Volume 25 Number 1
Fighting chronic wound infection – one model at a time
Zlatko Kopecki and Allison Cowin
Keywords biofilm, Chronic wounds, Infection, animal models.
Abstract
Wound infections are a serious medical problem for patients with non-healing chronic wounds and burn injuries. The healing of these wounds is often compromised by colonisation of many different bacteria, predisposing patients to life-threatening infections. Infected wounds continue to represent a complex problem for both health professionals and patients. Bacterial infections are a critical component of hard-to-heal wounds, often leading to biofilm formation, inhibition of innate inflammatory responses and resistance to traditional therapeutics. Over the last 20 years, tremendous progress has been made in understanding the intricacies of wound biofilm pathology and bacteria–host interactions. This has been achieved by the development of in vitro and in vivo models of wound infection. This review will discuss the challenges in the development of wound infection models and will focus on different in vivo models of cutaneous wound infection, highlighting advantages and clinical applicability of each model. It will also describe the development of novel bioluminescent models of cutaneous wound infection in vivo, which may help to revolutionise the future testing of novel antimicrobial therapeutics for the treatment of wounds.
Introduction
Chronic wounds represent a silent epidemic affecting a substantial portion of the global population and place a significant economic burden on the healthcare system. In Australia alone, chronic wound treatments are estimated to cost over $2.85 billion annually1. These estimates are often understated, and do not include the impact on patient lifestyle, financial security and overall wellbeing, which collectively place an immeasurable burden on these most vulnerable people. Despite the significant progress that has been made in understanding chronic wound infections and the improved treatment modalities, the incidence of infected chronic wounds is still on the rise, in part due to the growing rate of other chronic diseases that can impact on wound healing, including obesity, diabetes mellitus, and perivascular disease2.
Human skin provides a formidable barrier to environmental pathogens; however, in patients with chronic wounds, burn injuries, the elderly, and those suffering from skin blistering diseases, including epidermolysis bullosa (EB), there is impairment to the skin’s barrier and often a compromised immune system, which leads to severe infection and sepsis3. Effective management of infection in chronic wounds focuses on aggressive debridement of devitalised tissue and control of patient co-morbidities that may further delay wound healing as well as the use of systemic antibiotics in the presence of cellulitis; however, these approaches are often not successful4. Furthermore, studies have shown that between 55% and 80% of patients admitted to burn units develop hospital-acquired infections4. In addition, sepsis is the leading cause of infant mortality in patients with different EB subtypes and up to 24% of patients with junctional EB die from sepsis by 15 years of age5. Alarmingly, infection of skin wounds by pathogenic bacteria that are resistant to multiple classes of antimicrobials accounts for massive morbidity and mortality in humans worldwide and the development of new antimicrobials is hampered by a lack of technologies aimed at preclinical assessment of novel therapies that might combat infections and bacterial biofilm formation6.
The lack of adequate in vivo models of wound infection has made it difficult to investigate bacterial wound infections6. As human studies are logistically and ethically prohibitive, the use of animal models for preclinical testing of new localised or systemic therapies is the best approach to treat the problem of chronic wound infection. An established theory of modern medicine is that no in vitro, in situ or in silico model can adequately account for the complex host defence mechanisms and interactions between a host and a pathogen that is encountered in a live animal model7. Developing an in-depth understanding of bacterial biofilms, infections and potential therapeutic treatments requires in vivo models that can be utilised to understand the complex interactions between the bacteria and the host, while overcoming the challenges of strain diversity and differences between local and systemic infection manifestations7.
This review will describe some of the most common and latest in vivo models of cutaneous wound infection under development, focusing on Streptococcus pyogenes (S. pyogenes) and Staphylococcus aureus (S. aureus) as examples of some of the most common bacteria encountered in patients with chronic wounds8. Advances in technologies have allowed the development of in vivo models to become more sophisticated and these novel models will also have a direct impact on our understanding and treatment of infected human wounds.
