Volume 24 Number 3
An economic evaluation of chronic wound management in a tertiary hospital
Bien-Keem Tan, Esther Wan Xian Tan, Si-Jack Chong, Yee-Yee Chang, Colin Song and Vernon J Lee
Abstract
Aim: The study aims to determine the cost of chronic wound management in a tertiary hospital in Singapore.
Background: Given the impact of chronic wound care on health care expenditure, wound prevention is a priority in public health initiatives. Holistic wound management can reduce the incidence of chronic wounds and will potentially reduce wound care costs.
Methodology: This is a retrospective analysis over a two-year period (1 January 2012 to 31 December 2013) in a tertiary institution specialising in wound care. Five common chronic wound categories were included, namely: diabetic, pressure, venous, ischaemic and unspecified chronic skin ulcers. Their diagnosis-related group (DRG) code served as an identifier for patients suffering from these conditions. The cost of hospitalisation of each patient was individually calculated. Total cost consisted of direct and indirect costs, and are represented in Singapore dollars (SGD).
Results: Four hundred and seventy patients were admitted in this study. The mean age was 68 years and mean length of stay was 13.2 days. The annual direct cost amounted to a gross sum of SGD 4.59 million and net payable sum was SGD 1.98 million after subsidies. Annual total indirect costs (for example, income loss due to hospitalisation stay and sick leave) amounted to SGD 0.86 million (mean of SGD 3,600 per patient). Of the patients, 23% had the primary diagnosis of ‘diabetes mellitus with foot ulcer due to multiple causes’, followed by 16.1% ‘pressure injuries unspecified’, and 13.4% ‘varicose veins of lower extremities with ulcer’. The direct gross cost per annum was highest for diabetic foot ulcers, followed by pressure injuries, and then ischaemic ulcers (SGD 1.68 million, SGD 0.63 million and SGD 0.43 million, respectively).
Conclusion: Chronic wounds present a substantial economic burden on society. We recommend early medical interventions through regular screening by specialised wound teams and evaluating the cost-effectiveness of these interventions in the local setting.
Introduction
Chronic wounds are defined as wounds that do not heal after a period of three months1. They can be classified into wounds resulting from chronic disease (for example, diabetes mellitus, venous disease, peripheral vascular disease), and pressure injuries. An ageing population has an increased incidence of major chronic diseases, such as cardiac disease, diabetes as well as wounds2,3. In Singapore, wound care has yet to be fully established as a major focus in chronic disease management. It has been reported that health care professionals and the society in general are not fully cognisant of the medical complications and social burden of chronic wounds4. Furthermore, wounds are not viewed as high priority relative to other conditions because they are not considered life-threatening.
One approach to define the gravity of this problem is to consider the clinical and economic burden imposed by chronic wounds. Studies in the United States, Australia and France on the cost of wound care have demonstrated the economic burden of chronic wounds and their management4-6. For instance, the US spends US$10 billion annually on chronic wound care7. In addition, pressure injuries were estimated to cost between £1.76 and £2.64 billion from 2005 to 2006 in the UK8.
Our study aims to provide an overview of the costs of managing chronic wounds in Singapore. It describes both the direct and indirect costs of wound management. We estimate the costs of chronic wound management in patients admitted to the Singapore General Hospital (SGH) and the information will serve as a reference for future interventions.
Methodology
Singapore is a country in South-East Asia with a population of 5.47 million9. This study was conducted in the SGH, the largest acute tertiary hospital and national referral centre. The hospital has a capacity of 1,500 beds and receives approximately 20% of all wound patients in Singapore annually. The Singhealth Institutional Review Board approved this study prior to its initiation.
Five common chronic wounds were identified, namely: diabetic ulcers; pressure injuries; chronic ulcers of the skin; venous leg ulcers; and ischaemic ulcers. This was determined by interviewing various medical personnel in the wards. Each type of wound is assigned a diagnosis-related group (DRG) code that serves as an identifier for the diagnosis of the patient.
