Volume 25 Number 1
Developing an Australian skin risk assessment and management tool for neonates
Margaret M Broom, Wendy A Burton, Leanne M Ehrlich, Ann Marie Dunk and Mohamed E Abdel-Latif
Keywords Neonates, skin injuries, risk assessment, pressure injuries, neonatal intensive care unit.
Abstract
Background: Skin injuries are a common occurrence in neonatal units. Currently there are few tools that meet the specific needs of the neonatal population. To assist nurses in identifying neonates at risk and improve neonatal skin care, a working group developed a neonatal skin risk assessment and management tool (SRAMT) for their unit.
Setting: University-affiliated tertiary neonatal intensive care unit (NICU).
Design: Single-site prospective longitudinal study (2010–2014).
Method: The study was conducted over three phases: 1) skin injuries incidence study; 2) development of the SRAMT; and 3) post-implementation review of the SRAMT.
Results: The SRAMT lists eight risk categories: gestational age, sensory perception, activity/mobility, moisture, respiratory support, visual examination, blood collection and nutrition and these categories are graded from low (grade 1) to extreme risk (grade 4). The tool also provides assessment and management guidelines. Evaluation of the SRAMT showed a reduction in neonates who acquired skin injuries pre SRAMT from 37/60(61.7%), compared to post 12/30(40%), (OR 0.41 95% CI 0.17–1.02; p-value 0.085).
Conclusion: The study has shown introducing a skin risk assessment tool may reduce the incidence of skin injuries and standardise skin management in the NICU. Future research will be undertaken to validate the SRAMT and assess its suitability across neonatal units.
Background
There is a growing awareness that immobilised and acutely ill neonates are at risk of medically induced skin injuries within the neonatal intensive care units (NICU)1. Skin injuries may lead to local or systemic infection, fluid and electrolyte imbalances, and temperature instability2. Skin breakdown through various injuries can result in pain, infection, disfigurement, and mortality as well as increased costs, length of stay and risk of litigation2.
Premature infants have development anatomic and physiologic differences to adult skin, which often puts them at a higher risk of skin injuries. At birth the skin of an infant is at least 30% thinner than adult skin1. A premature infant’s skin is more fragile and less mature than full-term infant skin as there are only two to three layers of protective stratum corneum and fewer fibrils connecting the skin layers1.
In addition, there are both intrinsic and extrinsic factors to consider3. Neonatal immobility due to sedation or muscle relaxing agents, impaired tissue perfusion (with cooling or dehydration), surgery, sepsis and malnutrition are some known intrinsic factors3. Extrinsic factors that are commonly cited as placing a neonate at high risk of an injury include medical devices such as continuous positive air pressure (CPAP) equipment, tapes used for line and tube securement (shearing, friction and stripping injuries), cleaning agents (chemical injury) and pressure injuries from monitoring probes and electrodes3,4. Over the past 10 years the gestation and acuity of neonates surviving, but requiring medical support for extended periods, has increased, thus escalating the need for nursing staff to prioritise skin care practices in the NICU environment4.
In 2007 Baharestani and Ratcliff highlighted that although there is no agreement on which risk factors contribute to pressure injury development in neonates, there is agreement that prevention lies in early risk identification3. They also identified that there were 10 published paediatric pressure injury risk assessment scales at that time, of which only three had been tested for sensitivity and specificity: Braden Q scale, Glamorgan Scale and Neonatal Skin Risk Assessment Scale (NSRAS)3. International literature reporting injury rates from 47% to 75% with the highest prevalence in children with chronic illness and those supported by medical devices7. However, premature infants are at great risk of many other types of skin injuries and all causations should be considered when introducing a risk assessment tool that covers all types of skin injury1,2. Pressure injury risk assessment tools or scales ideally should have high sensitivity and specificity, good predictive value, clear definition of terms, and should be easy to use8. Validated tools are difficult to find and are mainly focused on the adult population with some variations having been made to accommodate the paediatric population; however, tools designed for the neonatal population are minimal and none focus on the Australian healthcare context8.