Challenges in developing wound infection and biofilm models
Bacterial biofilms have an inherent defence and survival mechanism that includes: avoidance of host inflammatory cells, resistance to antibiotics, and dynamic cell to cell communication pathways, all of which allow their continuing presence in non-healing wounds2. The moist and nutritionally supportive microenvironment of the wound bed matrix is ideal for the formation of bacterial biofilms, creating a destructive and sustainable environment which impairs wound repair2. Studies have demonstrated that the presence of a biofilm and bioactive compounds secreted by bacteria inhibit key wound healing mechanisms including cell proliferation and migration6. The presence of bacteria in chronic, hard-to-heal wounds, has been confirmed by both imaging and more sophisticated molecular sampling techniques which shows that the amount of bacteria in a wound is often underestimated and that most often wounds contain a mixed population of multiple bacterial species2. These bacterial biofilms present a major obstacle in the development of wound infection models that represent infection of chronic human wounds both histologically and immunologically.
Models of cutaneous wound infection
The infected subcutaneous ulcer/air sac model is one of the most widely used models for the analysis of S. pyogenes and other streptococci that results in localised inflammatory lesions with robust levels of bacteria cell proliferation and inflammatory cell recruitment7. In this model, 106–108 CFU (colony forming units) of the S. pyogenes strain of interest are injected into tissue, which results into a well-defined area of infection by 8–12 hours post infection. The lesion formed is infiltrated by neutrophils and leads to formation of an abscess, which by 18–24 hours post-infection ulcerates and develops a wound covered by an eschar (Figure 1)7. By day 8 the lesion begins to resolve and by day 14 the wound is healed. This model has been widely used in different strains of mice including BALB/c, C57BL6 strains and many other transgenic and knock-out variants7.
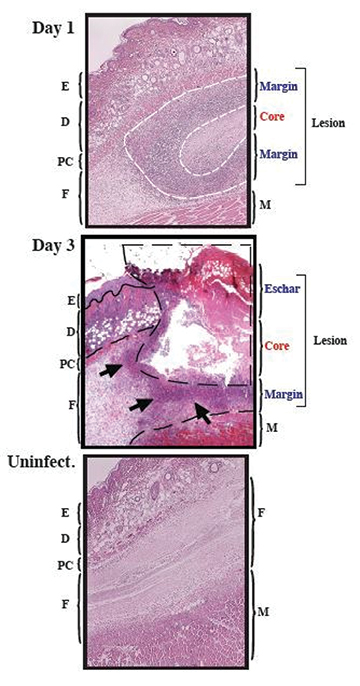
Figure 1: Murine subcutaneous ulcer model of wound infection
Representative histological Haematoxylin and Eosin stained images of S. pyogenes HSC5 infection at day 1 and day 3 post infection following subcutaneous inoculation of 1x106 CFUs into flank skin of SKH1 mice. Uninfected control section illustrates the histological differences and the development of the infected ulcer in these mice SKH1 mice. By day 1 of inoculation, a well-defined subcutaneous lesion is formed consisting of a necrotic core containing the bacteria and a margin infiltrated with inflammatory cells. E = epidermis. D = dermis, PC = panniculus carnosus. F = fascia. M = muscle. Magnification = 40x. White dotted line = lesion margin, black dotted line = abscess/wound outline, black arrows = inflammatory cell infiltrate. Figure adapted from reference 7 and modified with permission.