For diabetic ulcers we used the code ‘diabetic foot with ulcers: E1473’, for pressure injuries we used codes ‘pressure injuries unspecified which type: L899’, ‘pressure injuries stage III: L892’, and ‘pressure injuries stage IV: L893’. Pressure injuries stages III and IV were highlighted for our research because it fulfilled our criteria of a chronic wound, whereas stages I and II did not. Under the wound category chronic ulcers of the skin we used the code ‘chronic ulcers of the skin: L984’, venous leg ulcers we used ‘varicose veins with ulcers: I830’, under ischemic leg ulcers we used ‘peripheral vascular disease with ulceration: I7023’.
Patients with the relevant DRG codes with at least one of the five chronic wound types in the hospital databases were included in the cost analysis. We included all patients in the two-year period from 1 January 2012 to 31 December 2013 in our study. The patients recruited were aged 21 years and above and had been an inpatient during the period of the study, suffering from at least one of the chronic wounds listed above.
This is a retrospective cost evaluation of chronic wounds in Singapore. A list of resources consumed was drawn up based on consulting health care professionals (podiatrists, nursing managers and physicians) involved in wound care. They include: general treatment, surgical costs, specialist consultations, hospitalisation and laboratory investigations. Additional costs following treatment were also considered, which included both pharmaceutical and wound care costs.
All records were electronically captured via the SGH Information and Technology Database. These records included information on patient demographics (age, sex, race), length of stay, diagnostic tests (X-ray, computed tomography scan, magnetic resonance imaging, diagnostic angiogram and so on), interventions (antibiotic use, endovascular revascularisation, open revascularisation, amputations and so on), and inpatient charges incurred by each patient. Codes used to extract information were billing codes used for reimbursements. Through the DRG codes we were able to obtain a list of patient identification numbers from the Medical Records Office (MRO). Individual costs for the patients were then calculated.
Total cost consisted of direct costs and indirect costs. Direct costs included hospital stays, inpatient surgical treatment (if any), and inpatient costs such as medications, wound dressing, laboratory investigation costs, and consultations. In order to extract these costs from the IT database, the codes of wound dressings, medications and surgical tables were used. A methodology of top-down gross costing was used to define the direct costs for each item. We presented the direct costs in two categories: total gross cost and net payable cost. Total gross cost is the sum of resources incurred by the patient over his/her entire hospital stay; whereas net payable costs is the out-of-pocket payment after subsidies, insurance coverage, and Government Goods and Services Tax (GST).Total gross cost included mean gross cost per patient and the annualised sum of all patients, while net payable cost included net payable cost per patient and the annualised sum of all patients.
Indirect costs consisted of two components: income loss during hospitalisation and income loss during sick leave. In calculating the loss in income due to hospitalisation, we took the median gross monthly income from work of full-time employed Singapore residents as reported by the Ministry of Manpower (MOM) in June 201410. We took data from MOM as we assumed that individuals that fall into the older age groups earn less than the general population or may not be working at all. We did not consider other costs such as carer costs as we were unable to ascertain the number of patients who needed carers. To avoid overinflating costs, we elected to be conservative in our estimate
The patients in the study were stratified according to their corresponding age groups similar to that used by the MOM. For income loss due to sick leave, an estimate of one month loss of income per patient was used.
IBM Statistical Package for the Social Sciences (SPSS) Version 21.0 software was used for our analysis. Descriptive statistics of patient demographics, cost per hospital episode per individual and total cost per individual were demonstrated. All costs are represented in 2014 Singapore dollars (1 SGD=0.887 A$), using an inflation rate of 3%.
Results
Four hundred and seventy patients were admitted with the primary diagnosis of a chronic wound over the two-year study period 2012–2013 in the SGH. (Mean age of 68 years, 51.1% female.) The mean length of stay was 13.2 days in the two-year period and the median was 7.
We subdivided our patients into the admitting speciality of care shown in Figure 1. We observed that these chronic wound patients were admitted to 25 different specialities over the two-year period. The majority of patients were admitted into the internal medicine speciality (n=224, 31.4%) and general surgery speciality (n=110, 15.4%).