Since the prevention and management of pressure injuries is one of the 10 Australian Commission on Safety and Quality in Health Care (ACSQHC) Standards, the profile of skin pressure-related injury has taken a higher profile throughout health services, notably so within the NICUs8. Thus, in accordance with ACSQHC recommendations: 1) the use of a risk assessment framework to investigate and reduce the frequency and severity of a variety of skin injuries and 2) regular skin inspection and best practice guidelines, NICUs across Australia are currently undertaking the process to implement a neonatal skin assessment risk tool7.
In 2009 the Australian Capital Territory (ACT) Quality Health Service Committee recommended the introduction of a skin risk assessment tool sensitive to the needs of the neonatal population5,6. A Skin Care Working Group (SCWG) was formed to explore the current literature to find a tool that would be suitable for the unit. A literature review undertaken highlighted three tools for consideration: the Neonatal Skin Condition Scale, Braden Q Scale (BQS) and Neonatal Skin Risk Assessment Scale as described below:
Neonatal Skin Condition Scale
The Neonatal Skin Condition Scale considers dryness, erythema and skin breakdown on a scale of 1–9; the higher the score the greater the risk of infection6. Validated for reliability on a large scale, the Neonatal Skin Condition Scale may assist in grading skin condition and risk of infection, but does not consider the intrinsic and extrinsic risks known to cause skin injuries in neonates admitted to an NICU6.
Braden Q Scale
The Braden Q Scale (BQS) is a modified version of the adult Braden Scale tested for sensitivity and specificity in paediatric population from three weeks to eight years of age9. Due to the lack of neonatal scales, many NICUs are implementing the BQS. It consists of seven identifiers, with each being graded on a one- to four-point rating scale: mobility, activity, sensory perception, moisture, friction/shear and nutrition and tissue oxygenation/perfusion9. Scores range from 7 to 28 with paediatrics scoring <16 at risk of skin breakdown9.
Neonatal Skin Risk Assessment Scale
The Neonatal Skin Risk Assessment Scale is a revised version of the adult Braden Scale10. It has six identifiers, each ranked on a score of one to four: general physical condition (based on gestational age); mental state (responsiveness); mobility (based on anticipated movements); activity (radiant warmer or a crib); nutrition (IV, bottle or breastfeeding); and moisture10. Scoring each neonate from six (high risk) to 24 (low risk)10. In 2013 the Neonatal Skin Risk Assessment Scale was updated to include parameters that address the added risks (humidification and completely bed bound) associated with caring for extremely premature neonates in an NICU10. This scale had only been trialled on 32 neonates raising questions about the validity10.
After completing an extensive review of the three scales, the SCWG considered implementing the BQS. The SCWG held workshops for nurses at which they were asked to score neonates using the BQS. Nurses attending the workshops reported the BQS was not sensitive to the nuances of the neonatal population in the NICU. Nurses stated the score did not assist them in identifying neonates at high risk or outline strategies to reduce the risk of skin injury. Literature published during the development period of the skin risk assessment and management tool (SRAMT) reported similar findings on the limitations of the BQS’s use in the neonatal population2,11,12. An example of this was the 2015 Delphi study undertaken by Vance et al. that highlighted the need for a risk assessment tool that focuses on the iatrogenic and traumatic skin issues significant to
neonates1. Following the assessment of the three available tools by senior nursing staff, the decision was taken to conduct a study that covered the design and testing of a skin risk assessment tool specifically constructed for neonates admitted to the NICU.
This study aimed to assess the incidence of skin injury in the study NICU, develop a SRAMT and review its impact on the incidence of skin injury post implementation.
Methodology
Study definition
All neonatal skin injuries, whether pressure induced or from iatrogenic causes, will be referred to as a ‘skin injury’ in the study.
Study Design
A prospective descriptive cohort study was undertaken in three phases over a four-year period from 2010–2014.
