore it is impossible to determine whether pain assessment tools are simply measuring pain prevalence more accurately or are asking the patient leading questions. With 75% of participants in our study reporting an absence of present pain, it also may be that pain assessment tools SF-MPQ and SF-BPI which incorporate the dimension of pain timing could possibly be providing a more comprehensive and accurate result than a singular question.
This study also queries the accuracy of pain assessment within clinical practice. None of our study participants reported pain when questioned by the podiatrist during their regular appointment immediately after the interview, yet had reported pain to the researcher. This discrepancy has been reported in literature before; with poor concordance found between investigator-recorded pain scores and data reported on hospital observation charts23. No definitive reasons have been found for these differences. Past literature has suggested that patients may be more hesitant to report wound pain due to the unwillingness to upset the clinician and the fear of addiction from prescription of stronger analgesia24,25. Bengtsson et al.5 suggested some patients were reluctant to talk to doctors for fear they would recommend amputation. In the outpatient group fear of inpatient admission is another possibility. It has also been suggested patients and clinicians use different terms to describe pain. The inability to measure pain directly means assessment relies largely on an individual’s reported subjective experience26. The difference in patients’ perception of pain to the clinicians’ as well as the assumption that wound pain is normal may also be reasons why patients do not report pain to clinicians5,25. The inability to accurately determine wound pain presence in a clinical setting indicates poorer management outcomes of wound pain25.
It is interesting to note that despite high usage of analgesia, participants did report wound pain to the researcher. This is consistent with findings from previous studies, indicating that in current practice, wound pain management appears to be inadequate2,4,5. Wound pain is complex and can have both nociceptive and neuropathic aetiologies9. It is well known that pharmacological agents useful for the management of nociceptive pain may be ineffective in the management of neuropathic pain25. Therefore the ability to differentiate between wound pain aetiologies is crucial in providing holistic wound management.
Low mean pain intensity scores were reported in our sample. This was consistent across all pain measures; including present pain, average pain and pain in the last 24 hours. Our results were considerably lower than Bradbury and Price4 who reported a mean VAS score of 26.4 ± 24.6 across the total sample. It should be noted that while analgesia usage remained similar across studies, participants with foot infection were included in this study, which is a known confounding factor of wound pain1,4. Bengtsson et al.3 reported mean VAS scores for people with continuous wound pain only, which made findings difficult to compare. There is no current agreement to determine the minimal clinical important difference of VAS wound pain levels, although the World Union of Wound Healing Societies’ consensus suggests ≥ 40 mm during or after dressing changes is indicative of moderate wound pain requiring immediate pain management review21.
Contrasting with previous research, our study found neuropathic foot ulcers to be more painful than neuroischaemic ulcers4. Neuroischaemic DRFU have also been associated with longer healing times and poorer HRQoL outcomes than neuropathic DRFU27,28. The inability to perform statistical analyses means we are unable to confirm whether there is a statistically significant difference between wound aetiologies.
No participants within our study had an ischaemic ulcer. This is consistent with the study by Bradbury and Price4, who found only one participant of a sample size of 28 had an ischaemic ulcer. In the literature, ischaemic DRFU are considered more rare than other wound aetiology types; this is possibly due to the high prevalence rates of peripheral neuropathy within the diabetic foot population11,27.
The effect of wound pain on HRQoL appears to be in keeping with the lower pain intensity scores. It was difficult to compare the results with previous studies due to the lack of homogeneity between outcome measures. In our study, 53% of participants reported their wound pain was brief or intermittent, while only 7% reported they had continuous pain. In previous studies, continuous pain or pain present most or all of the time was much more prevalent which associated with worse effects on HRQoL. The SF-MPQ descriptor “tender” was the most common sensory pain quality, while “punishing-cruel” was the most common affective pain quality on the SF-MPQ. This differs from previous research where “aching” and “tiring-exhausting” were the most common sensory and affective descriptors, respectively4.
Limitations
A number of limitations can be identified in this study. The small sample size meant statistical analyses were unable to be conducted to determine if real differences are likely to exist between the groups and therefore restricted the generalisability of this study into clinical practice. The area served by the health institution is multicultural, with many patients unable to speak or read English, which excluded them from the study, thus impacting the sample size.