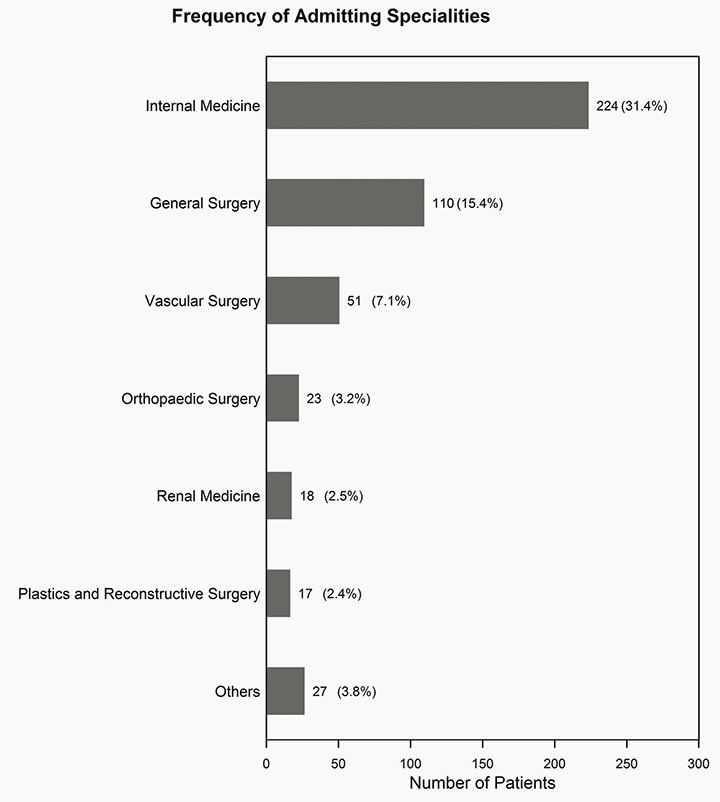
Figure 1: Frequency of admitting specialty description 2012–13
We analysed the number of patients that fell under each ICD-10 diagnosis description. The majority of patients that were admitted fell under the diagnosis ‘Unspecified diabetes mellitus with foot ulcer due to multiple causes’ (n=165, 23.1%), followed by patients with the diagnosis ‘Decubitus ulcer and pressure area unspecified’ (n=115, 16.1%), and then ‘Varicose veins of lower extremities with ulcer’ (n=96, 13.4%) (Figure 2).
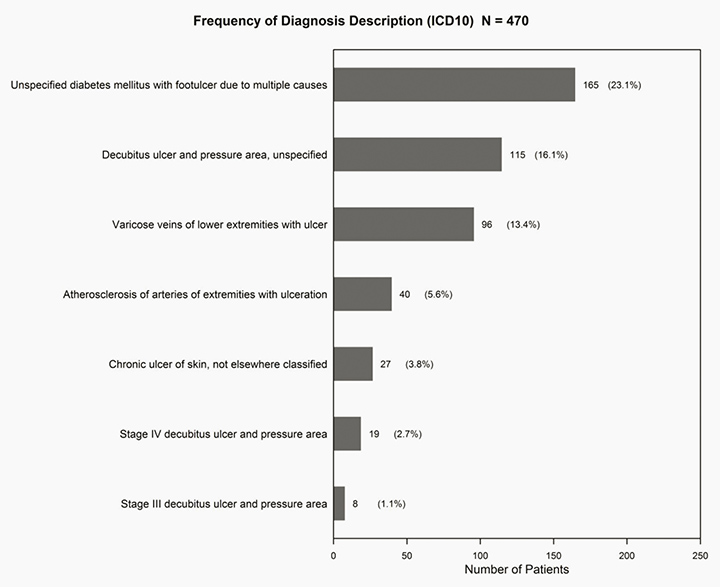
Figure 2: Frequency of patients per diagnosis description 2012–13
We classified the direct cost of chronic wounds according to each diagnosis description (Table 1), and noted that the highest total gross amount fell under the diagnosis category of ‘Unspecified diabetes mellitus with foot ulcer due to multiple causes’ amounting to around SGD 1.68 million. This was followed by ‘Decubitus ulcer and pressure area unspecified’ summing to SGD 627,000 per year and then ‘Arteriosclerosis of the arteries of extremities with ulceration’ costing SGD 427,000 per year.