Phase 1: Skin injury incidence study
This was a single-centre, prospective cohort study done over a six-week period between June and July 2010. On a weekly basis during the six-week study period or when a skin injury occurred, a study data form developed by the SCWG was completed on each neonate by the nurse allocated to their care. Neonates may have been assessed one to six times if they remained inpatients for the duration of the study period. Data collected included: gestational age, birth weight, age at assessment as well as type and causation of injury.
Phase 2: Development of the SRAMT
In 2011–12, during the development of the tool, a literature review was carried out by members of the study team. Searches were undertaken using databases such as MEDLINE, CINHAL, guided by search terms such as: skin tool, pressure injuries, skin integrity, neonates and intensive care. The SRAMT was formulated to assess a neonate’s risk of skin injury via inclusion of eight subscales that considered factors such as: medical devices, intravenous access, blood collection and respiratory equipment based on the result of the literature review and skin injury incidence study results.
During the development of the tool, the SCWG held consultative meetings with senior staff during which mock scenarios of neonates of acuity ranging from intensive care to special care, with different types of technical support and clinical requirements were set up. Senior nursing staff scored each neonate then graded the neonates as low, moderate, high or extreme risk. Several rounds of grading were held until a group consensus was gained, that neonates’ scores reflected their risk of skin injury. It was also suggested a table of preventative and treatment strategies should be attached to the tool to standardise skin care in the NICU.
Phase 3: Post implementation review of the SRAMT
Clinical notes of all neonates (n=30) discharged during one month period in 2013 were reviewed for the completion of the scale, skin injuries, preventative measures or treatment of skin injuries recorded and documented as per guidelines. Using a data collection sheet created by the SCWG, two members of the SCWG independently reviewed each file and conferred on their results to double-check the data. Data collected included: skin injuries, preventative measures, treatment of skin injuries recorded and number of times scale was completion during admission. Data was manually entered into an Excel spreadsheet and exported to SPSS for analysis (Figure 1).
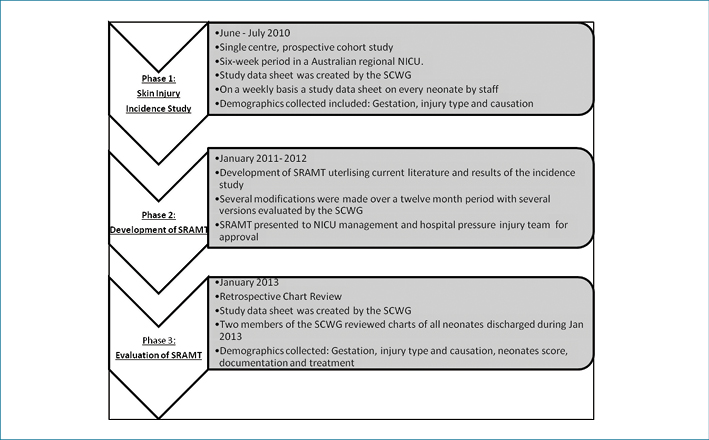
Figure 1: Outline of study methodology
Setting
This study was undertaken in a University-affiliated tertiary NICU in regional Australia. The catchment area of the NICU is approximately 6840 km2, encompassing the ACT as well as areas of the adjacent jurisdiction that borders the ACT. The NICU provides intensive and special care for 700 neonates per annum, born between 24 and 44 weeks’ gestation.
Participants
All neonates admitted to the NICU during both study periods were included. As skin assessment is part of normal care provided, no neonates needed to be excluded from the study. Parental consent was not required.
Data collection and analysis
Data collected throughout the study was collated and checked by the SCWG. It was manually transcribed to Excel and to SPSS for analysis. Descriptive analysis was undertaken to assess types and causation of injuries. Chi-squared testing was completed to evaluate statistical significance (p= 0.05).
Ethics approval
The project received Australian Capital Territory Human Research Ethics Committee approval.
