Volume 24 Number 4
A systematic review of the literature addressing asepsis in wound management
Emily Haesler, Lyn Thomas, Pam Morey and Judith Barker
Keywords wound cleansing, Asepsis, aseptic non-touch technique, handwashing, infection control.
Abstract
There has been extensive ongoing debate on the application of aseptic technique in wound management over the previous decades and changes to the way in which theory is applied to clinical practice have occurred regularly. Clinicians often express confusion over the way various techniques should be applied, particularly when practising in clinical settings in which maintenance of strict asepsis is inherently difficult (for example, community-based wound management). Wound cleansing, use of open but unused wound dressings and storage of wound management equipment are frequent issues on which clinicians request guidance. A systematic review using Joanna Briggs Institute methods was undertaken in order to establish the current state of the scientific literature on this topic and inform the development of recommendations for practice in this field. All levels of evidence were included in the review, including opinion papers. Findings from the 20 quantitative studies were reported in narrative summary and findings from 37 qualitative research papers were aggregated in a thematic synthesis. Although high-level evidence on wound cleansing solutions was identified, the review concluded that there is a paucity of scientific literature on most topics related to asepsis in wound care.
Introduction
Wound infection has a large impact on individuals and the health care system. Precise incidence rates are difficult to determine due to the many types of wounds and various methods of diagnosing and tracking wound infection. As many as 60% of chronic wounds have infection in the form of demonstrated presence of surface bacteria or invasive biofilm1,2. Rates of surgical site infection vary substantially based on surgical site3; however, recent estimates suggest 10–12% of all surgical wounds become clinically infected4. Infection rate in lacerations is cited at 5%5, and rate of biofilm in all acute wounds is approximately 6%2.
Facility-acquired wound infection is of particular concern given the increasing significance of antibiotic-resistant bacteria. Infection control procedures are first-line strategy to prevent infection spread6. Given the impact of wound infection and significance of infection control practices in reducing its incidence, it is important that clinicians understand the implementation of infection control procedures when managing wounds. Historically, there have been major changes to aseptic theory in wound management7-9. Surveys indicate clinicians experience confusion about how to implement aseptic technique and other infection control principles10,11.
Within Australia, the introduction of a standard on health care-associated infection12 and publication of a national infection control policy13 led to a demand for updated wound management procedures. Wounds Australia established a working party to develop clinical guidance on procedures associated with prevention and control of wound infection. To inform the development of this document, a systematic review (SR) was undertaken.
Aims
The objective of this review was to identify the contemporary evidence addressing topics associated with aseptic technique and infection control in wound management. Specific aims were to identify evidence related to cleaning considerations when performing a wound procedure, techniques for wound cleansing, environmental considerations in performing wound management and ways in which wound dressings can be handled and stored aseptically.
Review methods
The review was undertaken using methods published by the Joanna Briggs Institute (JBI)14,15. An initial search was conducted in MEDLINE, CINAHL, EMBASE, Current Contents and the Cochrane library. All papers published in English up to October 2015 that related to topics outlined in the aims were eligible for inclusion. All research designs, qualitative research and opinion papers were eligible for inclusion; however, news items, letters and conference abstracts were excluded. Papers related to aseptic technique in the operating room, intravenous therapy or catheterisation were excluded. Search terms and MESH headings included: asepsis, non-touch technique, aseptic technique, steriliz/sation, disinfection, microbial and bacterial contamination, hospital, healthcare and community-acquired infection. These terms were used in combination with terms associated with wound care, wound dressings, equipment storage, cleansing, and equipment recycling. The working party reviewed the search strategy to ensure it captured the intended literature. On review of the evidence it was noted by the working party that significant changes in theory and practice have occurred in the field of aseptic technique. It was determined that inclusion would be limited to papers published between January 2000 to October 2015 in order that the review findings reflect contemporary knowledge. References cited in included manuscripts were also considered for inclusion.
All papers meeting inclusion criteria were critically appraised by two independent reviewers using the JBI suite of appraisal tools. For randomised controlled trials (RCTs) and pseudo-RCTs, critical appraisal evaluated randomisation, blinding, allocation concealment, withdrawals, comparability and equivalent treatment of participants, outcome measurement and statistical analysis14. Consistent with JBI appraisal, RCTs and pseudo-RCTs were ranked as high quality or lower15. For descriptive studies and case series, process for randomisation, sample inclusion, outcome measurement, management of confounders, participant withdrawal and data analysis were evaluated14. These studies received a ranking of low or very low quality15. For interpretive and critical research, congruity of philosophies, methodology, research methods and analysis was evaluated, as well as reflexivity14. Qualitative research was ranked as high quality or lower15. Textual and opinion papers were evaluated based on source and logic of opinion and arguments, focus, referencing and support from peers14 and ranked as low or very low quality15.
Data extraction used standardised JBI tools. Quantitative results were not appropriate for meta-analysis as they generally addressed different topics, had heterogeneous methods, or were meta-analyses. These results are reported in a narrative format. Qualitative studies and opinion papers were analysed to identify themes, concepts and meanings within the research14, with identification of primary findings that were grouped in categories based on similarity in meaning. The categories were meta-aggregated in syntheses.
