Volume 24 Number 4
Chronic wound infection for the 21st century: perspective review
Matthew Malone, Annie Walsh
Abstract
The way we view bacteria and how they cause infection has changed significantly since the era of Louis Pasteur (France, 1822–1895) and Robert Koch (Germany, 1843–1910), early pioneers of modern vaccination. This review article will present a reflective perspective on the thoughts of today’s leading researchers and clinicians, in addition to reviewing evidence on chronic wound infection, both past and present.
Introduction
This reflective article starts at a trattoria (pasta restaurant) in Florence, where Dr Randy Wolcott, Professor Gregory Shultz and I (MM) were enjoying dinner following a successful day at the World Union of Wound Healing Societies 2016 Congress (Figure 1). I found myself mesmerised listening to their wealth of knowledge and ideas about the role of bacteria in chronic, non-healing wounds. After two hours of mostly listening, I felt that I had learned more about chronic wound infection than I had learned from the many textbooks I had read on the subject. In this article, we will traverse the history of how science, and my two Florentine dinning companions, Dr Wolcott and Professor Shultz, viewed infection.

Figure 1: Leading biofilm experts from left to right, Professor Gregory Schultz, Dr Randy Wolcott, Matthew Malone
During the development of microbiology, the general understanding of the role microbes play in human health and disease has been as planktonic, or free-floating, single-cell organisms. Seminal works by Pasteur and Koch in the mid-1800s paved the way in the field of microbiology; their methods still play an important role in today’s laboratories.
Current ‘culture-dependent’ approaches, involving growing bacteria in a laboratory using nutrient broth, rely on many different growth mediums. Notwithstanding the vast array of suitable mediums available, approximately 1% of all known bacteria are cultivatable by this method, which means that approximately 99% of all known bacteria cannot be identified using the simple and relatively convenient ‘culture-dependent’ approach that has been the ‘gold’ standard for approximately 150 years. In addition, some microorganisms grow very well under the strict conditions of a lab, whilst others do not grow at all. This means that some microorganisms may be overrepresented when cultured, and may not be the most clinically important microbes.
Koch used this technique to identify pathogens of infection. He postulated that the pathogenic microorganism must be found in abundance in all organisms suffering from the same disease, but should not be found in healthy organisms. The microorganism must then be isolated from the diseased organism and grown in pure culture. The cultured microorganism should cause disease when introduced into a healthy organism, and the microorganism must be reisolated from the inoculated, diseased experimental host and identified as being identical to the original specific causative agent. Applying these same theories, Dr Wolcott told us at dinner that he recently published a paper showing that he was able to take the microbiota of human chronic wounds complicated by biofilm, inoculate them on to mice that had an acute wound, to find the same microbiota readily form a biofilm and cause a chronic, non-healing wound.
This result has provided some of the first evidence to show that the microorganisms in chronic wounds are a cause of delayed healing and chronic infection. We will discuss in depth biofilms and the microbiome later in this article.
Chronic wounds: delayed healing from the perspective of the microbe
As we know, chronic wounds are a major cause of decreased quality of life, morbidity and mortality. Chronic wounds include diabetic foot ulcers (DFU), pressure injuries (PI), venous leg ulcers (VLU), non-healing surgical wounds (NHSW) and many other types. Each of these chronic wounds possesses its own unique underlying aetiology. Though we will not elaborate on the various aetiologies, we will explain what is meant by ‘chronic’. Human skin has a wonderfully complex engineering design involving an array of micro processes, one of which is the formation of an outer protective shell that acts as a barrier to external threats. In addition, the skin plays an integral role as an immunological interface that modulates the microorganism ecosystem that constitutes the skin flora1. A partnership between the host and the microbe is essential for the propagation of mutual benefits (symbiosis and/or commensalism) and this may afford protection against invasion from more pathogenic bacteria. In some skin wounds, a breakdown of this ‘partnership’ can result in the wound failing to heal in a timely fashion, despite optimal intervention, and this we define as a chronic wound.