One of the challenges of this model is that the ulcer will have an irregular border. This makes the model more relevant to human infected wounds; however, it makes the scientific conclusions about healing rate and bacterial virulence hard to interpret. However, useful parameters of assessment include changes in weight, numbers of CFUs that are recoverable from the wound and numbers of mice that do not develop an infection or ulcer, as well as time to maximum ulcer area and time to healing of the resulting wound7. The air sac model is an extension of this model whereby an air pouch is initially created under the skin by injection of air prior to infection and bacteria are subsequently injected into this air sac. The advantage of this model is that it allows researchers to recover host inflammatory cells for analysis by lavage compared to the subcutaneous ulcer model7. A disadvantage of these two models is that they do not histologically reproduce some of the features of human cutaneous disease including impetigo, pyoderma, cellulitis or necrotising fasciitis7. These differences are attributed to the anatomical differences between a mouse and human skin, with mouse skin having a significantly higher density of hair follicles; as well as variations in bacterial virulence between species9. Clear strengths of this model, however, include simplicity, high-throughput and low cost, and ability to use mice of different genetic backgrounds. In addition, as bacteria grow in a defined wound-like environment it reproduces the dynamic host environment that is remodelled by both host immunity and bacterial metabolism10.
The impetigo model of superficial wound-like infection is also a popular model used to study superficial infections of the skin11. In this model, humanised mice are created by grafting human epidermal tissue from neonatal foreskin onto the flanks of immune-compromised SCID mice which are known to have no adaptive immune responses, meaning that the graft is not rejected. The engrafted tissue is then superficially wounded using cross-wise cuts with a scalpel blade. Bacteria, typically S. pyogenes, are then added to the superficial wounds, which are then occluded with a dressing11. This results in a superficial wound infection with erosion of the stratum corneum and infiltration of murine polymorphonuclear lymphocytes, leading to pus formation11. Virulence is measured by a semi-quantitative visual assessment of the histopathology or by determining the number of CFU to monitor bacterial growth. The extent of damage has been shown to correlate with the magnitude of streptococcal growth11. The limitations of this model include the high technical skill required to conduct the model as well as a source of human tissue. In addition, while the human skin is infected in this model, it still lacks human-specific targets of important virulence factors7.
Excisional wound infection models have been widely studied in models of S. aureus cutaneous infection. S. aureus is a commensal bacteria of the human skin, nares and gastrointestinal tract; however, it is also a leading cause of cutaneous wound infections, bacteraemia, sepsis, pneumonia and endocarditis12. Therapeutic options for both cutaneous and invasive S. aureus infections are becoming limited due to rising antimicrobial resistance, hence testing of new antimicrobial agents using relevant animal models is particularly important13. The pathological hallmark of S. aureus infection is the formation of an abscess or lesion. This was originally demonstrated by Ogston and colleagues, who isolated the pus from a surgical wound infection and showed that its injection into subcutaneous tissue of experimental animals led to abscess formation in guinea pigs and mice14. Most S. aureus cutaneous wound infections models today focus on developing infection in excisional wounds topically; however, original models involved inducing the skin infection in mice by injecting a suspension of 107–109 CFU S. aureus subcutaneously. Within 24 hours, bacteria elicit an inflammatory response and cause localised swelling, which, over a time course of 5–7 days, can become 30–100 mm2 in size15. Abscess lesions can rupture and become an open wound or may be resolved over a 7–9 day period. Depending on the strain of S. aureus and the production of the α-haemolysin (α-toxin), subcutaneous lesions can be associated with superficial dermonecrosis, which heals at a similar rate to the resolution of the abscess lesion16,17. This model of cutaneous skin infection is often modified to examine the contribution of specific skin cells or tissue structures and immune cells. This model has also evolved to include inducing damage to the skin, that is, removal of superficial keratinocytes, creation of a full-thickness excisional wound using a punch biopsy (6 mm2) and creation of a full-thickness incisional wound or burn injury using heat. These wounds are then inoculated with 107–109 CFU of bacteria to induce infection4. In addition, some protocols involve inoculating the bacteria along with implanted foreign material (suture, dextran beads, cotton dust), allowing researchers to examine the effects of bacterial infection in different wound environments and reduce the pathological dose of bacteria required to cause a pathological lesion17,18. Along the same lines as wound infection mouse models, inoculation of partial-thickness wounds in pigs with S. aureus results in the formation of biofilm-like structures only 48 hours post-inoculation19. In addition, mouse models of S. aureus infection of surgically implanted medical devices and catheters have been developed20,21. The most popularly used one is the implanted chamber model often used in mice and rabbits22-24.
