Volume 24 Number 4
IWII Wound infection in clinical practice consensus document 2016 update
Terry Swanson, Emily Haesler, Donna Angel and Geoff Sussman
Keywords debridement, Wound infection continuum, biofilm, wound cleansing, wound infection management.
Abstract
The International Wound Infection Institute (IWII) is a volunteer group of interdisciplinary health professionals dedicated to advancing and improving practice relating to the prevention and control of wound infection. The second edition of Wound infection in clinical practice is an update of the first edition published in 2008 and was endorsed by the World Union of Wound Healing Societies (WUWHS). The original document was authored by leading experts who were in wound management, many of whom formed the inaugural committee of the IWII.
For the second edition, the IWII collaborative team undertook a comprehensive review of contemporary literature, including systematic reviews and meta-analyses when available. In addition, the team conducted a formal Delphi process to reach consensus on wound infection issues for which scientific research was minimal or lacking. This rigorous process provided a document with an update on the science and expert opinion regarding prevention, diagnosis and control of wound infection. The updated document outlines new definitions relevant to wound infection, presents new paradigms and advances in the management and diagnosis of a wound infection, and highlights controversial areas of discussion. The intent is to provide a practical, updated resource that is easy to use and understand.
Introduction
The Wound Infection Institute (WII) was formed formally in 2008 prior to the World Union Wound Healing Societies (WUWHS) conference in Toronto; however, the name was changed to the International Wound Infection Institute (IWII) in 2009 due to the launch of the WII games.
Prior to the Institute’s formation, a group met in Budapest in 2006 through an unrestricted grant by Smith & Nephew, with the aim of determining best practice for wound infection of various aetiologies. The 2006 participants were from 23 countries and most were health care professionals with disciplines related to wound management or the science of wound infection.
This volunteer group of scientists and health care professionals was dedicated to advancing and improving practice relating to the prevention and control of wound infection. In 2008 an expert group, many of whom were founding members of the inaugural IWII committee, authored the first edition of Wound infection in clinical practice (2008). In 2014 it was acknowledged that practice and knowledge had advanced and the document required an update, leading to the publication of the second edition of Wound infection in clinical practice in November 2016.
Work on the second edition commenced in earnest in May 2015, when many of the team members met in London to attend a development meeting. At this meeting the strategic plan was developed and implementation was commenced shortly after.
For the second edition, the IWII collaborative team undertook a comprehensive review of contemporary literature, including systematic reviews and meta-analyses when available. In addition, the team conducted a formal Delphi process to reach consensus on wound infection issues for which scientific research was minimal or lacking. This rigorous process provided a document with an update on the science and expert opinion regarding the prevention, diagnosis and control of wound infection. This edition outlines new definitions relevant to wound infection, presents new paradigms and advancements in the management and diagnosis of a wound infection, and highlights controversial areas of discussion. The intent of this new consensus document is to provide a practical, updated resource that is easy to use and understand regarding the concepts of wound infection.
The complete document of IWII Wound infection in clinical practice is free and can be downloaded from the IWII website http://www.woundinfection-institute.com.
Processes
To update the document, the group undertook a comprehensive targeted literature search to identify recent evidence related to management of wound infection. The best available evidence was reviewed and selected by the experts to inform the development of each section in the second edition document.
Because the science is not complete in this field, the expert group undertook a Delphi process to reach consensus on areas for which there is ongoing debate. A formal process was undertaken using the previously published RAND/UCLA Appropriateness Method1, through which the experts could collectively judge the available knowledge and reach agreement on the document content. The Delphi process was conducted over three consensus voting rounds using a web-based platform, and provided opportunity for the participants to review the current state of the science, vote on their level of agreement with statements related to the science, provide rationale for their opinion that was fed back to the group in successive voting rounds and to ask and answer questions of each other. Individual responses provided in the Delphi process were anonymous and a facilitator moderated the written discussion and calculated voting outcomes using the RAND/UCLA methods.
Definitions
One of the aims of the IWII 2016 consensus document was to update definitions and to provide new ones. As discussed, a Delphi process was conducted and the following three definitions were provided after three rounds:
Acute wound:
An acute wound is a wound with an aetiology that occurs suddenly, either with an aetiology that occurs suddenly, either with or without intention, but then heals in a timely manner.
