Volume 24 Number 2
Immune regulation by the peripheral lymphatics and its implications for wound healing and infection control in lymphoedema
David G Hancock, Tessa M Potezny and Patrick M White
Abstract
Lymphoedema is complex disorder with high disease morbidity characterised initially by progressive fluid accumulation and subsequently by altered tissue fibrosis and fat deposition. Primary lymphoedema is the result of congenital conditions that affect how lymph vessels are formed, whilst the inciting event in secondary lymphoedema is classically the disruption of normal lymphatic flow in the context of surgery or trauma. In addition to the altered fluid and fat homeostasis, lymphoedema is characterised by immune deficits that typically manifest as an increased susceptibility to infection and altered wound healing in the affected site. In contrast to the common perception of the lymphatics as a passive conduit for fluid, waste products, and immune cells, the altered immune homeostasis in lymphoedema patients suggests that the lymphatics play a more active role in the immune response. Indeed, lymphatic dysfunction appears to be a global phenomenon in all immune-related diseases. In this review, we highlight papers that support an active role for the lymphatics in immunity and link this evidence to the observed deficits in wound healing and immune surveillance present in lymphoedema.
Introduction
The peripheral lymphatic system is made up of a complex network of lymphatic vessels that connect local tissue sites with secondary lymphoid organs. The peripheral lymphatics play an essential role in the regulation of fluid balance, in the transport of fatty acids from the gastrointestinal tract, and the trafficking of immune cells to and from the periphery. Specifically, the transport of dendritic cells to lymphoid organs for the generation of adaptive immune responses and the drainage of local immune mediators for the maintenance of local immune homeostasis have been the primary immune roles of the lymphatic system described to date1,2.
Alterations in the peripheral lymphatics, as is seen with specific genetic deficiencies or peripheral lymphatic destruction, can lead to primary and secondary lymphoedema, respectively. Lymphoedema was originally considered a circulatory condition characterised initially by abnormal fluid distribution, with the progression to fat deposition/fibrosis in its later stages. However, there is also evidence of concurrent immune dysfunction in lymphoedema, suggesting that lymphoedema could also be classified as a functional immune disorder. The immune dysfunction present in lymphoedema typically manifests in excessive fibrosis, local inflammation, poor wound healing, and an increased susceptibility to infections and new malignancies3-5. Indeed, these consequences are a major contributor to the extremely high disease morbidity and poor quality of life consistently observed in patients with lymphoedema as well as being the primary reason for hospitalisation in this patient population3.
However, the mechanisms leading to the immune deficits in lymphoedema remain poorly understood. While the role of the peripheral lymphatic system in immune trafficking is well described, it may be that the lymphatics play additional roles in regulating the phenotype and function of local immune populations.
While the incidence of lymphoedema is relatively low, the insights gained from understanding the progression from primary lymphatic disruption to the observed patterns of altered immune homeostasis has important consequences for progressing our understanding of the complexity of immunological disease in humans. Indeed, de novo lymphangiogenesis, the formation of new lymphatic vessels, has been identified in the majority of human inflammatory conditions, including psoriasis, renal inflammation, and chronic airway inflammation6,7. In addition to the production of new lymphatic vessels, the phenotype of the lymphatic vessels present can also be altered as compared to controls, suggesting altered lymphatic function contributes to the pathogenesis of these diseases8,9. Finally, peripheral lymphatic vessels also play key roles in transplant rejection and tumour metastases10. Therefore, it appears clear that lymphatic function has strong implications for a wide range of immune-based conditions, supporting evidence of an active role of the lymphatics in immunity.
In this review, we attempt to consolidate the evidence surrounding lymphatic-mediated immune responses to provide mechanistic insights into the progression from disrupted lymphatic vessel flow to altered wound healing and immune clearance in lymphoedema.
Lymphatic regulation of the immune response
The peripheral lymphatics system has been implicated in the regulation of immune cell migration and activation, as well as the clearance of local immune mediators and waste products from the effector sites, all of which are influenced by de novo lymphangiogenesis1,2,11-14. While these functions have been described in a number of primarily in vitro or model organism studies, there remains a lack of strong mechanistic links between these functions and the observed immune deficits in lymphoedema or other immune conditions with lymphatic involvement.