Results
Phase 1: Skin injuries incidence study
Sixty neonates (25 to 41 weeks gestation at birth, median 35 weeks) were assessed in the six-week study period. Results showed 37 infants were identified as having one or more skin injuries 61.7% (95% CI: 49.0–72.9). Some babies acquired >1 injury during the study period, with 54 injuries in total (Table 1). Two main causal groups were identified: 1) medical devices: intravenous, blood collection and CPAP equipment (46%); and 2) factors related to routine skin care: dry skin, excoriated buttocks, positioning of the neonate, accounting for 39% of skin injuries.
Table1: Comparison of pre- and post-study groups
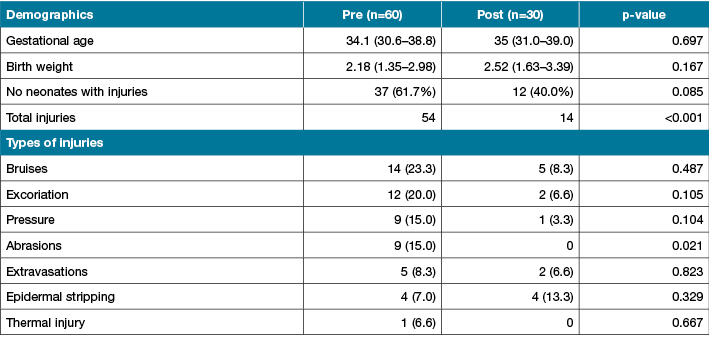
NB: Some babies acquired >1 injury during the study period
Phase 2: Development of the SRAMT
The study team developed the SRAMT. The tool is divided into three sections. The first section is a skin risk assessment score which staff use to predict the neonates’ risk of skin injury. In the second section, skin assessment guidelines provide staff with a care plan dependent on the neonates’ level of risk. The third section outlines strategies to prevent and manage skin injuries, as outlined below:
Section 1: Skin risk assessment score
In the first section, a staff member scores the neonates’ skin injury risk using eight risk categories: 1) gestational age; 2) sensory perception; 3) activity/mobility; 4) moisture; 5) respiratory support; 6) visual examination; 7) blood collection; and 8) nutrition. Each risk category is graded from 1 to 4 with a description to assist staff in scoring (1 being the lowest and 4 being the highest score) (Table 2).
Table 2: Skin risk assessment score
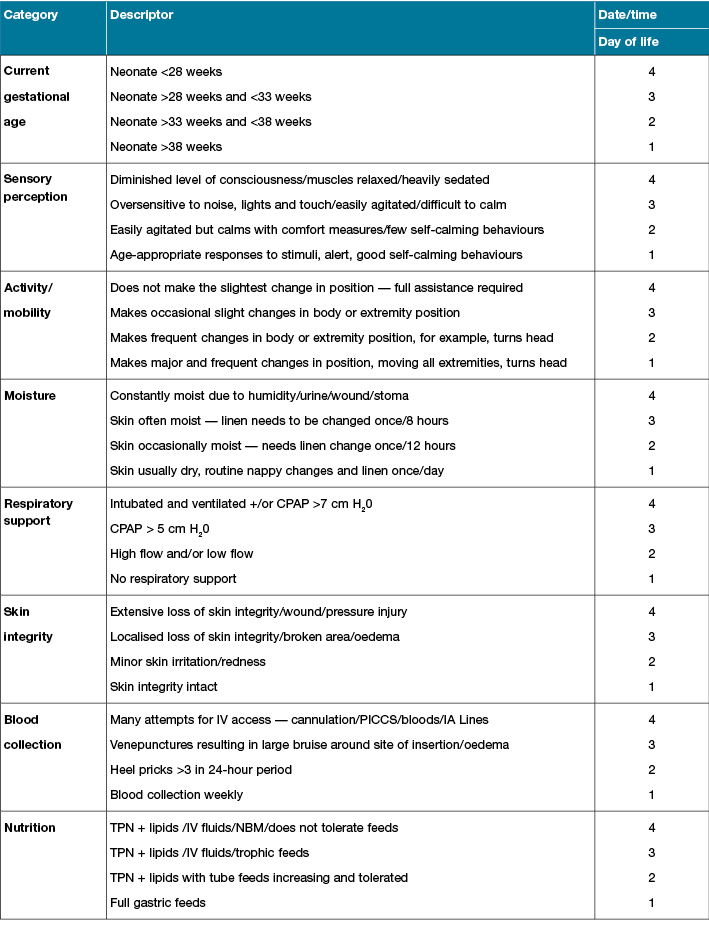
Section 2: Skin assessment guidelines
The assessment guidelines in Section 2 aim to standardise the frequency of care and documentation in the NICU. It provides staff with a care plan and the documentation requirements according to the neonates’ level of risk (Table 3).