From colonisation to infection
Once the skin is breached, microorganisms residing on the skin surface have access to its underlying soft tissues. Bacteria will colonise the tissue and, given certain favourable conditions, bacterial replication will occur with the possibility of ensuing infection. Contiguous access by the microbes to deeper structures and failure to control the consequential spread of infection can lead to extensive damage to host tissue and bone.
Some bacteria that contaminate and colonise wounds originate from the surrounding skin flora but other sources of bacteria include the host’s endogenous mucous membranes such as the GI tract or nares, and the environment. Wounds on the skin can present an optimal environment for microorganisms as skin provides warmth, moisture and nutrition for the micro visitor and especially if devitalised tissue is present in the wound bed2. The longer a wound remains open, the greater the chances of a more diverse and abundant bacterial colonisation; with the type, depth, location, level of perfusion and the efficacy of the host immune response dictating the niche of colonising bacteria3,4.
Bacterial presence in wounds and wound infection are not always co-concurrent. Treating clinicians must be conscious of this to ensure the appropriate use of antimicrobials and adjunct therapies. Whilst all wounds contain bacteria, colonisation refers to the specific scenario in which bacteria are multiplying but the sum of their actions are not enough to elicit an immune response4. Clinical infection is the term given to the situation where bacterial organisms proliferate within a wound and cause a substantial level of tissue damage, which, in turn, induces a host response accompanied by inflammation5. The diagnosis of infection has been promoted by expert groups as a ‘clinical diagnosis’ when three or more of the following symptoms are present: inflammation, erythema, local tenderness or pain, warmth or purulent discharge5,6.
In some people with co-morbidities such as diabetes, the overt clinical signs of infection may be diminished or absent, and this may be due to the failure to exhibit an inflammatory response7,8. This has led to a clinical view that some chronic wounds may have ‘secondary signs’ of infection that include but are not limited to malodour, delayed wound closure and poor quality wound bed tissue7,9.
The fine line between colonisation and infection can be clinically challenging and some clinicians have adopted more quantitative measures to differentiate potential ‘healthy’ colonisation from pathogenic infection by relying on the density of bacteria present per gram of tissue. Greater than 10^5 colony forming units (cfu) of bacteria per gram of tissue has been widely used as a key indicator of potential ‘bio-burden’ as the causative factor associated with delayed wound healing. This numerical indicator is based largely on early evidence from various wound aetiologies10 and further incorporated by others11. However, differences of opinion among experts persists over whether a burden >105 cfu of bacteria per gram of tissue is required to cause wound infection.
Kingsley (2003) proposed a wound infection continuum model that placed an emphasis on the progression from colonisation of bacteria within a wound through to infection (Figure 2)12. An important component of the wound continuum model is the concept of “critical colonisation” that refers to the multiplication of organisms within a wound without invasion or interfering with wound healing. Whilst the concept of critical colonisation is still the centre of much debate it is often used by clinicians to explain delayed wound healing in the absence of any overt clinical signs of infection and other wound delaying variables. This concept is of importance for clinicians as chronic wounds with critical colonisation may benefit from local and/or topical treatments such as antimicrobial wound dressings and wound debridement, rather than systemic management with the use of antibiotics.

Figure 2: ION Torrent personal genome machine used in our research facility
The role of microbiota in chronic wounds
Within the last decade, researchers have investigated the role and impact of microorganisms on human health and disease through a pioneering enterprise defined as the human microbiome project, which aims to identify, through DNA sequencing, all known microbes residing on the human host including bacterial, viral, fungal and archaea.
Within this human microbiome project, a consortium of universities and scientific institutions, nearly 80 in number, has collaboratively mapped the microbial make-up of the human body using molecular genomic methods. The project has created reference databases that have laid a foundation to accelerate infectious disease research13. The project, and the development of ‘culture-independent’ molecular techniques of microbe identification over the past few decades, have identified and mapped the microbial flora of skin and reports now suggest that researchers have identified between 81% and 99% of all microorganism genera in healthy adults13.