Regulation of cellular migration
The migration of dendritic cells from an infected tissue site to the draining lymph node is the essential first step in the initiation of T cell and B cell responses to peripheral infections. Thus, the lymphatics role in modulating the migration and function of dendritic cells has important consequences for adaptive immunity.
In response to inflammation, dendritic cells are known to upregulate the expression of a range of different chemokine receptors and integrins, which facilitate their migration through the lymphatics to the draining lymph nodes15. The most prominent molecules involved in dendritic cell migration through the lymphatics appear to be the chemokine receptor CCR7 (recognising lymphatic expressed CCL21) and the integrin LFA-1 (recognising lymphatic expressed ICAM-1)2,11,13,16-18, although a number of other molecules have been implicated19,20. However, given that the lymphatics have been shown to upregulate the expression of the chemokine CCL21 and integrin ICAM-1 in response to the same infectious signals that induce the expression of their partner molecules on dendritic cells, the activation programs of both dendritic cells and the lymphatics appear inseparable and equally important in this initiation step for adaptive immunity. In further support of the active role the lymphatics play in this process, lymphatic activation can induce the structural reorganisation and formation of ICAM-1 enriched microvilli structures, which optimise dendritic cell attachment and migration17.
While, the CCL21-CCR7 axis has been strongly linked with dendritic cell migration through the lymphatics, it is also important to note that CCL21 is an important chemoattractant for recruiting local macrophages to sites of inflammation (without inducing their migration through the lymphatics to the draining lymph nodes)21.
Given that T cells and neutrophils can also traffic through the peripheral lymphatics system and migrate in response to chemokine gradients, the lymphatics likely play a similar active role in inducing the migration and local recruitment of these local cell populations22-24. For example, various T cell subsets also express CCR7 and thus their migration or local recruitment is likely to be influenced by lymphatic expressed CCL2123.
Regulation of cellular activation
While the role of the lymphatics system in regulating cell migration is well established, there is strong circumstantial evidence to suggest an additional role in regulating cellular activation. However, these mechanisms have largely not been confirmed in vivo and as such the relative importance of this lymphatic function remains unclear.
While playing a primary role in inducing cellular migration, it is important to note that chemokine and integrin signalling additionally acts to induce cell activation25,26. For example, mice deficient in CCL21 (which signals through the chemokine receptor CCR7), not only show deficits in dendritic cell migration, but important additional defects in dendritic cell maturation, proliferation, differentiation, endocytosis function and overall survival25. Similarly, lymphatic expressed ICAM-1 (which signals through the integrin partner LFA-1) has been shown to downregulate dendritic cell expression of CD86 and other maturation markers, which in turn reduces the dendritic cell’s ability to activate T cell responses26. Thus cell migration and activation are clearly linked, and as such, the lymphatics are clearly important regulators of this process.
In addition to the expression of molecules involved in cellular migration, the lymphatics can also express a wide range of poly-functional pro-inflammatory and anti-inflammatory molecules13,27. For example, lipopolysaccharide has been shown to induce the expression of IL-6 in lymphatic endothelial cells in vitro13. IL-6 is an extremely important immune regulator that has been shown to regulate the activation and effector function of neutrophils, macrophages, and dendritic cells, among a range of other functions28. In addition, IL-6 has been clearly shown to contribute to the autoimmune driven inflammation in disease like rheumatoid arthritis, and as such has been successfully targeted with the IL-6R antibody, Tociluzimab28. While a large number of immune cell populations have been shown to express IL-6 including fibroblasts, macrophages, neutrophils, and T-cells, the observation of lymphatic IL-6 expression strongly suggests that the lymphatics are contributing to immune cell activation and the progression of the immune response. Indeed, this mechanism of immune regulation can be extended to a large number of other immune mediators that the lymphatics express in response to stimulation29.
Regulation of Immune Homeostasis through Soluble Mediators and Antigen
As the primary conduit system draining fluid and waste tissues from peripheral sites, the lymphatics play an important role in maintaining the homeostasis of these factors. While the production of cytokines and chemokines (produced by both lymphatic endothelial cells and other immune populations) shape and direct the local immune response, the removal of these mediators is equally important for preventing chronic inflammation or aberrant signalling pathways1,12.