Table 1: Total direct costs for each diagnosis description (ICD10)
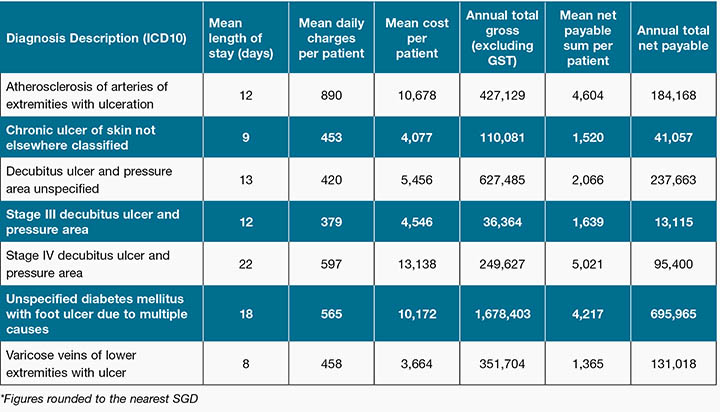
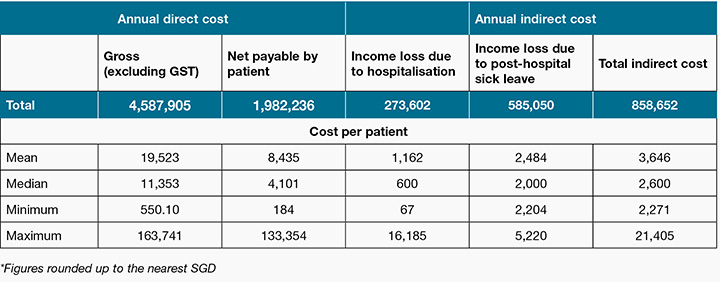
Table 2 shows the total costs per annum. The annual direct gross cost for chronic wounds was SGD 4.59 million and the net payable sum for patients was SGD 1.98 million, less than half the annual direct gross cost. Total indirect costs per year was SGD 859,000, which consisted of adding both income loss due to hospitalisation and sick leave, which amounted to SGD 274,000 and SGD 585,000 respectively. Therefore, the overall cost of chronic wounds per year in the SGH was around SGD 5.45 million.
Table 2: Total direct and indirect costs due to hospitalisation
Discussion
The objective of this study was to evaluate the cost of chronic wounds in an inpatient hospital setting. Similar studies exist11-13 but this is the first to be carried out in Singapore looking at the full spectrum of chronic wounds. It will form the basis for more specific economic studies to formulate policies and guide interventions. This would allow us to compare with the potential effectiveness of early wound interventions, and the potential savings vis-a-vis the costs of interventions.
By analysing epidemiological data based on current best practices, we aim to evaluate the economic impact of treating chronic wounds. This study is unique in that it does not focus solely on diabetic ulcers14-16, but included chronic wounds such as pressure, ischaemic and chronic venous ulcers. Hence, our sampled population was more diverse, and this is reflected in the 25 varied medical and surgical disciplines these patients were admitted under (Figure 1). Our data shows a large proportion of female patients (51.1%), which were not previously described or observed in other local reports. This could be the subject of future studies.