Identified research
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram16 is presented in Figure 1. The searches initially identified over 2,000 potential studies that was reduced to 57 papers that met inclusion criteria and were critically appraised. As indicated in Figure 1, most studies were excluded in the first review of the flagged references due to having insufficient focus on the topic of this review.
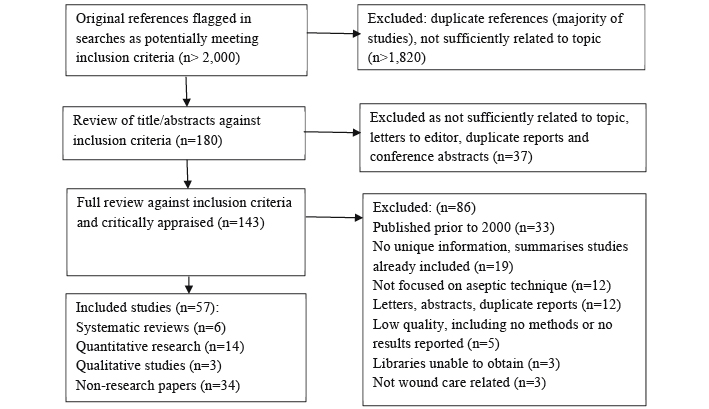
Figure 1: PRISMA review flow
Of the 57 included papers, 14 were quantitative research papers17-30, six were SRs31-36, three were qualitative research papers10,11,37 and 34 papers were non-research articles7-9,38-68. The quantitative research consisted of six RCTs18,21,23,25,26,29 (level 1.c evidence) that were of low or moderate quality15. There was one very low quality before/after study22 (level 2.d evidence), five observational studies17,19,20,28,30 (level 2 and 3 evidence), one very low quality cohort study24 (level 3.c evidence) and a very low quality cross-sectional study27 (level 4.b evidence). The SRs31-36 (level 1 evidence) ranged in quality from low to very high and the qualitative research10,11,37 (level 3 evidence) was of moderate to high quality. The majority of findings in this review arose from textual papers providing low and very low quality evidence. Table 1 presents summaries of the research papers.
Quantitative results from the literature
Cleansing solutions and technique
SRs and studies exploring irrigation fluids received the most attention in quantitative research. Details of the included studies, including their quality and level of evidence, are provided in Table 1 in the supplementary material accessible at www.woundsaustralia.com.au/journal/2404.php. As the individual studies18,19,21,23,26,29 in Table 1 were included in identified SRs, only SR results are reported below; however, none of the individual RCTs established significant differences in infection rates between wounds cleansed with sterile solutions versus tap water18,19,21,23,26,29.
A high quality SR36 compared sterile saline (n=326) to tap water (n=257) for cleansing lacerations, acute and chronic wounds. Pooled results from two RCTs showed no significant difference in wound infection rates, with tap water slightly less likely to result in infection (odds ratio [OR] 0.79, 95% confidence interval [CI] 0.36 to 1.72, p=0.55)36.
A moderate quality Cochrane SR33 compared cleansing methods for lacerations, open fractures, chronic and surgical wounds. Infection rate in all wound types (3 RCTs) was not significantly different between tap water cleansing and no cleansing (relative risk [RR] 1.06, 95% CI 0.07 to 16.50, p=not significant [ns]). There was no difference in infection rates in all acute sutured wounds (3 RCTs) between tap water versus sterile saline irrigation (RR 0.66, 95% CI 0.42 to 1.04, p=ns). Cost-effective analyses favoured tap water33. This Cochrane review reached the same conclusions as earlier systematic reviews by the same research team34,35.
A low quality SR compared tap water to sterile saline. Significantly more wounds cleansed with sterile saline became clinically infected (saline 7.1% versus tap water 4.3%, RR 0.62, 95% CI 0.39 to 1.01, p=0.05). There was no significant difference in wounds with positive cultures (saline 3.1% versus tap water 4.4%, RR 1.53, 95% CI 0.79 to 2.99, p=0.21)31.
A low quality SR compared bathing to no bathing for post-surgical foot wounds. Normal hygiene groups showered at 1–5 days postoperative (n=1,639). Patients abstaining from foot hygiene waited until sutures/staples removal (n=511). There were no significant differences in surgical site infection rates in any study32.
Although confounding factors are noted (for example, administration of saline at cooler temperatures than water) when controlled for these factors the outcomes did not change. Findings from the high-level evidence31,33-36 indicated no increase in wound infection rates associated with cleansing wounds in tap water.
Reuse of wound dressing products
A moderate quality observational study investigated rate of contamination of opened hydrogel products. The products were opened after handwashing, using clean gloves and away from direct patient care. After 28 days, one package from 60 random samples returned a positive bacterial culture. The sample collection technique may not reflect clinical practice17. A very low quality observational study reported contamination rates for opened dressings and reusable equipment stored in different containers in patients’ homes. After 14 days, 75% of samples (n=21) were contaminated30. Another low quality observational study investigated contamination rates for randomly selected multi-use saline flasks stored in hospital settings. Approximately half of the samples were found to be contaminated20.
