Volume 24 Number 2
Could hydrocephalus shunts have a role in the treatment of lymphoedema?
Jemima Bell and Neil Piller
Keywords lymphoedema, hydrocephalus, shunt, intralymphatic pressure.
Abstract
Lymphoedema is a consequence of impaired lymphatic drainage. Available treatment options vary in efficacy and impact on the individual. Whilst all are useful in reducing the extent and impact of lymphoedema, there are confounding factors such as patient compliance, financial and physical costs and unpredictably variable outcomes. There seems to be no single treatment that is affordable, effective and sustainable for patients with lymphoedema.
This review introduces the novel idea of a modified hydrocephalus shunt as a surgical alternative to treat (and perhaps prevent) lymphoedema.
Hydrocephalus shunts allow cerebrospinal fluid (CSF) to circumvent an obstruction during periods of impaired absorption, removing the build-up of fluid that causes hydrocephalus, working on a low pressure system.
Lymphatic pumping pressures in failing arm lymphatics have been recorded as approximately 25 mmHg1. In early stage leg lymphoedema, pressures have been recorded around 70mmHg (diastolic) and 100mmHg (systolic), whilst late stage lymphoedema has been recorded as 20mmHg, though often these lymphatics are very difficult to cannulate.
As hydrocephalus shunts work on pressures as low as 15-25mmHg at flow rates as low as 5ml/hr it is plausible that they could be used to facilitate normal flow, but importantly prevent retrograde flow of lymph in failing or failed lymphatics, thereby reducing lymphoedema.
Introduction
The lymphatic system is a low pressure vascular network that has a major role in the maintenance of fluid homeostasis and immune system regulation and defence in the human body2 and is often at risk of damage through illness, surgery, radiotherapy, infection and day-to-day life.
There is a net, low-level filtration of plasma from both the arterial and venous ends of capillary beds, resulting in accumulation of plasma in the interstitium3. This ultra-filtrate comprises fluid, proteins and small molecules which are able to filter out of the capillaries and into the interstitium4 and this interstitial fluid is then taken up into the lymphatic system by the terminal lymphatic capillaries5.
Lymphoedema is a process of either congenital (primary lymphoedema) or acquired (secondary lymphoedema) abnormalities of the lymph vessels that result in impaired lymphatic drainage and fluid accumulation in the tissues6,7. Damage to lymphatic vessels can result in degenerative changes such as hyperplasia of endothelial cells, thickening of sub-endothelial collagen fibres, decreased number of collagen fibres and eventual total occlusion of the lymphatic lumen8. These changes result in an initial deceleration of flow, which causes overloading of the lymphatics and increased intra-lymphatic pressure with subsequent dilation and incompetency of unidirectional valves and retrograde lymph flow. Eventually, due to lack of contractility of lymphatics there are no effective pressures produced by the lymphatics sufficient to propel lymph and as the outflow for lymph is blocked, muscle contractions are unable to empty excess lymph8. This reduction in lymph flow often predisposes patients to poor wound healing, causing further complications.
In developed countries, the greatest cause of lymphoedema is obstructive lymphoedema caused mainly from the treatment of cancer; such as those patients who have received mastectomies and lumpectomies6. Outside of developed nations the most common cause of lymphoedema is lymphatic filariasis, which affects approximately 120 million people worldwide6,9. There are functional, psychosocial and emotional aspects of lymphoedema which impact greatly on the quality of life of sufferers, some of which are associated with the treatment regimens prescribed10.
Current treatment options
As with most interventions, a multidisciplinary approach to the treatment and management of lymphoedema is most effective, currently referred to as complete decongestive physiotherapy or treatment6,11. For lymphoedema, current research dictates that an approach that involves exercise and movement, swelling reduction and maintenance, skin care, risk avoidance and management of pain and psychosocial issues offers the best outcomes for lymphoedema sufferers6. There is currently an array of non-surgical and surgical treatment options available that offer varying degrees of relief for patients.
Physical therapy combined with compression is considered the standard treatment aiming to reduce fluid outflow into the interstitium by reducing venous pressure and flow11. This has been shown to reduce the size of the oedema by 40–60%, with compression garments achieving a size reduction of 46% individually6. However, this treatment is merely a maintenance treatment, not a cure, and one which relies heavily on long-term patient participation and compliance6.
Manual lymphatic drainage is a form of massage developed with the aim of directing fluid towards functioning lymphatics and thereby bypassing damaged or obstructed lymphatics in order to drain an area of congestion6. Extensive studies have demonstrated that it is not beneficial as a stand-alone treatment, and that if done inappropriately (that is, too forcefully) can actually damage lymphatic vessels and increase capillary filtration, which will only worsen oedema. As such, it is a treatment that should only be performed by trained operators, be that therapists, caregivers or the patients themselves6,11.