Biofilm formation is a hallmark of chronic wound infection, and establishment of a good chronic wound model of infection is controversial and dependent on the research question. Besides inflicting a wound, maintaining a biofilm infection within a model for a certain period of time to classify the model as a chronic wound model remains challenging. Studies have attempted to address this problem by using different approaches, including: use of preconditioned animals (mutant breeds or by induction of a pathogenesis, for example diabetes25, preformation of the biofilm in vitro prior to wound inoculation26, or placement of a dressing material to maintain the wound moisture and facilitate biofilm formation27. In these models, utilising a well-described model of streptozotocin-induced diabetes, infection was caused in excisional wounds of diabetic mice by inoculation with ~107 CFUs of exponential phase S. aureus which were allowed to grow for 48 hours. Signs of chronic wound infection including purulent discharge, redness and swelling were subsequently observed28.
To achieve more clinically relevant chronic wound models of infection, studies have also tried to address the problem of contraction, by using dressings as a mechanical barrier to wound contraction in streptozotocin-induced diabetic mice with excisional full-thickness infected wounds29,30. Additionally, a rabbit dermal ulcer model has been developed, whereby full-thickness dermal wounds which closely resemble human chronic wounds and the underlying cartilage acts as a natural splint preventing contraction and allowing healing by epithelisation and granulation31,32. Finally, a recent study has developed a chronic wound infection model using alloxan (a thiol reagent) to induce insulin-dependent diabetes mellitus in New Zealand rabbits which were subsequently subjected to dorsal wounds inoculated with a mixed bacterial suspension of S. aureus and P. aeruginosa33. Wound chronicity was assessed at day 5 post inoculations by measurement of excessive inflammation and infection accompanied by clinical signs of chronic wound infection, namely exudate formation, wound degradation, epithelial bridges, discolouration of the wound bed, abscess formation and bad odour33.
New imaging methods for analysing wound infection
Improved visualisation and advanced imaging techniques have allowed the development of novel models of chronic wound infection with particular focus on “real-time” monitoring of the severity of infection and biofilm formation4. In addition, the development of bacterial strains engineered to be constitutively bioluminescent (mostly by insertion of the luxCDABE operon from Photorhabdus luminescens and Vibrio harveyi) have further enabled the development of novel models of wound infection. Bacterial luciferase operon can also be used for these applications as it is engineered for constitutive light emission at 490 nm12. Combined with the development of highly sensitive imaging procedures, it allows for direct and continuous monitoring and quantification of bacterial biofilm infections, by quantification of radiance levels, over a period of time, either using full-thickness wound injury (Figure 2), partial-thickness burn injury (Figure 3)4 or subcutaneously implanted catheters34 and surgical meshes35. A recent study using FVB/N mice investigated the effect of using a breathable dressing, allowing oxygen in and moisture vapour out, on excisional wounds inoculated with a mixed population of bioluminescent methicillin-resistant S. aureus and P. aeruginosa (107 CFU), on biofilm development, mimicking a clinically relevant chronic wound environment36. This study demonstrated that the use of a dressing allows development of a dense, mucous-like biofilm formation bulging the dressing outward, correlating with higher infection load and higher bioluminescent signal compared to uncovered wounds (Figure 4)36. In this model, the use of a wound dressing did not interfere with the detection of the bioluminescent signal and this model, while not in a physiologically chronic state (for example diabetes), offers a constant, longer lasting local wound infection consistent with chronic wounds, without the spontaneous clearing of bacteria that occurs when the wound dries out36.