It is estimated that SGH receives 20% of patients who need wound care in Singapore. The annual total gross direct cost for chronic wound management in SGH totalled 4.59 million SGD, with a net payable sum of 1.98 million in a two-year period from 2012 to 2013. If we extrapolate this, public hospital costs may amount to 23 million SGD a year; hence additional resources should be devoted to this problem and to finding solutions to it. The gross cost of 4.59 million SGD annually is likely to be an underestimation because only patients with the primary diagnosis of chronic wounds on admission were included in the study. Patients with a secondary diagnosis of chronic wounds or those who had developed the condition while in hospital were excluded. Moreover, while dressing materials were included into the cost, other expenses such as the cost of training wound care nurses were not considered. From our calculations, there was a cost-shift of SGD 2.61 million annually onto the health care system (gross costs of 4.59 minus net payable 1.98 million).
An economic study focusing on diabetic foot ulcers was carried out by Nather et al. from National University Hospital (NUH), another tertiary hospital in Singapore. The authors showed that the mean hospitalisation cost per patient over the years 2002–2007 was SGD 7,89017. Our study which included other types of chronic wounds gave a mean hospitalisation fee of SGD 8,435 per patient (2012–2013).
Previous studies have attempted to predict the changes necessary to sustain wound care services. Hjort and Gottrup from Denmark projected an increasing annual demand for services over the period 2009–202018. Similarly Dowsett and Bielby forecasted an annual increase of 1–2% in the cost of wound care services in the UK19. These projections suggest that fundamental changes need to be made on how wound care is delivered in order to reconcile capacity with future demand. Future changes broadly include: firstly, preventive measures; secondly, nurse-led programmes to provide comprehensive wound care; thirdly, enhanced therapies which accelerate wound healing, for example, topical negative pressure therapies; and fourthly, accurate diagnosis and treatment of wound infection.
Primary prevention is the best cost-saving measure. In terms of pressure injury prevention, we have contributed to the 2012 Pan-Pacific Clinical Practice Guideline20 — an initiative by Australia, New Zealand, Hong Kong and Singapore. The guideline included a comprehensive risk assessment and prevention plan by identifying high-risk patients, assessing existing pressure injuries and their subsequent treatment.
Nurse-led wound services contribute to effective wound treatment as they offer timely treatment through their clinics and ward rounds. They also devote more time to wound care and counselling. In the UK, some hospitals have started a ‘harm-free care’ agenda such as the SSKIN care bundle21 to eliminate category 3 and 4 pressure injuries. UK wound care nurses have also initiated a nurse-led leg ulcer service that focused specifically on patients with healed ulcers and it has showed a reduction in recurrence rates from 18–20% to 5.8%22. In our hospital, the wound team refers to a wound management book written by nurse clinicians. This guidebook with its illustrations is a reference for nurses on how to assess wounds, recognise aetiologies and institute treatment23. It covers all aspects of wound care including recognition of infection, management of stomas and external fixators, improving nutrition and the indications for hyperbaric oxygen therapy (HBOT). These services can be accessed by both outpatients and inpatients. By creating a chronic wound protocol and a specialised chronic wound team, care is streamlined and health care costs can potentially be lowered, although the upfront cost may initially be higher due specialised dressings and treatment, for example, HBOT.
A myriad of enhanced wound healing therapies are available, and these have been miniaturised to be ultra-portable24-26. In Singapore, negative pressure wound therapy has become a standard treatment, which is covered by Medisave27 — a national medical savings scheme. It promotes wound healing by improving local blood flow28, inducing granulation and angiogenesis29-30, as well as reducing oedema31.
The limitations of this study are that it is retrospective, and the overall cost of chronic wound care is likely to be underestimated. Given the chronic nature of these type of wounds, economic modelling of a longer term will provide a clearer depiction of the cost of intervention and its impact on society. For instance, an intervention may be more costly in the beginning months, yet might provide cost savings in the long run due to fewer additional interventions and follow-ups. Being a retrospective study, the data were derived from the information technology system. The costs were underestimated because some potentially relevant cost components such as medications, specialised wound materials and other accessories were not readily available. Chronic wound patients may also have been overlooked if they were admitted to the hospital for other co-morbidities. Hence, to prevent this from affecting our sample size, we considered all patients, instead of relying on random sampling. In addition, the study only considered inpatient costs, and therefore does not include patients who entered the health care system for wound care as outpatients — such as patients visiting the hospital for frequent wound dressings and treatment. This further emphasises the conservatism of our estimate.