Pneumomassage, or intermittent pneumatic compression (IPC) involves compression supplied by an external pump and compression stockings to reduce oedema6. This form of intervention is limited by patient compliance, risk of lymph obstruction, and lack of suitability for those with renal or congestive heart failure, and active metastatic disease. There is also a risk of damage to more superficial structures if the pressures in the pump are set too high6.
Liposuction in the treatment of lymphoedema aims to remove fat in the affected limb in order to reduce the appearance and symptoms of lymphoedema6. It is indicated when other, non-surgical treatment options have been exhausted and there is significant discomfort in the affected limb due to weight12. It is, however, not a treatment option for patients with pitting oedema and requires high levels of patient compliance post-surgery with the use of compression garments and wound care6.
Anastomoses can either be lymphatic to lymphatic (lymphatic-lymphatic bypass), lymphatic to vein (lymphaticovenous bypass) or lymph node to veins and arteries (microvascular lymph node transfer)6. These interventions aim to bypass the obstructed lymphatic. Whilst these surgical interventions have been shown to reduce lymph clearance time, there are potential complications, such as the development of lymphoedema at the site of lymphatic harvest and large surgical scars, combined with the fact that there is limited standardised data on the efficacy of such interventions6.
Hydrocephalus shunts
Hydrocephalus shunts traditionally allow cerebrospinal fluid (CSF) to circumvent an obstruction in its normal pathway during periods of impaired absorption, removing the build-up of CSF that causes hydrocephalus. These shunts usually comprise three components: an inflow catheter which drains CSF directly from the ventricles or subarachnoid space; a valve which regulates flow and pressure within the catheter; and an outflow catheter, which connects either to the peritoneal cavity, heart or other drainage site and allows CSF to flow out into this space (Figure 1)13.
Figure 1: Main components of a hydrocephalus shunt14
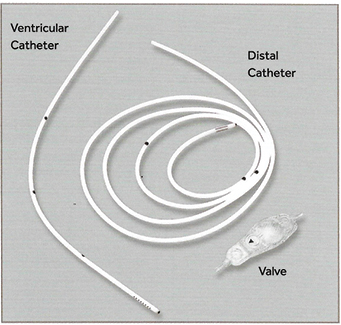
In order to assess whether the use of hydrocephalus shunts would be appropriate in the treatment of lymphoedema, two brands of hydrocephalus shunts commonly used in Australia were considered: Medtronic® and Codman®. Both companies boast an array of shunt systems with catheters designed for various specifications and valves that may operate under single or adjustable pressures15-17. Comparing the pressure-flow rate settings of the Codman CERTAS® Plus Programmable valve and the Medtronic™ Strata™ adjustable valves it can be seen that both operate similarly under very low pressures and flow rates (Figure 2)15,17.
As demonstrated by Figure 2, these adjustable valves operate under pressures as low as 15 mmHg and flow rates as low as 5 mL/min15,17.
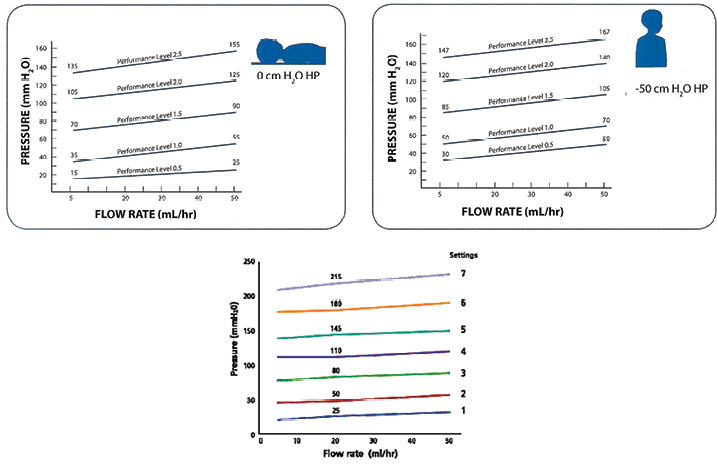
Figure 2: Top are the specifications for the Medtronic™ Strata™ adjustable valves and below are the settings for the Codman® CERTAS® Plus Programmable valve15,17.
Hydrocephalus shunts and intralymphatic pressures
There is limited data available with regard to the pressures acting within human lymphatics; however, studies by Modi et al. and Olszewski and Engeset have shed some light on lymphatic flow rates and pressures under various circumstances. In this review, end lymphatic pressures are being focussed on, as this data will be most relevant to the potential use of a hydrocephalus shunt as treatment.