The lymphatics are also involved in the active removal of cytokines and chemokines from circulation via the expression of scavenger receptors like D6, which internalise chemokines and pro-inflammatory cytokines1,12. Importantly, mice deficient in the scavenger receptor D6, showed grossly exaggerated inflammatory responses at local sites and altered patterns of immune cell migration and recruitment30, a general pattern sharing some similarities with the immune deficits in lymphoedema3-5.
Finally, the passive transport of antigen to the secondary lymphoid organs is important for maintaining self-tolerance (to self-antigens only expressed in the periphery) or generating immune responses (to pathogenic antigens), separate from the dendritic cell-mediated transport of these antigens1,12.
Regulation of Immune Homeostasis through Lymphangiogenesis
De novo lymphangiogenesis appears to be a critical step in many inflammatory contexts for promoting fluid drainage and immune cell migration from the site of inflammation, and as such is frequently observed in human disease31,32. Lymphangiogenesis is classically induced by the canonical vascular endothelial growth factors (VEGFs)-A, -C, and -D. Importantly, these VEGF molecules are commonly produced by activated macrophages, T cells, mast cells, and dendritic cells in response to a diverse range of immunogenic stimuli6,33-35, which provides a clear explanation for the observed changes in lymphangiogenesis in the majority of human immune-mediated diseases. Furthermore, lymphangiogenesis can also be induced by a range of secondary immune mediators (for example, IL-10, TFGβ) either directly or indirectly via the upregulation of VEGF expression by other immune cells36,37. Similarly, lymphangiogenesis can also be negatively regulated by immune mediators, including the Th2 cytokines IL-4 and IL-1338.
Most importantly, lymphangiogenesis is not always associated with favourable outcome, as the pathogenesis of ocular inflammation, transplant rejection, and tumour metastasis are often driven by undesired lymphangiogenesis10,39. Furthermore, it has been suggested that chronic inflammation can drive a disordered process of lymphangiogenesis, which actually impairs immune cell migration and fluid drainage when compared to the structured process of lymphangiogenesis in an appropriate inflammatory response40,41.
Stimulus-Specific Lymphatic Activation
The immune system allows for appropriate responses to distinct pathogens through the generation of stimulus-specific effector programs, such as the classical division between Th1 versus Th2 versus Th17 T cell responses. A given immune response is established through the integration of primary (to pathogens) and secondary (to immune cytokines/chemokines) activation signals. It is the capacity of these immune cells to generate the appropriate stimulus-specific response that determines whether the immune response effectively controls the pathogen or results in the development of an inappropriate response leading to chronic inflammation or autoimmunity. Thus to be considered an active regulator of the immune response, the lymphatics need to generate stimulus-specific effector programs in response to distinct stimuli.
Lymphatic endothelial cells express functional toll-like receptors (TLRs) 1–6 and 9 and can thus respond to a range of pathogenic stimuli including lipopolysaccharide (LPS) (via TLR4) or lipoteichoic acid (via TLR2); both major constituents of the bacterial cell wall10,13,42-44. Importantly, as with other cell populations distinct patterns of cytokines and chemokines are induced when lymphatic endothelial cells are stimulated in vitro with different TLR ligands10,13,43,44. For example, lipopolysaccharide (signalling via TLR4) induced the upregulated expression of the chemokine CCL20 in lymphatic endothelial cells in vitro, while stimulation with heat-killed Listeria monocytogenes (signalling primarily through TLR2) did not10.
Consistent with their response to a number of diverse pathogenic stimuli, lymphatic endothelial cells can also respond to a number of key immune mediators including adrenomedullin, chemokine (C-X-C Motif) ligand 12 (CXCL12), high-mobility group box 1 (HMGB1), histamine, hypoxia inducible factor-alpha (HIF-1α), interferon alpha (IFNα), IFNβ, IFNγ, IL-1β, IL-4, IL-6, IL-8, IL-13, IL-20, IL-27, oncostatin M, retinoic acids, thrombin, transforming growth factor beta (TFGβ), and tumour necrosis factor alpha (TNFα)18,37,45-60. Importantly, the responses to these secondary activation signals also appear to be stimulus-specific. One paper described unique effector responses observed to TNFα, IL-1β, or IFNγ stimulation in vitro, including the selective upregulation of the cell adhesion molecule E-selectin, in response to stimulation with IFNγ, but not to stimulation with TNFα or IL-1β47. Consistent with the pathogen-specific regulation of CCL20, lymphatic endothelial cell stimulation with TNFα or oncostatin M also induced the expression of CCL20 in vitro, while stimulation with IL-1β did not10.