Wound dressing practice
In a low quality RCT, leaving surgical wounds uncovered after surgery (n=235) was compared to wound dressings applied in the operating theatre (n=216). Patients were reviewed after seven days for clinical signs of infection and no significant difference in infection rates was found (exposed wounds 1.7% versus covered wounds 1.4%, p=ns)25.
A very low quality cohort study compared sterile (n=1,070 admissions) and clean (n=963 admissions) dressing procedures for surgical wounds. The outcome measure was positive wound culture established by wound swab. There was no significant difference in surgical site infection rates (0.84% versus 0.83%, p=ns) and the clean procedure was faster (10 minutes versus 13 minutes)24.
Aseptic technique education and behaviours
A very low quality before/after study investigated an education program delivered to medical students. The course was based on principles associated with handwashing and dressing procedures. After 10 weeks, there was a significant decline (p<0.001) in the ratio of students who were able to achieve a pass mark in the assessment, indicating the education had no prolonged influence on practice. Poor role modelling and lack of resources were identified as contributing to poor outcomes22. A very low quality observational study reported clinical practice amongst nurses in community settings. Practice was established through direct observation and validated in interviews with participants. As many as 40% of nurses did not engage in handwashing before a procedure28.
Qualitative results from the literature
Three hundred and eighty-six findings were extracted from qualitative studies and non-research articles. Using the JBI ratings15, 60 of the findings were rated as unequivocal, 218 were rated as credible and 20 findings were rated as unsupported, generally where an assertion was made without any supporting reference. These findings were grouped in 65 categories and aggregated into 23 syntheses that are reported in full in Figure 2 in the supplementary material accessible at www.woundsaustralia.com.au/journal/2404.php.
Current evidence base
Synthesis 1: Research on wound cleansing and aseptic technique is insufficient and that which is available is poorly translated into practice.
There is a lack of research on aseptic techniques9,65. Inconsistencies in terminology and practice guidance, and ongoing change to theory interpretation has a negative impact on compliance7-9. The need for more research on aseptic technique, including translation to different clinical settings was highlighted38,44,48.
Handwashing practices
Six categories aggregated into two syntheses represented textual findings on handwashing.
Synthesis 2: Liquid alcohol rub, antimicrobial hand wash or soap and water can be used for washing hands. When hands are visibly soiled, use soap and water.
Articles referred to three handwashing solutions: alcohol-based rubs, antiseptic/antimicrobial hand washes and soaps/detergents. Alcohol rub has broad spectrum activity51 and is quick to apply without the need for water8,40,45,50,51,55,57,59. A small risk of fire from alcohol exposed to a heat source before complete evaporation45 and potential for dry hands are reported. Some texts suggest alcohol-based hand rubs are not appropriate when hands are visibly soiled40,55. Antiseptic or antimicrobial hand wash with water is suggested for cleaning visibly dirty hands40,45,51,55 although it may be more expensive or cause irritation51. Antimicrobial-impregnated towels are an alternative for visibly clean hands, but not a replacement for soap and water40,45,55. Opinion articles agreed that soap and water is appropriate for visibly soiled hands40,49,51,55,64.
Synthesis 3: Handwashing should occur before and after patient contact, regardless of the use of gloves, and consist of vigorous rubbing for at least 15 to 30 seconds.
Hands should be washed before/after patient contact, or after contact with body fluids, to prevent cross-contamination7,40,55,56,64. Use of gloves does not preclude the need to wash hands40,55-57,59 because hands may become contaminated when removing gloves56. Handwashing should be a vigorous process covering all hand surfaces using soap and water or an alcohol rub40,55,59. Most papers suggested that handwashing should take at least 15 seconds40,55; however, one suggested at least 30 seconds59.
Gloves and personal protective equipment
Three syntheses related to the use and selection of gloves, and one related to other personal protective equipment.
Synthesis 4: Gloves are required to prevent contamination and cross-infection; however, they do not replace routine handwashing.
Textual findings highlighted that the primary purpose of gloves is to prevent contamination, between the patient and the nurse, or cross-infection between different anatomical sites on the same patient40,44,51,55,59. It was suggested that gloves are worn when there is a risk of coming into contact with bodily fluids or non-intact skin40,55; when removing old wound dressings45; and for invasive activities51. Use of gloves does not preclude handwashing45,57, regardless of the implementation of double-gloving45. Findings suggested gloves be removed immediately following care40,55.
Synthesis 5: Selection of gloves is guided by the procedure to be performed, risk of contamination, latex allergies and cost.
Synthesis 6: Sterile gloves are required for surgical aseptic non-touch technique, surgery and invasive aseptic procedures and clean gloves are for non-sterile procedures/standard aseptic non-touch technique.
Level of expected direct contact with susceptible sites44,53,57 should guide glove selection. Latex allergy influences glove choice44,50,59, and some texts identified the increased cost of sterile gloves as a factor in selection59,62. There was agreement that sterile gloves are required for sterile procedures44,48,51,57,61. Within the literature ‘sterile procedures’ referred to invasive activities44,51 surgical procedures44, aseptic technique44, aseptic non-touch technique (ANTT) requiring direct contact with key parts61, and delivering sterile pharmaceuticals44. Clean, non-sterile gloves were suggested for removing old wound dressings48, performing clean procedures48 and performing procedures that do not require direct contact with the key parts61.