It is important to note that in healthy individuals, the rhythmic contractions of the lymphatics are the main factor in the generation of pressures sufficient to produce lymph flow, and therefore contractions of surrounding musculature, and external massage and compression of the lymphatics of healthy individuals do not greatly increase flow as the lymphatics are usually empty between pulsations8,18,19 and the pulsations alone are sufficient to propel lymph. Whilst rhythmic contractions of surrounding leg muscles do not increase the mean pressure of normal lymphatics between pulsations, it does create a slight increase in pulse pressure of 0.5–3 mmHg superimposed on the intrinsic pressures produced in both horizontal and upright healthy patients8,20. These contractions also increase the frequency of pulsations8,18,20.
In individuals with obstructive lymphoedema, spontaneous contractions are irregular and ineffective, whilst muscle contractions, external compression of lymphatics through the use of elastic stockings and massage can produce lymph flow19.
Several studies have demonstrated that a systolic pumping pressure of 5–25 mmHg in normal leg lymphatics is required for lymphatic pulsations to begin to occur8,18,20, with most healthy lymphatics reaching systolic pumping pressures of 29–37 mmHg, sufficient to propel lymph, with a tendency towards higher pressures in larger vessels8,20. Diastolic pressures were usually around 0 mmHg between pulsations, during which time there is no flow of lymph8,18,20. In the upright position, normal leg systolic pressures were approximately 44.7 mmHg8,20; however, there have been other studies that have recorded no significant difference between supine and standing healthy leg pressures20.
In early-stage lymphoedematous legs, Olszewski demonstrated that there was an increase in both diastolic pressure (70 mmHg) and systolic pressure (100 mmHg). Muscular contractions then elevated these pressures as high as 200 mmHg. In this early staging of lymphoedema, both the spontaneous lymphatic contractions and the contractions of surrounding musculature were sufficient to propel lymph; however, this was not always propelled proximally due to failed valves8. It is hypothesised that the dramatic increase in intra-lymphatic pressure with muscle contractions in these cases is due to the lymphatics being full of lymph in between lymphatic pulsations19.
In advanced-stage lymphoedematous legs, Olszewski recorded end pressures as low as 20 mmHg with no recorded lymph flow due to incompetent valves; however, these lymphatics were often difficult to cannulate, if at all possible8. Muscular contractions did not help to increase lymph flow in these patients, neither did manual massage of the foot, compression bandaging at a pressure of 40 mmHg and pneumatic massage, as only rudimentary pulse waves could be detected due to fibrotic changes in the lymphatics21.
Modi et al. demonstrated that the average pumping pressures in arms of healthy individuals was 39 mmHg (±14 mmHg) whilst average pumping pressures in lymphoedematous arms was 24 mmHg (±19 mmHg)1. This study had a small population size (16 people in each the healthy and lymphoedematous group), and patients were not separated and observed based on lymphoedema stage.
Taking into consideration these pressures, and the pressures under which hydrocephalus shunts work, it is our hypothesis that a hydrocephalus shunt, modified in size to be suitably inserted into a damaged lymphatic could take the place of the dilated, incompetent lymphatic valves. Then with the aid of local muscle contractions and, if necessary, massage, to force the lymph past the valves, will act in such a way as to prevent retrograde lymph flow, reducing and possibly reversing the process of lymphoedema and giving the lymphatics an opportunity to maintain normal pumping.
Von der Weid and colleagues have been researching the impact of lymph stasis on inflammation and hypothesise that stasis of lymph caused by impaired lymph drainage leads to inflammation22. Further studies have furthered this line of thought and hypothesised that this lymph stasis not only leads to inflammation, but also to fat accumulation and fibrosis and that these three attributes cause and exacerbate each other in a vicious cycle22. As a result, the use of modified hydrocephalus shunts in the treatment of lymphoedema may help to break this cycle, preventing lymph stasis and therefore also inflammation, fat accumulation and fibrosis, which, in turn, will improve lymph flow itself.