Table 3: Skin assessment guidelines
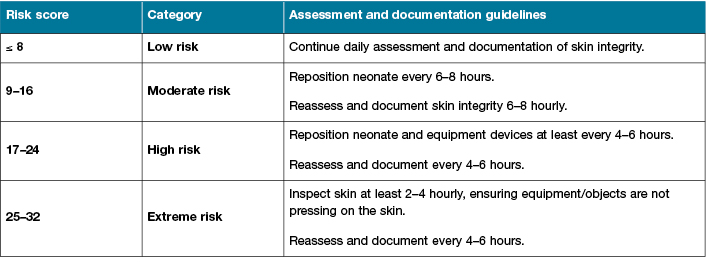
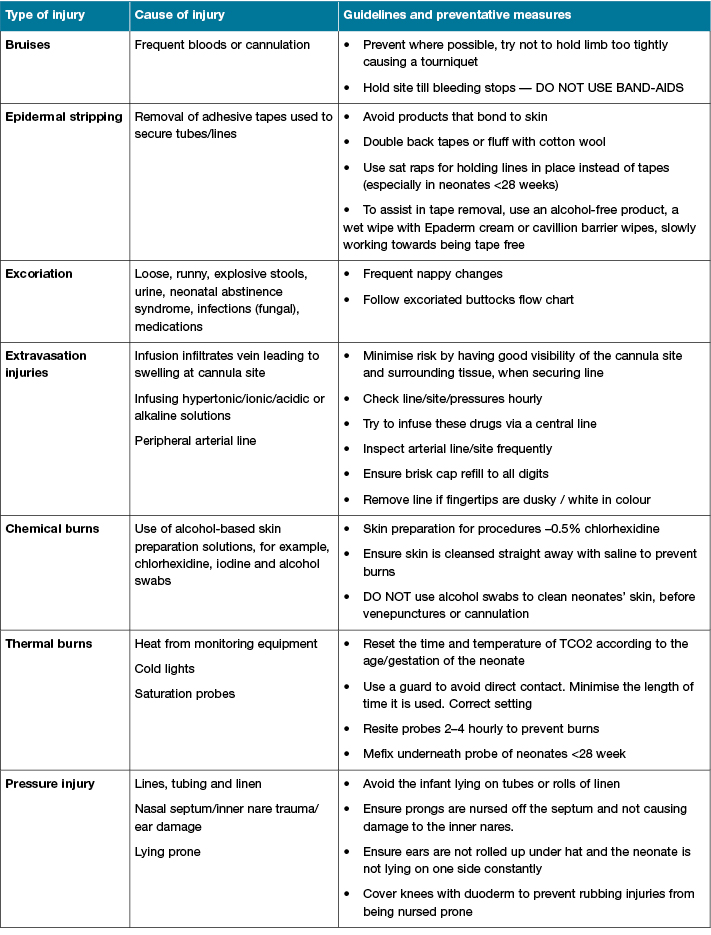
Section 3: Skin management guidelines
Section 3 lists skin injury types, causes and preventative measures to guide skin management. Table 4 provides staff with strategies to reduce the risk of or treat skin injuries. The table was constructed by a member of the SCWG utilising current skin management guidelines. Prior to submission, an update on the literature available revealed a recently published article by Vance et al. also included a table with many similarities to the table in our tool1.