This stimulus-dependent specificity is also observed in more physiological models of inflammation. For example, large differences in the transcriptional expression of key chemokines and integrins were observed in lymphatic endothelial cells isolated from a mouse model of oxazolone-induced contact hypersensitivity versus a mouse model of Complete Freund’s Adjuvant-induced inflammation61. Given the diverse immunoregulatory roles of the lymphatics (as discussed above), it seems likely that the different lymphatic activation programs in these two models is at least partially contributing to the gross differences in inflammation, cell activation/migration, and levels of oedema additionally observed in these models61. However, the relative contribution of the lymphatics to these differences, as compared to the effect of other cell populations (for example, macrophages) that are differentially activated in these two models is difficult to assess.
It should also be noted that over 1000 genes were differentially expressed in the inflammation-activated lymphatics in these models61. Given that we have only established the relevance of a few chemokines and adhesion molecules in this list, these results strongly highlight our relatively poor understanding of lymphatic function.
Clinical Significance of Lymphatic Function
While a number of diverse immune functions have been suggested for the peripheral lymphatics (as discussed above), the majority of these studies have been performed in model systems and their importance in clinical disease is less clear. However, there is strong indirect evidence to suggest that the in vitro observations of lymphatic immune function are relevant in vivo.
In a study that compared gene expression in lymphatic endothelial cells isolated from lymphoedema skin and normal controls, over 2500 genes were found to be differentially regulated, including important pro-inflammatory genes IL-6, IL-8, and IL-3214. It should be noted that this study would have ideally compared gene expression in lymphoedema to gene expression in ‘normal’ inflammation, in order to better assess which genes were specifically dysregulated in response to the unique inflammatory context of lymphoedema. Lymphatic filariasis (also known as elephantiasis) is a tropical disease caused by the filarial parasites Wuchereria bancrofti (90% of cases), Brugia malayi, and Brugia timori that presents with a clinical picture remarkably similar to lymphoedema62. In a gene expression study assessing the effects of lymphatic endothelial cell stimulation with Brugia malayi, key immunological molecules related to lymphangeogenesis and immune cell migration/activation were shown to be differentially expressed as compared to controls14. Finally a large number of differentially expressed genes have been observed in a study assessing lymphatic endothelial cell gene expression in type 2 diabetic patients, whose deficits in wound healing and immunity share some characteristics with those in lymphoedema patients63. Thus, these three gene expression studies all showed the differential expression of a large number of immune-related genes in different contexts where there is known lymphatic and immune dysfunction. While not directly assessed, when considered in the context of the in vitro studies (as discussed above), these results imply that the altered pattern of immune molecule expression is contributing to the altered immune homeostasis in a clinical context.
Alterations in the lymphatics system, especially in the phenotype of lymphatic vessels and in patterns of de novo lymphangiogenesis, have also been observed in the majority of human immune diseases6-9. As an example, the autoimmune, inflammatory skin condition psoriasis is characterised by a number of changes in the peripheral lymphatics system that together imply a role for the lymphatics in the pathogenesis of this disease. The immune pathogenesis in psoriasis is not fully understood, but thought to be driven by T cells polarised towards a Th-1 or Th-17 phenotype64,65. Interestingly, the lymphatics have been shown to be able to regulate their expression of key immune mediators in a stimulus-specific manner in response to the majority of cytokines that have been implicated in the pathogenesis of psoriasis64,65, including IL-2754 or TNFα47. In addition, increased tissue levels of VEGF, with corresponding increases in lymphatic vessel density, have been frequently observed in the skin of psoriasis patients32,66. Finally, in a mouse model of psoriasis, systemic VEGF blockade significantly improved the levels of inflammation, further implying a direct immunoregulatory role for the lymphatics67. Thus, while these mechanisms have not been conclusively established, these results strongly suggest that the lymphatics are in fact playing an important role in psoriasis and related immune-mediated conditions.