Lymphatic diameters vary, depending on their location in the body. Branches in the head and neck have recorded diameters of 0.1 mm in the anterior cervical branches to 1 mm in the supraclavicular and supratrapezoid branches23,24. In the upper limb, lymphatic diameters have been recorded as small as 0.2–0.5 mm in the fingers and hands, and as large as 1.2 mm in the arm above the elbow23. Lymphatics of the lower limb range in diameter from 0.2 mm in the toes and foot to 2.2 mm in the deep branches of the thigh23-26. Despite these small diameters, cannulas with an outer diameter of 1.1 mm have been used to cannulate lymphatics of the foot in the study by Stranden and Kramer27. Similarly Olszewski and Engeset cannulated leg lymphatics of diameters ranging between 0.1 and 0.4 mm using a polyethylene P60 Adams Clay cannula20,28. As the diameters of the catheters currently used with the CODMAN® and Medtronic® hydrocephalus shunts currently range between 0.7 mm and 1.4 mm inner diameter and 2.2 mm and 2.8 mm outer diameter17,29, some adjustments will be needed to produce catheters of smaller diameter, suitable for use with the lymphatics. However, it is promising that catheterisation of vessels this size has already been accomplished. The catheters used by Medtronic® and CODMAN® also have features such as x-ray detectable dots and impregnation with antimicrobials17,29, which may also be appropriate for use in the lymphatics.
Conclusion
Lymphoedema is a condition that affects a wide number and variety of patients, often causing a significant impact on the quality of life of its sufferers. Whilst there are a variety of treatment options available currently, none are curative and most impact the patient’s quality of life and require a high level of compliance.
Damage to the lymphatics resulting in incompetency of unidirectional valves and subsequent retrograde flow of lymph is a key issue in the development of lymphoedema8. This paper introduces the hypothesis that the insertion of a shunt, with a functioning valve, joined on either end to functioning lymphatics, will help to bypass lymphatics with incompetent valves; thereby providing a replacement functional valve and system which should improve the removal of lymph from the drainage site of the damaged lymphatic. Despite the limited amount of data present on human intra-lymphatic pressures in patients with lymphoedema, the current research available suggests that the use of a modified hydrocephalus shunt, inserted into ineffective lymphatics could reduce and possibly reverse the process of lymphoedema. In patients with lymphoedema secondary to the treatment of cancer (that is, lumpectomies, mastectomies and lymph node removal) the focus of the treatment would be to improve lymph drainage from these damaged lymphatic areas and allow lymph flow through scarred tissue once cancer treatment has ceased, as the insertion of a shunt prior to cancer removal would be ineffective. In patients suffering lymphatic filariasis, the aim would be to determine the areas of damaged or dysfunctional lymphatics and cannulate through these. Whilst adjustments to the size of the catheters currently used in hydrocephalus shunts would need to be made for them to be appropriate for use in the lymphatics, the fact that researchers have been able to catheterise the lymphatics already demonstrates that this is achievable. Figure 2 illustrates the low flow/pressure environment that the shunts currently operate under and demonstrate that it is conceivable that a correctly sized shunt could be appropriate for use when the lymphatics and their valves are dysfunctional.
It is hypothesised that the patients who are most likely to benefit will be those with early-stage lymphoedema. Generally speaking, in this group there are reasonable lymph pump pressures, pressure conduction through the tissues due to compression and muscle contraction is relatively good8. Also their lymphatics are easier to cannulate, (as is now evidenced by the tendency to move microsurgical interventions from the late stage of lymphoedema to the earlier ones) and the pressures generated will be more than adequate to pass through the shunt valve. However, for those with advanced lymphoedema where the lymphatics are difficult to cannulate8, there may be an indication for larger/longer areas of shunt bypass in order to find viable lymphatics to connect to. This would not only have a major impact on the quality of life of individual sufferers, but greatly impact the health care burdens of countries worldwide.
Trials of this process remain to be undertaken but the process seems feasible and benefits could be significant.
Conflicts of interest
None.
Author(s)
Jemima Bell*
BMedSc, MD Candidate
School of Medicine, Flinders University SA, Australia
Email bell0247@flinders.edu.au
Tel 0466519361
Neil Piller†
PhD, BSc (Hons), FACP
Director of the Lymphoedema Research Unit
Flinders University, SA, Australia
Email neil.piller@flinders.edu.au
Tel +61 8 82044711
* Corresponding author
† Supervising co-author
References
- Modi S, Stanton AW, Svensson WE, Peters AM, Mortimer PS, Levick JR. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol 2007;583(1):271–285.
- Byung-Boong L, Bergan JB, Rockson SG. Lymphoedema: A concise compendium of theory and practice. London: Springer-Verlag; 2011.
- Hansen KC et al. Lymph formation, composition and circulation: a proteomics perspective. 1460–2377 (Electronic).
- Michelini S, Amore M, Tapia L, Pattarone G, Mercado D, Torrisi J, Savetsky I, Gardenier J, Mehrara B. Embryology. In: Lymphedema: Complete Medical and Surgical Management. Peter Neligan, Jaume Masia, Neil Piller (eds). India: Taylor & Francis Group; 2015.