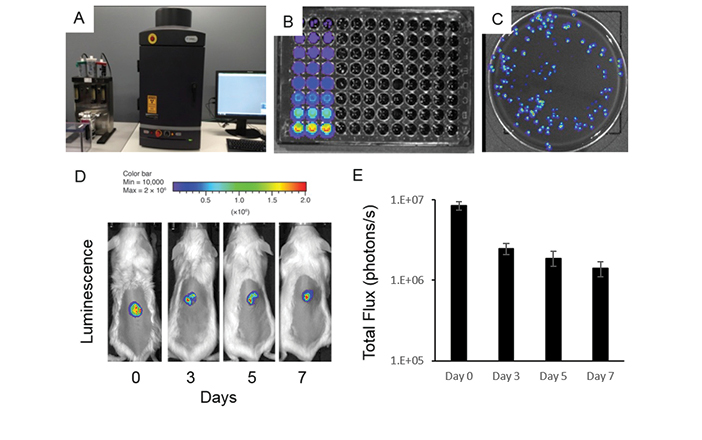
Figure 2: Novel bioluminescent model of excisional wound infection
A–C IVIS Lumina XRMS Series III Live Animal BioPhotonic imaging system (Caliper LifeSciences) allows for accurate and sensitive detection of live, actively metabolising bacteria in a high range of dilutions and individual growing bacterial colonies.
D–E Representative image of relative luminescence and total flux of S. aureus-infected excisional wounds on back of wild-type BALB/c mice over a period of seven days allowing for live in vivo assessment of wound infection and/or preclinical testing of antimicrobial agents. While bacterial load decreases after day 0 of inoculation, the bioluminescent signal is consistent over a period of seven days and sensitive enough to reflect even small changes in the bacterial infection load of wounds in vivo.
One of the main advantages of in vivo models of wound infection using bioluminescent strains of bacteria is that these models allow for daily or twice-daily monitoring of bacterial load by visualisation of bacteria in live animals. This temporal or spatial monitoring of the progression of infection offers a significant advantage over more traditional models and uses fewer animals and experimental time points37. Bioluminescent models of wound infection allows in vivo imaging, whole organ studies and can be used to quantify viable wound bacterial burden up to seven days post-infection (Figure 3). Importantly, the measure of radiance and total flux bioluminescence using the IVIS Lumina XRMS Series III Live Animal BioPhotonic imaging system or similar imaging systems closely correlates with the rate of wound healing and bacterial CFU counts obtained at end time points (Figure 2). Bioluminescent models of in vivo wound infection also uses bacteria that are stably luminescent even in the absence of antibiotic selection37. An additional advantage of these bioluminescent models is that they allow the unprecedented preclinical testing of novel antimicrobials (Figure 5). Additionally, there are now increasing numbers of different bacterial strains commercially available at an affordable price, allowing researchers to utilise these models. The only disadvantage of these models is the requirement of an expensive biophotonic imaging system which can cost in excess of $400,000. Nevertheless, these novel and reproducible models of wound infection allow the monitoring of wound infection in vivo over a seven-day period and facilitate the testing of antimicrobial compounds on their ability to treat wound infection.
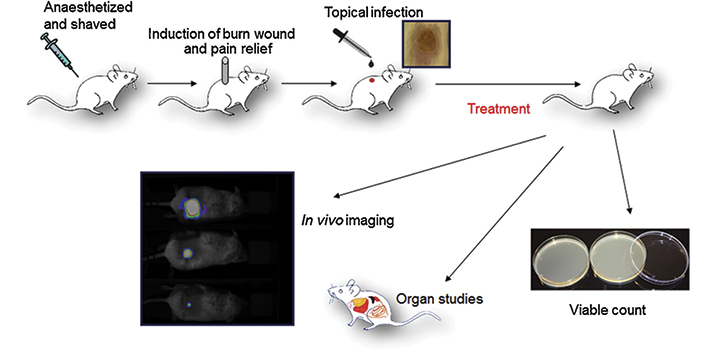
Figure 3: Novel bioluminescent model of burn injury wound infection
Schematic illustration of cutaneous burn injury wound infection model using a bioluminescent strain of Pseudomonas aeruginosa. The luminescent bacteria are continuously monitored between the treatments and over a period of time and the viable count is analysed in the wound as well as inner organs at the end of the study. Assessment of the bacterial load in blood and organs allows for monitoring of systemic sepsis development, which can occur depending on the type of bacteria and amounts of CFUs used in wound inoculations. Figure adapted from reference 4 with permission.