Synthesis 7: Wearing appropriately selected personal protective equipment helps to reduce the risk of cross-infection from exposure to body fluids or airborne contamination.
Personal protective equipment is designed to reduce the risk of contamination for both the patient and clinician57,64. When protective equipment is used, the clinician is protected from body fluid exposure (for example, blood splashes)51,57 and the patient is protected from the clinician as a source of infection risk59. Textual findings focused on using plastic aprons51,59 with selection of equipment based on the level of risk of body fluid exposure64.
Wound management environment
Two syntheses addressed the general and specific environment in which wound management is conducted.
Synthesis 8: Actions should be taken to reduce airborne and other infection risks in the home and hospital to ensure wound care is conducted in a clean environment.
The requirement for a clean environment, free from airborne and other infection risks was described51,61,64. The risk posed by carpets, soft furnishings and pets was reported64. Strategies to reduce environmental risk included reporting infection risk in the home to authorities64, ensuring there are cleaning routines that incorporate the ventilation and water supplies64; reducing airborne infection by closing windows, reducing foot traffic and turning off fans64; leaving the wound exposed for the shortest time64; and disposing of waste promptly and appropriately64.
Synthesis 9: A wound management field can be established on a clean surface in a space at low risk of environmental contaminants. Once established, introduction of contaminated external objects should be avoided.
The importance of establishing a sterile local field on a clean surface8,57,64 was discussed. Strategies for establishing a sterile field in a clean environment included using a visually clean dressing trolley57 or cleaning a hard surface with a broad spectrum disinfectant64. In the community, a plastic apron or lid could be used8,64. Considering the wound, an extension of the wound management field was suggested7, as was ensuring the wound management field remains sterile57. Findings were consistent that objects external to the wound and field should not contaminate the wound management field7,57,61. Clinicians could use either a critical aseptic field into which only sterile equipment is introduced (for example, for an invasive or extensive procedure) or a general aseptic field in which key parts are individually protected within the field (for example, for a simple wound procedure)61.
Cleansing solutions and technique
Eighteen categories were aggregated into six syntheses related to wound cleansing.
Synthesis 10: An ideal wound cleanser should adequately clean the wound, not cause cell damage or sensitivity and have a long shelf life.
The principle of doing no harm and preventing infection were highlighted as guiding the choice of wound cleanser9. Consideration to the toxicity of a cleanser and its potential to cause sensitivity was highlighted42,63. Using an expired product should be avoided57 by selecting a wound cleanser with a long shelf life42,63. The ability to effectively remove organic material and reduce bioburden are other considerations42,63.
Synthesis 11: An assessment should be conducted by the interdisciplinary team to determine if a wound bed should be cleansed, and if so, the cleansing process to use.
Not all wound beds require cleansing as a wound may heal without disruption if there are no visual contaminants or signs of infection39,58. The wound management team could work together to determine the best approach for individual patients9,39,58.
Synthesis 12: Normal saline, potable tap water, sterile water and low concentration antimicrobial solutions are safe and effective wound cleansers. Antiseptics are not a good choice for wound cleansing.
Sterile saline is an isotonic solution that has no impact on tissue repair processes42,46; therefore it is a safe and traditional option62, particularly in hospital environments46 or for vulnerable wounds9. Tap water, sterile water and normal saline were all reported as safe; however, none of these solutions reduces bioburden in the wound42,46,56,57,67. Antimicrobial solutions reduce bioburden63,67, although concentrations should be selected carefully in light of potential cell toxicity63,67. Use of skin cleaners and antiseptics in wound cleansing is warned against39,42,58,63. Cell toxicity42,58,63, potential carcinogenicity41, insufficient contact time with the wound to effectively reduce bacteria levels39 and association with antibiotic-resistant bacteria39 were concerns.
Synthesis 13: Apply a wound cleanser at a lukewarm temperature with consideration to the potential for cross-infection and using low pressure to irrigate the wound bed.
Irrigation at a low pressure (4 to 15 pounds per square inch) using a syringe or faucet tubing is suggested for promoting debris removal without disrupting granulating tissue42,56,62,65. Applying fluid at lukewarm temperature avoids vasoconstriction that lowers tissue healing capacity9,45,46,54,62,66. The potential for cross-infection between patients, or contaminated water from dirty body areas flowing over a wound are considerations when washing in a shower9,54,58. Directing fluid flow appropriately when irrigating54 was noted as another strategy to prevent cross-infection.
Synthesis 14: Good quality tap water is a cost-effective option for cleansing dirty wounds, chronic wounds and wounds with closed or sutured edges, although it may cause pain.
Benefits and disadvantages of tap water were discussed39,42,46,54,56,58,62,66. Water was noted as acceptable for sutured39, sacral/perineal56, open traumatic56, and chronic56 wounds, and wounds with sealed edges39. Ensuring high quality water is important54,56,62,66, although commentators noted that in cities with monitored and drinkable tap water it is sufficiently safe for wounds54,56,62,66. Texts suggested using running tap water for at least 30 seconds62 or soaking wounds in a bucket58. Higher, constant pressure62, large fluid volumes62, patient satisfaction66 and reduced time66 are advantages of water. Lack of additional equipment (for example, syringes) contributes to the cost-effectiveness of water46,54,62,66. However, there is potential that water may cause pain due to increasing osmotic pressure46.