Chronic wound:
A chronic wound is a wound that has a slow progression through the healing phases or delayed, interrupted or stalled healing due to intrinsic and extrinsic factors that impact on the individual and their wound. A chronic, non-healing wound could be suggestive of a biofilm infection, provided holistic evaluation has excluded or corrected underlying pathologies such as ischaemia.
During the Delphi process, there was much discussion regarding the concept of the time frame normally associated with the definition of a chronic wound and agreement could not be achieved after three rounds. The outcome of the Delphi process was to exclude the time frame from the definition, reflecting the recent understanding that non-healing of a healable wound that is non-malignant, regardless of time frame, should be considered chronic. The new paradigm of a chronic non-healing wound equating to presence of biofilm is discussed in the biofilm section.
Biofilm:
Both current science and agreement reached during the Delphi process suggest that an accurate definition of biofilm identifies that it is a structured community of microbes with genetic diversity and variable gene expression (phenotype) that creates behaviours and defences used to produce unique infections (chronic infection). Biofilms are characterised by significant tolerance to antibiotics and biocides whilst remaining protected from host immunity.
The second edition of the document contains a comprehensive glossary that allows for the novice and generalist to gain greater understanding of terminology that may be new or updated in recent years.
The Wound Infection Continuum
It is well acknowledged that it is more than the presence of bacteria that leads to adverse events in wounds, therefore the wound infection continuum has been updated to recognise that microbes other than bacteria are associated with wound infection. The importance to the development of wound infection of microbial virulence (as well as numbers) is also acknowledged2-7. The stages in the wound infection continuum describe the gradual increase in the number and virulence of microorganisms, together with the response they invoke within the host. The wound infection continuum is a conceptualised framework to provide greater understanding of the impact microbes may have on a wound (Figure 1)3.
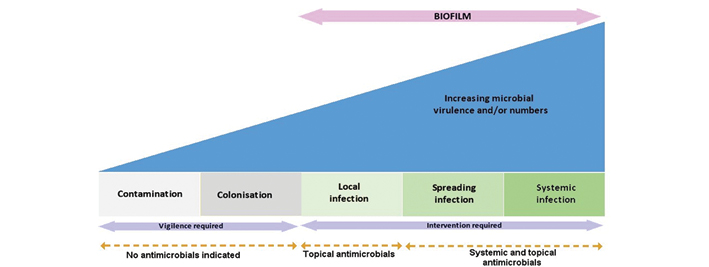
Figure 1: IWII Wound Infection Continuum8-10.
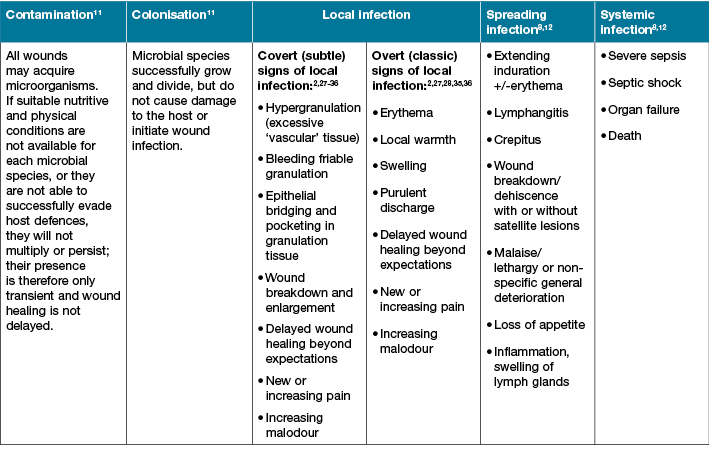
The IWII version of the wound infection continuum may spark debate as the term critical colonisation has been removed from this continuum. Consensus by the expert panel was that the term critical colonisation has previously been poorly defined and understood, and that local infection and the covert signs and symptoms can be identified by experienced clinicians2-3 and are provided in Table 1.