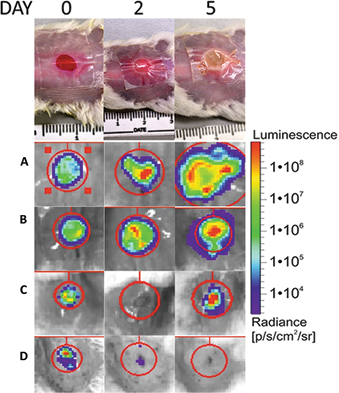
Figure 4: Use of wound dressing creates a better environment for development of more clinically relevant biofilm infected wounds in vivo
Top panel shows a visual representation of biofilm formation in excisional wounds infected with mixed population of S. aureus and P. aeruginosa covered with a TegadermTM dressing over a five-day period. Bioluminescent radiance as a measure of bacterial wound infection was analysed over a five-day period in: (A) covered non-treated infected wounds; (B) P. aeruginosa infected Rosa Bengal treated wounds; (C) S. aureus infected TLD1411 treated wounds; (D) uncovered non-treated infected wounds. Use of wound dressings in (A) clearly facilitates development of more chronic infection environment compared to uncovered infected wounds presented in (D). This model is useful for preclinical assessment of different antimicrobial treatments as shown in (B) and (C). Figure adapted from reference 36 with permission.
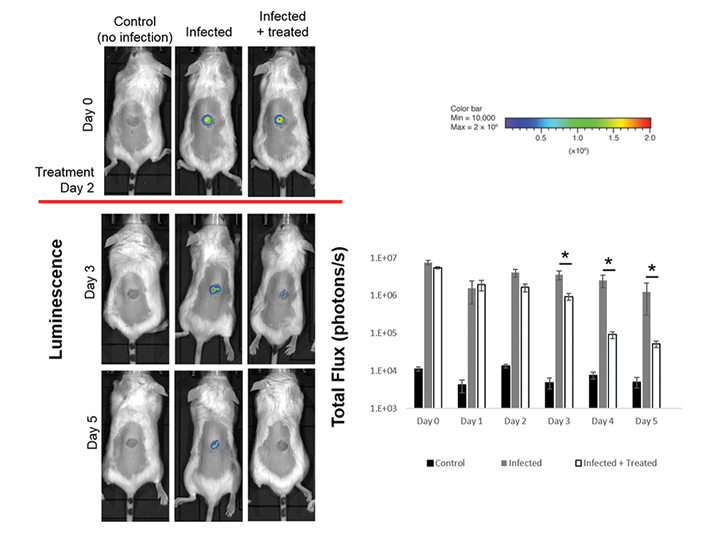
Figure 5: Use of bioluminescent models of wound infection for preclinical testing of novel antimicrobials
Excisional punch biopsy wounds were created on the back of Balb/c mice and inoculated with bioluminescent S. aureus (1x106 CFUs) on day 0 of the experiment. IVIS Lumina XRMS Series III Live Animal BioPhotonic imaging system was used for daily analysis of the bacterial burden and treatment with test antimicrobial started on day 2 of the experiment. Representative images of relative luminescence and total flux of S. aureus infected excisional wounds analysed illustrate the benefits of using this model for preclinical testing of novel antimicrobials. Control uninfected mice have background levels of signal detection, highlighting the sensitivity of the system model used which needs to be considered when designing experiments.