Table 4: Skin management guidelines
Phase 3: Post-implementation review of the SRAMT
Clinical notes of all neonates (n=30) discharged during a one-month period in 2013 were reviewed by two members of the SCWG, each member independently reviewed each file and conferred on their results to double-check the data. Data collected included: completion of the scale, skin injuries, preventative measures or treatment of skin injuries recorded and documented as per guidelines. The evaluation of the SRAMT showed a reduction in neonates who acquired skin injuries from 37/60 (61.7%) neonates to 12/30 (40%) (Table 1).
In 2014, post evaluation, the SCWG made several adjustments to refine the tool to meet the needs of the neonatal population cared for in the NICU. Our review also identified the need for several format modifications to make the tool easier to complete and clarify assessment guidelines for staff. The revised SRAMT was reviewed by NICU staff and management. The SRAMT was accepted by the hospital forms committee and is now part of the daily care plan for all neonates admitted to the NICU.
Discussion
We have undertaken a prospective descriptive cohort study to develop an SRAMT for neonates. The study has described three phases: assessing the need for a skin risk assessment tool in the NICU, developing a skin risk and management tool, and then a chart review to assess if implementing the tool has reduced injury frequency. The results of this study also provide further evidence that skin injuries are common in neonates and the main cause is medical devices4. Results have also identified the need for a tool that considered not only factors directly related to pressure injuries but includes items to address iatrogenic risks babies are exposed to during an admission to an NICU1,4. Previously it has been suggested (due to there being no well-validated risk assessment scales for the neonatal population) that clinical judgement may be the most efficient method of identifying injury risk rather than a tool. This study has shown implementing a skin risk assessment tool may assist in reducing skin injuries in the NICU1,3,9.
Study results have shown the importance of collecting evidence to assess the need for implementing a new tool or practice in an NICU. Results also highlighted the importance of nurses being encouraged to undertake research to improve clinical care and provide evidence for change14. This study is an example of improving care based on evidence through nurses participating in research.
Just as the acuity and needs of the neonates requiring care in an NICU has dynamically changed over the past 20 years, so has the causation and management of neonatal skin injuries. There continue to be gaps in the research undertaken relating to prevention and treatment of neonatal skin injuries4. Previously neonatology has focused on life-saving technologies and treatment for neonates but now we are transitioning to the next phase in neonatal care. A challenge for nurses caring for neonates is reducing the risk of iatrogenic injuries, predominately caused by the equipment or treatment required to support a neonate admitted to an NICU15,16.
This study has demonstrated the usefulness of the SRAMT in predicting a neonate’s category of risk, which prompts the development of the nursing care plan to reduce skin injuries and promote appropriate skin care.
Limitations
This was a pragmatic study that grew from a clinical issue in one NICU and we acknowledge it is just the first step in the development of the SRAMT. The small sample size and the need for further statistical evidence may limit transferability of the tool to other NICUs or neonatal populations. We encourage other researchers to test the SRAMT in their units and report the if changes they find are necessary to meet the needs of their NICU’s population.
Future Research
The next stage of our study is to undertake powered methodology such as logistic regression and predictive validity testing to assess the capability of the tool in predicting skin injury. Currently the cut-off points and scores have been developed by a group of senior nurses; however, future work to provide high-powered statistical evidence is core to validating the tool as suitable for use across neonatal populations and different NICUs.
Conclusion
There continues to be a gap in the research undertaken relating to the prevention and treatment of neonatal skin injuries; more specifically, a tool to predict neonates’ risk of skin injuries. We have shown how implementing the SRAMT has reduced the number and severity of skin injuries in the study NICU. Further research is required to validate the SRAMT and investigate its benefit in other NICU populations.
Acknowledgements
We would like to thank to the nurses who have assisted with data collection throughout the study and Janine McEwan who assisted with the review of the SRAMT. This study was assisted through funding from a Nurse Practice Development Grant from the Nursing and Midwifery Office of the Chief Nurse ACT Health.
Conflict of interest
Nothing to declare.