Table 1: Signs and symptoms associated with stages of the wound infection continuum
Biofilm
Biofilm was discussed at the WII first meeting in 2006 and was acknowledged in the first edition of Wound infection in clinical practice; however, significant scientific and clinical understanding of biofilm and the correlation with chronic wound healing has emerged since 200813-16. Although we have had advances in knowledge through the emerging science from the laboratory, we do not have a complete understanding of wound biofilm in the clinical context. The inclusion of biofilm in the wound infection continuum recognises the growing understanding and acceptance of the role of biofilm and the requirement to visit old paradigms and practices regarding biofilm identification, prevention and management17,18.
The identification of biofilm via visual indicators is an important concept for clinicians as this may lead to early identification and treatment19,20. Although this concept is supported in theory, we do not have conclusive evidence. The IWII expert panel reached agreement on the following factors as potential clinical indicators suggesting biofilm presence in a wound:
- failure of appropriate antibiotic treatment;
- recalcitrance to appropriate antimicrobial treatment;
- recurrence of delayed healing on cessation of antibiotic treatment;
- delayed healing in spite of optimal wound management and health support;
- increased exudate/moisture;
- low level chronic inflammation;
- low level erythema;
- poor granulation/friable hypergranulation; and
- secondary signs of infection.
Diagnosis of wound infection
All health care professionals should be aware of the clinical signs and symptoms (s&s) of wound infection, as stated in Table 1. The expert group reached agreement that local infection includes both overt/classic signs and symptoms, as well as covert or secondary signs of symptoms that may be subtle. These s&s of wound infection are in addition to the indicators of biofilm presented above. Early detection and management of wound infection may save a limb and lives. Unfortunately, diagnostic investigations for confirmation of the type of microbes that are causative agents and their virulence are limited and in many cases are inadequate21. In addition, inadequate collection and interpretation of specimens and results often reduces accuracy in diagnosis. Compounding this is that biofilm cannot be identified easily without advanced microscopy.
The second edition of Wound infection in clinical practice provides clinicians with guidance on when to take a wound culture, which culturing technique is most effective and information regarding emerging diagnostic techniques.
Holistic management of a patient with a wound infection
We have long understood that considerations of the individual, their wound and their environment can contribute to the development of a wound infection. A comprehensive, holistic approach is essential to accurately diagnose and treat an individual with a wound infection. Three main principles of patient-centred care for effective management of a wound infection are:
- optimising the host response;
- reducing the number and/or virulence of microorganisms in the wound; and
- optimising the wound healing environment.
Prevention of wound infection is fostered through good infection control and prevention practices by the health care professional, individuals with wounds and their carers.
Several tables are provided in the IWII Wound infection in clinical practice consensus document 2016, including an update on topical management for a wound infection. There is emphasis on wound cleansing and information regarding types of solutions, cytotoxicity and effects on biofilm. A section on topical management reviews the current antimicrobial treatments and their biofilm efficacy.
Topical antimicrobial therapy
This section reviews the terminology of antimicrobial therapy and discusses current practices in regard to managing wound infection.
Another controversy is the use of topical antibiotics, and their risks and benefits are discussed within this section. As previously noted, bacteria are not the only microbe that are commonly found in wounds; therefore topical antifungal therapy is also addressed.
Wound Bed Preparation
The principles of wound bed preparation, summarised in the acronym of TIME (tissue, infection/inflammation, moisture balance, edge of wound), have been the standard of care since the early 2000s for any open wound, regardless of aetiology12,22. In 2008, Wolcott provided us with biofilm-based wound care (BBWC)23, which places emphasis on debridement, therapeutic wound cleansing and topical antimicrobials with the intent of preventing biofilm and, if present, in disrupting immature, mature and dispersed biofilm.
It has been demonstrated that debridement provides a window of opportunity in which biofilm is more susceptible to the topical and systemic management strategies previously discussed24. The updated document provides detail on the types of debridement and their efficacy in preventing and treating biofilm.
Cleansing of the wound with a wound infection is now recommended at each dressing change, to decrease the bioburden and improve the wound environment. This takes time; however, cleansing is such a key component to good wound care that it must be a priority.
The growing understanding of the efficacy of surfactants to facilitate separation of loose, non-viable tissue and disruption of biofilm has led to the recommendation that they be used for cleansing of wounds at risk of or with a wound infection12.