Synthesis 15: Precautions can be taken to reduce the risk of potential contamination of water sources.
Another disadvantage is the potential for contaminated tap water9,41. One commentator suggested a risk of acquiring virulent pathogens or biofilm from hospital water41. This risk may be higher for immunocompromised patients41. However, precautions can be taken41,42,56. Water filters41, running taps for a few minutes before using the water42 and evaluating the water storage and delivery before use9 were suggested.
Selecting wound care technique and equipment
Four syntheses addressed selection of wound care techniques and equipment.
Synthesis 16: Selection of sterile/surgical ANTT or clean/standard ANTT is determined by the level of risk posed to the patient by his or her health status, the environment, factors associated with the wound and the type of wound management procedure being performed.
The infection risk from the surrounding environment is one consideration in selecting a wound management technique7,37,44,57,60,61,68. Findings illustrated that both the health care setting7,37,44,68 and the storage of equipment68 influences the ability to maintain a sterile or aseptic environment. The complexity of the procedure is a contributing factor, for example extensive debridement, wound packing and necessity to touch key parts were considered more invasive and requiring greater precautions44,51,57,60,61,68. Patient-related factors (for example, immune status) may also contribute to the risk of infection from a dressing procedure7,44,57,60,68. The chronicity, depth and location of the wound also contribute to selection of a technique7,44,50,56,57,68. Rigorous asepsis was considered to be inappropriate for chronic wounds50,56,57,68.
Synthesis 17: Simple wound management procedures on low-risk patients can be performed with non-sterile but clean equipment, solutions and gloves. More complex procedures or procedures in higher risk patients require surgical aseptic non-touch technique, using sterile gloves, solutions and equipment.
Textual findings referred to clean technique/standard ANTT and aseptic technique/surgical technique/surgical ANTT. The first technique is appropriate for routine dressing changes without surgical conservative debridement and simple procedures lasting less than 20 minutes37,61,68. This technique was reported to involve a clean surface, non-sterile gloves and clean equipment and irrigation fluids (for example, tap water)37,61,68. The surgical ANTT requires sterile gloves and equipment and a sterile irrigation fluid, with a strict aseptic field37,56,57,61,68. The findings suggested this procedure was appropriate for patients at high infection risk, wounds requiring surgical conservative debridement, complex/invasive procedures with many key parts or procedures lasting longer than 20 minutes56,57,61,68. One commentator suggested that this should be standard practice56.
Synthesis 18: Wound management equipment should be single use or cleaned with alcohol preparations. Using cleansers and wound dressings in smaller packages reduces waste and contamination risk.
Ensuring products are cleaned appropriately via sterilisation, disinfection or decontamination is important57,61,64. Using alcohol preparations or wipes and vigorously rubbing equipment to remove visual soiling cleans reusable products61,64, although single-use products may be easier, especially in community settings64. Wastage of excess products was noted as a concern, especially from dressing packs with pre-selected materials that are not always appropriate for the procedure8,11. Selecting smaller packages to reduce waste or risk of contamination from reusing products was suggested45,65.
Synthesis 19: When performing surgical ANTT the wound management field must remain free of non-sterile items, including equipment, cleansing fluids and gloved hands that have touched a non-sterile object.
Commentary highlighted the importance of all sterile equipment being free from potentially contaminated objects, including water that had touched surrounding skin during washing or forceps that had touched the wound bed7,8,43,51,68. One text referred to a dirty hand or forceps/a clean hand or forceps7. The difficulty clinicians have in manoeuvring forceps was raised7,56, and using a gloved hand for parts of a procedure was proposed as an optional wound management method7,8,56, if the potentially contaminated hand could be maintained away from the wound management field7,68.
Managing patients with known infection
Synthesis 20: Extra infection control precautions should be taken for people with known infection.
One opinion article addressed infection control for patients with known methicillin-resistant Staphylococcus aureus (MRSA)49. Findings indicated that clinicians should take additional precautions by thoroughly disinfecting surfaces, putting down plastic sheeting and using disposable equipment when possible49. Reusable equipment could be cleaned immediately49. Double-bagging waste products before disposal may reduce cross-infection49. Diligence is required in handwashing and the use of personal protective equipment49.
Product storage
Synthesis 21: Wound management products should be stored in dry, clean environments to reduce risk of contamination and cleansers should be dated on opening and discarded if visually contaminated or according to organisation policy.
Storing dressing products in a clean, dry space was suggested56,64. Wound cleansers should be dated and refrigerated on opening, although there is no set time frame after which they should be disposed45. One commentator mentioned discarding fluids if there is visual contamination, or to follow the organisation policy45. Risks posed by storing gloves in a manner that attracts mould or contamination were noted50,62.
Structural support
Two syntheses summarised eight categories related to structural support for aseptic technique and infection control.
Synthesis 22: Staff education that incorporates skills practice, simulation learning, theoretical knowledge update and procedures for different clinical settings is essential in promoting best practice in aseptic technique and infection control.