- Guyton A, Hall J. The Microcirculation and the Lymphatic System: Capillary Fluid Exchange, Interstitial Fluid, and Lymph Flow. In: Textbook of Medical Physiology; 2015.
- Champaneria M, Neligan P. Lymphedema: Lack of Solutions to a Clinical Problem. In: Lymphedema: Complete Medical and Surgical Management. Peter Neligan, Jaume Masia, Neil Piller (eds). India: Taylor & Francis Group; 2015.
- Campisi CC, Ryan M, Campisi C. Multiple Lymphaticovenous Anastomoses and Multiple Lymphatic-Venous-Lymphatic Anastomoses. In: Lymphedema: Complete Medical and Surgical Management. Peter Neligan, Jaume Masia, Neil Piller (eds). India: Taylor & Francis Group; 2015.
- Olszewski WL. Contractility patterns of normal and pathologically changed human lymphatics. Ann N Y Acad Sci 2002;979(1):52–63.
- WHO. Lymphatic filariasis. In: Fact Sheet 102. WM Centre (ed). 2015; p. May.
- Armer J, Hulett JM, Cormier J, Stewart B, Wanchai A, Cromwell K. Lymphedema and Its Impact on Quality of Life. In: Lymphedema: Complete Medical and Surgical Management. Peter Neligan, Jaume Masia, Neil Piller (eds). India: Taylor & Francis Group; 2015.
- Piller N. Conservative Treatments for Lymphedema. In: Lymphedema: Complete Medical and Surgical Management. Peter Neligan, Jaume Masia, Neil Piller (eds). India: Taylor & Francis Group; 2015.
- Brorson H. Liposuction. In: Lymphedema: Complete Medical and Surgical Management. Peter Neligan, Jaume Masia, Neil Piller (eds). India: Taylor & Francis Group; 2015.
- Hydrocephalus Association. Shunt Systems Overview; 2015. Available from: http://www.hydroassoc.org/shunt-systems/.
- Medtronic. Hydrocephalus Therapy: Living with Hydrocephalus. In: Medtronic (ed). Australia; 2015.
- Medtronic. Strata™ Family of Adjustable Valves, Innovative Technology for Hydrocephalus. In: Medtronic (ed). Australia; 2010.
- Medtronic. PS Medical™ Delta™ Valves and Shunts: The Fixed Pressure System with Siphon Control. In: Medtronic Australasia, Medtronic (ed). Australia; 2011.
- Neuro C. CODMAN CERTAS® Plus Programmable Valve, CODMAN CERTAS® Tool Kit. D Synthes (ed). Massachusetts, USA: Codman Neuro; 2013.
- Olszewski W, Engeset A. Studies on the Lymphatic Circulation of Humans. In: Experimental Biology of the Lymphatic Circulation. Elsevier Science Publishers; 1985.
- Olszewski W, Bryla P. Lymph and Tissue Pressures in Patients with Lymphedema During Massage and walking with elastic support. Lymphology 1994;27:512–516.
- Olszewski W, Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol 1980;239(6):H775–83.
- Olszewski W. Contractility patterns of human leg lymphatics in various stages of obstructive lymphedema. Ann N Y Acad Sci 2008;1131(1):110–118.
- von der Weid P-Y. Studying inflammation-induced lymphatic dysfunction to better understand lymphedema. Pathways; 2016(Winter).
- Pan W-R. Changing Concepts in Lymphatic Pathways. In: Lymphedema: Complete Medical and Surgical Management. Peter Neligan, Jaume Masia, Neil Piller (eds). India: Taylor & Francis Group; 2015
- Pan WR, Le Roux CM, Briggs CA. Variations in the lymphatic drainage pattern of the head and neck: further anatomic studies and clinical implications. Plast Reconstr Surg 2011;127(2):611.
- Pan WR, Wang DG, Levy SM, Chen Y. Superficial lymphatic drainage of the lower extremity: anatomical study and clinical implications. Plast Reconstr Surg 2013;132(3):696–707.
- Tourani SS, Taylor GI, Ashton MW. Anatomy of the superficial lymphatics of the abdominal wall and the upper thigh and its implications in lymphatic microsurgery. J Plast Reconstr Aesthet Surg 2013;66.
- Stranden E, Kramer K. Lymphatic and transcapillary forces in patients with edema following operation for lower limb atherosclerosis. Lymphology 1982;15.
- Olszewski W, Engeset A. Intrinsic contractility of leg lymphatics in man preliminary communication. Lymphology 1979;12.
- Medtronic. CSF-Drainage and Monitoring Products: External Drainage and Monitoring Kits. In: Medtronic (ed); 2013.