Although the pain assessment tools were administered by a single researcher (AD), clinician questioning regarding pain was conducted by several different podiatrists, which could be associated with inconsistencies in questioning technique. It is also worth noting the differences between wound assessment of outpatients and inpatients within the Diabetes Foot Service. Inpatients were not routinely questioned by clinicians regarding wound pain as a standard component of wound assessment, whereas outpatients were.
While no participants were specifically prescribed analgesia for their wound pain, 80% of the study sample reported using analgesia medication for another complaint. This may cause a possible underestimation of wound pain within our study sample.
A lack of research into a valid and reliable pain assessment tool for DRFU currently means findings have questionable rigour. In our study, anecdotally, it was observed many participants found it difficult to describe the pain in their DRFU. While the SF-MPQ was generally well understood, many participants reported other terms including “tense”, “discomfort”, “pinprick” and “tingling”. For participants who were describing ulcers at amputation sites terms including “imaginative pain” and “feels like I still got toes” were used. The effect of phantom limb pain was not evaluated as a part of this study. Four participants had difficulty completing the VAS scale due to poor eyesight and fine motor control. These participants reported no pain on VRS, yet had a score of 1–5 mm on the VAS scale. While an imprecision of ±20 mm in post-operative settings has been reported, no research has investigated the accepted margin of error in wound pain29. With retinopathy and cataracts being common diabetes complications, it should be considered whether the VRS is a more reliable assessment tool than the VAS in this population demographic.
Conclusion
This preliminary study indicates that wound pain appears to be present in people with neuropathic and neuroischaemic DRFU within the hospital setting. This finding is important as wound pain is associated with delayed wound healing and poorer HRQoL6,30. Therefore the ability to identify both the presence and the aetiology of wound pain is critical for providing holistic wound care. This study highlights that in current clinical practice wound pain in DRFU is possibly not being accurately assessed or managed.
There appear to be differences between clinician and researcher reported pain prevalence. Clinician questioning regarding the presence of pain appears to lead to an underassessment of pain prevalence when compared to the use of formal pain assessment tools.
This leads us to ask the question why this is happening — Is it due to the patients’ fear of the consequences of reporting pain? Are clinicians simply not asking the right questions? Are clinicians not acknowledging that pain is an integral part of wound management? Or is it that they do not know how to manage DRFU pain?
A future study with a much larger sample size conducted in both the hospital and community setting is needed to establish whether there is a statistically significant and clinically important difference in pain prevalence, intensity and nature between wound aetiologies of neuropathic, neuroischaemic and ischaemic wounds. This bigger study will determine whether we need to develop a specific pain assessment tool for DRFU or whether existing pain assessment tools are appropriate and we need to focus more on establishing guidelines for assessment and management of wound pain.
Acknowledgements
The authors would like to thank the staff at Western Health Diabetes Foot Service for their assistance in patient assessment of foot ulcers. None of the authors disclose any financial or personal relationships with other people or organisations that could influence this work.
Author(s)
Ashlea M Dickinson
BHthSc, MPodPrac
ISIS Primary Care, Melbourne, Vic, Australia
Nicoletta Frescos*
BAppSci(Pod), MPH
Discipline of Podiatry, College of Science,
Health and Engineering, La Trobe University, Melbourne, Vic, Australia
Tel +61 409 577 055
Email n.frescos@latrobe.edu.au
Julia C Firth
BAppSci(Pod), MHS
Department of Diabetes Foot Service,
Western Health, Melbourne, Vic, Australia
Peter S Hamblin
FRACP
Endocrinology & Diabetes, Western Health, Melbourne, Vic, Australia;
Department of Medicine, Melbourne Medical School — Western Precinct, The University of Melbourne, St Albans, Vic, Australia
* Corresponding author
References
1. Sibbald RG, Armstrong DG, Orsted HL. Pain in diabetic foot ulcers. Ostomy Wound Manage 2003;49(4 Suppl):24–9.
2. Ribu L, Rustoen T, Birkeland K, Hanestad BR, Paul SM, Miaskowski C. The prevalence and occurrence of diabetic foot ulcer pain and its impact on health-related quality of life. Pain 2006;7:290–9.
3. Obilor HN, Adejuno PO. Assessment of diabetic foot ulcer-related pain and its implications to quality of life. Wound Practice & Research 2015;17:124–131.