Importance of education was highlighted8,11,38,39,44,49-51,57,59,64. Some references suggested that clinicians develop ritualistic practice and may not fully understand theoretical concepts8,11,39. Qualitative studies indicated that community nurses experience frustration and have fatalistic attitudes11 that may influence the way in which they perform wound care10. Ongoing reinforcement of knowledge and skills through regular education using simulation learning53,57, visual feedback (for example, dye in handwashing exercises)59, hands-on practice with feedback64 and didactic lectures53 is suggested.
Synthesis 23: Best practice in aseptic technique and infection control procedures is promoted through development of facility policies, regular risk surveillance, annual auditing of staff practice, engaging with staff and patients and provision of acceptable hand hygiene products.
Regular risk surveillance7,50,59,64 promoting a culture of clinicians identifying risks50, and root cause analysis64 were highlighted as promoting quality improvement. Engaging with staff and patients by working with a wound champion to promote best practice38,64, empowering patients to ask clinicians about hand hygiene59 and ensuring adequate staffing levels may promote best practice51. Commentators proposed incorporating annual handwashing audits into quality improvement programs8,38,40,55,59. The importance of local policies and procedures was raised8,48,61, especially for topics for which there is insufficient evidence to make recommendations48. Finally, provision of products that are acceptable to clinicians (for example, low allergen) may promote handwashing40,55.
Discussion
There was general agreement between the quantitative and qualitative/textual findings in this review. Use of potable tap water for irrigation received the most attention, and findings from SRs, RCTs and non-research articles were in agreement that for many wound types, cleansing with good-quality, lukewarm tap water does not increase risk of wound infection18,20,21,23-26,29,32-36,39,42,46,54,56,58,62,66. This evidence should be considered when selecting appropriate and cost-effective wound management techniques.
Limited evidence was available on other topics of interest. No significant evidence was identified regarding strategies for managing opened wound dressing packages and minimal commentary on the advantages and risks of this practice was identified. One moderate quality study17 suggested reusing hydrogel products may be safe if the product was dispensed in controlled conditions. A very low quality study suggested contamination of opened wound dressings is an issue in community settings30 and commentators suggested using smaller packages to reduce waste45,65. There was also limited evidence on methods of performing aseptic technique, with technique details often derived from unreferenced opinion sources61. There is a strong need for well-designed studies exploring these issues.
There was a paucity of evidence on environmental factors in conducting wound care identified in the available literature. There was agreement that the environment in which wound care is conducted should be clean51,61,64, with guidance generally focused on strategies to address the potential risk from airborne contamination51,61,64. Practical solutions for maintaining asepsis in home care settings were provided8,51,61,64; however, the evidence supporting these practices was at best minimal.
This review is not without limitations. Foremost, the literature search was limited to journal articles. Reports12 and guidelines13,69 also inform this topic; however, these resources are developed from the existing body of evidence and are not specific to wound management. The search terms used for this review focused specifically on asepsis in wound management. It is probable that evidence on some included topics is available in the broader literature. Except for evidence related to irrigation, the evidence was primarily from non-research texts of low and very low quality and this should be considered when evaluating the adoption of the suggestions into practice. It should be noted that theory and translation in this field has changed substantially over time. The reviewers attempted to identify a cut-off date in order to exclude outdated concepts; however, given that much of the findings were opinion, some ideas may be anachronous.
Conclusions
The findings of this systematic review highlighted the lack of high-level evidence in many clinical areas associated with aseptic wound management practice. There is a need for further research in this field to establish with certainty the procedures that are necessary to prevent and control wound infection. Until such research exists, guidance based on the current evidence base, evidence derived from other clinical procedures (for example, intravenous therapy), broader guidelines12,13,69 and expert opinion is required to assist facilities in developing local policies and procedures.
Acknowledgements
Sue Atkins and Liz Howse undertook second review of some papers included in this systematic review.
Wounds Australia contributed to the funding of the systematic review.
Author(s)
Emily Haesler*
PhD, BN, PostGradDipAdvNurs(Gerontics)
Adjunct Associate Professor
Curtin University, School of Nursing, Midwifery and Paramedicine, WA, Australia
Honorary Associate
La Trobe University, Australian Centre for Evidence Based Aged Care, Vic, Australia
Email: Emily.Haesler@curtin.edu.au
Tel +61 2 6244 2946
Lyn Thomas
RN, NP, BHlthSci(Nurs), MNP
Community and Aged Care Services,Greater Newcastle Sector, Hunter New England Local Health District, NSW, Australia
Pam Morey
MN, NP, STN, PhD(C)
Nurse Practitioner, Advanced Wound Assessment Service, Silver Chain
Nurse Practitioner Course Coordinator
Curtin University, WA, Australia
Judith Barker
RN, NP, BHlthSc(Nurs), MNP
Nurse Practitioner — Wound Management
Adjunct Associate Professor, University of Canberra, Synergy: Research Centre for Nursing and Midwifery Practice, Canberra
Canberra Hospital, ACT Health, ACT, Australia
* Corresponding author
References
- Ngo QD, Vickery K, Deva AK. The effect of topical negative pressure on wound biofilms using an in vitro wound model. Wound Repair Regen 2012;20(1):83–90.