Linking Lymphatic Damage to the Immune Pathogenesis of lymphoedema
Secondary lymphoedema is generally initiated by local lymphatic damage following radiation or surgery, but results in a number of global immune deficits, including defects in immune surveillance (susceptibility to infection and malignancy) and local immune homeostasis (abnormal fibrosis, inflammation, and wound healing)3,4. While these immune deficits are commonly observed in lymphoedema patients, the mechanisms of disease progression following an initial lymphatic insult are poorly understood. However, the active immune roles of the peripheral lymphatics (as discussed in this review) may provide an insight into the pathogenesis of lymphoedema (Figure 1).
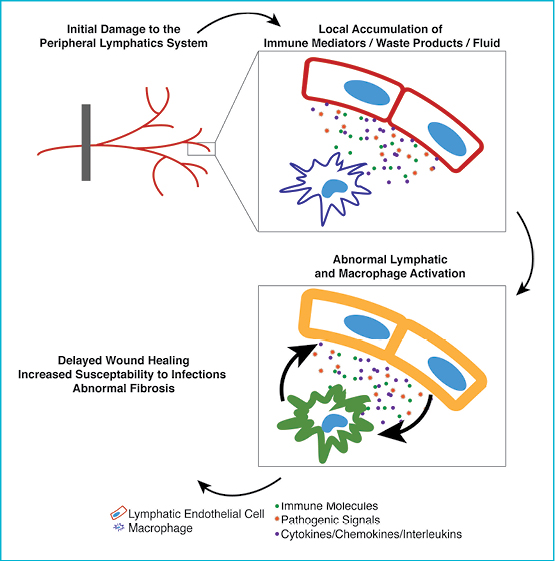
Figure 1
The primary result of lymphatic vessel damage following interventions like surgery or radiotherapy is a gross reduction in drainage function. This results in the local accumulation of fluid, waste products, immune mediators (cytokines and chemokines), and immune cells that are unable to effectively transit to lymphoid organs. The irregular build up of these molecules appears to be the initiating factor driving the altered activation programs in the lymphatic endothelial cells and in other immune cell populations (for example, macrophages) observed in lymphoedema and chronic inflammation14,61. However, given that the lymphatics are known to respond to immune mediators produced by macrophages (including TNFα, IL-1β, and IFNγ)47 and produce immune mediators that regulate the function of macrophages (including IL-6 and CCL21)13, it is likely that abnormal lymphatic activation perpetuates the abnormal activation of macrophages, and visa versa. Indeed, while the accumulation of these molecules may initially result in an excessive, but otherwise normal, pattern of inflammation, it is likely that prolonged exposure and persistent immune cell activation results in the disordered and abnormal inflammation characteristic of lymphoedema.
Given that both macrophages and the lymphatics system have been critically implicated in the normal wound healing process68-71, it is not surprising that wound healing is severely compromised in the grossly abnormal immune microenvironment of lymphoedema72,73. Specifically, IL-10 and TGFβ have both been linked to the abnormal wound healing observed in lymphoedema and both have been shown to modulate the phenotype and function of both macrophages and lymphatic endothelial cells50,74,75. Similarly, abnormal lymphatic endothelial cell activation and subsequent abnormal modulation of dendritic cell function, coupled with gross defects in the ability of dendritic cells to migrate to lymphoid organs through the disrupted lymphatics, likely explains the increased susceptibility to infection in lymphoedema.
Conclusions
This review has focused on highlighting the lymphatics as an active and integrated component of the immune response. While the initiating process in secondary lymphoedema may be disruption of lymphatic flow and the accumulation of fluid, waste products, and immune mediators, these initial processes are likely to drive a dysregulated cycle of abnormal lymphatic and immune cell activation, that affects the normal capacity of these cells to mediate wound healing and immune clearance.
Author(s)
David G Hancock*
PhD, MD Candidate
Lymphoedema Research Unit, Department of Surgery, School of Medicine, Flinders University, SA, Australia
Email hanc0130@flinders.edu.au
Phone +61 8 8204 4903
Tessa M Potezny
MD Candidate
Lymphoedema Research Unit, Department of Surgery, School of Medicine, Flinders University, SA, Australia
Patrick M White
BSc, MD Candidate
Lymphoedema Research Unit, Department of Surgery, School of Medicine, Flinders University, SA, Australia
* Corresponding author
References
- Hansen KC, D’Alessandro A, Clement CC, Santambrogio L. Lymph formation, composition and circulation: a proteomics perspective. Int Immunol 2015;27(5):219–27.