Dr Wolcott and colleagues are the pioneers in applying molecular DNA sequencing to chronic human wounds; their research has increased our understanding of the chronic wound microbiome. During our two-hour dinner, Dr Wolcott explained the science behind DNA sequencing and chronic wound microbiome in simple but insightful terms. Clinicians must hold a basic understanding of these new approaches, and the increasing knowledge provided on the microbiome, in order to realise the clinical benefits. We will endeavour to proceed in those same simple and insightful terms.
We, at the Ingham Institute for Applied Medical Research (Sydney, Australia) recently published an in-depth review article detailing how new molecular approaches may change the future of managing infection14, and currently our research group is using these techniques to better define diabetic foot infection from clinical samples. These new approaches start in much the same way as conventional culture methods where the clinician obtains a sample from the chronic wound. Many clinicians obtain cultures through cotton swabs, but tissue biopsy or debridement material are considered the optimal techniques to collect diabetic foot ulcer samples6. As tissue biopsy is the preferred sample source for our research group, we will briefly discuss work flow required for DNA sequencing of a biopsy sample.
Tissue samples are frozen at below 80 degrees immediately after removal. Analysis is completed in large batches due to cost efficiencies. An adequate amount of DNA must be extracted from the tissue sample using specialist kits to break down the tissue and any biofilm. The DNA material is then placed on a special chip that is inserted into a genome machine. These genome machines can now be bench top size, and are often referred to as ‘next generation’ sequencing platforms (Figure 2). The machines allow rapid multi-analysis of DNA sequences, with some sequencing sets generating over 600GB of data. Following the identification of the DNA sequences (Figure 3), the sequences are required to be loaded into a pipeline or database such as the US-based National Center for Biotechnology Information’s (NCBI) GenBank. These databases hold millions of DNA reads that subsequently match DNA sequences to those of known microorganisms.
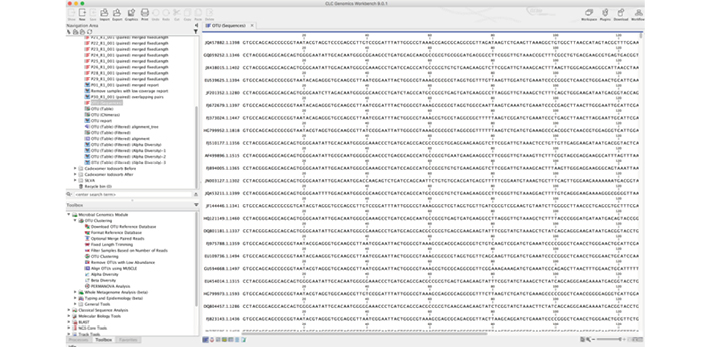
Figure 3: Software allows the visualisation of the DNA sequences, which relate to specific genera of species
Once this has been applied, analysis can be undertaken on software designed to look at microbial ecology such as QIIME. The microbiome data from chronic wound biopsy is analysed to provide information on three important components of the chronic wound infection, being: bacterial loads; ‘community’ structure, specifically richness of species, abundance and diversity; and, the presence of pathogens. To date, there have been a few published papers on chronic wound infection employing the above approach3,15-20.
DNA sequence data is large and complex, which makes analysis and clinical relevance difficult. ‘Large and complex’ is always a challenge in clinical settings, which seeks simple, convenient and low-cost techniques to ensure automatic and effective general use.
We will now briefly touch on those “few published papers” on molecular-based studies. The first report in the literature on the microbiome of DFUs was undertaken by Dowd et al. reporting on 10 chronic DFUs using multiple genomic approaches that included: partial ribosomal amplification and pyro-sequencing (PRAPS); full ribosomal amplification, cloning and Sanger sequencing (FRACS); density gel electrophoresis (DGGE); and, Sanger sequencing (PRADS)15. Facultative and strict anaerobic Gram-positive cocci formed the majority of sequences with genus-level identification highlighting the predominance of Staphylococcus, in addition to Peptoniphilus, Anaerococcus, Rhodopseudomonas, Enterococcus, Veillonella, Bacteroides, Clostridium and Finegoldia.