- James GA, Swogger E, Wolcott R et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16(1):37–44.
- Sandy-Hodgetts K, Carville K, Leslie G. Determining risk factors for surgical wound dehiscence: a literature review. Int Wound J 2015;12:265–75.
- Leaper D, Ousey K. Evidence update on prevention of surgical site infection. Curr Opin Infect Dis 2015;28(2):158–63.
- Roodsari GS, Zahedi F, Zehtabchi S. The risk of wound infection after simple hand laceration. World J Emerg Med 2015: DOI 10.5847/wjem.j.1920–8642.2015.01.008.
- Sarwat T, Rashid M, Rastogi V, Chander Y. A comparative study of antibiogram of Pseudomonas aeruginosa in hospital- and community-acquired infections. Int J Curr Microbiol App Sci 2015;1:286–91.
- Gillespie BM, Fenwick C. Comparison of the two leading approaches to attending wound care dressings. Wound Practice & Research 2009;17(2):84.
- Unsworth J. District nurses’ and aseptic technique: where did it all go wrong? Br J Community Nurs 2011;16(1):29–34.
- Watret L, McClean A. Cleansing diabetic foot wounds: tap water or saline? Diabetic Foot Journal 2009;12(3):5p.
- Hallett CE. Infection control in wound care: a study of fatalism in community nursing. J Clin Nurs 2000;9(1):103–109.
- Unsworth J, Collins J. Performing an aseptic technique in a community setting: fact or fiction? Prim Health Care Res Dev 2011;12(1):42–51.
- Australian Commission on Safety and Quality in Health Care (ACSQHC), Standard 3: Preventing and Controlling Healthcare Associated Infection. Safety and Quality Improvement Guide. Sydney: ACSQHC: 2012.
- National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare. Commonwealth of Australia, 2010.
- Joanna Briggs Institute. Reviewers’ Manual 2014. Adelaide: Joanna Briggs Institute, 2014.
- Joanna Briggs Institute, Reviewers’ Manual 2014: Summary of Findings Tables for Joanna Briggs Systematic Reviews. Adelaide: Joanna Briggs Institute, 2014.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6(6):e1000097.
- Aras PS, Sussman G. The clinical contamination of amorphous hydrogels. Primary Intention 2000;8(4):137–40.
- Bansal BC, Wiebe RA, Perkins SD, Abramo TJ. Tap water for irrigation of lacerations. Am J Emerg Med 2002;20(5):469–472.
- Fellows J, Crestodina L. Home-prepared saline: a safe, cost-effective alternative for wound cleansing in home care. J Wound Ostomy Continence Nurs 2006;33(6):606–609.
- Gouveia JCF, Miguéns CIM, Nogueira CLS, Alves MIP. Is it safe to use saline solutions to clean wounds? EWMA J 2007;7(2):7–12.
- Griffiths RD, Fernandez RS, Ussia CA. Is tap water a safe alternative to normal saline for wound irrigation in the community setting? J Wound Care 2001;10(10):407–411.
- Jackson D, Wall D, Bedward J. The sociocultural contribution to learning: why did my students fail to learn Aseptic Non-Touch Technique? Multidimensional factors involved in medical students’ failure to learn this skill. Med Teach 2012;34(12):e800–12.
- Lakshmi R, Andrews R, Chumber S. A study to compare the effectiveness of normal saline vs tapwater in irrigation of chronic wounds. Int J Nurs Educ 2011;3(1):19–21.
- Lawson C, Juliano L, Ratliff CR. Does sterile or nonsterile technique make a difference in wounds healing by secondary intention? Ostomy Wound Manage 2003;49(4):56.
- Merei JM. Pediatric clean surgical wounds: is dressing necessary? J Pediatr Surg 2004;39(12):1871–3.
- Moscati RM, Mayrose J, Reardon RF, Janicke DM, Jehle DV. A multicenter comparison of tap water versus sterile saline for wound irrigation. Acad Emerg Med 2007;14(5):404–409.
- Poole M, Coughlan A. Use of sterile dressing packs: challenging tradition. Nurs Times 2002;98(25):59–60.
- Ribu E, Haram R, Rustøen T. Observations of nurses’ treatment of leg and foot ulcers in community health care. J Wound Ostomy Continence Nurs 2003;30(6):342–350.
- Valente JH, Forti RJ, Freundlich MS, Zandieh SO, Crain EF. Wound irrigation in children: saline solution or tap water? Ann Emerg Med 2003;41(5):609–16.
- Zwanziger PJ, Roper S. Bacterial counts and types found on wound care supplies used in the home setting. J Wound Ostomy Continence Nurs 2002;29(2):83–87.
- Bee TS, Maniya S, Fang ZR et al. Wound bed preparation — cleansing techniques and solutions: a systematic review. Singapore Nursing Journal 2009;36(1):16.
- Dayton P, Feilmeier M, Sedberry S. Does postoperative showering or bathing of a surgical site increase the incidence of infection? A systematic review of the literature. Foot Ankle Surg 2013;52(5):612–614.