- Russo E, Teijeira A, Vaahtomeri K, Willrodt AH, Bloch JS, Nitschke M et al. Intralymphatic CCL21 Promotes Tissue Egress of Dendritic Cells through Afferent Lymphatic Vessels. Cell Rep 2016;14(7):1723–34.
- Rockson SG. The lymphatics and the inflammatory response: lessons learned from human lymphedema. Lymphat Res Biol 2013;11(3):117–20.
- Ruocco V, Schwartz RA, Ruocco E. Lymphedema: an immunologically vulnerable site for development of neoplasms. J Am Acad Dermatol 2002;47(1):124–7.
- Lund AW, Medler TR, Leachman SA, Coussens LM. Lymphatic Vessels, Inflammation, and Immunity in Skin Cancer. Cancer Discov 2016;6(1):22–35.
- Aebischer D, Iolyeva M, Halin C. The inflammatory response of lymphatic endothelium. Angiogenesis 2014;17(2):383–93.
- Seeger H, Bonani M, Segerer S. The role of lymphatics in renal inflammation. Nephrol Dial Transplant 2012;27(7):2634–41.
- Mori M, Andersson CK, Graham GJ, Lofdahl CG, Erjefalt JS. Increased number and altered phenotype of lymphatic vessels in peripheral lung compartments of patients with COPD. Respiratory Research 2013;14(1):65.
- Bouta EM, Li J, Ju Y, Brown EB, Ritchlin CT, Xing L et al. The role of the lymphatic system in inflammatory-erosive arthritis. Semin Cell Dev Biol 2015;38:90–7.
- Pegu A, Qin S, Fallert Junecko BA, Nisato RE, Pepper MS, Reinhart TA. Human lymphatic endothelial cells express multiple functional TLRs. J Immunol 2008;180(5):3399–405.
- Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med 2011;208(10):2141–53.
- Santambrogio L, Stern LJ. Carrying yourself: self antigen composition of the lymphatic fluid. Lymphat Res Biol 2013;11(3):149–54.
- Sawa Y, Ueki T, Hata M, Iwasawa K, Tsuruga E, Kojima H et al. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J Histochem Cytochem 2008;56(2):97–109.
- Ogunbiyi S, Chinien G, Field D, Humphries J, Burand K, Sawyer B et al. Molecular characterization of dermal lymphatic endothelial cells from primary lymphedema skin. Lymphat Res Biol 2011;9(1):19–30.
- Teijeira A, Russo E, Halin C. Taking the lymphatic route: dendritic cell migration to draining lymph nodes. Semin Immunopathol 2014;36(2):261–74.
- Johnson LA, Jackson DG. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int Immunol 2010;22(10):839–49.
- Teijeira A, Garasa S, Pelaez R, Azpilikueta A, Ochoa C, Marre D et al. Lymphatic Endothelium Forms Integrin-Engaging 3D Structures during DC Transit across Inflamed Lymphatic Vessels. J Invest Dermatol 2013.
- Teijeira A, Palazon A, Garasa S, Marre D, Auba C, Rogel A et al. CD137 on inflamed lymphatic endothelial cells enhances CCL21-guided migration of dendritic cells. FASEB J 2012;26(8):3380–92.
- Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 2012;37(2):276–89.
- Johnson LA, Jackson DG. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J Cell Sci 2013;126(Pt 22):5259–70.
- Kang S, Lee SP, Kim KE, Kim HZ, Memet S, Koh GY. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood 2009;113(11):2605–13.
- Chakraborty S, Zawieja SD, Wang W, Lee Y, Wang YJ, von der Weid PY et al. Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. Am J Physiol Heart Circ Physiol 2015;309(12):H2042–57.
- Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol 2013;13(5):309–20.
- Rigby DA, Ferguson DJ, Johnson LA, Jackson DG. Neutrophils rapidly transit inflamed lymphatic vessel endothelium via integrin-dependent proteolysis and lipoxin-induced junctional retraction. J Leukoc Biol 2015;98(6):897–912.
- Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity 2005;22(4):493–505.
- Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol 2009;183(3):1767–79.
- Miller CN, Hartigan-O’Connor DJ, Lee MS, Laidlaw G, Cornelissen IP, Matloubian M et al. IL-7 production in murine lymphatic endothelial cells and induction in the setting of peripheral lymphopenia. Int Immunol 2013.
- Schaper F, Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev 2015;26(5):475–87.
- Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest 2014;124(3):943–52.
- Di Liberto D, Locati M, Caccamo N, Vecchi A, Meraviglia S, Salerno A et al. Role of the chemokine decoy receptor D6 in balancing inflammation, immune activation, and antimicrobial resistance in Mycobacterium tuberculosis infection. J Exp Med 2008;205(9):2075–84.
-
Liao S, von der Weid PY. Lymphatic system: an active pathway for immune protection. Semin Cell Dev Biol 2015;38:83–9.
-
Varricchi G, Granata F, Loffredo S, Genovese A, Marone G. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J Am Acad Dermatol 2015;73(1):144–53.
- Dieterich LC, Seidel CD, Detmar M. Lymphatic vessels: new targets for the treatment of inflammatory diseases. Angiogenesis 2014;17(2):359–71.
- Huggenberger R, Ullmann S, Proulx ST, Pytowski B, Alitalo K, Detmar M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med 2010;207(10):2255–69.
- Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol 2000;156(5):1499–504.
- Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y et al. Transforming growth factor-beta induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int 2012;81(9):865–79.
- Hos D, Bucher F, Regenfuss B, Dreisow ML, Bock F, Heindl LM et al. IL-10 Indirectly Regulates Corneal Lymphangiogenesis and Resolution of Inflammation via Macrophages. Am J Pathol 2016;186(1):159–71.
- Savetsky IL, Ghanta S, Gardenier JC, Torrisi JS, Garcia Nores GD, Hespe GE et al. Th2 cytokines inhibit lymphangiogenesis. PloS One 2015;10(6):e0126908.
- Chauhan SK, Dohlman TH, Dana R. Corneal Lymphatics: Role in Ocular Inflammation as Inducer and Responder of Adaptive Immunity. J Clin Cell Immunol 2014;5.
- Kim H, Kataru RP, Koh GY. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol 2012;33(7):350–6.
- Liao S, von der Weid PY. Inflammation-induced lymphangiogenesis and lymphatic dysfunction. Angiogenesis 2014;17(2):325–34.
- Zampell JC, Elhadad S, Avraham T, Weitman E, Aschen S, Yan A et al.. Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am J Physiol Cell Physiol 2012;302(4):C709–19.
- Sawa Y, Tsuruga E. The expression of E-selectin and chemokines in the cultured human lymphatic endothelium with lipopolysaccharides. J Anatomy 2008;212(5):654–63.
- Sawa Y, Tsuruga E, Iwasawa K, Ishikawa H, Yoshida S. Leukocyte adhesion molecule and chemokine production through lipoteichoic acid recognition by toll-like receptor 2 in cultured human lymphatic endothelium. Cell Tissue Res 2008;333(2):237–52.
- Breslin JW. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction. Lymphat Res Biol 2011;9(1):3–11.
- Bryant-Hudson KM, Chucair-Elliott AJ, Conrady CD, Cohen A, Zheng M, Carr DJ. HSV-1 Targets Lymphatic Vessels in the Eye and Draining Lymph Node of Mice Leading to Edema in the Absence of a Functional Type I Interferon Response. Am J Pathol 2013.
- Chaitanya GV, Franks SE, Cromer W, Wells SR, Bienkowska M, Jennings MH et al. Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in vitro. Lymphat Res Biol 2010;8(3):155–64.
- Choi I, Lee S, Kyoung Chung H, Suk Lee Y, Eui Kim K, Choi D et al. 9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-cis retinoic acid for secondary lymphedema. Circulation 2012;125(7):872–82.
- Choi I, Lee YS, Chung HK, Choi D, Ecoiffier T, Lee HN et al. Interleukin-8 reduces post-surgical lymphedema formation by promoting lymphatic vessel regeneration. Angiogenesis 2013;16(1):29–44.
- Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A et al. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 2008;295(5):H2113–27.
- Hammer T, Tritsaris K, Hubschmann MV, Gibson J, Nisato RE, Pepper MS et al. IL-20 activates human lymphatic endothelial cells causing cell signalling and tube formation. Microvasc Res 2009;78(1):25–32.
- Jin D, Otani K, Yamahara K, Ikeda T, Nagaya N, Kangawa K. Adrenomedullin reduces expression of adhesion molecules on lymphatic endothelial cells. Regul Pept 2011;166(1–3):21–7.