Author(s)
Margaret M Broom*
PhD
Department of Neonatology, Centenary Hospital for Women and Children, Canberra Hospital
ACT, Australia
Australian Catholic University, Australia
Department of Neonatology
Centenary Hospital for Women and Children
PO Box 11, Woden, ACT 2606, Australia
Tel +61 2 6174 7565
Email Margaret.Broom@act.gov.au
Wendy A Burton
BA Nursing
Department of Neonatology, Centenary Hospital for Women and Children, Canberra Hospital
ACT, Australia
Leanne M Ehrlich
BA Nursing
Department of Neonatology, Centenary Hospital for Women and Children, Canberra Hospital
ACT, Australia
Ann Marie Dunk
RN, BHlthSc (Nurs), MNurs (Research)
Tissue Viability Unit, Canberra Hospital
Adjunct Associate Professor, University of Canberra
ACT, Australia
Mohamed E Abdel-Latif
FRACP, MRCPCH, AFRACMA, MPaeds, MPH, MScEpi, MD
Department of Neonatology, Centenary Hospital for Women and Children, Canberra Hospital
ACT, Australia
Medical School, College of Medicine, Biology & Environment, Australian National University, Acton, ACT, Australia
* Corresponding author
References
- Vance DA, Demel S, Kirksey K, Moynihan M, Hollis K. A Delphi study for the development of an infant skin breakdown risk assessment tool. Adv Neonatal Care 2015;15(2):150–157.
- Kottner J, Hauss A, Schlüer AB, Dassen T. Validation and clinical impact of paediatric pressure ulcer risk assessment scales: A systematic review. Int J Nurs Stud 2013;50:807–818.
- Baharestani MM, Ratcliff CR. Pressure ulcers in neonates and children. Adv Skin Wound Care 2007;20(4):208–220.
- August DL, Edmonds L, Brown DK, Murphy M, Kandasamy Y. Pressure injuries to the skin in a neonatal unit: Fact or fiction. J Neonatal Nurs 2014;20:129–137.
- Huffines B, Logsdon MC. The Neonatal Skin Risk Assessment Scale for predicting skin breakdown in neonates. Issues Compr Pediatr Nurs 1997;20(2):103–114.
- Lund CH, Osborne JW. Validity and reliability of the neonatal skin condition score. J Obstet Gynecol Neonatal Nurs 2004;3:320–327.
- Australian Commission on Safety and Quality in Health Care, National Safety and Quality Health Service Standards (September 2012). Sydney: ACSQHC; 2012.
- National Pressure Ulcer Advisory panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Perth, Australia: Cambridge Media; 2014.
- Curley MAQ, Razmus IS, Roberts KE, Wypij D. Predicting pressure ulcer risk in pediatric patients: The Braden Q Scale. Nurs Res 2003;52:22–33.
- Dolack M, Huffines B, Stikes R. Updated Neonatal Skin Risk Assessment Scale. Kentucky Nurse 2013;61(4):6–8.
- Grosvenor J, O’Hara M, Dowling M. Skin injury prevention in an Irish neonatal unit: An action research study. J Neonatal Nurs 2016;22(4):185–195. DOI: 10.1016/j.jnn.2016.01.004.
- Lu Y-F, Yang Y, Wang Y, Gao L-Q, Qiu Q, Li C, Jin J. Predicting pressure ulcer risk with the Braden Q Scale in Chinese pediatric patients in ICU. Chinese Nursing Research 2015;2(1):1–5. DOI: http://dx.doi.org/10.1016/j.cnre.2015.01.002.
- Scheans P. Neonatal pressure ulcer prevention. Neonatal Network 2015;34:126–132.
- Mulhall A. Nursing, research, and the evidence. Evid Based Nurs 1998;1:4–6.
- Nist MD, Rodgers EA, Ruth BM et al. Skin rounds: a quality improvement approach to enhance skin care in the neonatal intensive care unit. Adv Neonatal Care 2016;16(5):S33–S41.
- Johnson DE. Extremely preterm infant skin care: a transformation of practice aimed to prevent harm. Adv Neonatal Care 2016;16(5):S26–S32.