In a further study by Dowd et al., bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) was employed to sample 40 chronic infected DFUs from a range of locations on the foot and ankle16. The authors hypothesised that a single major pathogen would be associated with all wounds; therefore, DNA reads for each DFU were reported individually and not pooled. This allowed the compilation of community profiles for each DFU including the accurate identification of the number of samples each genera were detected, and the average percentage each genus contributed to its positive sample. Results identified each DFU contained a rich diversity of microorganisms with Corynebacterium, Bacteroides, Peptoniphilus, Finegoldia spp., Anaerococcus and Streptococcus all being present in the majority of DFUs.
Gardner and colleagues profiled the microbiome of 52 individuals with non-infected DFUs and were the first to restrict the sampling of patients to a homogenous sample of DFUs (neuropathic DFUs only)3. In characterising the microbiome of their sample, Staphylococcus was identified as the most common and abundant genus in 49 of 52 DFU samples. At a species-level, the majority of sequences belonged to the common pathogen S. aureus (96.5%), an unsurprising finding, considering the highly documented role of this microorganism in diabetes-related foot infection.
Further analysis of microbial diversity in Gardner and colleagues’ 52 DFUs reported, on average, 30 different microorganisms per DFU (range 7–64) in comparison to lab culture that detected on average 4 species per DFU (p <0.0001). Comparisons of the relative abundance of each species using lab culture identified the overestimation in the abundance of Staphylococcus spp. (0.47 vs. 0.32, p = 0.0001) and the underestimation of anaerobes (0.11 vs. 0.18, p = 0.0063) in comparison to DNA sequencing. By lab culture, anaerobes were identified as the predominant organisms in only 6 of the 52 DFUs (12%), a finding consistent with the known limitations of this method, particularly in the identification of slow-growing, fastidious anaerobic organisms.
This finding is certainly true across most DNA sequence studies, including our own research on the microbiome of DFUs. In particular, the Clostridiales family XI (Anaerococcus, Peptoniphilus and Finegoldia) has been frequently identified. These are obligate anaerobes and do not grow under routine lab culture. In fact, most studies utilising DNA sequences have concluded that anaerobes are greatly under-appreciated. The conundrum is to determine if anaerobes play a pathogenic role in the delayed healing of wounds or as pathogens of infection. In most DNA studies, the obligate anaerobe bacteria identified only constitute a small percentage of the overall bacterial load when viewed at a single-species level. Only when all the obligate anaerobe bacteria are combined is it seen that they contribute collectively as a major player.
To clarify this farther, if we obtain a tissue sample and find 10,000 DNA sequences, we would look to determine how many of those sequences belonged to a specific species. When S. aureus is present, for example, we often find that it contributes as a major player and may individually account for 5,000 of the 10,000 (hypothetical) DNA sequences, being 50% of the 10,000 total reads. We would determine Staphylococcus as the major pathogen as it is a known pathogen of infection and also because it makes up 50% of the total bacterial load in this hypothetical example. We also find in this hypothetical wound, members of the Clostridiales family XI (Anaerococcus, Peptoniphilus and Finegoldia) which each make up 1,000 reads, giving 3,000 reads in total of the 10,000 total sequencing reads, a bacterial load of 30%. As an anaerobic group, they contribute to 30% of the abundance of all microorganisms in the wound, although individually they contribute much less. Nevertheless, in this hypothetical scenario, it remains uncertain if their contribution to infection is greater as a group than the sum of their contributions individually: Do their interactions have a catalytic effect on each other? Also, should therapy include both aerobic Gram-positive cocci (S. aureus) and obligate anaerobes?