Our study included all aspects of chronic wound treatment (hospitalisation, wound dressing, surgery and so on). Therefore, the outcomes of the study can be directly extrapolated to Singapore’s population. Another benefit of computing cost data on a per-patient as opposed to an aggregate basis is that it provides estimates for health care policy makers’ disease models.
In public health, primary prevention reduces patient morbidity and overall economic burden. Yet, future policy makers have to consider the concept of discounting — costs that are incurred in the present have a higher value than cost incurred in the future. A minor fluctuation in the discount rate could reduce the cost-effectiveness of these surgical interventions over conservative measures. Lastly, benefits of any medical interventions cannot be solely measured by economic gains. Social gains and improvement of public health are equally important and a cost-utility analysis can be done to explore these aspects further. Such studies should be done in countries looking to assess the cost of wound care to their health care systems, and to determine the cost-effectiveness of investing in prevention programmes.
Conclusion
The aim of this study has been to determine the inpatient cost associated with the management of chronic wounds in the SGH. A detailed computation of patients with relevant ICD codes produced an annual overall cost of 5.45 million SGD with a mean hospitalisation fee of SGD 8,435 per patient. It is evident that chronic wounds present a substantial economic burden unto society. Early medical follow-up coupled with increased, coordinated and regular screening by multidisciplinary health care teams will optimise treatment of chronic wounds and subsequently have significant economic ramifications. Yet, only by considering outpatient costs will we be able to attain a more holistic view of this challenge; and this should be carried out in subsequent studies.
Acknowledgements
We would like to thank the Chief Finance Officer Mr Wong Loong Kin and Deputy Finance Officer Mr Paul Wong for their contributions in this study.
There are no sources of financial support for this paper.
Author(s)
Bien-Keem Tan*
MBBS (Singapore), FRCS (Edin), Department of Plastic, Reconstructive & Aesthetic Surgery, Singapore General Hospital, Singapore
Email bienkeem@gmail.com
Esther Wan Xian Tan
MBBS (Singapore)
Singapore General Hospital, Singapore
Email esthertanwx@gmail.com
Si-Jack Chong
MBBS (Singapore), MRCS (Edin), MMed (Surg)
Department of Plastic, Reconstructive & Aesthetic Surgery, Singapore General Hospital, Singapore
Email chong_si_jack@hotmail.com
Yee-Yee Chang
Division of Nursing, Singapore General Hospital
Singapore
Email chang.yee.yee@sgh.com.sg
Colin Song
MBBS (Rand), FRCS (Edin), Department of Plastic, Reconstructive & Aesthetic Surgery, Singapore
Email colinsong@capeclinic.com
Vernon J Lee
MBBS, PhD, MPH, MBA
Saw Swee Hock School of Public Health, National University of Singapore, Singapore
Email vernonljm@hotmail.com
* Corresponding author
References
- Mustoe TA, O’Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg 2006;117:35S–41.
- Günter CI, Machens HG. New strategies in clinical care of skin wound healing. Eur Surg Res 2012;49(1):16–23.
- Augustin M. Cumulative life course impairment in chronic wounds. Curr Probl Dermatol 2013;44:125–129.
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17(6):763–771.
- Whitlock E, Morcom J, Spurling G, Janamian T, Ryan S. Wound care costs in general practice. A cross-sectional study. Aust Fam Physician 2014;43(3):143–146.
- I Girod, P Valensi, C Laforet, T Moreau-Defarges, P Guillon, F Baron. An economic evaluation of the cost of diabetic foot ulcers: results of a retrospective study on 239 patients. Diabetes Metab 2003;29:269–277.
- Swanson L. Solving stubborn-wound problem could save millions, team says. CMAJ 1999;160:556.
- Posnett J, Franks PJ. Skin breakdown: the silent epidemic. Hull: The Smith and Nephew Foundation, 2007.