4. Bradbury S, Price P. The impact of diabetic foot ulcer pain on patient quality of life. Wounds UK 2011;7:32–49.
5. Bengtsson L, Jonsson M, Apelqvist J. Wound-related pain is underestimated in patients with diabetic foot ulcers. J Wound Care 2008;17(10):433–5.
6. Downe A. What does primary research reveal about pain associated with leg ulcers? Wounds UK 2012;8:26–37.
7. Woo KY, Sibbald RG. Chronic wound pain: a conceptual model. Adv Skin Wound Care 2008;21:175–88.
8. Hogg FRA, Peach G, Price P, Thompson MM, Hinchliffe RJ. Measures of health-related quality of life in diabetes-related foot disease: a systematic review. Diabetologia 2012;55:552–65.
9. Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K. Quality of life in people with their first diabetic foot ulcer: a prospective cohort study. J Am Podiatr Med Assoc 2009;99(5):406–14.
10. Bowers K, Barrett S. Wound-related pain: features, assessment and treatment. J Prim Health Care 2009;19:37.
11. Apelqvist J, Bakker K, van Houtum WH, Schaper NC. Practical guidelines on the management and prevention of the diabetic foot. Diabetes Metab Res Rev 2008;24(Suppl.1):S181–7.
12. Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004;20(Suppl.1):S90–5.
13. Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 2001;24(1):84–8.
14. National evidence-based guideline on prevention, identification and management of foot complications in diabetes (part of the guidelines on management of type 2 diabetes). Melbourne, Australia 2011;1–55.
15. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007;33(Suppl.1):S1–S75.
16. Melzack R. The short-form McGill pain questionnaire. Pain 1987;30:191–7.
17. Cleeland CS. The Brief Pain Inventory: User Guide. Houston, Texas: The University of Texas M.D. Anderson Cancer Centre, 2009.
18. Caraceni A, Cherny N, Fainsinger R, Kaasa S, Poulain P, Radbruch L et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an expert working group of the European Association of Palliative Care. J Pain Symptom Manage 2002;23(3):239.
19. Grimmer-Somers K, Vipond N, Kumar S, Hall G. A review and critique of assessment instruments for patients with persistent pain. J Pain Res 2009;2:21–47.
20. Ferreira KA, Teixeira MJ, Mendonza TR, Cleeland CS. Validation of brief pain inventory to Brazilian patients with pain. Support Care Cancer 2011;19(4):505–11.
21. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS pain), Numeric Rating Scale for Pain (NRS pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63(Suppl.11):S240–S52.
22. World Union of Wound Healing Societies. Principles of best practice: minimising pain at wound dressing-related procedures. A consensus document. Toronto, Ontario, Canada, 2004.
23. Rockett MP, Simpson G, Crossley R, Blowey S. Characteristics of pain in hospitalized medical patients, surgical patients, and outpatients attending a pain management centre. Br J Anaesth 2013;110(6):1017.
24. Frescos N, Nay R, Fetherstonhaugh D. A focus group study into health practitioners perceptions and experience of assessment and management of chronic wound pain. Australian Wound Management Association Conference; 2012; Sydney, Australia 2012.
25. Doughty DB. Strategies for minimizing chronic wound pain. Adv Skin Wound Care. 2006; 19(2):82–5.
26. Part III: Pain Terms, A Current List with Definitions and Notes on Usage. In: Merskey H, Bogduk N, editors. Classification of Chronic Pain. Second ed. Seattle: IASP Press; 2011, 209–14.
27. Moura Neto A, Zantut-Wittmann DE, Fernandes TD, Nery M, Parisi MC. Risk factors for ulceration and amputation with diabetic foot at risk: study in a cohort of 496 patients. Endocrine 2013 Aug;44(1):119–24.
28. Apelqvist J, Elgzyri T, Larsson J, Londahl M, Nyberg P, Thorne J. Factors related to outcome of neuroischemic/ischemic foot ulcer in diabetic patients. J Vasc Surg 2011;53:1582–8.
29. DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg 1998;86:102–6.
30. Woo K, Sibbald G, Fogh K, Glynn C, Krasner D, Leaper D et al. Assessment and management of persistent (chronic) and total wound pain. Int Wound J 2008;5(2):205–15.