- McKimmie CS, Singh MD, Hewit K, Lopez-Franco O, Le Brocq M, Rose-John S et al. An analysis of the function and expression of D6 on lymphatic endothelial cells. Blood 2013;121(18):3768–77.
- Nielsen SR, Hammer T, Gibson J, Pepper MS, Nisato RE, Dissing S et al. IL-27 inhibits lymphatic endothelial cell proliferation by STAT1-regulated gene expression. Microcirculation 2013.
- Qiu Y, Chen Y, Fu X, Zhang L, Tian J, Hao Q. HMGB1 promotes lymphangiogenesis of human lymphatic endothelial cells in vitro. Med Oncol 2012;29(1):358–63.
- Sugaya M, Fang L, Cardones AR, Kakinuma T, Jaber SH, Blauvelt A et al. Oncostatin M enhances CCL21 expression by microvascular endothelial cells and increases the efficiency of dendritic cell trafficking to lymph nodes. J Immunol 2006;177(11):7665–72.
- Zhuo W, Jia L, Song N, Lu XA, Ding Y, Wang X et al. The CXCL12-CXCR4 chemokine pathway: a novel axis regulates lymphangiogenesis. Clin Cancer Res 2012;18(19):5387–98.
- Al-Kofahi M, Becker F, Gavins FN, Woolard MD, Tsunoda I, Wang Y et al. IL-1beta reduces tonic contraction of mesenteric lymphatic muscle cells, with the involvement of cycloxygenase-2 and prostaglandin E2. Br J Pharmacol 2015;172(16):4038–51.
- Zampell JC, Yan A, Avraham T, Daluvoy S, Weitman ES, Mehrara BJ. HIF-1alpha coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J 2012;26(3):1027–39.
- Shin K, Kataru RP, Park HJ, Kwon BI, Kim TW, Hong YK et al. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat Commun 2015;6:6196.
- Vigl B, Aebischer D, Nitschke M, Iolyeva M, Rothlin T, Antsiferova O et al. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 2011;118(1):205–15.
- Bennuru S, Nutman TB. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: implications for pathogenesis. PLoS Pathog 2009;5(12):e1000688.
- Haemmerle M, Keller T, Egger G, Schachner H, Steiner CW, Stokic D et al. Enhanced lymph vessel density, remodeling, and inflammation are reflected by gene expression signatures in dermal lymphatic endothelial cells in type 2 diabetes. Diabetes 2013;62(7):2509–29.
- Shibata S, Tada Y, Kanda N, Nashiro K, Kamata M, Karakawa M et al. Possible roles of IL-27 in the pathogenesis of psoriasis. J Invest Dermatol 2010;130(4):1034–9.
- Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol 2012;51(4):389–95; quiz 95–8.
- Henno A, Blacher S, Lambert C, Colige A, Seidel L, Noel A et al. Altered expression of angiogenesis and lymphangiogenesis markers in the uninvolved skin of plaque-type psoriasis. Br J Dermatol 2009;160(3):581–90.
- Schonthaler HB, Huggenberger R, Wculek SK, Detmar M, Wagner EF. Systemic anti-VEGF treatment strongly reduces skin inflammation in a mouse model of psoriasis. Proc Natl Acad Sci U S A 2009;106(50):21264–9.
- Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 2011;13:e23.
- Leoni G, Neumann PA, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune-epithelial interactions. Mucosal Immunol 2015;8(5):959–68.
- Martinez-Corral I, Olmeda D, Dieguez-Hurtado R, Tammela T, Alitalo K, Ortega S. In vivo imaging of lymphatic vessels in development, wound healing, inflammation, and tumor metastasis. Proc Natl Acad Sci U S A 2012;109(16):6223–8.
- Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 2007;170(4):1178–91.
- Macdonald JM. Wound healing and lymphedema: a new look at an old problem. Ostomy Wound Manage 2001;47(4):52–7.
- Mallon EC, Ryan TJ. Lymphedema and wound healing. Clin Dermatol 1994;12(1):89–93.
- Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG et al. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol 2010;177(6):3202–14.
- Kimura T, Sugaya M, Blauvelt A, Okochi H, Sato S. Delayed wound healing due to increased interleukin-10 expression in mice with lymphatic dysfunction. J Leukoc Biol 2013;94(1):137–45.