Antibiotic therapy
Antibiotics must be used in combination with TIME or BBWC due to virulence and tolerance factors of biofilms to antibiotics and antimicrobials12,25,26. Inappropriate antibiotic prescribing and patterns of use is contributing to antibiotic resistance and has become a significant international concern37. Combining preparation of the wound for application of a carefully selected wound dressing with wound cleansing and debridement and systemic management will improve outcomes.
Future Developments
As the need for improved diagnostics and treatment strategies continues, revision of old and development of new concepts will emerge. Point of care diagnostics is a promising area, with ability to determine the presence of bacteria and where within the wound bed they reside. Determination of the best strategies to disrupt and prevent biofilm is imperative and early indicators are positive.
Summary
Clinicians and managers must take time to provide optimal wound management. Understanding that a wound dressing procedure is not just a routine task but is a key component of assessment, diagnosis and the development of regimens that optimise and promote wound healing is an imperative. The goal for the wound care team, including the individual with a wound, is prevention of wound infection and optimal outcomes. The second edition of the Wound Infection in clinical practice, endorsed by the IWII, has been developed to provide the wound care team with resources to help meet these goals.
Acknowledgements
The authors of the 2016 document:
Terry Swanson, South West Healthcare, Warrnambool, Australia. Donna Angel, Royal Perth Hospital, Perth, Australia. Geoff Sussman, Monash University, Melbourne, Australia. Rose Cooper, Cardiff Metropolitan University, Cardiff, Wales, UK. Emily Haesler, Curtin University and Australian National University, Canberra, Australia. Karen Ousey, Institute of Skin Integrity and Infection Prevention, Huddersfield, UK. Keryln Carville, Silver Chain Group and Curtin University, Perth, Australia. David Keast, Lawson Health Research Institute, London, Canada. Greg Schultz, University of Florida, Gainesville, Florida, USA. David Leaper, Wound Healing Research Unit, Cardiff University, Wales, UK. Jacqui Fletcher, Independent Wound Consultant, UK. Lindsay Kalan, University of Pennsylvania, Philadelphia, Pennsylvania, USA. Joyce Black, University of Nebraska Medical Center Omaha, Nebraska, USA. Evan Call, EC Service and Weber State University, Centerville, Utah, USA. Gojiro Nakagami, University of Tokyo, Tokyo, Japan.
Unrestricted grant from:
Convatec, B Braun, Smith & Nephew and Medline.
External review:
Professor Emeritus George Rodeheaver.
Author(s)
Terry Swanson*
NPWM, RN, MHSc, FAWMA, FMACNP
Chair of Consensus Document Project and Immediate Past Chair of IWII
South West Healthcare, Warrnambool, Vic, Australia
Email: tswanson@swh.net.au
Tel: 0419 597 560
Emily Haesler
PhD, BN, PGradDipAdvNurs(Gerontics)
Adjunct Associate Professor, Curtin University Department of Nursing, Midwifery and Paramedicine
Visiting Fellow, Australian National University, ANU Medical School Academic Unit of General Practice
Donna Angel
NPWM, RN, BN, PGradDip(Clin Spec), MHSc
Royal Perth Hospital, Perth, WA, Australia
Geoff Sussman
OAM, Associate Professor
Faculty of Medicine, Nursing and Health Sciences Monash University, Vic, Australia
* Corresponding author
References
- Fitch K et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND, 2001.
- Collier M. Recognition and management of wound infections. World Wide Wounds, 2004.
- Eberlein T. Critical colonisation and local infection — current therapy by use of polihexanide. 2006.
- Siddiqui AR, Bernstein JM. Chronic wound infection: Facts and controversies. Clin Dermatol 2010;28(5):519–26.
- Davis SC, Ricotti C, Cazzaniga A, Welsh E et al. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen 2008;16(1):23–9.
- Ramage G, Robertson SN, Williams C. Strength in numbers: antifungal strategies against fungal biofilms. Int J Antimicrob Agents 2014;43(2):114–20.
- Leake JJ, Dowd SE, Wolcott RD, Zischkau AM. Identification of yeast in chronic wounds using new pathogen-detection technologies. J Wound Care 2009;18:103–8.
- World Union of Wound Healing Societies (WUWHS). Principles of best practice: Wound infection in clinical practice. An international consensus. London: MEP Ltd, 2008.