When summarising the work undertaken in the field of microbiome of chronic wounds, there are still many unanswered questions. First, in my opinion, many microbiome data studies do not indicate sufficient clinical relevance; our management of infection has not benefited from these studies. Furthermore, though the data has provided us with an extended view of ‘who is there?’, we still have to decipher ‘who is doing what?’. Potential applications such as microbial meta genomics, meta transcriptomics and meta proteomics may improve infection identification. Transcriptome and proteome data are capable of identifying expressed biological signatures such as RNA transcripts or proteins, respectively, which control metabolic activities in microbial communities. In this respect, transcriptome or proteome analysis may characterise not only an infection’s microbial diversity: Which microorganisms are present? but also its functional potential: What are they capable of doing and how?
Biofilms
Evidence-based knowledge on the properties of biofilms has emerged from filtered microbial studies of aquatic environments in the medical field such as dental plaque. This data has provided evidence that microorganisms have a natural tendency to associate with surfaces, and with each other, and prefer a sessile (stationary, slow growth) lifestyle (Figure 4). A significant proportion of this work was conducted on environmental samples and has provided a platform for the contemporary medical models that we have come to understand as microbial biofilms.
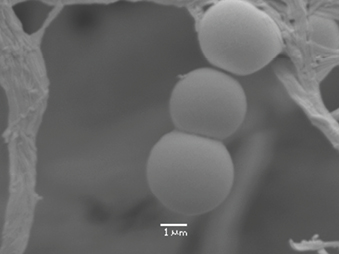
Figure 4: Coccoid-shaped microorganisms that demonstrate both attachment to a surface and attachment to each other
Biofilms are now a major area of research for both clinicians and scientists. This research has increased significantly in the past decade due to advancements in emerging technologies and techniques applicable to the study of bacterial populations in situ. Using light and electron microscopy, in combination with specific probes to define cell surface structures, William Costerton (1934–2012), a microbial ecologist, pioneered the understanding of the role that biofilms played in human health and disease.
Costerton’s early enterprising work focused on environmental models, but his work quickly encompassed the medical arena along the lines: How does a bacterium know whether it is in a urinary catheter or an alpine stream? This question hypothesises that the bacterium would grow as a biofilm on both surfaces regardless21. Costerton and his colleagues were also the first to propose the role of Pseudomonas aeruginosa biofilms in the sputum of cystic fibrosis patients22, notwithstanding strong opposition from their medical peers at the time, ‘par for the course’ in the journey of new ideas. The concept of biofilms in human health and disease is now universally accepted in periodontal disease and dental caries23, cystic fibrosis22,24,25, in-dwelling medical device infections26, Otitis media and other upper respiratory infections27,28 and chronic wounds29,30.
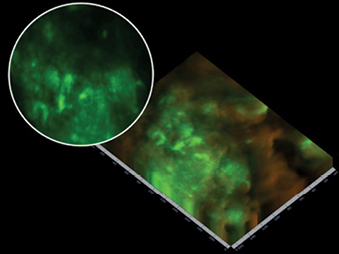
Figure 5: Partial nucleic acid fluorescent in situ hybridisation (PNA-FISH), identified microbial aggregates with EPS (biofilm) production on a wound surface. The light green patches are areas of increased focal microbial aggregates
The definition of biofilm has also changed over the years to reflect increasing knowledge. Early definitions included bacteria attached to surfaces, encapsulated in a self-produced matrix or glycoclayx and tolerant to antimicrobials31,32. Recently, the International Wound Infection Institute (IWII) proposed that a biofilm be defined as: “A structured community of microbes with genetic diversity and variable gene expression (phenotype), which creates behaviours and defences used to produce unique infections (chronic infection) with characteristics of significant tolerance to antibiotics and biocides whilst also being protected from host immunity.”