Conclusion
Chronic wound infections represent a significant health and financial burden to both patients and health service providers. Developing new approaches and therapeutics to kill bacteria but not damage the wound tissue is important. Several models of wound infection exist and through the advent of bioluminescent bacteria and new imaging technologies, the real-time assessment of the development and treatment of infection and biofilm formation is becoming possible. In vivo animal models of wound infection are a valuable research tool that will aid our understanding of the pathogenesis of bacteria and biofilm formation as well as help the preclinical assessment of potential new wound treatments.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
AJC is supported by the NHMRC Senior Research Fellowship (GNT#1102617). ZK is supported by a Future Industries Institute Foundation Fellowship.
Author(s)
Zlatko Kopecki*
PhD
Future Industries Institute, University of South Australia, Adelaide, SA, Australia
Email zlatko.kopecki@unisa.edu.au
Tel +61 8 8302 6384
Allison Cowin
PhD
Future Industries Institute, University of South Australia, Adelaide, SA, Australia
* Corresponding author
References
- Graves N, Zheng H. Modelling the direct health care costs of chronic wounds in Australia. Wound Practice & Research 2014;22(1):20–33.
- Seth AK, Geringer MR, Hong SJ, Leung KP, Mustoe TA, Galiano RD. In vivo modeling of biofilm-infected wounds: a review. J Surg Res 2012;178(1):330–8.
- van der Kooi-Pol MM, Duipmans JC, Jonkman MF, van Dijl JM. Host-pathogen interactions in epidermolysis bullosa patients colonized with Staphylococcus aureus. Int J Med Microbiol 2014;304(2):195–203.
- Bjorn C, Noppa L, Naslund Salomonsson E, Johansson AL, Nilsson E, Mahlapuu M et al. Efficacy and safety profile of the novel antimicrobial peptide PXL150 in a mouse model of infected burn wounds. Int J Antimicrob Agents 2015;45(5):519–24.
- Fine JD, Eady RA, Bauer EA, Bauer JW, Bruckner-Tuderman L, Heagerty A et al. The classification of inherited epidermolysis bullosa (EB): Report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol 2008;58(6):931–50.
- Jeffery Marano R, Jane Wallace H, Wijeratne D, William Fear M, San Wong H, O’Handley R. Secreted biofilm factors adversely affect cellular wound healing responses in vitro. Sci Rep 2015;5:13296.
- Watson Jr ME, Neely MN, Caparon MG. Animals models of Streptococcus pyogenes Infection. Ferretti JJ, Stevens DL, Fischetti VA, editors. Oklahoma City: National Center for Biotechnology Information, The University of Oklahoma Health Sciences Center; 2016.
- Mills M, Estes MK. Physiologically relevant human tissue models for infectious diseases. Drug Discov Today 2016.
- Cusumano ZT, Watson ME, Jr., Caparon MG. Streptococcus pyogenes arginine and citrulline catabolism promotes infection and modulates innate immunity. Infection Immunity 2014;82(1):233–42.
- Loughman JA, Caparon MG. A novel adaptation of aldolase regulates virulence in Streptococcus pyogenes. EMBO J 2006;25(22):5414–22.
- Scaramuzzino DA, McNiff JM, Bessen DE. Humanized in vivo model for streptococcal impetigo. Infection immunity 2000;68(5):2880–7.
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 2010;120(5):1762–73.
- Xiong YQ, Willard J, Kadurugamuwa JL, Yu J, Francis KP, Bayer AS. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother 2005;49(1):380–7.
- Ogston A. Micrococcus poisoning. J Anat Physiol 1882;17(Pt 1):24–58.
- Malachowa N, Kobayashi SD, Braughton KR, DeLeo FR. Mouse model of Staphylococcus aureus skin infection. Methods Mol Biol 2013;1031:109–16.
- Kennedy LA, Gill JA, Schultz ME, Irmler M, Gordin FM. Inside-out: the changing epidemiology of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2010;31(9):983–5.
- Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 2012;15(1):92–9.
- Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature 2013;503(7476):397–401.
- Davis SC, Mertz PM. Determining the effect of an oak bark formulation on methicillin-resistant Staphylococcus aureus and wound healing in porcine wound models. Ostomy Wound Manage 2008;54(10):16–8, 20, 2–5.
- Reglinski M, Sriskandan S. The contribution of group A streptococcal virulence determinants to the pathogenesis of sepsis. Virulence 2014;5(1):127–36.
- Schlievert PM. Chitosan malate inhibits growth and exotoxin production of toxic shock syndrome-inducing Staphylococcus aureus strains and group A streptococci. Antimicrob Agents Chemother 2007;51(9):3056–62.
- Arrecubieta C, Asai T, Bayern M, Loughman A, Fitzgerald JR, Shelton CE et al. The role of Staphylococcus aureus adhesins in the pathogenesis of ventricular assist device-related infections. J Infect Dis 2006;193(8):1109–19.
- Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI et al. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PloS One 2010;5(9):e12580.
- Snowden JN, Beaver M, Smeltzer MS, Kielian T. Biofilm-infected intracerebroventricular shunts elicit inflammation within the central nervous system. Infection Immunity 2012;80(9):3206–14.
- Goldufsky J, Wood SJ, Jayaraman V, Majdobeh O, Chen L, Qin S et al. Pseudomonas aeruginosa uses T3SS to inhibit diabetic wound healing. Wound Repair Regen 2015;23(4):557–64.
- Zhao G, Hochwalt PC, Usui ML, Underwood RA, Singh PK, James GA et al. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen 2010;18(5):467–77.
- Brackman G, Coenye T. In vitro and in vivo biofilm wound models and their application. Adv Exp Med Biol 2016;897:15–32.
- Sahu K, Sharma M, Dube A, Gupta PK. Topical antimicrobial photodynamic therapy improves angiogenesis in wounds of diabetic mice. Lasers Med Sci 2015;30(7):1923–9.
- Watters C, Everett JA, Haley C, Clinton A, Rumbaugh KP. Insulin treatment modulates the host immune system to enhance Pseudomonas aeruginosa wound biofilms. Infection Immunity 2014;82(1):92–100.
- Watters C, DeLeon K, Trivedi U, Griswold JA, Lyte M, Hampel KJ et al. Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Med Microbiol Immunol 2013;202(2):131–41.
- Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen 2009;17(3):354–9.
- Gurjala AN, Geringer MR, Seth AK, Hong SJ, Smeltzer MS, Galiano RD et al. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen 2011;19(3):400–10.
- Gongora J, Diaz-Roa A, Ramirez-Hernandez A, Cortes-Vecino JA, Gaona MA, Patarroyo MA et al. Evaluating the effect of Sarconesiopsis magellanica (Diptera: Calliphoridae) larvae-derived haemolymph and fat body extracts on chronic wounds in diabetic rabbits. J Diabetes Res 2015;2015:270253.
- Vuong C, Kocianova S, Yu J, Kadurugamuwa JL, Otto M. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. J Infect Dis 2008;198(2):258–61.
- Engelsman AF, van der Mei HC, Francis KP, Busscher HJ, Ploeg RJ, van Dam GM. Real time noninvasive monitoring of contaminating bacteria in a soft tissue implant infection model. J Biomed Mater Res B Appl Biomater 2009;88(1):123–9.
- Fila G, Kasimova K, Arenas Y, Nakonieczna J, Grinholc M, Bielawski KP et al. Murine model imitating chronic wound infections for evaluation of antimicrobial photodynamic therapy efficacy. Front Microbiol 2016;7:1258.
- Plaut RD, Mocca CP, Prabhakara R, Merkel TJ, Stibitz S. Stably luminescent Staphylococcus aureus clinical strains for use in bioluminescent imaging. PloS One 2013;8(3):e59232.