- Singapore Statistics. http://www.singstat.gov.sg/statistics/browse-by-theme/population-and-population-structure
- Ministry of manpower. http://stats.mom.gov.sg/Pages/Occupational-Wages-Tables2014.aspx
- Redekop WK, McDonnel J, Verboom P, Lovas K, Kalo Z. The cost-effectiveness of Apligraf treatment of diabetic foot ulcers. Pharmacoeconomics 2003;21:1171–83.
- Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with Promogram in four European countries. J Wound Care 2002;11:70–4.
- Foglia E, Restelli U, Napoletano AM, Coclite D, Porazzi E, Bonfanti M et al. Pressure ulcers management: an economic evaluation. J Prev Med Hyg 2012;53:30–6.
- Hicks CW, Selvarajah S, Mathioudakis N, Perler BA, Freischlag JA, Black JH 3rd, Abularrage CJ. Trends and determinants of costs associated with the inpatient care of diabetic foot ulcer. J Vasc Surg 2014 Nov;60(5):1247–54.
- Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 2010 Sep–Oct;100(5):335–41.
- Cawich SO, Islam S, Hariharan S, Harnarayan P, Budhooram S, Ramsewak S et al. The economic impact of hospitalization for diabetic foot infections in a Caribbean nation. Perm J 2014 Winter;18(1):e101–4.
- Nather A, Siok Bee C, Keng Lin W, Xin-Bei Valerie C, Liang S, Tambyah PA, Jorgensen A, Nambiar A. Value of team approach combined with clinical pathway for diabetic foot problems: a clinical evaluation. Diabet Foot Ankle 2010;1. doi:10.3402/dfa.v1i0.5731.
- Hjort A, Gottrup F. Cost of wound treatment to increase significantly in Denmark over the next decade. J Wound Care 2010 May;19(5):173–4, 176, 178, 180, 182, 184.
- Dowsett C, Bielby A, Searle R. Reconciling increasing wound care demands with available resources. J Wound Care 2014 Nov;23(11):552, 554, 556–8 passim.
- http://www.awma.com.au/publications/2012_AWMA_Pan_Pacific_Guidelines.pdf
- NHS East and Midlands. SSKIN: five steps to prevent and treat pressure ulcers. http://nhs.stopthepressure.co.uk/SSKIN/sskin.htm (accessed July 2014)
- Dowsett CA. Treatment and prevention of recurrence of venous leg ulcers. Wounds UK 2011;7(1):115–119.
- Singapore General Hospital (SGH). Wound Care Manual.
- Brandon T. A portable, disposable system for negative-pressure wound therapy. Br J Nurs 2015 Jan 22–Feb 11;24(2):98, 100–6. doi: 10.12968/bjon.2015.24.2.98.
- Hurd T, Trueman P, Rossington A. Use of a portable, single-use negative pressure wound therapy device in home care patients with low to moderately exuding wounds: a case series. Ostomy Wound Manage 2014 Mar;60(3):30–6.
- Banasiewicz T, Bobkiewicz A, Borejsza-Wysocki M. Portable VAC therapy improve the results of the treatment of the pilonidal sinus — randomized prospective study. Pol Przegl Chir 2013 Jul;85(7):371–6. doi: 10.2478/pjs-2013-0056.
- https://www.moh.gov.sg/content/moh_web/home/costs_and_financing/schemes_subsidies/medisave.html
- Schintler MV. Negative pressure therapy: theory and practice. Diabetes Metab Res Rev 2012;28:S72–7.
- Kopp J, Hoff C, Rosenberg B, von Buelow S, Kilpadi DV, Schroeder W et al. Application of VAC-therapy upregulates growth factor levels in neuropathic diabetic foot ulcers. Wound Repair Reg 2003;11:0.007.
- Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA et al. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006;56:418–22.
- Lu X, Chen S, Li X. The experimental study of the effects of vacuum-assisted closure on edema and vessel permeability of the wound. Chin J Clin Rehab 2003;7:1244–5.