Biofilms are frequently identified from in vitro observations through methods such as partial nucleic acid fluorescent in situ hybridisation (PNA-FISH) or scanning electron microscopy (SEM). PNA-FISH describes a technique that combines a fluorescent probe (these can be species-specific or universal) viewed under confocal laser scanning microscopy. This technique helps to depict the spatial organisation of microbial cells rather than visualising actual biofilm architecture. The PNA-FISH technique can be combined with DNA sequencing to determine the exact microbiome of residing microbes in a biofilm sample. SEM allows direct visualisation of microbial aggregates and any EPS produced (Figure 6). It is the preferred visualisation technique to confirm the presence of biofilm through other techniques: light microscopy and transmission electron microscopy.
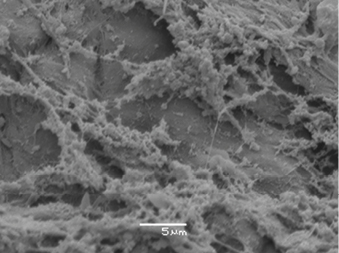
Figure 6: Scanning electron microscope identifies biofilm aggregates surrounded by a sticky, string-like substance (this is the EPS) attached to the wound bed of a chronic, non-healing DFU
Clinical significance of bacterial biofilms in chronic wounds
Early work by Costerton and colleagues33 identified that bacteria growing on medical devices existed within a biofilm, and that they exhibited a remarkable tolerance to both host defences and antimicrobial therapy. A plethora of in vitro biofilm models have indicated that bacterial biofilms can withstand antimicrobial concentrations 100 to 1000 times higher than that of planktonic counterparts34-36. However, to date, no single causative mechanism has been identified to explain biofilm resistance/tolerance and resistance (recalcitrance) to antimicrobials. It has been suggested that a combination of factors may contribute, but not exclusively, to biofilm recalcitrance, being: slow or incomplete permeation of antimicrobials through extracellular polymeric substances (EPS)34; altered microenvironment and niches within biofilms promoting slow growth rates and adaptive stress response37,38: efflux pumps39; and, the role of ‘persisters’40, dormant microbial cells that can survive the antimicrobial treatments that kill the majority of their genetically identical siblings.
How do biofilms impede wound healing?
The mechanisms of biofilm impairment on wound healing remain ambiguous. Existing data suggest a chronic wound is kept in either a ‘vicious’ inflammatory state or subject to localised low-oxygen tensions. The pathways behind this are not clear, but several systemic and local factors may contribute to the occurrence and maintenance of the wound chronicity.
Biofilms sustain hyper-inflammation
In a review article, Wolcott and colleagues41 presented a detailed hypothesis suggesting that once a biofilm community becomes established, their stubbornness and resistance to many treatments propagates hyper-inflammation. Specifically, they propose that biofilm phenotype bacteria produce proteases that inhibit and destroy extracellular matrix. In addition, the chronic wound environment also exhibits host-derived proteases. Together, this may over-fill a chronic wound with a proteolytic mix of proteases, elastases and gelatinases, commonly referred to as matrix metalloproteinases (MMP). Concurrently, biofilm adherence to the wound bed may also inhibit the release of the natural suppressors of MMPs, tissue inhibitors of MMPs (TIMPS). This scenario may, therefore, sustain a perpetual state of hyper-inflammation.
Wolcott and colleagues also put forward the possibility of biofilms to ‘bait’ the immune system through releasing planktonic bacteria41. They suggest that the presence of anaerobic bacteria play a key role, releasing a cell wall constituent lipopolysaccharide, a potent inflammatory inducer. In an animal model of cystic fibrosis patients, biofilm forming P. aeruginosa were shown to undergo lipopolysaccharide modifications that induced greater inflammatory responses in mice42. No human in vivo data exist to support this aspect for chronic wounds.
Biofilms may contribute to localised areas of low-oxygen tension within a wound
Early microelectrode studies of aerobic in vitro biofilm models found discrete areas within biofilm that had significant oxygen depletion37. This suggested that areas of biofilm, ‘housing’ micro-niches favouring differing microorganisms, may explain how the presence of anaerobes in mixed-species biofilms exist, contribute and cooperate with aerobic neighbours.