- Fernandez R, Griffiths R. Water for wound cleansing. Cochrane Database Syst Rev 2012(2).
- Fernandez R, Griffiths R, Ussia C. Effectiveness of solutions, techniques and pressure in wound cleansing. JBI Reports 2004;2(7):231–270.
- Fernandez RS, Griffiths RD, Ussia C. Wound cleansing: which solution, what technique? Primary Intention 2001;9(2):51.
- Queirós P, Santos E, Apóstolo J, Cardoso D, Cunha M, Rodrigues M. The effectiveness of cleansing solutions for wound treatment: a systematic review. JBI Database of Systematic Reviews & Implementation Report 2014;12(10):121–151.
- Selim P, Bashford C, Grossman C. Evidence-based practice: tap water cleansing of leg ulcers in the community. J Clin Nurs 2001;10(3):372–379.
- Aziz AM. Variations in aseptic technique and implications for infection control. Br J Nurs 2009;18(1):26–31.
- Blunt J. Wound cleansing: ritualistic or research-based practice? Nurs Stand 2001;16(1):33–36.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee. Society for Healthcare Epidemiology of America. Association for Professionals in Infection Control. Infectious Diseases Society of America. Hand Hygiene Task Force. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol 2002;23(12 Suppl):S3–40.
- Clark AP, John LD. Legal and ethical. Nosocomial infections and bath water: any cause for concern? Clin Nurse Spec 2006;20(3):119–123.
- Cunliffe PJ, Fawcett TN. Wound cleansing: the evidence for the techniques and solutions used. Prof Nurse 2002;18(2):95–99.
- Ellis T. CPD: understanding the act of contamination in wound dressing procedure ... including commentary by Dreimanis D and Rice J. Collegian 2004;11(3):39–42.
- Flores A. Sterile versus non-sterile glove use and aseptic technique. Nurs Stand 2008;23(6):35–39.
- Friedman MM. Infection control update for home care and hospice organizations. Home Healthc Nurse 2003;21(11):753–760.
- Gannon R. Wound cleansing: sterile water or saline? Nurs Times 2007;103(9):44–46.
- Gilmour D. Is aseptic technique always necessary? Journal of Community Nursing 2000;14(4):32.
- Gray M, Doughty DB. Clean versus sterile technique when changing wound dressings. J Wound Ostomy Continence Nurs 2001;28(3):125–128.
- Grossman S, Mager DD. Managing the threat of methicillin-resistant Staphylococcus aureus in home care. Home Healthc Nurse 2008;26(6):356–366.
- Hampton S. Nurses’ inappropriate use of gloves in caring for patients. Br J Nurs 2003;12(17):1024.
- Hart S. Using an aseptic technique to reduce the risk of infection. Nurs Stand 2007;21(47):43–8.
- Karch AM, Karch FE. ‘Clean’ vs ‘sterile’: are sanitary napkins and diapers acceptable as wound dressings? Am J Nurs 2001;101(4):25–25.
- Lewis G. ANTT clinical competencies for nursing students ... aseptic non touch technique (ANTT). Australian Nursing Journal 2009;17(4):39–41.
- O’Neill D. Can tap water be used to irrigate wounds in A&E? Nurs Times 2002;98(14):56.
- World Health Organisation. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Switzerland: WHO, 2009.
- Parker L. Applying the principles of infection control to wound care. Br J Nurs 2000;9(7):394–404.
- Pegram A, Bloomfield J. Wound care: principles of aseptic technique. Mental Health Practice 2010;14(2):14–18.
- Platt C. Wound cleansing: is tap water best? Primary Health Care 2005;15(5):27–30.
- Preston RM. Aseptic technique: evidence-based approach for patient safety. Br J Nurs 2005;14:540–46.
- Rowley S, Clare S. ANTT: a standard approach to aseptic technique. Nurs Times 2011;107(36):12–14.
- Rowley S, Clare S, Macqueen S, Molyneux R. ANTT v2: an updated practice framework for aseptic technique. Br J Nurs 2010;19(Supp 1):S5–11.
- Schremmer RD. New concepts in wound management. Clin Pediatr Emerg Med 2004;5(4):239–245.
- Spear M. Wound care management. Wound cleansing: solutions and techniques. Plast Surg Nurs 2011;31(1):29–31.
- Swanson J, Jeanes A. Infection control in the community: a pragmatic approach. Br J Community Nurs 2011;16(6):282–288.
- Towler J. Cleansing traumatic wounds with swabs, water or saline. J Wound Care 2001;10(6):231–234.
- Whaley S. Tap water or normal saline for cleansing traumatic wounds? Br J Community Nurs 2004;9(11):471–476.
- Wilkins RG, Unverdorben M. Wound cleaning and wound healing: a concise review. Adv Skin Wound Care 2013;26(4):160–163.
- WOCN Wound Committee. Clean vs. sterile dressing techniques for management of chronic wounds: a fact sheet. J Wound Ostomy Continence Nurs 2012;39(2S):S30–s34.
- Rowley S, Clare S. ANTT Core Clinical Guidelines 2016, Association for Safe Aseptic Practice: http://antt.org/ANTT_Site/about.html.