- Edwards R, Harding KG, Bacteria and wound healing. Curr Opin Infect Dis 2004;17(2):91–6.
- Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 2009;49(10):1541–9.
- Cooper R. Understanding wound infection, in Identifying criteria for wound infection. European Wound Management Association Position Document. Cutting K, Gilchrist B, Gottrup F, editors. London: MEP Ltd, 2005.
- Leaper DJ, Schultz G, Carville K, Fletcher J et al. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J 2012;9(Suppl 2):1–19.
- James GA, Swogger E, Wolcott R et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16(1):37–44.
- Kirketerp-Møller K, Jenson PO, Fazli M et al. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol 2008;46(8):2712–22.
- Bjarnsholt T, Kirketerp-Møller K, Jensen PO et al. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen 2008;16(1):2–10.
- Han A, Zenilman J, Melendez JH et al. The importance of a multifaceted approach to characterizing the microbial flora of chronic wounds Wound Repair Regen 2011;19(5):532–41.
- Swanson T, Grothier L, Schultz G. Wound Infection Made Easy. Wounds International, 2014.
- Swanson T, Keast DH, Cooper R et al. Ten top tips: identification of wound infection in a chronic wound. Wounds Middle East 2015;2(1):20–5.
- Hurlow J, Blanz E, Gaddy JA. Clinical investigation of biofilm in non-healing wound by high resolution microscopy techniques. J Wound Care WUWHS Suppl 2016;25(9):S11–S22.
- Metcalf DG, Bowler PG & Hurlow J. A clinical algorithm for wound biofilm identification. J Wound Care 2014;23(3):137–142.
- Copeland-Halperin LR, Kaminsky AJ, Bluefeld N, Miraliakbari R. Sample procurement for cultures of infected wounds: a systematic review. J Wound Care 2016;25(4):S4–S10.
- Schultz GS, Barillo DJ, Mozingo DW, Chin GA. Wound bed preparation and a brief history of TIME. Int Wound J 2004;1(1):19–32.
- Wolcott RD, Rhoads DD. A study of biofilm-based wound management in subjects with critical limb ischaemia. J Wound Care 2008;17(4):145–55.
- Wolcott RD, Rumbaugh KP, James G et al. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care 2010;19(8):320–8.
- Gürgen M. Excess use of antibiotics in patients with non-healing ulcers. EWMA J 2014;14(1):17–22.
- O’Meara S, Al-Kurdi D, Ologun Y, Ovington LG. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2014(1).
- Gardner SE and Frantz RA, Wound bioburden and infection-related complications in diabetic foot ulcers. Biol Res Nurs, 2008. 10(1): 44-53.
- Gardner SE, Franz RA, and Doebbeling BN, The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen, 2001. 9(3): 178-86.
- Gardner SE, Frantz RA, Park H, and Scherubel M, The inter-rater reliability of the clinical signs and symptoms checklist in diabetic foot ulcers. Ostomy Wound Manage, 2007. 53(1): 46-51.
- Kingsley AR, The wound infection continuum and its application to clinical practice. Ostomy Wound Manage, 2003. 47(suppl A): S1-S.
- Cutting KF, White RJ, Maloney P, and Harding KD, Clinical identification of wound infection: A Delphi approach, in European Wound Management Association Position Document: Identifying criteria for wound infection, Calne S, Editor. 2005, MEP Ltd.: London.
- Joseph WS and Lipsky BA, Medical therapy of diabetic foot infections. J Am Podiatr Med Assoc, 2010. 100(5): 395-400.
- Cutting KF and Harding KG, Criteria for identifying wound infection. J Wound Care, 1994. 3(4): 198-20.
- White RJ, Cutting KF, and Kingsley A, Critical colonisation: clinical reality or myth? Wounds UK, 2005. 1(1): 94-5.
- Stotts NA and Hunt TK, Managing bacterial colonization and infection. Clin Geriatr Med, 1997. 13: 565-73.
- Galpin JE, Chow AW, Bayer AS, and Guze LB, Sepsis associated with decubitus ulcers. Am J Med, 1976. 61: 346-50.
- World Health Organization (WHO). Antimicrobial resistance: global report on surveillance. Geneva, Switzerland: WHO, 2014.