Further studies employing microelectrodes with controlled low-strength material (CLSM) have identified micro-domains with different biochemical environments including alterations in pH and oxygen43. Recent data by James and colleagues38 has provided further evidence to support a concept of localised low-oxygen tensions contributing to wound chronicity. Using oxygen micro-sensors and transcriptomics (examining microbial metabolic activities) to study in-situ biofilms, the authors identified steep oxygen gradients and induced oxygen-limitation stress responses from bacteria. Additionally, their use of transcriptomics indicated that the metabolic activities of the biofilm, and the recruitment of cells that consume oxygen for host-defensive processes, were the primary pathways of oxygen depletion. Taken collectively, this data supports the concept of a biofilm establishing and maintaining localised low-oxygen tensions in a wound and contributing to chronicity.
Summary
The progression of wound infection in a person who is immune-compromised, diabetics for example, can lead to devastating outcomes such as lower extremity amputation. For this reason, the early clinical identification of infection, masked signs of infection and/or the presence of biofilm is crucial. To direct targeted therapy, clinicians need to understand the interaction of microorganisms in the wound-healing process. Molecular DNA-based approaches can identify many ‘hidden’ microorganisms, but notwithstanding the recent rapid progress and application of DNA sequencing, we have only been enlightened with the broader view telling us which microorganisms are ‘in play’, not how or why they ‘play’, yet. However, hope springs eternal in the accumulation of evidence-based medical knowledge, and though current interpretations, and the clinical implications of additional bacteria within samples, remain unclear, further DNA-based research should provide the ‘eureka’ platform we now seek, if only to allow us to stand firmly and gaze up at the newly revealed heights of the complexities of life’s micro architecture. Importantly for now, the concept of biofilms and their involvement as contributors to chronic, non-healing wounds, and their role in the pathogenesis of chronic infection, herald a significant and positive shift in therapeutic paradigms. This shift needs to be handled cautiously with collaboration between antimicrobial stewardship, wound care clinicians and the broader medical community.
How will DNA sequencing and the microbiome change the way we practise at the coal face of our patients’ needs? How do we practise safely and effectively with the known unknowns, let alone the Rumsfeldian “unknown unknowns”?
There is much to learn, much to understand, and, quite frankly, we do not have all the answers. The comforting thought for me is that we are not alone, and the other members, like my dining companions recently in Florence, Dr Wolcott and Professor Schultz, as well as the many authors we have referred to in this article, are on the case.
Author(s)
Matthew Malone*
BSc (Hons), MSc, PhD candidate, FFPM RCPS (Glasg)
Head of Department Podiatric Medicine,
High Risk Foot Service Liverpool Hospital,
South Western Sydney Local Health District
Liverpool Diabetes Collaborative Research Unit, Inghams Institute of Applied Medical Research
Molecular Microbiology Research Group,
Western Sydney University, Faculty of Medicine
Liverpool Hospital, Elizabeth Street, Liverpool, NSW 2170, Australia
Email Matthew.malone@sswahs.nsw.gov.au
Annie Walsh
BHSc Pod (Hons), MWoundCare
Senior Podiatrist, High Risk Foot Service Liverpool Hospital, Liverpool Hospital, South Western Sydney Local Health District
Liverpool Diabetes Collaborative Research Unit, Inghams Institute of Applied Medical Research
Liverpool Hospital, Elizabeth Street, Liverpool, NSW 2170, Australia
Email Annie.walsh@sswahs.nsw.gov.au
* Corresponding author
References
- Borkowski AW, Gallo RL. The coordinated response of the physical and antimicrobial peptide barriers of the skin. J Invest Dermatol 2011;131(2):285–287.
- Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14(2):244–269.
- Gardner SE et al. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes 2013;62.
- Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14(2):244–269.
- Lipsky BA et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 2016;32:45–74.
- Lipsky BA et al. 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections. Clin Infect Dis 2012;54(12):e132–e173.
- Gardner SE, Hillis SL, Frantz RA. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biological Research for Nursing 2009;11(2):119–128.
- Gardner SE, Frantz RA. Wound bioburden and infection-related complications in diabetic foot ulcers. Biol Res Nurs 2008;10.
- Cutting KF, White RJ. Criteria for identifying wound infection — revisited. Ostomy Wound Manage 2005;51.
- Robson MC. Wound infection: a failure of wound healing caused by an imbalance of bacteria. Surg Clin N Am 1997;77(3):637–650.
- Browne AC, Vearncombe M, Sibbald RG. High bacterial load in asymptomatic diabetic patients with neurotrophic ulcers retards wound healing after application of Dermagraft. Ostomy Wound Manage 2001;10(47):44–49.
- Kingsley A. The wound infection continuum and its application to clinical practice. Ostomy Wound Manage 2003;49(7A Suppl):1–7.
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012;486(7402):207–214.
- Malone M et al. Can molecular DNA-based techniques unravel the truth about diabetic foot infections? Diabetes Metab Res Rev 2016:n/a–n/a.
- Dowd SE et al. Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol 2008;8.
- Dowd SE et al. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS One 2008;3.
- Rhoads DD et al. Comparison of culture and molecular identification of bacteria in chronic wounds. Int J Mol Med Sci 2012;13(3):2535–2550.
- Price LB et al. Community analysis of chronic wound bacteria using 16S rRNA gene-based pyrosequencing: impact of diabetes and antibiotics on chronic wound microbiota. PLoS One 2009; 4.
- Wolcott RD et al. Evaluation of the bacterial diversity among and within individual venous leg ulcers using bacterial tag-encoded FLX and titanium amplicon pyrosequencing and metagenomic approaches. BMC Microbiology 2009;9:226–226.
- Wolcott RD et al. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen 2015;n/a–n/a.
- Costerton JW et al. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol 1981;8(4):303–338.
- Lam J et al. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infection and Immunity 1980;28(2):546–556.
- Marsh PD, Bradshaw DJ. Dental plaque as a biofilm. J Ind Microbiol 1995;15(3):169–175.
- Costerton JW. Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol 2001;9(2):50–52.
- Bjarnsholt T et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 2009;44(6):547–558.
- Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 2001;33(8):1387–1392.
- Hall-Stoodley L et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006;296(2):202–211.
- Boase S et al. The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect Dis 2013;13(1):1–9.
- James G et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16(1):37–44.
- Bjarnsholt T et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008;16(1):2–10.
- Carpentier B, Cerf O. Biofilms and their consequences, with particular reference to hygiene in the food industry. J Appl Bacteriol 1993;75(6):499–511.
- Costerton JW et al. Bacterial biofilms in nature and disease. Ann Rev Microbiol 1987;41(1):435–464.
- Costerton JW, Irvin RT, Cheng KJ. The bacterial glycocalyx in nature and disease. Ann Rev Microbiol 1981;35(1):299–324.
- Walters MC et al. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 2003;47(1):317–323.
- Anwar H, Costerton JW. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob Agents Chemother 1990;34(9):1666–1671.
- Machado I et al. Antimicrobial pressure of ciprofloxacin and gentamicin on biofilm development by an endoscope-isolated Pseudomonas aeruginosa. ISRN Biotechnol 2013;10.
- de Beer D et al. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol Bioeng 1994;43(11):1131–1138.
- James GA et al. Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen 2016;n/a–n/a.
- Soto SM. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013;4(3):223–229.
- Brooun A, Liu S, Lewis K. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 2000;44(3):640–646.
- Wolcott R, Rhoads D, Dowd S. Biofilms and chronic wound inflammation. J Wound Care 2008;17(8):333–341.
- Ciornei CD et al. Biofilm-forming Pseudomonas aeruginosa bacteria undergo lipopolysaccharide structural modifications and induce enhanced inflammatory cytokine response in human monocytes. Innate Immunity 2010;16(5):288–301.
- Lawrence JR et al. In situ evidence for microdomains in the polymer matrix of bacterial microcolonies. Can J Microbiol 2007;53(3):450–458.



